Conflict of interest
Dr. Ahmet Kağan Özdemir does not report any conflict of interest. Dr. Sera Kayhan does not report any conflict of interest. Dr. Seray Külcü Çakmak does not report any conflict of interest.
Dear editor,
Coronavirus disease 2019 (COVID‐19) can cause various cutaneous manifestations that were identified since the outbreak. 1 With the era of vaccination, cutaneous side effects of vaccines will likely be frequently encountered. Mild reactions like localized redness and swelling have been detected in vaccines reaching phase 3 trials; besides, rarer adverse effects such as injection site urticaria, maculopapular rash, reaction to dermal fillers due to COVID‐19 vaccines have been reported. 2 COVID‐19 vaccine‐associated herpes zoster (HZ) has been described for the two most used vaccine types recently: Bostan and Yalici‐Armagan reported a 78‐year‐old man with thoracic HZ after administration of an inactivated vaccine, and Tessas and Kluger reported a 44‐year‐old man with cervical HZ after administration of a mRNA COVID‐19 vaccine. 3 , 4 In this case report, we present two immunocompetent young adults developing herpes zoster after inactivated severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) vaccination.
First case was a 23‐year‐old otherwise healthy woman who attended to our dermatology outpatient clinic with itchy and painful rash on her lower back. Her symptoms first appeared a day after she was vaccinated with inactivated SARS‐CoV‐2 vaccine. On examination, grouped vesicles on an erythematous base were noted. Lesions were confined in a single unilateral dermatome. She reported pain and itch which had started on the same day with rash. She was diagnosed with HZ and commenced on brivudine 125 mg tablet once daily for 7 days. Second case was a 21‐year‐old otherwise healthy man who presented with painful blisters at his abdomen (Figure 1). He was vaccinated with inactivated SARS‐CoV‐2 vaccine two days before his symptoms first appeared. On examination, painful eruption of grouped vesicles on an erythematous base in a single unilateral dermatome was observed. He was diagnosed with HZ, and valacyclovir 1 gr three times daily for seven days and analgesics were prescribed. Both patients stated that they had never experienced COVID‐19‐related symptoms since the beginning of pandemic, and they were well at the time of admission. After 14 days, both patients’ lesions disappeared completely; moreover, they did not experience neither COVID‐19‐related symptoms nor postherpetic neuralgia during that period.
Figure 1.
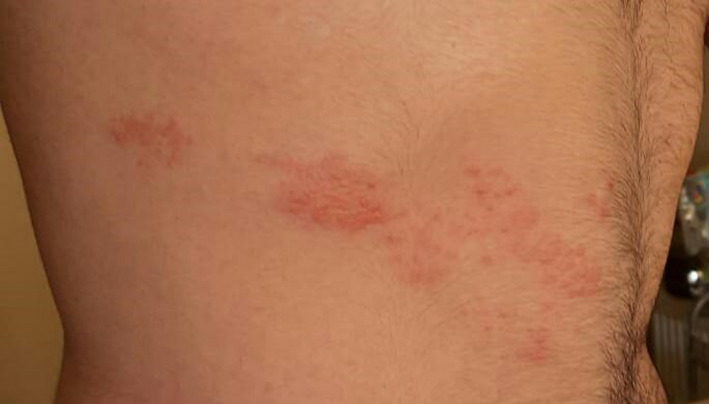
Painful vesicles on an erythematous base in a dermatomal configuration on abdomen.
HZ is caused by reactivation of latent varicella zoster virus (VZV) in the sensory dorsal root ganglion. Age, stress, immunocompromised status and immunosuppressive drugs are risk factors for virus reactivation, mostly because these conditions lead to a reduction in VZV‐specific T cells. 5 COVID‐19‐associated HZ has been reported previously, and the median time from hospitalization to HZ beginning was found as 5,5 days in series mainly involving older adults. 6 The mechanism of SARS‐CoV‐2 induced HZ is yet to be discovered, but virus’s ability to deplete CD8+ T cells may reduce VZV‐specific T cells throughout infection. 6
This case report describes highly possible inactivated SARS‐CoV‐2 vaccine‐related HZ in two healthy young adults. As HZ is rare in young and otherwise healthy adults, vaccine itself could be the trigger, but it is impossible to rule out reactivation of VZV by chance. Vaccines may cause immunomodulation and lead to herpes virus reactivation. 7 Recently a case report of disseminated HZ after recombinant zoster vaccine was published, in which authors hypothesized that the immunostimulatory adjuvant in the vaccine could act as a physiologic stressor on the patient’s immune system, as a consequence patient’s cell‐mediated immunity towards VZV fell below the threshold of containment and permitted zoster reactivation. 8 Short delay after vaccination, young age and previous reports 3 , 4 are arguments that support our cases’ link between HZ and vaccination. COVID‐19 vaccine‐associated HZ was not reported in published vaccine studies, 9 , 10 and case reports may help to define this possible side effect.
Funding source
None.
Acknowledgements
The patients in this manuscript have given written informed consent to publication of their case details.
References
- 1. Genovese G, Moltrasio C, Berti E, Marzano AV. Skin manifestations associated with COVID‐19: current knowledge and future perspectives. Dermatology 2021; 237: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rice SM, Ferree SD, Atanaskova Mesinkovska N, Shadi KA. The art of prevention: COVID‐19 vaccine preparedness for the dermatologist. Int J Womens Dermatol 2021; 7: 209–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bostan E, Yalici‐Armagan B. Herpes zoster following inactivated COVID‐19 vaccine: a coexistence or coincidence? J Cosmet Dermatol 2021; 20: 1566–1567. [DOI] [PubMed] [Google Scholar]
- 4. Tessas I, Kluger N. Ipsilateral Herpes Zoster after the first dose of BNT162b2 mRNA COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e620–e622. 10.1111/jdv.17422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koshy E, Mengting L, Kumar H, Jianbo W. Epidemiology, treatment and prevention of herpes zoster: a comprehensive review. Indian J Dermatol Venereol Leprol 2018; 84: 251–262. [DOI] [PubMed] [Google Scholar]
- 6. Tartari F, Spadotto A, Zengarini C et al. Herpes zoster in COVID‐19‐positive patients. Int J Dermatol 2020; 59: 1028–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walter R, Hartmann K, Fleisch F, Reinhart WH, Kuhn M. Reactivation of herpesvirus infections after vaccinations? Lancet 1999; 353: 810. [DOI] [PubMed] [Google Scholar]
- 8. Housel LA, McClenathan BM. Herpes zoster after recombinant zoster vaccine: A first case report. J Allergy Clin Immunol Pract 2020; 8: 772–774.e1. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Zeng G, Pan H et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18–59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis 2020; 21: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]


