Abstract
Chitosan is an abundant organic polysaccharide, which can be relatively easily obtained by chemical modification of animal or fungal source materials. Chitosan and its derivatives have been shown to exhibit direct antiviral activity, to be useful vaccine adjuvants and to have potential anti‐SARS‐CoV‐2 activity. This thorough and timely review looks at the recent history of investigations into the role of chitosan and its derivatives as an antiviral agent and proposes a future application in the treatment of endemic SARS‐CoV‐2.
Keywords: adjuvant, antimicrobial, antiviral, chitosan, coronavirus, COVID, nanoparticles, vaccine
INTRODUCTION
Chitosan is a linear polysaccharide composed of randomly distributed β‐(1 → 4)‐linked d‐glucosamine and N‐acetyl‐d‐glucosamine (Figure 1). The chain length of chitosan is variable, but most chitosan products exhibit a molecular weight range of 3800–200,000 Daltons.
FIGURE 1.
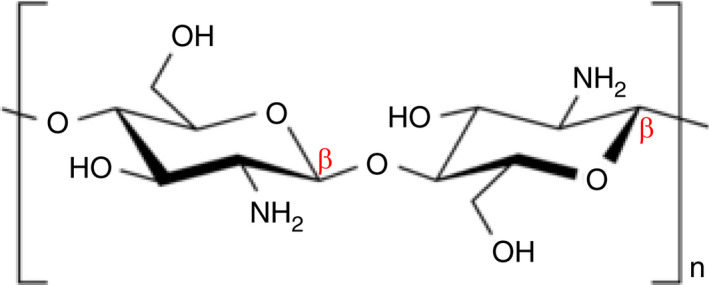
Schematic diagram of the basic molecular structure of deacetylated chitosan, where n indicates the repeat of the formula, resulting in the molecular weight range of 3800–20,000 Daltons (Qniemiec; https://commons.wikimedia.org/wiki/File:Chitosan_chair.png)
Most chitosan of commercial use is synthesized via the alkaline treatment (e.g. NaOH) and deacetylation of chitin extracted from shrimp shells. However, increasingly, chitosan can also be synthesized from fungal chitin grown in industrial quantities.
Chitosan has several uses, among which are biocontrol in horticulture and agriculture (Devappa et al., 2021), wine finings (Ivić et al., 2021), wound dressings to staunch bleeding (Amirian et al., 2021), bioprinting (Costantini et al., 2021), weight loss (Qinna et al., 2013) and as an edible antimicrobial film in food production (Amor et al., 2021). Increasingly, research has been undertaken as to the role of chitosan as a drug delivery agent and vaccine adjuvant (Idris et al., 2021).
Among the historical and current uses for chitosan is a continuing suggestion in the literature that it possesses antimicrobial properties (Amor et al., 2021). A literature survey of the potential antimicrobial properties of chitosan dating back to the 1960s has revealed sporadic interest in this characteristic as evidenced by the work of several groups. Papavizas et al. (1962) studied the use of chitosan in the inhibition of the plant pathogen Rhizoctonia solani. Muzzarelli et al. (1990) studied the potential antimicrobial activity of N‐carboxybutyl chitosan as a wound dressing against a panel of test micro‐organisms including Staphylococcus aureus, Streptococcus spp., Enterobacter faecalis and Candida spp. They found considerable antimicrobial activity across all the groups of micro‐organisms tested and suggested a complex mode of action resulting in cell membrane disruption and subsequent loss of membrane function. Omura et al. (2003) showed that the antimicrobial activity of chitosan against Bacillus subtilis, Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa was dependent upon the degree of acetylation and the molecular weight of the chitosan used. Benhabiles et al. (2012) studied chitin, chitosan and several chitosan oligomers of shrimp origin (Parapenaeus longirostris) as antibacterial agents against a panel of Gram‐positive and Gram‐negative bacteria, including P. aeruginosa, Salmonella typhimurium, Vibrio cholerae and B. subtilis. They observed significant activity against all tested bacteria. The use of chitosan as a plant protective agent against fungi, yeasts and bacteria led to research into its anti‐bacteriophage activity (Pospieszny et al., 1991) and subsequently into its general antiviral activity, which was summarized in an earlier review by Chirkov (2002). This review undertakes a logical assessment of chitosan's antiviral activity, commencing with its anti‐bacteriophage activity, then plant viruses and finally animal viruses. It is this latter activity which underpins our current interest.
In addition to chitosan's proven antimicrobial and antiviral properties, it also has a role in the development of antiviral vaccines due to its adjuvant properties (Van der Weken et al., 2021). Additionally, the use of chitosan in the development of nanoparticle vaccines to treat arbovirus disease has been suggested by de Souza et al. (2021). In the last 12–18 months, such suggestions have led to the investigation of chitosan as a potential treatment for SARS‐CoV‐2 (the aetiological agent of COVID‐19) (Gurunathan et al., 2020; Pyrć et al., 2020). It is our long‐standing interest in chitosan as a pharmaceutical agent and these latter observations pertaining to chitosan and SARS‐CoV‐19 that have led us to reconsider the antiviral properties of chitosan in this timely review. The potential role of chitosan in the therapy of viral infections is summarized in Figure 2. This will form the basis of the subsequent discussions in this review.
FIGURE 2.
Schematic diagram illustrating the potential role of chitosan in controlling viral infections
ANTIVIRAL ACTIVITY OF CHITOSAN AND ITS DERIVATIVES
Previous research has shown that marine polysaccharides and oligosaccharides derived from chitosan, carrageenans, alginates and fucans have proven antiviral activity (He et al., 2019; Wang et al., 2012). Chitosan antiviral activity in plants has been thoroughly investigated by Chirkov (2002), Davydova et al. (2011) and He et al. (2019). They found that chitosan can suppress viral infections in many plant species belonging to different families. Chitosan was shown to affect the hypersensitivity response to plant viral infections, as well as preventing the systemic spreading of the virus in the plant (Chirkov, 2002; Davydova et al., 2011). Some reports indicated that chitosan has no direct antiviral activity, but instead it stimulates the immune response of the plant and improves its general defence mechanisms (Iriti & Varoni, 2015; Iriti & Varoni, 2016). Ai et al. (2012) studied the antiviral activity of chitosan from the larvae of houseflies (Musca domestica). They used chitosan to treat silkworm larvae (Bombyx mori L.) infected with B. mori nucleopolyhedrosis virus (BmNPV occlusion‐derived virus [ODV]). Results showed that the larval mortality rate decreased significantly, thus indicating that chitosan has BmNPV ODV antiviral bioactivity. They also reported that chitosan can inhibit Autographa californica nucleopolyhedrovirus (AcMNPV) and BmNPV infections as well as providing helpful information about antiviral breeding technology for insect and plant pathology (Ai et al., 2012). Only a few studies on the antiviral activity of chitosan in animals have been reported. The earlier review by Chirkov (2002) suggests that chitosan inhibits viral infections in animal cells, but gives little evidence. However, since then, several studies have reported that chitosan has antiviral activity against human cytomegalovirus strain AD169 and H1N1 influenza A virus (Divya et al., 2017; Jarach et al., 2020). Intranasal preparation of chitosan was administered to BALB/c mice to stimulate the innate immune system against H7N9 Influenza viral infection that is normally highly pathogenic to humans. Results showed that chitosan was effective in protecting the mice against this virus and three other virus strains tested. These studies highlight the promising potential of chitosan as an anti‐influenza agent (Mohammadi et al., 2020; Popescu et al., 2020; Zheng et al., 2016). Hassan et al. (2016) tested the antiviral activity of chitosan against Rift Valley Fever virus (RVFV), Herpes Simplex‐1 (HSV‐1) and Coxsackie viruses. Results showed a reduction in the infectivity titres of the three viruses (Hassan et al., 2016). In conclusion, it is obvious that chitosan has antiviral activity in various biological systems. Properties of chitosan polymers determine its efficacy in viral suppression. Chitosan concentration, molecular weight, degree of acetylation and amination, as well as chemical modification play an important role in its antiviral properties (Chirkov, 2002). Low concentrations (0.1 mg/ml) of high molecular weight chitosan have been shown to suppress potato virus X (PVX) and tobacco mosaic virus (TMV) in a third to a half of treated potato and tomato plants, whereas higher concentrations of chitosan solution (1 mg/ml) suppressed the viral infection in 80% of the treated plants. These results illuminate the effect of concentration on antiviral activity of chitosan (Kulikov et al., 2006). The results of these and other studies are summarized in Table 1. Many studies show that chitosan antiviral activity increases as its molecular weight decreases (Davydova et al., 2011; Iriti & Varoni, 2015; Kulikov et al., 2006). On the other hand, data from other studies indicate that high molecular weight chitosan possesses higher antiviral properties against PVX infection in potato plants (Chirkov et al., 2001, 1998). Davydova et al. (2011) reported that antiviral activity is weakly affected by its degree of acetylation (Davydova et al., 2011). Divya et al. (2017) suggest that the antimicrobial properties of chitosan are greatly affected by the repeated amino groups on the backbone of the polymer structure. Other researchers reported that deaminated chitosan has a higher antiviral activity (Chirkov, 2002). This controversy has led researchers to study the structure of chitosan and its associated antiviral activity relationship more precisely. Many chemical modifications have been performed on the chitosan backbone, resulting in the production of different polymeric derivatives that possess novel antiviral activity (He et al., 2019; Mori et al., 2013; Wang et al., 2012; Zhou, Hu, et al., 2020; Zhou, Shuxiang, et al., 2020). He et al. (2019) studied the antiviral activity of 6‐amine chitosan derivatives using haemagglutination tests. They found that these derivatives have more antiviral activity, especially those with a bromine ion. 6‐deoxy‐6‐bromo‐N‐phthaloyl chitosan reduced the observed haemagglutination titre of virus to zero. On the other hand, the real‐time polymerase chain reaction (RT‐PCR) results indicated that amino‐modified chitosan stimulates the immune response as well as inhibiting human immunodeficiency virus (HIV) viral transcription (He et al., 2019). Many studies have shown the effectiveness of sulphonated chitosan derivatives as antiviral agents. Two groups have shown that sulphated synthetic galactomannans possess anti‐HIV activity with low cytotoxicity (Budragchaa et al., 2015; Dimassi et al., 2018). N‐carboxymethylchitosan N,O‐sulphate has also exhibited activity against HIV by inhibition of HIV‐reverse transcriptase (Dimassi et al., 2018). It has been suggested that the viral inhibitory activity of anionic derivatives depends upon the position of the sulphate group on the chitosan backbone and whether it is at O2 and/or O3 positions in the glucosamine residue (Chirkov, 2002). The antiviral activity of guanidinylated chitosan hydrochloride against TMV has been evaluated. Results indicate a higher inhibitory rate for chitosan hydrochloride than for chitosan and a subsequent increase in plant resistance against TMV HIV (Hu et al., 2009). A fluorinated chitosan oligomer decreases viral replication from the norm to 40% in the infected cells, hence confirming the antiviral activity of chitosan derivatives (Kim et al., 1999). Figure 3 summarizes the proven antiviral activity of chitosan against several human viruses.
TABLE 1.
Summary of the studies for the investigation of chitosan and chitosan derivatives as antiviral agents
| Virus type |
Type of Chitosan a Type of virus host used |
Chitosan concentration/dose | Antiviral activity | ET | TºC | pH | References |
|---|---|---|---|---|---|---|---|
| BmNPV ODV |
The chitosan was prepared from larvae of housefly Fresh leaves of mulberry—53rd silk worm larva for BmNPV The Sf9 cells were used for AcMnPV |
0.5 mg/ml chitosan | The virus titre TCID50/ml declined significantly from 2.0 × 107 to 2.8 × 106 | 24 h | 37 | 7.4 | Ai et al. (2012) |
| AcMNPV | |||||||
| 1.0 mg/ml | The virus titre TCID50/ml declined from 2.0 × 107 to 3.3 × 105 | 1 h | 28 | 7.4 | |||
| HCMV strain AD169 |
Chitosan of medium molecular weight (viscosity 200–800 cP, 1% w/v in 1% v/v acetic acid), with a degree of deacetylation of 85% Human embryonic lung fibroblasts |
4.5 mg chitosan/8.8–14 mg Fostcanet antiviral drug used as nanoparticle delivery system | IC50 40–58 µM for nanoparticle delivery system compared to free Fostcanet 41 μM. The system maintained the activity of Fostcanet and could be useful in improving blood residence time and prolongation of contact of the drug with CMV‐infected cells. | 3–5 days | 37 | Acid to neut. | Russo et al. (2014) |
| H1N1 Influenza A virus |
Chitosan (average molecular weight 54 kg/mol, deacetylation ratio 84%). Madin–Darby Canine Kidney cells |
10 mg/ml chitosan with Ag nanoparticles | No antiviral activity was observed for chitosan alone; however, Ag nanoparticles bound to chitosan showed significant antiviral activity | 1 h | RT | 5.2–6.3 | Mori et al. (2013) |
| H7N9 |
0.4% (w/v) solution of chitosan was prepared in sodium acetate solution at a concentration of 25mmol/L Mice intranasal dosing |
100 μg chitosan dose intranasal route mice | The lung virus titres (log TCID50/ml) decreased from 6.63 to 3.38 | 7 days | 37 | 5.0 | Zheng et al. (2016) |
| RVFV |
The degree of deacetylation (DDA) of chitosan was 80.5% dissolved in 0.1 M acetic acid. Vero cells |
60μg/ml chitosan | The log cycle difference was 1.6 with reduction of 24.9% | 24 h | 37 | Acid | Hassan et al. (2016) |
| HSV‐1 | One log cycle difference and the reduction percent was 18.8% | ||||||
| Coxsackie virus | One log cycle difference and the reduction percent was 26.1% | ||||||
| Potato Virus X |
Crab chitosan low molecular weight chitosan 2.2 and 1.2 kDa with deacetylation degree of 85% Leaves of Phaseolus vulgaris L. cultivar Contender bean plant |
100 µg/ml chitosan solution was sprayed on leaves | Complete suppression of virus infection after applying chitosan on the leaves using enzyme immunoassay test | 14 days | RT | 5.8 | Kulikov et al. (2006) |
| Tobacco Mosaic Virus | |||||||
| Newcastle disease virus |
6‐deoxy‐6‐bromo‐N‐phthaloyl chitosan derivative Virus was inoculated in embryonated chicken SPF eggs |
1 g/L chitosan derivative dissolved in physiological saline | Reduced the hemagglutination titre of virus to zero | 72 h | 37 | Neut. | He et al. (2019) |
| Tobacco mosaic virus |
Guanidine derivatives of chitosan with molecular weight 210 kDa The deacetylation degree was determined as 91.6% N. glutinos and N. tobacum plants |
1 mg/ml chitosan derivative solution used on plants |
The inhibitory effect was evaluated using special Light Bioculturer to investigate the local lesions on half leaves The chitosan derivative resulted in 52% of inhibitory effect |
12 h | RT | 7.2 | Hu et al. (2009) |
| Hepatitis C virus |
Curcumin chitosan nanocomposite Human hepatoma cells Huh7 |
20 µg/ml of 4% of curcumin encapsulated into chitosan nanocomposite |
Real‐time PCR test antiviral activity High antiviral activity against entry of HCV into Huh7 cells by almost 100%reduction in viral titre |
24 h | 37 | NA | Loutfy et al. (2020) |
| HSV‐1 and HSV‐2 |
Acyclovir loaded in 1% w/w chitosan in acetic acid (medium MW, deacetylation 75–85%) nanoemulsion Applied on African green monkey kidney (Vero) cells |
The loading capacity of the drug was about 8.5% |
Virus yield reduction assay showed IC50 values against HSV‐1 at 48 h was 0.012 µM for acyclovir‐loaded nanoemulsion and 0.156 µM for free acyclovir IC50 obtained against HSV‐2 determined at 24 h post‐infection were 0.100 µM for acyclovir‐loaded nanoemulsion and 1.608 µM for free acyclovir |
24 h 48 h | 37 | Acid | Donalisio et al. (2018) |
| HIV‐1 |
Chitosan–zinc stabilized (degree of acetylation (DA)~4%, average molar mass (Mw)~5.8 × 105 g/mol) and chondroitin sulphate polyelectrolyte complex for delivery of Tenofovir Human peripheral blood mononuclear cells |
Chitosan 0.1%–0.2% w/v in acetic acid solution | IC50 was decreased from 4.35 µmol/L for aqueous Tenofovir to 1.95 µmol/L for Tenofovir‐loaded polyelectrolyte complex nanoparticles | NA | 37 | 4–5.5 | Wu et al. (2016) |
| Feline Infectious Peritonitis virus |
20 µM Curcumin loaded in low molecular weight chitosan (75%–85%) deacetylated CrFK cell culture |
Different concentrations of curcumin were added to a fixed concentration of chitosan nanoparticles in ratios of 0.5:1, 0.75:1 and 1:1 | Cur‐CS nanoparticles exhibited antiviral effects, with an SI value three times higher than that of curcumin | 24 h 48 h | 37 | 3.0 | Ng et al. (2020) |
| Hepatitis A virus |
Polyquaternary phosphonium oligochitosans/nanosilver Vero cells – Hepatitis A virus Feline calici virus – Crandell Reese feline kidney cells Coxsackie virus B4‐Hep 2 cell lines |
100 μl/ml polyquaternary phosphonium oligochitosans/nanosilver | Maximum viral percent reduction was 41.4% | 5 days | 37 | 3.0–9.0 | Sofy et al. (2019) |
| Norovirus/Feline calici virus | Reduction 80.6% | ||||||
| Coxsackie virus B4 | Reduction 84.0% |
Abbreviations: AcMNPV, Autographa californica nucleopolyhedrovirus; NA , no available information; ET, exposure time; T°C, Temperature in °C; RT, room temperature; h, hour.
Chitosan quality was not fully described in terms of source, molecular weight, degree of deacetylation, purity, percent ash and protein content.
FIGURE 3.
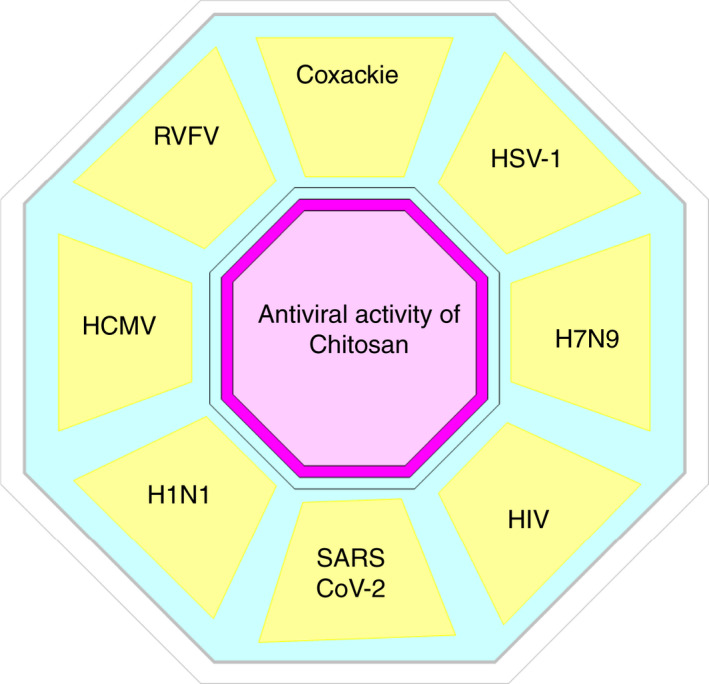
The tested and proven antiviral activity of chitosan against several human viruses, where HSV‐1 is herpes simplex virus type 1; H7N9 is an avian‐origin influenza A virus; HIV is human immunodeficiency virus; SARS CoV‐2 is severe acute respiratory syndrome Coronavirus‐2; H1N1 is pandemic 2009 influenza A virus; HCMV is human cytomegalovirus and RVFV is Rift Valley fever Virus
PROPOSED MECHANISMS OF ACTION OF CHITOSAN AND ITS DERIVATIVES AS AN ANTIVIRAL AGENT
In the last few years, there has been great interest in the development of novel drugs and the re‐purposing of currently available antiviral medications to fight novel viral infections. This development is partially due to most novel viral infections having no associated licensed antiviral drug or vaccine (Pardi and Weissman, 2020). Although the efforts to produce vaccines are ongoing, there is still a need for prophylactic intervention against novel viral infections, especially as some of the more promising vaccine technologies neutralize the systemic virus, but not viral particles within the nasal epithelia (Sahebnasagh et al., 2020; Pyrć et al., 2020). In this context, great interest has been directed towards natural biopolymer compounds as natural antiviral candidates (Randazzo et al., 2018; Sofy et al., 2019). Here, we review the proposed mechanisms of action of chitosan and its derivatives used to prevent or limit viral infection in general and COVID‐19 infections in particular. Recently, the antiviral activity of chitosan and its derivatives has been studied by several groups (Donalisio et al., 2020; Hamed et al., 2020; He et al., 2019; Paul et al., 2019; Taubner et al., 2020; Wang et al., 2020). Despite these extensive published reports, the exact antiviral mechanism of chitosan and its derivatives is not fully understood (Matica et al., 2019). Moreover, several factors may contribute to the antiviral action of any novel antiviral agent. In addition to the inherent physical, chemical and biological properties of chitosan, its biodegradability, biocompatibility, antimicrobial, antiviral, non‐toxicity, cost‐effectiveness, mucoadhesiveness and eco‐friendly nature make it a promising platform for the design and development of new derivatives and delivery/or target systems for viruses (Abd El‐Hack et al., 2020). Unfortunately, the poor aqueous solubility, low bioavailability and low thermal and mechanical stability of chitosan limit the widespread application of unmodified, native chitosan (Sofy et al., 2019; Lu et al., 2020). The surface functionalization of chitosan (i.e. the development of novel chitosan derivatives) may be used to tackle such limitations. By aiming to improve its physicochemical and biological properties and consequently, expand its range of applications (Riva et al., 2011). The activity of chitosan is dependent on many factors, such as type and species of micro‐organism, molecular weight and degree of deacetylation of chitosan, as well as the chemical structure and functionalization/derivatization of chitosan molecules (Goy et al., 2009; Hosseinnejad and Jafari, 2016). Here, we review the published proposed mechanisms of the antiviral activity of chitosan (Figure 4). A good understanding of the stages of viral infection to a host cell is crucial to understand the mechanisms of the antiviral activity of chitosan and its derivatives thereof. Traditionally, the viral life cycle is of four major steps: (i) attachment and entry into a target cell (Stages 1 and 2 in Figure 4), (ii) replication of the viral genome (Stages 3, 4, 5 and 6 in Figure 4), (iii) maturation of viral proteins and genome packaging into the infectious progeny (Stage 7 in Figure 4) and (iv) egress and dissemination to the next target cell (Stage 8 in Figure 4) (Jones et al., 2020).
FIGURE 4.
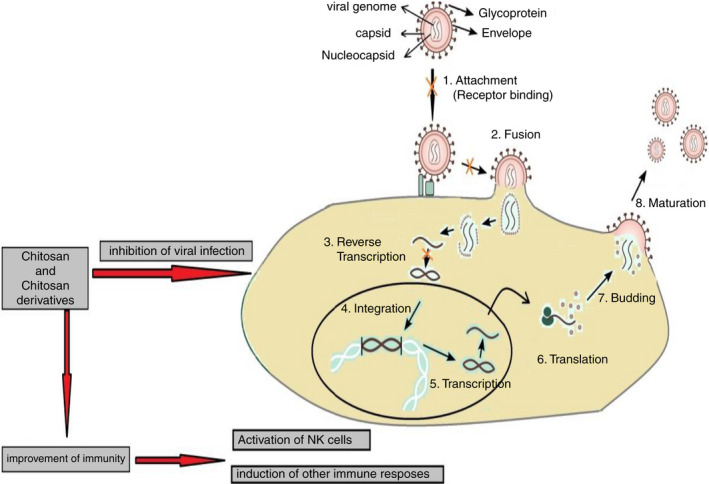
Schematic diagram of the proposed mechanisms of antiviral activity of chitosan (Adapted from Shi et al., 2017). The viral life cycle is of four major steps: (i) attachment and entry into a target cell (Stages 1 and 2), (ii) replication of the viral genome (Stages 3, 4, 5 and 6), (iii) maturation of viral proteins and genome packaging into the infectious progeny (Stage 7) and (iv) egress and dissemination to the next target cell (Stage 8)
Direct killing of the virus
Some studies have reported that some polysaccharides can directly enter the infected host cell and kill viruses in situ (Chen & Huang, 2018; Shi et al., 2017). The electrostatic interaction between the polycationic positive charge of chitosan and the negatively charged surface of the virus can inhibit the infectious ability of the virus and/or directly kill the virus by disrupting its protective membrane (Mansouri et al., 2004; Raafat & Sahl, 2009). N‐(2‐hydroxypropyl)‐3‐trimethylammonium chitosan chloride (HTCC), is a cationically modified chitosan derivative that exhibits effective inhibition of SARS‐CoV‐2 and Middle Eastern Respiratory Syndrome Coronavirus (MERS‐CoV) replication. The analysis of the mechanism of interaction revealed a complex formation between HTCC polymer and S‐protein of the virus, which blocks viral entry into the host cell (Milewska et al., 2021, 2020, 2016).
Inhibiting viral adsorption and subsequent host cell invasion
The initial step in the viral infectious life cycle is adsorption to the host cell through binding to the cell surface by electrostatic interaction, which is followed by subsequent viral invasion of the host cell. The viral invasion process is often associated with the endocytosis of the virus, the fusion of the viral particle with the host cell membrane and the subsequent translocation of the virus into the host cell. Gao et al., (2018) claimed that 3,6‐sulphated chitosan directly inhibits human papillomavirus (HPV) by binding to the viral capsid proteins and thus, blocking virus–host cell adsorption (Gao et al., 2018). Other sulphated chitosan‐oligosaccharide derivatives have been reported to block the interaction between HIV‐1gp120 and CD4+ cell surface receptors, which inhibits virus–host cell fusion and subsequent viral entry into the host cell. Moreover, sulphated polysaccharides can interact with the positively charged regions of cell surface glycoproteins, leading to a shielding effect on these regions, and thus preventing the binding of viruses to the cell surface (Artan et al., 2010; Gao et al., 2018). Sosa et al. (1991) showed that the N‐carboxymethylchitosan N,O‐sulphate, a polymer derived by the sulfation of N‐carboxymethyl chitosan, inhibits HIV‐1 adsorption to the cell surface receptor CD4+ and reverse transcription of the viral genome (Sosa et al., 1991). Water‐soluble chitosan is shown to decrease the infectivity of both feline calicivirus (FCV‐F9) and bacteriophage MS2 (an infectious agent of E. coli and other members of the Enterobacteriaceae), but having minimal effects on murine norovirus (MNV‐1). Chitosan oligosaccharide was found to be less effective in decreasing the infectivity of FCV‐F9 and MS2 than was water‐soluble chitosan. For MNV‐1, higher molecular weight chitosan, higher concentrations and longer incubation times may be necessary to cause a reduction in viral titres (Davis et al., 2012; Su et al., 2009).
Chitosan as a prophylactic antiviral agent (effects on immune system)
The mucous membrane that covers, for example, the nasal cavity and the respiratory system is a major part of the human immune system (Parkin and Cohen, 2001). Such mucosal membranes act as a mechanical and chemical barrier that limits the invasion and colonization of the body by foreign pathogens (Sperandio et al., 2015). In addition to this local effect, the mucosal membranes are interlinked with the lymphatic system, and thus are known as the mucosal immune system. The mucosal immune system has two major parts, the innate system and the adaptive system (Dunkelberger & Song, 2010). The innate system comprises various recognition molecules and natural killer cells, while the adaptive system comprises various antigen‐presenting cells and the T and B lymphocytes (Ryan et al., 2001). Most infectious agents enter the body at mucosal surfaces and therefore mucosal immune responses function as a first line of defence (Ryan et al., 2001). From the aforementioned discussion regarding the importance of the mucosal membranes as a part of the immune system, mucosal membranes have been a target for local immunization that could prevent the penetration and replication of the virus in the mucosa at a very early stage of host infection. Challenges of such local immunization compared with injection vaccination have been addressed and reviewed elsewhere (Holmgren et al., 2003). Intranasal delivery of vaccines is one of the most extensively tested methods to elicit both mucosal and systemic immune responses (Arulanandam et al., 2001; Cossette et al., 2020; Jackson & Herbst‐Kralovetz, 2012; Ku et al., 2020; Morabito et al., 2018; Perrone et al., 2009; Terauchi et al., 2018; Zheng et al., 2020; Zhou, Hu, et al., 2020; Zhou, Shuxiang, et al., 2020). The intranasal delivery of vaccines offers the possibility of self‐administration, higher patient compliance, and reduces the risk of cross‐infection by blood‐borne pathogens, especially in cases of mass vaccination (Jackson & Herbst‐Kralovetz, 2012). Because of the advantages of mucosal vaccination over injectable vaccination, much attention has been addressed to the development of effective and safe adjuvants specific for this route of vaccine administration. In this context, chitosan alone or its derivatives demonstrates a universal value as a prophylactic antiviral agent. To explore the universality of chitosan‐induced protective effects, the chitosan intranasal vaccine was tested against two strains of influenza virus, influenza A virus (H1N1) and avian influenza virus (H9N2). The data obtained provide strong evidence that intranasal administration of chitosan can induce an effective immune protection against diverse strains of influenza viruses (Sui, Chen, Fang, et al., 2010; Sui, Chen, Wu, et al., 2010; Zheng et al., 2016). Bedford et al. (2020) described an effective chitosan‐hydrogel‐based vaccine as a vaccination strategy for depositing highly protective local immunity within the nasal cavity mucosa and preventing the progress of severe lower respiratory tract infections. Such formulation provided effective protection against influenza virus and could easily be adapted to elicit nasal tissue‐resident memory (Trm) CD8+ T cells that are reactive against other relevant respiratory viruses, including SARS‐CoV‐2 (Bedford et al., 2020). In another study where native chitosan was administered intranasally to mice, Zheng et al. (2016) revealed a potent activation of mucosal immune responses through a significant enhancement of the infiltration of leukocytes into the bronchoalveolar lavage and elevated levels of pro‐inflammatory cytokines in the bronchus/lung tissues. Such action was able to completely protect the mice from otherwise lethal infectious doses of H7N9 influenza virus (Sui, Chen, Wu, et al., 2010). Of the tested chitosan derivatives, the RT‐PCR result showed that the expression level of tumour necrosis factor (TNF‐α) and interferon‐beta (IFN‐β) increased significantly, as a result of the application of the amino‐modified chitosan (6‐deoxy‐6‐bromo‐N‐phthaloyl chitosan), which was the result of the stimulation of immune response. Moreover, chitosan was used as an adjuvant in other types of vaccines. Chitosan augmented the efficacy of the nasally administered influenza matrix protein 2 (M2) without transmembrane matrix protein‐2 (sM2) and matrix protein 1 (M1). Such vaccines completely protected mice against the homologous virus and partially against the heterologous influenza A (H1N1) and avian influenza A (H5N1) viruses (Sui, Chen, Fang, et al., 2010; Sui, Chen, Wu, et al., 2010).
CHITOSAN NANO‐SYSTEMS AND CHITOSAN DERIVATIVES FOR THE DELIVERY OF ANTIVIRAL AGENTS
In recent years, nanotechnology has been applied to increase the effectiveness of chitosan as an antiviral agent (Gu et al., 2017; Rikta, 2019; Singh et al., 2019). Chitosan nanoparticles have improved physical characteristics, and can be used as carrier molecules to enhance the antiviral activity of certain antiviral agents. The antiviral activity of curcumin chitosan nanoparticles against hepatitis C virus (HCV) in human hepatoma cells was evaluated by Loutfy et al. (2020). Hepatitis C virus core protein expression confirmed 100% inhibition of viral entry and replication for both chitosan nanoparticles and for curcumin chitosan nanoparticles (Loutfy et al., 2020). These studies also evaluated the effect of chitosan nanoparticles in increasing the safety profile of the Sofosbuvir, which is a potent anti‐HCV agent. This work resulted in an in‐use dose reduction and a subsequent decrease in drug side effects and an improved antiviral efficiency (Loutfy et al., 2020). The solubility and oral bioavailability of an inhibitor of hepatitis B virus called Bay 41‐4109 was improved through formulation as chitosan nanoparticles for delivery. Results showed negligible toxicity and enhanced drug uptake (Xue et al., 2015). Anti‐retroviral agents used in the treatment of acquired immuno‐deficiency syndrome have drawbacks such as poor bioavailability and host cytotoxicity. The use of chitosan as a nano‐carrier for Saquinavir increases the bioavailability of the drug by increasing loading potential and cell targeting (Ramana et al., 2014). Frank et al. (2017) succeeded in the formulation of a semisolid preparation of the antiviral agent imiquimod for the treatment of vaginal papillomavirus (HPV) infections. They used chitosan to undertake nano‐encapsulation of the drug to increase the contact time with the vaginal tissue through mucoadhesion, which results in increased drug penetration (Frank et al., 2017). Liang et al. (2020) exhibited that 3,6‐sulphated chitosan inhibits HPV via direct binding to the viral capsid, which blocks subsequent viral adsorption to the host cell surface (Liang et al., 2020). Gómez‐Mascaraque et al. (2016) encapsulated (–)‐epigallocatechin gallate in electro‐sprayed chitosan capsules to prolong its antiviral activity against MNV. This sustained the drug release and protected it from degradation under host physiological conditions (Gómez‐Mascaraque et al., 2016). A nano‐emulsion of acyclovir was developed via encapsulation within chitosan nanospheres for topical administration of the skin for the treatment of HSV infections. This increased the local concentration of the drug. An in vitro assessment of acyclovir‐loaded chitosan nanospheres against HSV‐1 and HSV‐2 strains indicated a higher antiviral activity when compared to that of free acyclovir (Donalisio et al., 2018). Chitosan crosslinked tripolyphosphate nanoparticles encapsulated with acyclovir were evaluated as ocular drug delivery systems. Results were promising, since these nanoparticles have the ability to intimately contact the corneal and conjunctival surfaces with negligible associated toxicity (Calderón et al., 2013). Chitosan‐coated liposomes have been prepared to contain the novel antiviral agent Triazavirin. These liposomes were then assessed for their stability for 3 months and any changes in their mucoadhesive properties (Kozhikhova et al., 2018). Other researchers have evaluated the anti‐retroviral activity of Tenofovir disoproxil against HIV‐1 infection using zinc‐stabilized chitosan–chondroitin sulphate nano‐complex carriers. Results of the in vitro assessment of antiviral effects of this nanoparticle formulation exhibited a reduction in HIV‐1infection. Cytotoxicity studies also showed that this nano‐complex system is non‐toxic to humans (Wu et al., 2016). In in vitro tests, a total inhibition of HIV‐1 infection was obtained by chitosan–hyaluronan nano‐poly‐electrolyte complex surface decorated with anti‐α4β7 IgA (Wu et al., 2020). This group also found that chitosan‐based colloidal poly‐electrolyte complexes can be used as anti‐retroviral drug delivery systems against HIV infections (Wu et al., 2020). Curcumin has a previously confirmed antiviral activity (Mathew & Hsu, 2018) and is a natural photosensitizer. Randazzo et al. (2016) exhibited activity of photoactivated curcumin against norovirus surrogates, feline calicivirus (FCV) and MNV. Their results showed that photoactivated curcumin at 50 µg/ml reduced FCV titres by almost 5 log10 cycles after incubation at 37℃ for 30 min. A lower antiviral activity (0.73 log10 cycle TCID50/ml reduction) was reported for MNV. Such results indicate that photoactivated curcumin can be used as an alternative natural additive to reduce viral contamination (Randazzo et al., 2016). However, curcumin suffers from poor bioavailability. Ng et al. (2020) developed a delivery system of curcumin‐encapsulated chitosan nanoparticles and they assessed their antiviral activity against feline infectious peritonitis virus (FIPV) in cats. Results showed an improvement in the oral bioavailability of curcumin and a decrease in the immune‐related proteins in FIPV infections in the treated cats (Ng et al., 2020). Mori et al. (2013) exhibited significant antiviral activity of silver chitosan nanoparticles preparations against H1N1 Influenza A virus. However, the increase in activity was directly proportional to the concentration of silver nanoparticles and inversely proportional to the size of the particles. They suggested that silver chitosan nanoparticles interact with viruses and so exhibit antiviral activity (Jarach et al., 2020; Mori et al., 2013). Sofy et al. (2019) stabilized silver nanoparticles using polyquaternary phosphonium oligochitosans. This system has a synergistic action against enteric viruses such as FCV, Hepatitis A virus and Coxsackievirus (CoxB4) (Sofy et al., 2019).
Other researchers have suggested that many properties of chitosan make it a promising candidate for use in the delivery of antiviral drugs. For example, Sahraei et al. (2020) report that the antimicrobial drug chloroquine acts by increasing the pH of intracellular vacuoles, and altering protein degradation pathways through acidic hydrolases. On the other hand, other researchers have modified chloroquine into hydroxychloroquine, which is more soluble, less toxic and easier to attach to other compounds, such as chitosan. Hydroxychloroquine‐encapsulated chitosan nanoparticles have an increased pH and subsequently, this increases its antiviral activity against SARS‐CoV‐2 (Ejeromedoghene et al., 2020). Figure 5 summarizes the advantages of using chitosan as an antiviral nanodelivery system.
FIGURE 5.
Schematic diagram summarizing the potential advantages of chitosan as antiviral drug nanodelivery systems
CHITOSAN‐BASED ANTIVIRAL VACCINE FORMULATIONS
Many studies have investigated the use of adjuvants as a strategy to increase the immune response to selected vaccines including those for influenza. Chitosan has been used as an adjuvant for vaccine delivery because it has well‐known biosafety, biocompatibility and mucosal adsorption‐promoting properties (Şenel, 2019; Xing et al., 2018). Chitosan is capable of significantly enhancing cellular immune responses of nasally administered influenza, pertussis and diphtheria vaccines (Mohammed et al., 2017; Read et al., 2005). Norovirus virus‐like particle antigen has been formulated as a spray dried powder with chitosan and a monophosphoryl lipid immunity enhancer. Phase 1 clinical study results exhibited an induction of IgA secretions in the mucosal tissues after intranasal chitosan adjuvant vaccine administration (Mohammed et al., 2017). A complex coacervation method has been used to encapsulate a DNA vaccine in chitosan nanoparticles to be tested against swine influenza in mice. Results showed better immune responses in immunized mice with a prolonged release of the plasmid DNA compared to the DNA vaccine alone (Zhao et al., 2011). Gu et al. (2017) used the same method to prepare chitosan nanoparticles encapsulating siRNA (small interfering RNA) and conjugated with antibody. They assessed the antiviral activity of this dual system to deliver siRNA across the blood–brain barrier to inhibit HIV replication. Results exhibited an increase in cellular accumulation of siRNA and gene silencing compared to non‐modified chitosan nanoparticles and single antibody modified chitosan nanoparticles (Gu et al., 2017). A nasal vaccine consisting of pegylated chitosan microparticles loaded with Bordetella bronchiseptica dermonecrotoxin (a virulence factor of atrophic rhinitis causative agent) was prepared. Results on murine RAW264.7 cells exposed to B. bronchiseptica dermonecrotoxin‐loaded pegylated chitosan microspheres suggested that this system is a promising vaccine delivery systems (Xing et al., 2018). Yu et al. (2019) developed alginate chitosan‐coated nanocomposites to be used as oral vaccine delivery system for proteins. Release studies indicated the protein protection effect of chitosan against acidic environment. As well as flow cytometry analysis showed enhancement of protein internalization into human Caco2 and macrophage cells (Yu et al., 2019). A vaccine of Hepatitis B surface antigen was developed using glycol‐chitosan nanoparticles to be delivered via an intranasal route, to enhance mucosal immune responses. In vitro studies exhibited an increase in mucosal uptake while anti‐Hepatitis B surface antigen antibody titre test in mice confirmed the immunogenicity of the system. The suggested explanation for this observation was that glycol‐chitosan nanoparticles have better mucoadhesive properties, which results in a prolongation of the nasal residence time and a subsequent increase in vaccine antigen uptake by B cells (Pawar & Jaganathan, 2016). Other researchers loaded the surface antigen of hepatitis B (HBsAg) into alginate‐coated chitosan nanoparticles anchored with lipopolysaccharide for immunity enhancement. An alginate coat protected the chitosan nanoparticles from acidic conditions in the stomach. The system showed a significant increase in IgA at mucosal secretions and IgG antibodies in systemic circulation. Results were promising for oral mucosal vaccination (Saraf et al., 2020). AbdelAllah et al. (2020) used alginate‐coated chitosan nanoparticles as an adjuvant to deliver hepatitis A vaccine. The prepared system exhibited an increase in the rate of seroconversion, antibody level and the splenocyte proliferation (AbdelAllah et al., 2020). A killed/inactivated swine influenza virus antigen was loaded in chitosan nanoparticles, which were co‐administered with the adjuvant poly (I:C) via the nasal route. An increase in haemagglutination inhibition titres was observed, as well as the proliferation of antigen‐specific interferon‐gamma (IFNγ) secreting T‐helper/memory and γδ T cells was induced. Th1 (IFNγ, IL‐6 and IL‐2) and Th2 (IL‐10 and IL‐13) cytokine mRNA expression in the tracheobronchial lymph nodes was observed to increase. The virus load in the nasal passages and microscopic lung lesions were observed to be partially reduced. These results indicate that the chitosan nano‐vaccine induces a cross‐protective immune response in host cells (Renu et al., 2020). Kim et al. (2020) immunized mice with chitosan particles encapsulated with ovalbumin. IgG2 titre and interferon‐gamma production were observed to be enhanced (Kim et al., 2020). Yang et al. (2020) evaluated chitosan and hydroxypropyltrimethyl ammonium chloride chitosan as effective vaccine adjuvants. Humoral immunity levels of immunized chickens were lower than the control population, but the cellular immunity levels were improved (Yang et al., 2020). Chitosan and trimethyl chitosan nanoparticles were stabilized using negatively charged sodium alginate. The modified particles were loaded with inactivated PR8 influenza virus and evaluated as adjuvants for mucosal vaccine delivery. In this experiment, immunized mice exhibited higher IgG2a and IgG1 antibody titres for the non‐stabilized chitosan nanoparticles compared to those for the trimethyl chitosan nanoparticles. Alginate‐coated chitosan nanoparticles exhibited a decrease in antibody titres with a lower immune response compared to the alginate‐coated trimethyl chitosan nanoparticles (Mosafer et al., 2019).
CHITOSAN IN VACCINE PREPARATIONS AGAINST CORONAVIRUSES
The emergence of the recent pandemic strain of coronavirus, SARS‐CoV‐2, has resulted in urgent interest in the development of vaccine delivery systems and adjuvants for the treatment of the associated disease (COVID‐19). Previous studies on the role of chitosan for such uses indicate that the cationic polymer chitosan may be used as a successful biodegradable vaccine delivery adjuvant and that this characteristic may be applicable to SARS‐CoV‐2. Bande et al. (2020) studied the use of chitosan as a vaccine adjuvant in the treatment of poultry infectious bronchitis caused by an avian coronavirus. Chitosan–saponin nanoparticles were used to encapsulate a DNA vaccine and subsequently, their immunogenicity was determined against two infectious bronchitis viruses, M41 and CR88. There was an observed increase in the mucosal IgA and an expression of interferon‐gamma related genes, indicating an improvement of antibody‐ and cell‐mediated responses. Their results confirmed that both chitosan and saponin can be used as adjuvants or immunomodulators that enhance the immune responses of the host (Bande et al., 2020). Sun et al. (2009) prepared biotinylated chitosan nanoparticles encapsulated with bovine coronavirus N‐protein. They evaluated the immunization efficacy of their formula against SARS‐CoV‐2. The researchers used bifunctional fusion protein vector to achieve dendritic cell selective targeting. The increase in the mucosal IgA and systemic IgG against N‐protein indicating that chitosan is a promising gene delivery vehicle (Mohammadi et al., 2020; Sun et al., 2009). Chitosan nanoparticles were used for intranasal delivery of plasmid DNA encoding nucleocapsid SARS‐CoV‐2. This stimulates the secretion of the SARS‐CoV‐2 spike protein that will compete with live coronavirus for binding to human ACE2 receptors. Results indicated the production of high levels of IgA and IgG in mice. Chitosan prevents DNA vaccine degradation and its cationic nature increases binding to the negatively charged DNA. Furthermore, the mucoadhesive properties of chitosan encourage its use as a delivery adjuvant to mucosal surfaces (Ejeromedoghene et al., 2020; Tatlow et al., 2020).
PATENTED APPLICATIONS FOR ANTIVIRAL CHITOSAN
There are three approaches to the use of chitosan in the antiviral applications, as shown in Table 2 and which are considered here:
TABLE 2.
Summary of patents using chitosan in antiviral preparations
| Use | Mechanism | Process | Activity | References |
|---|---|---|---|---|
| (I) Antiviral activity by physical mixtures | Mixing with organics | Polyvinylpyrrolidone, gelatine, photo‐initiator, sodium sulphate, ethanol, and acrylate glycidyl ether, etc. | Chitosan hydrogel antiviral liquid gloves | Qingliang et al. (2020) |
| Chitosan mixed with grapefruit seed extract | Antiviral disinfectant activity against influenza virus, parainfluenza virus and rotavirus | Yong‐Chul et al. (2018) | ||
| Chitosan succinate | Antiviral gel | Azat Rashidovich et al. (2010) | ||
| Chitosan oligosaccharide pesticide | Against resistant watermelon virus disease and hot pepper virus diseases | Zhang et al. (2012) | ||
| Chitosan and virus insecticide raw powder | Raw powder promotes the infection of insects with the virus so as to guarantee the insect killing | Zhenpu et al. (2017) | ||
| Chitosan in combination of heparin or heparin sulphate | Prevention or treatment of infections in mammal including man caused by herpes virus | Olle et al. (2001) | ||
| Mixing with inorganics | Copper‐containing chitosan fibres | Field of sanitary protection articles | Xianming et al. (2020) | |
| Chitosan particles with nickel ion | Adsorb enterovirus | Ya‐Ching et al. (2009) | ||
| (II) Antiviral activity by chemistry | Chitosan derivatives | Changing the structure of chitosan | Excellent antiviral action | Yonghong et al. (2004) |
| Chitosan–arginine derivative | Against influenza A (H1N1), vaccinia virus (Copenhagen strain), herpes simplex 1 (SP7 strain) and encephalomyocardidits virus (B strain) | Baker and Wiesmann (2020) | ||
| (III) To prepare vaccines | Attenuated live vaccine | Chitosan microsphere | Against Porcine epidemic diarrhoea virus | Qigai et al. (2019); Fulai et al., (2018) |
| Chitosan derivatives N‐2‐hydroxypropyldimethylethyl ammonium chloride chitosan/carboxymethyl chitosan | Newcastle disease attenuated live nanoparticle vaccines | Kai and Wang (2015) | ||
| Nanoparticles | Influenza virus attenuated live vaccine | Ze (2012) | ||
| Chitosan adjuvant intranasally | Pandemic influenza | Holdings (2001) |
Chitosan/disinfectant mixtures
The first approach is the use of disinfectant chitosan mixed with various other materials as a potential antiviral disinfectant. These materials may be classified as organic or inorganic compounds. Chitosan/organic compositions include mixing of the partially acrylated chitosan with the following organic materials: polyvinylpyrrolidone, gelatine, a photoinitiator, fumed silica, mutton fat ester, coconut diethanolamide, fatty alcohol polyoxyethylene ether sodium sulphate, ethanol and acrylate glycidyl ether to produce a chitosan hydrogel. This hydrogel may then be used in an antiviral spray and as antiviral liquid gloves (Qingliang et al., 2020). These preparations show remarkable antibacterial and antiviral effects. Chitosan may also be mixed with grapefruit seed extract, which has been described as having antiviral disinfectant activity with significant inhibitory activity against influenza virus, parainfluenza virus and rotavirus. These compositions exhibited high safety profiles towards the human body, thus encouraging its utilization as a safe antiviral agent (Yong‐Chul et al., 2018). Chitosan succinate has been included in a composition containing human leukocytic interferon and p‐methyl hydroxyl benzoate (nipagin) to form an antiviral gel (Rashidovich et al., 2010). Chitosan oligosaccharide pesticide combinations have also been used to treat the normally highly resistant watermelon virus and hot pepper virus diseases. The addition of chitosan oligosaccharide to pesticides has apparently solved the problem of virus disease resistance and decreased the necessary concentration of pesticide residues after antiviral processing of seeds and plants (Zhang et al., 2012). A study by Zhenpu et al. (2017) also described the combination of chitosan and virus insecticide powder. The formula contains chitosan, virus raw powder, stabilizer and surfactant. Chitosan's role in this formulation is to induce the plant to generate chitinase, which damages the peritrophic membranes of insects and subsequently promotes the infection of insects with the virus so as to guarantee insect killing. In addition, chitosan protects the function of the insect virus so that the virus is more stable in field conditions (Zhenpu et al., 2017). Chitosan in combination with heparin or heparan sulphate has been shown to prevent herpes virus infections in mammals, including humans (Olle et al., 2001). Chitosan/inorganic compositions have also been reported to have antiviral activity. In a study by Xianming et al. (2020), a filter was prepared from copper‐containing chitosan fibres, which was used for sanitary protection of various articles. These antiviral filters have excellent filtering effect on bacteria and viruses, and can additionally kill the bacteria and viruses trapped by the filter. The filter can also be used on daily basis for protection against respiratory disease infectious particles (Xianming et al., 2020). Another application describes a composite of chitosan particles with nickel ions (Ni2+). The chitosan in this application has a degree of N‐deacetylation greater than 60%. It was reported that such chitosan composite particles are able to adsorb enterovirus, in particular enterovirus 71 (Ya‐Ching et al., 2009).
Disinfectant chitosan in the preparation of antiviral agents
The second approach is the application of disinfectant chitosan and chitin derivatives in the preparation of antiviral agents. Such antiviral activity has been proven with RNA viruses, DNA viruses and retroviruses. Such derivatives have exhibited positive results in the prevention and treatment of viral diseases, coupled with insignificant adverse effects (Yonghong et al., 2004). Baker and Wiesmann (2020) investigated the antiviral activity of chitosan–arginine derivatives, which were shown to exhibit broad antiviral activity. The assay test for loss in infectivity via standard plaque assay showed a twofold to threefold reduction in viral particle titre against influenza A (H1N1), vaccinia virus (Copenhagen strain), herpes simplex 1 (SP7 strain) and encephlomyocardidits virus (B strain). Thus, soluble chitosan–arginine derivatives inactivate both enveloped and non‐enveloped viruses (Baker & Wiesmann, 2020).
Chitosan and live vaccines
The third approach is the use of chitosan to prepare live vaccines. Due to its antiviral activity, chitosan has been employed to prepare attenuated live virus vaccine. Attenuated live vaccine chitosan microspheres were prepared for porcine epidemic diarrhoea virus (PEDV). The advantage of chitosan microspheres was that the PEDV antigen activity was preserved, the immune effect of the vaccine was improved and the preparation can be stored for prolonged periods with an associated ease of transportation (Qigai et al., 2019). A process of inactivation of PEDV using chitosan has also been described elsewhere (Fulai et al., 2018). The inactivation process includes treatment of the virus with chitosan and the addition of different concentrations of acid or alkaline solutions, formaldehyde, peroxyacetic acid and chlorine‐containing disinfectants in a specific temperature range. Park et al. (2015) showed that chitosan has a significant viral inactivation activity. Chitosan derivatives have also been used to form virus‐loaded nanoparticles, for example, live Newcastle disease virus encapsulated in N‐2‐hydroxypropyl dimethylethyl ammonium chloride chitosan nanoparticles (Kai and Wang, 2015). The nanoparticle method solved problems related to low encapsulation ratios and drug‐loading capacities of vaccine antigens and poor adsorption capabilities on the mucosal surface. Chitosan as an adjuvant in the preparation of influenza virus attenuated live vaccine has also been reported (Ze, 2012). Chitosan adjuvant enhances the immunogenicity of the vaccine, induces the human body to generate a stronger immune response, effectively reduces the required amount of the vaccine and effectively enlarges the immunized population within an existing production capacity and technology. This technique could have important social impact and a real application value for pandemic influenza. An older study by Holdings (2001) describes a pharmaceutical product comprising an intranasal dispensing device adapted to deliver a vaccine comprising of an influenza virus antigen and chitosan as adjuvant. This device was found to be useful for producing a protective IgA mucosal immune response in humans (Holdings, 2001).
NEW APPLICATIONS OF CHITOSAN IN THE COVID‐19 BATTLE
SARS‐CoV‐2 is the causative factor for COVID‐19 pandemic. The virus is transmitted between humans mainly via inhalation of infectious respiratory droplets (Kannan et al., 2020). Healthcare providers are the most highly exposed group regarding the hazard of SARS‐CoV‐2 transmission. SARS‐CoV‐2 consists of negatively charged RNA as its genetic material, enclosed within a positively charged protein capsid, which is further enveloped in material taken from the host cell membranes (Cascella et al., 2020; Mousavizadeh & Ghasemi, 2020). The virus has a particle size of 70–90 nm and carries a positive zeta‐potential. Hathout and Kassem (2020) suggested that positively charged chitosan polymers may be used in production of positive, large surface area nanofibres. These nanofibres could then be incorporated into fabrics for the production of protective clothes for healthcare providers. Such fibres would create an electrostatic repulsion between the chitosan nanofibres and the positively charged viral particles and hence, decrease the viral load on the protective clothes (Baji et al., 2020; Hathout & Kassem, 2020). N‐(2‐hydroxypropyl)‐3‐trimethylammonium chitosan chloride with an average molecular weight of 250 kDa and degree of substitution between 50% and 80% exhibits a strong interaction with the recombinant ectodomain of the S‐protein of HCoV (human coronavirus, a SARS‐CoV‐2 analogue). This interaction blocks the S‐protein–host cellular interaction and subsequent viral infection (Milewska et al., 2016; Rasul et al., 2020). N‐palmitoyl‐N‐monomethyl‐N,N‐dimethyl‐N,N,N‐trimethyl‐6‐O‐glycolchitosan with a concentration of 10–100 µg/ml has been shown to decrease SARS‐CoV‐2 infection in A549ACE2+ human lung cells and Vero E6 cells (kidney epithelial cells from an African green monkey (Chlorocebus sp.)) by 3–4 log values. This observation can be explained as a result of electrostatic binding to the virus that prevents the viral entry into the human airway epithelial cells (Pyrć et al., 2020).
THE LIMITATIONS OF CHITOSAN AS AN ANTIVIRAL AGENT
Chitosan is obtained from chitin. Much of chitin is present in seafood Crustacea, mainly shrimps, prawns, crabs and lobsters, where it occurs as a significant component in the shells/exoskeletons (Gerente et al., 2007). Chitin is also present in numerous other organisms and has been isolated from fungi and insect species, such as beetles, silkworms and crickets (Wang et al., 2020). Various chemical and physical procedures have been used in the preparation of chitosan from chitin. This processing significantly affects the final chemistry of the chitosan and the product quality. The variability of chitosan source and process of preparation has led to production of wide range of chitosan polymers with different physicochemical properties, such as differences in degree of deacetylation, molecular weight, crystallinity, and residual ash and protein contents. Such variability in chitosan physicochemical properties could lead to inconsistent and conflicting reports regarding their biological and antiviral performance (Hamilton et al., 2007; Iriti & Varoni, 2015; Iriti & Varoni, 2016).
CONCLUSIONS
It is apparent from the literature cited above, that chitosan and its derivatives are molecules of great interest in the development and delivery of vaccines in the prevention and treatment of various viral infections of humans. Most particularly, recent evidence suggests that chitosan and its derivatives may play a crucial role in the treatment of SARS‐CoV‐2. The ability of chitosan to be both a vaccine adjuvant and an antiviral agent in its own right is critical in our study of this molecule and the development of formulations for both vaccines and direct antiviral agents (e.g. antiviral nasal sprays). The apparent success of the SARS‐CoV‐2 vaccination programmes in several nations is tempered by the knowledge that the virus has already mutated into several, more infectious if equally morbid and fatal variants. At the time of writing (May 2021), GISAID (Global Initiative on Sharing Avian Influenza Data) have identified nine global clades (S, O, L, V, G, GH, GR, GV and GRY) of SARS‐CoV‐2 (https://www.gisaid.org/phylodynamics/global/nextstrain/Globalphylogeny, updated by Nextstrain. GISAID. 18 May 2021. Retrieved 21 May 2021). Such results indicate that SARS‐CoV‐2 is likely to evolve into an endemic form in the near future. This will mean that we will require a continuing effort to treat this disease (COVID‐19) on a seasonal basis and in a fashion similar to that we undertake with seasonal influenza. Such efforts must consider the use of differing vaccine adjuvants and antiviral protocols. We believe that chitosan and its derivatives have a vital role to play in this campaign.
CONFLICT OF INTEREST
The authors are not aware of any actual or potential conflicts of interest associated with this work.
AUTHORS’ CONTRIBUTIONS
All authors made an original contribution to the planning and structure of this review. All authors actively engaged with the writing and preparation of the review.
ACKNOWLEDGEMENTS
The authors wish to thank Deanship of Research & Graduate Studies at the University of Petra for funding part of this work under the research grant (12/4/2020).
Jaber, N. , Al‐Remawi, M. , Al‐Akayleh, F. , Al‐Muhtaseb, N. , Al‐Adham, I.S.I. & Collier, P.J. (2022) A review of the antiviral activity of Chitosan, including patented applications and its potential use against COVID‐19. Journal of Applied Microbiology, 132, 41–58. 10.1111/jam.15202
Contributor Information
Ibrahim S. I. Al‐Adham, Email: ialadham@uop.edu.jo, Email: pip.collier@yahoo.com.
Phillip J. Collier, Email: pip.collier@yahoo.com.
REFERENCES
- Abd El‐Hack, M.E. , El‐Saadony, M.T. , Shafi, M.E. , Zabermawi, N.M. , Arif, M. , Batiha, G.E. et al. (2020) Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. International Journal of Biological Macromolecules, 164, 2726–2744. [DOI] [PubMed] [Google Scholar]
- AbdelAllah, N.H. , Gaber, Y. , Rashed, M.E. , Azmy, A.F. , Abou‐Taleb, H.A. & AbdelGhani, S. (2020) Alginate‐coated chitosan nanoparticles act as effective adjuvant for hepatitis A vaccine in mice. International Journal of Biological Macromolecules, 152, 904–912. [DOI] [PubMed] [Google Scholar]
- Ai, H. , Wang, F. , Xia, Y. , Chen, X. & Lei, C. (2012) Antioxidant, antifungal and antiviral activities of chitosan from the larvae of housefly, Musca domestica L. Food Chemistry, 132(1), 493–498. [DOI] [PubMed] [Google Scholar]
- Amirian, J. , Zeng, Y. , Shekh, M.I. , Sharma, G. , Stadler, F.J. , Song, J. et al. (2021) In‐situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydrate Polymers, 251, 117005. 10.1016/j.carbpol.2020.117005. [DOI] [PubMed] [Google Scholar]
- Amor, G. , Sabbah, M. , Caputo, L. , Idbella, M. , De Feo, V. , Porta, R. et al. (2021) Basil essential oil: composition, antimicrobial properties, and microencapsulation to produce active chitosan films for food packaging. Foods, 10, 121. 10.3390/foods10010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artan, M. , Karadeniz, F. , Karagozlu, M.Z. , Kim, M.‐M. & Kim, S.‐K. (2010) Anti‐HIV‐1 activity of low molecular weight sulfated chitooligosaccharides. Carbohydrate Research, 345(5), 656–662. [DOI] [PubMed] [Google Scholar]
- Arulanandam, B.P. , Lynch, J.M. , Briles, D.E. , Hollingshead, S. & Metzger, D.W. (2001) Intranasal vaccination with pneumococcal surface protein A and interleukin‐12 augments antibody‐mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infection and Immunity, 69(11), 6718–6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baji, A. , Agarwal, K. & Oopath, S.V. (2020) Emerging developments in the use of electrospun fibres and membranes for protective clothing applications. Polymers, 12(2), 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, M. & Wiesmann, W. (2020) Chitosan‐derivative compounds and methods of controlling microbial populations. US patent publication no. 20200299417. Available from: https://patents.google.com/patent [Accessed 12th February 2021].
- Bande, F. , Arshad, S.S. , Bejo, M.H. , Omar, A.R. , Moeini, H. , Khadkodaei, S. et al. (2020) Development and immunogenic potentials of chitosan‐saponin encapsulated DNA vaccine against avian infectious bronchitis coronavirus. Microbial Pathogenesis, 149, 104560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford, J.G. , Caminschi, I. & Wakim, L.M. (2020) Intranasal delivery of a chitosan‐hydrogel vaccine generates nasal tissue resident memory CD8+ T cells that are protective against influenza virus infection. Vaccines, 8(4), 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhabiles, M.S. , Salah, R. , Lounici, H. , Drouiche, N. , Goosen, M.F.A. & Mameri, N. (2012) Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocolloids, 29(1), 48–56. 10.1016/j.foodhyd.2012.02.013. [DOI] [Google Scholar]
- Budragchaa, D. , Bai, S. , Kanamoto, T. , Nakashima, H. , Han, S. & Yoshida, T. (2015) Synthetic galactomannans with potent anti‐HIV activity. Carbohydrate Polymers, 130, 233–242. [DOI] [PubMed] [Google Scholar]
- Calderón, L. , Harris, R. , Cordoba‐Diaz, M. , Elorza, M. , Elorza, B. , Lenoir, J. et al. (2013) Nano and microparticulate chitosan‐based systems for antiviral topical delivery. European Journal of Pharmaceutical Sciences, 48(1–2), 216–222. [DOI] [PubMed] [Google Scholar]
- Cascella, M. , Rajnik, M. , Cuomo, A. , Dulebohn, S.C. & Di Napoli, R. (2020) Features, evaluation and treatment coronavirus (COVID‐19). Statpearls [internet]. StatPearls Publishing. In Press. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK554776/. [PubMed] [Google Scholar]
- Chen, L. & Huang, G. (2018) The antiviral activity of polysaccharides and their derivatives. International journal of biological macromolecules, 115, 77–82. [DOI] [PubMed] [Google Scholar]
- Chirkov, S.N. (2002) The antiviral activity of chitosan. Applied Biochemistry and Microbiology, 38, 1–8. 10.1023/A:1013206517442. [DOI] [Google Scholar]
- Chirkov, S.N. , Il’ina, A.V. , Surgucheva, N.A. , Letunova, E.V. , Varitsev, Y.A. , Tatarinova, N.Y. et al.(2001) Effect of chitosan on systemic viral infection and some defense responses in potato plants. Russian Journal of Plant Physiology, 48(6), 774–779. [Google Scholar]
- Chirkov, S.N. , Surguchova, N. , Gamzazade, A.I. , Abdulabekov, I.M. & Pospieszny, H.A. (1998) Comparative efficiency of chitosan derivatives as inhibitors of viral infection in plants. Doklady Rossi Akademii Nauk, 360, 271–273. [Google Scholar]
- Cossette, B. , Kelly, S.H. & Collier, J.H. (2020) Intranasal subunit vaccination strategies employing nanomaterials and biomaterials. ACS Biomaterials Science & Engineering, 7(5), 1765–1779. 10.1021/acsbiomaterials.0c01291. [DOI] [PubMed] [Google Scholar]
- Costantini, M. , Barbetta, A. , Swieszkowski, W. , Seliktar, D. , Gargioli, C. & Rainer, A. (2021) Photocurable biopolymers for coaxial bioprinting. In: Rainer, A. & Moroni, L. (Eds.) Computer‐aided tissue engineering. methods in molecular biology, 2147. New York, NY: Humana. 10.1007/978-1-0716-0611-7_4. [DOI] [PubMed] [Google Scholar]
- Davis, R. , Zivanovic, S. , D’Souza, D.H. & Davidson, P.M. (2012) Effectiveness of chitosan on the inactivation of enteric viral surrogates. Food Microbiology, 32(1), 57–62. [DOI] [PubMed] [Google Scholar]
- Davydova, V.N. , Nagorskaya, V.P. , Gorbach, V.I. , Kalitnik, A.A. , Reunov, A.V. , Solov’eva, T.F. et al. (2011) Chitosan antiviral activity: dependence on structure and depolymerization method. Applied Biochemistry and Microbiology, 47(1), 103–108. [PubMed] [Google Scholar]
- de Souza, G.A.P. , Rocha, R.P. , Gonçalves, R.L. , Ferreira, C.S. , de Mello Silva, B. , de Castro, R.F.G. et al. (2021) Nanoparticles as vaccines to prevent arbovirus infection: a long road ahead. Pathogens, 10, 36. 10.3390/pathogens10010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devappa, V. , Sangeetha, C.G. & Jhansirani, N. (2021) Bioprospecting of diseases of horticultural crops in India. In: Singh, K.P. , Jahagirdar, S. & Sarma, B.K. (Eds.) Emerging trends in plant pathology. Singapore: Springer. 10.1007/978-981-15-6275-4_20. [DOI] [Google Scholar]
- Dimassi, S. , Tabary, N. , Chai, F. , Blanchemain, N. & Martel, B. (2018) Sulfonated and sulfated chitosan derivatives for biomedical applications: a review. Carbohydrate Polymers, 202, 382–396. [DOI] [PubMed] [Google Scholar]
- Divya, K. , Vijayan, S. , George, T.K. & Jisha, M.S. (2017) Antimicrobial properties of chitosan nanoparticles: mode of action and factors affecting activity. Fibres and Polymers, 18(2), 221–230. [Google Scholar]
- Donalisio, M. , Argenziano, M. , Rittà, M. , Bastiancich, C. , Civra, A. , Lembo, D. et al. (2020) Acyclovir‐loaded sulfobutyl ether‐β‐cyclodextrin decorated chitosan nanodroplets for the local treatment of HSV‐2 infections. International Journal of Pharmaceutics, 587, 119676. [DOI] [PubMed] [Google Scholar]
- Donalisio, M. , Leone, F. , Civra, A. , Spagnolo, R. , Ozer, O. , Lembo, D. et al. (2018) Acyclovir‐loaded chitosan nanospheres from nano‐emulsion templating for the topical treatment of herpesviruses infections. Pharmaceutics, 10(2), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkelberger, J. & Song, W.C. (2010) Complement and its role in innate and adaptive immune responses. Cell Research, 20, 34–50. 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- Ejeromedoghene, O. , Oderinde, O. , Egejuru, G. & Adewuyi, S. (2020) Chitosan‐drug encapsulation as a potential candidate for COVID‐19 drug delivery systems: a review. Journal of the Turkish Chemical Society Section A: Chemistry, 7(3), 851–864. [Google Scholar]
- Frank, L.A. , Chaves, P.S. , D'Amore, C.M. , Contri, R.V. , Frank, A.G. , Beck, R.C.R. et al. (2017) The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: increasing penetration and adhesion of imiquimod in vaginal tissue. European Journal of Pharmaceutics and Biopharmaceutics, 114, 202–212. [DOI] [PubMed] [Google Scholar]
- Fulai, S. , Feng, Y. & Han, B. (2018) Chitosan virus inactivating process and applications thereof. Chinese patent publication no. 108570117. Available from: https://patents.google.com/patent [Accessed 12th February 2021].
- Gao, Y. , Liu, W. , Wang, W. , Zhang, X. & Zhao, X. (2018) The inhibitory effects and mechanisms of 3, 6‐O‐sulfated chitosan against human papillomavirus infection. Carbohydrate polymers, 198, 329–338. [DOI] [PubMed] [Google Scholar]
- Gerente, C. , Lee, V.K.C. , Cloirec, P.L. & McKay, G. (2007) Application of chitosan for the removal of metals from wastewaters by adsorption—mechanisms and models review. Critical Reviews in Environmental Science and Technology, 37(1), 41–127. [Google Scholar]
- Gómez‐Mascaraque, L.G. , Sanchez, G. & López‐Rubio, A. (2016) Impact of molecular weight on the formation of electrosprayed chitosan microcapsules as delivery vehicles for bioactive compounds. Carbohydrate Polymers, 150, 121–130. [DOI] [PubMed] [Google Scholar]
- Goy, R.C. , de Britto, D. & Assis, O.B.G. (2009) A review of the antimicrobial activity of chitosan. Polímeros, 19(3), 241–247. [Google Scholar]
- Gu, J. , Al‐Bayati, K. & Ho, E.A. (2017) Development of antibody‐modified chitosan nanoparticles for the targeted delivery of siRNA across the blood‐brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Delivery and Translational Research, 7(4), 497–506. [DOI] [PubMed] [Google Scholar]
- Gurunathan, S. , Qasim, M. , Choi, Y. , Do, J.T. , Park, C. , Hong, K. et al. (2020) Antiviral potential of nanoparticles ‐ can nanoparticles fight against coronaviruses? Nanomaterials, 10(9), 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed, A.A. , Abdelhamid, I.A. , Saad, G.R. , Elkady, N.A. & Elsabee, M.Z. (2020) Synthesis, characterization and antimicrobial activity of a novel chitosan schiff bases based on heterocyclic moieties. International Journal of Biological Macromolecules, 153, 492–501. [DOI] [PubMed] [Google Scholar]
- Hamilton, V. , Yuan, Y. , Rigney, D.A. , Chesnutt, B.M. , Puckett, A.D. , Ong, J.L. et al. (2007) Bone cell attachment and growth on well‐characterized chitosan films. Polymer International, 56(5), 641–647. [Google Scholar]
- Hassan, M.I. , Mohamed, A.F. , Taher, F.A. & Kamel, M.R. (2016) Antimicrobial activities of chitosan nanoparticles prepared from Lucilia cuprina maggots (Diptera: Calliphoridae). Journal of the Egyptian Society of Parasitology, 46(3), 563–570. [PubMed] [Google Scholar]
- Hathout, R.M. & Kassem, D.H. (2020) Positively charged electroceutical spun chitosan nanofibres can protect health care providers from COVID‐19 infection: an opinion. Frontiers in Bioengineering and Biotechnology, 8, 885. 10.3389/fbioe.2020.00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Xing, R. , Liu, S. , Qin, Y. , Li, K. , Yu, H. et al. (2019) The improved antiviral activities of amino‐modified chitosan derivatives on Newcastle virus. Drug and Chemical Toxicology, 44(4), 335–340. 10.1080/01480545.2019.1620264. [DOI] [PubMed] [Google Scholar]
- Holdings, M. (2001) A vaccine composition comprising an influenza virus antigen and chitosan as adjuvant, useful for producing a protective IgA mucosal immune response. New Zealand patent publication no. NZ292953. Available from: https://patents.google.com [Accessed 12th February 2021].
- Holmgren, J. , Czerkinsky, C. , Eriksson, K. & Mharandi, A. (2003) Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine, 21, S89–S95. [DOI] [PubMed] [Google Scholar]
- Hosseinnejad, M. & Jafari, S.M. (2016) Evaluation of different factors affecting antimicrobial properties of chitosan. International Journal of Biological Macromolecules, 85, 467–475. [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Cai, J. , Du, Y. , Lin, J. , Wang, C. & Xiong, K. (2009) Preparation and anti‐TMV activity of guanidinylated chitosan hydrochloride. Journal of Applied Polymer Science, 112(6), 3522–3528. [Google Scholar]
- Idris, F. , Ting, D.H.R. & Alonso, S. (2021) An update on dengue vaccine development, challenges, and future perspectives. Expert Opinion on Drug Discovery, 16(1), 47–58. 10.1080/17460441.2020.1811675. [DOI] [PubMed] [Google Scholar]
- Iriti, M. & Varoni, E.M. (2015) Chitosan‐induced antiviral activity and innate immunity in plants. Environmental Science and Pollution Research, 22(4), 2935–2944. [DOI] [PubMed] [Google Scholar]
- Iriti, M. & Varoni, E.M. (2016) Chitosan‐elicited plant innate immunity: focus on antiviral activity. Research Progress in Oligosaccharins (pp. 65–81). New York, NY: Springer. [Google Scholar]
- Ivić, I. , Kopjar, M. , Jakobek, L. , Jukić, V. , Korbar, S. , Marić, B. et al. (2021) Influence of processing parameters on phenolic compounds and color of cabernet sauvignon red wine concentrates obtained by reverse osmosis and nanofiltration. Processes, 9, 89. [Google Scholar]
- Jackson, E.M. & Herbst‐Kralovetz, M.M. (2012) Intranasal vaccination with murabutide enhances humoral and mucosal immune responses to a virus‐like particle vaccine. PLoSOne, 7(7), e41529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarach, N. , Dodiuk, H. & Kenig, S. (2020) Polymers in the medical antiviral front‐line. Polymers, 12(8), 1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J.E. , Le Sage, V. & Lakdawala, S.S. (2020) Viral and host heterogeneity and their effects on the viral life cycle. Nature Reviews Microbiology, 19(4), 272–282. 10.1038/s41579-020-00449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai, Z. & Wang, X. (2015) Preparation method for N‐2‐hydroxypropyldimethylethyl ammonium chloride chitosan/carboxymethyl chitosan Newcastle disease virus nanoparticles. Chinese patent publication no. CN104784685. Available from: https://patents.google.com [Accessed 12 February 2021].
- Kannan, S. , Ali, P.S.S. , Sheeza, A. & Hemalatha, K. (2020) COVID‐19 (Novel Coronavirus 2019)‐recent trends. European Review for Medical and Pharmacological Sciences, 24(4), 2006–2011. [DOI] [PubMed] [Google Scholar]
- Kim, C.H. , Shin, C.G. , Shin, K.S. & Son, T.I. (1999) Preparation of trifluoroacetyl chitosan derivatives with antiviral activity. Applied Chemistry for Engineering, 10(4), 599–602. [Google Scholar]
- Kim, S.H. , Ryu, Y.C. , Wang, H.M.D. & Hwang, B.H. (2020) Optimally fabricated chitosan particles containing ovalbumin induced cellular and humoral immunity in immunized mice. Biotechnology and Bioprocess Engineering, 25(5), 681–689. [Google Scholar]
- Kozhikhova, K.V. , Ivantsova, M.N. , Tokareva, M.I. , Shulepov, I.D. , Tretiyakov, A.V. , Shaidarov, L.V. et al. (2018) Preparation of chitosan‐coated liposomes as a novel carrier system for the antiviral drug Triazavirin. Pharmaceutical Development and Technology, 23(4), 334–342. [DOI] [PubMed] [Google Scholar]
- Ku, M.‐W. , Bourgine, M. , Authie, P. , Lopez, J. , Nemirov, K. , Moncoq, F. et al. (2020) Intranasal vaccination with a lentiviral vector strongly protects against SARS‐CoV‐2 in mouse and golden hamster preclinical models. bioRxiv. In Press 10.1101/2020.07.21.214049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikov, S.N. , Chirkov, S.N. , Il’ina, A.V. , Lopatin, S.A. & Varlamov, V.P. (2006) Effect of the molecular weight of chitosan on its antiviral activity in plants. Applied Biochemistry and Microbiology, 42(2), 200–203. [PubMed] [Google Scholar]
- Liang, L. , Ahamed, A. , Ge, L. , Fu, X. & Lisak, G. (2020) Advances in antiviral material development. ChemPlusChem, 85(9), 2105–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutfy, S.A. , Elberry, M.H. , Farroh, K.Y. , Mohamed, H.T. , Mohamed, A.A. , Mohamed, E.B. et al. (2020) Antiviral activity of chitosan nanoparticles encapsulating curcumin against hepatitis C virus genotype 4a in human hepatoma cell lines. International Journal of Nanomedicine, 15, 2699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lu, M. , Yu, S. , Wang, Z. , Xin, Q. , Sun, T. , Chen, X. et al. (2020) Zwitterionic choline phosphate functionalized chitosan with antibacterial property and superior water solubility. European Polymer Journal, 134, 109821. 10.1016/j.eurpolymj.2020.109821 [DOI] [Google Scholar]
- Mansouri, S. , Lavigne, P. , Corsi, K. , Benderdour, M. , Beaumont, E. & Fernandes, J.C. (2004) Chitosan‐DNA nanoparticles as non‐viral vectors in gene therapy: strategies to improve transfection efficacy. European Journal of Pharmaceutics and Biopharmaceutics, 57(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Mathew, D. & Hsu, W.L. (2018) Antiviral potential of curcumin. Journal of Functional Foods, 40, 692–699. [Google Scholar]
- Matica, M.A. , Aachmann, F.L. , Tøndervik, A. , Sletta, H. & Ostafe, V. (2019) Chitosan as a wound dressing starting material: antimicrobial properties and mode of action. International Journal of Molecular Sciences, 20(23), 5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewska, A. , Chi, Y. , Szczepanski, A. , Barreto‐Duran, E. , Dabrowska, A. , Botwina, P. et al. (2021) HTCC as a polymeric inhibitor of SARS‐CoV‐2 and MERS‐CoV. Journal of Virology, 95(4), 10.1128/JVI.01622-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewska, A. , Chi, Y. , Szczepanski, A. , Barreto‐Duran, E. , Liu, K. , Liu, D. et al. (2020) HTCC as a highly effective polymeric inhibitor of SARS‐CoV‐2 and MERS‐CoV. BioRxiv. In Press. 10.1101/2020.03.29.014183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewska, A. , Kaminski, K. , Ciejka, J. , Kosowicz, K. , Zeglen, S. , Wojarski, J. et al. (2016) HTCC: broad range inhibitor of coronavirus entry. PLoSOne, 11(6), e0156552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi, Z. , Eini, M. , Rastegari, A. & Tehrani, M.R. (2020) Chitosan as a machine for biomolecule delivery: a review. Carbohydrate Polymers, 256, 117414– 10.1016/j.carbpol.2020.117414. [DOI] [PubMed] [Google Scholar]
- Mohammed, M.A. , Syeda, J. , Wasan, K.M. & Wasan, E.K. (2017) An overview of chitosan nanoparticles and its application in non‐parenteral drug delivery. Pharmaceutics, 9(4), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito, K.M. , Ruckwardt, T.J. , Bar‐Haim, E. , Nair, D. , Moin, S.M. , Redwood, A.J. et al. (2018) Memory inflation drives tissue‐resident memory CD8+ T cell maintenance in the lung after intranasal vaccination with murine cytomegalovirus. Frontiers in Immunology, 9, 1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, Y. , Ono, T. , Miyahira, Y. , Nguyen, V.Q. , Matsui, T. & Ishihara, M. (2013) Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Research Letters, 8(1), 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosafer, J. , Sabbaghi, A.H. , Badiee, A. , Dehghan, S. & Tafaghodi, M. (2019) Preparation, characterization and in vivo evaluation of alginate‐coated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian Journal of Pharmaceutical Sciences, 14(2), 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavizadeh, L. & Ghasemi, S. (2020) Genotype and phenotype of COVID‐19: their roles in pathogenesis. Journal of Microbiology, Immunology and Infection, 54(2), 159–163. 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzarelli, R. , Tarsi, R. , Filippini, O. , Giovanetti, E. , Biagini, G. & Varaldo, P.E. (1990) Antimicrobial properties of N‐carboxybutyl chitosan. Antimicrobial Agents and Chemotherapy, 34, 2019–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, S.W. , Selvarajah, G.T. , Hussein, M.Z. , Yeap, S.K. & Omar, A.R. (2020) In vitro evaluation of curcumin‐encapsulated chitosan nanoparticles against feline infectious peritonitis virus and pharmacokinetics study in cats. BioMed Research International, 2020. 10.1155/2020/3012198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olle, L. , Back, M. & Bergstroem, T. (2001) Use of heparin or heparan sulphate in combination with chitosan for the prevention or treatment of infections caused by herpes virus. United States patent publication no. 6207653 (B1). Available from: https://patents.google.com [Accessed 12th February 2021].
- Omura, Y. , Shigemoto, M. , Akiyama, T. , Saimoto, H. , Shigemasa, Y. , Nakamura, I. et al. (2003) Antimicrobial activity of chitosan with different degrees of acetylation and molecular weights. Biocontrol Science, 8(1), 25–30. [Google Scholar]
- Papavizas, G.C. , Davey, C.B. & Woodard, R.S. (1962) Comparative effectiveness of some organic amendments and fungicides in reducing activity and survival of Rhizoctonia solani in soil. Canadian Journal of Microbiology, 8(6), 915–922. 10.1139/m62-119. [DOI] [Google Scholar]
- Pardi, N. & Weissman, D. (2020) Development of vaccines and antivirals for combating viral pandemics. Nature Biomedical Engineering, 4, 1128–1133. 10.1038/s41551-020-00658-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.P. , Koh, M.Y. , Sung, P.S. , Kim, K. , Kim, M.S. , Lee, M.S. et al. (2015). Inactivation efficiency of DNA and RNA viruses during chitin‐to‐chitosan conversion. Macromolecular Research, 23(6), 505–508. [Google Scholar]
- Parkin, J. & Cohen, B. (2001) An overview of the immune system. The Lancet, 357(9270), 1777–1789. [DOI] [PubMed] [Google Scholar]
- Paul, P. , Kolesinska, B. & Sujka, W. (2019) Chitosan and its derivatives‐biomaterials with diverse biological activity for manifold applications. Mini Reviews in Medicinal Chemistry, 19(9), 737–750. [DOI] [PubMed] [Google Scholar]
- Pawar, D. & Jaganathan, K.S. (2016) Mucoadhesive glycol chitosan nanoparticles for intranasal delivery of hepatitis B vaccine: enhancement of mucosal and systemic immune response. Drug Delivery, 23(1), 185–194. [DOI] [PubMed] [Google Scholar]
- Perrone, L.A. , Ahmad, A. , Veguilla, V. , Lu, X. , Smith, G. , Katz, J.M. et al. (2009) Intranasal vaccination with 1918 influenza virus‐like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. Journal of Virology, 83(11), 5726–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu, R. , Ghica, M.V. , Dinu‐Pîrvu, C.E. , Anuța, V. , Lupuliasa, D. & Popa, L. (2020) New opportunity to formulate intranasal vaccines and drug delivery systems based on chitosan. International Journal of Molecular Sciences, 21(14), 5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospieszny, H. , Chirkov, S. & Atabekov, J. (1991) Induction of antiviral resistance in plants by chitosan. Plant Science, 79(1), 63–68. 10.1016/0168-9452(91)90070-O. [DOI] [Google Scholar]
- Pyrć, K. , Milewska, A. , Duran, E.B. , Botwina, P. , Lopes, R. , Arenas‐Pinto, A. et al. (2020) SARS‐CoV‐2 inhibition in human airway epithelial cells using a mucoadhesive, amphiphilic chitosan that may serve as an anti‐viral nasal spray. bioRxiv. In Press. 10.1101/2020.12.10.413609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qigai, H. , Zhang, C. , Chen, F. , Wu, M. , Fan, S. , He, D. et al. (2019) Preparation method and application of attenuated live vaccine chitosan microsphere for porcine epidemic diarrhea virus. Chinese patent publication no. 109172817. Available from: https://patents.google.com [Accessed 12th February 2021].
- Qingliang, Y. , Yang, C. , Wang, R. , Xiao, B. , Jiang, Z. , Xu, H. & Wang, J. (2020) Chitosan hydrogel, preparation method of chitosan hydrogel, antiviral spray and antiviral liquid gloves. Chinese patent publication no. 111529762. Available from: https://patents.google.com [Accessed 12th February 2021].
- Qinna, N.A. , Akayleh, F.T. , Al Remawi, M.M. , Kamona, B.S. , Taha, H. & Badwan, A.A. (2013) Evaluation of a functional food preparation based on chitosan as a meal replacement diet. Journal of Functional Foods, 5(3), 1125–1134. [Google Scholar]
- Raafat, D. & Sahl, H.‐G. (2009) Chitosan and its antimicrobial potential–a critical literature survey. Microbial Biotechnology, 2(2), 186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramana, L.N. , Sharma, S. , Sethuraman, S. , Ranga, U. & Krishnan, U.M. (2014) Evaluation of chitosan nanoformulations as potent anti‐HIV therapeutic systems. Biochimica et Biophysica Acta (BBA)‐General Subjects, 1840(1), 476–484. [DOI] [PubMed] [Google Scholar]
- Randazzo, W. , Aznar, R. & Sánchez, G. (2016) Curcumin‐mediated photodynamic inactivation of norovirus surrogates. Food and Environmental Virology, 8(4), 244–250. [DOI] [PubMed] [Google Scholar]
- Randazzo, W. , Fabra, M.J. , Falcó, I. , López‐Rubio, A. & Sánchez, G. (2018) Polymers and biopolymers with antiviral activity: potential applications for improving food safety. Comprehensive Reviews in Food Science and Food Safety, 17(3), 754–768. [DOI] [PubMed] [Google Scholar]
- Rashidovich, K. , Bikbov, M.M. & Sibirjak, S.V. (2010) Antivirus agent with human leukocytic human interferon based on chitosan succinate. Russian patent publication no. 2404796. Available from: https://patents.google.com [Accessed 12th February 2021].
- Rasul, R.M. , Zakaria, Z. , Shah, K. , Chee, C.F. , Dabbagh, A. , Abd Rahman, N. et al. (2020) A review on chitosan and its development as pulmonary particulate anti‐infective and anti‐cancer drug carriers. Carbohydrate Polymers, 250, 116800– 10.1016/j.carbpol.2020.116800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, R.C. , Naylor, S.C. , Potter, C.W. , Bond, J. , Jabbal‐Gill, I. , Fisher, A. et al. (2005) Effective nasal influenza vaccine delivery using chitosan. Vaccine, 23(35), 4367–4374. [DOI] [PubMed] [Google Scholar]
- Renu, S. , Feliciano‐Ruiz, N. , Ghimire, S. , Han, Y. , Schrock, J. , Dhakal, S. et al. (2020) Poly(I:C) augments inactivated influenza virus‐chitosan nanovaccine induced cell mediated immune response in pigs vaccinated intranasally. Veterinary Microbiology, 242, 108611– 10.1016/j.vetmic.2020.108611. [DOI] [PubMed] [Google Scholar]
- Rikta, S.Y. (2019) Application of nanoparticles for disinfection and microbial control of water and wastewater. In: Nanotechnology in water and wastewater treatment. Elsevier, pp. 159–176. [Google Scholar]
- Riva, R. , Ragelle, H. , des Rieux, A. , Duhem, N. , Jérôme, C. & Préat, V. (2011) Chitosan and chitosan derivatives in drug delivery and tissue engineering. Chitosan for biomaterials II, pp 19–44. Berlin, Heidelberg, Germany: Springer. [Google Scholar]
- Russo, E. , Gaglianone, N. , Baldassari, S. , Parodi, B. , Cafaggi, S. , Zibana, C. et al. (2014) Preparation, characterization and in vitro antiviral activity evaluation of foscarnet‐chitosan nanoparticles. Colloids and Surfaces B: Biointerfaces, 118, 117–125. [DOI] [PubMed] [Google Scholar]
- Ryan, E.J. , Daly, L.M. & Mills, H.G. (2001) Immunoregulators and delivery systems for vaccination by mucosal routes. Trends in Biotechnology, 19, 293–304. [DOI] [PubMed] [Google Scholar]
- Sahebnasagh, A. , Saghafi, F. , Avan, R. , Khoshi, A. , Khataminia, M. , Safdari, M. et al. (2020) The prophylaxis and treatment potential of supplements for COVID‐19. European Journal of Pharmacology, 887, 173530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahraei, Z. , Shabani, M. , Shokouhi, S. & Saffaei, A. (2020) Aminoquinolines against coronavirus disease 2019 (COVID‐19): chloroquine or hydroxychloroquine. International Journal of Antimicrobial Agents, 55(4), 105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraf, S. , Jain, S. , Sahoo, R.N. & Mallick, S. (2020) Lipopolysaccharide derived alginate coated Hepatitis B antigen loaded chitosan nanoparticles for oral mucosal immunization. International Journal of Biological Macromolecules, 154, 466–476. [DOI] [PubMed] [Google Scholar]
- Şenel, S. (2019) Current status and future of chitosan in drug and vaccine delivery. Reactive and Functional Polymers, 147, 104452– 10.1016/j.reactfunctpolym.2019.104452 [DOI] [Google Scholar]
- Shi, Q. , Wang, A. , Lu, Z. , Qin, C. , Hu, J. & Yin, J. (2017) Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydrate Research, 453, 1–9. [DOI] [PubMed] [Google Scholar]
- Singh, K. , Mishra, A. , Sharma, D. & Singh, K. (2019) Antiviral and antimicrobial potentiality of nano drugs. In: Applications of targeted nano drugs and delivery systems (pp. 343–356). Elsevier. [Google Scholar]
- Sofy, A.R. , Hmed, A.A. , Abd El Haliem, N.F. , Zein, M.A.E. & Elshaarawy, R.F. (2019) Polyphosphonium‐oligochitosans decorated with nanosilver as new prospective inhibitors for common human enteric viruses. Carbohydrate Polymers, 226, 115261. [DOI] [PubMed] [Google Scholar]
- Sosa, M.A. , Fazely, F. , Koch, J.A. , Vercellotti, S.V. & Ruprecht, R.M. (1991) N‐carboxymethyl chitosan‐N, O‐sulfate as an anti‐HIV‐1 agent. Biochemical and Biophysical Research Communications, 174, 489–496. [DOI] [PubMed] [Google Scholar]
- Sperandio, B. , Fischer, N. & Sansonetti, P.J. (2015) Mucosal physical and chemical innate barriers: lessons from microbial evasion strategies. Seminars in Immunology, 27(2), 111–118. 10.1016/j.smim.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Su, X. , Zivanovic, S. & D'Souza, D.H. (2009) Effect of chitosan on the infectivity of murine norovirus, feline calicivirus, and bacteriophage MS2. Journal of Food Protection, 72(12), 2623–2628. [DOI] [PubMed] [Google Scholar]
- Sui, Z. , Chen, Q. , Fang, F. , Zheng, M. & Chen, Z. (2010) Cross‐protection against influenza virus infection by intranasal administration of M1‐based vaccine with chitosan as an adjuvant. Vaccine, 28(48), 7690–7698. [DOI] [PubMed] [Google Scholar]
- Sui, Z. , Chen, Q. , Wu, R. , Zhang, H. , Zheng, M. , Wang, H. et al. (2010) Cross‐protection against influenza virus infection by intranasal administration of M2‐based vaccine with chitosan as an adjuvant. Archives of Virology, 155(4), 535–544. [DOI] [PubMed] [Google Scholar]
- Sun, Q. , Li, X. , Che, X. , Chang, J. , Yang, Y. & Yu, L. (2009) Evaluation on the immune efficiency of bovine coronavirus N protein‐loaded chitosan microspheres. Zhongguo Yufang Shouyi Xuebao/Chinese Journal of Preventive Veterinary Medicine, 31(11), 882–886. [Google Scholar]
- Tatlow, D. , Tatlow, C. , Tatlow, S. & Tatlow, S. (2020) A novel concept for treatment and vaccination against Covid‐19 with an inhaled chitosan‐coated DNA vaccine encoding a secreted spike protein portion. Clinical and Experimental Pharmacology and Physiology, 47(11), 1874–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubner, T. , Marounek, M. & Synytsya, A. (2020) Preparation and characterization of hydrophobic and hydrophilic amidated derivatives of carboxymethyl chitosan and carboxymethyl β‐glucan. International Journal of Biological Macromolecules, 163, 1433–1443. 10.1016/j.ijbiomac.2020.07.257. [DOI] [PubMed] [Google Scholar]
- Terauchi, Y. , Sano, K. , Ainai, A. , Saito, S. , Taga, Y. , Ogawa‐Goto, K. et al. (2018) IgA polymerization contributes to efficient virus neutralization on human upper respiratory mucosa after intranasal inactivated influenza vaccine administration. Human Vaccines and Immunotherapeutics, 14(6), 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Weken, H. , Cox, E. & Devriendt, B. (2021) Advances in oral subunit vaccine design. Vaccines, 9, 1. 10.3390/vaccines9010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Meng, Q. , Qi, L. , Jinbao, L. , Mo, Z. , Zheng, J. et al. (2020) Chitosan derivatives and their application in biomedicine. International Journal of Molecular Sciences, 21(2), 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Wang, S.X. & Guan, H.S. (2012) The antiviral activities and mechanisms of marine polysaccharides: an overview. Marine Drugs, 10(12), 2795–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, D. , Ensinas, A. , Verrier, B. , Primard, C. , Cuvillier, A. , Champier, G. et al. (2016) Zinc‐stabilized chitosan‐chondroitin sulfate nanocomplexes for HIV‐1 infection inhibition application. Molecular Pharmaceutics, 13(9), 3279–3291. [DOI] [PubMed] [Google Scholar]
- Wu, D. , Zhu, L. , Li, Y. , Zhang, X. , Xu, S. , Yang, G. et al. (2020) Chitosan‐based colloidal polyelectrolyte complexes for drug delivery: a review. Carbohydrate polymers, 238, 116126. [DOI] [PubMed] [Google Scholar]
- Xianming, S. , Qi, Y. , Li, J. , Hao, Y. & Zhang, D. (2020) Anti‐virus filter layer prepared from copper‐containing chitosan fibres and application of anti‐virus filter layer. Chinese patent publication no. 111235871. Available from: https://patents.google.com [Accessed 12th February 2021].
- Xing, L. , Fan, Y.T. , Zhou, T.J. , Gong, J.H. , Cui, L.H. , Cho, K.H. et al. (2018) Chemical modification of chitosan for efficient vaccine delivery. Molecules, 23(2), 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, M. , Hu, S. , Lu, Y. , Zhang, Y. , Jiang, X. , An, S. et al. (2015) Development of chitosan nanoparticles as drug delivery system for a prototype capsid inhibitor. International Journal of Pharmaceutics, 495, 771–782. [DOI] [PubMed] [Google Scholar]
- Ya‐Ching, L. , Wu, S.‐C. & Cheng, Y.‐C. (2009) A kind of chitosan particles is capable for virus adsorption. Taiwan patent publication no. 200927201. Available from: https://patents.google.com [Accessed 12th February 2021].
- Yang, Y. , Xing, R. , Liu, S. , Qin, Y. , Li, K. , Yu, H. et al. (2020) Chitosan, hydroxypropyltrimethyl ammonium chloride chitosan and sulfated chitosan nanoparticles as adjuvants for inactivated Newcastle disease vaccine. Carbohydrate Polymers, 229, 115423. [DOI] [PubMed] [Google Scholar]
- Yong‐Chul, S. , Piao, Z. , Kim, Y.‐S. & Kim, A.‐S. (2018) Antiviral composition containing chitosan and grapefruit seed extract as active ingredients. Chinese patent publication no. 108339017. Available from: https://patents.google.com [Accessed 12th February 2021].
- Yonghong, Z. , Fu, M. & Cao, F. (2004) Application of chitin, chitosan and their derivatives in preparing antiviral. Chinese patent publication, no. 1548056, Available from: https://patents.google.com [Accessed 12th February 2021].
- Yu, X. , Wen, T. , Cao, P. , Shan, L. & Li, L. (2019) Alginate‐chitosan coated layered double hydroxide nanocomposites for enhanced oral vaccine delivery. Journal of Colloid and Interface Science, 556, 258–265. [DOI] [PubMed] [Google Scholar]
- Ze, C. (2012) Application of chitosan as adjuvant in preparation of influenza virus attenuated live vaccine. Chinese patent application no. 102824635. Available from: https://patents.google.com [Accessed 12th February 2021].
- Zhang, S. , Hongxia, L. , Yusheng, Z. , Dingding, C. , Bin, X. , Lifen, D. et al. (2012) Chitosan oligosaccharide composition for resisting hot pepper virus disease as well application and method thereof. Chinese patent publication no. 102365949. Available from: https://patents.google.com [Accessed 12th February 2021].
- Zhao, K. , Shi, X. , Zhao, Y. , Wei, H. , Sun, Q. , Huang, T. et al. (2011) Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in chitosan nanoparticles. Vaccine, 29(47), 8549–8556. [DOI] [PubMed] [Google Scholar]
- Zheng, H. , Li, P. , Jianliang, L. , Zhongwang, Z. , Yuanyuan, W. , Wenfa, H. et al. (2020) Comparison of immune responses in guinea pigs by intranasal delivery with different nanoparticles‐loaded FMDV DNA vaccine. Microbial Pathogenesis, 142, 104061. [DOI] [PubMed] [Google Scholar]
- Zheng, M. , Qu, D. , Wang, H. , Sun, Z. , Liu, X. , Chen, J. et al. (2016) Intranasal administration of chitosan against influenza A (H7N9) virus infection in a mouse model. Scientific Reports, 6(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhenpu, L. , Zhang, X. , Liu, Y. , Zhang, J. , Li, P. , Feng, W. et al. (2017) Virus insecticide suspension agent containing chitosan and preparation method thereof. Chinese patent publication no.107087639. Available from: https://patents.google.com [Accessed 12th February 2021].
- Zhou, J. , Hu, Z. , Zabihi, F. , Chen, Z. & Zhu, M. (2020) Progress and perspective of antiviral protective material. Advanced Fiber Materials, 2(3), 123–139. 10.1007/s42765-020-00047-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Shuxiang, L. , Shuai, B. , Ning, L. , Yuhai, B. , Wenjun, L. et al. (2020) Long‐lasting protective immunity against H7N9 infection is induced by intramuscular or CpG‐adjuvanted intranasal immunization with the split H7N9 vaccine. International Immunopharmacology, 78, 106013. [DOI] [PubMed] [Google Scholar]


