Abstract
Purpose
To study the long-term safety of genipin treatment using a vacuum device with or without epithelial cells at different crosslinking times.
Methods
Twenty-five healthy New Zealand white rabbits were separated into five treatment groups: 0.25% genipin with epithelial cells for 5 minutes (G1), 0.25% genipin without epithelial cells for 5 minutes (G2), 0.25% genipin without epithelial cells for 10 minutes (G3), ultraviolet A–riboflavin collagen crosslinking (UVA), and controls (C). Before and 2, 4, 6, and 8 weeks after crosslinking treatment, anterior segment optical coherence tomography (ASOCT), in vivo confocal microscopy (IVCM), and the Pentacam system were used to evaluate the right eyes.
Results
A demarcation line (DL) was observed in the corneal stroma in the G2, G3, and UVA groups. The DL depths in the G2 and G3 groups were stable but decreased in the UVA group over time. The density of keratocytes in these groups increased. Endothelial cell density was decreased in the UVA group. There were no differences in the endothelium before and after treatment in the G1, G2, G3, and C groups. The densitometry, as determined using the Pentacam system, significantly increased in the G2, G3, and UVA groups and was positively correlated with keratocyte densities.
Conclusions
A vacuum ring assisting local genipin immersion crosslinking without corneal epithelium can activate the keratocytes in the corneal stroma and was safe enough for the thin cornea.
Translational Relevance
Genipin can not only crosslink the collagen fibers but also activate the keratocytes and even may promote collagen fiber secretion.
Keywords: genipin, crosslinking, keratocytes, endothelium
Introduction
Over the past 20 years, strengthening the biochemical properties of the cornea and producing new chemical bonds by corneal crosslinking have become one of the most effective treatments to stop progression of keratoconus, corneal ectasia after refractive corneal surgery,1 and even in the treatment of some forms of resistant infectious keratitis.2 This clinical method includes light-initiated crosslinking, such as the ultraviolet (UV)–riboflavin crosslinking,3–5 Rose Bengal and green light method,6–8 and chemical-initiated crosslinking, such as genipin.9–11
Genipin, obtained from geniposide, induces intramolecular and intermolecualr crosslinking of cyclic structures within collagen fibers by spontaneously reacting with the amino acid chains or proteins.12 As it demonstrates superior biocompatibility,13,14 lower cytotoxicity,9,15,16 and better crosslinking property,11,13 it has been used in crosslinking on cornea11 and sclera.17
Genipin crosslinking is still at the experimental stage. The previous study and our study showed that genipin could significantly strengthen the stiffnesss of porcine cornea and sclera in vivo and vitro.18–21 Corneal collagen crosslinking induced with genipin on porcine cornea in vitro produced a significant increase in biomechanical strength and resistance to bacterial collagenase,13,22 and the effect of crosslinking increased with dose.13 It was also found that genipin was similar to ultraviolet-riboflavin crosslinking (UV-CXL). The cytotoxic effect in the endothelial cells is similar between UV-CXL and genipin.9 In vivo, genipin induced corneal flattening in rabbit eyes after 60 days, with a mean flattening of the corneas of 4.4 D.11 In conclusion, genipin might have potential for management of corneal ectasia and keratoconus.
Although genipin has good prospects for corneal crosslinking, researchers and our previous study paid more attention to the short-term safety evaluation of genipin crosslinking. There have been fewer long-term evaluations about it. The modes of genipin administration are soaking cornea in vitro,13,22 eye drops in vivo,15,16 or topical injection.23 Genipin is a natural crosslinker for molecules with primary amino groups that is widely distributed in tissue. The crosslinking effect could act on tissues around cornea. In addition, some mysteries need to be solved, such as the epithelial cells’ effect during the crosslinking progress. Avila et al.9 said that genipin's effect was similar in corneas with or without epithelium with similar biomechanical effects, but he did not provide data to support his opinion. In his follow-up studies, the epithelial cells of experimental animal cornea were scarped during genipin crolinking. Therefore, the effect of epithelium on genipin crosslinking needs to be studied. Avila et al.11 used a vacuum device designed to prevent drops in the conjunctiva, providing a new approach for genipin crosslinking. But his vivo experiment observed only the slit-lamp evaluation and intraocular pressure, and he chose only 5 minutes as the work time. More details, such as the keratocytes and endothelium, need to be evaluated.
In this study, based on our previous research,15,16 we chose 0.25% genipin solution as the effective and safe work liquid. We used a topical soaking method with a vacuum ring to study the long-term effect of genipin crosslinking on rabbit cornea at different times with or without epithelia.
Materials and Methods
Animals
All of the animal experiments were performed in accordance with the Chinese Ministry of Science and Technology Guidelines on the Humane Treatment of Laboratory Animals (Vgkfcz-2006–398) and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. This study was approved by the Laboratory Animal Ethics Committee of Peking University First Hospital (J201425). Twenty-five healthy female New Zealand white rabbits (3.0–3.5 kg) were used in the study. All of the animals were provided by the Peking University First Hospital Animal Center. The animals were subdivided into five groups: 0.25% genipin crosslinking with epithelial cells for 5 minutes (G1 group), 0.25% genipin crosslinking without epithelial cells for 5 minutes (G2 group), 0.25% genipin crosslinking without epithelial cells for 10 minutes (G3 group), ultraviolet A–riboflavin collagen crosslinking (UVA group), and control group (C group, only scraped the epithelial cells), with five rabbits in each group. Right eyes were experimental eyes.
Genipin Crosslinking
Rabbits were anesthetized with intravenous injections of 5% pentobarbital. GP (Wako Pure Chemical Industry, Osaka, Japan) was dissolved in an isotonic medium (phosphate-buffered saline; ZSGB-BIO, Beijing, China) to concentrations of 0.25%. In the G1 group, corneal epithelial cells were kept. In the G2 and G3 groups, the right eye of each rabbit was deepithelialized. The experimental eyes were treated with 500 µL 0.25% genipin in a custom vehicle (Fig. 1) for a corresponding time at room temperature (20°C), using a vacuum device to prevent solution diffusing into the conjunctiva. After the surgery, the genipin solution was then removed by cotton swabs and the corneas were rinsed with 0.9% sodium chloride solution, followed by application of a levofloxacin gel (Sinqi Pharmaceutical, Shenyang, China) to the operated eye to protect the cornea from infection.
Figure 1.

Schematic diagram of genipin crosslinking administration.
UVA Crosslinking
Rabbits were anesthetized with an intravenous injection of 5% pentobarbital. The corneal epithelium of 8 mm was removed by scraping the corneal surface, and then 0.1% riboflavin (Sigma-Aldrich, Darmstadt, Germany) dissolved in 20% dextran (Adamas, Shanghai, China) was applied to the cornea as a droplet every 5 minutes for 30 minutes, followed by 30 minutes of UVA exposure (365 ± 5 nm, 3 mW/cm2) using a light-emitting diode (Lamplic Technology, Shenzhen, China). After surgery, 0.9% sodium chloride solution was used to wash the corneal surface and conjunctival sac. Levofloxacin gel was then applied to the operated eye to protect the cornea from infection.
Control Group
Rabbits were anesthetized in the same manner as in the other groups, and only the corneal epithelium was removed. Levofloxacin gel was then applied to the operated eye to protect the cornea from infection.
Measurements
Before and 1 day after the surgery, we observed rabbit corneas, and then we observed the animals every 2 weeks. All rabbits underwent anterior segment optical coherence tomography (ASOCT) (Heidelberg Engineering, Heidelberg, Germany), in vivo confocal microscopy (IVCM) (HRT3 RCM; Heidelberg Engineering), and Pentacam (Oculus, Optikgerate GmbH, Wetzlar, Germany) scan in vivo to evaluate changes in corneal morphology before and after genipin and UVA crosslinking treatment every 2 weeks. If a well-defined demarcation line (DL) was observed on the ASOCT images, the depth from the corneal surface to the DL at the center cornea was measured with the software on ASOCT. Three to five nonoverlapping images of the cornea stroma and endothelial cells were selected from IVCM for quantified analysis. The average cell count of keratocytes and endothelium cells was calculated with software on IVCM. Densitometry and corneal thinnest thickness were obtained by the software on Pentacam.
Statistical Analysis
The depth of DL, corneal stromal cell density, endothelial cell density, corneal densitometry, and corneal thinnest thickness had a normal distribution by the W test. The data were presented as the mean ± standard deviation using SPSS software (version 20; IBM Corp., Armonk, NY, USA). The differences among groups were assessed by one-way analysis of variance (Bonferroni analysis). Repeated-measures single-factor analysis of variance was used to analyze the differences before and after the treatment, and multiple comparisons were performed using the Least-Significant difference (LSD) method. The correlation analysis used the Pearson method. P<0.05 indicated a statistically significant difference.
Results
Changes of Operated Eyes
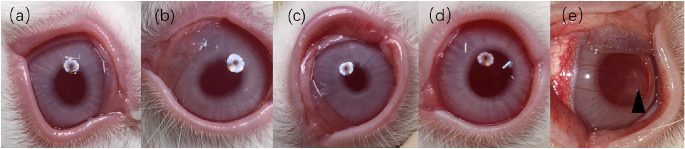
One week after surgery, no obvious eyelid conjunctival edema or congestion was seen in the G1, G2, G3, and C groups. The corneal epithelium had recovered in the G2 and G3 groups. Conjunctival congestion and edema were obvious in the UVA group. At 8 weeks postoperatively, the corneal transparency of G1, G2, G3, and C groups was good. The corneas of G1, G2, and G3 groups had mild blue staining. Most of the UVA group returned to normal. In one experimental animal, due to the slow healing of the corneal epithelium, corneal scarring (Fig. 2) was formed until the end of the follow-up.
Figure 2.
Anterior segmental images of experimental animals in each group 8 weeks after treatment. No congestion, edema, and secretions were found in the conjunctiva of G1 (a), G2 (b), G3 (c), and control (d). In the G1, G2, and G3 groups, mild corneal blue staining was seen at 8 weeks after surgery. In the UVA group (e), corneal scar formation was observed at the eighth week in an experimental animal (▲).
ASOCT
Under ASOCT, 8 weeks after treatment, the corneas of group C showed as a smooth arch, with uniform thickness, intact epithelium, uniform grayish white matrix, and smooth endothelial surface (Fig. 3d). There was no significant difference between the G1 and C groups. However, high-reflective crosslinking demarcation lines in the corneal stroma could be seen in the experimental animals of G2, G3, and UVA groups (Fig. 3), of which the G3 group was more reflective and the border slightly blurred.
Figure 3.

The performance of each experimental group under ASOCT 8 weeks after treatment. There was no significant difference in performance between group G1 (a) and group C (d). Crosslinking demarcation lines (▲) were visible in groups G2 (b), G3 (c), and UVA (e), but the crosslinking demarcation lines of group G3 showed significant reflection.
After determining the midpoint of the pupil, we chose the ASOCT tomographic image that passed through the midpoint of the pupil or closest to the midpoint. We used the ASOCT software to measure the distance from the epithelium to the crosslink line or cross-band boundary in the middle of the pupil. Demarcation line depth (DL depth, unit: µm) was measured at the center of the cornea at each time point, shown in Table 1. Only the DL could be seen in the G2, G3, and UVA groups. In-depth comparison among three groups showed the following: G2>UVA>G3. There was a statistically significant difference in DL depth at weeks 6 and 8.
Table 1.
DL Depth of Each Group at Each Time Point
| 2 Weeks (µm) | 4 Weeks (µm) | 6 Weeks (µm) | 8 Weeks (µm) | |
|---|---|---|---|---|
| G2 | 240.20 ± 36.19 | 209.60 ± 47.13 | 225.80 ± 42.28 | 239.20 ± 37.53 |
| G3 | 196.8 ± 32.38 | 169.80 ± 21.94 | 158.20 ± 8.56 | 164.00 ± 19.00 |
| UVA | 227.40 ± 48.35 | 199.80 ± 40.45 | 193.00 ± 37.66 | 171.60 ± 40.56 |
| P (between groups) | 0.293 | 0.236 | 0.004* | 0.018* |
P < 0.05 compared among each group.
From the change of DL depth, we found that the change of the crosslinking line in the G2 group was relatively stable, and the DL depth was still maintained at 239.20 ± 37.53 µm at the eighth week. The DL depth of the G3 group decreased slightly compared with the previous one and remained at 164.00 ± 19.00 µm. In the UVA group, as time progressed, the DL gradually became shallower, and the DL depth decreased from 227.40 ± 48.35 µm in the second week to 171.60 ± 40.56 µm in the eighth week.
IVCM
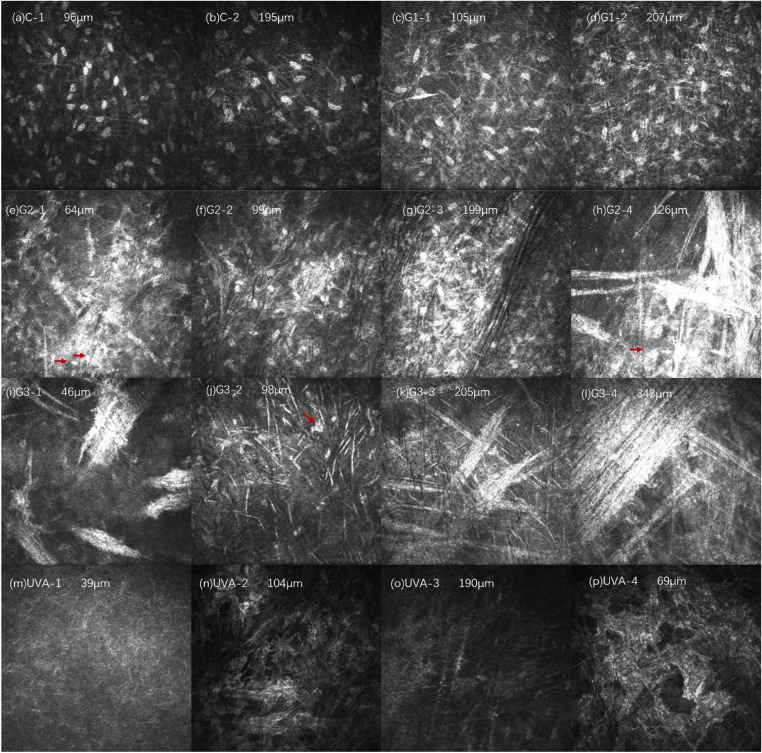
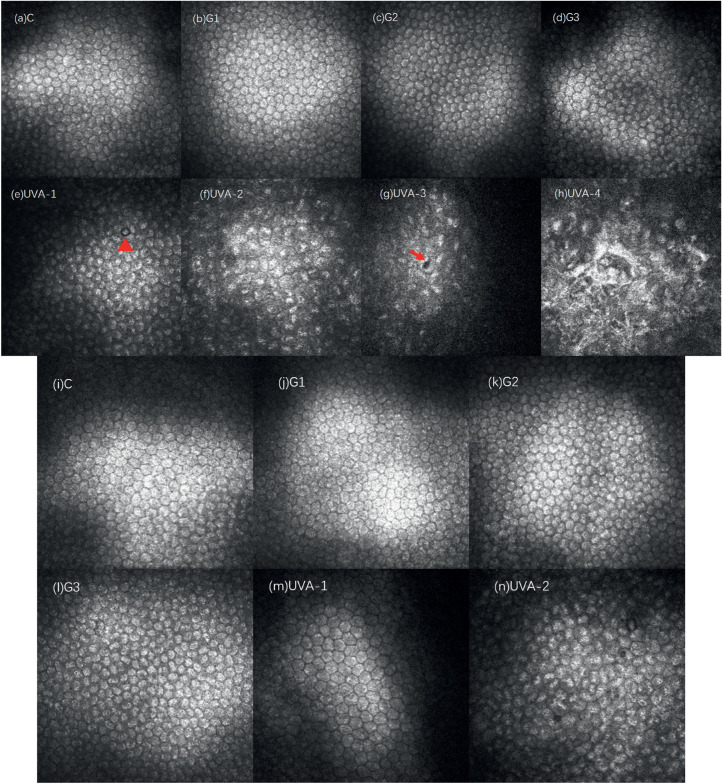
The corneal stroma structure and stromal cell morphology of each experimental group were observed 2 weeks after treatment, as shown in Figure 4. In the C group, the distribution of the superficial stromal cells was slightly uneven, and the number of cells was relatively increased. No obvious abnormalities were found in the deep stromal structure (Figs. 4a, 4b). In the G1 group, the background reflection of the superficial and deep stroma of the cornea increased, the density of stromal cells increased, and the reflection of the filamentous highly reflective structure between the cells increased slightly (Figs. 4c, 4d). There was no significant difference between groups C and G1. In the G2 group, the background reflection of the superficial and deep cornea increased, and the density of stromal cells increased and aggregated into highly reflective cell clusters. A large number of irregular high-reflective structures could be seen in the matrix, which could have been thick rods or clouds, with unclear boundaries. Vaguely visible matrix cells (indicated by the arrow in Fig. 4h) were indistinguishable from the surrounding highly reflective materials. In the G3 group, the background reflection of the superficial and deep stroma increased, and a large number of amorphous high-reflective structures could be seen in the stroma, which could be like pine needles, thick rods, or broad bands, with unclear borders and faintly visible stromal cells (Fig. 4j, arrow showed in the picture), and it was not easy to distinguish them from the surrounding highly reflective materials. As the depth increased, the arrangement of the highly reflective materials gradually became more organized. In the UVA group, the background reflection of the corneal stroma gradually darkened from light to deep, the stroma was a relatively uniform low-reflective structure, and the cell structure was invisible. Occasionally, a structure similar to the activated morphology of stromal cells (Fig. 4p) was observed, but the nucleus of the stromal cells was not seen.
Figure 4.
Changes in corneal stroma in each group 2 weeks after treatment. Compared with the normal corneal structure, the density of stromal cells in group C (a, b) increased slightly. In group G1 (c, d), there were more filamentous hyperreflective structures between stromal cells. In the G2 group, the stromal cells increased in density and aggregated into highly reflective cell clusters (e–g). A large number of amorphous highly reflective structures (h) were visible, and stromal cells were faintly visible (indicated by the arrow in h). In the G3 group, a large number of amorphous high-reflective structures could be seen in the shallow (i, j) and deep (k, l) stroma, and stromal cells could be seen (indicated by arrows in j). The shallow (m, n) and deep (o) stroma of UVA had a relatively uniform low-reflective structure. Occasionally, a structure similar to the stromal cell activation morphology was not seen (p).
In summary, 2 weeks after treatment, no stromal cells activation was observed in the G1 and C groups. A large number of highly reflective structures and stromal cell aggregation were seen in the G2 and G3 groups. The shallow and deep stroma of the UVA group were relatively low reflective, and stromal cell structure was missing.
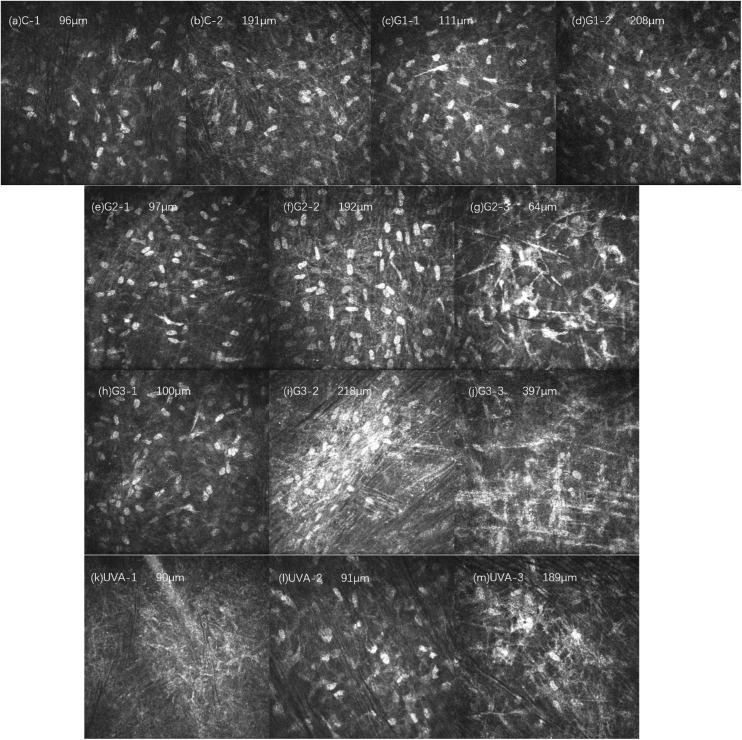
The corneal stroma structure and stromal cell morphology change at 8 weeks after treatment of each experimental group are shown in Figure 5. The corneal stroma structures of groups C and G1 basically returned to normal. In the G2 group, the density of corneal stromal cells increased, stromal cells with high-reflective masses were visible in the superficial layer, and rod-like and filamentary high-reflective structures were seen between the stromal cells. In the G3 group, the density of corneal stromal cells increased, and the superficial stromal structure was basically normal. In the deep layer, high-reflective clusters formed by the aggregation of stromal cells were visible, and rod-like and filamentary high-reflective structures were seen between the stromal cells. In the UVA group, areas with no cell structure and relatively normal cell structures were both observed, but the number of cells was less than that in the G2 and G3 groups. High-reflective cell clusters were observed in the deep matrix.
Figure 5.
Changes in corneal stroma in each group 8 weeks after treatment. The corneal stroma structure of group C (a, b) and group G1 (c, d) basically returned to normal. In the G2 group (e, f, g), the density of the corneal stromal cells increased, and in the superficial layer (g), a hyperreflective group of stromal cells was seen. In the G3 group (h, i, j), the density of corneal stromal cells increased, and deep (i, j) visible hyperreflective clusters formed by the aggregation of stromal cells, and rod-like and filamentary highly reflective structures were seen between the stromal cells. In the UVA group, a cell-free structure area (k) and a relatively normal area (l) could both be observed, and a deep reflective cell mass (m) could be observed in the deep matrix.
As the acellular structure area was still observed at the eighth week in the UVA group, the stromal cell count was difficult, and only the G1, G2, G3, and C groups were counted for stromal cell density. According to the ASOCT measurement of the crosslinking line depth range, 100 µm (about half DL depth) and 200 µm (about full DL depth) were selected as target depths for stromal cell density counting. We selected an image in the range of the target depth ±20 µm for each experimental animal, preferably the smaller absolute value of the target depth. We then chose three to five pictures for each target depth of each experimental animal and calculated the average value to represent the stromal cell density.
The corneal stromal cell densities and their change values before and after treatment for 100 µm and 200 µm depth in each group of experimental animals are shown in Tables 2 and 3. Comparing the groups, there was no significant difference in the density of corneal stromal cells at 100 µm and 200 µm depth before treatment, and there were statistically significant differences in the density of corneal stromal cells at 100 µm and 200 µm depth after treatment (100 µm: P = 0.001; 200 µm: P = 0.004).
Table 2.
Corneal Stromal Cell Density at 100 µm Depth Before and After Treatment at 8 Weeks
| Before (/mm2) | After (/mm2) | P | |
|---|---|---|---|
| G1 | 471.79 ± 52.22 | 452.74 ± 36.93 | 0.131 |
| G2 | 408.53 ± 35.16 | 551.07 ± 73.28 | 0.028† |
| G3 | 364.07 ± 42.75 | 595.52 ± 90.42 | 0.003† |
| C | 367.33 ± 60.64 | 386.25 ± 67.46 | 0.037† |
| P | 0.224 | 0.001‡ |
P < 0.05 compared to the cell density before the treatment.
P < 0.05 compared among the G1, G2, G3, and C groups.
Table 3.
Corneal Stromal Cell Density at 200 µm Depth Before and After Treatment at 8 Weeks
| Before (/mm2) | After (/mm2) | P | |
|---|---|---|---|
| G1 | 358.58 ± 60.50 | 390.98 ± 44.93 | 0.061 |
| G2 | 384.04 ± 46.23 | 515.72 ± 99.51 | 0.023† |
| G3 | 349.68 ± 44.37 | 477.98 ± 78.53 | 0.015† |
| C | 310.66 ± 34.87 | 342.23 ± 31.40 | 0.142 |
| P | 0.146 | 0.004‡ |
P < 0.05 compared to the cell density before the treatment.
P < 0.05 compared among the G1, G2, G3, and C groups.
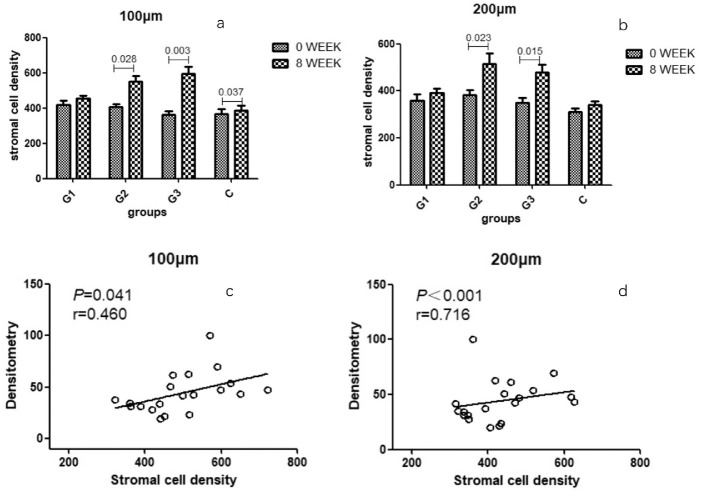
Corneal stromal cell density changes in each group are shown in Figures 6a and 6b. In the G1 group, at 100 µm and 200 µm depth, there was no significant change in the density of corneal stromal cells before and after treatment at 8 weeks. In the G2 group at 100 µm and 200 µm depth, the density of corneal stromal cells increased significantly before and after treatment, and the difference was statistically significant (100 µm: P = 0.028; 200 µm: P = 0.003). In the G3 group, at 100 µm and 200 µm depth, the density of corneal stromal cells increased significantly before and after treatment, and the difference was statistically significant (100 µm: P = 0.023; 200 µm: P = 0.015). In group C at a depth of 100 µm, the density of corneal stromal cells before treatment and after treatment was mildly significant, and the difference was statistically significant (P = 0.037).
Figure 6.
Stromal cell density of each group and the correlation analysis. (a) Before the treatment. (b) After treatment. (c) Correlation analysis of stromal cell density and densitometry at 100 µm. (d) Correlation analysis of stromal cell density and densitometry at 200 µm.
The changes of corneal endothelial cells in each group at 2 weeks were shown in Figures 7a–h. The corneal endothelial cells in groups C, G1, and G2 were arranged closely, and their morphology was basically normal. The border of corneal endothelial cells in group G3 was slightly blurred, but relatively normal hexagonal cell could be seen. The corneal endothelial cells in the UVA group showed different individual characteristics. Relatively normal cell morphology could be observed, but the boundary of the endothelial cells was blurred. The enlarged, damaged, and expanded endothelial cells could be seen (Fig. 7e). In some subjects, endothelial cells were significantly damaged, the enlarged hexagonal morphology of endothelial cells disappeared, and the endothelial surface was uneven (Fig. 7f). The depression left after the endothelial cells were damaged and detached could be observed (indicated by the arrow in Fig. 7g). In severe cases, the morphology of endothelial cells was completely absent, and only the uneven reflective interface was seen (Fig. 7h). The changes of corneal endothelial cells in each group after treatment at 8 weeks are shown in Figures 7i–n. The corneal endothelial cells in groups C, G1, and G2 were arranged closely, and their morphology was basically normal. The border of corneal endothelial cells in group G3 was slightly blurred, but relatively normal hexagonal cell could be seen. In the UVA group, corneal endothelial cells could be relatively normal, but some endothelial cells had been significantly damaged. The cells were swollen and enlarged, hexagonal morphology disappeared, and the endothelial surface was uneven (Fig. 7n).
Figure 7.
Corneal endothelial cells in each group after treatment at 2 weeks and 8 weeks. At 2 weeks, the corneal endothelial morphology and structure of the G1 (a), G2 (b), and G3 (c) groups were basically normal, and the borders of the corneal endothelial cells in the G3 group were slightly blurred. The UVA group showed diverse performances: damaged and swollen endothelial cells (e, ▲); endothelial cells with enlarged hexagonal morphology disappeared, and the endothelial surface was uneven (f); depressions left after endothelial cells were damaged and shed (g); endothelial cell morphology was not complete, only bumpy reflective interface (h). At 8 weeks, the morphology and structure of corneal endothelial cells in groups C (i), G1 (j), and G2 (k) were basically normal, and the border of corneal endothelial cells in group G3 (l) was slightly blurred. In the UVA group, relatively normal cell morphology was seen (m), but endothelial cells were still damaged, swollen, and enlarged (n).
Corneal endothelial cell density of each group before and after treatment is shown in Table 4. Comparing these groups, there was no significant difference in corneal endothelial cell density counts before treatment, and there was a statistically significant difference in corneal endothelial cell density after treatment (P < 0.001). Before and after treatment, the G1, G2, G3, and C groups had no significant changes in endothelial cell density. But in the UVA group, 8 weeks after the treatment, the corneal endothelial cell density decreased significantly compared with that before treatment. The difference was statistically significant (P = 0.010).
Table 4.
Corneal Endothelial Cell Density Before and After 8 Weeks of Treatment
| Before (/mm2) | After (/mm2) | P | |
|---|---|---|---|
| G1 | 2705.44 ± 582.30 | 2764.67 ± 167.25 | 0.328 |
| G2 | 2754.39 ± 300.55 | 2928.25 ± 299.23 | 0.254 |
| G3 | 3090.22 ± 244.28 | 3079.77 ± 361.64 | 0.339 |
| C | 2779.21 ± 174.57 | 2886.43 ± 158.73 | 0.684 |
| UVA | 2686.13 ± 340.80 | 2243.28 ± 39.28 | 0.010† |
| P | 0.338 | <0.001‡ |
P < 0.05 compared to the cell density before the treatment.
P < 0.05 compared among the G1, G2, G3, and C groups.
Pentacam
The corneal optical densitometry of each group before and 8 weeks after treatment is shown in Table 5. The densitometry of G2, G3, and UVA groups increased significantly 8 weeks after treatment, and the difference was statistically significant. There was no significant change in the densitometry of the G1 and C groups 8 weeks after treatment.
Table 5.
Densitometry of Each Group Before and After 8 Weeks of Treatment
| Before (µm) | After (µm) | P | |
|---|---|---|---|
| G1 | 26.62 ± 5.49 | 25.38 ± 5.67 | 0.422 |
| G2 | 24.58 ± 3.40 | 49.38 ± 8.23 | 0.006† |
| G3 | 28.14 ± 4.45 | 66.34 ± 20.29 | 0.020† |
| C | 34.60 ± 3.15 | 35.32 ± 4.54 | 0.770 |
| UVA | 28.36 ± 3.56 | 74.74 ± 22.80 | 0.010† |
P < 0.05 compared to the cell density before the treatment.
Correlation analysis of stromal cell density and densitometry at 8 weeks after treatment in each group found that stromal cell density at 100 µm and 200 µm depth had a positive correlation with densitometry. The corresponding P value and correlation coefficient r are shown in Figures 6c and 6d.
The thinnest corneal thickness measured by Pentacam before and 8 weeks after treatment in each group is shown in Table 6. Comparing the groups, the thickness of the thinnest part of the cornea before treatment was not statistically significant, and the thickness of the thinnest part of the anterior cornea after treatment was statistically significant (P = 0.002). After treatment in the G3 and UVA groups, the thinnest part of the cornea became significantly thinner, and the difference was statistically significant (G3: P = 0.031; UVA: P = 0.009). The thinnest part of the cornea in group C was slightly thickened, and the difference was statistically significant (P = 0.046). There was no significant difference between the G1 and G2 groups before and after treatment.
Table 6.
Thinnest Corneal Thickness Before and After Treatment
| Before (µm) | After (µm) | P | |
|---|---|---|---|
| G1 | 309.10 ± 20.02 | 363.60 ± 30.88 | 0.061 |
| G2 | 301.40 ± 50.76 | 331.20 ± 11.71 | 0.296 |
| G3 | 329.20 ± 24.95 | 251.20 ± 67.36 | 0.031† |
| C | 331.20 ± 32.54 | 352.80 ± 43.59 | 0.046† |
| UVA | 316.20 ± 39.26 | 277.00 ± 45.03 | 0.009† |
| P | 0.629 | 0.002‡ |
P < 0.05 compared to the thinnest corneal thickness before the treatment.
P < 0.05 compared among the groups.
Discussion
Genipin, an aglycone derived from an iridoid glycoside called geniposide, present in the fruit of Gardenia jasminoides Ellis, has long been used as a traditional Oriental medicine for the treatment of hepatic disorders and inflammatory diseases.24 Sung et al.25,26 found that genipin-crosslinked gelatin mixtures have better biocompatibility and lower cytotoxicity, induce less inflammatory response, and recover sooner than common chemical crosslinking agents such as formaldehyde, glutaraldehyde, and epoxy resin. The research on the application of genipin crosslinking mainly includes several aspects. On one hand, genipin-crosslinked biomacromolecular materials have potential pharmaceutical applications, especially in controlling drug delivery from diverse formulations.27 One the other hand, genipin-crosslinked acellular biological tissues or biomacromolecules can be used as bioreplacement materials, such as using genipin-crosslinked acellular porcine corneal stroma for cosmetic corneal lens implants,28 genipin-fixed vascular29 and pericardium30 as grafts, and genipin crosslinking of cartilage to enhance resistance to biochemical degradation and mechanical wear. In addition, in ophthalmology, genipin also has been used to directly crosslink biological tissues to enhance their biomechanical properties. Studies have found that crosslinking the sclera and corneal tissue can strengthen the biomechanical strength of the sclera and the cornea.11,18–21 By this, it can prevent the pathologic growth of the axial axis and the expansion of the corneal tissue, as well as provide new treatment options for pathologic myopia and corneal dilatation diseases.
At present, direct soaking is the main method to complete the genipin crosslinking progress on biomaterials. Researchers have mainly considered the biocompatibility, duration, cytotoxicity, and inflammatory response of crosslinked materials. For these aspects, genipin has shown excellent crosslinking ability and safety.27,28,31–34 Genipin crosslinking includes ex vivo crosslinking and in vivo crosslinking. For ex vivo corneal and scleral tissues, researchers usually choose the direct immersion method.13,18,35 For in vivo crosslinking, researchers will choose different methods depending on the location of the tissue. Liu and Wang23 and Wang and Corpuz21 injected genipin solution directly under Tenon's capsule for scleral crosslinking. In our previous study, we used genipin as a droplet to crosslink the deepithelialized cornea.15,16 These methods show that genipin has broad application prospects in improving the biomechanical properties of sclera and cornea, but using genipin for direct crosslinking needs to consider many aspects. The direct immersion method is easy to operate in vitro, but it is difficult to achieve crosslinking in vivo. Proteins and amino acids are the biochemical structures for genipin crosslinking. Therefore, the crosslinking effect is not selective. Droplet and injection will inevitably expose surrounding nontarget tissues to the genipin solution, causing stimulation of surrounding tissues and crosslinking of nontarget tissues, with obvious side effects. In addition, the exudation from the conjunctiva exudate can dilute the drug concentration, but evaporation of the ocular surface can concentrate the drug concentration. Therefore, the predictability of the crosslinking effect is poor, as the genipin effect is concentration dependent.13 Avila et al.11 used a vacuum device to prevent drops in the conjunctiva and increase the permeability of the drug. Since the genipin solution did not contact other surrounding tissues and the crosslinking time was only 5 minutes, the side reaction was slight. This method provides a new approach for genipin crosslinking. In this study, a corneal vacuum ring was used as an auxiliary drug delivery device. The genipin solution was limited to the center of the cornea within 8 mm, so that genipin was only in full contact with the cornea. At the end of the treatment, the liquid was fully dried with a cotton swab first, and then the negative pressure ring was removed and flushed with saline to maximize the isolation of the genipin solution from nontarget tissues. The entire crosslinking process is only 5 to 10 minutes, with less pain. Therefore, the ocular surface reaction is slight after surgery, and the corneal epithelium recovers quickly. The classic UV-riboflavin crosslinking method takes about an hour, and the postoperative epithelial recovery is slow. In our study, one animal eventually developed a corneal scar due to the delayed healing of the epithelium. Therefore, compared with UV-riboflavin crosslinking, this genipin crosslinking method is superior to traditional UV-riboflavin crosslinking methods for patient tolerance and postoperative recovery.
In the clinic, after crosslinking by UV riboflavin, the presence of a crosslinking boundary in the corneal stroma can be seen under a slit lamp, ASOCT, and a corneal confocal lens.36 Under the slit lamp, it appears as a gray-white dividing line in the corneal stroma. Under ASOCT, it shows a high-reflective arc-shaped band in the stroma. Under confocal microscopy, it is generally considered a stroma transition zone with and without stroma cells. The depth may indicate the position and degree that can be achieved by crosslinking.36 In this study, ASOCT found that after genipin crosslinking, a high-reflective arc-shaped band could appear similar to UV-riboflavin crosslinking. Moreover, the DL depth of the G2, G3, and UVA groups was similar. Dynamic observation of the DL depth found that the G2 and G3 groups were relatively stable, and the UVA group gradually became shallower. This also helps confirm the effectiveness of genipin crosslinking. The composition of the DL after genipin crosslinking is currently unknown. We found a large number of highly reflective structures by IVCM, which is speculated to be related to the DL, but histologic examination and molecular biological testing are required in the future.
Under IVCM, we found that 2 weeks after treatment, a large number of highly reflective cell clusters and highly reflective amorphous substances were seen in the corneal stroma of the G2 and G3 groups, showing various forms and other highly reflective structures (corneal stroma cells, nervous) not easy to distinguish. At the end of observation, highly reflective cell clusters and rod-like and filament-like highly reflective structures could still be seen in the G2 and G3 groups. Similarly, 8 weeks after treatment, we also found highly reflective stromal cell clusters in the deep stroma in the UVA group. Mazzotta et al.37 found similar changes in corneal confocal microscopy scans of patients after UV-riboflavin crosslinking and speculated that such highly reflective substances may represent new collagen fibers and extracellular matrix produced by restored stromal cells ingredient. Since the superficial and middle corneal stromal cells quickly die and disappear after UV-riboflavin crosslinking, it takes 2 to 3 months for the stromal cells to recover from deep to shallow.38 Therefore, most of these highly reflective structures were first found in the deep layers of the stroma and later, which is consistent with our findings. Corneal stromal cells can secrete collagen fibers and proteoglycans, assist in the assembly of collagen fibers, assist in the formation of collagen laminae, and play an important role in the formation of the corneal stroma and the maintenance of the stroma structure.39 Corneal stromal cells are still present 24 hours after genipin crosslinking.15 In this study, stromal cells were mixed in highly reflective materials. Therefore, we speculate that the highly reflective amorphous material observed in the genipin crosslinking group is related to corneal stromal cells, which may be neocollagenous fibers or matrices produced by activated stromal cells, which are stimulated by crosslinking. For the highly reflective substances observed in the genipin crosslinking group, no research has elucidated the composition of such structures. Further research is needed from the perspectives of biochemistry, biomechanics, and ultrastructure.
Haze after corneal crosslinking is a common side effect after UV-riboflavin crosslinking. Greenstein40 found that the haze densitometry after crosslinking was inversely related to postoperative vision. However, haze does not persist after crosslinking, and most of this haze disappears 6 to 12 months after lamellar remodeling.40,41 Densitometry measurement using Pentacam is currently used to find haze after crosslinking of UV riboflavin, which is difficult to discern by the eye.42 Avila et al.,11 who used Pentacam to measure corneal densitometry of rabbit cornea ex vivo after genipin solution crosslinking, found that the densitometry of the cornea was concentration dependent on genipin solution. In this study, Pentacam was used to find the changes in corneal optical density of each group before and after 8 weeks of treatment. It was found that the densitometry of the G2, G3, and UVA groups increased significantly after 8 weeks. In addition to crosslinking surgery, a similar haze occurs after Photo Refractive Keratectomy (PRK). Studies have shown that haze after PRK is associated with corneal stromal cell–mediated damage repair. During this process, the density of stromal cells increases, generating new extracellular matrix components.43 Some studies suggest that haze is also associated with corneal stromal cells after crosslinking.40 Corneal stromal cells contain crystalline proteins, which have the same refractive index as corneal stroma. Changes in the crystal protein and its refractive index in activated corneal stromal cells have caused the scattering of light to form haze.44 We analyzed the correlation between corneal stromal cell density and densitometry value and found that there is a positive correlation between corneal stromal cell density and densitometry value. We speculate that corneal stromal cells may play an important role in the genipin crosslinking process.
The safety of various crosslinking methods for corneal endothelial cells is the focus of our attention. UV riboflavin crosslinking has the possibility of damaging corneal endothelial cells.45,46 Therefore, for progressive keratoconus patients and corneal dilatation patients after refractive surgery with a corneal thickness less than 400 µm, UV-riboflavin crosslinking is generally not recommended.47 The thinnest corneal thickness of the UVA group before treatment in this study was only 316.20 ± 39.26 µm, and the G2 and G3 groups were 301.40 ± 50.76 µm and 329.20 ± 24.95 µm, respectively. After treatment, the corneas in the G3 and UVA groups became thinner: 251.20 ± 67.36 µm and 277.00 ± 45.03 µm, respectively. Studies have suggested that the thinning of the cornea after crosslinking may be due to the denser corneal stroma and lamellar compression due to the new fiber connections.48 IVCM found that the density of corneal endothelial cells in each genipin-crosslinked group had no significant change compared with that before treatment, but the corneal endothelial cells in the UV riboflavin crosslinked group decreased significantly. Morphologically, the corneal endothelial cells were not significantly abnormal after 2 weeks of treatment in each genipin group. In the UV riboflavin crosslinked group, obvious morphologic changes of endothelial cells were seen, including swelling and expansion of endothelial cells, which is consistent with the damaged morphology of UV riboflavin crosslinked endothelial cells observed under scanning electron microscopy in the previous study.15 Up to 8 weeks after treatment, normal endothelial cell morphology was seen in all groups treated with genipin, while endothelial cells in the UV riboflavin crosslinked group still had edema and enlargement. Therefore, for thin cornea, especially ultra-thin cornea less than 350 µm, the genipin crosslinking method has irreplaceable advantages.
Because corneal stromal cells play an important role in the formation of the corneal stroma and the maintenance of the stroma structure,37 we counted corneal stromal cells in each experimental group 8 weeks after treatment and selected target depths of 100 µm (approximately half the depth of the crosslinking line) and 200 µm (approximately the depth of the full crosslinking line). In the UVA group, there were still areas with no cell structure in the corneal stroma at 8 weeks after operation, and it was difficult to count the stroma cells, so it was not included in the statistical scope. At 8 weeks after treatment, the density of corneal stromal cells in the G2 and G3 groups at 100 µm and 200 µm depth increased significantly compared with that before treatment. This further illustrates that genipin crosslinking has no toxic effect on corneal stromal cells. It is well known that UV riboflavin crosslinking has a clear injury effect on corneal stromal cells, and this significant change was also observed in our study. In contrast, genipin is safer for the cellular components of the corneal stroma. In the previous study, we found that vacuole-like structures appeared in the stromal cells observed under transmission electron microscopy 24 hours after genipin crosslinking.15 The cell was expanded and deformed due to the vacuole structure, but the cell membrane and organelle structure were normal. This study also found increased corneal stromal cell density after genipin crosslinking. Therefore, we infer that genipin may have the effect of activating corneal stromal cells, and this effect begins to occur 24 hours after crosslinking, but its specific mechanism needs to be further explored.
Avila et al.9 mentioned that the crosslinking effect of genipin was not affected by the presence of corneal epithelial cells, but they did not give any experimental data, and subsequent studies have removed corneal epithelium. In this experiment, an unscratched epithelial group was set up to study the effect of corneal epithelium on genipin crosslinking. The results showed that no crosslinking lines were observed in ASOCT examination, no changes in densitometry were observed in Pentacam examination, and no changes in stroma and cellular components were observed under IVCM. The corneal epithelium is rich in lipids, and fat-soluble substances are easy to pass. Genipin is an iridoid glycoside, which contains multiple chemical groups such as hydroxyl and carboxyl groups in the molecule and is easily soluble in water.49,50 Therefore, it can be judged that the 0.25% genipin solution cannot penetrate the corneal epithelium into the corneal stroma to crosslink in a short time.
In conclusion, this study adopted a vacuum ring local immersion crosslinking method, using UV riboflavin crosslinking as an effective control, and observed the corneal stroma structure by immersing 0.25% genipin solution for 5 minutes after epithelial removal. From the influence of cell components, this study further confirmed the safety of genipin crosslinking, especially in the field of thin corneal crosslinking. This method takes a short time, has strong operability, and has good application prospects. At the same time, we also found that corneal stromal cells may play an important role in genipin crosslinking, leading the way for further exploration of genipin corneal crosslinking. This study still has some limitations. The most important point is that this study mainly focuses on morphologic observations. The mechanisms for many morphologic changes after genipin crosslinking are unclear. Including the DL observed under ASOCT, the composition of highly reflective materials under the confocal microscope, and the increase in densitimetry found by Pentacam. More studies, such as histology, cell biology, and molecular biology research are needed.
Acknowledgments
Supported by the Beijing Natural Science Foundation (7192210), Scientific Research Seed Fund of Peking University First Hospital (2020SF07), and National Natural Science Foundation of China (11372011). The funding organizations had no role in the design or conduct of this research.
Disclosure: W. Song, None; Y. Cheng, None; X. Yan, None; S. Yang, None
References
- 1.O'Brart DPS.Corneal collagen crosslinking for corneal ectasias: a review. Eur J Ophthalmol. 2017; 27: 253–269. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Tao XC, Zhang J, et al.. A review of collagen cross-linking in cornea and sclera. J Ophthalmol. 2015; 2015: 289467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregor W, Eberhard S, Theo S.. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003; 135: 620–627. [DOI] [PubMed] [Google Scholar]
- 4.Tomita M, Mita M, Huseynova T.. Accelerated versus conventional corneal collagen crosslinking. J Cataract Refract Surg. 2014; 40: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 5.Hassan Z, Szalai E, Modis L Jr, Berta A, Nemeth G. Assessment of corneal topography indices after collagen crosslinking for keratoconus. Eur J Ophthalmol. 2013; 23: 635–640. [DOI] [PubMed] [Google Scholar]
- 6.Bekesi N, Kochevar IE, Marcos S. Corneal biomechanical response following collagen cross-linking with rose bengal-green light and riboflavin-UVA. Invest Ophthalmol Vis Sci. 2016; 57: 992–1001. [DOI] [PubMed] [Google Scholar]
- 7.Zhu H, Alt C, Webb RH, Melki S, Kochevar IE.. Corneal crosslinking with rose bengal and green light: efficacy and safety evaluation. Cornea. 2016; 35: 1234–1241. [DOI] [PubMed] [Google Scholar]
- 8.Fadlallah A, Zhu H, Arafat S, Kochevar I, Melki S, Ciolino JB.. Corneal resistance to keratolysis after collagen crosslinking with rose bengal and green light. Invest Ophthalmol Vis Sci. 2016; 57: 6610–6614. [DOI] [PubMed] [Google Scholar]
- 9.Avila MY, Gerena VA, Navia JL.. Corneal crosslinking with genipin, comparison with UV-riboflavin in ex-vivo model. Mol Vis. 2012; 18: 1068–1073. [PMC free article] [PubMed] [Google Scholar]
- 10.Dias J, Diakonis VF, Lorenzo M, et al.. Corneal stromal elasticity and viscoelasticity assessed by atomic force microscopy after different cross linking protocols. Exp Eye Res. 2015; 138: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avila MY, Narvaez M, Castaneda JP.. Effects of genipin corneal crosslinking in rabbit corneas. J Cataract Refract Surg. 2016; 42: 1073–1077. [DOI] [PubMed] [Google Scholar]
- 12.Lim HG, Kim SH, Choi SY, Kim YJ.. Anticalcification effects of decellularization, solvent, and detoxification treatment for genipin and glutaraldehyde fixation of bovine pericardium. Eur J Cardiothorac Surg. 2012; 41: 383–390. [DOI] [PubMed] [Google Scholar]
- 13.Avila MY, Navia JL.. Effect of genipin collagen crosslinking on porcine corneas. J Cataract Refract Surg. 2010; 36: 659–664. [DOI] [PubMed] [Google Scholar]
- 14.Lai JY.Biocompatibility of genipin and glutaraldehyde cross-linked chitosan materials in the anterior chamber of the eye. Int J Mol Sci. 2012; 13: 10970–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song W, Tang Y, Qiao J, et al.. The comparative safety of genipin versus UVA-riboflavin crosslinking of rabbit corneas. Mol Vis. 2017; 23: 504–513. [PMC free article] [PubMed] [Google Scholar]
- 16.Song W, Tang Y, Qiao J, et al.. The short-term safety evaluation of corneal crosslinking agent-genipin. Ophthalmic Res. 2019; 62: 141–149. [DOI] [PubMed] [Google Scholar]
- 17.Xue A, Zheng L, Tan G, et al.. Genipin-crosslinked donor sclera for posterior scleral contraction/reinforcement to fight progressive myopia. Invest Ophthalmol Vis Sci. 2018; 59: 3564–3573. [DOI] [PubMed] [Google Scholar]
- 18.Yuan W, Songlin Y, Haili L, Xiaoming Y, Fan S.. Effect of collagen crosslinking on porcine sclera with different methods. Chin J Exp Ophthalmol. 2013; 31: 168–171. [Google Scholar]
- 19.Taixiang L, Junshu W, Yuwei G, Bin Y, Zheng W. Change of biomechanical properties in porcine sclera treated with genipin. Chin J Optometry Ophthalmol Vis Sci. 2014; 16: 274–278. [Google Scholar]
- 20.Taixiang L, Zheng W.. Efficiency and safety of crosslinking of scleral collagen using genipin in rabbit. Recent Adv Ophthalmol. 2015; 35: 619–622. [Google Scholar]
- 21.Wang M, Corpuz CC.. Effects of scleral cross-linking using genipin on the process of form-deprivation myopia in the guinea pig: a randomized controlled experimental study. BMC Ophthalmol. 2015; 15: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gharaibeh AM, Saez V, Garcia N, Bataille L, Alió JL.. Optimizing genipin concentration for corneal collagen cross-linking: an ex vivo study. Ophthalmic Res. 2018; 60: 100–108. [DOI] [PubMed] [Google Scholar]
- 23.Liu TX, Wang Z.. Biomechanics of sclera crosslinked using genipin in rabbit. Int J Ophthalmol. 2017; 10: 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim ES, Jeong CS, Moon A.. Genipin, a constituent of Gardenia jasminoides Ellis, induces apoptosis and inhibits invasion in MDA-MB-231 breast cancer cells. Oncol Rep. 2012; 27: 567–572. [DOI] [PubMed] [Google Scholar]
- 25.Sung HW, Huang DM, Chang WH, Huang LL, Tsai CC, Liang IL.. Gelatin-derived bioadhesives for closing skin wounds: an in vivo study. J Biomater Sci Polym Ed. 1999; 10: 751–771. [DOI] [PubMed] [Google Scholar]
- 26.Sung HW, Huang DM, Chang WH, Huang RN, Hsu JC.. Evaluation of gelatin hydrogel crosslinked with various crosslinking agents as bioadhesives: in vitro study. J Biomed Mater Res. 1999; 46: 520–530. [DOI] [PubMed] [Google Scholar]
- 27.Balamurugan M, Rajesh S, Manogaran E. ‘Genipin’—the natural water soluble cross-linking agent and its importance in the modified drug delivery systems: an overview. Curr Drug Deliv. 2014; 11: 139–145. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Zhou Q, Zhu J, et al.. Using genipin-crosslinked acellular porcine corneal stroma for cosmetic corneal lens implants. Biomaterials. 2012; 33: 7336–7346. [DOI] [PubMed] [Google Scholar]
- 29.Chang Y, Hsu C-K, Wei H-J, et al.. Cell-free xenogenic vascular grafts fixed with glutaraldehyde or genipin: in vitro and in vivo studies. J Biotechnol. 2005; 120: 207–219. [DOI] [PubMed] [Google Scholar]
- 30.Chang Y, Tsai C-C, Liang H-C, Sung H-W.. In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin). Biomaterials. 2002; 23: 2447–2457. [DOI] [PubMed] [Google Scholar]
- 31.Somers P, De Somer F, Cornelissen M, et al.. Genipin blues: an alternative non-toxic crosslinker for heart valves? J Heart Valve Dis. 2008; 17: 682–688. [PubMed] [Google Scholar]
- 32.Gu Z, Zhang X, Yu X, Li L, Xu Y, Chen Y.. Biocompatibility of genipin-fixed porcine aorta as a possible esophageal prosthesis. Mater Sci Eng C. 2011; 31: 1593–1601. [Google Scholar]
- 33.McGann ME, Bonitsky CM, Jackson ML, Ovaert TC, Trippel SB, Wagner DR.. Genipin crosslinking of cartilage enhances resistance to biochemical degradation and mechanical wear. J Orthop Res. 2015; 33: 1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grolik M, Szczubialka K, Wowra B, et al.. Hydrogel membranes based on genipin-cross-linked chitosan blends for corneal epithelium tissue engineering. J Mater Sci Mater Med. 2012; 23: 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu TX, Luo X, Gu YW, Yang B, Wang Z.. Correlation of discoloration and biomechanical properties in porcine sclera induced by genipin. Int J Ophthalmol. 2014; 7: 621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khandelwal SS, Randleman JB.. Current and future applications of corneal cross-linking. Curr Opin Ophthalmol. 2015; 26: 206–213. [DOI] [PubMed] [Google Scholar]
- 37.Mazzotta C, Traversi C, Baiocchi S, et al.. Corneal healing after riboflavin ultraviolet-A collagen cross-linking determined by confocal laser scanning microscopy in vivo: early and late modifications. Am J Ophthalmol. 2008; 146: 527–533. [DOI] [PubMed] [Google Scholar]
- 38.Mazzotta C, Balestrazzi A, Traversi C, et al.. Treatment of progressive keratoconus by riboflavin-UVA-induced cross-linking of corneal collagen: ultrastructural analysis by Heidelberg Retinal Tomograph II in vivo confocal microscopy in humans. Cornea. 2007; 26: 390–397. [DOI] [PubMed] [Google Scholar]
- 39.Chen S, Mienaltowski MJ, Birk DE.. Regulation of corneal stroma extracellular matrix assembly. Exp Eye Res. 2015; 133: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenstein SA, Fry KL, Bhatt J, Hersh PS.. Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia: Scheimpflug and biomicroscopic analysis. J Cataract Refract Surg. 2010; 36: 2105–2114. [DOI] [PubMed] [Google Scholar]
- 41.Alnawaiseh M, Rosentreter A, Eveslage M, Eter N, Zumhagen L.. Changes in corneal transparency after cross-linking for progressive keratoconus: long-term follow-up. J Refract Surg. 2015; 31: 614–618. [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez R, Lopez I, Villa-Collar C, Gonzalez-Meijome JM.. Corneal transparency after cross-linking for keratoconus: 1-year follow-up. J Refract Surg. 2012; 28: 781–786. [DOI] [PubMed] [Google Scholar]
- 43.Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV.. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology. 2000; 107: 1235–1245. [DOI] [PubMed] [Google Scholar]
- 44.Jester JV, Moller-Pedersen T, Huang J, et al.. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J Cell Sci. 1999; 112(pt 5): 613–622. [DOI] [PubMed] [Google Scholar]
- 45.Holopainen JM, Kari K.. Transient corneal thinning in eyes undergoing corneal cross-linking. Am J Ophthalmol. 2011; 152: 533–536. [DOI] [PubMed] [Google Scholar]
- 46.Kymionis GD, Kounis GA, Portaliou DM, et al.. Intraoperative pachymetric measurements during corneal collagen cross-linking with riboflavin and ultraviolet A irradiation. Ophthalmology. 2009; 116: 2336–2339. [DOI] [PubMed] [Google Scholar]
- 47.Parker JS, van Dijk K, Melles GR.. Treatment options for advanced keratoconus: a review. Surv Ophthalmol. 2015; 60: 459–480. [DOI] [PubMed] [Google Scholar]
- 48.Toprak I, Yildirim C.. Scheimpflug parameters after corneal collagen crosslinking for keratoconus. Eur J Ophthalmol. 2013; 23: 793–798. [DOI] [PubMed] [Google Scholar]
- 49.Nickerson MT, Farnworth R, Wagar E, Hodge SM, Rousseau D, Paulson AT.. Some physical and microstructural properties of genipin-crosslinked gelatin-maltodextrin hydrogels. Int J Biol Macromol. 2006; 38: 40–44. [DOI] [PubMed] [Google Scholar]
- 50.Koo HJ, Song YS, Kim HJ, et al.. Antiinflammatory effects of genipin, an active principle of gardenia. Eur J Pharmacol. 2004; 495: 201–208. [DOI] [PubMed] [Google Scholar]