Abstract
In its wake, the COVID‐19 pandemic has ushered in a surge in the number of cases of mucormycosis. Most cases are temporally linked to COVID‐19; hence, the entity is described as COVID‐19‐associated mucormycosis (CAM). The present systematic review was undertaken to provide an up‐to‐date summary of the hitherto available literature on CAM. PubMed, Scopus and Google Scholar databases were systematically searched using appropriate keywords till 14 May 2021, to identify case reports/case series pertaining to mucormycosis in patients with COVID‐19. Relevant data extracted included demographic characteristics, comorbidity profile, clinical category of mucormycosis, glucocorticoid use, treatment offered and patient outcome. We identified 30 case reports/case series, pooling data retrieved from 99 patients with CAM. Most cases were reported from India (72%). The majority of the patients was male (78%) and had diabetes mellitus (85%). A prior history of COVID‐19 was present in 37% patients with mucormycosis developing after an initial recovery. The median time interval between COVID‐19 diagnosis and the first evidence of mucormycosis infection or CAM diagnosis was 15 days. Glucocorticoid use was reported in 85% of cases. Rhino‐orbital mucormycosis was most common (42%), followed by rhino‐orbito‐cerebral mucormycosis (24%). Pulmonary mucormycosis was observed in 10 patients (10%). The mortality rate was 34%; the use of adjunct surgery, which was undertaken in 81% of patients, was associated with better clinical outcomes (p < .001). In conclusion, CAM is an emerging problem necessitating increased vigilance in COVID‐19 patients, even those who have recovered. CAM portends a poor prognosis and warrants early diagnosis and treatment.
Keywords: COVID‐19, COVID‐19‐associated mucormycosis, Mucormycosis
1. INTRODUCTION
As the novel coronavirus disease (COVID‐19) continues to rampage, an abrupt increase in the number of opportunistic fungal infections has been observed. Globally, several cases of mucormycosis have been described in patients with COVID‐19, 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 an entity being described as COVID‐19‐associated mucormycosis (CAM). Although a causal link between COVID‐19 and mucormycosis remains unearthed, multiple factors including glucocorticoids, worsening of blood glucose control and viral‐induced lymphopenia have been implicated in the development of mucormycosis in patients with COVID‐19.
Hitherto, the available literature on CAM is heterogeneous in terms of patient characteristics and clinical outcomes. A review of the literature published in April 2021 did provide a collated summary; however, it had included only 43 cases. 9 Subsequently, there has been an upsurge in the number of cases of CAM that have been reported from all across the world. 10 , 11 , 12 , 13 , 14 , 15
Hence, the present systematic review was undertaken to provide an up‐to‐date summary of the available literature on CAM.
2. METHODS
This systematic review was conducted and reported according to the Preferred Reporting Items for Systematic reviews and Meta‐analyses (PRISMA) statement. 16
2.1. Search strategy
Two investigators (RP and BS) independently performed a systematic search of the literature across the PubMed, Scopus and Google Scholar databases from inception till 14 May 2021, using the following keywords interposed with appropriate Boolean operators: ‘COVID‐19’ OR ‘SARS‐CoV‐2’ AND ‘mucormycosis’. The language was restricted to English only. The references of relevant reviews and retrieved articles were also screened for potentially eligible articles. For missing data, the corresponding authors of the potentially eligible studies were contacted wherever possible.
2.2. Eligibility and exclusion criteria
Eligibility criteria were set as follows:
Case reports and case series with a diagnosis of mucormycosis were included
Patients must have had a proven diagnosis of COVID‐19 either prior to or at the time of development of mucormycosis
Patients having co‐infections of mucormycosis and aspergillosis were also included
Exclusion criteria were set as follows:
Patients having fungal infections other than mucormycosis were excluded
Patients without a confirmed diagnosis of COVID‐19 (or serological evidence of prior COVID‐19) were excluded
Reviews, systematic reviews, comments and editorials
2.3. Data extraction
Two investigators (RP and MB) independently scanned titles and/or abstracts to exclude duplicate studies and studies that failed to meet the aforementioned eligibility criteria. Potentially eligible studies were full‐text assessed. Any discrepancies between the aforementioned investigators were solved by discussion, consensus or arbitration by a third senior investigator (SKB). Studies hence selected were reviewed, and the following data were extracted from full‐text reports for further assessment: study characteristics, number of patients reported, patient characteristics and comorbidities, present or prior history of COVID‐19, the severity of COVID‐19, the time from COVID‐19 to mucormycosis diagnosis, presence or absence of diabetic ketoacidosis, the clinical category of mucormycosis, the fungal species isolated from clinical specimens, whether corticosteroids had been used for the management of COVID‐19, use of other treatment modalities (like remdesivir, tocilizumab, convalescent plasma, antivirals and antibiotics), antifungal drug(s) used, any adjunct surgery performed for mucormycosis, investigations (blood glucose, C‐reactive protein, ferritin, D‐dimer and creatinine) and reported patient outcome (alive, deceased, or lost to follow‐up).
For the sake of uniformity, irrespective of the category assigned by the authors, wherever possible, we tried to classify the severity of COVID‐19 as follows 17 :
Mild: Individuals who have any of the various signs and symptoms of COVID‐19 but who do not have shortness of breath, dyspnoea or abnormal chest imaging
Moderate: Individuals who show evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation (SpO2) ≥ 94% on room air at sea level
Severe: Individuals who have SpO2 < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mm Hg, respiratory frequency >30 breaths/min, or lung infiltrates >50%
Critical: Individuals who have respiratory failure, septic shock and/or multiple organ dysfunction
Where the reported clinical data were insufficient, the disease severity designated by the authors was used for the present systematic review.
We also tried to decipher the time interval between COVID‐19 diagnosis and the onset of symptoms of mucormycosis. Mucormycosis was considered ‘concurrent’ if evidence of infection was present at the time of diagnosis of COVID‐19. Reports where there was no clarity in the timing of onset of symptoms (as in patients with pulmonary mucormycosis complicating an underlying severe/critical COVID‐19 disease), we considered the time when a diagnosis of mucormycosis was made.
2.4. Assessment of study quality and risk of bias
As all the included studies were either case reports or case series, a formal assessment of study quality and risk of bias was not performed.
2.5. Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) 23.0 software program (SPSS Inc, Chicago, IL, USA). Kolmogorov‐Smirnov test was used to check the normality of the extracted data. Normally distributed data were expressed as mean ± standard deviation (SD), while non‐parametric data were expressed in median (interquartile range, IQR). Categorical data were expressed as proportions (%). The comparison of demographic and other parameters between groups (alive vs. deceased) was made using independent samples t test (for continuous variables) or Pearson chi‐square test/Fisher's exact test (for categorical variables). A p value <.05 was considered to be statistically significant.
3. RESULTS
Following a thorough literature search and meticulous study selection process, we included 30 case reports/case series in our systematic review, pooling data retrieved from 99 patients with CAM. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 10 , 11 , 12 , 13 , 14 , 15 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 The study selection process has been summarised in the PRISMA flowchart (Figure 1). The patient details extracted from the case reports/case series have been described in Tables S1 and S2.
FIGURE 1.
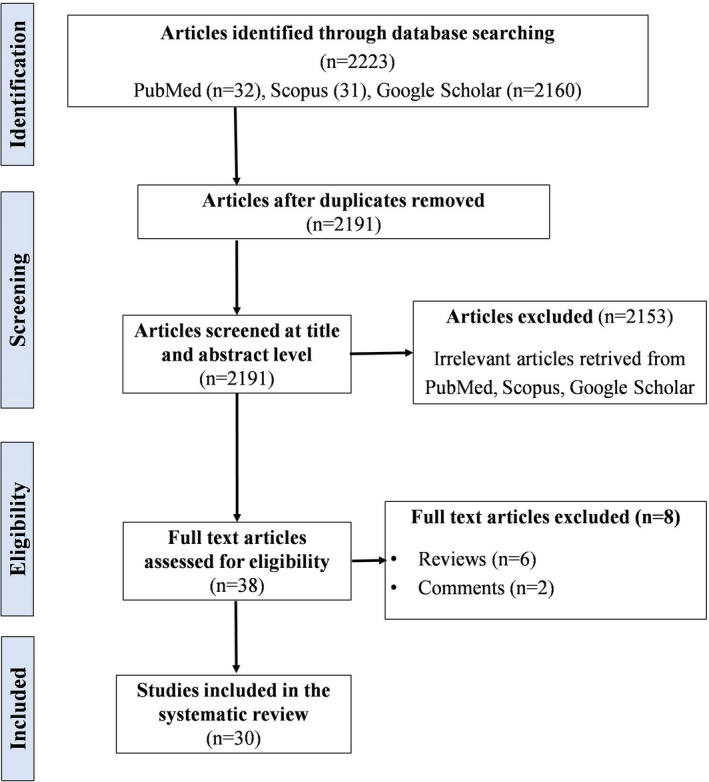
Preferred Reporting Items for Systematic reviews and Meta‐analyses (PRISMA) flowchart showing the study selection process
Out of these 30 studies, 21 were individual case reports, 1 , 2 , 3 , 4 , 5 , 6 , 7 , 10 , 11 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 29 , 31 while 9 were case series reporting 2, 12 , 28 , 32 6, 8 , 15 10, 13 , 30 17 cases 33 and 23 cases, 14 respectively. Most of the cases were reported from India (71 cases, 72%) followed by the United States of America (10 cases, 10%), Egypt (6 cases, 6%), Iran (3 cases, 3%), Brazil (2 cases, 2%) and Chile (2 cases, 2%). One case each was reported from the United Kingdom, France, Italy, Austria and Mexico. Only 14 cases of CAM (14%) were reported in 2020, while the rest of the cases (86%) were reported in 2021.
The analysed data have been summarised in Table 1. Of note, there was a noticeable gender bias, with 78% of the cases being male. Diabetes mellitus was present in the majority of the cases (85%). Hypertension was the second most prevalent comorbidity after diabetes mellitus. Only four patients (4%) did not have any comorbidity. Most of the patients had a present history of COVID‐19 (63%); nevertheless, the rest (37%) had a prior history of COVID‐19 with mucormycosis developing after an initial recovery. Although COVID‐19 was severe/critical in most patients (67%), mucormycosis developed even in patients with mild/moderate disease (24%). The median time interval between COVID‐19 diagnosis and the first evidence of mucormycosis infection or CAM diagnosis was 15 days; however, mucormycosis developed even as late as 42 days and 90 days following COVID‐19 diagnosis. 8 , 26
TABLE 1.
Summarising the characteristics of the patients included in the systematic review
| Parameter | Value |
|---|---|
|
Age (years) a Mean ± SD |
52.6 ± 13.9 |
| M:F | 7:2 |
| Presence of diabetes mellitus | 84 (85%) |
| Type of diabetes mellitus b | |
| T2D | 25 (78%) |
| T1D | 2 (6%) |
| New‐onset/newly diagnosed | 5 (16%) |
| Other comorbidities c | |
| HTN | 28 (58%) |
| Heart disease d | 6 (12%) |
| CKD/ESRD | 8 (17%) |
| Obesity | 3 (6%) |
| Asthma | 2 (4%) |
| Hypothyroidism | 3 (6%) |
| Haematological malignancy | 2 (4%) |
| Obstructive sleep apnoea | 1 (2%) |
| Chronic liver disease | 1 (2%) |
| Atrial fibrillation | 1 (2%) |
| History of COVID‐19 | |
| Present | 62 (63%) |
| Prior | 37 (37%) |
| COVID‐19 severity e | |
| Mild | 7 (13%) |
| Moderate | 6 (11%) |
| Moderate to severe | 5 (9%) |
| Severe | 16 (30%) |
| Critical | 20 (37%) |
| Time interval between COVID‐19 diagnosis and evidence of mucormycosis infection or diagnosis (days) f | |
| Median (interquartile range) | 15 (8‐19) |
| Presence of diabetic ketoacidosis g | 20 (29%) |
| Clinical category of mucormycosis | |
| Rhino‐orbital | 41 (42%) |
| Nose/paranasal sinus | 16 (16%) |
| Rhino‐cerebral | 4 (4%) |
| Rhino‐orbito‐cerebral | 24 (24%) |
| Oral | 1 (1%) |
| Pulmonary | 10 (10%) |
| Gastrointestinal | 1 (1%) |
| Cutaneous | 1 (1%) |
| Disseminated | 1 (1%) |
| Causative Mucorale h | |
| Rhizopus sp | 18 (85%) |
| Mucor sp | 2 (10%) |
| Lichtheimia sp | 1 (5%) |
| Use of glucocorticoids for management of COVID‐19 i | 82 (85%) |
| Use of broad‐spectrum antibiotics during management of COVID‐19 j | 40 (87%) |
| Use of amphotericin B for treatment of mucormycosis k | 87 (95%) |
| Use of adjunct surgery for mucormycosis | 80 (81%) |
| Outcome | |
| Alive | 63 (64%) |
| Deceased | 33 (33%) |
| Lost to follow‐up | 3 (3%) |
Calculated from 76 patients in whom individual data were available.
Data on the type of diabetes were available in 32 patients.
Data on comorbidities other than diabetes mellitus were available in 72 patients of whom 48 had ≥1 comorbidities and 24 had none.
Includes ischaemic heart disease, coronary artery disease, ischaemic cardiomyopathy.
COVID‐19 severity was reported in 54 patients.
Based on data available from 23 patients. COVID‐19 and mucormycosis was deemed concurrent in 17 patients, while in 3 patients, mucormycosis was diagnosed postmortem.
Presence or absence of diabetic ketoacidosis was reported in 70 patients.
Data on causative Mucorale was available from 21 patients.
Data on use of glucocorticoids for the management of COVID‐19 was available from 96 patients.
Data on the use of one or more broad‐spectrum antibiotics during the management of COVID‐19 were available from 46 patients.
Data on the use of one or more antifungal agents were available from 92 patients.
The use of glucocorticoids for the management of COVID‐19 was observed in 85% of the cases. Parenteral dexamethasone was the most commonly used glucocorticoid. The dose and duration of the glucocorticoid were infrequently reported across the included articles, hence, could not be analysed. Rhino‐orbital mucormycosis was most common (42%), followed by rhino‐orbito‐cerebral mucormycosis (24%). Pulmonary mucormycosis was observed in 10 patients (10%). The predominant fungal organism identified was Rhizopus sp. Mixed mould infection (mucormycosis and aspergillosis) was reported in 4 patients. 10 , 23 , 32 , 33 Surgery, as an adjunct to antifungal therapy, was undertaken in 81% of the patients. The biochemical parameters, namely HbA1c, blood glucose, CRP, ferritin, D‐dimer and creatinine, were scarcely reported and excluded from the analysis.
The final outcome was reported in 96 cases, while 3 patients were lost to follow‐up. Out of these 96 cases, 33 (34%) were deceased, while 63 (66%) were alive. Table 2 depicts the differences in the clinical profile of patients who were alive compared to those who were deceased. Notably, patients with pulmonary mucormycosis showed a trend towards poor outcomes as compared to patients with rhino‐orbital/rhino‐cerebral/rhino‐orbito‐cerebral mucormycosis (p = .084). Besides, the use of adjunct surgery was found to be associated with better clinical outcomes (p < .001).
TABLE 2.
Showing comparison of patients who were alive vs. those who were deceased
| Parameter | Alive (n = 42) | Deceased (n = 33) | p value |
|---|---|---|---|
|
Age (years) Mean ± SD |
53.2 ± 12.8 | 51.7 ± 15.5 | .645 |
| M:F | 33:9 | 28:5 | .489 |
| Presence of diabetes mellitus | 37 (88%) | 25 (76%) | .161 |
| History of COVID‐19 | |||
| Present | 30 (71%) | 27 (82%) | .296 |
| Prior | 12 (29%) | 6 (18%) | |
| COVID‐19 severity a | |||
| Mild and moderate | 19 (68%) | 19 (83%) | .336 |
| Severe and critical b | 9 (32%) | 4 (17%) | |
| Clinical category of mucormycosis c | |||
| Pulmonary mucormycosis | 3 (7%) | 7 (23%) | .084 |
| ROM/RCM/ROCM | 38 (93%) | 23 (77%) | |
| Use of adjunct surgery for mucormycosis | |||
| Yes | 38 (90%) | 18 (54%) | <.001 |
| No | 4 (10%) | 15 (46%) |
Abbreviations: RCM, Rhino‐cerebral mucormycosis; ROCM, Rhino‐orbito‐cerebral mucormycosis; ROM, Rhino‐orbital mucormycosis; SD, Standard deviation.
Analysed with data available from 51 patients.
Patients with moderate to severe disease have also been considered as having severe disease for the statistical analysis.
Analysed with data available from 71 patients. Other sites of mucormycosis have not been included in the analysis.
4. DISCUSSION
The present systematic review that had included 99 cases of CAM reported till 14 May 2021 provides an updated summary of this unique clinical entity. The majority of the cases were reported from India. Most of the patients were male with an underlying history of diabetes mellitus and had received glucocorticoids for the management of COVID‐19. Patients with CAM had a high mortality rate (34%); the use of adjunct surgery for mucormycosis was associated with improved clinical outcomes.
In its wake, the COVID‐19 pandemic has ushered in a surge in the number of cases of mucormycosis. For example, a retrospective observational study from a tertiary care hospital in Egypt reported 12 cases of rhino‐orbito‐cerebral mucormycosis (ROCM) identified over 6 months (25 March 2020 to 25 September 2020). The number of cases identified was much higher than those presenting in the prior 3 years during the corresponding 6‐month interval (1 case in 2017, 2 in 2018 and 1 in 2019). 15 Similarly, a prospective single‐centre observational study conducted in India from August 2020 to December 2020 identified 23 cases of invasive mucormycosis of the paranasal sinuses. 14 This is in stark contrast to a report published in 2015 from two large medical institutes in Mumbai, India that encompassed only 20 cases over a period of 3 years. 34 The majority of the cases have been temporally linked to COVID‐19; hence, the entity is described as COVID‐19‐associated mucormycosis and abbreviated as CAM or CAMCR. 9 , 29
Several observations have emerged from the present systematic review. We found a drastic geographical disparity, with most cases being reported from India (72%). Nevertheless, India has the highest burden of mucormycosis globally, with an estimated prevalence of 140 cases per million population. 35 Besides, India is home to nearly 77 million people with diabetes, with a nationwide prevalence of 7.3%. 36 , 37 Diabetes mellitus being a major risk factor for mucormycosis, 35 , 38 , 39 , 40 a spurt in the number of cases of CAM from India is not surprising.
As would be expected, most of the patients had underlying diabetes mellitus (85%). Existing data suggest that individuals who lack phagocytes or have impaired phagocytic function are at higher risk of mucormycosis. 41 Neutrophils are critical for inhibiting the proliferation of fungal spores. Besides, both mononuclear and polymorphonuclear phagocytes of a normal host kill Mucorales by generating reactive oxygen species and cationic peptides, namely defensins. 42 , 43 Hyperglycemia, as seen in patients with uncontrolled diabetes mellitus, leads to phagocyte dysfunction, impaired chemotaxis and defective intracellular killing by oxidative and non‐oxidative mechanisms. 44
Although data on the degree of glycemic control were infrequently reported across all the included studies, it is expected that COVID‐19 might have further worsened the glucose profile of the patients with diabetes, thereby further predisposing them to mucormycosis. Multiple mechanisms have been implicated in the genesis or worsening of dysglycaemia in patients with COVID‐19. Of note, SARS‐CoV‐2 can infect and replicate in the human islet cells, 45 leading to β‐cell damage and reduced endogenous insulin secretion. 46 , 47 Besides, the plethora of cytokines, as seen in patients with COVID‐19, can lead to worsening of insulin resistance. 47 Usually elevated in patients with severe COVID‐19, interleukin‐6 (IL‐6) causes insulin resistance by impairing the phosphorylation of insulin receptor and insulin receptor substrate‐1. 48 Elevated IL‐6 levels have been observed even in one‐third of patients with mild COVID‐19 that can contribute to dysglycaemia in such patients. 49 Lastly, drugs used in the management of COVID‐19, namely glucocorticoids, lopinavir‐ritonavir and remdesivir can further worsen glucose control and predispose to mucormycosis. 47
Apart from uncontrolled hyperglycemia, free unbound iron in serum plays an essential role in the pathogenesis of mucormycosis. Diabetic ketoacidosis, an important risk factor for mucormycosis, is associated with increased serum levels of free iron secondary to acidosis and proton‐mediated displacement of ferric iron from transferrin. 50 Likewise, severe COVID‐19 is a hyper‐ferritinemic state. High serum ferritin leads to excess intracellular iron that generates reactive oxygen species and resultant death of hepatocytes. Cytokines, especially IL‐6, stimulate ferritin synthesis and downregulate iron export resulting in intracellular iron overload, further aggravating the process. 51 Following tissue damage, ferritin is released into the bloodstream; circulating ferritin loses part of the inner iron content, producing high levels of free iron. 52 , 53
Another possible link between COVID‐19 and mucormycosis is the development of ketoacidosis. Ketonemia and ketoacidosis have been observed in patients with COVID‐19, even in the absence of diabetes mellitus. 54 Apart from raising free iron levels, acidosis impairs phagocytic activity predisposing to mucormycosis. 44
The use of glucocorticoids is a known risk factor for the development of mucormycosis. 41 Glucocorticoid‐induced immunosuppression, hyperglycaemia and lymphopenia predispose to the pathogenesis of mucormycosis. The rampant use of glucocorticoids in patients with COVID‐19 has undoubtedly contributed to the upsurge in the number of cases of CAM. 9 , 29 , 33 Other factors that predispose COVID‐19 patients to the development of CAM include viral‐induced lymphopenia and endotheliitis. Widespread endothelial damage, as seen in patients with COVID‐19 might promote adhesion and penetration of Mucorales to the endothelium. Endothelial adhesion and penetration are critical early steps in the pathogenesis of mucormycosis. 9 The likely association between COVID‐19 and mucormycosis has been schematically represented in Figure 2.
FIGURE 2.
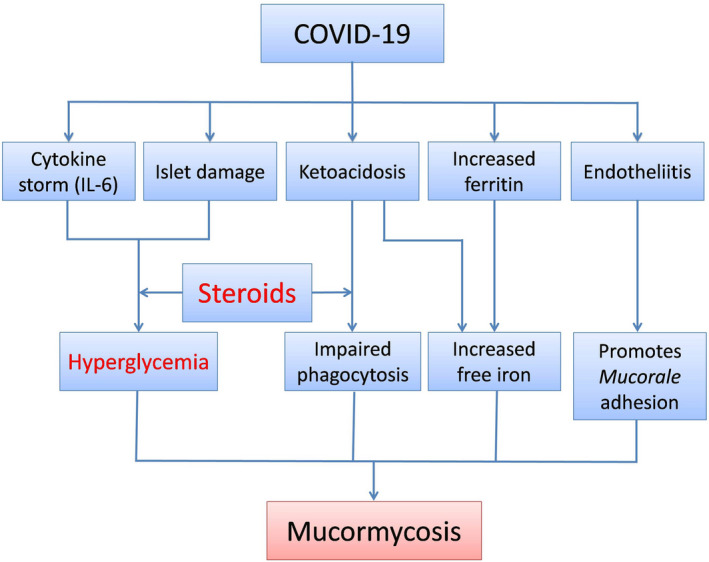
Schematic diagram showing the likely association between the novel coronavirus disease (COVID‐19) and mucormycosis. IL‐6, Interleukin‐6
Another indirect association between the concomitant surge in COVID‐19 and mucormycosis is the dissemination of fungal spores via water used in oxygen humidifiers. Indeed, hospital water is a potential reservoir for fungi including Mucorales. 55 Transmission of mucormycosis via water in oxygen humidifiers could be a potential reason for the disproportionate increase in the number of CAM cases in a developing country like India.
We found that in nearly one‐third of the cases of CAM, there was a prior history of COVID‐19. This necessitates continued vigilance and regular follow‐up even after recovery from COVID‐19, especially those with uncontrolled blood glucose and who had been on high‐dose glucocorticoid therapy of significant duration. The present systematic review suggests that extra caution should be exercised and a high index of suspicion for CAM should be kept, especially for the first 15 days following the diagnosis of COVID‐19.
We observed a mortality rate of 34%, which is similar to that reported in the literature in patients with mucormycosis not associated with COVID‐19. 56 Thus, although COVID‐19 predisposes an individual to mucormycosis, it does not appear to alter the prognosis in such patients. Pulmonary mucormycosis, which was observed in 10% of the cases, was associated with a trend towards poor outcomes as compared to rhino‐orbital/rhino‐cerebral/rhino‐orbito‐cerebral mucormycosis. The possible reason being a delay in the diagnosis of pulmonary mucormycosis. 57 This is more likely in patients with severe/critical COVID‐19, in whom the underlying cytokine‐mediated acute respiratory distress syndrome (ARDS) may mask the symptoms of pulmonary mucormycosis, thereby causing significant delay in diagnosis. However, pulmonary mucormycosis should be kept high on the cards in COVID‐19 patients who redevelop cough, expectoration and worsening of respiratory distress while recovering from ARDS.
Regarding the management of CAM, we found that surgery (sinonasal debridement in most cases) as an adjunct to antifungal therapy was associated with higher survival rates. The debridement surgery needs to be performed before the infection spreads to other adjoining areas, particularly the brain. 58 Thus, timely debridement is essential in improving patient outcomes in CAM; however, surgery may be challenging in a patient infected with SARS‐CoV‐2 and demands extra precautions and the use of barrier draping techniques by the operating surgeons. 25 , 59
The present systematic review happens to be the largest hitherto available data on CAM. Grey literature search on Google Scholar database helped us identify cases that we would have missed if we only restricted our search to PubMed. Besides, to the best of our knowledge, this is the only systematic review on CAM that has been performed in accordance with the PRISMA statement. Nonetheless, we humbly acknowledge the limitations of our review. First, in the absence of a denominator, it was not possible to calculate the incidence of CAM in patients with COVID‐19. Second, in the absence of a control group (ie COVID‐19 without CAM), we could not delineate the risk factors that could predict the development of CAM in COVID‐19 patients. Third, we were not able to assess attributable mortality of CAM again because of lack of appropriate controls. Fourth, data of dose and duration of glucocorticoid therapy and parameters of glycaemic control (blood glucose, HbA1c) were infrequently reported, hence, they could not be included in the systematic review.
In conclusion, the present systematic review provides a collated summary of all the patients of CAM reported globally. Most of the cases were reported from India, had an underlying history of diabetes mellitus and had received glucocorticoids during the management of COVID‐19, thereby necessitating judicious use of this drug amid the pandemic. Unfortunately, patients with CAM exhibited a high mortality rate, although the use of adjunct surgery in addition to antifungal therapy was associated with improved clinical outcomes.
CONFLICTS OF INTEREST
None to declare.
AUTHOR CONTRIBUTIONS
Rimesh Pal: Conceptualization (equal); data curation (equal); formal analysis (equal); methodology (equal); writing–original draft (equal). Birgurman Singh: Data curation (equal). Sanjay Bhadada: Conceptualization (equal); supervision (lead); writing–review and editing (equal). Mainak Banerjee: Formal analysis (equal); methodology (equal); writing–review and editing (equal). Ranjitpal Singh Bhogal: Data curation (equal); formal analysis (equal); writing–review and editing (equal). Neemu Hage: Data curation (equal); writing–review and editing (equal). Ashok Kumar: Formal analysis (equal); writing–review and editing (equal).
Supporting information
Table S1
Table S2
ACKNOWLEDGEMENT
None.
Pal R, Singh B, Bhadada SK, et al. COVID‐19‐associated mucormycosis: An updated systematic review of literature. Mycoses. 2021;64:1452–1459. 10.1111/myc.13338
Funding information
None.
REFERENCES
- 1. Placik DA, Taylor WL, Wnuk NM. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS‐CoV‐2 pneumonia. Radiol Case Rep. 2020;15:2378‐2381. 10.1016/j.radcr.2020.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Werthman‐Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID‐19. Am J Emerg Med. 2021;42:264.e5‐264.e8. 10.1016/j.ajem.2020.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pasero D, Sanna S, Liperi C, et al. A challenging complication following SARS‐CoV‐2 infection: a case of pulmonary mucormycosis. Infection. Published online. 2020. 10.1007/s15010-020-01561-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. do Monte Junior ES, dos Santos MEL, Ribeiro IB, et al. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID‐19 patient: a case report. Clin Endosc. 2020;53:746‐749. 10.5946/ce.2020.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mekonnen ZK, Ashraf DC, Jankowski T, et al. Acute invasive rhino‐orbital mucormycosis in a patient with COVID‐19‐associated acute respiratory distress syndrome. Ophthal Plast Reconstr Surg. 2021;37:e40‐e80. 10.1097/IOP.0000000000001889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta S, Pandey A. Rhino‐orbital mucormycosis associated with COVID‐19. Cureus. Published online. 2020. 10.7759/cureus.10726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID‐19: a post‐mortem study. Lancet Microbe. 2020;1:e245‐e253. 10.1016/S2666-5247(20)30115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sen M, Lahane S, Lahane T, Parekh R, Honavar S. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021;69:244. 10.4103/ijo.IJO_3774_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID‐19 converge: the perfect storm for mucormycosis. J Fungi. 2021;7:298. 10.3390/jof7040298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bellanger A‐P, Navellou J‐C, Lepiller Q, et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS‐CoV‐2) patient. Infect Dis Now. Published online. 2021:S2666991921000300. 10.1016/j.idnow.2021.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maini A, Tomar G, Khanna D, Kini Y, Mehta H, Bhagyasree V. Sino‐orbital mucormycosis in a COVID‐19 patient: a case report. Int J Surg Case Rep. 2021;82:105957. 10.1016/j.ijscr.2021.105957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veisi A, Bagheri A, Eshaghi M, Rikhtehgar MH, Rezaei Kanavi M, Farjad R. Rhino‐orbital mucormycosis during steroid therapy in COVID‐19 patients: a case report. Eur J Ophthalmol. Published online 2021:112067212110094. 10.1177/11206721211009450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mishra N, Mutya VSS, Thomas A, et al. A case series of invasive mucormycosis in patients with COVID‐19 infection. Int J Otorhinolaryngol Head Neck Surg. 2021;7:867. 10.18203/issn.2454-5929.ijohns20211583 [DOI] [Google Scholar]
- 14. Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. Published online 2021:1‐6. 10.1017/S0022215121000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fouad Y, Abdelaziz T, Askoura A, et al. Spike in rhino‐orbital‐cerebral mucormycosis cases presenting to a tertiary care center during the COVID‐19 pandemic. Front Med (Lausanne). Published online. 2021. 10.3389/fmed.2021.645270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. NIH COVID‐19 Treatment Guidelines . https://www.covid19treatmentguidelines.nih.gov/overview/clinical‐spectrum/. Accessed May 14, 2021
- 18. Khan N, Gutierrez CG, Martinez DV, Proud KC. A case report of COVID‐19 associated pulmonary mucormycosis. Arch Clin Cases. 2020;07:46‐51. 10.22551/2020.28.0703.10172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alekseyev K, Didenko L, Chaudhry B. Rhinocerebral Mucormycosis and COVID‐19 Pneumonia. J Med Cases. 2021;12:85‐89. 10.14740/jmc3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zurl C, Hoenigl M, Schulz E, et al. Autopsy proven pulmonary mucormycosis due to Rhizopus microsporus in a critically Ill COVID‐19 patient with underlying hematological malignancy. J Fungi. 2021;7:88. 10.3390/jof7020088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waizel‐Haiat S, Guerrero‐Paz JA, Sanchez‐Hurtado L, Calleja‐Alarcon S, Romero‐Gutierrez L. A case of fatal rhino‐orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID‐19. Cureus. Published online. 2021. 10.7759/cureus.13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanwar A, Jordan A, Olewiler S, Wehberg K, Cortes M, Jackson BR. A fatal case of Rhizopus azygosporus pneumonia following COVID‐19. J Fungi. 2021;7:174. 10.3390/jof7030174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson AK, Ghazarian Z, Cendrowski KD, Persichino JG. Pulmonary aspergillosis and mucormycosis in a patient with COVID‐19. Med Mycol Case Rep. 2021;32:64‐67. 10.1016/j.mmcr.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Revannavar SM, Supriya PS, Samaga L, Vineeth VK COVID‐19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep. 2021;14:e241663. 10.1136/bcr-2021-241663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saldanha M, Reddy R, Vincent MJ. Title of the article: paranasal mucormycosis in COVID‐19 patient. Indian J Otolaryngol Head Neck Surg. Published online. 2021. 10.1007/s12070-021-02574-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khatri A, Chang K‐M, Berlinrut I, Wallach F. Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient – case report and review of literature. J Med Mycol. 2021;31:101125. 10.1016/j.mycmed.2021.101125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karimi‐Galougahi M, Arastou S, Haseli S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID‐19). Int Forum Allergy Rhinol. Published online. 2021:alr.22785. 10.1002/alr.22785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dallalzadeh LO, Ozzello DJ, Liu CY, Kikkawa DO, Korn BS. Secondary infection with rhino‐orbital cerebral mucormycosis associated with COVID‐19. Orbit. Published online 2021:1‐4. 10.1080/01676830.2021.1903044 [DOI] [PubMed] [Google Scholar]
- 29. Garg D, Muthu V, Sehgal IS, et al. Coronavirus disease (Covid‐19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186:289‐298. 10.1007/s11046-021-00528-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sarkar S, Gokhale T, Choudhury S, Deb A. COVID‐19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69:1002. 10.4103/ijo.IJO_3763_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pauli MA, de Pereira L, Monteiro ML, de Camargo, AR , Rabelo GD. Painful palatal lesion in a patient with COVID‐19. Oral Surg Oral Med Oral Pathol Oral Radiol. Published online. 2021:S2212440321001607. 10.1016/j.oooo.2021.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rabagliati R, Rodríguez N, Núñez C, Huete A, Bravo S, Garcia P. COVID‐19–Associated Mold Infection in Critically Ill Patients. Chile. Emerg Infect Dis. 2021;27:1454‐1456. 10.3201/eid2705.204412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moorthy A, Gaikwad R, Krishna S, et al. SARS‐CoV‐2, uncontrolled diabetes and corticosteroids—an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi‐centric analysis. J Maxillofac Oral Surg. Published online. 2021. 10.1007/s12663-021-01532-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kolekar JS. Rhinocerebral mucormycosis: a retrospective study. Indian J Otolaryngol Head Neck Surg. 2015;67:93‐96. 10.1007/s12070-014-0804-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019;5:26. 10.3390/jof5010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. IDF Diabetes ATLAS Ninth Edition . 2019. https://diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e‐final‐web.pdf#page=42&zoom=auto Accessed May 12, 2020.
- 37. Anjana RM, Deepa M, Pradeepa R, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population‐based cross‐sectional study. Lancet Diabetes Endocrinol. 2017;5:585‐596. 10.1016/S2213-8587(17)30174-2 [DOI] [PubMed] [Google Scholar]
- 38. Prakash H, Ghosh AK, Rudramurthy SM, et al. A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Med Mycol. 2019;57:395‐402. 10.1093/mmy/myy060 [DOI] [PubMed] [Google Scholar]
- 39. Corzo‐León DE, Chora‐Hernández LD, Rodríguez‐Zulueta AP, Walsh TJ. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: epidemiology, diagnosis, and outcomes of reported cases. Med Mycol. 2018;56:29‐43. 10.1093/mmy/myx017 [DOI] [PubMed] [Google Scholar]
- 40. Bhansali A. Presentation and outcome of rhino‐orbital‐cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004;80:670‐674. 10.1136/pgmj.2003.016030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54:S16‐S22. 10.1093/cid/cir865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waldorf AR. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol Ser. 1989;47:243‐271. [PubMed] [Google Scholar]
- 43. Diamond RD, Haudenschild CC, Erickson NF. Monocyte‐mediated damage to Rhizopus oryzae hyphae in vitro. Infect Immun. 1982;38:292‐297. 10.1128/IAI.38.1.292-297.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123‐1129. 10.1128/IAI.38.3.1123-1129.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Müller JA, Groß R, Conzelmann C, et al. SARS‐CoV‐2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3:149‐165. 10.1038/s42255-021-00347-1 [DOI] [PubMed] [Google Scholar]
- 46. Pal R, Banerjee M. COVID‐19 and the endocrine system: exploring the unexplored. J Endocrinol Invest. 2020;43:1027‐1031. 10.1007/s40618-020-01276-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pal R, Bhadada SK. COVID‐19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab Syndr Clin Res Rev. 2020;14:513‐517. 10.1016/j.dsx.2020.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rehman K, Akash MSH, Liaqat A, Kamal S, Qadir MI, Rasul A. Role of interleukin‐6 in development of insulin resistance and type 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr. 2017;27:229‐236. 10.1615/CritRevEukaryotGeneExpr.2017019712 [DOI] [PubMed] [Google Scholar]
- 49. Costela‐Ruiz VJ, Illescas‐Montes R, Puerta‐Puerta JM, Ruiz C, Melguizo‐Rodríguez L. SARS‐CoV‐2 infection: the role of cytokines in COVID‐19 disease. Cytokine Growth Factor Rev. 2020;54:62‐75. 10.1016/j.cytogfr.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ibrahim AS, Spellberg B, Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis. 2008;21:620‐625. 10.1097/QCO.0b013e3283165fd1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perricone C, Bartoloni E, Bursi R, et al. COVID‐19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol Res. 2020;68:213‐224. 10.1007/s12026-020-09145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pretorius E, Kell DB. Diagnostic morphology: biophysical indicators for iron‐driven inflammatory diseases. Integr Biol. 2014;6:486‐510. 10.1039/C4IB00025K [DOI] [PubMed] [Google Scholar]
- 53. Habib HM, Ibrahim S, Zaim A, Ibrahim WH. The role of iron in the pathogenesis of COVID‐19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother. 2021;136:111228. 10.1016/j.biopha.2021.111228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. covid ‐19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22:1935‐1941. 10.1111/dom.14057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rammaert B, Lanternier F, Zahar J‐R, et al. Healthcare‐associated mucormycosis. Clin Infect Dis. 2012;54:S44‐S54. 10.1093/cid/cir867 [DOI] [PubMed] [Google Scholar]
- 56. Serris A, Danion F, Lanternier F. Disease entities in mucormycosis. J Fungi. 2019;5:23. 10.3390/jof5010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556‐569. 10.1128/CMR.18.3.556-569.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Turner JH, Soudry E, Nayak JV, Hwang PH. Survival outcomes in acute invasive fungal sinusitis: a systematic review and quantitative synthesis of published evidence: survival outcomes in invasive fungal sinusitis. The Laryngoscope. 2013;123:1112‐1118. 10.1002/lary.23912 [DOI] [PubMed] [Google Scholar]
- 59. Tsagkovits A, Ioannidis D, Rokade A. The microscope drape method to reduce aerosolisation during endoscopic sinus and skull base surgery in the COVID era. How i do it. Eur Arch Otorhinolaryngol. 2021;278:573‐576. 10.1007/s00405-020-06441-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


