Dear Editor,
The Philippines remains one of the countries with the highest number of new COVID‐19 cases in the Western Pacific region. 1 The Philippine government granted emergency use authorizations for inactivated SARS‐COV‐2 (Sinovac) and recombinant ChAdOx1‐S (AstraZeneca) vaccines. Early trials of these vaccines have described reactions ranging from injection site reactions 2 , 3 to generalized urticaria. 3
We report 20 healthcare workers who developed cutaneous reactions after receiving their first dose of either Sinovac or AstraZeneca from 1 March 2021 to 31 March 2021. Seven patients received Sinovac, while 13 patients received AstraZeneca. Their median age was 37 years (range: 24–57 years). Fifteen (75%) were female, and five (25%) were male. All patients were seen by either the authors or other dermatologists. All seven patients who developed localized injection site reactions received AstraZeneca (Fig. 1a,b). These were all delayed reactions, appearing more than 24 h postvaccination, and none reported symptoms of anaphylaxis. Of the 13 patients who developed distant site reactions, defined as cutaneous reactions that are distributed beyond the injection site, six received AstraZeneca, and seven received Sinovac. Six patients developed immediate cutaneous reactions: three who either had urticaria, angioedema or petechiae had anaphylactic symptoms; one experienced angioedema and transient focal neurologic deficits; and two had generalized macules and patches and urticaria but without anaphylactic symptoms. Other cutaneous reactions that appeared more than 24 h postvaccination included urticaria, angioedema, erythematous macules, patches, papules, vesicles, purpuric patches and pityriasis rosea (PR)‐like eruption (Table 1; Fig. 1c‐f).
Figure 1.
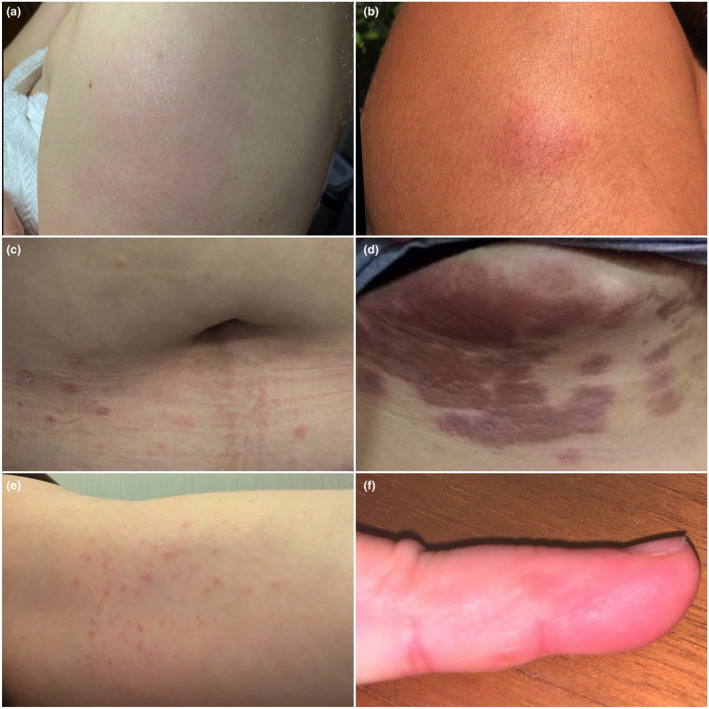
Cutaneous reactions to COVID‐19 vaccines. Erythematous patches on the injection site (a‐b). Erythematous papules and plaques with collarette scaling on the trunk (c). Purpuric patches on the right inframammary area contralateral to the injection site (d). Erythematous macules and papules inner arm of the injected arm (e). Pruritic vesicles on the lateral aspects of the 5th digit of the injected arm (f).
Table 1.
Cutaneous reactions to AstraZeneca and Sinovac vaccines distant to the injection site: characteristics, associated signs and symptoms, and management
| Distant site reactions | |||||||
|---|---|---|---|---|---|---|---|
| Patient | Age/Sex |
COVID‐19 Vaccine |
Allergy History |
Onset postvaccination | Duration of reaction |
Cutaneous reactions and associated signs and symptoms |
Management |
| 1 | 57/F | SV |
Peanuts, shrimp paste, NSAIDs, aspirin, cotrimoxazole, prednisolone + chlorphenamine maleate |
15 min | <1 d |
Angioedema on the left eye and left earlobe Metallic taste, lip numbness 9 h postvaccination: wheals developed on the left side of the neck, associated with a coughing spasm |
Betamethasone + Loratadine Cetirizine |
| 2 | 54/M | SV |
Etoricoxib Allopurinol |
1–2 h | 1 d |
Petechiae on the chest and abdomen (20% TBSA) Laryngeal spasm, hoarseness, dizziness |
IM epinephrine IM diphenhydramine Methylprednisolone Bilastine |
| 3 | 29/F | AZ | None | 3 h | 3 d |
Angioedema on the upper lip Fever, myalgia, arthralgia 1‐day postvaccination: angioedema on the upper lip, hoarseness, shortness of breath |
IM epinephrine IM diphenhydramine Bilastine |
| 4 | 54/F | AZ | None | 15 min | 14 d | Wheals on both arms and legs |
Cetirizine Hydrocortisone cream |
| 5 | 36/M | SV | None | <30 min | 3 d |
Macules and patches, generalized (80% TBSA) |
Cetirizine |
| 6 | 54/F | SV | Shellfish | 5 h | <1 h |
Angioedema on both 2nd digits, generalized pruritus Elevated BP, tachypnoea, right upper arm weakness, slurring of speech |
IV hydrocortisone, IM diphenhydramine Betamethasone + loratadine |
| 7 | 25/F | SV | None | Day 1 | 1 d |
Wheals on arms and legs with burning sensation, angioedema on the wrist, dermatographism Injection site pain and numbness |
IM diphenhydramine Prednisone Bilastine Ebastine +betamethasone |
| 8 | 28/F | AZ | Ceftriaxone | Day 1 | 14 d |
Purpuric patches on the right inframammary area spreading to the left with a needle‐prick sensation Injection site tenderness, arm pain and heaviness |
Clobetasol propionate |
| 9 | 53/F | SV |
Penicillin NSAIDs |
Day 1 | 14 d |
Vesicles on ipsilateral* 5th digit, macules and papules on ipsilateral* arm, axilla and chest, severe pruritus on ipsilateral* arm on the day of vaccination |
Prednisone Loratadine, cetirizine Clobetasol |
| 10 | 47/F | AZ | Nalbuphine | Day 2 | 2 d |
Macules on ipsilateral arm and upper chest (5% TBSA) Arm pain, myalgia |
Bilastine |
| 11 | 24/F | AZ | None | Day 3 | 14 d |
Pityriasis rosea‐like eruption on trunk and extremities with pruritus Injection site pain and heaviness |
Cetirizine Betamethasone valerate |
| 12 | 37/F | AZ | Shrimps | Day 3 | 4 d |
Erythematous macule on injection site, angioedema on the right eye Arm pain and heaviness, runny nose, fever, headache, increase blood pressure |
Prednisone Cetirizine |
| 13 | 31/F | SV | None | Day 4 | 7 d |
Erythematous macules and patches on trunk, axillae, inguinal areas (18% TBSA) Headache, fever before the cutaneous eruption |
Bilastine |
AZ, AstraZeneca; SV, Sinovac; and d, day *Ipsilateral to the vaccination site
Hypersensitivity to vaccines is often caused by excipients rather than the vaccine antigen. For AstraZeneca, the most likely cause is polysorbate, 4 whereas, for Sinovac, aluminium hydroxide may be causative. 3 Both have been implicated in hypersensitivity reactions. 4 Most vaccination reactions are classified as type I (immediate) or type IV (delayed) hypersensitivity responses. Type I responses usually occur within the first four hours and result from mast cell activation and degranulation, exemplified by anaphylaxis. Type IV responses are delayed, commonly within hours or days after exposure. 5 In our cases, the onset of localized injection site reactions was suggestive of type IV hypersensitivity. These were similarly reported in other mRNA vaccines. 6 , 7 The distant site reactions were either immediate or delayed. Three of the six patients who had immediate cutaneous reactions had anaphylactic symptoms either concomitantly or within hours. While urticaria and angioedema are common in anaphylactic reactions, the petechial rash was notable in one of our cases. None of those who had delayed cutaneous reactions developed anaphylactic symptoms. A PR‐like eruption, previously reported following vaccination (Moderna and Pfizer COVID‐19 vaccines, as well as influenza and hepatitis vaccines) and COVID‐19 infection, may be related to a T‐cell mediated response to the viral epitope rather than HHV‐6 and HHV‐7 reactivation associated with true PR. 7 , 8 , 9 , 10
Most cutaneous reactions observed in this case series were self‐limited. However, for patients presenting with immediate cutaneous reactions within hours postvaccination, it may be prudent to monitor for further development of anaphylactic symptoms in the next 24 h for immediate control and intervention. For those presenting with cutaneous reactions 24 h postvaccination, supportive management and reassurance seem sufficient. As the widespread vaccination of COVID‐19 vaccines continues worldwide, we anticipate more data regarding their side effect profile, including the mechanism and pathophysiology of such side effects. The benefits versus risks from the vaccines support their use and significant role in putting an end to the pandemic.
Conflict of interest
The authors declare no conflicts of interest.
Funding sources
None.
IRB approval status
Not applicable.
Acknowledgements
The patients in this manuscript have given written informed consent to the publication of their case details.
References
- 1. World Health Organization . Coronavirus Disease 2019 (COVID‐19) External Situation Report #46 [Internet]. 2021. Available from: https://www.who.int/westernpacific/internal‐publications‐detail/covid‐19‐situation‐report‐for‐the‐western‐pacific‐region‐46‐17‐march‐2021‐‐‐23‐march‐2021
- 2. Ramasamy MN, Minassian AM, Ewer KJ et al. Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): a single‐blind, randomised, controlled, phase 2/3 trial. Lancet 2020; 396: 1979–1993. Available from https://www.thelancet.com/journals/lancet/article/PIIS0140‐6736(20)32466‐1/fulltext [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Y, Zeng G, Pan H et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18–59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21: 181–192. 10.1016/S1473-3099(20)30843-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kounis NG, Koniari I, de Gregorio C et al. Allergic reactions to current available COVID‐19 vaccinations: pathophysiology, causality, and therapeutic considerations. Vaccines 2021; 9: 221. 10.3390/vaccines9030221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McNeil MM, DeStefano F. Vaccine‐associated hypersensitivity. J Allergy Clin Immunol 2018; 141: 463–472. 10.1016/j.jaci.2017.12.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wei N, Fishman M, Wattenberg D, Gordon M, Lebwohl M. “COVID arm”: a reaction to the Moderna vaccine. JAAD Case Reports 2021; 10: 92–95. 10.1016/j.jdcr.2021.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. 10.1016/j.jaad.2021.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cyrenne BM, Al‐Mohammedi F, DeKoven JG, Alhusayen R. Pityriasis rosea‐like eruptions following vaccination with BNT162b2 mRNA COVID‐19 Vaccine. J Eur Acad Dermatol Venereol 2021; 35: e546–e548. 10.1111/jdv.17342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drago F, Ciccarese G, Rebora A, Parodi A. Human herpesvirus‐6, ‐7, and Epstein‐Barr virus reactivation in pityriasis rosea during COVID‐19. J Med Virol 2021; 93: 1850–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen JF, Chiang CP, Chen YF, Wang WM. Pityriasis rosea following influenza (H1N1) vaccination. J Chinese Med Assoc 2011; 74: 280–282. [DOI] [PubMed] [Google Scholar]


