The significant impact of the COVID‐19 pandemic on public health, the economy and society required rapid action and the development of vaccines in an unprecedented time frame. While traditional vaccine development may take 15 years or more, vaccine development for SARS‐CoV‐2 has been reduced to 12‐18 months with an accelerated timeline. 1
Phase 1/2 clinical trials of the inactivated vaccine candidate CoronaVac COVID‐19 vaccine showed that this vaccine is safe and tolerable, and phase 3 clinical trials were conducted in Brazil, Turkey and Indonesia. 2 Announced emergency use authorization for CoronaVac on 13 January 2021 in Turkey. 3 Vaccination was initiated primarily in healthcare workers and higher risk groups. The vaccine was given in two doses (days 0 and 28).
Here, we present a case series of 6 patients who developed a cutaneous reaction after CoronaVac COVID‐19 vaccination of healthcare workers from a single centre. The demographic data of the patients and the clinical course of the cutaneous reactions are detailed in Table 1.
Table 1.
The demographic data of the patients and the clinical course of the cutaneous reactions
| Case age /sex | Cutaneous manifestation | Distribution | The clinical course of the lesions during the vaccination process | Medical history | PCR proven COVID‐19 infection |
|---|---|---|---|---|---|
| 45/F |
Erythematous dusky macules and papules and targetoid lesions Erythematous patch |
Symmetric, trunk, upper and lower limbs Upper palate |
Onset 3 days after the first dose, improved but recurred 1 day after the second dose with increased severity | Skin rashes with NSAID | No |
| 45/F |
Two oval thin plaques with a peripheral collarette scaling reminiscent of a herald patch Multiple scaly erythematous plaques |
Right breast and scapula Symmetric, along skin cleavage lines on trunk and upper limbs |
Onset 4 days after the first dose, gradually partially resolved but reactivated again 4 days after the second dose. | Unremarkable | Yes |
| 29/F | Linear weals | Along the shape of the scratching and rubbing areas | Onset 4 h after the first dose. She refused second dose. | Penicillin and metal allergy, polymorphic light eruption | No |
| 32/F |
Weals Angioedema |
Trunk, upper and lower limbs Both eyes(periorbital) |
Onset 12 weeks after the first dose. Persisted with worsening after the second dose. | Hashimoto thyroiditis | Yes |
| 48/F |
Weals Angioedema |
Trunk, upper and lower limbs Both eyes(periorbital) and lips |
Onset 4 h after the first dose. Persisted with worsening after the second dose. | Asthma, allergic rhinoconjunctivitis, latex and metal allergy, Hashimoto thyroiditis | No |
| 26/F | Weals | Ears and upper limbs | Onset 2 h after the second dose, improved within a week. | Unremarkable | No |
| Histopathology | Diagnosis | Treatment |
Follow‐up period/ current situation |
|---|---|---|---|
| Parakeratosis, spongiosis, lymphocytic exocytosis, parabasal layer vacuolar changes, apoptotic keratinocytes in the dermis and moderate mononuclear inflammation in the dermis | Erythema multiforme major | Oral antihistamine, systemic and topical corticosteroid |
8 weeks/ resolution |
| Focal parakeratosis with exocytosis of lymphocytes, spongiosis in the epidermis, and extravasated red blood cells in the dermis | Pityriasis rosea | Topical corticosteroid |
8 weeks/ resolution |
| None | Symptomatic dermographism | Oral antihistamine | 12 weeks/ improvement |
| None | Chronic spontaneous urticaria | Oral antihistamine and systemic corticosteroid |
9 weeks/ resolution |
| None | Chronic spontaneous urticaria | Oral antihistamine and omalizumab 300 mg/4 weeks |
12 weeks/ improvement |
| None | Acute urticaria | None |
1 week/ resolution |
F, Female.
One patient developed a maculopapular rash one week after the initial vaccination and resolved spontaneously within one week. One day after the second vaccination, the rashes recurred with atypical targetoid lesions, more extensive skin involvement and an erythematous patch on the upper palate. Histological examination revealed interface dermatitis (Figure 1a–c). There was initial concern about possible progression to Steven Johnson syndrome, and however, as there was no further mucosal involvement or skin necrolysis, the final diagnosis was erythema multiforme major, and she had good clinical recovery with systemic corticosteroid.
Figure 1.
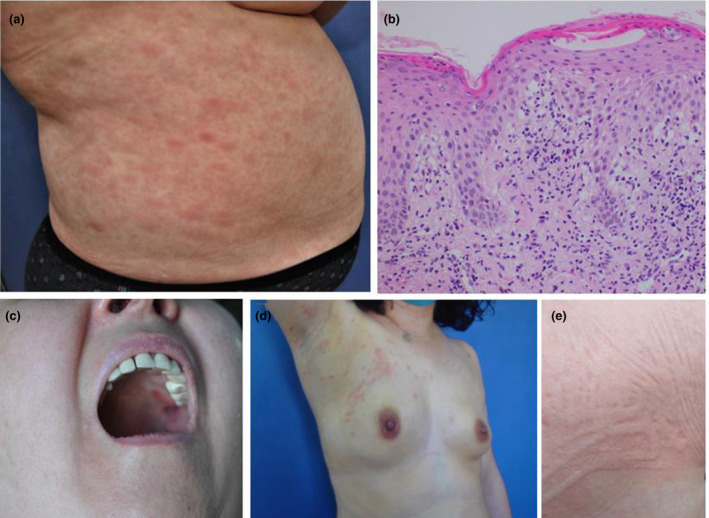
(a‐c) In a patient with erythema multiforme major, clinical presentation of dusky red macules and papules, targetoid lesions on the trunk and erythematous plaque on the upper palate, and histopathological appearance of parakeratosis, spongiosis, lymphocytic exocytosis, parabasal layer vacuolar changes, moderate mononuclear inflammation in the dermis (HE, ×200). (d) In a patient with pityriasis rosea, multiple erythematous scaly papules located along the skin cleavage lines with herald patch on the right breast. (e) In a patient with symptomatic dermographism, weals along the shape of the scratching and rubbing areas
One patient developed erythematous scaly papules located along the skin cleavage lines with two plaques resembling the herald patch on the trunk 4 days after the first dose of vaccine (Figure 1d). The morphological appearance of the lesions and histopathological findings were consistent with classical pityriasis rosea. The rashes faded within three weeks, but reactivated 4 days after the second vaccination, and all lesions resolved completely within 8 weeks.
Three patients presented with symptoms of urticaria after the first vaccination and one patient after the second vaccination (Figure 1e). None of the 4 patients had a prior history of urticaria. Three of the patients were subsequently diagnosed with chronic urticaria as symptoms had persisted for more than 6 weeks.
In comprehensive history evaluations, there was no other condition (e.g. infection or use of other medication) explaining the cause of skin rash in any patient.
Vaccines are the most important intervention against preventable infections in the protection of public health, but vaccine‐related adverse events are a common problem in clinical practice. Fortunately, serious acute or delayed onset systemic reactions are extremely rare. The most common reactions after immunization are local reactions and non‐immediate skin reactions such as delayed urticaria or maculopapular eruptions. Delayed reactions are generally considered to be self‐limiting conditions that do not contraindicate the administration of booster doses of the same vaccine. 4 , 5 Delayed cutaneous reactions were evident in these 6 patients who developed cutaneous adverse events among the 4257 vaccinated healthcare workers. Except for one patient with acute urticaria, other patients applied to our outpatient clinic because they suffered from severe or prolonged skin rash and itching. However, the actual incidence of cutaneous reactions is possibly higher as patients with mild self‐limiting symptoms may not have applied or sought medical care.
CoronaVac vaccine‐related cutaneous adverse events have been reported very few, and cutaneous reactions following inactivated CoronaVac vaccine have been well documented in this series. As vaccination studies continue, cutaneous reactions are also likely to continue to occur. Therefore, it is very important for dermatologists to recognize and manage skin rashes associated with the CoronaVac COVID‐19 vaccine and inform patients.
Conflict of interest
All authors (EA, BÖ, ÖE, MOÖ and Nİ) have nothing to disclose.
Funding sources
None.
Acknowledgement
The patients in this manuscript have given written informed consent to the publication of their case details.
References
- 1. Krammer F. SARS‐CoV‐2 vaccines in development. Nature 2020; 586: 516–527. [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y, Zeng G, Pan H et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18–59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021; 21: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mallapaty S. China COVID vaccine reports mixed results—what does that mean for the pandemic? Nature 2021. [DOI] [PubMed] [Google Scholar]
- 4. Caubet J‐C, Ponvert C. Vaccine allergy. Immunol Allergy Clin 2014; 34: 597–613. [DOI] [PubMed] [Google Scholar]
- 5. McNeil MM, DeStefano F. Vaccine‐associated hypersensitivity. J Allergy Clin Immunol 2018; 141: 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]


