Abstract
Background
Increased frequency of using alcohol‐based hand sanitizers (ABHS) by consumers during COVID times have resulted in increased incidences of skin issues on palms.
Objective
(1) To quantify skin damage with increased usage frequency of ABHS by consumers and (2) To evaluate Virgin Coconut Oil (VCO) as natural prophylactic agent to counter the adverse effects.
Methods
In‐home usage study was carried out with 60 volunteers for a 15‐day intervention—Control Group: 6 applications per day of ABHS and Test Group: Overnight VCO use (6–8 drops) followed by 6× usage per day of ABHS. This leg included dermatological evaluation and WHO Self‐Assessment Scale for skin health. Another leg of measurement included non‐invasive instrumental study (Moisture & TEWL Probes, Tape Strip for protein content and IR spectroscopy for protein & lipid content) on forearm of 12 subjects (25–60 years age) with and without VCO application and repeated alcohol exposure.
Results
In‐home usage study established consumer experiencing skin protective effect of VCO in the context of ABHS onslaught. 25% increase in perceived moisture content was recorded for VCO users, using WHO Self‐Assessment Scale. Instrumental studies confirmed an increase in TEWL and decrease in lipids & protein content. Overnight VCO application resists the extraction which builds up with repeated application.
Conclusions
Current work provides evidence of compromised hand skin barrier with ABHS daily usage. Overnight VCO application helps prepare the skin for next day alcohol use. Based on the findings, a regimen of overnight VCO application on hands as a natural prophylactic is recommended.
Keywords: alcohol‐based hand sanitizers, skin damage, skin lipid extraction, skin moisturization, virgin coconut oil
1. INTRODUCTION
Epidemic infections in the form of bacterial or viral pathogens have always posed serious challenges to global public health threat.1 Recent among those is the emergence of the dangerous Severe Acute Respiratory Syndrome Novel Coronavirus 2 (SARS‐nCoV‐2) associated with causing Coronavirus Disease 2019 (COVID‐19)—a global pandemic declared by the World Health Organization in early 2020. COVID‐19—first reported in Wuhan district of China in December 2019—have infected more than 80 million people by the end of 2020 and resulted more than 2 million casualties.2 With only recent development of vaccines and planned immunization program across the world still in infancy, the most effective method still being prevention aimed at reducing the transmission. COVID‐19 has been hailed as a “Black Swan” event with far‐reaching implications in social, economic, and behavioral domains.3 Like any other black swan event, it has caused unexpected and rapid shifts in existing lifestyles & preferences.
One of the many changes triggered by COVID‐19 has been the resurgence of interest in the personal hygiene segment. Earliest recommendation to prevent the spread of SARS‐nCOV‐2, as with previous contagious pathogens, was frequent and effective hand hygiene practices. World Health Organization (WHO) guidelines4 for maintaining hand hygiene is frequent hand wash using soap and water for at least 20 s especially after going to the bathroom, before eating, and after coughing, sneezing, or blowing one's nose. When soap and water are not available, the Food and Drug Administration (FDA) recommends sanitizing non‐visibly soiled hands with an alcohol‐based hand sanitizer (ABHS) containing 80% v/v ethanol or 75% v/v isopropanol. ABHS is widely considered to be effective to reduce or eliminate bacterial/viral load, but with variable compliance rates.5 The alcohols—ethanol, isopropanol, and n‐propanol—as used for disinfection are commonly applied in the form of hand rub rinses, gels, and foams.
1.1. Exponential increase in ABHS usage
Google Trends analysis helps quantify the magnitude of the emerging interest about ABHS in the general public. Google Trends is an online tool which help analyze the search volume of given keywords on Google platform.6 Figure 1 exhibits the search trend for the keyword “hand sanitizer” worldwide. A nominal yet constant volume of Google searches on this topic was shown until February 2020, post which a sudden spike (100× increase) in the interest was recorded. This search trend also correlated with the increased search of the word “COVID‐19,” thus linking the two terms. The search interest has also been substantiated with the product launch activity as well as the product sales volumes over the last one year. Figure 2 captures the global product launches under the Hand Sanitizer category from Mintel GNPD tool.7 Also, as confirmed by Nielsen report, the sale of ABHS increased by 300% and 470% in last week of February 20 and 1st week of March 20, respectively.8 Although the volume of searches on “hand sanitizer” has tapered down toward the end of 2020, one can expect the awareness generated of the importance of hand hygiene using ABHS will remain in the future usage behavior and will become a necessary part of the personal hygiene practices, even post‐COVID era.
FIGURE 1.
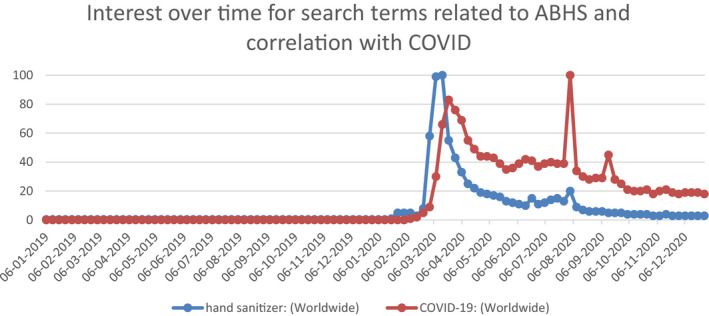
Google Trends Worldwide Search Volumes for the Search words “hand sanitizer” and “COVID‐19,” extracted on January 4, 2021
FIGURE 2.
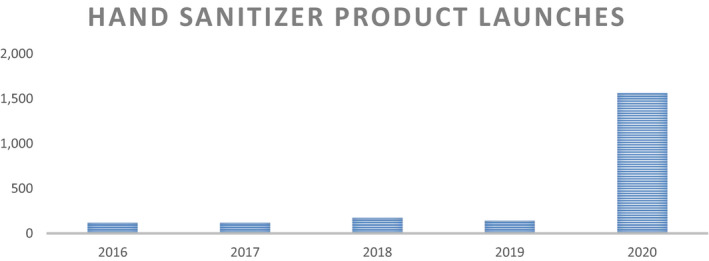
Product launches under Hand Sanitizer Categories worldwide, data extracted from product launches in Mintel GNPD for the defined time period
1.2. Emergent hand skin concerns in COVID times
The practice of using ABHS several times daily for hand disinfection has been a part of regime of healthcare workers for a long time. Upto 100 applications of ABHS per day may be necessary for full hand hygiene compliance.9 It has also been reported that skin irritation and negative skin tolerance remain a major reason for low compliance rates for ABHS usage among healthcare workers.9 There are two major types of skin reactions associated with hand hygiene. The first and most common type—Irritant Contact Dermatitis—includes symptoms that include dryness, irritation, itching, and even cracking and bleeding. The second type of skin reaction—Allergic Contact Dermatitis—is rare and represents an allergy to ingredients (like fragrance, preservatives, and emulsifiers) in a hand hygiene product.
In general public, the increased and sustained frequency of ABHS and other hand hygiene solutions, in the wake of COVID‐19 spread, have resulted in challenges related to skin health.10 As seen, again, from Google Trends analysis the increase in worldwide search volumes of “dry hand” and “hand cream” has coincided with the emergence of COVID‐19 (Figure 3) and more so toward the end of 2020 when the after effects of repeated exposure have reached a certain degree of skin barrier threshold. Also, in an August 20 consumer study, comprising 2534 Internet users by IPSOS/Mintel, about 44% subjects stated that hand sanitizers make their hands dry.11 Organizations like American Academy of Dermatology Association (AAD) and Centers for Disease Control (CDC) recommends that people apply hand moisturizer and/or ointment immediately after washing or sanitizing hands to keep the hand skin hydrated. AAD further professes the use of hand moisturizer onto your fingertips & nails since dry, cracked skin makes it easier for bacteria and other germs to get inside the body.12
FIGURE 3.
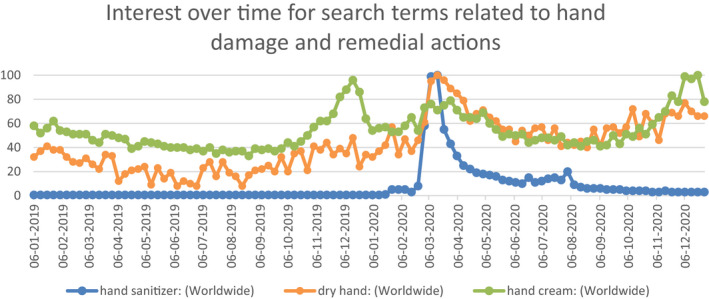
Google Trends worldwide search volumes for terms “dry hand” and “hand cream,” as extracted on January 4, 2021. Search term “hand sanitizer” is kept for reference
1.3. Alcohol‐induced skin damage
The ability of alcohols to denature proteins, impact enzyme activities, kill epithelial cells, impair barrier integrity, etc., has been reported, and their irritancy in this respect depends on alcohol chain length and hydrocarbon content together with their octanol‐water partition coefficients.13 Various scientific studies have explored the impact of alcohols on skin ranging from microbial count methods to cell‐based assays13, 14, 15 to physicochemical investigations16, 17 to dermatologist forward assessments.18, 19 The skin‐related parameters and impact of alcohol on the same are summarized in Table 1. Skin tolerability decreases with increase in alcohol concentration and increase in alcohol chain length. Repeated usage of ABHS is expected to have impact on skin texture and palmar pain.20 This has been known to result from Barrier Integrity loss as well as imbalance in microbial population. Usage of hand sanitizers incorporated with glycerin, monolaurin, etc., is known to provide positive shift in the skin health markers.17, 20
TABLE 1.
Alcohol Impact on Skin Parameters compiled from various literature studies9, 13, 17, 20, 40 under different clusters
| SC Microbiome | Keratinocytes expression | Physicochemical changes | Qualitative in‐vivo | Quantitative in‐vivo |
|---|---|---|---|---|
|
|
|
|
|
|
Denaturation of
|
|
|
|
|
Abbreviations: EtOH, Ethanol; IC50, Half‐maximal inhibitory concentration; IFN‐γ, Interferon gamma; IL, Interleukin; IPA, Iso‐propyl alcohol; NMF, Natural Moisturizing Factor; n‐PA, n‐propyl alcohol; SC, Stratum Corneum; TEWL, Trans‐Epidermal Water Loss; TGF‐α, Transforming growth factor‐alpha.
1.4. Coconut oil as skin therapeutic
Topical application of coconut oil is known to improve skin barrier as well as serve as anti‐microbial shield with added benefits in terms of moisturization increase, collagen boost, and anti‐aging. Various studies have been reported in literature regarding the topical coconut oil application for management of skin conditions like Xerosis (Dry Skin) and Atopic Dermatitis (eczema).21, 22, 23, 24 Table 2 summarizes scientific studies demonstrating therapeutic, skin barrier improvement benefits of topical coconut oil application.
TABLE 2.
In‐vivo studies with topical coconut oil application for improvement in skin barrier
| Study | Aim | Study design | Patients, n | Dose | Findings |
|---|---|---|---|---|---|
| Escuadro‐Chin et al (2019)21 | Determine the efficacy and safety of virgin coconut oil compared to mineral oil for the treatment of senile xerosis | Assessor‐blinded, randomized controlled trial | 148 participants (59males,89females) | Participants were instructed to apply the test oil twice daily to the legs for 2 weeks |
43% in the VCO group had no visible signs of leg xerosis versus 22.4% in the mineral oil group. 74% in VCO group have >1 point decrease in Overall Dry Skin Score (ODSS) versus 34% in the mineral oil group VCO showed significantly greater skin hydration, skin lipid content and quality of life score VCO group showed 32.1% (26/81) treatment success compared to 8.9% (6/67) in the mineral oil group |
| Nangia et al (2008)22 | Assess efficacy of topical virgin coconut oil in reduction of TEWL in very‐low‐birthweight neonates | Randomized trial | 74 preterm very‐low birth weight neonates | 7‐d treatment: application of 4 ml of coconut oil to whole body twice daily; control, no treatment |
Significantly lower TEWL in coconut oil group than controls Topical VCNO use in increases plasma levels of monolaurin and decreases incidence of late‐onset sepsis (blood infection) |
| Evangelista et al (2014)23 | Compare effect of topical virgin coconut oil with that of mineral oil in patients with atopic dermatitis | Assessor‐blinded, randomized controlled trial | 117 children (aged 1–13 years) | 8‐week treatment: application of 5 ml of coconut or mineral oil to all body surfaces twice daily |
Significantly lower SCORAD index in coconut oil than mineral oil group. Greater decrease in TEWL and increase in skin capacitance in coconut oil than mineral oil group |
| Verallo‐Rowell et al (2008)24 | Compare effects of topical virgin coconut oil with those of topical virgin olive oil on Staphylococcus aureus colonization and atopic dermatitis parameters | Randomized controlled trial | 52 adults | 4‐week treatment: application of 5 ml coconut or olive oil to affected areas twice daily | Significantly lower Staphylococcus aureus colonization and SCORAD index in VCO than olive oil group |
In the present study, we evaluated virgin coconut oil as a prophylactic against the hand skin damage due to excessive exposure of alcohol in the form of increased ABHS usage. We employed both instrumental and in‐home usage studies to evaluate various parameters of interest.
2. MATERIALS AND METHODS
2.1. Materials
70% Ethanol in water solution was prepared using laboratory grade 99% pure ethanol and HPLC analytical grade water. ABHS was prepared in laboratory wherein the alcohol content was adjusted to 70% v/v along with polymers for rheology modification (viscosity __ cP) and propylene glycol as emollient. Safety and efficacy tests were done with the ABHS and found to be as per the standards available. Commercially, available VCO under the brand name of Coco SoulTM was procured and used for the studies. Standard D‐Squame® tape (2.2 cm in diameter, 3.8 cm2) was obtained from CuDerm Corporation (Dallas, TX, USA). Micro BCA™ Protein Assay Kit (Thermo Scientific™ make) and Tumour Necrosis Factor alpha ELISA Kit‐RAB0476 (Sigma Aldrich make) were used for protein and cytokine markers quantification. Cytation™ 3 Cell Imaging Multi‐Mode Reader (BioTek® Instruments, Inc.) was used to read the solution absorbance in quantifying protein content. TEWL was measured using a Tewameter® TM 300 (Courage & Khazaka GmbH, Cologne, Germany). Skin Moisture Content was measured using a Corneometer® CM 825 (Courage & Khazaka GmbH, Cologne, Germany). Infrared Spectroscopy measurements were done using the Alpha Attenuated Total Reflectance Infrared (ATR‐IR) Spectrophotometer with ZnSe crystal (Bruker, Germany).
2.2. Volunteer recruitment
Experimentation was conducted in three phases and the recruitment was done accordingly:
Phase I: Tape Stripping for biomarkers analysis and Skin Parameteric Probes Testing was done on 12 healthy volunteers (5females,7males) selected in the age group of 18–60 years.
Phase II: In‐home usage study (15‐dayinterventionperiod) was done with 60 volunteers (43% males, 57% females; All Asian) aged 18–60 years. The protocol was approved by the expert dermatologist and carried out under their supervision from October 2020 to November 2020. All 30 subjects in control group completed the study; while only 28 subjects in VCO‐treated group completed the study duration (2dropouts)
Phase III: A mechanistic study was done using ATR‐IR probe on 5 volunteers (3 males, 2 females; All Asian) aged 20–45 years with (10‐dayinterventionperiod)
Informed consent was obtained, and a participant information leaflet was supplied to all the volunteers prior to the studies. None of the volunteers had any history of skin disease. All the volunteers were asked, apart from daily washing, not to apply any moisturizer or cosmetic product to the study treatment sites 15 days prior to and during the study.
2.3. Tape stripping and probe measurements
Mid‐volar forearms were delineated to provide three well‐separated circular sites (3.14 cm2 area each) on each arm (total 6 sites). The sites were cleaned with distilled water before equilibrating the subjects under constant temperature (25°C) and humidity (60% RH) conditions for 30 min. Intra‐subject differences were minimized by balancing and randomizing application across forearms. VCO as test formulation and distilled water as placebo were applied at a dose of 0.1 ml/cm2 over the sites on either forearm (selected randomly) using a needleless syringe. Gentle massaging and enough residence time (~30 min) are provided to ensure the test and placebo formulations are absorbed into the sites. The three marked sites were administered with (1) Distilled Water (Placebo)—1× application, (2) 70% Ethanol Solution—1× application, and (3) 70% Ethanol Solution—6× applications each interspaced by 1 h (each application of 0.1 ml/cm2 dose). Tewameter and Corneometer probes were used in accordance with the instrumental protocols as described elsewhere15 on each of the three sites to measure the TEWL and Moisture Content, respectively. Standard D‐Squame® tape was applied to the forearm sites at a constant pressure as described elsewhere.25 Six consecutive tape strippings were performed for all the selected areas at the center of application sites and stored in Eppendorf tubes for further processing. The study design is captured in Figure 4 for visual reference.
FIGURE 4.
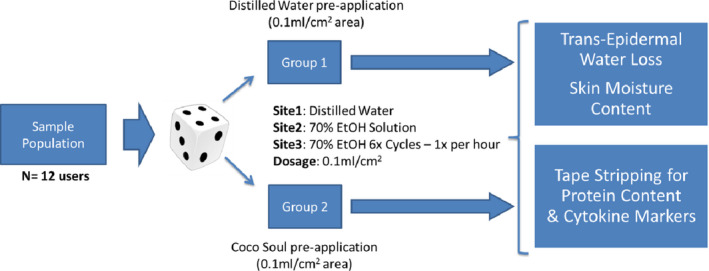
Phase I Study Design Schematic
2.4. Protein content and inflammation assay
The tape strips were solubilized in 1 ml of phosphate‐buffered saline (PBS) solution and sonicated on an ice bath for 10 min to undergo cell lysis. The sonicated solution was centrifuged at 5600 g for 10 min at 4°C, and samples from the cell supernatant were used for protein content analysis and detection of the pro‐inflammatory cytokine.
The protein content was quantified using colorimetric method with Micro BCA™ Protein Assay Kit and compared with standard bovine serum albumin (BSA). The reagents were prepared using manufacturer's protocol and microplate procedure was followed from the instruction manual. Briefly, in a 96 well plate, 150 µl of sample or BSA standard was reacted with 150 µl of working reagent containing bicinchoninic acid (BCA) and Cu+2 Ions and incubated for 2 h at 37°C. Absorbance of the solution post‐incubation was taken at 562 nm using Cytation™ 3 Cell Imaging Multi‐Mode Reader for samples and standard. The standard curve obtained from various dilutions of BSA was used to determine the concentration of protein in µg/ml.
Inflammation marker TNF‐α was measured using Human Tumour Necrosis Factor alpha ELISA Kit‐RAB0476 following manufacturer's instructions. The value of TNF‐α present was reported in picograms/ml of sample solution.
2.5. In‐home usage study
The home‐use study was a randomized, controlled trial wherein all the subjects were randomly assigned (as per computer‐generated randomization list) to either one of the two treatment arms in 1:1 ratio. Control group (Group I) was provided with the ABHS formulated (70% v/v Ethanol) and recommended to apply 6 times per day on palms as a regular hand hygiene product. Test Group (Group II) used VCO (4‐8drops) on their hands and palms before going to bed in addition to the ABHS usage 6 times per day. Study duration was 15 days, and assessments were done on Day 0 and Day 15. Self‐assessment was done by volunteers using hand skin self‐assessment tool (prescribed by World Health Organization26;) pre‐ and post‐intervention. Questionnaire‐based assessment for moisturization, hand feel, and safety was done using a curated set of questions for VCO group, in consultation with the dermatologist expert. Phase II Study schematic (refer Figure 5) summarizes the design of the in‐home usage study.
FIGURE 5.
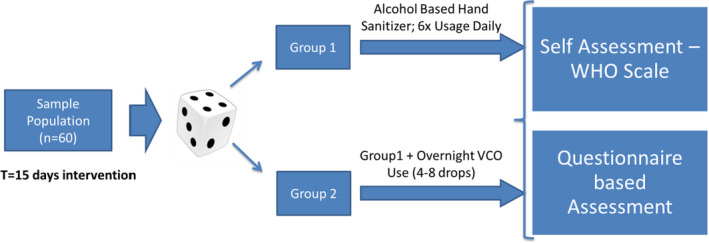
Phase II In‐Home Usage Study Design Schematic
2.6. Infrared spectroscopy measurements
The lower portion of the palm is identified as the region for non‐invasive investigation using the Fourier Transform IR spectrophotometer equipped with an ATR accessory. The delineated area was cleaned with distilled water and cotton swab before equilibrating the subjects under constant temperature (25°C) and humidity (60% RH) conditions for 30 min. At the end of calibration time, the IR reference scan was taken by placing the palm region adjacent to the ZnSe crystal for 2 min. The IR spectra recorded were the average of 50 scans, which required a data collection over the period of 2 min. Post that, ABHS was applied on the lower portion of the palm in generous amount (1 ml/cm2) and the region is wrapped with a cotton gauge & tape. At the end of 15 min of closed contact and 5 min of calibration time, the IR reference scan is taken again to record the post‐exposure spectrum. After noise correction and normalization, the area under the curve for peaks identified for different components and compared for difference between reference & post‐exposure spectra. The volunteers were provided with VCO samples and recommended to apply 4–8 drops on their hands and palms before going to bed for 15 days. The IR scans of pre‐ and post‐exposure were taken at Day 0, Day 1, and Day 15.
2.7. Statistical analysis
Minitab® version 18 and Microsoft® Excel Office 2007 were used to analyze the data. Normality was assessed using the Kolmogorov‐Smirnov statistical test. Parametric statistical tests (one‐way between‐group ANOVA and paired t test to compare means) were used to investigate statistical differences between treated and untreated sites as well as control and test groups for in‐home usage study. A probability of p < 0.05 was considered statistically significant. All results are presented as mean and standard error of the mean.
3. RESULTS
3.1. TEWL measurement
The epidermal water loss values for initial and post‐intervention for the three sites (1× Distilled Water, 1× 70% Ethanol Solution, and 6× 70% Ethanol Solution) are shown in Figure 6. As expected, TEWL values increase significantly with ethanol treatment in control scenario (no pre‐treatment). With VCO pre‐treatment, the change in TEWL values is insignificant even with 6 applications of ethanol solution. Percent change in TEWL values from initial readings is shown in Figure 7.
FIGURE 6.
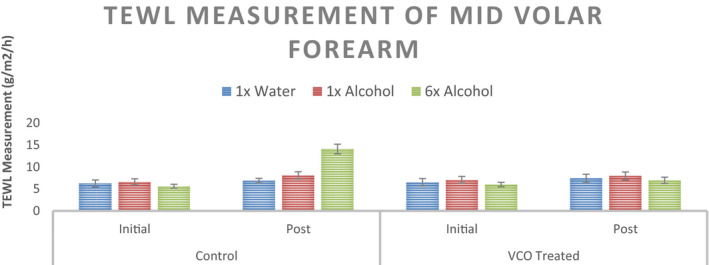
TEWL measurement of the mid volar forearm at three sites with different stimuli for the untreated control and VCO pre‐treatment (n = 12, mean ± SE)
FIGURE 7.
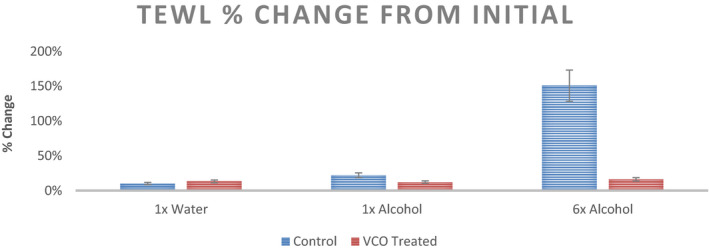
Percent change from initial readings in TEWL recorded at three sites with different stimuli for the untreated control and VCO pre‐treatment (n = 12, mean ± SE)
3.2. Corneometer measurement
Moisture content for initial and post‐intervention for the three sites (1× Distilled Water, 1× 70% Ethanol Solution, and 6× 70% Ethanol Solution) are shown in Figure 8. No significant changes in the Corneometer values are observed for either untreated control or VCO pre‐treatment scenario. Percent change in Corneometer values from initial readings is shown in Figure 9.
FIGURE 8.
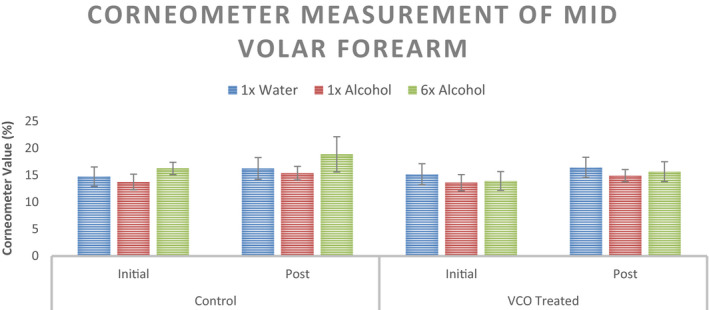
Corneometer measurement of the mid volar forearm at three sites with different stimuli for the untreated control and VCO pre‐treatment (n = 12, mean ± SE)
FIGURE 9.
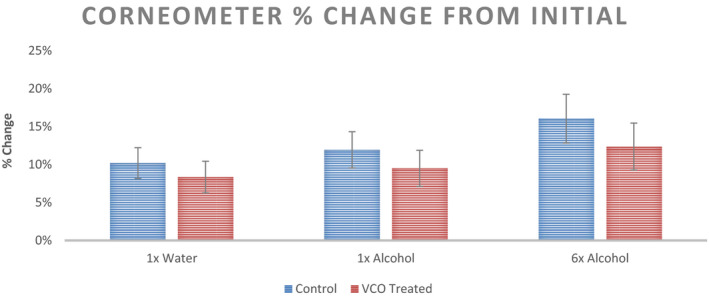
Percent change from initial readings in Corneometer values recorded at three sites with different stimuli for the untreated control and VCO pre‐treatment (n = 12, mean ± SE)
3.3. Stratum corneum protein content
Average stratum corneum (SC) protein content from 6 tape strips from the subjects in two groups—control and VCO pretreted—is captured in Figure 10. As it can be seen, untreated group observes an increase in protein content stripped out with increase in ethanol exposure of the SC. More the ethanol exposure, easier it is to strip out the protein from the SC. The differences are significantly different with 1× Ethanol cycle and, for 6× cycles. However, for VCO pre‐treatment scenario the protein content stays relatively stable (insignificant change). This suggests the SC resistance maintenance by VCO even under the ethanol onslaught.
FIGURE 10.
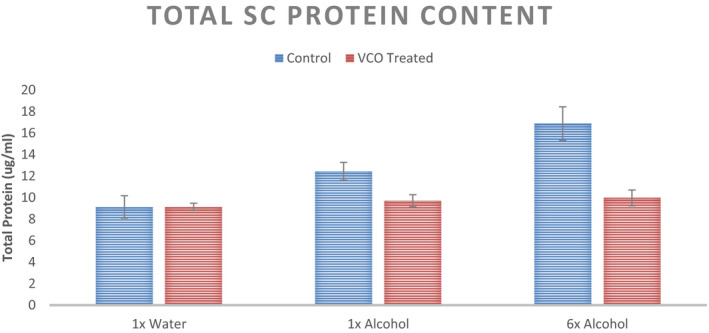
SC Protein Content from the tape strip of the mid volar forearm at three sites with different stimuli for the untreated control and VCO pre‐treatment (n = 12, mean ± SE)
3.4. Cytokine content
Inflammation marker—cytokine level—in the form of a cytokine (TNF‐α) content was measured from the tape strips from different sites. Average TNF‐α content (in Pg/ml) from 6 tape strips from the subjects in two groups—control and VCO pretreated—is captured in Figure 11. On untreated areas, the exposure to alcohol leads to an increase in the cytokine level and hence inflammation. Significant increase in inflammation marker in SC was observed for 6× cycles of ethanol solution exposure (p < 0.05). However, for VCO pretreated areas, exposure to alcohol did not result in any significant change in the cytokine content (p > 0.05).
FIGURE 11.
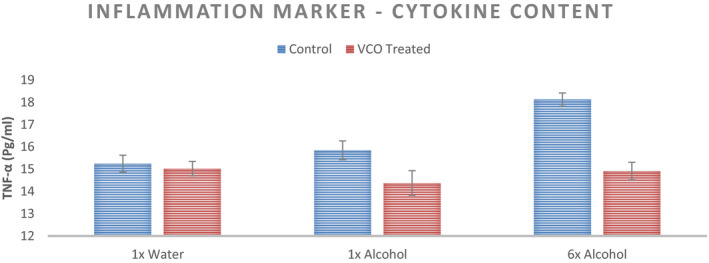
TNF‐α Content from the tape strip of the mid volar forearm at three sites with different stimuli for the untreated control and VCO pre‐treatment (n = 12, mean ± SE)
3.5. In‐home usage study
WHO Skin Assessment Scale comprises of 4 vectors—Appearance, Intactness, Moisture Content, and Sensation—rated on a 7‐point scale (1beinglowerand7indicateshealthystate). Day 0 and Day 15 ratings of the two groups—Control Group (Untreated) and VCO Overnight Treatment Group—are summarized in Figure 12. Except moisture content, the other three vectors have changed insignificantly over the duration of study (t = 15 days). For moisture content, the ratings indicate a significant negative shift toward dry hands for control group (p < 0.05) and a significant positive shift for the VCO‐treated group (p < 0.05). The percent change in ratings over the intervention period for the two groups is shown in Figure 13.
FIGURE 12.
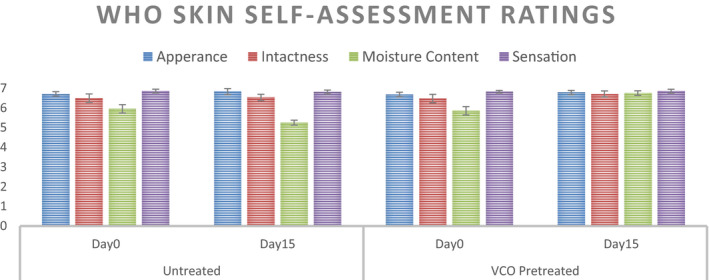
WHO Skin Self‐Assessment Scale Ratings pre‐ and post‐ABHS usage for t = 15 days for the untreated control group and VCO pre‐treatment (n = 30 for Control while n = 28 for VCO Group, mean ± SE)
FIGURE 13.
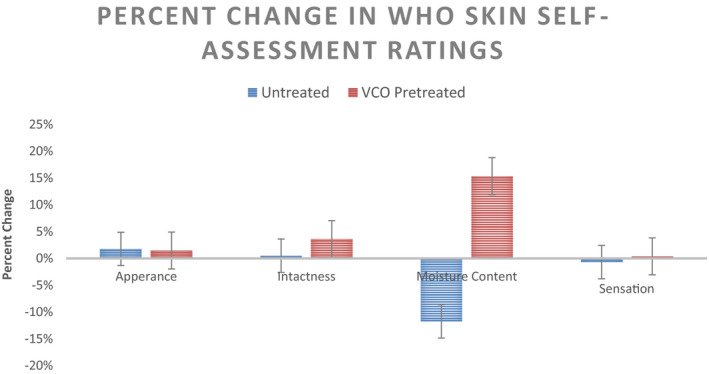
Percentage Change in WHO Skin Self‐Assessment Scale Ratings pre‐ and post‐ABHS usage for t = 15 days for the untreated control group and VCO pre‐treatment (n = 30 for Control while n = 28 for VCO Group, mean ± SE) Pre‐treatment
VCO group was also administered a hand feel perception questionnaire. The responses recorded are summarized in Figure 14. Various statements which were ratified by all 28 volunteers of the VCO group at the end of day 15 are compiled in Figure 15. In terms of protection perceived, the average hours of protection were estimated at 11 ± 1.7 h. Figure 16 captures the histogram of the perceptible protection duration in hours by VCO.
FIGURE 14.
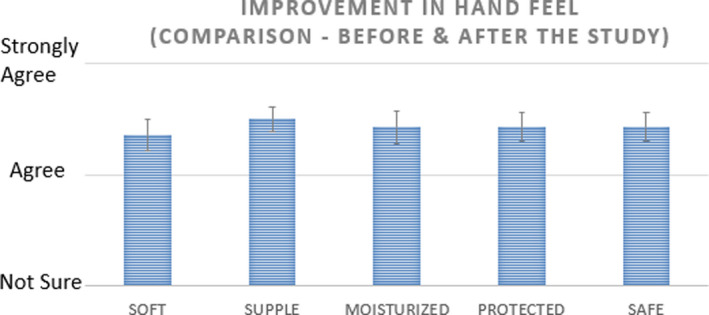
Perception‐based improvement for VCO group at the end of Day 15 for Sensorial Parameters as a part of Q’re Assessment (Improvement Rating for n = 28 subjects, mean ± SE)
FIGURE 15.

Statements agreed by VCO Group Subjects post the intervention period
FIGURE 16.
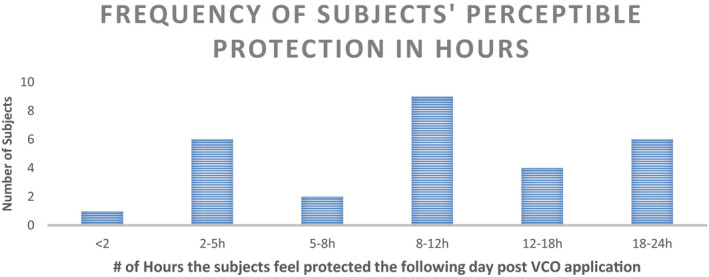
Histogram of the perceptible protection from ABHS damage in hours due to VCO pre‐treatment post the intervention period
3.6. Infrared spectroscopy measurement
IR spectra for lower palm are analyzed for integrated intensity around (1) the lipid peaks characterized with CH2, CH3 stretch, and C=O ester stretch as well as (2) the protein peak at 1540 cm−1. Average integrated intensity of the identified peaks is mentioned in Figure 17. Percentage change in the integrated intensities between pre‐ and post‐ABHS exposure spectra is captured in Figure 18. As it can be seen, the percent change is higher for the untreated state and the change reduces with progressive VCO application with a significant (p < 0.05) change in all 3 peak intensities at the end of 15 days of VCO usage.
FIGURE 17.
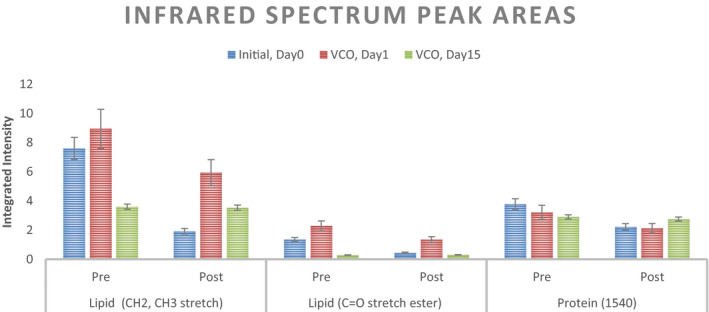
Average Integrated intensity for the peaks from the IR spectra (n = 5; mean ± SE)
FIGURE 18.
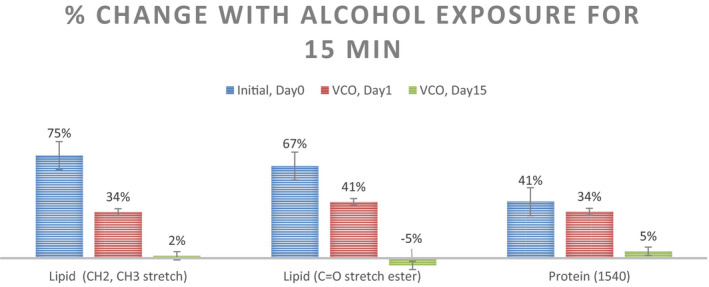
Percent change in the average Integrated intensities for the peak from the IR spectra (n = 5; mean ± SE)
4. DISCUSSION
The main barrier component of skin (located in the stratum corneum, SC) comprises multiple layers of intercellular lipids (predominantly ceramides, cholesterol, and free fatty acids) in a gel phase.27 Ethanol is known to partition into the skin and enhance the permeation of both polar and nonpolar molecules.28 Multiple studies in the literature point to the lipid extraction, and thus, membrane damage, as the mechanistic action of ethanol.9, 29, 30, 31, 32 The same got validated in the present study with a substantial increase in transepidermal water loss with repeated application of 70% ethanol solution. The change in moisture content was not observed as the SC layer is known to act as “responding membrane”33 and the body supplies more water from deeper skin layers as the concentration in SC goes down. The biological markers from tape stripping suggest a significant increase in protein content which is a marker of loosened skin integrity and a significant increase in cytokine marker which suggests higher degree of inflammation. All these instrumental measurements on the skin of human subjects re‐validates the deleterious effect of repeated alcohol exposure to the skin.
Coconut oil is known to act on the skin via predominantly three mechanisms: (1) Occlusive Layer formation34 as the reinforced first line of defense against external aggressors like water, surfactant, and alcohol; (2) Internal Occlusion of the penetrated oil—that is, coating of the skin from inside35 —help avoid the loss of moisture and adds to the resistance from external aggressors; and (3) Penetration inside the skin and strengthening the lipids bilayer which is proven as increased resistance to alter the lipid packing structure from orthorhombic to hexagonal.36 As it can be seen from the present study, the pre‐application of virgin coconut oil on skin helps (1) maintain the transepidermal water loss, (2) maintain the moisture levels, (3) keeps in check the protein content from tape stripping, and (4) manages the cytokine content at the original levels. The data obtained confirm the protective benefits of coconut oil application on skin against repetitive exposure to 70% ethanol.
In‐home usage study represents the most practical and closest to consumer assessment for any cosmetics product evaluation.37 Thus, the 15‐day in‐home usage study—with the introduction of overnight application of VCO on palms & hands amidst the regular usage of ABHS during the day—is the opportunity to test in the consumer relevant environmental settings. WHO Skin Self‐Assessment Scale26 was used as the objective metric for recording the changes at the end of 15 days intervention period. A significant reduction (−11.8%) in moisture content was observed in the group with only the ABHS application while a significant positive shift (+15.4%) was recorded for the group with overnight VCO application. For appearance, intactness, and sensation, the shift was not significant. Further, the self‐assessment ratings for subjective hand feel parameters—like soft, supple, moisturized, protection, and safety—also exhibited a positive shift for all the subjects using VCO as overnight treatment. VCO application, as seen from the instrumental studies, helped reinforce the skin barrier against the harmful effects of ethanol penetration and thus, maintained skin barrier integrity both from inside and outside. The protective benefits of VCO regular application were confirmed in the statements ratified by the consumers which refers to VCO usage as remedy for dry hands, deep care, deep nourishment, overnight repair, overnight pampering of hands, protection for next day sanitization, etc. Perceptive assessment of the duration of protection was also included in the questionnaire. The subjects rated the VCO protection duration ranging from 2 to 24 h with an average protection of around 11 h.
Coconut oil skin protection mechanism of penetrating the stratum corneum and strengthening the skin lipids was also investigated in vivo using FTIR, inspired by similar studies in the past on the effect of ethanol on SC.38, 39 An exaggerated protocol of applying ethanol on the lower portion of palm and keeping it wrapped with cotton gauge for 15 min was administered to cause exaggerated damage to SC in terms of leaching out of lipids by alcohol. The average percent change in intensities of lipid peaks and protein peaks was substantial at Day 0 signaling the damaging effect of alcohol on untreated skin. Day 1 of the VCO treatment regime shows benefits by a significant reduction in the percent change for lipid peak intensities but no significant reduction is observed for protein peak. On day 15 of the VCO application, the protective benefits were seen to the best extent and no change in the lipid and protein peaks was observed before and after alcohol exposure. Thus, it establishes the progressive, reparative barrier strengthening of SC by the regular usage of coconut oil.
As 70% ethanol is known to take away the cellular lipids (particularly free fatty acids and ceramides) loosening the SC barrier, the regular usage of overnight coconut oil helps in barrier strengthening by penetration of VCO triglycerides in the SC layers and stabilization of the lipid structure. The exact contribution of the mechanistic pathways—internal and exclusion effect as well as the lipid strengthening aspects of VCO—in the improved skin protection is still to be ascertained.
5. CONCLUSION
Alcohol exposure on the skin leading to weakened skin barrier is known in the state‐of‐art. The mechanism of alcohol extracting the skin lipids and hence, weakening the lipid bilayers structure is known in detail. Present work provides evidence of alcohol compromising skin barrier integrity in terms of instrumental in vivo studies as well as in‐home usage studies to replicate the consumer assessment scenario. The current study also establishes the protective benefit of regular coconut oil application regime against the increased exposure of skin to ABHS during these COVID times. The work provides in vivo proof of the skin lipid strengthening aspects of regular VCO topical application. Based on the findings, a regimen of overnight VCO application on hands as a natural prophylactic against the increased frequency of ABHS usage is recommended.
CONFLICT OF INTEREST
Dr. Punit Saraogi received a study grant from Marico Limited for the consumer study described in this publication. All the other authors worked full‐time with Marico Limited covering the duration of the study described in this publication.
AUTHOR CONTRIBUTIONS
V.K., V.G., P.S., and S.M. conceived and planned the experiments. R.C. and S.C. carried out the laboratory studies with instruments. P.S. planned and supervised the in‐home usage study. R.C. contributed to the sample preparation. V.K., V.G., and P.S. contributed to the interpretation of the results. V.K. took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
ETHICAL APPROVAL
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed written consent was obtained from all the subjects for being included in the study.
ACKNOWLEDGEMENT
This work is supported and sponsored by the Marico Ltd. The work is carried out partly at the Marico Ltd R&D Centre at Mumbai, India; and at Everything Skin & Hair, Medical & Aesthetic Dermatology clinic, Mumbai India.
Saraogi P, Kaushik V, Chogale R, Chavan S, Gode V, Mhaskar S. Virgin coconut oil as prophylactic therapy against alcohol damage on skin in COVID times. J Cosmet Dermatol. 2021;20:2396–2408. 10.1111/jocd.14258
Funding information
The work described in this publication was supported by Marico Limited
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Madhav N, Oppenheim B, Gallivan M, Mulembakani P, Rubin E, Wolfe N. Chapter 17: Pandemics: Risks, Impacts, and Mitigation. In Jamison DT, Gelband H, Horton S. eds. Disease Control Priorities: Improving Health and Reducing Poverty. 3rd ed, Washington (DC): World Bank Publications; 2017:315‐345. [Google Scholar]
- 2.Polak SB, van Gool IC , Cohen D, Jan H, van Paassen J . A systematic review of pathological findings in COVID‐19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33(11):2128‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halliburton BC. 2020. COVID‐19 is a Black Swan. https://www.forbes.com/sites/forbesbooksauthors/2020/03/19/covid‐19‐is‐a‐black‐swan/?sh=401b4d617b4b. Accessed December 12, 2020.
- 4.World Health Organization . 14, Skin reactions related to hand hygiene. In WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care. Geneva, Switzerland: World Health Organization;2009. Available from: ttps://www.ncbi.nlm.nih.gov/books/NBK144008/. [PubMed] [Google Scholar]
- 5.US Food and Drug Administration . Q&A for Consumers | Hand Sanitizers and COVID‐19. www.fda.gov/drugs/information‐drug‐class/qa‐consumers‐hand‐sanitizers‐and‐covid‐1.
- 6.Choi H, Varian H. Predicting the present with Google Trends. Econ Rec. 2012;88:2‐9. [Google Scholar]
- 7.Solis E. Mintel global new products database (GNPD). J Bus Finance Librarian. 2016;21(1):79‐82. [Google Scholar]
- 8.Huddleston T, [J.]. 2020. The history of hand sanitizer—how the coronavirus staple went from mechanic shops to consumer shelves. www.cnbc.com/2020/03/27/coronavirus‐the‐history‐of‐hand‐sanitizer‐and‐why‐its‐important.htm.
- 9.Löffler H, Kampf G, Schmermund D, Maibach HI. How irritant is alcohol? Br J Dermatol. 2007;157(1):74‐81. [DOI] [PubMed] [Google Scholar]
- 10.Arora N. 2020. Hand sanitiser in Covid‐19 pandemic: How much is too much? www.hindustantimes.com/coronavirus‐crisis/hand‐sanitiser‐in‐covid‐19‐pandemic‐how‐much‐is‐too‐much/story‐ptqGLC12Ck7CV54mxvvheL.htm.
- 11.Rajani T. 2020. Attitudes towards home & personal hygiene ‐ Indian consumer ‐ 2020.
- 12.American Academy of Dermatology . Dry skin relief from COVID‐19 handwashing. www.aad.org/public/everyday‐care/skin‐care‐basics/dry/coronavirus‐handwashin.
- 13.Cartner T, Brand N, Tian K, et al. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A2 together with epidermal keratinocytes and skin irritation. Int J Cosmet Sci. 2017;39(2):188‐196. [DOI] [PubMed] [Google Scholar]
- 14.Mansour GN, El‐rafey DS. Ethyl glucuronide, ethyl sulfate and acetone as biomarkers for alcohol based hand sanitizers chronic exposure in health care workers. Ain‐Shams J Forensic Med Clin Toxicol. 2019;33(2):80‐91. [Google Scholar]
- 15.Maia Campos PM, Gonçalves GMS, Gaspar LR. In vitro antioxidant activity and in vivo efficacy of topical formulations containing vitamin C and its derivatives studied by non‐invasive methods. Skin Res Technol. 2008;14(3):376‐380. [DOI] [PubMed] [Google Scholar]
- 16.Tarka P, Gutkowska K, Nitsch‐Osuch A. Assessment of tolerability and acceptability of an alcohol‐based hand rub according to a WHO protocol and using apparatus tests. Antimicrob Resist Infect Control. 2019;8:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houben E, de Paepe K , Rogiers V. Skin condition associated with intensive use of alcoholic gels for hand disinfection: a combination of biophysical and sensorial data. Contact Dermatitis. 2006;54(5):261‐267. [DOI] [PubMed] [Google Scholar]
- 18.Kramer A, Bernig T, Kampf G. Clinical double‐blind trial on the dermal tolerance and user acceptability of six alcohol‐based hand disinfectants for hygienic hand disinfection. J Hosp Infect. 2002;51(2):114‐120. [DOI] [PubMed] [Google Scholar]
- 19.Ghazali DA, Thomas J, Deilhes E, et al. Design and validation of an anatomically based assessment scale for handwashing with alcohol‐based hand rub. Infect Control Hosp Epidemiol. 2018;39(8):1000‐1002. [DOI] [PubMed] [Google Scholar]
- 20.Abraham ERL, Verallo‐Rowell VM, Baello BQ. Testing of Lauricidin versus isopropyl alcohol for antisepsis of cutaneous hand microbes to prevent infection. Philipp J Microbiol Infect Dis. 2000;29:128‐135. [Google Scholar]
- 21.Escuadro‐Chin MO, Maaño MMC, Dofitas BL. Randomized assessor‐blinded controlled trial on the efficacy and safety of virgin coconut oil versus mineral oil as a therapeutic moisturizer for senile xerosis. Acta Medica Philippina. 2019;53(4):336. [Google Scholar]
- 22.Nangia S, Paul V, Chawla D, Deorari A. Topical coconut oil application reduces transepidermal water loss in preterm very low birth weight neonates: a randomized clinical trial. Pediatrics. 2008;121(Supplement 2):S139.2‐S139. [Google Scholar]
- 23.Evangelista MTP, Abad‐Casintahan F, Lopez‐Villafuerte L. The effect of topical virgin coconut oil on SCORAD index, transepidermal water loss, and skin capacitance in mild to moderate pediatric atopic dermatitis: a randomized, double‐blind, clinical trial. Int J Dermatol. 2014;53(1):100‐108. [DOI] [PubMed] [Google Scholar]
- 24.Verallo‐Rowell VM, Dillague KM, Syah‐Tjundawan BS. Novel antibacterial and emollient effects of coconut and virgin olive oils in adult atopic dermatitis. Dermatitis. 2008;19(6):308‐315. [PubMed] [Google Scholar]
- 25.Mohammed D, Matts PJ, Hadgraft J, Lane ME. Depth profiling of stratum corneum biophysical and molecular properties. Br J Dermatol. 2011;164(5):957‐965. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization . 2009. WHO guidelines on hand hygiene in health care: first global patient safety challenge clean care is safer care. World Health Organization, Geneva. [PubMed]
- 27.Proksch E, Brandner JM, Jensen J‐M. The skin: an indispensable barrier. Exp Dermatol. 2008;17(12):1063‐1072. [DOI] [PubMed] [Google Scholar]
- 28.Goates CY, Knutson K. Enhanced permeation of polar compounds through human epidermis. I. Permeability and membrane structural changes in the presence of short chain alcohols. Biochim Biophys Acta. 1994;1195:169‐179. [DOI] [PubMed] [Google Scholar]
- 29.Farkas A, Kemény L, Széll M, Dobozy A, Bata‐Csörgo Z. Ethanol and acetone stimulate the proliferation of HaCaT keratinocytes: the possible role of alcohol in exacerbating psoriasis. Arch Dermatol Res. 2003;295(2):56‐62. [DOI] [PubMed] [Google Scholar]
- 30.Ingólfsson HI, Andersen OS. Alcohol's effects on lipid bilayer properties. Biophys J. 2011;101(4):847‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patra M, Salonen E, Terama E, et al. Under the influence of alcohol: the effect of ethanol and methanol on lipid bilayers. Biophys J. 2006;90(4):1121‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thind R, O'Neill DW, Del Regno A, Notman R. Ethanol induces the formation of water‐permeable defects in model bilayers of skin lipids. Chem Commun (Camb). 2015;51(25):5406‐5409. [DOI] [PubMed] [Google Scholar]
- 33.Sparr E, Millecamps D, Isoir M, Burnier V, Larsson Å, Cabane B. Controlling the hydration of the skin though the application of occluding barrier creams. J R Soc Interface. 2012;10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzu‐Kai L, Zhong L, Santiago JL. Anti‐inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2018;19:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamatas GN, de Sterke J , Hauser M, von Stetten O , van der Pol A . Lipid uptake and skin occlusion following topical application of oils on adult and infant skin. J Dermatol Sci. 2008;50(2):135‐142. [DOI] [PubMed] [Google Scholar]
- 36.Mainkar A, Mhaskar S, Agarwal N, Mendelsohn R, Ibrahim M, Wiechers J. Stabilization of the orthorhombic phase of stratum corneum lipids by coconut oil leads to a clinical benefit. Int J Cosmet Sci. 2009;31(4):39. [Google Scholar]
- 37.Bloomfield SF, Aiello AE, Cookson B, O'Boyle C, Larson EL. The effectiveness of hand hygiene procedures in reducing the risks of infections in home and community settings including handwashing and alcohol‐based hand sanitizers. Am J Infect Control. 2007;35(10):S27‐S64. [Google Scholar]
- 38.Bommannan D, Potts R, Guy RH. Examination of the effect of ethanol on human stratum corneum in vivo using infrared spectroscopy. J Control Release. 1991;16:299‐304. [Google Scholar]
- 39.Kwak S, Brief E, Langlais D, Kitson N, Lafleur M, Thewalt J. Ethanol perturbs lipid organization in models of stratum corneum membranes: An investigation combining differential scanning calorimetry, infrared and (2)H NMR spectroscopy. Biochim Biophys Acta. 2012;1818(5):1410‐1419. [DOI] [PubMed] [Google Scholar]
- 40.Soltanipoor M, Stilla T, Riethmüller C, et al. Specific barrier response profiles after experimentally induced skin irritation in vivo. Contact Dermatitis. 2018;79(2):59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


