Conflict of interest
G. Fabbrocini acted as a speaker or consultant for Abbvie, Amgen, Eli Lilly, Janssen, Leo‐Pharma, Almyrall, Novartis and UCB. Maria Vastarella, Vincenzo Picone and Fabrizio Martora have no conflict of interest. None of the contributing authors has any conflict of interest, including specific financial interests of relationships and affiliation relevant to the subject matter or discussed materials in the manuscript.
Dear Editor,
The SARS‐CoV‐2 pandemic has plagued the world over the year. Many vaccines have been created to alleviate the morbidity and mortality associated with COVID‐19 and stop viral transmission. In Italy, the vaccination campaign with the recombinant adenoviral vector encoding the SARS‐CoV‐2 spike protein (AstraZeneca) started on 30 January 2021. The most described vaccine‐related side effects in the literature are fever, redness, pain and tenderness at the injection site, musculoskeletal pains and headache. 1 Here, we report three cases of patients that presented a reactivation of herpes zoster after the first dose of the vaccine (AstraZeneca).
In the first case, a 76‐year‐old woman presented to our dermatology department with tense vesicular lesions on an erythematous background placed on the right breast region. During anamnesis collection, it emerged that the patient received the first dose of the ChAdOx1 nCoV‐19 vaccine 7 days before the skin eruption. In the second case, a 79‐year‐old man presented the same manifestations placed over the right thigh 6 days after vaccination. Finally, a 70‐year‐old man showed the same manifestations located on the left side of the neck 10 days after vaccination. None of the 3 aforementioned cases had other symptoms associated with the rash. On dermatological physical examination, groups of tense vesicles, sometimes excoriated, on an erythematous background with dermatomal distribution have been objectives, with associated burning and itching symptoms (Fig. 1). Based on the clinical history and physical examination, a diagnosis of herpes zoster was made, and, according to guidelines, systemic antiviral therapy was prescribed in all cases resulting in the resolution of the manifestations.
Figure 1.
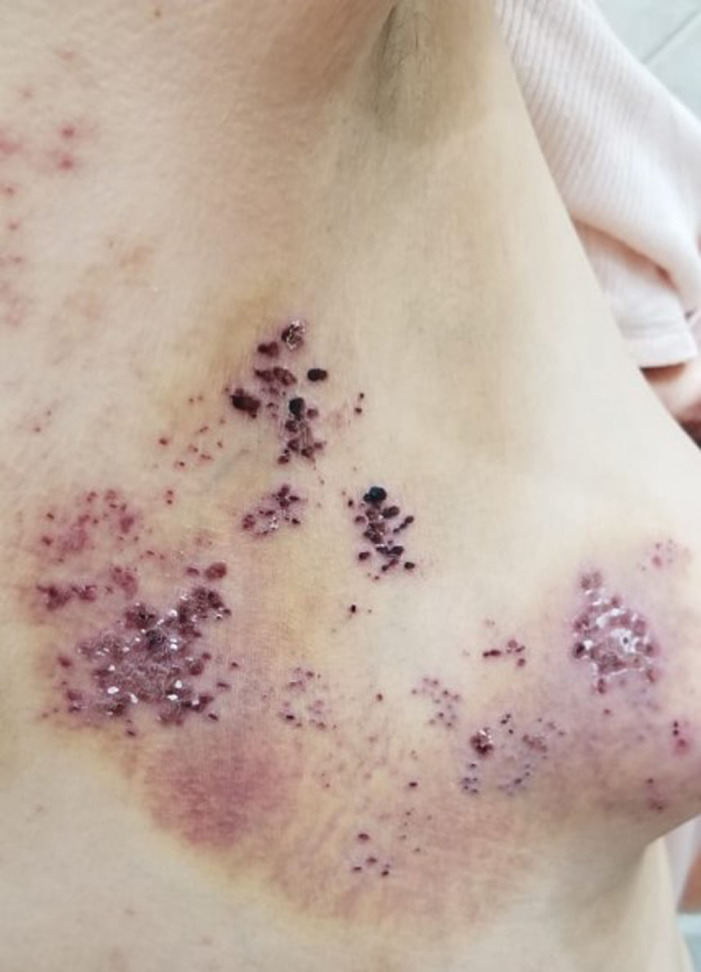
Tense vesicles and serocrust located on the right breast on an erythematous background with dermatomal distribution.
VZV is a DNA virus responsible for chickenpox, with a strong tropism for central nervous system cells. After the first infection, it remains latent in the cranial nerves or dorsal root ganglia. In situations of immunosuppression, trauma and fever, it can reactivate and cause shingles. The immune status of the host influences the natural history of herpes zoster. Moreover, age‐related immunosenescence is the major risk factor, with the disease‐related or iatrogenic immunosuppression as possible triggers for reactivation. As already described in the literature, infection with COVID‐19 can trigger a VZV reactivation, too. 2 SARS‐COV‐2 infection probably causes an immunosuppressive state secondary to a decrease in the quantity of T lymphocytes. This immunosuppressive state has also been shown to be responsible for reactivating other viruses, such as pityriasis rosea. 3
Moreover, as already described in the literature, vaccines can also trigger the reactivation of shingles. 4 Generally, the latency time is about 5 days, while in our experience, the mean latency of VZV reactivation after the ChAdOx1 nCoV‐19 vaccine was 7.6 days. Probably, the vaccine may cause some immunomodulation that allows VZV to escape from its latent phase. 4 , 5 However, based on these data, it is possible to imagine that mass vaccination on a global scale, due to the Covid19 pandemic, could naturally cause an increase in the number of shingles reactivation, especially in the elderly population. As there are still very few cases of this type described in the literature, it is essential to stress how much is still to be discovered regarding the pathophysiological mechanisms underlying the dermatological manifestations after the ChAdOx1 nCoV‐19 vaccine, so we find these observations noteworthy.
Funding sources
None.
Acknowledgement
The patients in this manuscript have given written informed consent to the publication of their case details.
References
- 1. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mMRNA COVID‐19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tartari F, Spadotto A, Zengarini C et al. Herpes zoster in COVID‐19‐positive patients. Int J Dermatol 2020; 59: 1028–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Merhy R, Sarkis AS, Stephan F. Pityriasis rosea as a leading manifestation of COVID‐19 infection. J Eur Acad Dermatol Venereol 2021; 35: e246–e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eid E, Abdullah L, Kurban M, Abbas O. Herpes zoster emergence following mRNA COVID‐19 vaccine. J Med Virol 2021; 93: 5231–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bostan E, Yalici‐Armagan B. Herpes zoster following inactivated COVID‐19 vaccine: a coexistence or coincidence? J Cosmet Dermatol 2021; 20: 1566–1567. [DOI] [PubMed] [Google Scholar]


