CONFLICT OF INTEREST
No conflict of interest exists.
AUTHOR CONTRIBUTIONS
Enji Ahmed: Conceptualization; Supervision; Writing‐original draft; Writing‐review & editing. Asmaa Abou‐Bakr: Resources; Writing‐original draft. Radwa R. Hussein: Data curation; Methodology. Ayman A. El‐Gawish: Writing‐original draft. Abou‐bakr E. Ras: Investigation; Supervision. Dalia M. Ghalwash: Supervision; Visualization; Writing‐review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/odi.13973.
The current COVID‐19 pandemic has thrown up yet an extra challenge for health care, with some cases of an uncommon fungal infection being described, which are associated with increased death rate. COVID‐19 patients are predisposed to developing fungal infections as mucormycosis, which has been described from those still battling, and those who recently convalesced, especially medically compromised patients. This could be attributed to the reduction in CD4+ T cells and CD8+ T cells causing immune suppression. The illness usually progresses rapidly in debilitated or immunocompromised patients. Recent rise in the incidence of rhinomaxillary mucormycosis has been noticed and has become an emergency presume (Krishna et al., 2021).
The rise of mucormycosis in COVID‐19 patients is predisposed by trauma, poorly controlled diabetes, the unnecessary use of corticosteroids, prolonged neutropenia, hemopoietic malignancies, hematopoietic stem cell transplant, organ transplant, and prolonged stays in the intensive care unit (Raut & Huy, 2021).
Oral lesions seen in COVID‐19 patients are more probably caused by co‐infections, adverse reactions, and immunity impairment instead of direct COVID‐19 infection. Not every disease that occurs in the pandemic times is due to COVID‐19 (Anaya‐Saavedra, 2021; Rawson et al., 2020).
In a recent systematic review, it was stated that 8% of patients suffered from fungal or bacterial co‐infection through hospitalization. Co‐infections mostly occur when the patients themselves are already loaded by the infective organism before the viral infection, when patients suffer from an underlying persistent infection, or when patients are hospitalized (Rawson et al., 2020).
We would like to report 21 post‐COVID‐19 patients (14 days after recovery) who had oral mucormycosis (11 [52.4%] males and 10 [47.6%] females) with mean age (58 ± 12) years with no significant difference.
Figure 1 is showing the descriptive data of palatal lesions among the post‐COVID‐19 patients with oral mucormycosis.
FIGURE 1.
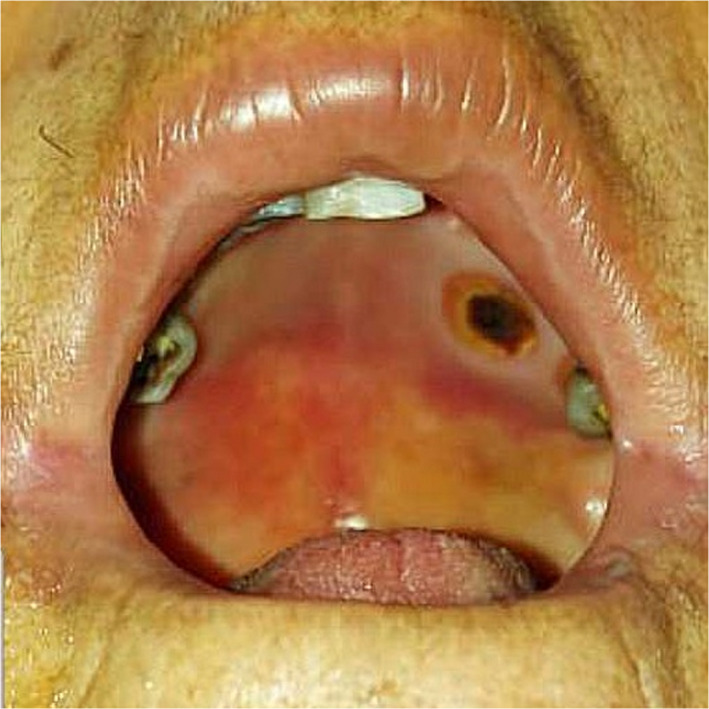
The left side of the hard palate showing deep necrotic ulcer
Oral manifestations of mucormycosis in COVID‐19 patients are usually seen in the palate and may include varying degrees of mucosal discoloration, swelling, ulcerations, superficial necrotic areas in the palate (Figure 1), bone exposure and necrosis with dark eschar formation (Brandao et al., 2020). Hence, palatal ulcerations could be the first presenting symptom, leading the patient to the dentist, who can be the first clinician to suspect an infection leading to the diagnosis of mucormycosis (Brandao et al., 2020; Amorim dos Santos et al., 2021).
Therefore, a non‐specific palatal ulcer could be considered as the presenting sign of mucormycosis, and it is necessary for a dental practitioner to be alert to initial signs and symptoms of this disease, specifically when evaluating the high‐risk patients. Early diagnosis of mucormycosis is critical, as treatment should start as soon as possible in an attempt to decrease mortality (Sanath et al., 2020).
The role of dentists is critical because mucormycosis primarily occurs around rhinomaxillary or rhinocerebral areas involving facial tissues, palate, alveolar bone, and mandibular bone. Therefore, dental professionals should be mindful of symptoms of mucormycosis. In addition to palatal lesions, atypical symptoms such as sinus pain, facial pain, unanticipated odontalgia of otherwise sound teeth, or patient deterioration after dental therapeutic interventions should alert clinicians to seek confirmation of the diagnosis and promptly start optimal treatment (Bains & Hosseini‐Ardehali, 2005).
Finally, it is noteworthy that although the recent rise of mucormycosis occurring concurrently with COVID‐19, it is not conceivable to state that there is a causal association between the two conditions, but this is rather due to the presence of many factors and conditions related to the pandemic setting that predispose to mucormycosis, thus increasing the susceptibility of COVID‐19 patients to co‐infections.
ACKNOWLEDGMENTS
We would like to acknowledge the contributions of the oral maxillo‐facial and ENT specialties for helping us to reach the final diagnosis.
REFERENCES
- Amorim dos Santos, J. , Normando, A. G. C. , Carvalho da Silva, R. L. , Acevedo, A. C. , De Luca Canto, G. , Sugaya, N. , . . . Guerra, E. N. S. (2021). Oral manifestations in patients with COVID‐19: a living systematic review. Journal of Dental Research, 100, 141–154. 10.1177/0022034520957289 [DOI] [PubMed] [Google Scholar]
- Anaya‐Saavedra, G. (2021). Oral manifestations accompanying and related to COVID‐19: Overlooking the obvious. Oral Diseases. 10.1111/odi.13857. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains, M. K. , & Hosseini‐Ardehali, M. (2005). Palatal perforations: Past and present. Two case reports and a literature review. British Dental Journal, 199(5), 267–269. 10.1038/sj.bdj.4812650 [DOI] [PubMed] [Google Scholar]
- Brandao, T. B. , Gueiros, L. A. , Melo, T. S. , Prado‐Ribeiro, A. C. , Alo Nesrallah, A. C. F. , Prado, G. V. B. , . . . Migliorati, C. A. (2020). Oral lesions in patients with SARS‐CoV‐2 infection: could the oral cavity be a target organ?. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics, 131, e45–e51. 10.1016/j.oooo.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna, S. D. , Raj, H. , Kurup, P. , & Juneja, M. (2021). Maxillofacial infections in Covid‐19 era‐actuality or the unforeseen: 2 Case reports. Indian Journal of Otolaryngology and Head & Neck Surgery, 17, 1–4. 10.1007/s12070-021-02618-5. Epub ahead of print. PMID: 34026593; PMCID: PMC8127475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut, A. , & Huy, N. T. (2021). Rising incidence of mucormycosis in patients with COVID‐19: Another challenge for India amidst the second wave? The Lancet Respiratory Medicine. 10.1016/S2213-2600(21)00265-4. Epub ahead of print. PMID: 34090607; PMCID: PMC8175046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson, T. M. , Moore, L. S. P. , Zhu, N. , Ranganathan, N. , Skolimowska, K. , Gilchrist, M. , Satta, G. , Cooke, G. , & Holmes, A. (2020). Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID‐19 antimicrobial prescribing. Clinical Infectios Diseases, 71, 2459–2468. 10.1093/cid/ciaa530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanath, A. K. , Nayak, M. T. , Sunitha, J. D. , Malik, S. D. , & Aithal, S. (2020). Mucormycosis occurring in an immunocompetent patient: A case report and review of literature. Ceskoslovenska Patologie, 56(4), 223–226. 10.4317/jced.53655 [DOI] [PubMed] [Google Scholar]


