Conflict of interest
Nothing to disclose: Dr Yates, Dr Dand, Prof. Langan, Dr. Norton, Dr. Tsakok, Dr. Yiu, Dr De La Cruz, Dr. Contreras, Ms. Vesty, Ms. Vincent, Mr. Bola Coker, Ms. Meynell, Dr. Lambert, Prof. Brown, Prof. Naldi. Prof. Barker reports grants and personal fees from Abbvie, grants and personal fees from Novartis, grants and personal fees from Lilly, grants and personal fees from J&J, from null, during the conduct of the study. Prof. Griffiths reports grants and personal fees from AbbVie, grants from Amgen, grants from BMS, grants and personal fees from Janssen, grants from LEO, grants and personal fees from Novartis, grants from Pfizer, grants from Almirall, grants and personal fees from Lilly, grants and personal fees from UCB Pharma, outside the submitted work. Prof. Jullien reports personal fees and non‐financial support from Abbvie, personal fees and non‐financial support from Novartis, personal fees and non‐financial support from Janssen‐Cilag, personal fees and non‐financial support from Lilly, personal fees and non‐financial support from Leo‐Pharma, personal fees and non‐financial support from MEDAC, personal fees and non‐financial support from Celgene, personal fees from Amgen, outside the submitted work. Dr. Capon reports consultancy fees from AnaptysBio, grants from Boheringer‐Ingelheim, outside the submitted work. Prof. Bachelez reports personal fees from Abbvie, personal fees from Janssen, personal fees from LEO Pharma, personal fees from Novartis, personal fees from UCB, personal fees from Almirall, personal fees from Biocad, personal fees from Boehringer‐Ingelheim, personal fees from Kyowa Kirin, personal fees from Pfizer, outside the submitted work. Prof. Gisondi reports personal fees from Abbvie, Amgen, Eli Lilly, Janssen, Novartis, Pierre Fabre, Sandoz, UCB, outside the submitted work. Dr. Galloway reports personal fees from Abbvie, personal fees from Sanofi, personal fees from Novartis, personal fees from Pfizer, grants from Eli Lilly, personal fees from Janssen, personal fees from UCB, outside the submitted work. Prof. Weinmann has presented talks for Abbvie, Abbott, Bayer, Chiesi, Boehringer Ingelheim, Roche and Merck. Dr. Mason reports personal fees from LEO Pharma and Novartis, outside the submitted work. Ms. Moorhead reports personal fees from Abbvie, personal fees from Celgene, personal fees from Janssen, personal fees from LEO Pharma, personal fees from Novartis, personal fees from UCB, outside the submitted work. Dr. Puig reports grants and personal fees from AbbVie, grants and personal fees from Almirall, grants and personal fees from Amgen, grants and personal fees from Boehringer Ingelheim, personal fees from Bristol Myers Squibb, personal fees from Fresenius‐Kabi, grants and personal fees from Janssen, grants and personal fees from Lilly, personal fees from Mylan, grants and personal fees from Novartis, personal fees from Pfizer, personal fees from Sandoz, personal fees from Sanofi, personal fees from Samsung‐Bioepis, grants and personal fees from UCB, outside the submitted work. Dr. Mahil reports departmental income from Abbvie, Celgene, Eli Lilly, Janssen‐Cilag, Novartis, Sanofi, UCB, outside the submitted work. Dr. Di Meglio reports grants and personal fees from UCB, personal fees from Novartis, personal fees from Janssen, outside the submitted work. Prof. Warren reports grants and personal fees from Abbvie, grants and personal fees from Celgene, grants and personal fees from Eli Lilly, grants and personal fees from Novartis, personal fees from Sanofi, grants and personal fees from UCB|, grants and personal fees from Almirall, grants and personal fees from Amgen, grants and personal fees from Janssen, grants and personal fees from Leo, grants and personal fees from Pfizer, personal fees from Arena, personal fees from Avillion, personal fees from Bristol Myers Squibb, personal fees from Boehringer Ingelheim, outside the submitted work. Prof. Smith reports grants from Abbvie, Sanofi, Novartis, and Pfizer and through consortia with multiple academic partners (psort.org.uk, BIOMAP‐IMI.eu), outside the submitted work. Dr. Torres reports grants and personal fees from AbbVie, Almirall, Amgen, Arena Pharmaceuticals, Biogen, Biocad, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Eli Lilly, Janssen, LEO Pharma, MSD, Novartis, Pfizer, Samsung‐Bioepis, Sandoz, during the conduct of the study. Dr. Waweru is on the Board of the International Federation of Psoriasis Associations who have received grants from Abbvie, Almirall, Amgen, Bristol Meyers Squibb, Boehringer Ingelheim, Celgene, Janssen, Leo Pharma, Eli Lilly, Novartis, Sun Pharma, Pfizer, and UCB, outside the submitted work. Mr. Urmston reports grants from Almirall, grants from Abbvie, grants from Amgen, grants from Celgene, grants from Dermal Laboratories, grants from Eli Lilly, grants from Janssen, grants from LEO Pharma, grants from T and R Derma, grants from UCB, outside the submitted work. Ms. McAteer reports grants from Abbvie, grants from Almirall, grants from Amgen, grants from Celgene, grants from Dermal Laboratories, grants from Eli Lilly, grants from Janssen, grants from LEO Pharma, grants from UCB, grants from T and R Derma, outside the submitted work.
Funding sources
We acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London, the NIHR Manchester Biomedical Research Centre and the Psoriasis Association. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. SKM is funded by a Medical Research Council (MRC) Clinical Academic Research Partnership award (MR/T02383X/1). ND is funded by Health Data Research UK (MR/S003126/1), which is funded by the UK MRC, Engineering and Physical Sciences Research Council; Economic and Social Research Council; Department of Health & Social Care (England); Chief Scientist Office of the Scottish Government Health and Social Care Directorates; Health and Social Care Research and Development Division (Welsh Government); Public Health Agency (Northern Ireland); British Heart Foundation; and Wellcome Trust. ZZNY is funded by an NIHR Academic Clinical Lectureship through the University of Manchester. CEMG is an NIHR Emeritus Senior Investigator and is funded in part by the MRC (MR/101 1808/1). CEMG and RBW are in part supported by the NIHR Manchester Biomedical Research Centre. SML is supported by a Wellcome senior research fellowship in clinical science (205039/Z/16/Z); this research was funded in whole or in part by the Wellcome Trust [205039/Z/16/Z]. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript (AAM) version arising from this submission. SML is also supported by Health Data Research UK (grant no. LOND1), which is funded by the UK MRC, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation and Wellcome Trust.
Editor,
Indirect excess morbidity in the COVID‐19 pandemic may arise from public health risk‐mitigation efforts such as stay‐at‐home orders and re‐purposing of healthcare services.1 Increased mental health disorders and shortfalls in the care of long‐term conditions are described.2, 3 We used global self‐reported cross‐sectional data to characterise the factors associated with worsening psoriasis in the pandemic, focussing on the impact of anxiety and depression.
Data from a cross‐sectional survey (PsoProtectMe 4) were extracted on 15th January 2021. After excluding participants self‐reporting COVID‐19, the association between mental health and worsening psoriasis was assessed using a fully adjusted logistic regression model including covariates selected a priori as potentially influential on psoriasis severity and anxiety/depression. Participants scoring ≥3 in GAD‐2 or PHQ‐2 defined a positive mental health screen.5
4043 people with psoriasis from 86 countries were included (Table 1). 1728 (42.7%) reported worsening psoriasis in the pandemic. A total of 3575 (88.4%) returned information on their mental health, with a greater proportion of those reporting worsening psoriasis having a positive mental health screen (814/1621, 50.2%) compared to those without worsening psoriasis (562/1954, 28.8%). A greater proportion of females reported worsening psoriasis (1322/2684, 49.3%) compared with males (406/1354, 30.0%).
Table 1.
Patient demographics and clinical characteristics stratified by disease state
| Total | Missing data | Non‐worsening disease | Worsening disease | P‐value | |
|---|---|---|---|---|---|
| N = 4,043 | N = 2,315 | N = 1,728 | |||
| Shielded | 2,224 (55.1%) | 9 (0.2%) | 1,240 (53.8%) | 984 (56.9%) | 0.045 |
| Advised to shield | 742 (33.6%) | 1,833 (45.3%) | 465 (37.5%) | 277 (28.6%) | <0.001 |
| Female gender | 2,684 (66.5%) | 5 (0.1%) | 1,362 (59.0%) | 1,322 (76.5%) | <0.001 |
| Age, mean (SD) | 47.2 (15.1) | 31 (0.8%) | 49.5 (15.3) | 44.2 (14.3) | <0.001 |
| White European ethnicity | 3,016 (74.6%) | 0 | 1,707 (73.7%) | 1,309 (75.8%) | 0.15 |
| BMI, mean (SD) | 27.6 (6.0) | 369 (9.1%) | 27.4 (5.8) | 28.0 (6.3) | 0.003 |
| Alcohol >14 units a week | 495 (13.8%) | 455 (11.3%) | 295 (15.0%) | 200 (12.3%) | 0.018 |
| Current smoker | 559 (15.8%) | 498 (12.3%) | 291 (15.0%) | 268 (16.7%) | 0.18 |
| Full time employed | 1,929 (47.7%) | 0 | 1,072 (46.3%) | 857 (49.6%) | 0.038 |
| Household number, mean (SD) | 2.8 (1.8) | 26 (0.6%) | 2.8 (1.7) | 2.9 (1.9) | 0.003 |
| Key worker | 1,131 (28.1%) | 22 (0.5%) | 595 (25.9%) | 536 (31.1%) | <0.001 |
| Psoriasis severity prior to COVID‐19 pandemic | 284 (7%) | <0.001 | |||
| Clear | 451 (12.0%) | 299 (14.6%) | 152 (8.9%) | ||
| Nearly clear | 767 (20.4%) | 463 (22.7%) | 304 (17.7%) | ||
| Mild | 989 (26.3%) | 477 (23.3%) | 512 (29.9%) | ||
| Moderate | 892 (23.7%) | 442 (21.6%) | 450 (26.2%) | ||
| Moderate‐severe | 480 (12.8%) | 273 (13.4%) | 207 (12.1%) | ||
| Severe | 180 (4.8%) | 90 (4.4%) | 90 (5.2%) | ||
| Systemic therapy | 522 (12.9%) | <0.001 | |||
| No systemic therapy | 1,980 (56.2%) | 938 (49.4%) | 1,042 (64.2%) | ||
| Standard systemic therapy | 560 (15.9%) | 309 (16.3%) | 251 (15.5%) | ||
| Targeted therapy | 981 (27.9%) | 652 (34.3%) | 329 (20.3%) | ||
| Non adherent to systemic therapy | 284 (18.4%) | 2507 (62%) | 114 (11.9%) | 170 (29.6%) | <0.001 |
| 1 or more comorbidity | 1,606 (39.7%) | 0 | 908 (39.2%) | 698 (40.4%) | 0.45 |
| Anxiety | 1,069 (30.1%) | 489 (12.1%) | 408 (21.0%) | 661 (41.0%) | <0.001 |
| Depression | 977 (27.5%) | 494 (12.2%) | 392 (20.1%) | 585 (36.5%) | <0.001 |
| Anxiety or depression | 1,376 (38.5%) | 468 (11.6%) | 562 (28.8%) | 814 (50.2%) | <0.001 |
Targeted therapy was defined as anyone taking TNF inhibitors (adalimumab, certolizumab, etanercept, infliximab), IL‐17 inhibitors (ixekizumab, secukinumab, brodalumab), IL‐23 inhibitors (guselkumab, risankizumab, ustekinumab; apremilast). Standard systemic therapy was defined as anyone taking acitretin, ciclosporin, or methotrexate and not taking a targeted therapy.
BMI, body mass index; SD, standard deviation.
A fully adjusted regression model for worsening psoriasis estimated an odds ratio (OR) 2.01 (95% CI, 1.72–2.34) for those with a positive screen for anxiety or depression compared to those without a positive screen (Fig. 1). Associations were also observed for female gender (OR, 1.82, 95% CI, 1.56–2.13); obesity (OR, 1.22, 95% CI, 1.09–1.36) and shielding (OR, 1.18, 95% CI, 1.03–1.35).
Figure 1.
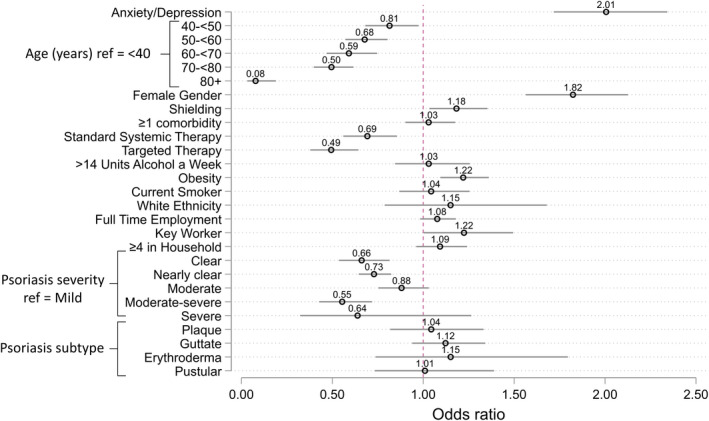
Fully adjusted model for associations with worsening psoriasis. Odds ratios for associations with worsening psoriasis. Anxiety/depression is defined as those who screened positive for either anxiety or depression. Obesity is defined as a BMI >30.
There were inverse associations with systemic therapy use [standard systemic OR 0.69 [95% CI, 0.56–0.86] and targeted therapy OR 0.49 (95% CI, 0.38‐0.64)]. Of 1541 (38.1%) participants receiving standard systemic ?or targeted therapies for psoriasis, 284 (18.4%) reported non‐adherence during the pandemic (Table 1). The commonest reason was concern regarding complications related to COVID‐19 (n = 217). Non‐adherence was associated with worsening psoriasis (OR, 2.90, 95% CI, 2.31–3.63). A positive mental health screen was more common in those reporting non‐adherence compared to those who were adherent (42.8% vs. 32.4%).
These data indicate a burden due to the COVID‐19 pandemic in people with psoriasis; worsening psoriasis is common and is associated with poor mental health. We find that in the subset on systemic therapy, non‐adherence is associated with worsening disease and is driven by concerns about immunosuppressant‐related risks of COVID‐19. This is an important observation since current guidelines (informed by reassuring data on drug‐related risks of severe COVID‐196) recommend continuing immunosuppression in people without COVID‐19 to maintain disease control.7
Our findings parallel data from the general population indicating an increased mental health burden during the pandemic, particularly in women.8 People with psoriasis – especially those with severe psoriasis, and women – have a high prevalence of anxiety and depression and may thus be particularly vulnerable to the adverse impact of the pandemic on mental health.9 Whilst men are known to be at greater risk of severe outcomes from COVID‐19, our data suggest that women may be more susceptible to indirect excess morbidity – poor mental health and worsening skin disease – than men.
The generalisability of results is limited given the self‐selecting bias of our study population towards UK white women. Individuals non‐adherent to treatment, with low computer literacy or less anxiety, may be disinclined to participate, which may introduce ascertainment bias.
Our data underscore the importance of holistic models of care and indicate a need to provide access to psychological support. In those with worsening psoriasis, possible non‐adherence should be explored. Evidence‐based communication around medication‐related COVID‐19 risks and behavioural approaches for supporting adherence may help address fears, anxieties and confusion.10 Attention given now to address this may mitigate a long‐lasting detrimental impact of the pandemic on health outcomes in people with psoriasis.
Acknowledgements
We are grateful to all the patients who have contributed to PsoProtectMe, the professional and patient organisations who supported or promoted PsoProtectMe and for the input of Prof Lars Iversen, Prof Nick Reynolds, Prof Joel Gelfand, Ms Christine Janus and Ms Melissa Sweeney through their vital contributions. We would also like to acknowledge the following individuals for help with translating the PsoProtectMe survey; Dr Haleema Alfailakawi, Dr Wisam Alwan, Dr Rosa Andres Ejarque, Dr Ines Barbosa, Ms Carmen Bugarin Diz, Ms Katarzyna Grys, Dr Mahira Hamdy El Sayed, Mr Tran Hong Truong, Mr Masanori Okuse, Ms Dagmara Samselska, Ms Isabella Tosi, Ms Ya‐Hsin Wang, and the Engine Group UK for their generous creative input and website expertise.
Data Availability Statement
The pre‐print for this manuscript has been archived in the MedRxiv server: https://www.medrxiv.org/content/10.1101/2021.05.04.21256507v1.
References
- 1.Zylke JW, Bauchner H. Mortality and morbidity: the measure of a pandemic. JAMA 2020; 324: 458–459. [DOI] [PubMed] [Google Scholar]
- 2.Pfefferbaum B, North CS. Mental health and the covid‐19 pandemic. N Engl J Med 2020; 383: 510–512. [DOI] [PubMed] [Google Scholar]
- 3.Wright A, Salazar A, Mirica M, Volk LA, Schiff GD. The invisible epidemic: neglected chronic disease management during COVID‐19. J Gen Intern Med 2020; 35: 2816–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahil SK, Yates M, Langan SMet al. Risk mitigating behaviours in people with inflammatory skin and joint disease during the COVID‐19 pandemic differ by treatment type: a cross‐sectional patient survey. Br J Dermatol 2021; 185: 80–90. 10.1111/bjd.19755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroenke K, Spitzer RL, Williams JBW, Löwe B. An ultra‐brief screening scale for anxiety and depression: the PHQ‐4. Psychosomatics 2009; 50: 613–621. [DOI] [PubMed] [Google Scholar]
- 6.Mahil SK, Dand N, Mason KJet al. Factors associated with adverse COVID‐19 outcomes in patients with psoriasis‐insights from a global registry‐based study. J Allergy Clin Immunol 2021; 147: 60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelfand JM, Armstrong AW, Bell Set al. National Psoriasis Foundation COVID‐19 task force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol 2020; 83: 1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce M, Hope H, Ford Tet al. Mental health before and during the COVID‐19 pandemic: a longitudinal probability sample survey of the UK population. Lancet Psychiat 2020; 7: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamb RC, Matcham F, Turner MAet al. Screening for anxiety and depression in people with psoriasis: a cross‐sectional study in a tertiary referral setting. Br J Dermatol 2017; 176: 1028–1034. [DOI] [PubMed] [Google Scholar]
- 10.Read S, Morgan J, Gillespie Det al. Chronic conditions and behavioural change approaches to medication adherence: rethinking clinical guidance and recommendations. Patient Prefer Adherence 2020; 14: 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The pre‐print for this manuscript has been archived in the MedRxiv server: https://www.medrxiv.org/content/10.1101/2021.05.04.21256507v1.


