Abstract
Objective
The current study explored pre‐pandemic sociodemographics, medical characteristics, social/family support, and mood symptoms, and current COVID‐19 experiences as predictors of mood, positive/negative diabetes‐specific experiences, and COVID‐19‐specific distress among parents of children with type 1 diabetes during the COVID‐19 pandemic. We hypothesized that parents from marginalized backgrounds, youth with higher pre‐pandemic A1c and no CGM use, parents with lower pre‐pandemic social/family support and more pre‐pandemic mood/anxiety symptoms, and those with more negative COVID‐19 experiences would have more depressive symptoms, fewer positive and more negative diabetes‐specific experiences, and more COVID‐19‐specific distress during the initial months of the pandemic.
Research Design and Methods
Participants were parents of early school‐age children with type 1 diabetes (n = 100; 65% non‐Hispanic, white, 92% mothers, 75% married; M child age = 6.74 ± 1.59 years) who had completed a behavioral intervention trial ≥6 months ago and were re‐contacted in June/July 2020 to report on their COVID‐19 pandemic experiences and parent psychosocial outcomes. Pre‐pandemic parent mood/anxiety symptoms, family/social support, and children's medical characteristics (CGM use; M A1C = 8.17% ± 1.40%) were assessed M = 1.45 ± 0.59 years prior.
Results
More pre‐pandemic social support predicted fewer depressive symptoms, more positive diabetes‐specific experiences, and less COVID‐19‐specific distress during the pandemic. More pre‐pandemic depressive symptoms predicted more depressive symptoms during the pandemic. More life disruptions due to the pandemic were associated with more negative diabetes‐specific experiences and more COVID‐19‐specific distress. Parents of color had more negative diabetes‐specific experiences.
Conclusions
Social support may be particularly important to assess and address through intervention. Pediatric diabetes care providers should monitor parent experiences in relation to children's diabetes management.
ClinicalTrials.gov identifier: NCT02527525.
Keywords: parent psychosocial functioning, parenting, type 1 diabetes
1. INTRODUCTION
Type 1 diabetes management in early‐school age children is particularly challenging due to developmental factors, such as unpredictable diet and physical activity, behavioral challenges, frequent illness, glycemic variability, high insulin sensitivity, and a potentially shortened honeymoon period. 1 , 2 Parents bear the burden of diabetes management, as early school‐age children are often unable to sense glucose variations or reliably conduct diabetes self‐management activities, resulting in more parenting stress. 1 , 3 Relatedly, managing diabetes in early school‐age children may be even more demanding on parents during the global COVID‐19 pandemic, given additional stressors including balancing work and childcare/school, concerns about contracting COVID‐19, and risk of financial instability. 4 Emerging research has identified parental stress as one of the most commonly endorsed psychological problems during the COVID‐19 pandemic. 4 , 5 , 6
Regarding diabetes‐specific experiences, a study of 34 families in Greece found that children with type 1 diabetes on insulin pumps and continuous glucose monitors (CGM) experienced increased glycemic variability and behavioral changes related to mealtime routines/schedules during pandemic lockdowns; however, no differences were found in blood glucose (BG) time in range between the COVID‐19‐related lockdown and pre‐pandemic levels. 7 On the other hand, a study of 22 families from Italy showed that parents were more involved in daily diabetes management in early school‐age children due to the “stay at home” order, and more involvement was associated with more BG time in range based on CGM data. 8 This early research on diabetes‐specific experiences of parents of youth with diabetes during the pandemic is illuminating; yet it is in its infancy, has been limited by small sample sizes, and has minimal focus on the unique psychosocial issues related to parenting early school‐age children with type 1 diabetes.
Different characteristics of parents of youth with type 1 diabetes may also be associated with varying psychosocial outcomes during the COVID‐19 pandemic. Pre‐existing anxiety and depression have been identified as risk factors for greater psychological distress in adults, broadly, and parents, specifically. 9 , 10 Social support is a well‐known buffer against stress, and recent data also show that social support protects against adverse psychological outcomes during the COVID‐19 pandemic in parents. 11 Sociodemographic factors may also be important. For example, initial research has reported that non‐Hispanic white individuals, women, and single individuals have reported more distress during the COVID‐19 pandemic, whereas older individuals and those with higher income have not experienced more distress. 12 Parents from communities of color are also more likely to experience social and economic inequities, unemployment due to the pandemic, and exposure to the virus, 10 , 13 which have all been linked to more distress. Considering the growing body of literature related to predictors of psychosocial functioning during the COVID‐19 pandemic among adults generally and parents specifically, similar patterns may apply for parents of children with type 1 diabetes, yet their experiences during the COVID‐19 pandemic remain unexplored in the current literature.
To fill this gap, the current study sought to prospectively examine pre‐pandemic sociodemographics/medical factors, pre‐pandemic parent mood/anxiety and social/family support, and current COVID‐19 experiences as predictors of parent mood, diabetes‐specific and COVID‐19‐specific experiences among parents of early school‐age children with type 1 diabetes during the initial months of the COVID‐19 pandemic.
We hypothesized that parents from marginalized backgrounds, youth with higher pre‐pandemic A1c and no CGM use, parents with more pre‐pandemic anxiety and depressive symptoms and lower social/family support, and those experiencing more significant impacts of the pandemic (e.g., increased family/school disruption, COVID‐19 exposure, illness or death in the family, stricter quarantine) would experience (1) more depressive symptoms, (2) more negative diabetes‐specific experiences, (3) fewer positive diabetes‐specific experiences, and (4) more COVID‐19‐specific distress during the initial months of the COVID‐19 pandemic. We refer to these four outcomes collectively as parent psychosocial outcomes.
2. METHODS
2.1. Procedures
Between 2015 and 2019, a cohort of 157 parents of children who were initially ages 1–6 diagnosed with type 1 diabetes for ≤8 weeks enrolled in a two‐site randomized clinical trial, where they were randomly assigned to either a stepped care behavioral intervention (n = 115) or usual care (n = 42). Original study inclusion/exclusion criteria and study procedures, including intervention specifics, are described elsewhere. 14 , 15 As part of the original trial, all participants completed assessments throughout the trial, including a “final assessment” at 15‐month post‐randomization.
During the initial months of the COVID‐19 pandemic (June and July, 2020), we re‐contacted this original cohort for further data collection to examine their functioning during the pandemic. Participants were eligible for this additional follow up if they had completed their “final assessment” for the original trial ≥6 months prior. The Institutional Review Board at two pediatric academic medical centers in the mid‐Atlantic and Southwest regions of the United States gave approval to email eligible participants from the original cohort to complete this additional survey without requiring additional documentation of informed consent. Text/email reminders were sent to those who did not reply within 2 weeks. Participants received $50 in appreciation of their time.
In total, 121 participants from the original cohort were eligible to complete the current COVID‐19 survey (collected in June/July 2020), and 100 completed it (83% response rate). The “pre‐pandemic” timepoint in the current study represents data collected from the “final assessment” of the original trial, which occurred about 1.45 ± 59 years prior (range 0.52–2.61 years; November 2017 to December 2019) (see Figure 1 for full study timeline).
FIGURE 1.
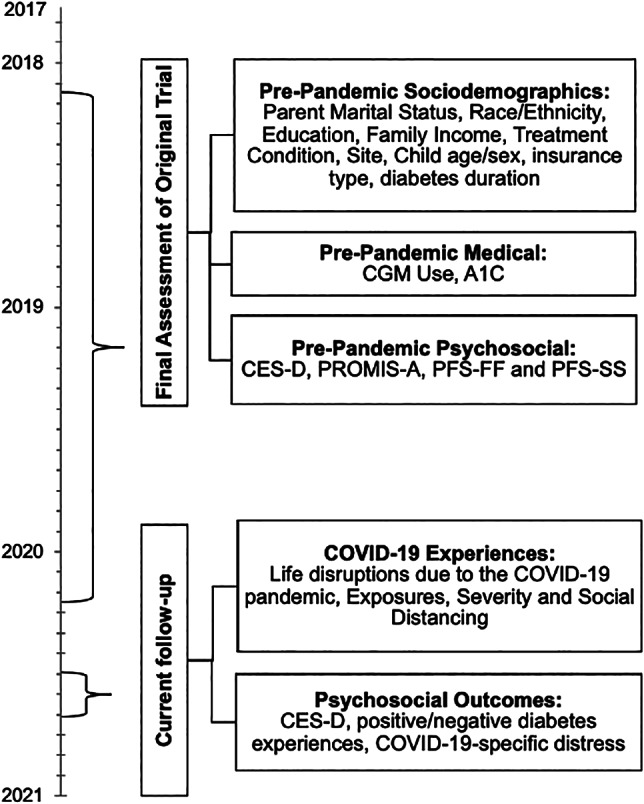
Study timeline. CES‐D, Center for Epidemiological Studies‐Depression Scale; CGM, continuous glucose monitor; PFS‐FF, Protective Factors Survey‐Family Functioning Subscale; PFS‐SS, Protective Factors Survey‐Social Support Subscale; PROMIS‐A, Patient Reported Outcomes Measurement Information System‐Anxiety
2.2. Participants
The current sample of 100 participants was 65% non‐Hispanic, white, 92% mothers, and 75% married; M child age = 6.74 (SD = 1.59; range 3–10 years).
2.3. Measures
2.3.1. Pre‐pandemic sociodemographics
As part of the original trial, parents self‐reported on sociodemographics at study entry, such as parent race/ethnicity, marital status, education, family income, child age/sex, and insurance type (Table 1 and Figure 1).
TABLE 1.
Descriptive data on sociodemographics, pre‐pandemic medical characteristics, and psychosocial functioning
| Sociodemographics | M | SD |
|---|---|---|
| Parent age at current follow‐up (years) | 36.41 | 6.83 |
| Child age at current follow‐up (years) | 6.74 | 1.59 |
| Type 1 Diabetes Duration at current follow‐up (years) | 2.95 | 0.54 |
| Time between “pre‐pandemic” and current follow‐up (years) | 1.45 | 0.55 |
| N | % | |
|---|---|---|
| Site | ||
| Mid‐Atlantic | 43/100 | 43.00 |
| Southwest | 57/100 | 57.00 |
| Treatment condition | ||
| Usual care | 32/100 | 32.00 |
| Stepped care | 68/100 | 68.00 |
| Parent role | ||
| Mothers | 92/100 | 92.00 |
| Parent race/ethnicity | ||
| Non‐Hispanic, White | 65/99 | 65.65 |
| Black/African American | 13/99 | 13.13 |
| Hispanic/Latinx | 11/99 | 11.11 |
| Asian/Asian American | 8/99 | 8.08 |
| Multiracial | 2/99 | 2.02 |
| Parent marital status | ||
| Married | 73/98 | 74.45 |
| Child sex | ||
| Female | 60/100 | 60.00 |
| Insurance | ||
| Public insurance | 32/99 | 32.32 |
| Income | ||
| ≥100,000 k+/year | 40/84 | 47.62 |
| Parent education | ||
| (≥College) | 54/100 | 54.00 |
| Pre‐pandemic medical characteristics | N/M | %/SD |
|---|---|---|
| CGM use | 63/100 | 63.00% |
| A1c (%) | 8.17 | 1.40 |
| A1c (mmol/mol) | 66.00 | 15.30 |
| Pre‐pandemic parent psychosocial functioning | M | SD |
|---|---|---|
| Pre‐pandemic CES‐D | 10.01 | 9.46 |
| Pre‐pandemic PROMIS‐A (t‐score) | 53.25 | 8.55 |
| Pre‐pandemic PFS‐FF | 5.76 | 0.93 |
| Pre‐pandemic PFS‐SS | 6.12 | 0.99 |
Abbreviations: CES‐D, Center for Epidemiological Studies‐Depression Scale; PROMIS‐A, Patient Reported Outcomes Measurement Information System‐Anxiety‐Short Form; PFS‐SS, Protective Factors Survey‐Social Support; PFS‐FF, Protective Factors Survey‐Family Functioning.
2.3.2. Pre‐pandemic medical characteristics
A1C values were collected at the pre‐pandemic timepoint as part of routine diabetes care and obtained from medical charts (M A1c = 8.17% ± 1.40%). CGM use at the pre‐pandemic timepoint was based on parent report to a one‐item, yes/no question, which was corroborated by medical record review and whether the study team had collected CGM data as part of the original trial.
2.3.3. Pre‐pandemic psychosocial functioning
Pre‐pandemic depressive symptoms were assessed using the 20‐item Center for Epidemiological Studies‐Depression Scale (CES‐D ≥ 16 = elevated). 16 Pre‐pandemic anxiety symptoms were assessed using the 7‐item Patient Reported Outcomes Measurement Information System Anxiety‐Short Form (PROMIS‐A). 17 Reliability was adequate for both (depression: α = 0.92; anxiety: α = 0.93). Pre‐pandemic family and social support were assessed using two subscales of the Protective Factors Survey: Family Functioning/Resiliency (PFS‐FF; 5 items, α = 0.89) and Social Support (PFS‐SS; 3 items, α = 0.88). 18
2.3.4. COVID‐19 pandemic experiences
In June/July 2020, parents completed the Pandemic Parenting—Type 1 Diabetes (PP‐T1D) survey, a newly developed, self‐report survey on parents' COVID‐19 experiences, COVID‐19‐specific distress and positive/negative diabetes‐specific experiences. A team of six PhD‐level clinical researchers with expertise in T1D psychosocial issues developed the items based on theory as well as their clinical and research experiences. Existing COVID‐19 psychosocial measures were also reviewed, and biostatisticians were consulted regarding item measurement. For each item, participants were prompted to report on their experiences “Since the COVID‐19 pandemic, I…” (see relevant PP‐T1D items in Appendix A).
Based on parent responses to the PP‐T1D survey regarding COVID‐19 experiences, we generated the following domains: “Life disruptions due to COVID‐19 pandemic” was defined as the total number of disruptions reported (range 0–6), including change in school, loss of job/hours, loss of child care, change in health insurance, moving, and other changes (Table 2). “COVID‐19 exposure (immediate family)” and “COVID‐19 exposure (non‐immediate family)” were defined as whether immediate or non‐immediate family members, respectively, had experienced any COVID‐19 symptoms, diagnoses, treatments, hospitalizations, or death. “COVID‐19 severity” was defined as whether any family members (immediate or non‐immediate) experienced severe outcomes due to contracting COVID‐19, such as treatments, hospitalizations or death (as opposed to only symptoms/diagnoses). “Strict COVID‐19 social distancing” describes whether parents reported strictly adhering to self‐quarantining (Appendix A).
TABLE 2.
Current COVID‐19 experiences and parent psychosocial outcomes during COVID‐19 pandemic
| Current COVID‐19 experiences | N | % |
|---|---|---|
| Life disruptions due to the COVID‐19 pandemic | ||
| Change for schooling over the next year | 48/99 | 48.48 |
| Loss of job/reduced hours | 33/99 | 33.33 |
| Loss/change of child care provider(s) | 13/99 | 13.13 |
| Change of health insurance | 8/99 | 8.08 |
| Moving family home | 4/99 | 4.04 |
| Other | 6/99 | 6.06 |
| None | 16/99 | 16.16 |
| COVID‐19 exposure (symptoms, diagnosis, treatment, hospitalization or death) | ||
| Immediate family | 7/99 | 7.07 |
| Non‐immediate family | 17/99 | 17.17 |
| COVID‐19 severity: immediate/non‐immediate family experienced COVID‐19 symptoms, diagnosis, treatment, hospitalization or death | 10/99 | 10.10 |
| Strictness regarding Social Distancing | ||
| None | 2/99 | 2.02 |
| Some | 40/99 | 40.40 |
| Strict | 57/99 | 57.57 |
| Parent psychosocial outcomes during the COVID‐19 pandemic (June/July 2020) | M | SD |
|---|---|---|
| CES‐D | 10.43 | 8.30 |
| Positive diabetes‐specific experiences | 10.69 | 3.37 |
| Negative diabetes‐specific experiences | 7.97 | 3.26 |
| COVID‐19‐specific distress | 31.16 | 9.21 |
Abbreviation: CES‐D, Center for Epidemiological Studies‐Depression Scale.
2.3.5. COVID‐19 pandemic parent psychosocial outcomes
In June/July 2020, parents again self‐reported on their current depressive symptoms using the CES‐D. They also completed items on the PP‐T1D about three domains of experiences during the initial months of the COVID‐19 pandemic: COVID‐19‐specific distress and positive and negative diabetes‐specific experiences during the COVID‐19 pandemic.
“COVID‐19‐specific distress” was the total score of 12 items measuring parents' own levels of anxiety, stress, depressive symptoms, eating, sleep, and activity specifically during the COVID‐19 pandemic (e.g., “have been more anxious than usual”; α = 0.84). “Positive diabetes‐specific experiences during the COVID‐19 pandemic” included parent ratings of three items: “found it easier to manage my child's diabetes,” “more time to monitor/manage my child's diabetes,” and “more time to make/eat healthy meals” (α = 0.79). “Negative diabetes‐specific experiences during COVID‐19 pandemic” included parent ratings of four items: “struggled to properly manage my child's diabetes,” “worried about having adequate access to my child's T1D supplies,” “noticed more fluctuations/variability in my child's blood glucose levels,” and “less access to my child's diabetes team” (α = 0.68). The latter two domains are referred to as “positive diabetes‐specific experiences” and “negative diabetes‐specific experiences,” respectively. Both were calculated by summing parents' perceptions of positive (three items) and negative (four items) aspects of their child's diabetes management during the COVID‐19 pandemic, respectively. For the PP‐T1D domains, higher scores represent more positive experiences, more negative experiences, and more COVID‐19‐specific distress (see Table 2 and Appendix A for additional information).
3. DATA ANALYTIC PLAN
All analyses were conducted in SPSS v.27. Sociodemographics, CGM use, and COVID‐19 experience variables (i.e., PP‐T1D domains: COVID‐19 exposure‐immediate family, COVID‐19 exposure‐non‐immediate family, COVID‐19 severity, and strict COVID‐19 social distancing) were dichotomized (Table 3). Pre‐pandemic depression/anxiety, social/family support, A1C, life disruptions due to COVID‐19 pandemic (PP‐T1D domain), current child age, diabetes duration and all parent psychosocial outcomes were continuous variables. Further, given that participants are from an original cohort who completed a two‐site randomized clinical trial, treatment condition and site were also explored as covariates.
TABLE 3.
Pearson's and point‐biserial correlations between sociodemographics, pre‐pandemic psychosocial, and medical variables, and current COVID‐19 experiences with parent psychosocial outcomes during the COVID‐19 pandemic (June/July 2020)
| CES‐D during pandemic | Positive diabetes experiences | Negative diabetes experiences | COVID‐19‐specific distress | |
|---|---|---|---|---|
| Sociodemographics | ||||
| Treatment condition | 0.09 | −0.10 | 0.04 | 0.11 |
| Site | −0.17 + | −0.19 + | 0.04 | −0.17 |
| Parent race/ethnicity | −0.02 | −0.01 | 0.30** | 0.17 + |
| Parent education | 0.13 | −0.02 | 0.08 | 0.21* |
| Family income | 0.05 | 0.01 | −0.11 | 0.14 |
| Child insurance type | −0.06 | −0.07 | −0.05 | 0.07 |
| Child sex | 0.02 | −0.15 | 0.06 | 0.08 |
| Child age | 0.14 | −0.07 | 0.12 | 0.14 |
| Diabetes duration | −0.12 | −0.14 | 0.02 | 0.01 |
| Pre‐pandemic medical characteristics | ||||
| CGM | 0.17 + | −0.02 | −0.01 | 0.22* |
| A1C | 0.06 | 0.16 | 0.16 | 0.03 |
| Pre‐pandemic psychosocial variables | ||||
| Pre‐pandemic CES‐D | 0.41** | −0.18 + | 0.13 | 0.17 + |
| Pre‐pandemic PROMIS‐A | 0.33** | −0.20* | 0.05 | 0.23* |
| Pre‐pandemic PFS‐FF | −0.16 | 0.28** | −0.07 | −0.02 |
| Pre‐pandemic PFS‐SS | −0.41** | 0.35** | −0.10 | −0.25* |
| Current COVID‐19 experiences | ||||
| COVID‐19 exposure (immediate family) | 0.10 | −0.14 | −0.02 | −0.03 |
| COVID‐19 exposure (non‐immediate family) | −0.20* | 0.05 | −0.05 | −0.04 |
| COVID‐19 severity (treat, hospitalized, died) | −0.14 | 0.08 | 0.02 | 0.04 |
| Number of life disruptions due to COVID‐19 pandemic | 0.14 | 0.10 | 0.22* | 0.27** |
| Strict social distancing | 0.10 | 0.07 | 0.22* | 0.23* |
Note: Treatment condition: 0 = usual care, 1 = stepped care; Site: 1 = mid‐Atlantic, 2 = Southwest; Parent Race: 1 = non‐Hispanic white, 2 = parents of color; Parent education: 0 = <college, 1= ≥college; Family income: 0 = <100,000$, 1= ≥100,000$; insurance status: 1 = public, 2 = private; child sex: 1 = female, 2 = male; CGM, continuous glucose monitor, 0 = no, 1 = yes; CES‐D, Center for Epidemiological Studies‐Depression Scale; PROMIS‐A, Patient Reported Outcomes Measurement Information System‐ Anxiety; PFS‐FF, Protective Factors Survey‐ Family Functioning Subscale; PFS‐SS, Protective Factors Survey‐ Social Support Subscale; Immediate/non‐immediate family exposure: 0 = no, 1 = yes; strict social distance: 1 = strictly, 0 = none/some.
p < 0.10;
p < 0.05;
p < 0.001.
First, bivariate analyses using Pearson's and point‐biserial correlations were conducted to evaluate associations among sociodemographic/medical characteristics, pre‐pandemic depressive/anxious symptoms and social/family support, and COVID‐19 experience variables with parent psychosocial outcomes (Table 3). Next, we tested multiple regression models to predict each parent psychosocial outcome variable: (1) current depressive symptoms, (2) positive and (3) negative diabetes‐specific experiences, and (4) COVID‐19‐specific distress. Any predictor variable with a marginal (p < 0.10) or significant (p < 0.05) bivariate association with a parent psychosocial outcome variable was included as a predictor in a multiple regression model predicting the relevant parent psychosocial outcome variable. Based on recommended cut‐offs for variance inflation factors and tolerance statistics, 19 no concerns with multicollinearity were present, and skew/kurtosis statistics were in the acceptable range.
4. RESULTS
4.1. Descriptive analyses
Of the 121 participants eligible to complete the June/July 2020 follow‐up, participants who completed the current follow‐up (n = 100) did not differ from those who declined it (n = 21) in terms of parent race/ethnicity, family income, and marital status (p > 0.05). However, compared to those who declined the current follow‐up (n = 21), completers (n = 100) were more likely to come from the southwest site (χ 2[1, N = 121] = 7.60, p < 0.01). Eligible participants who did (n = 100) and did not complete (n = 21) the current follow‐up did not differ by treatment condition or diabetes duration (p > 0.05). Further, independent samples t‐tests indicated dependent variables (depressive symptoms, positive/negative diabetes‐specific experiences, COVID‐19‐specific distress) did not differ by treatment condition (p > 0.05) at this time point; thus, analyses were conducted with the sample as a whole.
Regarding COVID‐19 experiences, 83.84% reported at least one COVID‐19 disruption, with change in school being the most common (48.48%). Moreover, 57.57% reported adhering to “strict” social distancing guidelines, and the majority had no COVID‐19 exposure during the initial months of the pandemic (see Table 2).
Rates of elevated CES‐D scores were similar pre‐pandemic (24.30%) and during the COVID‐19 pandemic (22.20%), and 9.10% of the whole sample were elevated at both points (see Tables 1 and 2).
4.2. Bivariate analyses
Results of all Pearson's and point‐biserial correlations among predictors and parent psychosocial outcome variables are presented in Table 3.
Parents of color experienced significantly more negative diabetes‐specific experiences and marginally more COVID‐19‐specific distress than non‐Hispanic, white parents. Parents with college education experienced significantly more COVID‐19‐specific distress than those without college education. Participants from the southwest site experienced marginally fewer depressive symptoms and fewer positive diabetes‐specific experiences than thoe from the mid‐Atlantic site. Parents whose children used CGM experienced marginally more parent depressive symptoms and significantly more COVID‐19‐specific distress.
More pre‐pandemic depressive symptoms were significantly correlated with more current depressive symptoms and marginally correlated with fewer positive diabetes‐specific experiences and more COVID‐19‐specific distress. Both lower pre‐pandemic social support and more pre‐pandemic anxiety were significantly correlated with more depressive symptoms, fewer positive diabetes‐specific experiences, and more COVID‐19‐specific distress. More pre‐pandemic family support was significantly correlated with more positive diabetes‐specific experiences.
More life disruptions due to the COVID‐19 pandemic and strict social distancing practices were each significantly correlated with more negative diabetes‐specific experiences and more COVID‐19‐specific distress. COVID‐19 exposure in non‐immediate family members was significantly correlated with fewer current depressive symptoms.
4.3. Multivariate analyses
See Table 4 for results of multiple regression analyses. For the model predicting depressive symptoms during the pandemic, fewer pre‐pandemic depressive symptoms and more pre‐pandemic social support predicted fewer depressive symptoms during the pandemic. For the model predicting positive diabetes‐specific experiences during the pandemic, more social support and being from the mid‐Atlantic (vs. southwest) site predicted more positive diabetes‐specific experiences. For the model predicting negative diabetes‐specific experiences during the pandemic, more COVID‐19 life disruptions and being a parent of color (compared to a parent of non‐Hispanic, white background) predicted more negative diabetes‐specific experiences. For the model predicting COVID‐19‐specific distress, more pre‐pandemic social support and fewer life disruptions due to the COVID‐19 pandemic were associated with less COVID‐19‐specific distress.
TABLE 4.
Multiple regression analyses predicting parent psychosocial outcomes during the COVID‐19 pandemic (June/July 2020)
| b | SE | β | p | |
|---|---|---|---|---|
| CES‐D (R 2 = 0.29) | ||||
| Site | −1.24 | 1.52 | −0.08 | 0.42 |
| Pre‐pandemic CESD | 0.28 | 0.12 | 0.32 | 0.02* |
| Pre‐pandemic PROMIS‐A | −0.05 | 0.21 | −0.04 | 0.81 |
| Pre‐pandemic PFS‐SS | −2.37 | 0.79 | −0.28 | <0.01* |
| Non‐immediate family exposure to COVID‐19 | −2.66 | 2.01 | −0.12 | 0.19 |
| CGM use | 2.03 | 1.61 | 0.12 | 0.21 |
| Positive diabetes‐specific experiences (R 2 = 0.22) | ||||
| Site | −1.65 | 0.63 | −0.24 | 0.01* |
| Pre‐pandemic CESD | 0.03 | 0.05 | 0.07 | 0.62 |
| Pre‐pandemic PROMIS‐A | −0.09 | 0.09 | −0.14 | 0.31 |
| Pre‐pandemic PFS‐FF | 0.71 | 0.37 | 0.19 | 0.06 |
| Pre‐pandemic PFS‐SS | 1.07 | 0.34 | 0.31 | <0.01* |
| Negative diabetes‐specific experiences (R 2 = 0.14) | ||||
| Parent race/ethnicity | 1.76 | 0.71 | 0.26 | 0.02* |
| COVID‐19 life disruptions | 0.85 | 0.42 | 0.20 | 0.04* |
| Strict social distancing | 0.68 | 0.69 | 0.10 | 0.33 |
| COVID‐19‐specific distress (R 2 = 0.23) | ||||
| Parent education | 2.19 | 1.79 | 0.12 | 0.22 |
| Pre‐pandemic PROMIS‐A | 0.17 | 0.16 | 0.10 | 0.30 |
| Pre‐pandemic PFS‐SS | −2.12 | 0.90 | −0.23 | 0.02* |
| COVID‐19 life disruptions | 3.09 | 1.14 | 0.26 | <0.01* |
| Strict social distancing | 2.40 | 1.77 | 0.13 | 0.18 |
| CGM use | 2.32 | 1.83 | 0.12 | 0.21 |
Note: b = unstandardized beta; SE = standard error; β = standardized beta; p = statistical significance; Site: 1 = mid‐Atlantic, 2 = Southwest; Parent race/ethnicity: 1 = non‐Hispanic white, 2 = parents of color; Parent Education: 0 = <college, 1= ≥ college; Immediate/Non‐immediate family exposure: 0 = no, 1 = yes; strict social distance: 1 = strictly, 0 = none/some. CES‐D, Center for Epidemiological Studies‐Depression Scale; PROMIS‐A, Patient Reported Outcomes Measurement Information System‐ Anxiety; PFS‐FF, Protective Factors Survey‐ Family Functioning Subscale; PFS‐SS, Protective Factors Survey‐ Social Support Subscale; CGM (continuous glucose monitor): 1 = yes, 0 = no. For the COVID‐19‐specific distress model, given the number of marginal and significant bivariate associations, only variables with significant associations (p < 0.05) were included in the multiple regression model.
5. DISCUSSION
This is one of the first prospective studies to examine predictors of parent psychosocial outcomes among parents of early school‐age children with type 1 diabetes in the initial months of the COVID‐19 pandemic. Results support emerging data showing that social support buffers against psychological distress specifically during the COVID‐19 pandemic, 20 which adds to the well‐documented stress‐buffering effects of social support. 21 Recently, social support has also been proposed as a salient predictor of psychological thriving, defined as growth (as opposed to just the absence of maladaptive outcomes) despite adversity. 21 Though improved health outcomes, such as increased BG time in range, have been reported during the COVID‐19 pandemic, 7 our study is the first to report positive diabetes‐specific psychosocial experiences during the COVID‐19 pandemic. Positive diabetes experiences were uniquely predicted by more pre‐pandemic social support, even when controlling for pre‐pandemic anxiety and depressive symptoms, pointing to a likely source of resilience for parents.
What might have accounted for these patterns? During turbulent times, social supports provide functions such as safety, protection, and relief; clarification of values/strengths; and reframing adversity. 21 Similarly, during neutral times, social supports provide different functions like growth opportunities, positive appraisals, life engagement. These functions lead to positive emotions/coping, positive appraisals of the event, as well as improvements at the biological (e.g., less brain activation in threat areas) and health behaviors (e.g., sleep, diet) level, which all support thriving . 21 Thus, pre‐pandemic social supports that helped parents thrive pre‐pandemic may have continued to help them manage the turbulence and stressors of the pandemic. Given that diabetes‐related challenges are common even pre‐pandemic, further research into the mechanisms specific to the type 1 diabetes population are needed.
Although social support predicted positive diabetes‐specific experiences, it was unrelated to negative diabetes‐specific experiences, even in bivariate analyses. Our definition of negative diabetes‐specific experiences emphasized challenges with access to diabetes supplies/medical team, given that this was a major concern during the initial months of the COVID‐19 pandemic 22 ; a conceptualization focused on challenges with day‐to‐day diabetes management may have yielded different results. Experiencing more life disruptions due to the COVID‐19 pandemic was related to more negative diabetes‐specific experiences, aligning with data showing that more stressful life events are associated with worse parent diabetes‐specific functioning. 23 Black, Hispanic, and other parents of color were also more likely to report more negative diabetes‐specific experiences. Critically, this survey overlapped with the peak of racial tension across the United States following the murders of George Floyd and Breonna Taylor. While our findings focused on diabetes‐specific negative experiences, it is quite plausible that the overlapping stresses of parenting and diabetes management during the pandemic and racial tension may have amplified the challenges faced by families of color in our study in ways we did not capture. 24 Also, the most commonly reported COVID‐19 disruption was closures/changes in school, which removes the opportunity to receive diabetes care assistance at school. Given findings from a previous study that parents of color reported more positive perceptions of type 1 diabetes management at school compared to parents of non‐Hispanic white backgrounds, 25 it is possible diabetes management among families of color may be particularly affected by COVID‐19 school closures. Deeper analysis of this important issue with implications for reducing health disparities, including understanding possible mechanisms, requires further study.
Contrary to our hypotheses, several sociodemographic and medical variables, such as family income, insurance type, child sex/age, diabetes duration, and A1c, were unrelated to parent psychosocial outcomes during the COVID‐19 pandemic, in neither bivariate nor multivariate analyses. While some studies have reported that those with lower socioeconomic status experience worse mental health during the pandemic, 26 other studies found the opposite. 27 Thus, identifying sociodemographic correlates of mental health outcomes during the COVID‐19 pandemic is complex, and perhaps requires more data variability in order to detect effects. Regarding medical characteristics, we found bivariate correlations between CGM use and more parent depressive symptoms and more COVID‐19‐specific distress (but associations were attenuated in multivariate analyses). Given that COVID‐19 stay‐at‐home‐orders are associated with limited physical activity and increased rates of unhealthy eating patterns, 28 it is possible that ongoing CGM feedback about BG levels may increase parent stress. Further, the benefits of CGM use (i.e., remote monitoring) for parent psychosocial outcomes may also be reduced while adhering to stay at home orders. 29
Overall, the current study had several strengths. It included prospective data of pre‐pandemic sociodemographics/medical information and parent social/family support and depression/anxiety; a more diverse sample than is typical for a type 1 diabetes sample; data collection that occurred 3–4 months into the COVID‐19 pandemic; and examination of multiple parent psychosocial outcomes.
However, the study is not without limitations, including the use of the newly developed PP‐T1D measure, which has not been validated and does not have established clinical cut‐offs. We developed this measure based on established literature in diabetes and current research about experiences of people during the early months of the pandemic. 22 The negative diabetes‐specific experiences domain had a focus on difficulty with access to supplies/medical team to reflect the concern of families during the initial months of the COVID‐19 pandemic 22 ; however, negative diabetes‐specific experiences likely changed as the pandemic progressed, so these items may not be as relevant later in the pandemic. As the original trial enrolled the “primary caregiver” of the child, and most primary caregivers of young children and/or children with type 1 diabetes are women/mothers, 1 , 30 the current study included only 6% fathers, which may limit our understanding of fathers' experiences during the pandemic. Though mothers continued to carry the majority of childcare during the pandemic, some data suggest an increase in father's contribution toward childcare during the COVID‐19 pandemic. 31 Thus, examining changes in paternal involvement specifically in children's T1D tasks during stay‐at‐home‐orders is an area for further inquiry. Additionally, our A1c variable was measured at the pre‐pandemic timepoint, as opposed to the pandemic timepoint, due to the lack of in‐person A1c data collection during the pandemic (resulting in ~75% missing A1c data), which was a common issue during the pandemic. 32 Lastly, the study was conducted based on knowledge of COVID‐19 during the initial months, and knowledge of the pandemic is continuously changing.
Future studies should continue to assess stability and change in psychosocial functioning across the stages of the COVID‐19 pandemic, as knowledge about the virus, social distancing restrictions, and vaccine developments evolve. Moreover, as the pandemic progresses, the chances of having an immediate or extended family member diagnosed with COVID‐19 increases, which is another consideration for future research. Clinically, given that several social support interventions have shown promising results among chronic illness groups, 33 including reduction in emergency room visits, the benefits of targeting and improving social support among families of youth with chronic illness like type 1 diabetes cannot be overstated. Future research should focus on dissemination and implementation of such interventions in real‐world settings.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Christine H. Wang contributed to the conceptualization of the current study and wrote the methods, results and discussion. Samantha A. Carreon and Jasmine Jones assisted with writing the introduction. John R. Barber assisted with statistical analyses, and KellyAnn Rooney assisted with data management/descriptive analyses. Carrie Tully and Maureen Monaghan contributed to the conceptualization of the original cohort and contributed to the editing the current manuscript. Randi Streisand and Marisa E. Hilliard are the PIs of the original study and contributed to conceptualization of the study and editing of the manuscript. All authors have read and approved the final manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.13255.
ACKNOWLEDGMENT
Funding was provided by National Institutes of Diabetes and Digestive and Kidney Diseases 1R01 DK102561 (PI: R Streisand).
APPENDIX A.
For the next few items, please consider your experience during the Coronavirus (COVID‐19) pandemic.
Using a scale of 1–5, please respond to the questions below.
1 = not at all
2 = slightly/rarely
3 = moderately/some of the time
4 = very/often
5 = extremely/almost all of the time
[Positive diabetes‐specific experiences]
Since the COVID‐19 pandemic, I…
Have found it easier to manage my child's diabetes.
Have had more time to monitor/manage my child's diabetes.
Have had more time to make/eat healthy meals.
[Negative diabetes‐specific experiences]
Since the COVID‐19 pandemic, I…
Have struggled to properly manage my child's diabetes.
Have been worried about having adequate access to my child's T1D supplies.
Have noticed more fluctuations/variability in my child's blood glucose levels.
Have less access to my child's diabetes team.
[COVID‐19‐specific distress]
Since the COVID‐19 pandemic, I…
Have been eating more frequently than usual.
Have been less physically active than usual.
Have been more anxious than usual.
Have experienced more variability in day‐to‐day mood than usual.
Have been more frightened by the news and media.
Have felt more irritable toward my family/loved ones than usual.
Have experienced difficulty sleeping.
Have been able to get more sleep. (reverse coded)
Have been more worried about family finances.
Have felt more vulnerable than usual.
Have worried more about my and others' health.
Have spent more time using social media/electronics.
[Life disruptions due to COVID‐19 pandemic]
1. Did any of the following life events occur as a result of COVID‐19? Select all that apply.
Loss of job or reduced hours
Moving family home
Change of health insurance
Loss or change of child care provider(s)
Planned change for schooling over the next year
Other____________________________________________________
[COVID‐19 exposure]
2. Have you, your family member or any significant person experienced any of the following as a result of COVID‐19: Had symptoms (but never tested), diagnosed, treated, hospitalized or passed away? If so, please check all that apply:
| Had symptoms of COVID‐19 | Diagnosed with COVID‐19 | Treated for COVID‐19 | Hospitalized due to COVID‐19 | Passed away from COVID‐19 | |
|---|---|---|---|---|---|
| Myself | |||||
| My child(ren) | |||||
| My significant other or child's co‐parent | |||||
| Extended family members | |||||
| Other significant person in my life |
[COVID‐19 social distancing]
3. Which of these is most true for you?
I have gone about my normal routines since learning about the novel coronavirus, or COVID‐19.
I tried to follow guidelines related to social distancing since learning about the novel coronavirus, or COVID‐19.
I strictly limited my interactions with others (self‐quarantined) since learning about the novel coronavirus, or COVID‐19.
Wang CH, Hilliard ME, Carreon SA, et al. Predictors of mood, diabetes‐specific and COVID‐19‐specific experiences among parents of early school‐age children with type 1 diabetes during initial months of the COVID‐19 pandemic. Pediatr Diabetes. 2021;22(7):1071‐1080. doi: 10.1111/pedi.13255
Funding information National Institutes of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: 1R01 DK102561
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Streisand R, Monaghan M. Young children with type 1 diabetes: challenges, research, and future directions. Curr Diabetes Rep. 2014;14:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdul‐Rasoul M, Habib H, Al‐Khouly M. 'The honeymoon phase' in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006;7:101‐107. [DOI] [PubMed] [Google Scholar]
- 3. Streisand R, Swift E, Wickmark T, Chen R, Holmes CS. Pediatric parenting stress among parents of children with type 1 diabetes: the role of self‐efficacy, responsibility, and fear. J Pediatr Psychol. 2005;30:513‐521. [DOI] [PubMed] [Google Scholar]
- 4. Carroll N, Sadowski A, Laila A, et al. The impact of covid‐19 on health behavior, stress, financial and food security among middle to high income Canadian families with young children. Nutrients. 2020;12:2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elbarbary NS, Dos Santos TJ, de Beaufort C, Agwu JC, Calliari LE, Scaramuzza AE. COVID‐19 outbreak and pediatric diabetes: perceptions of health care professionals worldwide. Pediatr Diabetes. 2020;21:1083‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. APA . Stress in AmericaTM 2020: A national mental health crisis. American Psychological Association October 2020. https://www.apa.org/news/press/releases/stress/2020/report-october. Accessed November 11, 2020.
- 7. Christoforidis A, Kavoura E, Nemtsa A, Pappa K, Dimitriadou M. Coronavirus lockdown effect on type 1 diabetes management οn children wearing insulin pump equipped with continuous glucose monitoring system. Diabetes Res Clin Pract. 2020;166:108307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schiaffini R, Barbetti F, Rapini N, et al. School and pre‐school children with type 1 diabetes during Covid‐19 quarantine: the synergic effect of parental care and technology. Diabetes Res Clin Pract. 2020;166:108302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asmundson GJG, Paluszek MM, Landry CA, Rachor GS, McKay D, Taylor S. Do pre‐existing anxiety‐related and mood disorders differentially impact COVID‐19 stress responses and coping? J Anxiety Disord. 2020;74:102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown SM, Doom JR, Lechuga‐Peña S, Watamura SE, Koppels T. Stress and parenting during the global COVID‐19 pandemic. Child Abuse Negl. 2020;110:104699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ren J, Li X, Chen S, Chen S, Nie Y. The influence of factors such as parenting stress and social support on the state anxiety in parents of special needs children during the COVID‐19 epidemic. Front Psychol. 2020;11:565393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holingue C, Badillo‐Goicoechea E, Riehm KE, et al. Mental distress during the COVID‐19 pandemic among US adults without a pre‐existing mental health condition: findings from American trend panel survey. Prev Med. 2020;139:106231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fortuna LR, Tolou‐Shams M, Robles‐Ramamurthy B, Porche MV. Inequity and the disproportionate impact of COVID‐19 on communities of color in the United States: the need for a trauma‐informed social justice response. Psychol Trauma. 2020;12:443‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hilliard ME, Tully C, Monaghan M, Wang J, Streisand R. Design and development of a stepped‐care behavioral intervention to support parents of young children newly diagnosed with type 1 diabetes. Contemp Clin Trials. 2017;62:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tully C, Clary L, Monaghan M, Levy W, Hilliard ME, Streisand R. Implementation and preliminary feasibility of an individualized, supportive approach to behavioral care for parents of young children newly diagnosed with type 1 diabetes. Cogn Behav Pract. 2021;28(2):293‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Measur. 1977;1:385‐401. [Google Scholar]
- 17. Irwin DE, Stucky B, Langer MM, et al. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res. 2010;19:595‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Counts JM, Buffington ES, Chang‐Rios K, Rasmussen HN, Preacher KJ. The development and validation of the protective factors survey: a self‐report measure of protective factors against child maltreatment. Child Abuse Negl. 2010;34:762‐772. [DOI] [PubMed] [Google Scholar]
- 19. Field AP. Discovering Statistics Using SPSS: (and Sex and Drugs and Rock 'n' Roll). 3rd ed. London: SAGE; 2009. [Google Scholar]
- 20. Lechner WV, Laurene KR, Patel S, Anderson M, Grega C, Kenne DR. Changes in alcohol use as a function of psychological distress and social support following COVID‐19 related university closings. Addict Behav. 2020;110:106527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feeney BC, Collins NL. New look at social support: a theoretical perspective on thriving through relationships. Pers Soc Psychol Rev. 2015;19:113‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DiMeglio LA, Albanese‐O'Neill A, Muñoz CE, Maahs DM. COVID‐19 and children with diabetes‐updates, unknowns, and next steps: first, do no extrapolation. Diabetes Care. 2020;43(11):2631‐2634. [DOI] [PubMed] [Google Scholar]
- 23. Stanek KR, Noser AE, Patton SR, Clements MA, Youngkin EM, Majidi S. Stressful life events, parental psychosocial factors, and glycemic management in school‐aged children during the 1 year follow‐up of new‐onset type 1 diabetes. Pediatr Diabetes. 2020;21:673‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu SR, Modir S. The outbreak that was always here: racial trauma in the context of COVID‐19 and implications for mental health providers. Psychol Trauma. 2020;12:439‐442. [DOI] [PubMed] [Google Scholar]
- 25. Herbert LJ, Clary L, Owen V, Monaghan M, Alvarez V, Streisand R. Relations among school/daycare functioning, fear of hypoglycaemia and quality of life in parents of young children with type 1 diabetes. J Clin Nurs. 2015;24:1199‐1209. [DOI] [PubMed] [Google Scholar]
- 26. Fong VC, Iarocci G. Child and family outcomes following pandemics: a systematic review and recommendations on COVID‐19 policies. J Pediatr Psychol. 2020;45:1124‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wanberg CR, Csillag B, Douglass RP, Zhou L, Pollard MS. Socioeconomic status and well‐being during COVID‐19: a resource‐based examination. J Appl Psychol. 2020;105:1382‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mattioli AV, Sciomer S, Cocchi C, Maffei S, Gallina S. Quarantine during COVID‐19 outbreak: changes in diet and physical activity increase the risk of cardiovascular disease. Nutr Metab Cardiovasc Dis. 2020;30:1409‐1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burckhardt M, Roberts A, Smith GJ, Abraham MB, Davis EA, Jones TW. The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: a randomized crossover trial. Diabetes Care. 2018;41:2641‐2643. [DOI] [PubMed] [Google Scholar]
- 30. Raley S, Bianchi SM, Wang W. When do fathers care? Mothers' economic contribution and fathers' involvement in child care. AJS. 2012;117(5):1422‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sevilla A, Smith S. Baby steps: the gender division of childcare during the COVID‐19 pandemic. Oxf Rev Econ Policy. 2020;36:S169‐S186. [Google Scholar]
- 32. Pierce JS, Gurnurkar S, Vyas N, Carakushansky M, Owens L, Patton SR. Feasibility of implementing a pediatric diabetes clinic via telehealth. Diabetes Spectrsc. 2021;34:ds200060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parry M, Watt‐Watson J. Peer support intervention trials for individuals with heart disease: a systematic review. Eur J Cardiovasc Nurs. 2010;9:57‐67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


