Abstract
Objective
The aim of the present case–control study was to evaluate the morphological aspects of the epithelial cells from the dorsum of the tongue and the expression of the SARS‐CoV‐2 Spike protein in these cells, in patients with and without COVID‐19 infection.
Methods
24 individuals with at least one symptom of COVID‐19 were recruited among inpatients from Hospital Universitário Pedro Ernesto (Rio de Janeiro, Brazil). 14 patients who tested positive for COVID‐19 by RT‐PCR were included in the case group, and 10 patients who tested negative were included in the control group. Cytological smears from the dorsum of the tongue were obtained from all patients and analyzed using immunohistochemistry directed against SARS‐CoV‐2‐Spike protein. Morphological changes in epithelial cells were analyzed using light microscopy.
Results
Immunohistochemistry showed that 71% of the COVID‐19 patients presented epithelial cells positive for the presence of the SARS‐CoV‐2 Spike protein, and all cells coming from patients in the control group were negative. Cytological analysis showed significant differences when comparing epithelial cells from COVID‐19‐positive and COVID‐19‐negative patients.
Conclusion
COVID‐19 may generate dimensional changes in tongue epithelial cells; however, further studies are necessary to understand how this happens.
Keywords: epithelial cells, RT‐PCR, SARS‐CoV‐2, spike protein
1. INTRODUCTION
SARS‐CoV‐2 is a new enveloped RNA beta‐coronavirus that causes a severe acute respiratory syndrome known as COVID‐19, declared a pandemic by the World Health Organization (WHO) in 2020, causing over 3 million deaths (Lu et al., 2020; Zou, Ruan et al., 2020). Human‐to‐human transmission of COVID‐19 occurs due to close contact with an infected person, exposure to respiratory or saliva droplets (Li et al., 2020; WHO, 2020).
Xu chen et al. (2020) have described that the SARS‐CoV‐2 receptor‐binding domain has a strong interaction with human ACE2 molecules, and this finding suggests that ACE2‐expressing cells may act as targets to SARS‐CoV‐2 infection (Zou, chen et al., 2020). Previous data have shown that ACE2 is highly expressed in the oral mucosa, especially in epithelial cells of the tongue and salivary glands, suggesting that these sites may act as a potential reservoir of the virus (Xu, Zhong et al., 2020; GTEx Portal). In addition, tongue ulcers have been associated with SARS‐CoV‐2 infection and have been suggested as a manifestation of the disease (Riad et al., 2020).
The aim of the present study was to evaluate the morphological aspects of the epithelial cells from the dorsum of the tongue and the expression of the SARS‐CoV‐2 Spike protein in these cells, in patients with and without COVID‐19 infection.
2. MATERIALS AND METHODS
Reporting of this case–control study follows the STROBE guidelines (Elm et al., 2007). The study was approved by the Ethical Committee from Pedro Ernesto University Hospital (CAAE‐31222820.6.0000.5259), all individuals included in the study gave a written consent to participate, and the study was performed in accordance with the Declaration of Helsinki (World Medical Association, 2013).
2.1. Sample size calculation
Sample size calculation using EPI Info™ software (Centers for Disease Control and Prevention, Atlanta, GA, U.S.) was based on the following estimates: α error of 0.05; β error of 0.20 (80% power). Frequency of SARS‐CoV‐2‐Spike protein expression in COVID‐19+ patients was arbitrarily set at 90% and in control group at 25%. Assuming an unmatched case–control study design with groups of equal size, these estimates into the equation yield a sample size of 20 individuals (10 per group).
2.2. Population
Twenty‐four individuals were recruited among inpatients from Hospital Universitário Pedro Ernesto (Rio de Janeiro, Brazil). All of them had at least one symptom of COVID‐19 (fever, coughing, headache, sore throat, diarrhea, or loss of taste/smell). Fourteen of these participants tested positive for COVID‐19 by RT‐PCR assay (kit SARS‐CoV‐2 Bio‐Manguinhos, Rio de Janeiro, Brazil) and were included in the case group, and 10 of the participants tested negative and were included in the control group. Table 1 shows demographic and clinical data such as age, gender, smoking status, distribution of symptoms, clinical status, and ethnicity from individuals in case and control groups.
TABLE 1.
List table showing age, gender, smoking status, distribution of symptoms, clinical status and ethnicity among individuals in case and control groups
| Patient | Group | Gender | Age | Smoking | Fever | Coughing | Headache | Sore throat | Diarrhea | Loss of taste or smell | Clinical status | Ethnicity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Case | Male | 57 | Yes | Yes | Yes | No | No | No | No | Moderate COVID−19 | Mixed |
| 2 | Case | Female | 75 | No | Yes | Yes | Yes | Yes | Yes | Yes | Moderate COVID−19 | Mixed |
| 3 | Case | Male | 70 | Yes | No | No | Yes | No | No | No | Moderate COVID−19 | Mixed |
| 4 | Case | Female | 53 | Yes | No | Yes | No | No | No | Yes | Moderate COVID−19 | Mixed |
| 5 | Case | Male | 79 | Yes | Yes | Yes | Yes | Yes | Yes | No | Moderate COVID−19 | Black |
| 6 | Case | Male | 47 | Yes | No | Yes | Yes | Yes | Yes | No | Moderate COVID−19 | Mixed |
| 7 | Case | Male | 61 | No | No | Yes | Yes | Yes | Yes | No | Moderate COVID−19 | Mixed |
| 8 | Case | Male | 55 | No | Yes | Yes | Yes | No | No | Yes | Moderate COVID−19 | Mixed |
| 9 | Case | Male | 67 | Yes | Yes | Yes | Yes | No | No | Yes | Moderate COVID−19 | Mixed |
| 10 | Case | Male | 28 | No | No | No | No | No | No | No | Mild COVID−19 | Caucasian |
| 11 | Case | Female | 83 | No | No | No | Yes | No | No | No | Moderate COVID−19 | Black |
| 12 | Case | Female | 23 | No | Yes | No | Yes | Yes | Yes | Yes | Moderate COVID−19 | Mixed |
| 13 | Case | Male | 46 | No | Yes | Yes | Yes | No | No | Yes | Moderate COVID−19 | Mixed |
| 14 | Case | Male | 32 | No | Yes | No | No | No | No | No | Mild COVID−19 | Mixed |
| 15 | Control | Female | 32 | No | No | No | Yes | No | No | No | Non‐COVID−19 | Mixed |
| 16 | Control | Female | 26 | No | No | No | Yes | No | No | No | Non‐COVID−19 | Mixed |
| 17 | Control | Male | 17 | No | No | No | Yes | No | No | No | Non‐COVID−19 | Caucasian |
| 18 | Control | Female | 24 | No | No | No | Yes | No | No | No | Non‐COVID−19 | Caucasian |
| 19 | Control | Male | 25 | No | No | No | Yes | No | No | No | Non‐COVID−19 | Black |
| 20 | Control | Female | 53 | Yes | No | No | Yes | No | No | No | Non‐COVID−19 | Mixed |
| 21 | Control | Male | 53 | Yes | No | No | Yes | No | No | No | Non‐COVID−19 | Mixed |
| 22 | Control | Female | 70 | Yes | No | No | Yes | No | No | No | Non‐COVID−19 | Mixed |
| 23 | Control | Female | 24 | No | No | No | Yes | No | No | No | Non‐COVID−19 | Caucasian |
| 24 | Control | Male | 25 | Yes | No | No | Yes | No | No | No | Non‐COVID−19 | Mixed |
2.3. Cytological and Immunohistochemical analysis
A cytological smear containing superficial epithelial cells from the dorsum of the tongue by scraping with a spatula was obtained from all patients. The cells were distended in six histological glass slides for each patient and fixed in 95º alcohol. Smears were stained with hematoxylin and eosin and Papanicolaou, and one histological slide from each patient was submitted to immunohistochemistry directed against SARS‐CoV‐2 Spike protein (mouse monoclonal antibody anti‐ SARS‐CoV‐2 Spike protein, clone 1A9, Invitrogen, Massachusetts, US, dilution 1:200) through the immunoperoxidase technique followed by diaminobenzidine as chromogen and counterstaining with Carazzi hematoxylin. The hematoxylin and eosin and Papanicolaou‐stained smears were analyzed by two examiners (BM and TG) in high power using light microscopy, and two desquamate epithelial cells from each patient were measured in four planes: maximum cell diameter, maximum perpendicular to this measure, maximum diameter of the nucleus, and maximum perpendicular to this measure. All measures were performed by using the Image J software (NIH, Bethesda, MD, USA). All immunohistochemical slides were analyzed by a single oral pathologist (FRP) and interpreted as positive or negative for the presence of SARS‐CoV‐2 Spike protein.
2.4. Statistical Analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS Statistics 20, IBM Corporation, Armonk, NY, USA). After verifying that the data were not normally distributed, non‐parametric Mann–Whitney (U) test was used. Significance level was set at 0.05.
3. RESULTS
An intraclass correlation coefficient to determine the agreement between the two examiners (BM and TG) on the four measured plans was calculated and ranged from 0.97 to 0.99. Cytological analysis showed significant differences in all measures when comparing epithelial desquamate cells derived from COVID‐19‐positive and COVID‐19‐negative patients (Table 2). Immunohistochemistry showed that 10 out 14 (71%) of the COVID‐19 patients presented epithelial cells positive for the presence of the SARS‐CoV‐2 Spike protein, and all cells coming from patients in the control group were negative (Figure 1). When comparing the cellular and nuclear measures in cells obtained from COVID‐19 patients expressing (10 patients) or not (4 patients) the SARS‐CoV‐2 Spike protein, no significant differences were found. In contrast, when comparing the cells obtained from COVID‐19 patients expressing the SARS‐CoV‐2 Spike protein with cells from all other patients that did not express the SARS‐CoV‐2 Spike protein (14 patients), the maximum perpendicular diameter of the cell was larger in the second group while no differences were found for the other three measures (Figure 2). No other morphological changes suggestive of viral infection (such as multinucleation, and nuclear hyperchromatism and vacuolization) were observed in cells obtained from COVID‐patients and in cells expressing SARS‐CoV‐2 Spike protein. No intraoral lesions were observed in this population.
TABLE 2.
Comparison of the cytological measures according to COVID‐19 infection and SARS‐CoV‐2 Spike protein expression
| Parameter |
COVID−19‐infected patients‐derived epithelial cells Mean (SD; ±95%CI) |
Non‐COVID−19 patients‐derived epithelial cells Mean (SD; ±95% CI) |
P‐value |
Patients expressing the SARS‐CoV−2 Spike protein Mean (SD; ±95%CI) |
Patients not expressing the SARS‐CoV−2 Spike protein Mean (SD; ±95%CI) |
P‐value | |
|---|---|---|---|---|---|---|---|
| N | 14 | 10 | ‐ | 10 | 14 | ‐ | |
| Maximum cell diameter (mm) | 18.460 (6.30; ±1.69) | 23.531 (7.95; ±2.55) | 0.001* | 18.900 (6.92; ±2.22) | 21.768 (7.62; ±2.04) | 0.104 | |
| Maximum perpendicular to maximum cell diameter (mm) | 16.983 (6.16; ±1.60) | 20.210 (7.18; ±2.3) | 0.018* | 16.392 (6.16; ±1.97) | 19.710 (6.88; ±1.85) | 0.015* | |
| Maximum nuclear diameter (mm) | 2.763 (654,21; ±0.26) | 3.267 (1.17; ±0.37) | 0.032* | 2.779 (891,4; ±0.28) | 3.112 (1.17; ±0.32) | 0.123 | |
| Maximum perpendicular to maximum nuclear diameter(mm) | 2.679 (935,2; ±0.25) | 3.120 (1.00; ±0.33) | 0.025* | 2.695 (920,5; ±0.29) | 2.983 (1.01; ±0.27) | 0.109 | |
statistically significant (p<0.05);SD‐ standard deviation; 95%CI ‐ Confidence Interval 95%Legends to Figures.
FIGURE 1.
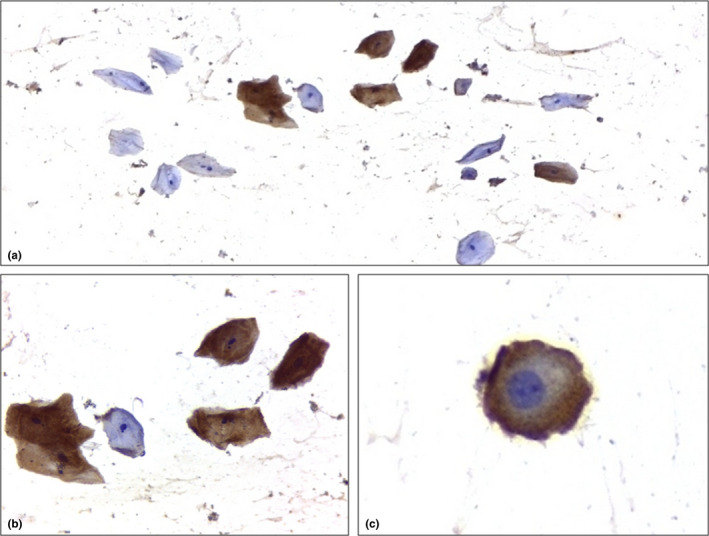
Immunoexpression of Spike protein in desquamated tongue epithelial cells (Immunoperoxidase; 1a, 1b and 1c, respectively, 100x, 400x and 1000x magnification)
FIGURE 2.
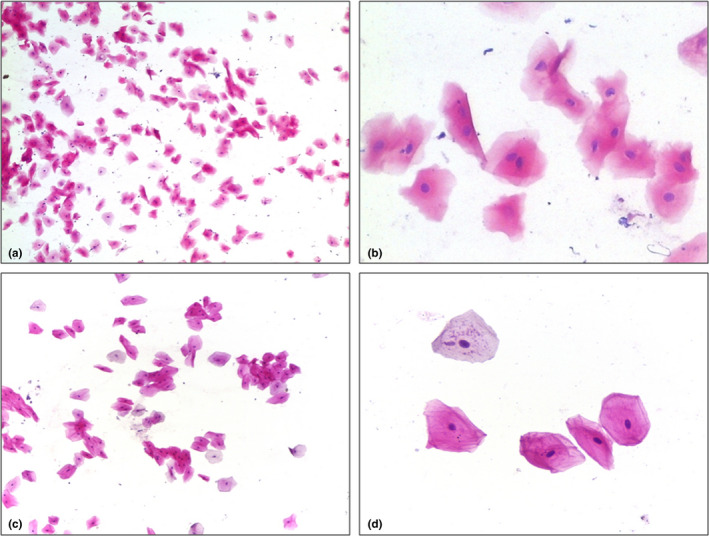
Cytological features of desquamated tongue epithelial cells derived from a COVID‐19‐infected patient (test group) (2a, 100x magnification; 2b, 400x magnification) in comparison with desquamated tongue epithelial cells derived from a non‐COVID‐19‐infected patient (control group) (2c, 100x magnification; 2d, 400x magnification)
4. DISCUSSION
In this study, morphological changes in epithelial cells were particularly associated with positivity of RT‐PCR assay in nasopharyngeal swabs, as this method is the gold standard for diagnosis of SARS‐CoV‐2 infection and is a much more sensitive detection technique than immunohistochemistry. It is possible that the lower sensitivity of immunohistochemistry assay may have influenced the results.
Expression of ACE2, furin, and TMPRSS2 has been demonstrated in tongue epithelial cells, which reinforces the perspective that the oral mucosa, and specifically this anatomical region, is an access route to SARS‐CoV‐2 infection (Zhong et al., 2020). It has been suggested that these features would explain the mechanism of dysgeusia in these patients and also that removing the tongue coating would reduce the risk of virus exposure (Sakaguchi et al., 2020).
The results of this study showed that the presence of SARS‐CoV‐2 Spike protein can be demonstrated in desquamated epithelial cells from the dorsum of the tongue, one of the cell‐based disease models for COVID‐19 (Mhaske et al., 2020). It also highlighted that epithelial cells obtained from the dorsum of tongue of COVID‐19 patients show morphological alterations when compared with COVID‐19‐negative patients, although no morphological alterations classically associated with other viral infections were observed, as previously demonstrated (Parada et al., 2021).
In this study, ethnic background, gender, proportion of smokers, and distribution of symptoms were similar in both groups. However, the mean age of the control group was lower (34.9 yrs) than in the case group (55,4 yrs), which may have act as a confounder.
There is few published information on cytological alterations in epithelial, endothelial and other cellular types associated with the presence of SARS‐CoV‐2 virus. Morphological changes also seen in other viral infections, such as the presence of multinucleated and/or ballooned keratinocytes, acantholytic cells, desquamated cells, amphophylic granular cytoplasm, hyperchromatic nuclei, as well as apoptotic cells, some of them with hialine cell membrane, have been described in cutaneous and pulmonar lesions associated with SARS‐CoV‐2 virus infection (Batah and Fabro, 2021; Rongioletti et al., 2021). The present results showed that oral mucosal epithelial cells from SARS‐CoV‐2‐infected patients showed no morphological changes typically associated with viral infections. However, they showed smaller cellular and nuclear diameter when comparing to non‐SARS‐CoV‐2‐infected patients. As these morphological changes are described during the early phases of apoptosis, further studies with larger samples are encouraged to analyze their relationship to the presence of SARS‐CoV‐2 in epithelial cells form normal oral mucosa.
In conclusion, COVID‐19 may generate dimensional changes in tongue epithelial cells; however, further studies are necessary to understand how this happens.
CONFLICT OF INTEREST
All authors gave their final approval and agreed to be accountable for all aspects of the work. All authors reported no conflict of interest.
AUTHOR CONTRIBUTIONS
Barbara Bruno Fagundes Marques: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing‐original draft; Writing‐review & editing. Taísa Coelho Guimarães: Conceptualization; Data curation; Investigation; Methodology; Validation; Visualization; Writing‐review & editing. Ricardo Guimaraes Fischer: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing‐review & editing. Justine Monteiro Monnerat Tinoco: Conceptualization; Investigation; Methodology; Project administration; Validation; Visualization; Writing‐original draft; Writing‐review & editing. Fábio Ramoa Pires: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Validation; Visualization; Writing‐original draft; Writing‐review & editing. Josué da Costa Lima Junior: Conceptualization; Investigation; Methodology; Project administration; Validation; Visualization; Writing‐review & editing. Roy H. Stevens: Conceptualization; Investigation; Methodology; Project administration; Validation; Visualization; Writing‐original draft; Writing‐review & editing. Eduardo Muniz Barretto Tinoco: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing‐original draft; Writing‐review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/odi.13988.
ACKNOWLEDGEMENTS
This study received financial support from Rio de Janeiro Foundation for Research Support (FAPERJ, E‐26/202.810/2018).
Marques, B. B. F. , Guimarães, T. C. , Fischer, R. G. , Tinoco, J. M. M. , Pires, F. R. , Lima Junior, J. C. , Stevens, R. H. , & Tinoco, E. M. B. (2021). Morphological alterations in tongue epithelial cells infected by SARS‐CoV‐2: A case–control study. Oral Diseases, 00, 1–6. 10.1111/odi.13988
Contributor Information
Barbara Bruno Fagundes Marques, Email: barbarafagmar@gmail.com.
Josué da Costa Lima Junior, Email: josuelimajr@gmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Batah, S. S. , & Fabro, A. T. (2021). Pulmonary pathology of ARDS in COVID‐19: a pathological review for clinicians. Respiratory Medicine, 176, 106239. 10.1016/j.rmed.2020.106239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GTEx Portal . Gene expression for ACE2 (ENSG00000130234.10). Broad Institute of MIT and Harvard. https://www.gtexportal.org/home/gene/ACE2#geneExpression [Google Scholar]
- Li, Q. , Guan, X. , Wu, P. , Wang, X. , Zhou, L. , Tong, Y. , Ren, R. , Leung, K. S. M. , Lau, E. H. Y. , Wong, J. Y. , Xing, X. , Xiang, N. , Wu, Y. , Li, C. , Chen, Q. I. , Li, D. , Liu, T. , Zhao, J. , Liu, M. , … Feng, Z. (2020). Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus‐Infected Pneumonia. The New England Journal of Medicine, 382(13), 1199–1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. O. , Wu, H. , Wang, W. , Song, H. , Huang, B. , Zhu, N. A. , Bi, Y. , Ma, X. , Zhan, F. , Wang, L. , Hu, T. , Zhou, H. , Hu, Z. , Zhou, W. , Zhao, L. I. , … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet, 395, 565–574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhaske, S. , Yuwanati, M. , Mhaske, A. , Desai, A. , Sarode, S. C. , & Sarode, G. S. (2020). Perspective on oral exfoliative cytology and COVID‐19. Oral Oncology, 107, 104858. 10.1016/j.oraloncology.2020.104858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada, D. , Peña, K. B. , Gumà, J. , Guilarte, C. , & Riu, F. (2021). Liquid‐based cytological and immunohistochemical study of nasopharyngeal swab from persons under investigation for SARS‐CoV‐2 infection. Histopathology, 78(4), 586–592. 10.1111/his.14257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad, A. , Kassem, I. , Hockova, B. , Badrah, M. , & Klugar, M. (2020). Tongue ulcers associated with SARS‐CoV‐2 infection: A case series. Oral Diseases, 10.1111/odi.13635 [DOI] [PubMed] [Google Scholar]
- Rongioletti, F. , Ferreli, C. , Sena, P. , Caputo, V. , & Atzori, L. (2021). Clinicopathologic correlations of COVID‐19‐related cutaneous manifestations with special emphasis on histopathologic patterns. Clinics in Dermatology, 39(1), 149–162. 10.1016/j.clindermatol.2020.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi, W. , Kubota, N. , Shimizu, T. , Saruta, J. , Fuchida, S. , Kawata, A. , Yamamoto, Y. , Sugimoto, M. , Yakeishi, M. , & Tsukinoki, K. (2020). Existence of SARS‐CoV‐2 entry molecules in the oral cavity. International Journal of Molecular Sciences., 21(17), 1–16. 10.3390/ijms21176000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm, E. , Altman, D. G. , Egger, M. , Pocock, S. J. , Gøtzsche, P. C. , Vandenbroucke, J. P. , & Initiative, S. T. R. O. B. E. (2007). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England), 370(9596), 1453–1457. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- World Health Organization (2020). Modes of transmission of virus causing COVID‐19: implications for IPC precaution recommendations Modes of transmission of the COVID‐19 virus. World Health Organization. https://www.who.int/news‐room/commentaries/detail/modes‐of‐transmission‐of‐virus‐causing‐covid‐19‐implications‐for‐ipc‐precaution‐recommendations [Google Scholar]
- World Medical Association (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA, 310(20), 2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- Xu, H. , Zhong, L. , Deng, J. , Peng, J. , Dan, H. , Zeng, X. , Li, T. , & Chen, Q. (2020). High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science, 12(1), 8. 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Chen, P. , Wang, J. , Feng, J. , Zhou, H. , Li, X. , Zhong, W. U. , & Hao, P. (2020). Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Science China Life Sciences, 63, 457–460. 10.1007/s11427-020-1637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, M. , Lin, B. , Pathak, J. L. , Gao, H. , Young, A. J. , Wang, X. , Liu, C. , Wu, K. , Liu, M. , Chen, J.‐M. , Huang, J. , Lee, L.‐H. , Qi, C.‐L. , Ge, L. , & Wang, L. (2020). ACE2 and Furin Expressions in Oral Epithelial Cells Possibly Facilitate COVID‐19 Infection via Respiratory and Fecal‐Oral Routes. Frontiers in Medicine, 7, 580796. 10.3389/fmed.2020.580796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, L. , Ruan, F. , Huang, M. , Liang, L. , Huang, H. , Hong, Z. , Yu, J. , Kang, M. , Song, Y. , Xia, J. , Guo, Q. , Song, T. , He, J. , Yen, H.‐L. , Peiris, M. , & Wu, J. (2020). SARS‐CoV‐2 Viral Load in Upper Respiratory Specimens of Infected Patients. The New England Journal of Medicine, 382(12), 1177–1179. 10.1056/NEJMc2001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, X. , Chen, K. , Zou, J. , Han, P. , Hao, J. , & Han, Z. (2020). Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Frontiers of Medicine, 14(2), 185–192. 10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


