Abstract
Whether people living with HIV (PLWH) are at greater risk of acquiring SARS‐CoV‐2 infection is currently unknown. Prospective serologic studies may allow seroincidence analyses, where all infections are accurately identified. Because of this, we evaluated the incidence of associated factors with and the clinical outcome of SARS‐CoV‐2 infection in PLWH in Southern Spain.
This prospective cohort study included PLWH from a Tertiary University Hospital in Southern Spain. Patients were enrolled in the study if (1) they had attended as outpatients our Unit from 1 August 2019 to 8 February 2020 and (2) had two subsequent evaluations from 9 February 2020 to 4 March 2021. SARS‐CoV‐2 infections were diagnosed by PCR, antigen detection or serology.
Seven hundred and nine PLWH were included in the study. Of them, 55 [7.8%, 95% confidence interval (95% CI) 5.9%–9.9%] patients developed SARS‐CoV‐2 infection. Between 18 May and 29 November 2020, the rate of seroconversion was 5.3% (95% CI: 3.1%–9.0%) for the general population in the area of Seville and 2.3% (95% CI: 1.3%–2.6%) for PLWH in this study (p = .001). After multivariable analysis, adjusted by age, sex, and risk factors for HIV infection, active tobacco use and CDC stage, active tobacco smoking was the only factor independently associated with lower risk of SARS‐Cov‐2 infection [Incidence rate ratio: 0.29 (95% CI 0.16–0.55) p < .001].
In conclusion, the incidence of SARS‐CoV‐2 infection among PLWH in Southern Spain during the ongoing pandemic was lower than that reported for the general population in the same area.
Keywords: COVID‐19, HIV, incidence, SARS‐CoV‐2 infection, serum antibodies
1. INTRODUCTION
SARS‐CoV‐2 infection pandemic has extensively involved Spain, with over 3.2 million confirmed cases by March 2021 after three waves. The first reported case in the country was diagnosed on9 February 2020 (Ministerio de Sanidad, 2020). COVID‐19 cases have been observed among people living with HIV (PLWH) in many countries across the world. Several studies suggested that the risk of SARS‐CoV‐2 infection for PLWH is similar to that of general population (Boulle et al., 2020; Braunstein et al., 2020; Del Amo et al., 2020). However, the evaluation of the incidence of SARS‐CoV‐2 infection in PLWH have been based on clinical reports. As in some cases SARS‐CoV‐2 infections are asymptomatic (Pollán et al., 2020) and diagnosis was limited by test shortage and health care system capacity surpassing, the incidence of SARS‐CoV‐2 infection among PLWH could have been underestimated. Thus, whether PLWH are at different risk of acquiring SARS‐CoV‐2 infection or not is currently unknown (Office of AIDS Research Advisory Council, 2021).
Similarly, the impact of HIV coinfection on the outcome of SARS‐CoV‐2 infection has not been clearly defined. PLWH, due to premature ageing and comorbidities (Ekong et al., 2020), could be at a higher risk of poorer COVID‐19 outcomes. In fact, after standardization to the age and sex distribution of Spain, the risk of death from COVID‐19 was slightly higher among PLWH than for the Spanish general population during the same period (Del Amo et al., 2020). In this same regard, HIV infection was associated with approximately a twofold increase in COVID‐19 mortality in a study from South Africa (Boulle et al., 2020). In these two studies, the use of tenofovir diphosphate (TDF) plus emtricitabine (FTC) or lamivudine was associated with a lower risk of severe COVID‐19 (Boulle et al., 2020; Del Amo et al., 2020). On the contrary, propensity‐matched analyses revealed no difference in COVID‐19 outcomes for PLWH, showing that higher mortality is driven by higher burden of comorbidities than in the general population (Hadi et al., 2020). More importantly, within the same cohort of veterans, HIV infection was not a factor related with outcomes (Park LS et al., 2020).
As with COVID‐19 incidence, the outcome data of SARS‐CoV‐2 infection among PLWH are based on reported clinical cases. This is a strong limitation for all the aforementioned studies, as milder cases, as well as those in which PCR for SARS‐CoV‐2 was not carried out, could have gone unnoticed. Serologic studies in which the diagnosis of SARS‐CoV‐2 infection is based on the detection of serum antibodies allow prospective seroincidence analyses, where all infections are accurately identified. In this manner, the above‐mentioned limitations may be overcome. Because of this, we undertook this study, with the purpose of providing insight on the actual incidence, associated factors and clinical outcome of SARS‐CoV‐2 infection in PLWH in Southern Spain.
2. METHODS
2.1. Design and study patients
This is a study conducted in a cohort of prospectively followed PLWH at the Unit of Infectious Diseases of a university hospital in Seville, Southern Spain. All patients from this cohort are seen at least every six months. At each visit, all patients undergo clinical evaluation and blood drawing for routine testing, as well as sample storage for future determinations. After February 2020, all patients were specifically questioned on respiratory or general symptoms suggesting COVID‐19 since the former visit. Serum samples from all patients were cryopreserved at −80°C at each visit. Patients enrolled in that cohort were included in the present study if they fulfilled the following criteria: (1) they had attended the outpatient clinic of our Unit from 1 August 2019 to 8 February 2020 as the day before the first case officially diagnosed in Spain and (2) had two subsequent evaluations from 9 February 2020 to 4 March 2021.
Patients were managed according to national protocols for suspected SARS‐CoV‐2 infection care in force (Ministerio de Sanidad, 2020, 2021). These protocols are common throughout Spain for the entire population. In accordance with them, antigen or RT‐PCR tests were performed among all individuals with symptoms suggestive of COVID‐19. Suggestive symptoms were acute respiratory infection of any severity that included, but it is not limited to, fever, cough or feeling of shortness of breath. Other symptoms such odynophagia, anosmia, ageusia, muscle pain, diarrhoea, thoracic pain or headache, among others were also considered suggestive symptoms of infection by SARS‐CoV‐2. The choice of test, SARS‐CoV‐2 Ag or RT‐PCR, depended on availability and the days of evolution of the symptoms. Additionally, close contacts of a positive case were identified and PCR tests were performed.
According to data available from the Spanish National Center of Epidemiology (www.cnecovid.isciii.es), the period of study was divided into three periods of time: Baseline or pre‐pandemic period, from 1 August 2019 to 8 February 2020; first pandemic period, corresponding to the first wave in Spain, from 9 February 2020 to 31 May 2020; second pandemic period, corresponding to the second and third pandemic waves in Spain, from 1 June 2020 to 4 March 2021.
2.2. Source of data on general population
For comparisons, data coming from the ENE‐COVID survey, a nationwide population‐based study to investigate seropositivity for SARS‐CoV‐2 in the non‐institutionalized Spanish population was used (Pollán et al., 2020). The study design in this survey includes successive follow‐up waves of data collection from random samples of the Spanish population. A first phase with three waves of data collection, from 27 April to 22 June 22 2020, and a second phase with one wave of data collection, from 16 November to 29 November 2020, have been completed (https://portalcne.isciii.es/enecovid19/). Data on the incidence of SARS‐CoV‐2 in the general population living in the province of Seville was gathered from the ENE‐COVID study reports (https://portalcne.isciii.es/enecovid19/informes/informe_cuarta_ronda_01.pdf). Detailed seroconversion rates and demographic data are provided as Supporting data.
2.3. SARS‐CoV‐2 infection diagnosis
A diagnosis of SARS‐CoV‐2 infection was established if, at least, one of the following criteria were met: (1) SARS‐CoV‐2 RNA or antigen was detected in nasopharynx exudate by PCR or immunochromatography, respectively and (2) seroconversion for SARS‐CoV‐2 antibodies was documented between the baseline (pre‐pandemic) and the follow‐up (intra‐pandemic) samples.
2.4. Classification of COVID‐19 severity
Severe COVID‐19 was defined as cases showing at least one of the following data: (i) dyspnoea, (ii) a respiratory rate of 30 or more breaths per minute, (iii) a blood oxygen saturation of 93% or less, (iv) a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (Pao2:Fio2) of less than 300 mm Hg or (v) infiltrates in more than 50% of the lung field (Wu & McGoogan, 2020).
2.5. Laboratory procedures
All serum samples were tested for serum SARS‐CoV‐2 antibodies by electro‐chemiluminescence immunoassay (ECLIA), which detects total serum antibodies (including IgG) against this agent (ELECSYST Anti‐SARS‐CoV‐2; Roche Diagnostic International). The reported sensitivity of this test 14 days after infection is 99.5% and the specificity for routine diagnostic is 99.8%. PCR analyses for SARS‐CoV‐2 RNA detection in naso‐pharynx exudate were performed by a commercially available procedure (Cobas SARS‐CoV‐2; Roche Diagnostic International), following the manufacturer's instructions. SARS‐CoV‐2 Ag was tested in naso‐pharynx exudate using a point‐of‐care commercially available procedure (Panbio COVID‐19 Ag Rapid Test Devic;, Abbott Rapid Diagnostics), following the manufacturer's instructions.
2.6. Statistical analysis
Continuous variables are expressed as median (Q1–Q3) and categorical variables as numbers (percentage). Cumulative incidences are provided along with 95% confidence interval (95% CI). Frequencies were compared by the chi‐square test or the Fisher's test, when there was at least one cell with an expected frequency lower than five. The Mann–Whitney U test was used for comparing continuous variables. Differences were considered significant for p values ≤.05. Poisson regression models were elaborated to assess the factors independently associated with acquisition of SARS‐CoV‐2 infection in PLWH. In those analyses, variables related to this condition in the bivariate analysis with a p value <.2, as well as age and sex, were included. These analyses were carried out using IBM SPSS 25.0 (IBM Corporation) and STATA 12.0 (StataCorp).
2.7. Ethics statement
The study was designed and conducted following the Helsinki declaration. The Ethics committee of the Hospital Universitario Virgen de Valme approved the study. All patients gave written informed consent to be entered in the cohort.
3. RESULTS
3.1. Features of the study population
Overall, 709 patients were included in this study. The main baseline features of the study population, as well as the antiretroviral drug combinations they were receiving, are shown in Table 1. Seven hundred and seven individuals (99.7%) were on antiretroviral therapy. Two of 339 patients receiving tenofovir‐including regimens were treated with TDF plus FTC. The remaining 337 patients on tenofovir‐based therapy were receiving tenofovir alafenamide (TAF) plus FTC. Twelve (1.7%) patients died during the study period. The cause of death from two (16.7%) out of the 12 patients was SARS‐CoV‐2 infection. Other causes of death were neoplasia, four (33.3%); cardiovascular events, two (16.7%); bacterial pneumonia, three (25%) and urinary sepsis, one (8.3%).
TABLE 1.
Characteristics of the study population (n = 709)
| Parameter | Value |
|---|---|
| Age, years † | 52 (43–57) |
| Male sex, n (%) | 572 (80.7) |
| HIV infection way, n (%) | |
| Drug use | 277 (39) |
| Sexual | 387 (54.6) |
| Other and unknown | 45 (6.3) |
| Active tobacco smokers, n (%) | 370 (52.2) |
| Daily alcohol intake ≥50 g, n (%) | 53 (7.5) |
| Active opiate use, n (%) | 48 (6·8) |
| CDC clinical category C, n (%) | 187 (26.4) |
| Nadir CD4 cell counts (cel/μl) [Link] , ‡ | 239 (77–400) |
| Baseline CD4 cell counts (cel/μl) † | 637 (414–844) |
| Baseline plasma HIV‐RNA <200 c/ml, n (%) | 664 (93.7) |
| ART combination § | |
| TAF or TDF/FTC‐based | 339 (47.8) |
| ABC/3TC‐based | 97 (13.7) |
| 3TC‐including dual therapy | 168 (23.7) |
| Nucleos(t)ide‐free | 103 (14.5) |
| Positive plasma HBsAg | 18 (2.5) |
| Positive plasma anti‐HCV | 314 (44.3) |
| Active HCV infection | 3 (0.4) |
| Charlson index, n (%) | |
| 0 | 206 (29.1) |
| 1‐2 | 300 (42.3) |
| 3‐5 | 145 (20.5) |
| ≥5 | 58 (8.2) |
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; FTC, emtricitabine; HBV, hepatitis B virus; TAF, tenofovir alafenamide fumarate; TDF, tenofovir disoproxil fumarate; 3TC, Lamivudine.
Median (Q1‐Q3).
Available data for 601 patients
707 patients on ART.
3.2. Incidence of SARS‐CoV‐2 infection
During the study period, 55 [7.8% (95% CI 5.9%–9.9%)] patients developed SARS‐CoV‐2 infection. The diagnosis of SARS‐CoV‐2 infection was determined by PCR in 21 (38.2%) by serology in 24 (43.6%) and by antigen test in 10 (18.2%) of the cases. The incidence of SARS‐CoV‐2 infection by study period is summarized in Figure 1.
FIGURE 1.
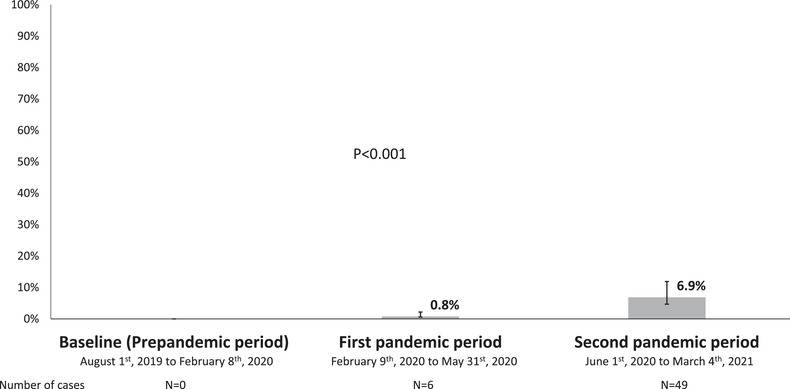
Incidence of SARS‐CoV‐2 infection by study period
No pre‐pandemic sample turned out to be positive for serum SARS‐CoV‐2 antibodies. Overall, 53 (7.5%) of 707 PLWH showed seroconversion during the follow‐up. Two (2.8%) patients did not have follow‐up serum samples because they died due to severe COVID‐19 during the study period. There was no seroreversion during the study. For PLWH with seroconversion, the median (Q1–Q3) time between the first positive serology and the end of follow up was 3.1 (1.4‐4‐6) months. Six (11.3%) of the 53 individuals could have a second serology test after the first positive one. All of them showed a positive follow‐up serology test. From 18 May 2020 to 29 November 2020, 70 [5.3% (95% CI 3.1%–9%] of 1326 the general individuals from the general population in the province of Seville, Southern Spain, showed seroconversion for SARS‐CoV‐2 antibodies, whereas 16 [2.3% (95% CI 1.3%–3.6%)] of 707 patients (p = .001) of PLWH in this study did it during the same period of time (Figure 2).
FIGURE 2.
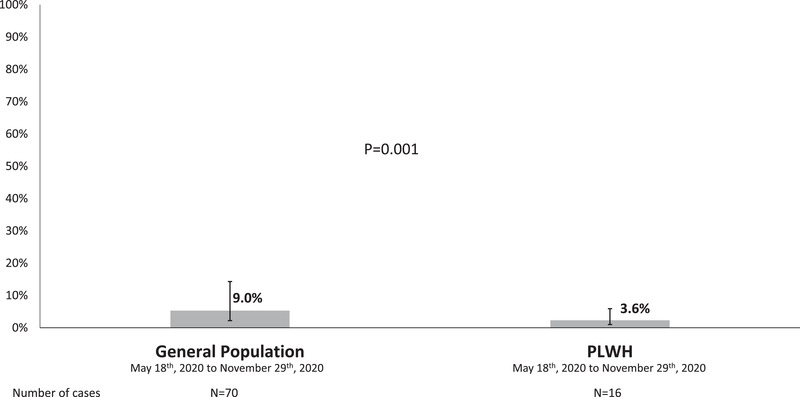
Seroincidence of SARS‐CoV‐2 infection between 18 May to 29 November 2020
3.3. Clinical outcomes of SARS‐CoV‐2 infection
Clinical outcomes of SARS‐CoV‐2‐infected patients are shown in Table 2. Six (10.7%) patients were admitted due to severe COVID‐19, two (33.3%) of them required intensive care unit admission and subsequently died. Three (50%) patients with severe COVID‐19 compared with five (10.2%) with asymptomatic or mild disease had previous diagnosis of AIDS (p = .009). The median (Q1–Q3) nadir CD4 cell counts were 151 (73‐279) for patients with severe COVID‐19 compared with 290 (88‐421) for those without severe COVID‐19 (p = .183). The Charlson index was ≤2 in one (16.7%) patient with severe COVID‐19 versus 39 (79.6%) patients with non‐severe COVID‐19 (p = .001).
TABLE 2.
Clinical outcome of patients with SARS‐CoV‐2 infection (n = 55)
| Clinical presentation | N (%) | Active smokers N (%) | CDC C category N (%) | Nadir CD4 counts † | Charlson index ≤2 N (%) |
|---|---|---|---|---|---|
| Asymptomatic infection | 24 (44) | 5 (9) | 3 (5.5) | 214 (62–413) | 22 (40) |
| Upper‐respiratory tract infections | 24 (44) | 8 (15) | 2 (3.6) | 300 (124–428) | 16 (29) |
| Extra‐respiratory symptoms ‡ | 1 (1.8) | 1 (1.8) | 0 | 514 | 1 (1.8) |
| Viral pneumonia* | 6 (11) | 0 | 3 (5.5) | 138 (46–271) | 1 (1.8) |
Median (Q1–Q3).
Extra‐respiratory symptoms: Abdominal pain and diarrhoea.
All six patients with pneumonia required hospital admission, two of them were transferred to the intensive care unit and ultimately died.
Twenty‐four [43.6% (95% CI 30.3%–57.7%)] PLWH with SARS‐CoV‐2 infection in this study were asymptomatic, compared with 642 out of 1689 [38% (95% CI 36%–40%)] among the Seville general population (p = .398). Regarding severe COVID‐19 needing hospital admission, 16% (95% CI 14%–18%) patients from the general population were admitted because of severe COVID‐19 (n/N = 267/1689) compared with six [11% (95% CI 4.1%–2.2%)] PLWH in this study (p = .325).
3.4. Factors associated with incident SARS‐CoV‐2 infection
Factors associated with SARS‐CoV‐2 infection are shown in Table 3. HIV way of transmission and smoking tobacco were associated with SARS‐CoV‐2 infection in the univariable study. Forty‐one (74.5%) patients with incident SARS‐CoV‐2 infection were non‐smokers at the time of the study. In the multivariable analysis, only non‐active tobacco smoking remained independently associated with incident SARS‐CoV‐2 infection.
TABLE 3.
Factors associated with SARS‐CoV‐2 infection among people living with HIV (PLWH)
| p Value | ||||
|---|---|---|---|---|
| Parameter | SARS‐CoV‐2 infection n (%) | Bivariate | Multivariable | Incidence rate ratio (95% CI) † |
| Sex | .563 | .877 | 1.1 (0.47–2.41) | |
| Male | 46 (8) | |||
| Female | 9 (6.6) | |||
| Age | .319 | .183 | 0.98 (0.96–1.01) ‡ | |
| <52 years | 30 (8.8) | |||
| ≥52 years | 25 (6.8) | |||
| Risk factor for HIV infection | .008 | .367 | 1.37 (0.69–2.73) | |
| MSM | 29 (11.3) | |||
| Non‐MSM | 26 (5.8) | |||
| Active tobacco use | <.001 | <.001 | 0.29 (0.16–0.55) | |
| Yes | 14 (3.8) | |||
| No | 41 (12.3) | |||
| Alcohol intake | .278 | ‐ | ‐ | |
| Abstinent | 30 (8.9) | |||
| Non‐abstinent | 25 (6.7) | |||
| Active opiate use | ||||
| Yes | 3 (6.3) | 1.000 | ‐ | ‐ |
| No | 52 (7.9) | |||
| CDC stage | .118 | .485 | 1.25 (0.67–2.33) | |
| A | 37 (9.1) | |||
| B or C | 18 (5.9) | |||
| CD4 nadir cell count | .488 | ‐ | ||
| <200 cell/ml | 19 (7.0) | ‐ | ||
| ≥200 cell/ml | 28 (8.5) | |||
| Baseline plasma HIV viral load | .216 | ‐ | ‐ | |
| <50 copies/ml | 53 (8.2) | |||
| ≥50 copies/ml | 2 (3.2) | |||
| TDF or TAF/FTC backbone | .727 | ‐ | ‐ | |
| Yes | 28 (8.3) | |||
| No | 27 (7.3) | |||
| Charlson index | ‐ | ‐ | ||
| ≤2 | 40 (7.9) | .830 | ||
| >2 | 15 (7.4) | |||
Abbreviations: FTC, emtricitabine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
95% confidence interval.
Entered as continuous variable in the multivariable analysis.
Available data for 601 patients.
The analysis of the relationship between SARS‐CoV‐2 infection and antiretroviral drugs or drug families did not show any association. Six (6.2%) subjects who were treated with abacavir (ABC) plus lamivudine (3TC)‐including combinations, 28 (8.3%) on TDF or TAF/FTC‐based regimens, 15 (8.9%) receiving 3TC‐including dual therapy and six (5.8%) patients on nucleos(t)ide free combinations developed SARS‐CoV‐2 infection.
4. DISCUSSION
The results of this study show that the incidence of SARS‐CoV‐2 infection among PLWH in Southern Spain during the ongoing pandemic was lower than that reported for the general population in the same area. In addition, the clinical outcome of SARS‐CoV‐2 infection is similar in the PLWH population considered as a whole and in the general population, although there is association between the degree of reached immunodeficiency and greater COVID‐19 severity.
The seroincidence of SARS‐CoV‐2 infection was significantly lower for PLWH than for the general population living in the province of Seville, Southern Spain. Three quarters of the participants in the first phase, conducted after the first wave in Spain, were also included in the second phase survey in the ENE‐COVID study (https://portalcne.isciii.es/enecovid19/informes/informe_cuarta_ronda.pdf). This provided fairly precise data on seroconversions among general population during the follow‐up waves, specifically between the first and second phases. We tested a prospective cohort of PLWH for antibodies against SARS‐CoV‐2 before the onset of the pandemic and during the pandemic. For comparisons, we evaluated seroconversions in the cohort selecting the same initial and final dates for data collection in the ENE‐COVID survey. Thus, the differences in SARS‐CoV‐2 infection seroincidence between PLWH and the general population reported herein can be regarded as accurate.
The reasons why the incidence of SARS‐CoV‐2 among PLWH is lower than in the general population in our area are unclear. Theoretically, this fact could have, at least, two explanations. First, antiretroviral therapy decreases the risk of acquiring SARS‐CoV‐2 infection. In our opinion, this is unlikely, as no association between the risk of SARS‐CoV‐2 and different antiretroviral combinations were found in the study population. The second reason could be that PLWH in our area are more aware of the risks associated with COVID‐19 that a great part of the general population and, consequently, were more compliant with general measures for the prevention of SARS‐CoV‐2 infection, such as mask wearing, social distance and hand hygiene. Given that PLWH included here are used to be very compliant with medical recommendations, we believe that the latter is the most conceivable reason underlying the lower incidence of SARS‐CoV‐2 infection in this population.
In our study, SARS‐CoV‐2 infection in PLWH was not significantly more severe than in the general population recruited in the ENE‐COVID survey. The rates of asymptomatic infection were similar, as were the proportion of individuals with severe COVID‐19. Contrary to other reports in PLWH (Bhaskaran et al., 2021; Davies, 2020; Geretti et al., 2020), COVID‐19 was not more severe in HIV infection compared with HIV‐uninfected persons. In our study, COVID‐19 severity depended on the Charlson index, compounded of comorbidities and age. Moreover, severe COVID‐19 in PLWH in our study was also associated with previous AIDS diagnosis. Nadir CD4 cell counts were numerically lower for patients with severe COVID‐19. Immunosuppression, that is current CD4 cell counts, was also associated with COVID‐19 outcomes in a previous study (Dandachi et al., 2020). Thus, in addition to age and comorbidities, the degree of attained immunosuppression, as reflected both by previous AIDS diagnosis and nadir CD4 cell counts, should be taken into account for COVID‐19 risk stratification among PLWH.
Surprisingly, active tobacco smoking was independently associated with a lower incidence of SARS‐CoV‐2 infection. However, this finding is in agreement with a recent report on a COVID‐19 outbreak on a French Navy nuclear aircraft carrier (Paleiron et al., 2021). Among crewmembers, current smoking was associated with a lower risk of developing SARS‐CoV‐2 infection. Smoking is characterized by inhalation and by repetitive hand‐to‐mouth movements, which may increase the chances of viral contamination. Moreover, nicotine induces angiotensin‐converting enzyme 2 (ACE‐2) overexpression in human bronchial epithelial cells (Russo et al., 2020). Because of these, increased incidence and/or severity of COVID‐19 could be expected. On the contrary, active smokers seem to be at a lower risk of SARS‐CoV‐2 infection than non‐active smokers. The underlying mechanisms involved in this paradoxical and apparent protective effect of tobacco smoking need to be clarified in further studies, as it may lead to open new ways for SARS‐CoV‐2 infection prevention or treatment. In any case, public‐facing messages about a potential protective effect of tobacco smoking on the risk of COVID‐19 must be avoided, as the health hazards associated with tobacco smoking largely surpass any theoretical benefit regarding risk for SARS‐CoV‐2 infection.
In this study, no association between antiretroviral drugs or combinations and the risk for SARS‐CoV‐2 infection was found. Previous studies have shown that TDF has potent antiviral effect against SARS‐CoV‐2, because it tightly binds the viral RNA‐dependent RNA polymerase (Elfiky, 2020). Because of this, clinical trials aimed to assess the efficacy of TDF, a tenofovir salt which reaches higher plasma concentration than TAF, both in the prevention and treatment of COVID‐19 were undertaken (Davis et al., 2020). Furthermore, two retrospective studies suggest an association between TDF plus FTC treatment and the prevention of SARS‐CoV‐2 acquisition, hospitalization or death associated with COVID‐19 (Boulle et al., 2020; Braunstein et al., 2020; Del Amo et al., 2020). In our study, only two patients were on TDF plus FTC, because most individuals in this cohort had been switched from TDF to TAF in the last few years. Consequently, we were unable to analyze the effect of TDF on the risk of SARS‐CoV‐2 infection.
This work may have some limitations. First, incidence comparisons were not age nor sex‐standardized. PLWH from this cohort are relatively younger and could bear less comorbidities than the general population and include a higher proportion of males. Nevertheless, the analysis of factors associated with incident SARS‐Cov‐2 infection did not show the risk of infection varied according to sex or Charlson index, which includes age and comorbidities. Second, the study results are applicable to settings where most patients are under effective antiretroviral therapy, with HIV replication suppression and high CD4 cell counts. The conclusions of the present study may not be valid for PLWH with advanced or untreated HIV infection. Third, HIV‐related immunosuppression might hamper immune response generated against SARS‐CoV‐2, and thus explain the finding of lower seroincidence of the study PLWH compared with the general population. Preliminary data highlighted that the vast majority of PLWH mount a functional adaptive immune response to SARS‐CoV‐2 infection. Furthermore, humoral response is comparable between PLWH with good immunovirological control and individuals without HIV infection and may persist 5–7 months following COVID‐19 disease (Alrubayyi et al., 2021). Nonetheless, incomplete immune reconstitution after starting ART might preclude the development of immunity to SARS‐CoV‐2 infection. In this study, the majority of patients showed HIV suppression and very few had a low CD4 cell count. Fourth, missing seroconversions due to infrequent sampling might explain the lower seroincidence of PLWH in the present study. However, patients were tested every 6 months, and very few were lost to follow‐up. Because of these, lack of SARS‐COV‐2 antibodies detection after COVID‐19 would be an unlikely event in the present study. Finally, we cannot rule out that other sociodemographic or behavioural factors are partially influencing our results. As a counterpart, this was a prospective cohort study, with pre‐planned visits and scheduled sera collection, which allows precisely estimating incident SARS‐CoV‐2 infection and the outcome of COVID‐19. Since prospective data on the incidence and risk of SARS‐CoV‐2 infection in PLWH are lacking (Office of AIDS Research Advisory Council, 2021), the present study fills part of this gap. These are the most important strengths of this study.
5. CONCLUSIONS
The incidence of COVID‐19 among PLWH in Seville, Southern Spain after one year of COVID‐19 pandemic was lower to that observed in the general population in the same geographical area. The severity of COVID‐19 was similar to that in patients without HIV infection and determined by comorbidities and age. Nevertheless, some HIV‐related factors, as historical immunosuppression, could influence the outcome of COVID‐19. The apparent protector role of tobacco smoking on the risk of SARS‐CoV‐2 infection deserves further evaluation, as it might lead to open ways to develop drugs for COVID‐19 treatment or prophylaxis.
CONFLICT OF INTEREST
Juan Macias has been an investigator in clinical trials supported by Bristol‐Myers Squibb, Gilead and Merck Sharp & Dome. He has received lectures fees from Gilead, Bristol‐Myers Squibb, and Merck Sharp & Dome, and consulting fees from Bristol Myers‐Squibb, Gilead and Merck Sharp & Dome. Juan A. Pineda reports having received consulting fees from Bristol‐Myers Squibb, Abbvie, ViiV Healthcare, Gilead, Merck Sharp & Dome and Janssen Cilag. He received research support from Bristol‐Myers Squibb, ViiV Healthcare, Abbvie, Merck Sharp & Dome, Janssen Cilag and Gilead and received lecture fees from Abbvie, Bristol‐Myers Squibb, ViiV Healthcare, Merck Sharp & Dome, Abbvie, Janssen Cilag and Gilead. Federico Garcia reports having received consulting fees from Abbvie, ViiV Healthcare, Gilead, Merck Sharp & Dome, Roche and Hologic & Qiagen. He received research support from ViiV Healthcare, Abbvie and Merck Sharp & Dome, and received lecture fees from Abbvie, ViiV Healthcare, Merck Sharp & Dome, Gilead, Roche, Hologic, Werfen and Intercept. Anaïs Corma‐Gomez received lecture fees from Gilead, Merck Sharp & Dome and Abbvie. The remaining authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Luis M Real and Marta Fernandez‐Fuertes had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Juan A. Pineda and Juan Macias. Acquisition, analysis or interpretation of data: Luis M. Real, Marta Fernandez‐Fuertes, Anaïs Corma‐Gomez, Federico Garcia, Ana Fuentes‐Lopez, Eva Torres, Samuel Bernal and Elena Rodriguez‐Pineda. Statistical analysis: Juan A. Pineda and Juan Macias. Drafting of the manuscript: Marta Fernandez‐Fuertes and Luis M. Real. Critical revision of the manuscript for important intellectual content: Luis M. Real, Marta Fernandez‐Fuertes, Elena Rodriguez‐Pineda, Ana Fuentes‐Lopez, Anaïs Corma‐Gomez, Pilar Rincon, Eva Torres, Nieves Fernandez, Samuel Bernal, Federico Garcia, Juan Macias and Juan A. Pineda. Obtained funding: Juan Macias, Federico Garcia and Juan A. Pineda. Study supervision: Juan A. Pineda and Juan Macias.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
This work was supported in part by the Instituto de Salud Carlos III (Project ‘PI16/01443’), integrated in the national I+D+i 2013–2016 and co‐funded by the European Union (ERDF/ESF, ‘Investing in your future’), by the Spanish Network for AIDS investigation (RIS) (www.red.es/redes/inicio) (RD16/0025/0010, RD16/0025/0040), as a part of the Nacional I+ D+I, ISCIII Subdirección General de Evaluación and the European Fund for Development of Regions (FEDER). Juan A. Pineda received a research extension grant from the Programa de Intensificación de la Actividad de Investigación del Servicio Nacional de Salud Carlos III (I3SNS). Federico Garcia received a research extension grant from the Programa de Intensificación de la Actividad de Investigación del Servicio Andaluz de Salud. Anaïs Corma‐Gomez received a Río Hortega grant from the Instituto de Salud Carlos III (grant number CM19/00251). Funding for open access charge: Universidad de Málaga/CBUA.
Fernandez‐Fuertes, M. , Corma‐Gomez, A. , Torres, E. , Rodriguez‐Pineda, E. , Fuentes‐Lopez, A. , Rincon, P. , Fernandez, N. , Garcia, F. , Bernal, S. , Real, L. M. , Macias, J. , & Pineda, J. A. (2022). Incidence of and factors associated with SARS‐CoV‐2 infection among people living with HIV in Southern Spain after one year of pandemic. Transboundary and Emerging Diseases, 69, e267–e275. 10.1111/tbed.14293
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in medRxiv at https://www.medrxiv.org/content/10.1101/2021.03.20.21253397v1, reference number MEDRXIV/2021/253397.
REFERENCES
- Alrubayyi, A. , Gea‐Mallorquí, E. , Touizer, E. , Hameiri‐Bowen, D. , Kopycinski, J. , Charlton, B. , Fisher‐Pearson, N. , Muir, L. , Rosa, A. , Roustan, C. , Earl, C. , Cherepanov, P. , Pellegrino, P. , Waters, L. , Burns, F. , Kinloch, S. , Dong, T. , Dorrell, L. , Rowland‐Jones, S. , McCoy, L. , & Peppa, D. (2021). Characterization of humoral and SARS‐CoV‐2 specific T cell responses in people living with HIV. Research Square. 10.21203/rs.3.rs-309746/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran, K. , Rentsch, C. T. , MacKenna, B. , Schultze, A. , Mehrkar, A. , Bates, C. J. , Eggo, R. M. , Morton, C. E. , Bacon, S. C. J. , Inglesby, P. , Douglas, I. J. , Walker, A. J. , McDonald, H. I. , Cockburn, J. , Williamson, E. J. , Evans, D. , Forbes, H. J. , Curtis, H. J. , Hulme, W. J. , … Goldacre, B. (2021). HIV infection and COVID‐19 death: A population‐based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV, 8, 24–32. 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulle, A. , Davies, M.‐A. , Hussey, H. , Ismail, M. , Morden, E. , Vundle, Z. , Zweigenthal, V. , Mahomed, H. , Paleker, M. , Pienaar, D. , Tembo, Y. , Lawrence, C. , Isaacs, W. , Mathema, H. , Allen, D. , Allie, T. , Bam, J.‐L. , Buddiga, K. , Dane, P. , … Tamuhla, T. (2020). Risk factors for coronavirus disease 2019 (COVID‐19) death in a population cohort study from the Western Cape province, South Africa. Clinical Infectious Diseases, ciaa1198. 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein, S. L. , Lazar, R. , Wahnich, A. , Daskalakis, D. C. , & Blackstock, O. J. (2020). Coronavirus disease 2019 (COVID‐19) Infection among people with human immunodeficiency virus in New York City: A population‐level analysis of linked surveillance data. Clinical Infectious Diseases, 72, e1021–e1029. 10.1093/cid/ciaa1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centro de Coordinación de Alertas y Emergencias Sanitarias . Ministerio de Sanidad (2020). Actualización no. 158. Enfermedad por el coronavirus (COVID‐19). [Google Scholar]
- Dandachi, D. , Geiger, G. , Montgomery, M. W. , Karmen‐Tuohy, S. , Golzy, M. , Antar, A. A. R. , Llibre, J. M. , Camazine, M. , Díaz‐De Santiago, A. , Carlucci, P. M. , Zacharioudakis, I. M. , Rahimian, J. , Wanjalla, C. N. , Slim, J. , Arinze, F. , Kratz, A. M. P. , Jones, J. L. , Patel, S. M. , Kitchell, E. , … Chow, J. (2020). Characteristics, comorbidities, and outcomes in a multicenter registry of patients with human immunodeficiency virus and coronavirus disease 2019. Clinical Infectious Diseases, ciaa1339. 10.1093/cid/ciaa1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, M. A. (2020). HIV and risk of COVID‐19 death: A population cohort study from the Western Cape Province, South Africa. MedRxiv. 10.1101/2020.07.02.20145185. [DOI] [Google Scholar]
- Davis, J. S. , Ferreira, D. , Denholm, J. T. , & Tong, S. Y. (2020). Clinical trials for the prevention and treatment of COVID‐19: Current state of play. Medical Journal of Australia, 213, 86–93. 10.5694/mja2.50673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Amo, J. , Polo, R. , Moreno, S. , Díaz, A. , Martínez, E. , Arribas, J. R. , Jarrín, I. , & Hernán, M. A. (2020). Incidence and severity of COVID‐19 in HIV‐positive persons receiving antiretroviral therapy: A cohort study. Annals of Internal Medicine, 173, 536–541. 10.7326/M20-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidance for COVID‐19 and People with HIV . (2021). DHHS panel on antiretroviral guidelines for adults and adolescents—A working group of the Office of AIDS Research Advisory Council (OARAC) . 1–9.
- Ekong, N. , Curtis, H. , Ong, E. , Sabin, C. A. , Chadwick, D. , Asboe, D. , Balasubramaniam, V. , Burns, F. , Chadwick, D. , Chaponda, M. , Churchill, D. , Delpech, V. , Ekong, N. , Freedman, A. , Kaide, E. , Kulasegaram, R. , Larbalestier, N. , Lowndes, K. , Mbewe, R. , … Vera, J. (2020). Monitoring of older HIV‐1‐positive adults by HIV clinics in the United Kingdom: A national quality improvement initiative. HIV Medicine, 21, 409–417. 10.1111/hiv.12842. [DOI] [PubMed] [Google Scholar]
- Elfiky, A. A. (2020). Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS‐CoV‐2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Science, 253, 117592. 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geretti, A. M. , Stockdale, A. J. , Kelly, S. H. , Cevik, M. , Collins, S. , Waters, L. , Villa, G. , Docherty, A. , Harrison, E. M. , Turtle, L. , Openshaw, P. J. M. , Baillie, J. K. , Sabin, C. A. , & Semple, M. G. (2020). Outcomes of coronavirus disease 2019 (COVID‐19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): A prospective observational study. Clinical Infectious Diseases, ciaa1605. 10.1093/cid/ciaa1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi, Y. B. , Naqvi, S. F. Z. , Kupec, J. T. , & Sarwari, A. R. (2020). Characteristics and outcomes of COVID‐19 in patients with HIV: A multicentre research network study. AIDS, 34, 3–8. 10.1097/QAD.0000000000002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministerio de Sanidad (2020). Estrategia de detección precoz, vigilancia y control de covid‐19. https://www.semg.es/images/2020/Coronavirus/20200709_COVID19_Estrategia_vigilancia_y_control_e_indicadores.pdf [Google Scholar]
- Ministerio de Sanidad . (2021). Estrategia de detección precoz, vigilancia y control De Covid‐19. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Estrategia_vigilancia_y_control_e_indicadores.pdf [Google Scholar]
- Paleiron, N. , Mayet, A. , Marbac, V. , Perisse, A. , Barazzutti, H. , Brocq, F.‐X. , Janvier, F. , Dautzenberg, B. , & Bylicki, O. (2021). Impact of tobacco smoking on the risk of COVID‐19: A large scale retrospective cohort study. Nicotine & Tobacco Research, 23(8), 1398–1404. 10.1093/ntr/ntab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, L. S. , Rentsch, C. T. , Sigel, K. (2020). AIDS 2020: 23rd International AIDS Conference Virtual. COVID‐19 Rate No Higher With HIV in Largest US HIV+/HIV‐ Cohort. https://www.natap.org/2020/IAC/IAC_105.htm.
- Pollán, M. , Pérez‐Gómez, B. , Pastor‐Barriuso, R. , Oteo, J. , Hernán, M. A. , Pérez‐Olmeda, M. , Sanmartín, J. L. , Fernández‐García, A. , Cruz, I. , Fernández de Larrea, N. , Molina, M. , Rodríguez‐Cabrera, F. , Martín, M. , Merino‐Amador, P. , León Paniagua, J. , Muñoz‐Montalvo, J. F. , Blanco, F. , Yotti, R. , Gutiérrez Fernández, R. , … Vázquez de la Villa, A. (2020). Prevalence of SARS‐CoV‐2 in Spain (ENE‐COVID): A nationwide, population‐based seroepidemiological study. Lancet, 396, 535–544. 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche Diagnostics (2020). Elecsys® Anti‐SARS‐CoV‐2 . https://diagnostics.roche.com/us/en/products/params/elecsys‐anti‐sars‐cov‐2.html.
- Russo, P. , Bonassi, S. , Giacconi, R. , Malavolta, M. , Tomino, C. , & Maggi, F. (2020). COVID‐19 and smoking: Is nicotine the hidden link? European Respiratory Journal, 55, 2001116. 10.1183/13993003.01116-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. , & McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. Journal of the American Medical Association, 323, 1239–1242. 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are openly available in medRxiv at https://www.medrxiv.org/content/10.1101/2021.03.20.21253397v1, reference number MEDRXIV/2021/253397.


