Abstract
Since the coronavirus disease 2019 (COVID‐19) outbreak, the nosocomial infection rate worldwide has been reported high. It is urgent to figure out an affordable way to monitor and alarm nosocomial infection. Carbon dioxide (CO2) concentration can reflect the ventilation performance and crowdedness, so CO2 sensors were placed in Beijing Tsinghua Changgung Hospital's fever clinic and emergency department where the nosocomial infection risk was high. Patients’ medical records were extracted to figure out their timelines and whereabouts. Based on these, site‐specific CO2 concentration thresholds were calculated by the dilution equation and sites’ risk ratios were determined to evaluate ventilation performance. CO2 concentration successfully revealed that the expiratory tracer was poorly diluted in the mechanically ventilated inner spaces, compared to naturally ventilated outer spaces, among all of the monitoring sites that COVID‐19 patients visited. Sufficient ventilation, personal protection, and disinfection measures led to no nosocomial infection in this hospital. The actual outdoor airflow rate per person (Q c) during the COVID‐19 patients’ presence was estimated for reference using equilibrium analysis. During the stay of single COVID‐19 patient wearing a mask, the minimum Q c value was 15–18 L/(s·person). When the patient was given throat swab sampling, the minimum Q c value was 21 L/(s·person). The Q c value reached 36–42 L/(s·person) thanks to window‐inducted natural ventilation, when two COVID‐19 patients wearing masks shared the same space with other patients or healthcare workers. The CO2 concentration monitoring system proved to be effective in assessing nosocomial infection risk by reflecting real‐time dilution of patients’ exhalation.
Keywords: Carbon dioxide, COVID‐19, nosocomial infection, ventilation
Practical Implications.
The present study explores an effective method to monitor and alarm nosocomial infection by reflecting real‐time dilution of patients’ exhalation.
In this study, aided with personal protection and disinfection measures, outdoor airflow rate per person of 15–18 L/(s·person) was sufficient to prevent nosocomial infection when there was only one COVID‐19 patient, and 21 L/(s·person) was sufficient during throat swab sampling.
This study provides evidence on affordably controlling nosocomial infection during the COVID‐19 pandemic using a real‐time CO2 concentration monitoring system.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) outbreak began in December 2019, and as 2020 ends, more than 80 million people had been infected. 1 Many nosocomial severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infections have emerged since January 2020. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 As of August 15, 2020, nearly 300 000 healthcare workers (HCWs) were confirmed to be infected with SARS‐CoV‐2 worldwide. 10 , 11 In Wuhan, an early study revealed that 41.3% of 138 patients were presumed to have been infected in hospital, including 40 HCWs (40/138; 29.0%) and 17 patients (17/138; 12.3%). 12 As of April 28, 2020, excluding HCW infections, the proportion of patient nosocomial infections with SARS‐CoV‐2 was 12.5% in 10 UK hospitals and 1 Italian hospital. 13 General patients attending hospitals are vulnerable to infection by the virus. 14 In brief, the scale of hospital‐acquired SARS‐CoV‐2 infection cannot be neglected.
In pandemics, the fast‐spreading diseases on top of other diseases, especially those requiring regular treatment and those that are seasonal, can cause overcrowding in hospitals and shortages of equipment needed to care for patients and HCWs. 15 , 16 , 17 A maximum capacity based on social distancing may be incorporated in hospital designs, 18 , 19 but is often not implemented in practice. Crowds lead not only to the violation of the minimum social distance to avoid short‐range transmission of respiratory droplets, 20 but also to inadequate dilution of fine droplets and droplet nuclei by the building ventilation system. 21
Recent studies have revealed that the release strength of SARS‐CoV‐2 from a patient can reach millions of copies per hour. 22 , 23 Hand hygiene and wearing masks can likely eliminate the transmission of most expiratory viruses. 24 , 25 , 26 , 27 , 28 Whether the remaining viruses, if any in the air, can cause infection depends on the critical infection dose, the intake and immune system of the susceptible person. 29 , 30 Poor indoor ventilation can lead to a high intake of the virus and potential infection. 31 , 32 , 33 , 34 , 35 The dose, intake, and immune response are unknown and potentially case‐dependent, which makes it challenging to identify the absolute infection risk caused by insufficient ventilation.
Confirmation of SARS‐CoV‐2 infection requires strong evidence incorporating computed tomography (CT) imaging features, epidemiology history, clinical manifestation, and pathogen serum examination. It is time‐consuming and fails to reflect the increasing nosocomial risk to HCWs and patients. 36
In this study, carbon dioxide (CO2) concentration was taken as an indicator of human exhalation and indoor ventilation performance, so sensors were placed in Beijing Tsinghua Changgung Hospital (Abbreviated as Changgung Hospital) at the end of January 2020. We also estimated the actual outdoor airflow rate per person during the presence of COVID‐19 patients. The experience and evidence may help hospitals and authorities to determine a strategy to minimize nosocomial SARS‐CoV‐2 infection.
2. METHODS
2.1. Study design
At present, reverse transcription‐polymerase chain reaction (PCR), droplet digital PCR, and isothermal amplification, among other methods, are applicable for quantitatively analyzing the environmental contamination of SARS‐CoV‐2 to demonstrate possible risks in less than 1 h. 37 , 38 , 39 , 40 , 41 , 42 , 43 A fluorescence spectrometer can measure viable microorganisms, 44 but the quantity is not correlated with the quantity of pathogens. Real‐time environmental surveillance technology of SARS‐CoV‐2 and many other pathogens is yet to come.
Unlike the abovementioned methods for direct detection, the CO2 concentration distribution can trace human exhalation in almost real time, and the difference between indoor and outdoor concentrations can reveal the dilution performance of ventilation for a known number of indoor occupants' exhalation if there are no other significant sources of CO2, such as combustion. 45 Thus, the CO2 concentration is an adequate index of the potential infection risk posed by poor ventilation in comparison with other methods, as shown in Table 1. If ventilation is supplied by a mechanical system and the flow rate is constant, the CO2 concentration indicates the crowdedness; if the number of occupants is known, it indicates the performance of ventilation.
TABLE 1.
Quantitative assessment method of nosocomial infection risk
| Type | Intent | Method | Lower limit/ Sensitivity | Correlation | Sampling analysis time | Price |
|---|---|---|---|---|---|---|
| Direct measurement | Detecting viral RNA (ORF1ab, N and E) in the air or on the surface | Quantitative RT‐PCR | 0.2 copies/μl (Sansure Biotech, Hunan, China) 33 | Strong: detect pathogens | 1.0 ~ 2.0 h (h) | $3 for SARS‐CoV−2 test kit& $50 000 for real‐time PCR system |
| ddPCR | 0.109 copies/μl for ORF1ab, 0.42 copies/μl for N (Bio‐Rad) 30 | 1.5 ~ 4.0 h | $ 200 000 (Bio‐Rad QX200) | |||
| Surrogate | Monitoring microorganisms in the environment | Cell culture and colony counting | ‐ | Weak: little correlation between culturable/ active microbes and pathogens | >24.0 h | $50 |
| ATP fluorescence detection | 1 × 10−16 mol/ATP (Hygiena EnSURE ATP Test Luminometer) | 1 s | $5,000 (Hygiena EnSURE ATP Test Luminometer) | |||
| Monitoring CO2 concentration | Infrared CO2 sensors | ±50 ppm (iBEEM) | Moderate: characterize exhaled breath | 5 min | $400 (iBEEM) |
Abbreviations: ATP, adenosine triphosphate; CO2, carbon dioxide; ddPCR, droplet digital PCR; E, the envelope protein; N, the Nucleocapsid protein gene; ORF1ab, open reading frame 1ab; RNA, ribonucleic acid; RT‐PCR, reverse transcription‐polymerase chain reaction.
2.2. Equipment and study conditions
A surveillance campaign was conducted at the Changgung Hospital from the beginning of the outbreak in January 2020. In accordance with symptoms of patients diagnosed with COVID‐19 and the patients’ whereabouts, we deployed 32 iBEEM sensors in areas with a high incidence of COVID‐19 patients (Figure 1). The sensors, whose measurement interval is 5 min, were placed approximately 0.8–1.2 m above ground and far from CO2 emission sources to collect real‐time ambient CO2 concentration. The measurement area covered the fever clinic (FC) and the emergency department which was in the outpatient building. The former adopts natural ventilation, the latter adopts mechanical ventilation.
FIGURE 1.
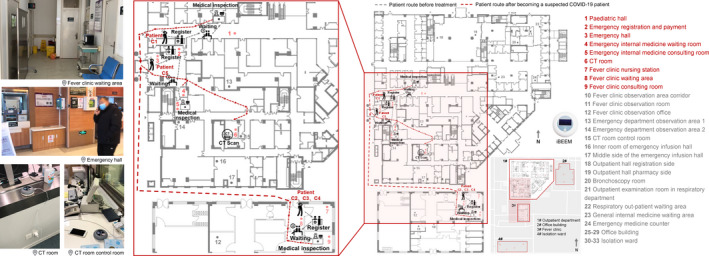
Carbon dioxide sampling locations within the hospital and the patient route in case of confirmed severe acute respiratory syndrome coronavirus 2 infection. aCT: computed tomography. Patient C1‐5: the confirmed COVID‐19 Patient 1–5. COVID‐19: coronavirus disease 2019. bGray dotted line: COVID‐19 patient route before treatment. Red dotted line: COVID‐19 patient route after becoming a suspected COVID‐19 patient. cIn February 2020, five COVID‐19 patients went to Changgung Hospital and were numbered as C1‐C5 according to the order of their registration time. According to medical records, the diagnosis process for confirmed patients is outpatient clinic, CT room, and isolation ward. Patient C1 went to the pediatrics department, emergency hall, CT room, and isolation ward. Patient C2‐C4 went to the fever clinic, emergency hall, CT room, and isolation ward. Patient C5 went to the emergency hall, emergency internal medicine, CT room, and isolation ward. dThe sensors were placed approximately 0.8–1.2 m above ground and far from CO2 emission sources. The measurement range of the sensor is calibrated between 400 and 1000 ppm, its accuracy is ±50 ppm and its measurement interval is 5 min
2.3. Current infection control strategies at Changgung Hospital
Changgung Hospital implemented masks, physical distancing, and other preventive measures to control nosocomial infection (Appendix S1A). With the infection control measures in place, neither HCWs nor patients were infected within Changgung Hospital throughout the COVID‐19 pandemic.
Five COVID‐19 patients went to Changgung Hospital in February 2020 and they were numbered as C1‐C5 according to the order of their registration time. Based on their medical records, they went to the pediatric hall, emergency hall, FC, and CT room (Figure 1). HCWs with adequate personal protective equipment (PPE) were in close contact with infectors in the CT room and FC nursing station for less than 5 min and in the FC consulting room and emergency internal medicine (EIM) consulting room for at least 5 min. Unconscious close contact of more than 5 min between infectors and susceptible people occurred in the pediatric hall, emergency hall, and FC waiting area. Prolonged or close contact indicates a higher infection risk. The ventilation performance of those areas is critical for diluting exhalation from the infectors. Therefore, this study focused on the ventilation performance of areas visited by COVID‐19 patients. This study was approved by the ethics commissions of Changgung Hospital and the sampling procedure did not obstruct routine medical care procedures at the hospital.
2.4. CO2 concentration analysis
Adequate ventilation can dilute the exhalation of confirmed patients in the hospital, reduce the residence time of the exhaled substances, and the possibility of other patients being exposed to the coronavirus. CO2 concentration has been used as an indicator to estimate ventilation rates and characterize ventilation for decades. If sufficiently ventilated and the occupancy is stable, the CO2 exhaled by people will be quickly diluted to a steady state. Based on the dilution equation, the theoretical upper limit of the CO2 concentration (C u) can be represented by Equation (1), as follows:
| (1) |
where Cu is the theoretical upper limit of the CO2 concentration (ppm); Co is the outdoor CO2 concentration (ppm); G m is the indoor personnel total CO2 generation rate when the number of people in the room reaches the maximum (m3/h) (Appendix S1B); and Q is the outdoor airflow rate (m3/h).
Each site's Cu varied (Table 2), so the CO2 dilution index (I d) and the risk ratio (R r) were used to briefly compare and evaluate all of the sites’ outdoor air ventilation performance.
TABLE 2.
Information on the monitoring sites
| Site | Area type | Area | Upper limit of occupants | Total generation rate of CO2 | Outdoor air | Theoretical upper limit of CO2 concentration | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Designed flow rate | Designed change rate | Required minimum change rate | ||||||||||
| A (m2) | N (person) a | G m (m3/h) | Q (m3/h) | n d (h−1) | n r (h−1) b | C u (ppm) c | ||||||
| Outpatient building | CT room | Consulting | 22.7 | 1.5 | 0.030 | 350 | 5 | 2 | 505 | |||
| Pediatric hall | Waiting | 139.2 | 61.0 | 0.952 | 1,750 | 4 | 963 | |||||
| Emergency hall | Waiting | 151.7 | 67.0 | 1.045 | 1.179 d | 2,100 | 3,150 d | 5 | 793 | |||
| EIM | Waiting room | Waiting | 12.6 | 5.0 | 0.078 | 350 | 9 | |||||
| Consulting room | Consulting | 19.2 | 3.0 | 0.056 | 700 | 12 | ||||||
| FC | Waiting area | Waiting | 25.1 | 11.0 | 0.211 | NV | NV | 6 | 867 | |||
| Nursing station | Consulting | 9.2 | 4.0 | 0.077 | 864 | |||||||
| Consulting room | Consulting | 8.6 | 4.0 | 0.077 | 902 | |||||||
Abbreviations: CO2, carbon dioxide; CT, computed tomography; EIM, emergency internal medicine; FC, fever clinic; NV, natural ventilation.
For the waiting area, N = int[A/d 2], where int[] is rounded down and d is the recommended interpersonal distance, which was 1.5 m. For the consulting area, N was set according to the room's actual usage pattern. CT room: before a CT scan, a doctor will be in the CT room to prepare the patient for the scan, and then the doctor will leave. The preparation and scan both take approximately 2 min, so the upper limit of the occupant number was set as 1.5 rather than 1.0. EIM consulting room: usually one patient with one relative and one doctor. FC nursing station and consulting room: usually 2 healthcare workers and one patient with one relative.
The required minimum outdoor air change rate was taken from the Code for design of general hospital (GB51039‐2014), Design code for heating ventilation and air conditioning of civil buildings (GB50736‐2012), Ventilation of health care facilities (ANSI/ASHRAE/ASHE standard 170–2017), and Code for design of infectious diseases hospital (GB50894‐2014).w
C u = C o+106 G m/Q, where C o is the outdoor air CO2 concentration, which was assumed to be 419 ppm (1 ppm = 10−6 mol/mol) for the outpatient building and 400 ppm for the FC. Sites in the outpatient building were mechanically ventilated, and their designed outdoor airflow rate (Q) as well as designed outdoor air change rate (n d) was listed. Because sites in the FC were naturally ventilated, their Q value was estimated by the required minimum outdoor air change rate (n r). Q = n r Ah, where h is the height of a room, which was 3 m in this study.
The monitored real‐time CO2 concentration in the emergency hall, EIM waiting room, and consulting room were significantly correlated with one another according to the Pearson correlation analysis [r = 0.596−0.841; p < 0.01 (2‐tailed)], which indicated that the indoor air was well‐mixed. The emergency hall, EIM waiting room, and consulting room were combined, so the total generation rate of CO2 was calculated separately and then added. The designed flow rate was also added to calculate the theoretical upper limit of the CO2 concentration.
The I d compares the ventilation effectiveness between the natural ventilation implemented in the FC and the mechanical ventilation applied in the outpatient building and is expressed as follows (Equation (2)):
| (2) |
where C is the measured real‐time CO2 concentration (ppm). I d > 1 indicates that the air ventilation is poorer than the designed/required condition, and thus the risk of nosocomial infection rises.
The R r is the time proportion of I d > 1, so it quantitatively describes how often the site had a poor CO2 dilution condition. If one site's long‐term R r exceeded 50%, namely, the CO2 concentration at one site exceeded the threshold over half of a long period, its outdoor air supply was deemed insufficient to dilute the occupants' exhalation during normal operation.
I d and R r in February (I d,f and R r,f, respectively) showed the comprehensive dilution conditions in late winter, whereas I d and R r during periods with the presence of COVID‐19 patients and in the first hour of their absence (I d,p and R r,p, respectively) indicated the risk of airborne nosocomial SARS‐CoV‐2 infection. The former was determined not only to provide a baseline for the latter, but also to determine the underlying risk of hospital‐acquired infection because COVID‐19 patients could arrive at any time.
Moreover, the actual outdoor airflow rate per person (Q c) can be calculated using equilibrium analysis according to American Society for Testing and Materials international standard D6245‐18 based on the assumptions as follow: (a) the CO2 concentration in the target zone is uniform; (b) the outdoor concentration is constant; (3) the outdoor air ventilation rate is constant and is expressed as follows (Equation (3)):
| (3) |
where g is the CO2 generation rate per person [m3/(h·person)], which was estimated by directly measured statistics 46 (Appendix S1B) for the lack of occupants’ body mass data; and Ce is the equilibrium CO2 concentration measured in the presence of COVID‐19 patients (ppm).
The implement of the Equation (3) should also meet the requirements below: (a) the target zone is free from air exchange with other indoor zones which has different CO2 concentration; (b) the average CO2 generation rate per person is constant and known; (c) the CO2 concentration in the target is at equilibrium.
3. RESULT
During our measurements at Changgung Hospital, only five COVID‐19 patients arrived for treatment. Nonetheless, during their presence and in the first hour of absence, we collected 510 CO2 concentration data points (Figure 1D). Furthermore, throughout February 2020, we obtained approximately 500 000 data points from all of the sites that the COVID‐19 patients had visited. Based on environmental data, we calculated I d and R r at mechanical ventilated areas (CT room, EIM, emergency hall) and natural ventilated areas (FC) to compare ventilation dilution condition, as shown in Figure 2. The CT room, EIM consulting room, and EIM waiting room had no outer windows or outer doors. However, the latter two rooms were directly connected to the emergency hall, in which an outer door was frequently opened, through open inner doors. According to the emergency department medical records (partially listed in Appendix S1C), approximately 90 patients were registered every day, so the emergency hall outer door could open hundreds of times each day. Cold air intrusion might greatly enhance the outdoor air supply in such an outer space. This may be due to the large temperature difference between indoor and outdoor.
FIGURE 2.
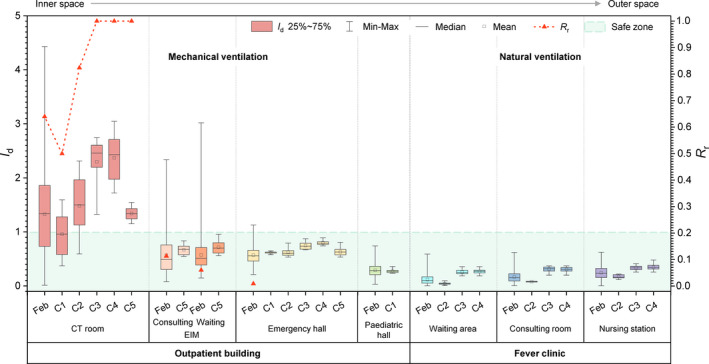
Carbon dioxide (CO2) dilution index (I d) and risk ratio (R r) distribution at monitoring sites. aCT: computed tomography. EIM: emergency internal medicine. bFeb displays the entire distribution at each site in February 2020. C1–C5 display the distribution during the presence of and the first hour of absence of the corresponding confirmed case at each site. cThe CO2 dilution index [I d = (C‐C o)/(C u‐C o)] aims to compare all of the sites’ ventilation conditions by nondimensionalizing the CO2 concentration, where C is the measured real‐time CO2 concentration, which was measured at eight sites (ppm); C o is the outdoor air CO2 concentration, which was 419 ppm for sites equipped with mechanical ventilation in the outpatient building and 400 ppm for sites relying on natural ventilation in the fever clinic (FC); C u is the theoretical upper limit of the CO2 concentration, which was 505, 963, and 793 ppm for the CT room, pediatric hall, and the remaining three sites in outpatient building, and 867, 902, and 864 ppm for the waiting area, consulting room, and nursing station in the FC, respectively. I d > 1 indicates that the ventilation condition is relatively poor compared with the designed/required condition, and thus the risk of hospital‐acquired infection increases. I d should be between 0 and 1 so that R r is defined as the proportion of I d > 1. Real‐time concentrations that were below the set outdoor air concentration were excluded when calculating I d. dThe top arrow shows that the sites were placed in order of closeness to the outdoors. The CT room, EIM consulting room, and waiting room had no outer windows/doors. However, the latter two rooms were directly connected to the emergency hall, which had an outer door, through an open inner door. The emergency hall, pediatric hall, and FC waiting area all had outer doors that were frequently opened. The FC consulting room had one open outer window, whereas the nursing station had two. eThe green rectangle represents the safe zone. The zone indicates that the ventilation condition is good
Generally, I d and R r were higher in mechanically ventilated inner spaces, and I d,p was usually higher than the mean value of the corresponding I d,f. In other words, natural ventilation dilutes airborne contaminants better than mechanical ventilation in Beijing in winter.
R r,p was 0 in the EIM consulting room, EIM waiting room, and emergency hall, but R r,f was 11%, 6%, and 1%, respectively. Dilution was occasionally insufficient in those areas throughout February, so the emergency hall outer door should be kept open if the occupancy cannot be controlled.
Based on the monitored real‐time CO2 concentration and equilibrium analysis method according to American Society for Testing and Materials international standard D6245‐18, the outdoor airflow rate per person Q c during the confirmed patients’ presence was estimated. The contact duration, distance, and types of PPE were different between HCWs and patients when they contacted with COVID‐19 patients. It means that HCWs and patients are not at the same risk of infection. Consequently, we sorted contact situations into four categories based on the type of contact object, type of PPE, contact distance, and contact duration (Figure D2; Table 3). The results showed variance depending on the occupancy and outdoor air supply state. The recommended value under each contact situation was determined by the minimum value considering that there was no nosocomial infection at Changgung Hospital.
TABLE 3.
Estimated actual outdoor airflow rate per person during coronavirus disease 2019 (COVID‐19) patients' presence
| Specific applicable scenarios: areas where COVID−19 patients would visit and remove their masks for less than 30 s | ||||
|---|---|---|---|---|
| Contact object | Normal patients | Healthcare workers | ||
| Type of PPE | Masks b | Level 2 PPE | ||
| Contact distance | Unconscious close contact c | >1 m distance kept | Close contact d | |
| Contact duration | >5 min | <5 min | >5 min (Consulting / throat swab sampling) e | <5 min |
| Qc [L/(s·person)] a | 18–42 | 15–20 | 17–30/21–38 | 15–58 |
Abbreviation: PPE, personal protective equipment.
Q c is the estimated actual outdoor airflow rate per person in the presence of COVID‐19 patients using equilibrium analysis [L/(s·person)] according to American Society for Testing and Materials international standard D6245‐18. Q c = 106 g/[3.6(C e‐C o)], where g is the carbon dioxide (CO2) generation rate per person [m3/(h·person)], which was estimated by directly measured statistics 46 (Appendix S1B) for the lack of occupants’ body mass data; C e is the equilibrium CO2 concentration measured in the presence of COVID‐19 patients (ppm); and C o is the outdoor CO2 concentration, which was 419 ppm for the outpatient building and 400 ppm for the fever clinic.
Changgung hospital requires patients to wear masks. Patients who come to the hospital for treatment wear disposable masks, medical surgical masks, and N95 or KN95 masks.
Patients diagnosed COVID‐19 at Changgung hospital waited in the fever clinic and emergency hall, and may have unconscious close contact with other patients. Unconscious close contact indicates that the distance between the patients confirmed COVID‐19 and the waiting patients is less than 1.0 m. Simultaneously, waiting patients are not aware of the presence of the infected around them. Patients are susceptible to contracting a nosocomial infection.
Healthcare workers in fever clinic and emergency internal medicine adopt secondary protection. In the medical process, healthcare workers have close contact (interpersonal distance less than 1.0 m) with COVID‐19 patients.
The throat swab was sampled immediately after a patient had been diagnosed as a COVID‐19 suspected case. The consulting process was supposed to last for more than 5 min, so the swab sampling process was sorted into the same categorize, but listed separately.
As expected, Q c was higher under contact situations in which the nosocomial infection risk was higher, such as situations with long‐term exposure and close contact. At Changgung Hospital, 15 L/(s·person) was sufficient for temporary contact (<5 min) with COVID‐19 patients, but social distancing should be maintained or the level of PPE should be increased. A Q c value of 17 L/(s·person) was sufficient to lower the nosocomial infection risk for long‐lasting close contact (>5 min) under the protection of level 2 PPE. With the protection of masks, including disposable masks, medical surgical masks, N95 masks, or other types of masks which patients can use to protect themselves, close contact was allowed under an outdoor airflow rate of 18 L/(s·person). However, these recommended values were reached provided that there was only one COVID‐19 patient in each space and everyone wore masks.
COVID‐19 patients took off their face masks when throat swab sampling, during which the emission rate of virus‐laden aerosols and droplets was supposed to be higher. Under the protection of level 2 PPE, an outdoor airflow rate of 21 L/(s·person) was sufficient to prevent SARS‐CoV‐2 hospital‐acquired infection in Changgung Hospital.
It should be noted that two COVID‐19 patients (C3 and C4) went to the FC simultaneously. Under such a circumstance, 36 L/(s·person) was sufficient for temporary close contact under level 2 PPE, whereas 42 L/(s·person) was sufficient for long‐term space sharing with unconscious close contact with masks. The recommended outdoor airflow rate per person was more than double with two COVID‐19 patients than that with one COVID‐19 patient, which indicates that the required outdoor airflow rate per person may increase linearly with the number of COVID‐19 patients in each space.
4. DISCUSSION
This is the first study to quantitatively provide a recommended outdoor airflow rate per person to prevent SARS‐CoV‐2 nosocomial infection based on a CO2 monitoring system set in an undesignated hospital where COVID‐19 patients were treated during the pandemic. However, our study still has some limitations.
First, due to the need to maintain patients' personal privacy, we had no access to surveillance video. Therefore, much background information was unknown, such as the actual occupancy inside each monitoring site, the type of masks and the way the patients wore them, the actual social distance that the patients kept, and the virus shedding pattern of COVID‐19 patients, including coughing and sneezing frequency. According to an official survey, approximately 98% of Beijing citizens wore face masks when they went to the hospital during the COVID‐19 pandemic, and 98% of those wore disposable face masks, medical surgical masks, and N95 or KN95 masks. 47 Therefore, we assumed that all of the patients and their relatives in Changgung Hospital wore masks.
Second, the outdoor CO2 concentration was estimated using data collected indoor when the target sites were unoccupied (Appendix S1B) rather than real‐time outdoor measurement. Considering that the outdoor CO2 concentration fluctuates with time, taking a specific value may result in overestimating of the Q c value on some days while underestimating on other days.
Third, the monitoring sites were not isolated from other sites with different CO2 concentration, which is required to use equilibrium CO2 analysis but was impossible during the hospital's daily operations. Furthermore, the airflow pattern between adjacent spaces during daily operation, the CO2 concentration in adjacent spaces, and the CO2 distribution inside most of the target sites were not determined. These would add uncertainty to the estimated outdoor airflow rates. Two sensors were set in the emergency hall due to its large area, and over 99% of the real‐time CO2 concentrations measured in February by these two sensors were within 10% of the mean value difference. Therefore, we assumed that the indoor air was also well‐mixed in other sites with a smaller area. We also ensured that each CO2 concentration period fluctuated within 10% (15%) of the mean value difference when calculating the outdoor airflow rate per person using equilibrium analysis in mechanically (naturally) ventilated sites.
Fourth, the occupants’ CO2 generation rate was estimated by directly measuring the CO2 generation of healthy people, 46 but over 50% of the occupants in Changgung Hospital were in poor health conditions during the pandemic. It is hard to say how the average CO2 generation rate per person at each target site would change without systematic and comprehensive researches about CO2 generation rate of different kinds of patients.
Finally, the potential nosocomial SARS‐CoV‐2 infection risk was represented via CO2 removal, rather than the more desirable infection risk or exposure risk, considering the lack of relative knowledge and measurement methods of real‐time virus emission as well as CO2 being a natural indicator of aerosol contaminants in human exhalation, but there is no solid evidence to prove that COVID‐19 patients would exhale virus‐laden aerosols at a constant rate.
As a result of not totally meeting the requirements of using equilibrium analysis method, uncertainty of Q c estimation existed and was related to the occupancy, outdoor CO2 concentration fluctuation, and indoor CO2 concentration distribution according to its calculation formula (Equation (3)). Daily average age‐gender distribution of registered patients was obtained from the medical records, and occupants’ activity level was estimated according to our observation in the hospital. However, the age‐gender distribution of patients’ relatives was not included, the difference of CO2 generation rate between healthy people and patients was unknown, and the real‐time age‐gender distribution as well as the real‐time activity level of occupants in each target site was unknown without the approach to surveillance video, which would add uncertainty to the calculation of CO2 generation rate per person. Measuring indoor CO2 concentration distribution is particularly difficult in that it is difficult to place and power several sensors in each target room of a normal operating hospital especially during such a pandemic. As mentioned before, the consistency of data measured by two sensors set in the spacious emergency hall led us to make the assumption that CO2 concentration is also uniform in other sites without simultaneously distributed measurements. Our placing one sensor at each target site (except the emergency hall) and collecting CO2 concentration data at the measurement interval of 5 min definitely was a proper solution under the existing conditions. Furthermore, outdoor CO2 concentration fluctuates slightly, which also brings uncertainty. The abovementioned factors all bring uncertainty to the estimation of Q c, but it is difficult to determine how and to what extent each factor influences the results.
Noticeably, the highest I d existed and R r,f reached 64% in the CT room, indicating a poorer dilution condition than designed, an increased nosocomial infection risk, and an urgent need to maintain its ventilation system. The outdoor air supply of the CT room was clearly insufficient for its daily operation. The number of occupants was strictly controlled because of the radiological hazard, but several patients would generally queue in the corridor near the frequently open CT room door, which might lead to excessive CO2 concentration to a certain extent. Moreover, the air exchange between the inside and outside of the CT room might have led to virus‐laden air spreading to the surrounding space. Thus, gathering outside the CT room should be avoided and the outdoor air supply should be enhanced both inside and outside it.
The CT room plays an important role in screening suspected cases during the COVID‐19 pandemic. Nosocomial infection prevention measures would not be sufficient to prevent contamination on the surfaces inside both the CT room and the CT gantry. 48 , 49 Changgung Hospital implemented disinfection policy for CT rooms (Appendix S1A) to prevent both touch and airborne contamination. The disinfection policy should be carefully followed, or cluster nosocomial infections may erupt similar to those in Qingdao Chest Hospital. 50
As an aid to clinical diagnosis of COVID‐19, the number of patients taking CT‐imaging may increase dramatically, of which many could be asymptomatic and infectious. We found that the dilution of CO2 was insufficient in the CT room. Though it may not reflect the fact that the circulating cooling airflow for the CT scanner, which can be up to 4,000 m3/h, may filter out some pathogen‐laden aerosols, the CT room still has a high risk of environmental contamination. A recent study found that the inner space of a CT scanner can contain SARS‐CoV‐2 because it takes in room air for cooling. 48 The CT room is rarely located in the outer zones of a hospital building complex because of radioactivity concerns. The CT scanner must have sufficient ventilation and thorough cleaning. In Changgung Hospital, the CT room was conducted a systematic protocol to clean the whole room after scanning a COVID‐19 patient. Surface wiping, ultraviolet germicidal irradiation, and dilution with door opening were implemented, to minimize the cross‐infection risk.
Indoor CO2 concentration represents the comprehensive effects of occupancy and the outdoor airflow rate. However, ventilation can be sufficient to maintain a relatively low CO2 concentration in an overcrowded room, which is dangerous for diseases that can be transmitted through close contact, such as COVID‐19. Therefore, the number of occupants and social distancing should be controlled in hospitals to prevent nosocomial infection, especially in a pandemic. On the basis of occupancy control measures, the outdoor air ventilation condition should be checked using the upper limit of the CO2 concentration as an indicator. Exceedance of the upper limit indicates that the outdoor air supply should be enhanced. For mechanically ventilated rooms, natural ventilation can be introduced if outer windows or doors are present and the ventilation system can be adjusted. For naturally ventilated rooms, outer windows or doors should remain wide open or mechanical ventilation measures should be taken. However, if the outdoor airflow cannot be enhanced, air purifiers can be introduced. 51 Furthermore, clinic spaces in which patients with aerosol infectious diseases are likely to be present should be located in the outer spaces of outpatient buildings to introduce natural ventilation quickly and easily at the beginning of a pandemic.
5. CONCLUSION
It is affordable and effective to deploy CO2 sensors to reveal nosocomial SARS‐CoV‐2 infection potential. The CO2 concentration and relative indicators can help HCWs understand the ventilation performance against a crowded hospital environment. Actual outdoor airflow rates per person with the presence of COVID‐19 patients in each site were estimated and sorted into four categories for reference for the design (adjustment) of ventilation systems in new (built) hospitals to prevent SARS‐CoV‐2 nosocomial infection. The conclusions are as follows:
(1) Ventilation conditions can be easily evaluated using the real‐time CO2 concentration as an indicator. We recommend that hospitals install a CO2 concentration monitoring system to provide a warning of the risk of nosocomial infection due to poor ventilation.
(2) According to this case study conducted in Changgung Hospital, outdoor airflow rate per person of 15–18 L/(s·person) was sufficient to prevent nosocomial infection when there was only one COVID‐19 patient at each site under different contact situations, and 21 L/(s·person) was sufficient during throat swab sampling. For where COVID‐19 patients would visit and remove their masks for less than 30 s, the outdoor airflow rate per person required to prevent nosocomial infection may increase linearly with the number of indoor COVID‐19 patients.
Furthermore, we suggest that further hospital environmental CO2 monitoring studies should collect more correlating information, including the real‐time outdoor CO2 concentration, the state of the building exterior envelope, the operating situation of mechanical ventilation systems, and the patients’ behavior.
CONFLICT OF INTEREST
No conflict of interest was declared.
AUTHOR CONTRIBUTIONS
BRL, MGL, and LL conducted conceptualization. YRL, YFL, HZ, and LL conducted data curation. YRL, YFL, and LL conducted formal analysis. MGL and LL conducted funding acquisition. YRL, YFL, HZ, BRL, and LL conducted methodology. HJX, MGL, and LL conducted project administration. JLL, ZZZ, HJX, and MGL conducted resources. HZ and BRL conducted software. BRL, MGL, and LL conducted supervision. JLL and ZZZ conducted validation. YRL and YFL conducted writing‐original draft. YRL, YFL, BRL, MGL, and LL conducted writing‐review and editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ina.12899.
Supporting information
Appendix S1
ACKNOWLEDGEMENT
The authors would like to thank Yizhou Bai, Zhe Zhao, Kaili Chen, Xiaohui Wang, and Jiao Wei from Beijing Tsinghua Changgung Hospital, for their thorough support in sensor deployment, equipment maintenance, and data sharing.
Lu Y, Li Y, Zhou H, et al. Affordable measures to monitor and alarm nosocomial SARS‐CoV‐2 infection due to poor ventilation. Indoor Air. 2021;31:1833–1842. 10.1111/ina.12899
Yiran Lu and Yifan Li contributed equally to this work.
Funding information
This study was supported by the National Natural Science Foundation of China (51778520) and Beijing Tsinghua Changgung Hospital COVID‐19 project (12020Z1003)
Contributor Information
Borong Lin, Email: linbr@tsinghua.edu.cn.
Minggui Lin, Email: linminggui309301@126.com.
Li Liu, Email: liuli_archi@tsinghua.edu.cn.
REFERENCES
- 1. World Health Organization (WHO) . WHO coronavirus disease (COVID‐9) dashboard. https://covid19.who.int/. Accessed February 6, 2020.
- 2. Meredith LW, Hamilton WL, Warne B, et al. Rapid implementation of SARS‐CoV‐2 sequencing to investigate cases of health‐care associated COVID‐19: a prospective genomic surveillance study. Lancet Infect Dis. 2020;20(11):1263‐1271. 10.1016/S1473-3099(20)30562-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khan KS, Reed‐Embleton H, Lewis J, Saldanha J, Mahmud S. Does nosocomial COVID‐19 result in increased 30‐day mortality? A multi‐centre observational study to identify risk factors for worse outcomes in patients with COVID‐19. J Hosp Infect. 2021;107:91‐94. 10.1016/j.jhin.2020.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID‐19 among front‐line health‐care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475‐e483. 10.1016/S2468-2667(20)30164-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richterman A, Meyerowitz EA, Cevik M. Hospital‐acquired SARS‐CoV‐2 infection: lessons for public health. JAMA. 2020;324(21):2155‐2156. 10.1001/jama.2020.21399 [DOI] [PubMed] [Google Scholar]
- 6. Suárez‐García I, de Aramayona M, López MJ, Sáez Vicente A, Lobo AP. SARS‐CoV‐2 infection among healthcare workers in a hospital in Madrid, Spain. J Hosp Infect. 2020;106(2):357‐363. 10.1016/j.jhin.2020.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heinzerling A, Stuckey MJ, Scheuer T, et al. Transmission of COVID‐19 to health care personnel during exposures to a hospitalized patient–Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):472‐476. 10.15585/mmwr.mm6915e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid‐19 in a long‐term care facility in king county. Washington. N Engl J Med. 2020;382(21):2005‐2011. 10.1056/NEJMoa2005412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS‐CoV‐2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081‐2090. 10.1056/NEJMoa2008457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhan M, Qin Y, Xue X, Zhu S. Death from Covid‐19 of 23 health care workers in china. N Engl J Med. 2020;382(23):2267‐2268. 10.1056/NEJMc2005696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erdem H, Lucey DR. Healthcare worker infections and deaths due to COVID‐19: a survey from 37 nations and a call for WHO to post national data on their website. Int J Infect Dis. 2021;102:239‐241. 10.1016/j.ijid.2020.10.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carter B, Collins JT, Barlow‐Pay F, et al. Nosocomial COVID‐19 infection: examining the risk of mortality. The COPE‐nosocomial study (COVID in Older People). J Hosp Infect. 2020;106(2):376‐384. 10.1016/j.jhin.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730‐1741. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 15. Ranney ML, Griffeth V, Jha AK. Critical supply shortages–the need for ventilators and personal protective equipment during the Covid‐19 pandemic. N Engl J Med. 2020;382(18):e41. 10.1056/NEJMp2006141 [DOI] [PubMed] [Google Scholar]
- 16. Lee IK, Wang CC, Lin MC, Kung CT, Lan KC, Lee CT. Effective strategies to prevent coronavirus disease‐2019 (COVID‐19) outbreak in hospital. J Hosp Infect. 2020;105(1):102‐103. 10.1016/j.jhin.2020.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boccia S, Ricciardi W, Ioannidis JPA. what other countries can learn from Italy during the COVID‐19 pandemic. JAMA Intern Med. 2020;180(7):927‐928. 10.1001/jamainternmed.2020.1447 [DOI] [PubMed] [Google Scholar]
- 18. Somsen GA, van Rijn C, Kooij S, Bem RA, Bonn D. Small droplet aerosols in poorly ventilated spaces and SARS‐CoV‐2 transmission. Lancet Respir Med. 2020;8(7):658‐659. 10.1016/S2213-2600(20)30245-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu L, Li Y, Nielsen PV, Wei J, Jensen RL. Short‐range airborne transmission of expiratory droplets between two people. Indoor Air. 2017;27(2):452‐462. 10.1111/ina.12314 [DOI] [PubMed] [Google Scholar]
- 20. Chen W, Zhang N, Wei J, Yen H, Li Y. Short‐range airborne route dominates exposure of respiratory infection during close contact. Build Environ. 2020;176:106859. 10.1016/j.buildenv.2020.106859 [DOI] [Google Scholar]
- 21. Morawska L, Tang JW, Bahnfleth W, et al. How can airborne transmission of COVID‐19 indoors be minimised? Environ Int. 2020;142:105832. 10.1016/j.envint.2020.105832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma J, Qi X, Chen H, et al. COVID‐19 patients in earlier stages exhaled millions of SARS‐CoV‐2 per hour. Clin Infect Dis. 2021;72(10):e652‐e654. 10.1093/cid/ciaa1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baric RS. Emergence of a highly fit SARS‐CoV‐2 variant. N Engl J Med. 2020;383(27):2684‐2686. 10.1056/NEJMcibr2032888 [DOI] [PubMed] [Google Scholar]
- 24. Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person‐to‐person transmission of SARS‐CoV‐2 and COVID‐19: a systematic review and meta‐analysis. Lancet. 2020;395(10242):1973‐1987. 10.1016/S0140-6736(20)31142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26(5):676‐680. 10.1038/s41591-020-0843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lotfinejad N, Peters A, Pittet D. Hand hygiene and the novel coronavirus pandemic: the role of healthcare workers. J Hosp Infect. 2020;105(4):776‐777. 10.1016/j.jhin.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ueki H, Furusawa Y, Iwatsuki‐Horimoto K, et al. Effectiveness of face masks in preventing airborne transmission of SARS‐CoV‐2. mSphere. 2020;5(5):e00637‐e720. 10.1128/mSphere.00637-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Worby CJ, Chang HH. Face mask use in the general population and optimal resource allocation during the COVID‐19 pandemic. Nat Commun. 2020;11(1):4049. 10.1038/s41467-020-17922-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adams JG, Walls RM. Supporting the health care workforce during the COVID‐19 global epidemic. JAMA. 2020;323(15):1439‐1440. 10.1001/jama.2020.3972 [DOI] [PubMed] [Google Scholar]
- 30. Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS‐CoV‐2 in two Wuhan hospitals. Nature. 2020;582(7813):557‐560. 10.1038/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- 31. Lu J, Gu J, Li K, et al. COVID‐19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26(7):1628‐1631. 10.3201/eid2607.200764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. 2020;382(16):1564‐1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo Z, Wang Z, Zhang S, et al. Aerosl and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital Wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26(7):1586‐1591. 10.3201/eid2607.200885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang JW, Li Y, Eames I, Chan PK, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100‐114. 10.1016/j.jhin.2006.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qian H, Miao T, Liu L, Zheng X, Luo D, Li Y. Indoor transmission of SARS‐CoV‐2. Indoor Air. 2021;31(3):639‐645. 10.1111/ina.12766 [DOI] [PubMed] [Google Scholar]
- 36. Wake RM, Morgan M, Choi J, Winn S. Reducing nosocomial transmission of COVID‐19: implementation of a COVID‐19 triage system. Clin Med. 2020;20(5):e141‐e145. 10.7861/clinmed.2020-0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Allen JG, Marr LC. Recognizing and controlling airborne transmission of SARS‐CoV‐2 in indoor environments. Indoor Air. 2020;30(4):557‐558. 10.1111/ina.12697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhagat RK, Davies Wykes MS, Dalziel SB, Linden PF. Effects of ventilation on the indoor spread of COVID‐19. J Fluid Mech. 2020;903:F1–F18. 10.1017/jfm.2020.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Esbin MN, Whitney ON, Chong S, Maurer A, Darzacq X, Tjian R. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID‐19 detection. RNA. 2020;26(7):771‐783. 10.1261/rna.076232.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou J, Otter JA, Price JR, et al. Investigating SARS‐CoV‐2 surface and air contamination in an acute healthcare setting during the peak of the COVID‐19 pandemic in London. Clin Infect Dis. 2020;ciaa905. 10.1093/cid/ciaa905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ahn JY, An S, Sohn Y, et al. Environmental contamination in the isolation rooms of COVID‐19 patients with severe pneumonia requiring mechanical ventilation or high‐flow oxygen therapy. J Hosp Infect. 2020;106(3):570‐576. 10.1016/j.jhin.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chia PY, Coleman KK, Tan YK, et al. Detection of air and surface contamination by SARS‐CoV‐2 in hospital rooms of infected patients. Nat Commun. 2020;11(1):2800. 10.1038/s41467-020-16670-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ong SWX, Tan YK, Chia PY, et al. Air, Surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) from a symptomatic patient. JAMA. 2020;323(16):1610‐1612. 10.1001/jama.2020.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghosh B, Lal H, Srivastava A. Review of bioaerosols in indoor environment with special reference to sampling, analysis and control mechanisms. Environ Int. 2015;85:254‐272. 10.1016/j.envint.2015.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rudnick SN, Milton DK. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air. 2003;13(3):237‐245. 10.1034/j.1600-0668.2003.00189.x [DOI] [PubMed] [Google Scholar]
- 46. Liu Y, Wang X, Li M, et al. Carbon dioxide generation rates of different age and gender under various activity levels. Build Environ. 2020;186:107317. 10.1016/j.buildenv.2020.107317 [DOI] [Google Scholar]
- 47. Sun CX, He B, Mu D, et al. Public awareness and mask usage during the COVID‐19 epidemic: a survey by China CDC new media. Biomed Environ Sci. 2020;33(8):639‐645. 10.3967/bes2020.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matos J, Paparo F, Mori M, et al. Contamination inside CT gantry in the SARS‐CoV‐2 era. Eur Radiol Exp. 2020;4(1):55. 10.1186/s41747-020-00182-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ye G, Lin H, Chen S, et al. Environmental contamination of SARS‐CoV‐2 in healthcare premises. J Infect. 2020;81(2):e1‐e5. 10.1016/j.jinf.2020.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xing Y, Wong GWK, Ni W, Hu X, Xing Q. Rapid response to an outbreak in Qingdao, China. N Engl J Med. 2020;383(23):e129. 10.1056/NEJMc2032361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao B, Liu Y, Chen C. Air purifiers: a supplementary measure to remove airborne SARS‐CoV‐2. Build Environ. 2020;177:106918. 10.1016/j.buildenv.2020.106918 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


