Abstract
Purpose
The role of extracorporeal membrane oxygenatio (ECMO) for rescue therapy of respiratory failure in critically ill coronavirus disease 2019 (COVID‐19) patients remains controversial. We aimed to evaluate the clinical outcomes of ECMO in the treatment of COVID‐19 compared with conventional ventilation support.
Methods
In this retrospective cohort study, data were collected on extremely critical patients with COVID‐19 from January 2020 to March 2020 in intensive care unit of a hospital in charge by national rescue team in Wuhan, China, the epicenter of pandemic. Patients were classified into the ECMO group and the conventional ventilation non‐ECMO group. Clinical characteristics, technical characteristics, laboratory results, mortality, and complications of the two groups were analyzed.
Results
88 patients with extremely critical COVID‐19 were screened; 34 received ECMO support and 31 received conventional ventilation support. Both groups had comparable characteristics at baseline in terms of age, gender, and comorbidities. Before ECMO or conventional therapy, patients in the two groups had sever acute respiratory distress syndrome with a mean partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) ratio of 69.6 and 75.4, respectively. At the time of reporting, patients in the ECMO had significantly lower in‐hospital mortality compared with the control group (58.8 vs. 93.5%, p = .001).
Conclusion
ECMO is shown to decrease the mortality of extremely critical ill COVID‐19 patients compared with the conventional treatment. Although complications occurred frequently, ECMO could still be a rescue therapy for the treatment of COVID‐19 during the pandemic.
Keywords: COVID‐19, ECMO, SARS‐CoV‐2
1. INTRODUCTION
In the late December, 2019, an unprecedented outbreak of a novel infection has emerged in Wuhan, China. The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was subsequently identified and rapidly led to a worldwide pandemic. Globally, as of 6:44 pm CET, 19 January 2021, there have been 94,124,612 confirmed cases of coronavirus disease 2019 (COVID‐19), including 2,034,527 deaths, reported to WHO.1 The majority of cases presented mild symptoms such as cough and fever, while there exists a proportion of severe or critical cases who presented, or developed to acute respiratory distress syndrome (ARDS), acute cardiac injury, shock, and multiple organ dysfunction syndrome (MODS), even death.2, 3 In an investigation in mainland China, the Chinese Center for Disease Control and Prevention reported that among a total of 72 314 case records, 81% of them were classified as mild, however, 14% were severe and 5% were critical.4 (p314)
According to the world health organization, mechanical ventilation, steroids, and some investigational treatment options, such as intravenous immunoglobulin and convalescent plasma could be considered for the management of severe and critically ill patients.5 And extracorporeal membrane oxygenation (ECMO) is recommended as a rescue therapy for COVID‐19 with refractory hypoxemia despite lung‐protective ventilation.6 However, to date, the experience of ECMO for COVID‐19 is very limited. Therefore, we report a retrospective cohort study on the effect of ECMO as a rescue therapy for extremely critical COVID‐19 patients during outbreak of the pandemic.
2. METHODS
2.1. Study design and patient selection
This was a retrospective study conducted in the intensive care unit (ICU) of a hospital in charge by national rescue team in Wuhan, China, the epicenter of pandemic between Jan 2020 and Mar 2020. This study was approved by the ethics committee of the Seventh Medical Center of Chinese PLA General Hospital (No. 2020‐023) and was registered at http://www.chictr.org.cn. (ID: ChiCTR2000032162). The study was performed in accordance with the approved guidelines and regulations of the participating institutions. The committee waived the need for informed consent. The data of the study have been opened at https://data.mendeley.com/datasets/wyb73tkf9z/3.
3. PARTICIPANTS
Patients were eligible for enrollment if meeting the following criteria: (1) Laboratory confirmed COVID‐19 cases tested by real‐time reverse‐transcriptase polymerase chain reaction assays using specimens from both the upper respiratory tract (nasopharyngeal and oropharyngeal) and lower respiratory tract (expectorated sputum, endotracheal aspirate, or bronchoalveolar lavage), according to the world health organization criteria.5 (2) Fulfilled the Berlin definition for ARDS.7 (3) Met ECMO initial criteria: (1) reversible respiratory failure with hypoxemia (partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2) ratio less than 50 mmHg for more than three hours or PaO2/FiO2 ratio less than 80 mmHg for more than 6 h; (2) Arterial blood pH less than 7.25 with a partial pressure of arterial carbon dioxide (PaCO2) of at least 60 mmHg for more than 6 h with respiratory rate more than 35 breaths per minute; (3) Plateau pressure more than 30‐35 cmH2O despite optimization of mechanical ventilation. (4) None of the following contraindications to ECMO: (1) mechanical ventilation at high settings (FiO2 > 0.9, plateau pressure greater than 30 cmH2O for ≥7 days; (2) major pharmacologic immunosuppression (absolute neutrophil count <400/mm3; (3) central nervous system hemorrhage that is recent or expanding; (4) nonrecoverable comorbidity, such as major central nervous system damage or terminal malignancy.8 Other adjunctive therapies, such as high frequency oscillatory ventilation and inhaled nitric oxide, were at the discretion of the attending physicians.
Patients meeting the above criteria were assigned to the ECMO group and underwent either venovenous or venoarterial percutaneous cannulation, while patients who met the above criteria but didn't receive ECMO due to limited resources during the outbreak were assigned to the control group and received conventional ventilation support.
The anticoagulation protocol was as follows: (a) Anticoagulation for initiation of ECMO: unfractioned heparin (50–100 unite/kg, 3000–5000 units intravenous bolus). (b) Initiation of continuous infusion anticoagulation was determined by the patient's clinical status based on post‐cannulation bleeding risks. (c) Continuous infusion anticoagulation: unfractioned heparin (8–20 unite/kg/h). Weaning from ECMO9 may be indicated when extracorporeal circulation support is less than 30% of total, native heart or lung function, which is judged by the treating physicians based on the clinical improvement of patients, such as adequate oxygenation and gas exchange reflected in arterial blood gas analysis and chest X‐ray. The procedure of weaning off the ECMO is to decrease flow in steps to 1 L/min at sweep FiO2 100% or decrease flow to 2 L/min then decrease sweep FiO2 to maintain arterial oxygen saturation (SaO2) > 95%. When SaO2 stable on these settings, trial off by clamping catheter and keeping the patient on the oxygenator at acceptable settings. Follow the patient SaO2 and PaCO2, ready for decannulation according to the lung function. For the ventilation protocol, SIMV and PCV mode is routinely adopted. Specific parameters are as follows: PC: 10–15 cmH2O, PEEP: 5–10 cmH2O, Respiratory rate: 10–15/min, FiO2: 30%–50%. Midazolam and remifentanil were used for sedation and analgesia, and muscle relaxants were used in the early stage. For the anticoagulation protocol, heparin was used. The antiviral drug was arbidol hydrochloride (umifenovir), a broad‐spectrum antiviral compound, using a gastric feeding tube (200 mg tid).10, 11
4. DATA COLLECTION
The medical records were collected retrospectively in standardized case report forms. Trained research team performed the data collection, including patient demographics, such as age and sex, predefined comorbidities, laboratory findings (leucocyte, hemoglobin concentration, liver function, kidney function, cardiac function, and blood gases), ventilator modes and settings (positive end‐expiratory pressure, plateau pressure, and FiO2), and outcomes.
5. STATISTICAL ANALYSIS
Continuous variables, expressed as means (with SD) or medians (with interquartile ranges), were compared with independent t test. Categorical variables were compared with the χ 2 or the Fisher's exact tests. The nonparametric values of the two groups were compared with the Mann–Whitney U test. All analyses were carried out using SPSS version 21 (IBM). A p value less than 0.05 was regarded as statistically significant.
6. RESULTS
From Jan 2020 to Mar 2020, a total of 88 patients with laboratory‐confirmed SARS‐CoV‐2 infection was admitted to the ICU of the hospital. Of them, 65 patients met the inclusion criteria and were included in the final analysis, 34 patients considered for the treatment of ECMO had ECMO support and 31 patients had conventional treatment (Figure 1). Table 1 shows the demographic characteristics of these patients at baseline. There were no statistically significant differences in the main clinical characteristics of patients between the two groups, including age, gender, and comorbidities (p > .05, respectively). All included patients in the two groups had severe ARDS with a mean PaO2/FiO2 ratio of 69.6 and 79.4, respectively. Both groups had similar plateau pressure, while patients in the control group had significantly lower PEEP. There were no significant differences in PaO2/FiO2 ratio and PaCO2 between the two groups. In the ECMO group, 28 of 34 patients (82.4%) were given vasoactive drugs during ECMO. Continuous renal replacement therapy and prone ventilation were used significantly more often in the ECMO group compared with the control group (64.7 vs. 38.7%, p = .036 and 73.5 vs. 16.1%, p = .000).
Figure 1.
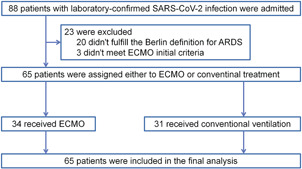
Flowchart of study enrollment. ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Table 1.
Clinical characteristics of patients with extremely critical COVID‐19 who received ECMO and those who received conventional ventilation non‐ECMO
| ECMO (n = 34) | non‐ECMO (n = 31) | p value | |
|---|---|---|---|
| Age median (IQR)‐years | 64.5 (56‐72) | 69.2 (65·2‐72·2) | 0.086 |
| Gender (Male)‐ no. (%) | 22 (64.7%) | 25 (80.6%) | 0.151 |
| Comobidities‐ no. (%) | |||
| Diabetes | 5 (14.7%) | 3 (9.7%) | 0.711 |
| Hypertension | 13 (38.2%) | 10 (32.3%) | 0.615 |
| Uremia | 0 (0%) | 2 (6.5%) | 0.224 |
| COPD | 0 (0%) | 1 (3.2%) | 0.477 |
| Emphysema | 0 (0%) | 1 (3.2%) | 0.477 |
| Heart disease | 0 (0%) | 2 (6.5%) | 0.224 |
| Pulmonary lobectomy | 0 (0%) | 1 (3.2%) | 0.477 |
| Postoperative Chemotherapy for colon cancer | 0 (0%) | 1 (3.2%) | 0.477 |
| After coronary bypass surgery | 0 (0%) | 1 (3.2%) | 0.477 |
| Hyperthyroidism | 2 (5.9%) | 0 (0%) | 0.493 |
| Cerebral infarction | 0 (0%) | 1 (3.2%) | 0.477 |
| Immunocompromised | 1 (2.9%) | 0 (0%) | 1.000 |
Note: Data are presented as median (minimum–maximum), number (%), or mean (SD). Data on plateau pressure were missing for six patients in the ECMO group and in 11 in the control group.
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; PaO2/FiO2, arterial oxygen tension to inspired oxygen fraction ratio; PEEP, positive end‐expiratory pressure; PaCO2, partial pressure of arterial carbon dioxide.
In the ECMO group, 31 of 34 patients (91.2%) received venovenous ECMO (VV ECMO). Of them, one patient crossed over from VV ECMO to venoarterial ECMO (VA ECMO) because of hemodynamic instability. Three patients were solely treated with VA ECMO.
The blood gas analysis and ventilatory settings data before ECMO and conventional ventilation between the two groups are shown in Table 2, both groups had similar modes of ventilation. Table 3 shows data of laboratory findings between the two groups, both groups had similar laboratory values in leucocyte, hemoglobin, alanine aminotransfease, lactic acid, and troponin I. While patients in the ECMO group had significantly lower values of creatinine and CK‐MB compared to the control group (p = .000 and p = .035, respectively).
Table 2.
Blood gas analysis and ventilatory settings data of patients with extremely critical COVID‐19 who received ECMO and those who received conventional ventilation non‐ECMO
| ECMO (n = 34) | non‐ECMO (n = 31) | p value | ||
|---|---|---|---|---|
| PaO2/FiO2 (mmHg) | 69.6 (30.1) | 75.4 (43.8) | 0.535 | |
| PEEP (cmH2O) | 11 (2.1) | 9.3 (3.4) | 0.042 | |
| Plateau pressure (cmH2O)a | 29.9 (9.1) | 33.8 (8.5) | 0.146 | |
| Arterial blood pH | 7.29 (0.12) | 7.31 (0.16) | 0.546 | |
| PaCO2 (mmHg) | 61.6 (17.0) | 52.8 (20.1) | 0.065 | |
| Mode of ventilation no. (%) | ||||
| PRVC | 3 (8.8%) | 0 (0%) | 0.240 | |
| PCV | 15 (44.1%) | 17 (54.8%) | 0.388 | |
| VCV | 8 (23.5%) | 12 (38.7%) | 0.185 | |
| APRV | 1 (2.9%) | 0 (0%) | 1.000 | |
| AC | 4 (11.8%) | 0 (0%) | 0.115 | |
| Use of CRRT‐ no. (%) | 22 (64.7%) | 12 (38.7%) | 0.036 | |
| Use of prone ventilation‐ no. (%) | 25 (73.5%) | 5 (16.1%) | 0.000 | |
Note: Data are presented as numbers (%).
Abbreviations: AC, assisted‐control ventilation; APRV, airway pressure release ventilation; COVID‐19, coronavirus disease 2019; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; PaO2/FiO2, arterial oxygen tension to inspired oxygen fraction ratio; PEEP, positive end‐expiratory pressure; PaCO2, partial pressure of arterial carbon dioxide; PCV, pressure‐controlled ventilation; PRVC, pressure‐regulatory volume control ventilation; VCV, volume‐controlled ventilation.
Data on plateau pressure was missing for six patients in the ECMO group and 11 in the control group.
Table 3.
Laboratory results
| ECMO (n = 34) | non‐ECMO (n = 31) | p value | |
|---|---|---|---|
| Leucocyte (*109/L) | 13.34 (11–16.57) | 12.1 (9.08–18.35) | 0.971 |
| Hemoglobin (mg/dl) | 108 (85.5–125.63) | 99 (85.25–123.75) | 0.642 |
| Creatinine (umol/L) | 58.5 (44–73.25) | 95.8 (70.25–231.75) | 0.000 |
| ALT (U/L) | 46.5 (34–73.3) | 52.8 (23.5–92.6) | 0.773 |
| Lactic acid (mmol/L) | 2.6 (1.8–3.94) | 3.9 (1.83–9.18) | 0.062 |
| CK‐MB (ug/L) | 19 (1.21–34.25) | 29.1 (19.8–40.85) | 0.035 |
| Troponin I (pg/ml) | 0.078 (0.027–0.22) | 0.11 (0.53–0.27) | 0.496 |
Note: Data are presented as median (minimum–maximum).
Abbreviations: ALT, alanine aminotransfease; ECMO, extracorporeal membrane oxygenation.
Table 4.
Outcomes of patients who received ECMO and those who received conventional ventilation non‐ECMO support
| ECMO (n = 34) | non‐ECMO (n = 31) | p value | |
|---|---|---|---|
| In‐hospital mortality no. (%) | 20 (58.8%) | 29 (93.5%) | 0.001 |
| Cause of death no. (%) | |||
| ARDS | 0 (0%) | 16 (51.6%) | 0.000 |
| MODS | 10 (29.4%) | 3 (9.7%) | 0.064 |
| Septic infection | 6 (17.6%) | 0 (0%) | 0.025 |
| Shock | 5 (14.7%) | 8 (25.8%) | 0.264 |
| Respiratory/heart failure | 4 (11.8%) | 0 (0%) | 0.115 |
| Bleeding | 4 (11.8%) | 0 (0%) | 0.115 |
| Arrhythmia | 0 (0%) | 1 (3.2%) | 0.477 |
| DIC | 0 (0%) | 1 (3.2%) | 0.477 |
Note: Data are presented as number (%).
Abbreviations: ARDS, acute respiratory distress syndrome; DIC, disseminated intravenous coagulation; ECMO, extracorporeal membrane oxygenation; MODS, multiple organ dysfunction syndrome.
Compared to the conventional ventilation non‐ECMO group, ECMO group had a significantly lower in‐hospital mortality (58.8 vs. 93.5%, p = .001). When analyzing the causes of death, less patients died of ARDS and more patients died of septic infection in the ECMO group as compared to the non‐ECMO group (0 vs. 51.6%, p = .000 and 17.6 vs. 0%, p = .025, respectively). Besides, there were no difference of other death causes between the two groups, including MODS, shock, respiratory/heart failure, bleeding, arrhythmia, and disseminated intravascular coagulation. Because the hospital costs of all the SARS‐CoV‐2 infected patients are covered by the Chinese government, we can't obtain the cost data of ECMO in the mainland of China.
ECMO‐related complications occurred in 28 of 34 (82.4%) patients. The main complications were bleeding, including gastrointestinal bleeding in four patients, intracranial bleeding in two patients, pulmonary hemorrhage in one patient, airway bleeding in one patient, and other bleeding events in 18 patients. Infectious complications occurred in eight patients. The main pulmonary complications were pneumothorax, which occurred in four patients. Three patients occurred oxygenator failure.
7. DISCUSSION
This retrospective cohort study aimed to compare the outcomes of ECMO and conventional ventilation for the treatment of extremely critical COVID‐19 patients. And the results suggested that extremely critical patients with COVID‐19 in the ECMO group had significantly lower in‐hospital mortality as compared to that of patients in the control group (58.8 vs. 93.5%, p = .001). And the mortality of patients with COVID‐19 who received ECMO in our study is in the context of the overall mortality in acute lung injury/ARDS, which ranges from 15% to 75%, with a pooled point estimate of 43% (95% confidential interval [CI] 0.40–0.46).12 Although a previous pilot pooled analysis of preliminary results in China has suggested that the effects of ECMO for ARDS caused by SARS‐CoV‐2 were not encouraging,13 a recent study implemented by Yang et al. showed that compared to the mechanical ventilation patients without ECMO support, patients with ECMO support had lower mortality rate with no significant difference (57.1 vs. 63.2%, p = .782).14 According to the data on ECMO in COVID‐19 patients in 90 institutions spanning 17 countries reported by the European Extracorporeal Life Support Organization (ELSO), death rate was 17.1% (95% CI: 0.13–0.21) in 333 patients with COVID‐19, which was much lower than that of our study. Besides, the authors also showed a significant association between death and age in those patients (odds ratio 4.80 [95% CI: 1.64–14.04], p = .004), and patient risk was significantly increased in patients aged greater than 60 years.15 To date, ECMO has been actively used to save COVID‐19 patients with extremely critical conditions.16
ECMO has been used as a rescue therapy in various patients with severe ARDS, and the survival rate were inconsistent, which may result from the different characteristics of patients at baseline, the disease severity, and the experience of ECMO centers. In a meta‐analysis including 13 studies with a total of 494 patients with severe H1N1 pneumonia and respiratory failure who received ECMO, the authors reported a overall mortality of 37.1% (95% CI: 0.30–0.45).17 A multicentre randomized controlled trial (the CESAR trial) by Peek et al. reported that patients with severe acute respiratory failure had improved survival rate without severe disability at 6 months after receiving ECMO support as compared to the conventional management group (63 vs. 47%, relative risk 0.69; 95% CI: 0.05–0.97, p = .03).18 Besides, in the EOLIA trial, patients with severe ARDS in the ECMO group had significantly lower mortality compared to the control group (35 vs. 46, relative risk 0.76; 95% CI: 0.55–1.04; p = .09).19 Furthermore, a retrospective cohort study recruiting Middle East respiratory syndrome coronavirus patients with refractory respiratory failure also indicated that the ECMO group had significantly lower in‐hospital mortality compared to the conventional group (65 vs. 100%, p = .02).20 In The Lancet, Zhou et al. reported that in‐hospital death of patients with COVID‐19 was associated with older age,21 and a higher percentage of men and a majority of patients with comorbidities were observed in several studies2, 3 (p2) , 21 – 23 In our study, the baseline characteristics of the two groups were comparable in terms of age, gender, and comorbidities, which may reduce the confounders of the study, although other confounders may still exist, such as the body mass index of the patients. In addition, for patients who received ECMO support, the sequential organ failure assessment scores and lung injury scores, ICU stay, and duration of mechanical ventilation probably contribute to the mortality. Besides, the results of the CESAR trial further suggest that referral and transfer to an ECMO center are associated with a reduction in mortality.18
In our study, VV ECMO was the most frequently used mode in patients with COVID‐19 due to its advantages of avoiding carotid cannulation and less vascular complications over VA ECMO. However, there was still patients who crossed over from VV ECMO to VA ECMO because of hemodynamic instability. Likewise, Ramanathan et al. suggested that for patients with COVID‐19 who develop cardiac failure, VA ECMO might be needed.23
COVID‐19 patients have various respiratory mechanical characteristics, which are divided into two types: Type L, characterized by low elastance, low ventilation‐to‐perfusion ratio, low lung weight and low recruitability and Type H, characterized by high elastance, high right‐to‐left shunt, high lung weight, and high recruitability.24 Most patients present early with type L, and that some transition to type H, potentially due to the synergistic effects of worsening COVID‐19 pneumonia and patient self‐inflicted lung injury.24 Respiratory characteristic‐oriented pulmonary protective ventilation strategy may benefit COVID‐19 patients most.25 For the response to PEEP differs on the basis of individual respiratory mechanics, an individualized strategy should be adopted.25 Specifically, higher PEEP for patients with high lung recruitment potential, while low PEEP is selected for low lung atelectasis recruitment potential patients, avoiding excessive PEEP aggravates the excessive expansion of alveolar space, resulting in respiratory‐related lung injury.26 Prone position can improve the ventilation and perfusion relationship, reduce the heterogeneity of pulmonary blood flow, improve oxygenation and reduce ventilator‐associated lung injury. Prone position ventilation is preferred for patients with low lung recruitment potential.27, 28 Individualized parameter adjustment according to the clinical situation will benefit the patients optimally.
In our study, complications including bleeding, infection, pneumothorax, and mechanical complications occurred during ECMO. According to the ELSO registry report,29 the most common complications of ECMO for adult respiratory failure were hemorrhage and infection, which is consistent with our study results. Bleeding complications are multifactorial. Management of bleeding includes preventative strategies to prevent bleeding, cessation of anticoagulation if significant bleeding occurs as well as transfusion support, antifibrinolytics, and local measures and surgical control where required.30 Some risk factors have been shown for the development of infection, including the duration of the ECMO run, the severity of illness in ECMO patients, the high risk of bacterial translocation from the gut, and ECMO‐related impairment of the immune system. Although antibiotic prophylaxis during ECMO is common practice, the ELSO Infectious Disease Task Force does not recommend the use of antimicrobial agents to prevent infectious complications during ECMO and advises not to prolong it beyond 48 h after cannulation, if performed. Generally, the application of antibiotic prophylaxis is in the judgement of the physicians based on the patients' condition.31
There are some limitations in our study: (1) First, single‐center study in Chinese patients and nonrandom; (2) Second, the sample in our study was small. (3) Third, limited number of variables were collected in the context of pandemic outbreak when clinicians were not able to collect the detailed data of parameters for each patient. (4) Fourth, follow‐up time of our study was short, some outcomes could not be observed. In spite of these limitations, this study still provides promise and meaningfulness to extremely critical patients with COVID‐19.
In summary, the present retrospective cohort study provided evidence to suggest that, in extremely critical COVID‐19 patients, the survival rate in the ECMO group was superior compared with that in the conventional ventilation group. Critically ill patients with COVID‐19 appear to develop MODS and shock,3 many factors could affect the outcomes. Whether ECMO has a role in those patients with very severe condition is still difficult to conclude due to limited experience worldwide. Understanding the risk‐to‐benefit ratio of performing ECMO in the clinical settings is essential.32 Generally, our study showed that ECMO might be a rescue therapy for extremely critical COVID‐19 patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
This study was approved by the ethics committee of the Seventh Medical Center of PLA general hospital (Beijing, China) (IRB No: 2020‐023).
ACKNOWLEDGMENTS
This study was supported by the grants of Beijing health and health science and technology achievements and appropriate technology promotion project (2018‐TG‐49) and the capital health research and development of special (2020‐2‐5093).
Li S, Xiong J, Du Z, et al. Extracorporeal membrane oxygenation (ECMO) for critically ill patients with coronavirus disease 2019 (COVID‐19): A retrospective cohort study. J Card Surg. 2021;36:3554‐3560. 10.1111/jocs.15833
Shuanglei Li and Jing Xiong contributed equally to this study.
Contributor Information
Xiaoyang Hong, Email: hongxyang81@163.com.
Yundai Chen, Email: yundchen301@163.com.
REFERENCES
- 1.World Health Organization . WHO coronavirus disease (COVID‐19) dashboard. 2021. https://covid19.who.int. Accessed January 20, 2021.
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W‐J, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease control and prevention. JAMA. 2020;323(13):1239‐1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Report of the WHO‐China Joint Mission on coronavirus disease (COVID‐19). 2019. https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019. Accessed January 20, 2021.
- 6.World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance, 25 January 2020. 2020. https://apps.who.int/iris/handle/10665/330854. Accessed January 20, 2021.
- 7.ARDS Definition Task F , Ranieri VM, Rubenfeld, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526‐2533. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 8.ELSO . Guidelines for adult respiratory failure. 2021. https://www.elso.org/Portals/0/ELSO%20Guidelines%20For%20Adult%20Respiratory%20Failure%201_4.pdf. Accessed January 20, 2021.
- 9.Grant AA, Hart VJ, Lineen EB, et al. A Weaning Protocol for Venovenous Extracorporeal Membrane Oxygenation With a Review of the Literature. Artif Organs. 2018;42(6):605‐610. 10.1111/aor.13087 [DOI] [PubMed] [Google Scholar]
- 10.Jie X, Hongmei Y, Ping F, Kuikui Z, Bohan Y, Rui M. Beneficial effect of Arbidol in the management of COVID‐19 infection. Aging. 2021;13(7):9253‐9264. 10.18632/aging.202867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prats‐Uribe A, Sena AG, Lai LYH, et al. Use of repurposed and adjuvant drugs in hospital patients with COVID‐19: multinational network cohort study. BMJ. 2021;373:n1038. 10.1136/bmj.n1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zambon M, Vincent J‐L. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120‐1127. 10.1378/chest.07-2134 [DOI] [PubMed] [Google Scholar]
- 13.Zeng Y, Cai Z, Xianyu Y, Yang BX, Song T, Yan Q. Prognosis when using extracorporeal membrane oxygenation (ECMO) for critically ill COVID‐19 patients in China: a retrospective case series. Crit Care. 2020;24(1):148. 10.1186/s13054-020-2840-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Cai S, Luo Y, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019‐induced acute respiratory distress syndrome: a multicenter descriptive study. Crit Care Med. 2020;48(9):1289‐1295. 10.1097/CCM.0000000000004447 [DOI] [PubMed] [Google Scholar]
- 15.Marullo AG, Cavarretta E, Biondi Zoccai G, et al. Extracorporeal membrane oxygenation for critically ill patients with coronavirus‐associated disease 2019: an updated perspective of the European experience. Minerva Cardioangiol. 2020;68(5):368‐372. 10.23736/S0026-4725.20.05328-1 [DOI] [PubMed] [Google Scholar]
- 16.ELSO . Full COVID‐19 registry dashboard. 2021. https://www.elso.org/Registry/FullCOVID19RegistryDashboard.aspx. Accessed January 20, 2021.
- 17.Sukhal S, Sethi J, Ganesh M, Villablanca PA, Malhotra AK, Ramakrishna H. Extracorporeal membrane oxygenation in severe influenza infection with respiratory failure: a systematic review and meta‐analysis. Ann Card Anaesth. 2017;20(1):14‐21. 10.4103/0971-9784.197820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351‐1363. 10.1016/S0140-6736(09)61069-2 [DOI] [PubMed] [Google Scholar]
- 19.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965‐1975. 10.1056/NEJMoa1800385 [DOI] [PubMed] [Google Scholar]
- 20.Alshahrani MS, Sindi A, Alshamsi F, et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8(1):3. 10.1186/s13613-017-0350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574‐1581. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID‐19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020;8(5):518‐526. 10.1016/S2213-2600(20)30121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gattinoni L, Chiumello D, Caironi P, et al. COVID‐19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099‐1102. 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant AA, Badiye A, Mehta C, et al. EMPROVING outcomes: evaluating the effect of an ultralung protective strategy for patients with ARDS treated with ECMO. J Card Surg. 2020;35(10):2495‐2499. 10.1111/jocs.14923 [DOI] [PubMed] [Google Scholar]
- 26.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end‐expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta‐analysis. JAMA. 2010;303(9):865‐873. 10.1001/jama.2010.218 [DOI] [PubMed] [Google Scholar]
- 27.Langer T, Brioni M, Guzzardella A, et al. Prone position in intubated, mechanically ventilated patients with COVID‐19: a multi‐centric study of more than 1000 patients. Crit Care. 2021;25(1):128. 10.1186/s13054-021-03552-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Zhang J, Feng H, Wan F, Zhang Y, Tan L. Prone positioning in intubated and mechanically ventilated patients with SARS‐CoV‐2. J Clin Anesth. 2021;71:110258. 10.1016/j.jclinane.2021.110258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63(1):60‐67. 10.1097/MAT.0000000000000475 [DOI] [PubMed] [Google Scholar]
- 30.Murphy DA, Hockings LE, Andrews RK, et al. Extracorporeal membrane oxygenation‐hemostatic complications. Transfus Med Rev. 2015;29(2):90‐101. 10.1016/j.tmrv.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 31.Biffi S, Di Bella S, Scaravilli V, et al. Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention. Int J Antimicrob Agents. 2017;50(1):9‐16. 10.1016/j.ijantimicag.2017.02.025 [DOI] [PubMed] [Google Scholar]
- 32.MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID‐19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020;323(13):1245‐1246. 10.1001/jama.2020.2342 [DOI] [PubMed] [Google Scholar]
- 33.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]


