Abstract
Many months after COVID‐19 vaccines were first authorised for public use, still limited supplies could only partially reduce the devastating loss of life and economic costs caused by the pandemic. Could additional vaccine doses have been manufactured more quickly some other way? Would alternative policy choices have made a difference? This paper provides a simple analytical framework through which to view the contours of the vaccine value chain. It then creates a new database that maps the COVID‐19 vaccines of Pfizer/BioNTech, Moderna, AstraZeneca/Oxford, Johnson & Johnson, Novavax and CureVac to the product‐ and location‐specific manufacturing supply chains that emerged in 2020 and 2021. It describes the choppy process through which dozens of other companies at nearly 100 geographically distributed facilities came together to scale up global manufacturing. The paper catalogues major pandemic policy initiatives – such as the United States' Operation Warp Speed – that are likely to have affected the timing and formation of those vaccine supply chains. Given the data, a final section identifies further questions for researchers and policymakers.
Keywords: COVID‐19, export restrictions, subsidies, supply chains, vaccines
1. INTRODUCTION
Pfizer. Moderna. AstraZeneca. Johnson & Johnson. In 2021, vaccines associated with these companies became the symbol of hope for a world desperate to end the COVID‐19 pandemic. The work of these firms likely saved millions of people's lives and reduced the suffering of hundreds of millions more. Yet, one of the most important retrospective questions to ask is whether vaccine makers could have done better. Given the nature of the pandemic and the state of the world in 2020, could more vaccine doses have been manufactured more quickly? Would alternative government policy choices have made a difference?
This paper details the process by which a number of COVID‐19 vaccines were manufactured. It shows how complex global supply chains emerged behind the scenes – in many instances nearly from scratch – to produce the billions of doses of vaccines that have become household names.
It is organised as follows. Section 2 provides a simple analytical framework through which to view the vaccine value chain. It identifies the five main steps critical to getting a new vaccine from start to finish: research and development; clinical trials; production of the drug substance and its formulation into drug product; ‘fill and finish’, or the assembly‐line process of putting a vaccine into millions of tiny vials; and then distribution. The ability to unbundle those first four functions affected how the pharmaceutical industry was organised heading into the pandemic. Splitting apart the third and fourth steps in particular – the heart of the vaccine manufacturing supply chain – ultimately affected how many doses were produced, where and how quickly.
The third section maps six key COVID‐19 vaccine candidates – the four identified above plus Novavax and CureVac – to essential elements of the manufacturing supply chains that emerged. Doing so requires the creation of a new database that links each vaccine to the firms, plants and geographic locations used to produce it, as well as to the timing of matches and other important events.1 Supply chains for most COVID‐19 vaccines were not pre‐determined – they evolved over time, with relationships often set between firms at arm's length, through a very choppy process. Behind the vaccine brands, dozens of other, lesser‐known companies at nearly 100 geographically dispersed facilities played critical roles.
Section 4 catalogues policy initiatives during the pandemic that are likely to have affected the formation of those supply chains. Understanding policy details is critical for evaluating their impact. For example, the United States made considerable public investments to accelerate the scaling‐up of manufacturing supply chains ‘at risk’ (i.e. in advance of any vaccine candidate clearing regulatory hurdles and for which there might have been zero payoff). Unlike others, the US approach also targeted many more upstream elements of the vaccine manufacturing supply chain, subsidising capacity expansion of key input suppliers, not simply downstream vaccine production facilities. Furthermore, policy surely affected the decision of many vaccine makers to establish parallel supply chains in different locations. For example, the highly subsidised contracts that vaccine makers signed with the US administration in mid‐2020 made clear that they would need to establish manufacturing facilities outside the United States if they wanted to simultaneously supply COVID‐19 vaccines to the rest of the world.
Given that demand for this completely new vaccine might reach 14 billion doses or more, could the manufacturing expansion have proceeded more quickly or on a larger scale in 2020 and 2021?2 The fifth section of the paper raises new questions for researchers to investigate, especially once more detailed data become available. Were the at‐risk public investments sufficient? Did pandemic‐era policy interventions miss subsidising enlargement of supplies of critical raw materials and equipment? In the face of extreme scarcity, were inputs and production capacity efficiently allocated and, in the light of newly emerging regulatory information on any particular vaccine, reallocated? Through which channels and how quickly did ‘learning‐by‐doing’ by vaccine manufacturers take place? Did the fact that supply chains crossed borders make coordination more difficult? Did international interdependence prevent vaccine nationalism from being worse than it was?
Before continuing, it is important to note that this analysis does not address the critical issues of vaccine demand and distribution, which are mentioned only briefly in the concluding Section 6. Other research has described the global public health and global economic benefits of an equitable vaccine allocation scheme, prioritising healthcare workers and vulnerable populations, as through the COVID‐19 Vaccines Global Access (COVAX) regime. COVAX was developed in early 2020 by the World Health Organization (WHO), Gavi (the Vaccine Alliance) and CEPI (the Coalition for Epidemic Preparedness Innovations) and aimed to coordinate vaccine manufacturing participants and to finance and procure enough COVID‐19 vaccine doses to administer to 20 per cent of the global population, including the world's poorest countries.3 Through mid‐2021, the ongoing effects of the pandemic meant that global limits to vaccine demand were unlikely to be a binding constraint on the main manufacturing supply chain issues of focus here.4
2. INDUSTRY ORGANISATION HEADING INTO THE PANDEMIC
Manufacturing vaccines is different from production of many of the small‐molecule drugs provided by the pharmaceutical industry.5 Unlike drugs given to sick patients, vaccines are typically provided to healthy individuals. Every year, vaccines are given to more than a billion people, necessitating their rigorous oversight. Sponsors must establish their safety and efficacy in multiple rounds of clinical testing. Working with manufacturers, they must demonstrate to national regulatory authorities that multiple sets of personnel can produce the vaccine consistently, according to clear and documented procedures, with multiple sources of equipment and raw materials, for an extended period of time without failure or interruption. Furthermore, the safety, effectiveness and quality of the vaccine continues to be closely regulated even after regulatory approval. Whereas the intellectual property for a small‐molecule drug might be adequately captured by a chemical compound alone, the technology for vaccines is equal part the production process.
Getting a new vaccine from beginning to end – from concept to delivering shots into the public's arms – requires five steps associated with five, largely separable, sets of fixed costs (Figure 1).
FIGURE 1.
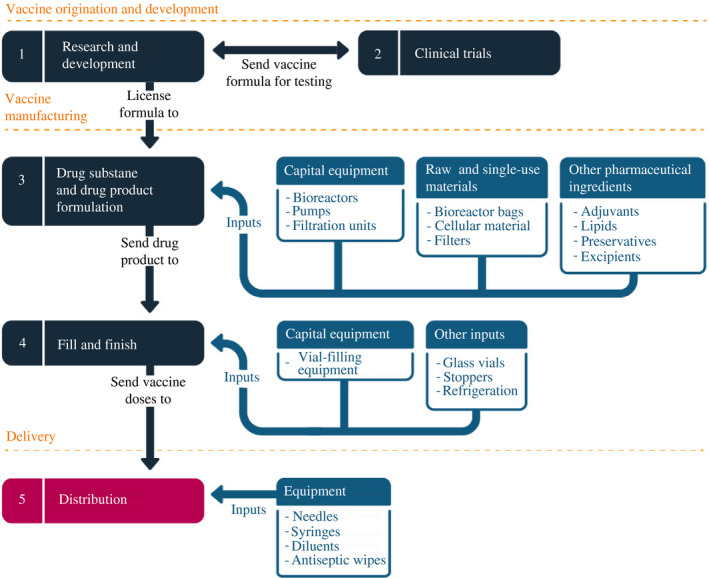
Vaccine manufacturing is a multistage process that requires extensive cooperation
Note: Stages and inputs depicted illustrate general vaccine production process and are not comprehensive
Source: Constructed by the authors
The first are the costs associated with the preclinical stage of research and development. Building on decades of scientific research and previous discovery, as well as new methods, scientists sought antigens – foreign substances that, when introduced into the body, induce an immune reaction – that triggered the same reaction as the virus does.
It normally takes years to identify vaccines, but things moved extraordinarily quickly in response to COVID‐19. China shared the genetic sequence of the novel coronavirus, named SARS‐CoV‐2, with the WHO in early January 2020. By 24 February 2020, for example, Moderna had already begun to ship its vaccine candidate off for Phase 1 clinical trials. By early April, BioNTech, Oxford, Janssen, Novavax and many other companies had all identified their leading COVID‐19 vaccine candidates.
The second step involved multiple rounds of clinical trials, which also proceeded at unprecedented speed. Trials start with relatively small numbers of healthy people – 45 in the cases of Pfizer and Moderna – to establish the safety of the candidate vaccine, as well as information as to whether it was triggering the desired immune response. Subsequent stages involve increasingly larger numbers of people, in order to generate preliminary estimates of safety, efficacy, dosage and adverse reactions. The critical phase 3 trial requires recruiting tens of thousands of people – who are randomly allocated (randomised) to be administered either the candidate vaccine or a control (a known comparator product, often a placebo) – and then tracking them over time to determine whether the vaccine was safe and effective. These clinical trials are performed according to protocols approved and overseen by national regulatory agencies and ethics committees. Smaller entities – such as biotech companies or universities – often lack the capacity to complete the costly late‐stage clinical trials necessary to support applications for marketing approval (licensure).
Before COVID‐19, clinical development of a novel vaccine had never been completed in less than 4 years, and it often took more than a decade. Development of some COVID‐19 vaccines occurred in a matter of months, thanks to innovative trial designs; the active support of national regulatory agencies, such as the US Food and Drug Administration (FDA); and financing and coordination support from the US National Institutes of Health (NIH), the WHO and others. In early December 2020, less than a year after public reports of the SARS‐CoV‐2 emerged, regulatory agencies – starting with the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom and the FDA in the United States – authorised the first COVID‐19 vaccines for expanded public use.
Manufacturing comes next, in two often separable steps. The first phase of manufacturing was creation of the drug substance and its formulation into a drug product.6 Scaling up production for the pandemic required plants capable of generating tens of millions, if not hundreds of millions, of doses a year. The fixed costs of such production facilities included creating and maintaining hyper‐clean rooms, acquiring specialised capital equipment such as bioreactors and filtration pumps, and employing skilled personnel able to transfer the technology behind the vaccine from a laboratory test tube to dedicated mass‐production lines. These facilities also required a number of critical and specialised variable inputs, including single‐use bioreactor bags, filters and cellular material. The process ultimately combined the drug substance with other pharmaceutical ingredients, such as excipients, adjuvants and preservatives, depending on the vaccine, to formulate a drug product.7 Mass volumes of some specialised ingredients were needed from other pharmaceutical companies, through separate nodes of a supply chain.
The fourth step of the entire process typically involved a separate manufacturing facility capable of receiving the drug product in order to ‘fill’ (squirt doses into vials) and ‘finish’ (cap the vials with stoppers and then label and package) the vaccine, so that it was ready for distribution. The fill‐and‐finish plants required specialised assembly‐line capital equipment, in addition to variable inputs like glass vials and stoppers. Materials were also needed for packaging and shipping, sometimes including cold storage.
The fifth and final stage was delivery. Upon receipt of the glass vials containing the vaccine at a distribution centre, skilled personnel would also need access to needles, syringes, antiseptic wipes and sometimes additional pharmaceutical ingredients. Some vaccines were shipped frozen and in concentrated form, requiring on‐site dilution. Only after the appropriate diluents were added could healthcare workers safely administer the appropriate dosage into the arms of people waiting to be inoculated.
Heading into the pandemic, the pharmaceutical industry employed a range of business models. At one extreme were legacy, integrated pharmaceutical companies, potentially performing each of those first four steps themselves. Table 1 lists the top 10 pharmaceutical firms by sales revenue over the last four decades. Although some companies were critical to certain supply chains during the pandemic, the integrated approach was hardly the dominant model.
TABLE 1.
Top 10 global pharmaceutical firms, by sales revenue, 1990–2020
| Ranking | 1990 | 2000 | 2010 | 2020 | 2020 revenues (billions of dollars) |
|---|---|---|---|---|---|
| 1 | Merck & Co. | Pfizer | Pfizer | Johnson & Johnson | 82.6 |
| 2 | Bristol‐Myers Squibb | GlaxoSmithKline | Novartis | Roche | 62.1 |
| 3 | Glaxo | Merck & Co. | Sanofi | Novartis | 48.7 |
| 4 | SmithKline Beecham | AstraZeneca | Merck & Co. | Merck & Co. | 48.0 |
| 5 | Ciba‐Geigy | Bristol‐Myers Squibb | GlaxoSmithKline | AbbVie | 45.8 |
| 6 | American Home Products | Novartis | Roche | GlaxoSmithKline | 43.8 |
| 7 | Hoechst | Johnson & Johnson | AstraZeneca | Bristol‐Myers Squibb | 42.5 |
| 8 | Johnson & Johnson | Aventis | Johnson & Johnson | Pfizer | 41.9 |
| 9 | Bayer | Pharmacia | Eli Lilly | Sanofi | 41.1 |
| 10 | Roche | American Home Products | Abbott | Takeda | 29.2 |
Sources: Pharmtech for 1990 and 2000, Statista for 2010 and Fierce Pharma for 2020. Companies in bold are involved in COVID‐19 vaccines described below.
The business model that much of the pharmaceutical industry had shifted towards over the previous 25 years involved fragmentation. As tariffs and other trade barriers had fallen globally, information and communications technology (ICT) developed, shipping and logistics efficiency increased, and protection of intellectual property rights steadily improved. The fact that trade could play a greater role in distributing pharmaceutical products globally meant that companies could operate fewer plants but at a larger scale.
At the same time, separability of these fixed costs contributed to breaking apart the vaccine production process. Firms could specialise in one step, leaving the remainder to be done by other firms through arm's length contracts. Furthermore, the dot.com boom increased the availability of venture capital. The genome project and other scientific advancements provided small biotech companies and university researchers with a starting point, which, coupled with the availability of external financing, meant that their new drug innovations could compete with those at the integrated pharmaceutical companies.8 Capitalising on those inventions also became less and less constrained by the need for scientists and innovators to have access to their own manufacturing facilities. Contract development and manufacturing organisations (CDMOs) could be hired to handle just the production, covering the third or fourth steps of the process shown in Figure 1.
Table 2 lists the top CDMOs by revenue in 2020. The revenues of the largest firms have grown over time, albeit remaining smaller than those of the top pharmaceutical companies (see Table 1). Some CDMOs have become global, operating plants in multiple countries and handling various parts of pharmaceutical production. Despite their relative anonymity, companies like Lonza and Catalent played incredibly important roles in manufacturing COVID‐19 vaccines during the pandemic. Finally, some major pharmaceutical companies listed in Table 1 – like Pfizer and GlaxoSmithKline (GSK) – had also developed business operations to offer CDMO‐like services to other firms, to better manage their own capacity.9
TABLE 2.
Top contract development and manufacturing organisations (CDMOs), by sales revenue in 2020
| Revenues (millions of dollars)/firms | Headquarters |
|---|---|
| 3 , 000–5 , 000 | |
| Lonza | Switzerland |
| Catalent | United States |
| Thermo Fisher Scientific (Patheon) | United States |
| 1 , 000–3 , 000 | |
| Fareva | France |
| Recipharm | Sweden |
| Wuxi AppTec/Bio | China |
| Siegfried | Switzerland |
| Delpharm | France |
| 750–1 , 000 | |
| Cambrex | United States |
| Albany Molecular Research (AMRI) | United States |
| Vetter | Germany |
| Aenova Group | Germany |
| Boehringer‐Ingelheim | Germany |
| Fujifilm Diosynth Biotechnologies (FDB) | Japan |
| 500–750 | |
| Ajinomoto | Japan |
| Almac Group | United Kingdom |
| Baxter Biopharma Solutions | United States |
Source: Constructed by the authors with data provided by Jim Miller at Drug, Chemical & Associated Technologies (Miller, 2021). Companies in bold are involved in the COVID‐19 vaccines described below.
3. SETTING UP VACCINE SUPPLY CHAINS IN THE MIDST OF A PANDEMIC
CEPI conducted a survey of global vaccine manufacturing capacity early in the pandemic, in an attempt to map the landscape of the resources that might be tapped (CEPI, 2020). By June 2020, its main takeaway was that existing vaccine manufacturing capacity was concentrated in India, Europe and North America (data for China were unavailable). The supply chains that emerged over the following year reflected this concentration.
According to the WHO, 291 COVID‐19 vaccine candidates were in the pipeline as of July 2021, including 184 in pre‐clinical development and 107 in clinical development.10 Six vaccines – Pfizer/BioNTech, Moderna, AstraZeneca/Oxford, Johnson & Johnson (Janssen), Sinopharm and Sinovac – had received regulatory approval for emergency use from the WHO, the FDA, MHRA and/or the European Union's European Medicines Agency (EMA) and were in widespread deployment around the world (Table 3). One other candidate – Novavax – seemed close (for that and other reasons, it is included in the analysis). A handful of other vaccine candidates – especially from India (Bharat Biotech) and Russia (Sputnik V) – had already been put into circulation domestically and in selected countries even before they received WHO emergency use listing. With the exception of Johnson & Johnson, each of these vaccines involved a two‐dose regimen. Other attempts – including by major industry players such as Merck and Sanofi/GSK, as well as CureVac – did not clear clinical trials. That so many candidates made it through so quickly is a scientific anomaly.
TABLE 3.
Dates key regulators authorised emergency use for various vaccines
| Vaccine |
FDA (US) |
EMA (EU) |
MHRA (UK) |
DCGI (India) |
China | Russia | WHO |
|---|---|---|---|---|---|---|---|
| Pfizer/BioNTech | 11 December 2020 | 21 December 2020 | 2 December 2020 | NA | NA | NA | 31 December 2020 |
| Moderna | 18 December 2020 | 6 January 2021 | 8 January 2021 | 29 June 2021 | NA | NA | 30 April 2021 |
| Johnson & Johnson a | 27 February 2021 | 11 March 2021 | 28 May 2021 | NA | NA | NA | 12 March 2021 |
| AstraZeneca | NA | 29 January 2021 | 30 December 2020 | 3 January 2021 | NA | NA | 15 February 2021 b |
| Sinopharm | NA | NA | NA | NA | 5 February 2021 | NA | 7 May 2021 |
| Sinovac | NA | NA | NA | NA | 31 August 2020 | NA | 1 June 2021 |
| Sputnik V | NA | NA | NA | 20 April 2021 | NA | 2 December 2020 | NA |
| Bharat Biotech | NA | NA | NA | 3 January 2021 | NA | NA | NA |
| Novavax | NA | NA | NA | NA | NA | NA | NA |
| CureVac | NA | NA | NA | NA | NA | NA | NA |
Dates are as of 15 July 2021.
Abbreviations: DCGI, Drugs Controller General of India; EMA, European Medicines Agency; FDA, Food and Drug Administration; MHRA, Medicines and Healthcare products Regulatory Agency; NA, Not authorised; WHO, World Health Organization.
Johnson & Johnson's vaccine was one dose, the others were all a two‐dose regimen.
The WHO ultimately issued an emergency use licence for the AstraZeneca vaccine from three sources: Serum Institute of India, SK bioscience and facilities in Europe.
Source: Constructed by the authors.
The geographic concentration of vaccine production was one reason why trade would play a substantial role in inoculating much of the global population. Most of Sub‐Saharan Africa, for example, as well as low‐ and middle‐income countries elsewhere, rely on imports, as they had little pre‐pandemic experience manufacturing vaccines locally. Trade was also critical because of the cross‐border nature of many vaccine supply chains that emerged during the pandemic, including trade in specialised inputs, the manufacturing of which was also characterised by the geographic concentration of suppliers.
Production of most of the COVID‐19 vaccines involved the establishment of multiple supply chains, partly out of fears that governments would resort to ‘vaccine nationalism’ – or the refusal to export doses, at least until their populations had been fully served.11 The possibility of this outcome was made obvious to pharmaceutical companies early in the pandemic, when the Trump administration demanded contractual terms that vaccines manufactured in the United States remain there, as the property of the US government. The United States was not alone: The UK government publicly adopted a similar strategy.12 Companies thus quickly learned that providing vaccines to other markets meant also manufacturing them from other markets.
Some of these vaccines also required additional nodes of production – separate mini‐supply chains – feeding into the main manufacturing supply chain illustrated in Figure 1. Pfizer/BioNTech and Moderna, for example, required massive volumes of lipid nanoparticles, and Novavax required a specialised adjuvant, a product that helps boost the body's immune response to the antigen.
Finally, companies would complain about limited availability of critical inputs throughout the pandemic. At times, there were too few single‐use bioreactor bags, filtration pumps, filters, skilled workers, financial capital and even partner companies with idle capacity to quickly scale up their production processes.
3.1. Pfizer/BioNTech
BioNTech – a biotech firm located in Mainz, Germany founded by Özlem Türeci, a child of Turkish immigrants, and Uğur Şahin, a Turkish immigrant – invented a messenger ribonucleic acid (mRNA) COVID‐19 vaccine early in 2020. On March 17, it announced a partnership with Pfizer in which the global pharmaceutical company would assist in clinical development and manufacturing for all markets outside of China. The two companies had a prior commercial relationship; in August 2018, for example, they had signed a collaborative agreement to develop mRNA‐based vaccines for the prevention of influenza. The Pfizer/BioNTech candidate would be the first vaccine to receive authorisation for emergency use by four of the main regulators, getting the nod from the MHRA, the FDA, the EMA and the WHO in December 2020 (see Table 3).
Pfizer and BioNTech had begun setting up their vaccine supply chains much earlier. Production would initially take place through a web of existing plants, most of them belonging to Pfizer (Figure 2).13 To start, Pfizer developed the first stage of the drug product (DNA plasmids) at a plant in Missouri. These plasmids were then frozen, packed and shipped to two plants – a Pfizer facility in Andover, Massachusetts and a BioNTech site in Mainz. At those plants, the DNA was turned into the mRNA – the active pharmaceutical ingredient. Bags of filtered mRNA were then sent to two additional sites for the last stage of formulation, fill and finish. The Andover mRNA was sent to a Pfizer plant in Michigan, and the Mainz mRNA was sent to a Pfizer facility in Puurs, Belgium. From there, the vaccine vials were packaged and distributed.
FIGURE 2.
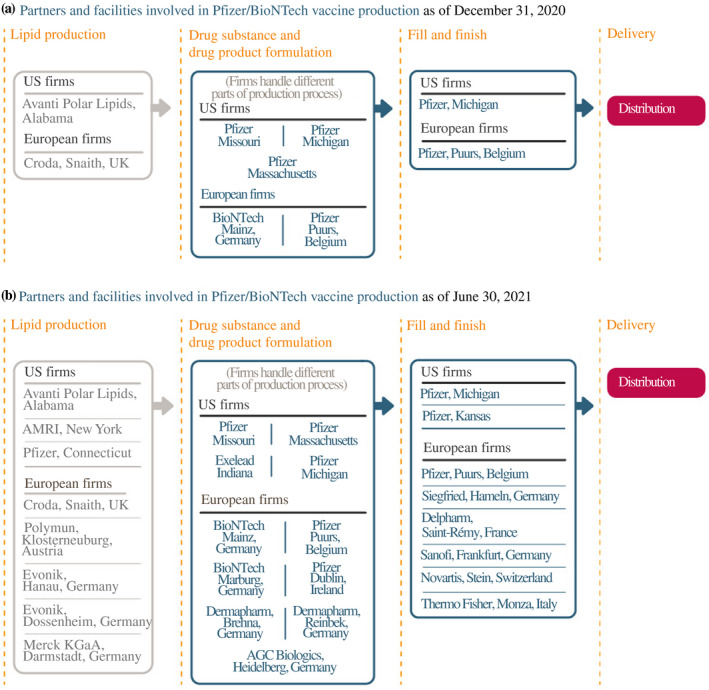
How Pfizer and BioNTech scaled up their manufacturing network
Source: Constructed by the authors based on firm announcements and media reports. See Table A1 in the accompanying database for timing and links to original sources
The formulation prepared at the facilities in Michigan and Belgium required vast supplies of lipid nanoparticles to combine with the mRNA. The lipids had their own specialised supply chains. BioNTech licensed technology from Acuitas, a Canadian firm, but the lipids were then manufactured at scale elsewhere. Pfizer's lipids were produced by Avanti Polar Lipids of Alabama, a subsidiary of the British company, Croda, under a five‐year contract signed in November. Croda also had a plant in Snaith in the United Kingdom; the Telegraph reported that it was the source for the essential lipid nanoparticles used by Pfizer in the Belgian plant.14 This finding is consistent with the data illustrating a sharp increase in UK exports of lipids, first to Belgium and then to Germany in early 2021 (Figure 3). The Financial Times later reported that this flow of exports from Britain was the input dependence that kept the European Commission from imposing export restrictions on vaccines in early 2021, including those involved in a dispute with AstraZeneca described below.15 BioNTech subsequently contracted with firms like Evonik and Merck KGaA to manufacture lipids at facilities within the European Union, not just the United Kingdom, perhaps out of growing concern that UK–EU tensions over the AstraZeneca vaccine would put their supply chains at risk.
FIGURE 3.
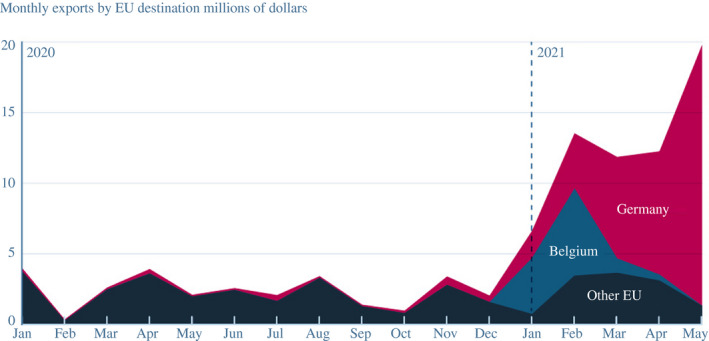
UK exports of lipid nanoparticles were critical to manufacturing the Pfizer/BioNTech vaccine in Europe
Note: EU, European Union. Converted from British pounds to US dollars using end‐of‐month exchange rates from Federal Reserve Economic Data
Source: UK Trade Info Overseas trade data table, commodity code 29225000
Given the early successes of the Pfizer/BioNTech vaccine, demand increased. The companies expanded each element of their US and European supply chains to increase capacity (see the bottom panel of Figure 2). Pfizer announced that it would manufacture lipid nanoparticles at one of its plants in Connecticut, and it added new vaccine formulation capacity in Michigan, as well as more fill and finish at another facility in Kansas. (It also signed up Exelead, a CDMO with experience producing lipid nanoparticles, to help scale up production.) For Europe, Pfizer began to use one of its plants in Ireland, and BioNTech's newly acquired plant from Novartis in Germany became operational in February 2021. BioNTech signed up other firms to formulate the mRNA active ingredients or produce lipids, as well as Siegfried, Delpharm, Sanofi, Novartis and Thermo Fisher to fill and finish in various plants across Europe, taking some of the load off the Pfizer facility in Belgium (which nevertheless also expanded capacity).
It would take much longer for the Pfizer/BioNTech vaccine to build capacity outside of the United States and Europe. Only in May 2021, for example, did BioNTech announce construction of a new manufacturing facility in Singapore, to be subsidised by the Singaporean government; the plant is not expected to become operational until 2023. Although BioNTech had disclosed a partnership with Shanghai Fosun Pharmaceutical to distribute its vaccine in China in March 2020, it took until May 2021 before they formally agreed for the joint venture to produce at a manufacturing facility owned by Fosun in China. And only in July 2021 did Pfizer and BioNTech strike a deal with the Biovac Institute in South Africa to use its Cape Town facility to fill and finish the vaccine supplied from plants in Europe for distribution across the African Union beginning in 2022.
Despite their extraordinary success, Pfizer and BioNTech ran into input shortages as they attempted to expand. As Uğur Şahin explained in an interview with Der Spiegel in January 2021,16 ‘We are currently trying to find new cooperation partners who will be able to produce the vaccine for us. But it's not as if there are unused, specialized factories sitting around the world that can start producing the vaccine tomorrow in the quality necessary’.
The companies also worried about running short of specific inputs for their existing production facilities. Unlike the other vaccine companies with which the US government contracted in 2020, Pfizer's first contract in July was not given a ‘priority rating’ under the Defense Production Act (DPA). Without the priority rating, Pfizer was not able to jump to the head of the line on supply acquisition, as discussed in Section 4.1. Pfizer reportedly struggled and requested US government help ‘to give the company better access to roughly nine specialised products it needs to make the vaccine’, including lipids.17 The Wall Street Journal later reported that initial shortages meant that ‘Pfizer figured out how to stretch scarce supplies of special filters needed for the vaccine production process by recycling them’.18 When asked in mid‐December 2020 whether Pfizer would request the US government to invoke the DPA on its behalf, CEO Albert Bourla said,19 ‘We are asking them, and I hope that they will do it very soon because, particularly in some components, we are running at critical supply limitations’. Pfizer's second contract with the US government, signed December 22, was granted a DPA priority rating. Then, on 5 February 2021, shortly after assuming office, the Biden administration announced that it was further ‘expanding the priority ratings for Pfizer to include filling pumps and tangential flow filtration skid units, critical components Pfizer needs to manufacture the COVID vaccine’.20
3.2. Moderna
Moderna is a Cambridge, Massachusetts, biotech start‐up founded in 2010. In collaboration with scientists at NIH, Moderna also invented an mRNA vaccine candidate. To support its Phase 2 and 3 trials, it first teamed with PPD, a contract research organisation. Moderna reportedly ran into hiccups with regulators along the way21 – one potential example of learning by doing for a company without much experience in vaccine trials – which may have slightly delayed its deployment. Nevertheless, Moderna received emergency use authorisation from the FDA on 18 December 2020.22
Moderna took a very different approach from Pfizer and BioNTech to create its manufacturing supply chain (Figure 4). Unlike those companies, it had to start from scratch. Moderna had a facility in Massachusetts for manufacturing smaller batches of its vaccine for clinical trials, but that plant was not large enough for commercial‐scale production. It teamed with Lonza, a global CDMO, signing a 10‐year strategic contract 1 May 2020. Lonza established production lines at a plant in New Hampshire, partly supported by US government funding, as well as at another facility in Switzerland for vaccine sales destined for outside the US market. (The Swiss facility did not appear to be subsidised at risk and was thus slower to come online.23) The mRNA nature of Moderna's vaccine also required large‐scale volumes of lipid nanoparticles, for which, Moderna collaborated with CordenPharma, another CDMO. Moderna had a prior relationship with CordenPharma, which could produce at sites in Colorado, Switzerland and France. The fill and finish for Moderna's vaccine was initially done by Catalent in the United States and in Spain by Rovi for the European supply chain.
FIGURE 4.
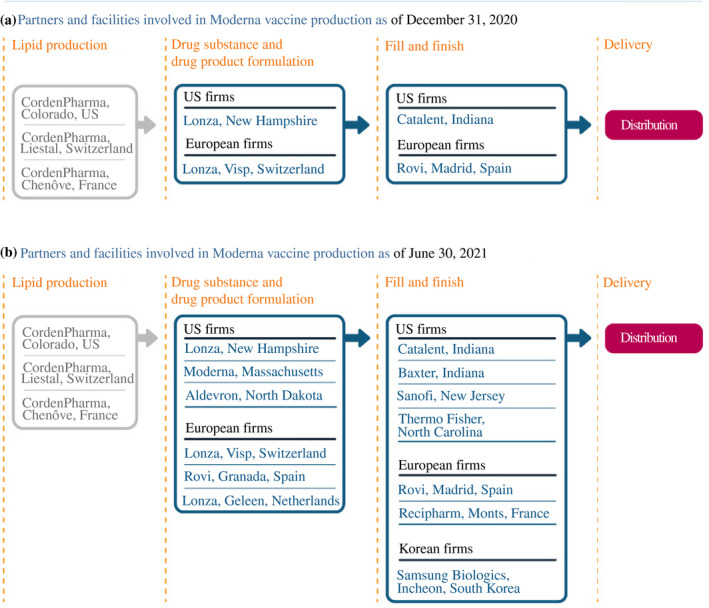
How Moderna scaled up its manufacturing network
Source: Constructed by the authors based on firm announcements and media reports. See Table A2 in the accompanying database for timing and links to original sources
Seeing early success, demand for its vaccine increased and Moderna also sought to expand. For drug substance in Europe, Moderna teamed with Rovi, at another of its facilities in Spain, and Lonza, at another plant in the Netherlands. In the United States, Moderna announced it would renovate its Massachusetts plant to increase its local manufacturing capacity. Fill and finish would expand to facilities run by Baxter, Sanofi, and Thermo Fisher for the US supply chain and Recipharm in France for Europe.24 There is evidence of a substantial increase in exports of vaccines from Switzerland to first Spain and then France in 2021, consistent with Moderna's drug product being exported to those two countries for fill and finish (Figure 5).
FIGURE 5.
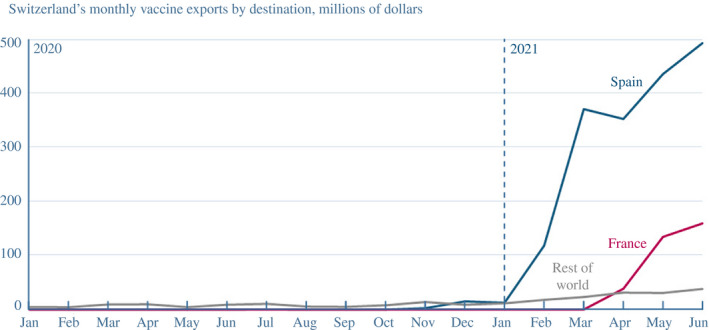
Keeping European supply chains open allowed Moderna to export its vaccine to Spain and France for fill and finish
Note: Converted from Swiss francs to US dollars using end‐of‐month exchange rates from Federal Reserve Economic Data
Source: Swiss Federal Customs Administration, commodity code 30022000
Moderna also publicly complained about input shortages hampering its ability to increase production, especially when shipments to the United Kingdom and Canada from its European supply chain were lower than expected in early 2021. CEO Stéphane Bancel stated unequivocally, ‘The bottleneck right now is people’, complaining that Modern's partner Lonza could not find enough local skilled workers to expand its European facilities. It asked the Swiss government to streamline work visas and sought to borrow specialist staff from other Swiss companies.25
3.3. AstraZeneca/Oxford University
AstraZeneca was at the heart of four controversies – each a case study of problems that can emerge when attempting to quickly scale up vaccine manufacturing.
The AstraZeneca vaccine story began in March 2020, when researchers at Oxford University publicly identified a vaccine candidate. Lacking large‐scale distributional experience, the academics tapped their personal connections by first touching base with Merck, a global pharmaceutical company headquartered in the United States. Those negotiations reportedly faltered, for a number of reasons, including the British government's concerns about tying up the vaccine exclusively with a US company, given the Trump administration's America First policy.
On April 30, Oxford partnered with AstraZeneca, a British–Swedish pharmaceutical company with global operations headquartered in Cambridge, England. In May, Oxford Biomedica signed up to produce the vaccine for clinical trials; in June, a Scottish plant (run by Symbiosis Pharmaceutical) agreed to do the fill‐and‐finish work. For commercial‐scale production, Cobra Biologics, UK, agreed to produce the drug product in England, and CP Pharmaceuticals was contracted to do fill and finish in Wales. (In January 2021, the Welsh facility was almost flooded, but disaster was averted.)
Despite this UK–centric supply chain – partially facilitated by the UK government, as described below – AstraZeneca's vaccine aspirations were global. But AstraZeneca would end up mostly coordinating multiple CDMOs into a global supply chain network rather than tapping its own facilities and operating as a globally integrated pharmaceutical company (Figure 6). That decision may have partially contributed to many of the challenges that emerged.
FIGURE 6.
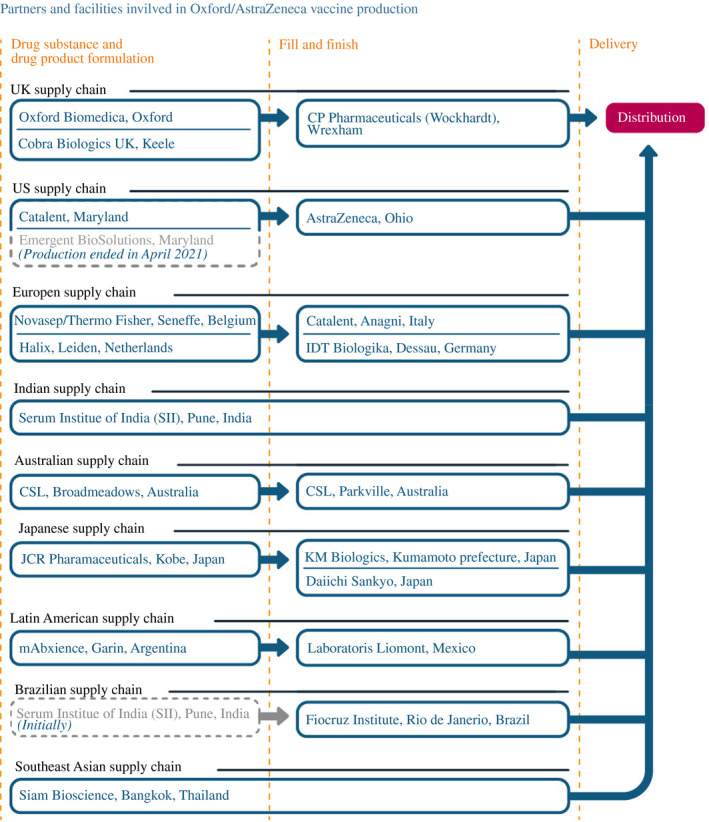
How AstraZeneca scaled up its manufacturing network
Note: As of 30 June 2021. The Novasep plant in Belgium was taken over by Thermo Fisher in January 2021
Source: Constructed by the authors based on firm announcements and media reports. See Table A3 in the accompanying database for timing and links to original sources
3.3.1. The clinical trial, data and public health controversies
On 9 September 2020, AstraZeneca paused all of its trials after a patient in its UK Phase 3 trial experienced an unexplained illness. Its UK trial resumed on September 12. Soon thereafter, trials began again in Brazil, South Africa, India and Japan. Only on October 23 did the FDA authorise resumption of the US Phase 3 trial. This delay was the first public sign of discord with US regulators.
On November 23, AstraZeneca released what it believed were positive results from two dosing regimens, with pooled data from different phases of trials taking place in different countries. The results, ultimately published in The Lancet on December 8, confused and sowed doubts among some regulators.26 Nevertheless, the United Kingdom authorised the vaccine for emergency use on December 30. India approved the vaccine for emergency use on 6 January 2021, and the EMA allowed its use across the European Union on January 29.
As the vaccine began to be rolled out, a handful of Europeans experienced a rare blood‐clotting condition, which led to a few deaths. Many countries – including France, Germany, Italy, Portugal and Spain – paused their vaccination campaigns while the EMA investigated the source of the side effects. Some countries eventually resumed distributing the vaccine, but some discontinued its use entirely.
AstraZeneca did not release its US Phase 3 trial results until March 22; when it did, it faced almost immediate rebuke. The US National Institute for Allergy and Infectious Diseases (NIAID), part of the NIH, indicated that the trial's independent data‐monitoring board had raised ‘concerns’ about the data AstraZeneca had chosen to highlight. As of July 2021, the vaccine had still not received emergency use authorisation in the United States.
3.3.2. The Serum Institute controversies
One troublesome element of AstraZeneca's global supply chain involved its partnership with the Serum Institute of India (SII), the largest vaccine manufacturer by volume in the world prior to the pandemic. In June 2020, AstraZeneca and SII formed a partnership, with SII committing to participate in the COVAX programme and promising to supply 400 million doses – of what it would call Covishield – by the end of the year in exchange for financial support from CEPI as well as Gavi. In an interview with the New York Times shortly thereafter, CEO Adar Poonawalla explained that SII was making at‐risk vaccine investment by relying on his family's own resources and not the Operation Warp Speed funding that manufacturers in the United States were receiving for scaling up their production at risk.27
Despite its promises, SII underdelivered. Determining how much was standard, manufacturing learning‐by‐doing vs. other shocks will be an important question for researchers to try to disentangle once data become available, but reports point to a number of mitigating factors. On 21 January 2021, SII's facility in Pune suffered a fire, killing five people. At the time, Poonawalla indicated that the fire would have no impact on supplies, tweeting ‘I would like to reassure all governments & the public that there would be no loss of #COVISHIELD production due to multiple production buildings that I had kept in reserve to deal with such contingencies at @SerumInstIndia’. Yet 2 months later, the Times of India reported that Poonawalla had broken contracts with Brazil, Morocco and Saudi Arabia, declaring force majeure and backtracking with a letter that indicated ‘Regrettably, a fire at one of our buildings has caused obstacles to the expansion of our monthly manufacturing output’.28
On February 20, Poonawalla indicated that SII's vaccine exports would fall further because of the Indian government. ‘Dear countries & governments’, he tweeted, ‘as you await #COVISHIELD supplies, I humbly request you to please be patient, @SerumInstIndia has been directed to prioritise the huge needs of India and along with that balance the needs of the rest of the world. We are trying our best’.29 On March 25, Gavi was forced to notify recipient countries in the COVAX programme of the stalled shipments from SII; on April 7, AstraZeneca served SII with a legal notice for vaccine delivery delays.30
Poonawalla then accused President Biden of imposing an ‘embargo of raw material exports’, suggesting that US policy was the cause of SII's delivery delays.31 Although input shortages likely affected SII, as it had other vaccine manufacturers, there was never a US export embargo.32 SII imports from vaccine suppliers operating in the United States had actually increased considerably in the 6 months from October 2020 to March 2021 (Figure 7).
FIGURE 7.
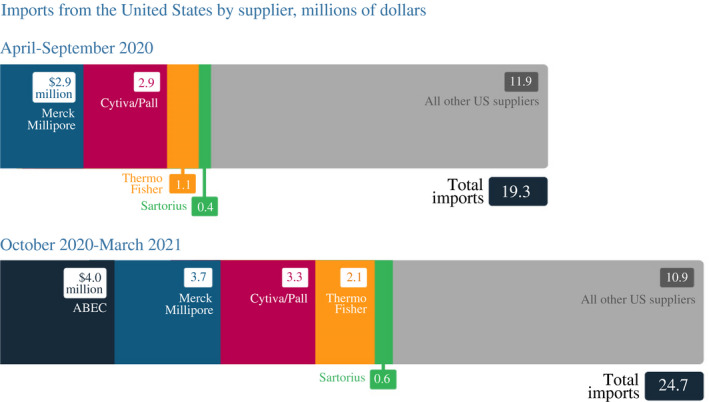
Serum Institute of India's imports of US vaccine material were up in October 2020–March 2021 from the previous 6 months
Source: Bown and Rogers (2021) with data from S&P Global Market Intelligence Panjiva
Poonawalla seemed to reverse course again in a stunning interview with the Financial Times on May 2. Instead of input shortages holding back production, he claimed, he had decided against expanding SII's production capacity earlier because ‘there were no orders, we did not think we needed to make more than 1 billion doses a year’.33 India was suffering perhaps the worst disease outbreak of anywhere in the world at that point, and Poonawalla temporarily escaped to London.
3.3.3. The Emergent BioSolutions and US market controversies
A second troublesome supply chain for AstraZeneca involved its US‐based production. Plans started quickly, however, and initially with high expectations. In June 2020, AstraZeneca signed an agreement with Emergent BioSolutions to produce its drug substance in Maryland, with funding from the US government, initially to produce investigational doses for use in its clinical trials. (In July 2020, an agreement was made for the Emergent facility to expand capacity from clinical to commercial scale.) In August, Catalent announced that it would also produce AstraZeneca's drug substance at a nearby Maryland facility. Fill and finish for the US‐manufactured product would be done at an AstraZeneca plant – potentially the only AstraZeneca facility put to early use for COVID‐19 vaccine production in its global supply chain – in Ohio. In late October, AstraZeneca signed a $1.6 billion contract with the US government under Operation Warp Speed.
Starting in March 2021, the New York Times ran a series of reports revealing quality control concerns at the Emergent facility. The lack of oversight resulted in tens of millions of manufactured vaccine doses having to be discarded. Cross‐contamination occurred, as the Maryland facility was also used to manufacture the Johnson & Johnson vaccine (as discussed in Section 3.4). In April 2021, the Biden administration pushed AstraZeneca production out of the Emergent plant, handing over its operation entirely to Johnson & Johnson and its quality control managers. Emergent then became subject to a Congressional inquiry, and in July, investors sued the company's executives for alleged insider trading.34 The lost doses may have not materially affected the US vaccine rollout, but the Emergent fiasco meant that fewer doses of the AstraZeneca vaccine were available for export to places that had authorised the vaccine for emergency use, including many poor countries.
3.3.4. Other European controversies
AstraZeneca's most public spat was perhaps with the European Union. It was caught in the crossfire of Brexit, the departure of Britain from the European Union that was finally nearing completion after 5 years of acrimonious, on‐and‐off negotiations.
Starting in June 2020, AstraZeneca began to establish an additional (outside the United Kingdom) supply chain across Europe. At a Belgian plant, Novasep would initially produce its drug substance. In December, a Halix facility in the Netherlands was signed up; in February, AstraZeneca signed with an IDT Biologika plant in Germany.35 Fill and finish for the European supply chain started with Catalent agreeing in June 2020 to use its plant in Italy. In January 2021, Insud Pharma in Spain signed on, as did IDT Biologika in April, when it convinced another customer (Merz Pharma) to release capacity previously booked to bottle another drug.
Set against this emerging supply chain, AstraZeneca's public controversy with the European Union began on 22 January 2021, when the company informed Brussels to expect delivery shortfalls. Coming less than a month after the formal completion of the bruising Brexit negotiations, and in the political context of a relatively more successful vaccination campaign taking place in Britain, the message raised suspicions at the European Commission that AstraZeneca was making good on delivery commitments to the UK at its expense.
On 28 January 2021, EU regulators raided the Belgian plant for inspections. The Wall Street Journal reported that AstraZeneca's low vaccine yields at the facility were the source of the shortfall.36 (Thermo Fisher had taken over operations of the plant in January as part of its buyout of Novasep's viral vector manufacturing business.) The next day, the Commission set up an EU‐wide export authorisation programme to determine how many vaccines produced in EU Member States were being exported and to where.
Also on January 29, the Commission invoked the Northern Irish protocol, which implemented a land border between Ireland and Northern Ireland. Within hours it reversed that politically explosive decision, but much of the damage had been done. Relations between Brussels and London had soured, and tension between AstraZeneca and Europe continued to build.
Fearful of vaccine shortages, the United Kingdom sought additional dosages of AstraZeneca vaccine from the company's other supply chains. MHRA, the UK regulatory agency, sent inspectors to the SII manufacturing site in India, and on February 23 the United Kingdom authorised the SII‐manufactured Covishield for domestic use.37 Shortly thereafter, the United Kingdom announced an expected shipment of 10 million doses of the AstraZeneca vaccine from SII to help overcome its shortfalls. Only 5 million doses were ultimately delivered before a new wave of disease caused the Indian government to shut down exports.
On the continent, the frustrations of EU Member States with AstraZeneca did not dissipate. On March 4, Italy refused to allow exports of 250,000 doses destined for Australia to leave the Catalent facility. Two weeks later, Italian military police raided the Italian plant, after EU Internal Market Commissioner Thierry Breton was alerted to accounting irregularities between AstraZeneca's promised doses and deliveries to the European Union.38
AstraZeneca's failure to meet delivery targets led the European Union to bring legal action against the company, on April 26. The European Commission ultimately decided against extending its vaccine contracts with AstraZeneca. Concerns with blood clots and contracts, as well as the existence of more effective alternatives from Moderna and Pfizer/BioNTech, all played a role. By mid‐2021, deployment of the AstraZeneca vaccine across the EU was dissipating.
3.3.5. The rest of the AstraZeneca global supply chain
Although AstraZeneca suffered growing pains with its US, Indian and European supply chains, as well as public health scares, its vaccine continued to play a global role in fighting the pandemic. The company contracted with numerous other partners to build out its supply chain elsewhere (see Figure 6).
In June 2020, Brazil's state‐run Fiocruz Institute announced that it would do fill and finish for AstraZeneca – for drug substance initially produced at SII – and eventually also manufacture the drug substance itself. Elsewhere in Latin America, the vaccine would be manufactured in Argentina (by mAbxience), with fill and finish done in Mexico, partially funded by the Carlos Slim Foundation.39 Siam Bioscience signed up in October to manufacture the vaccine for Thailand and other countries in Southeast Asia. For the Chinese market, Shenzhen Kangtai agreed to build capacity for annual production of 100 million doses.40 In February 2021, Kangtai indicated that it expected to be able to produce 400 million doses of the vaccine a year.
In Australia, CSL announced in August 2020 that it would produce drug substance at a plant in Broadmeadows, performing fill and finish locally at a plant in Parkville. In December, Japan's JCR Pharmaceuticals Company agreed to make the vaccine at a newly built plant in Kobe, with Daiichi Sankyo handling fill and finish. KM Biologics reportedly also signed up to do fill and finish.
3.4. Johnson & Johnson/Janssen
Johnson & Johnson was the first candidate to receive US government support for vaccines, in what later became known as Operation Warp Speed, in February and March 2020. Janssen Pharmaceutica, a Belgium‐based division of Johnson & Johnson, developed the vaccine in collaboration with Beth Israel Deaconess Medical Center of Boston, announcing the candidate on March 30. Initial manufacturing for clinical trials took place at a Johnson & Johnson plant in the Netherlands.
The supply chain began to develop in the United States in April, with collaboration announcements with Emergent BioSolutions to manufacture drug substance and Catalent to do fill and finish in Indiana (Figure 8). In July, the Catalent arrangement was expanded to include its Italian facility; in September, Grand River Aseptic Manufacturing (GRAM), in Michigan, was also contracted to provide fill and finish. In August, drug substance production started with a US government agreement to purchase 100 million doses. Facilitated by the US government, in March 2021, Johnson & Johnson also signed an agreement with Merck – first for fill and finish at a plant in Pennsylvania and eventually for manufacture of the drug substance at a Merck plant in North Carolina.
FIGURE 8.
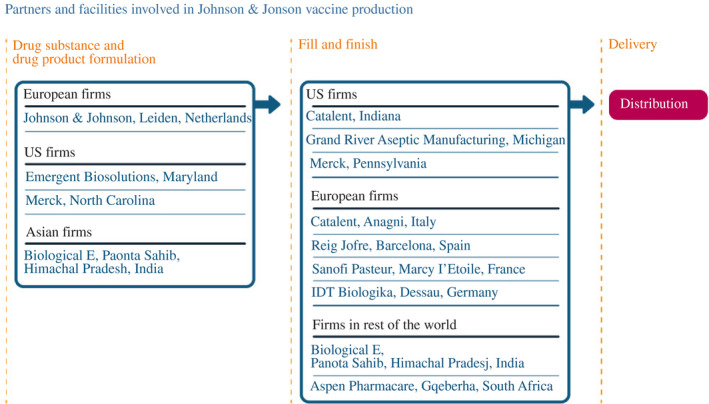
How Johnson & Johnson scaled up its manufacturing network
Note: As of 30 June 2021
Source: Constructed by the authors based on firm announcements and media reports. See Table A4 in the accompanying database for timing and links to original sources
In Europe, the supply chain was set up to receive drug substance from the Leiden plant. For fill and finish, Johnson & Johnson also made arrangements with Reig Jofre in Spain in December 2020, with Sanofi Pasteur in France in February 2021, and with IDT Biologika in Germany in March 2021. (Takeda gave up its previously booked capacity for 3 months to allow IDT Biologika to fill and finish the vaccine.) In March 2021, Johnson & Johnson signed an additional agreement with the Catalent facility in Italy to expand capacity.
Despite a seemingly successful setup of the US‐ and European‐based supply chains, the Johnson & Johnson vaccine ran into challenges. Like AstraZeneca, it had to temporarily pause its clinical trials in October 2020 after a participant fell ill.41 However, its trials resumed 2 weeks later, and in November 2020, drug substance was shipped from the Netherlands to GRAM in Michigan for fill and finish.42 The Leiden facility passed FDA inspection in January 2021, and the FDA authorised the vaccine for emergency use on February 27, making it the third vaccine available in the United States. After Catalent received FDA authorisation to ship from its Indiana plant on March 24, Johnson & Johnson began its US distribution.
A week later, the New York Times reported that 15 million doses of the Johnson & Johnson vaccine had been ruined at the same Emergent plant that was manufacturing the AstraZeneca vaccine. (The figure would later be updated to tens of millions of additionally contaminated doses.43) An early investigation blamed quality controls and cross‐contamination arising from producing two different vaccines at the same facility.44 Production of the Johnson & Johnson vaccine at the plant was halted and ultimately not allowed to resume until the end of July.45
Then, on April 13, FDA paused use of Johnson & Johnson's vaccine after six women who had taken it – out of 6.8 million doses administered – developed a rare blood‐clotting disorder. The United States resumed vaccine use on April 23, with a warning label about the risk of rare blood clots. In Europe, the Johnson & Johnson vaccine suffered a similar fate as the AstraZeneca vaccine, albeit without the political drama. While it had been put into use, the European Commission ultimately decided against renewing orders for more doses beyond 2021.
Outside of the United States and Europe, Johnson & Johnson had been active setting up additional production networks. The vaccine would potentially become important for inoculation campaigns in developing countries. In December 2020, Johnson & Johnson signed an agreement with Gavi to provide 500 million doses through the COVAX programme through 2022. In November 2020, South Africa's Aspen Pharmacare agreed to provide Johnson & Johnson with fill‐and‐finish services. Unfortunately, in June 2021, Aspen had to destroy contaminated doses that had inadvertently been shipped from the Emergent plant, waiting until late July to receive vaccine from the European plant to bottle instead.46 This slowed vaccination campaigns in South Africa and elsewhere.
In August 2020, Johnson & Johnson announced an agreement with Biological E. that would also allow the Indian company to mass produce the vaccine. That month, Biological E. purchased a manufacturing plant in Paonta Sahib in Himachal Pradesh from Akorn India, indicating plans to significantly expand its vaccine manufacturing capacity.47 Biological E. production did not scale up quickly, however, even having licensed the technology. In February 2021, Reuters reported that Biological E.'s managing director, Mahima Datla indicated plans to manufacture 600 million doses of the Johnson & Johnson vaccine in 2021.48 Shortly thereafter, Datla reported input shortages much like SII's Poonawalla, which she also blamed on US government use of the DPA.49 Although shortages of raw materials and equipment were likely, Biological E. also increased imports from US vaccine input suppliers considerably during the period, showing there was no US export ban (Figure 9).
FIGURE 9.
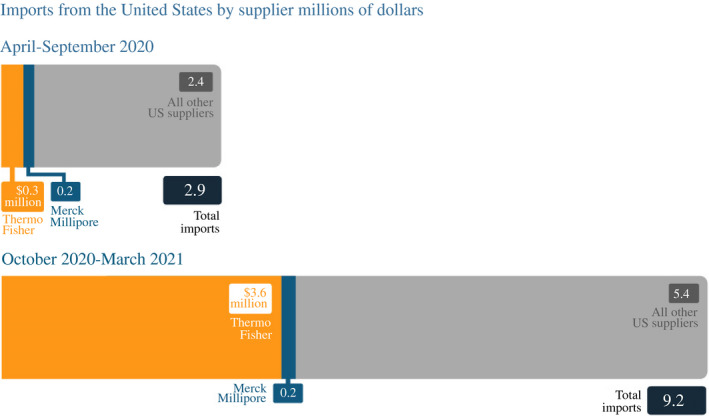
Biological E. imported more vaccine supplies from US companies in October 2020–March 2021 than in the previous 6 months
Source: Bown and Rogers (2021) with data from S&P Global Market Intelligence Panjiva
By May, the Times of India reported that delays had forced Biological E. to once again change its plans: It might import the Johnson & Johnson drug product for others to fill and finish starting in June or July, but it was unlikely to start production until September.50 As of July 2021, Indian regulators had not authorised the Johnson & Johnson vaccine for emergency use.
3.5. Novavax
Novavax is a Gaithersburg, Maryland company founded in 1987 to develop experimental vaccines. Like Moderna and BioNTech, it lacked experience prior to the pandemic in product development for commercial use. Unlike Moderna and BioNTech, Novavax was on the verge of bankruptcy, having sold its only factory in 2019. It needed considerable financial support from the US government, CEPI and others to help develop its candidate, which it identified on 8 April 2020.
The Novavax technology was closer to AstraZeneca and Johnson & Johnson than the Moderna and Pfizer/BioNTech vaccines. Its methods appeared easier to transfer than that of the mRNA‐based vaccines, making it an attractive candidate for plants in developing countries to eventually manufacture. The vaccine also had the benefit of not requiring the same cold‐storage requirements that made others challenging to deploy in remote areas.
The Novavax vaccine relied on a specialised adjuvant excipient from the soap‐bark tree of Chile – Matrix‐M – which helped stimulate a strong immune responses to the antigen. That adjuvant had other pre‐pandemic purposes, and Novavax originally manufactured it in Sweden. In June 2020, Novavax signed agreements with two other companies to manufacture the adjuvant at the scale needed for its expected vaccine sales. AGC Biologics would produce it at facilities in Denmark and Washington State, as would PolyPeptide Group in California and Sweden. Desert King, another California company, was tasked with acquiring the critical starting material of saponin.
The Novavax drug substance would be manufactured elsewhere, with a supply chain strategy similar to the AstraZeneca model (Figure 10). In May 2020, Novavax announced that it was using funding from CEPI to purchase a plant in the Czech Republic (formerly Praha Vaccines, a subsidiary of the Cyrus Poonawalla Group, the parent company of SII) that would allow it to manufacture an expected 1 billion doses of the drug substance. In the United States, vaccine for clinical trials was initially produced by Emergent BioSolutions.51 Fujifilm Diosynth Biotechnologies (FDB) eventually agreed to handle commercial‐scale manufacturing, at sites in Texas and North Carolina. Novavax also agreed to allow FDB to produce its vaccine at a UK plant, under an agreement with the UK government. Takeda signed on in August 2020 (finalised in February 2021) for Japanese production, with assistance from the government of Japan, as did SK bioscience in South Korea, with assistance from CEPI. In September 2020, Novavax signed similar agreements with Biofabri in Spain and SII in India. In February 2021, Novavax reached an agreement with the government of Canada to someday produce the vaccine at the National Research Council's Biologics Manufacturing Centre in Montreal.
FIGURE 10.
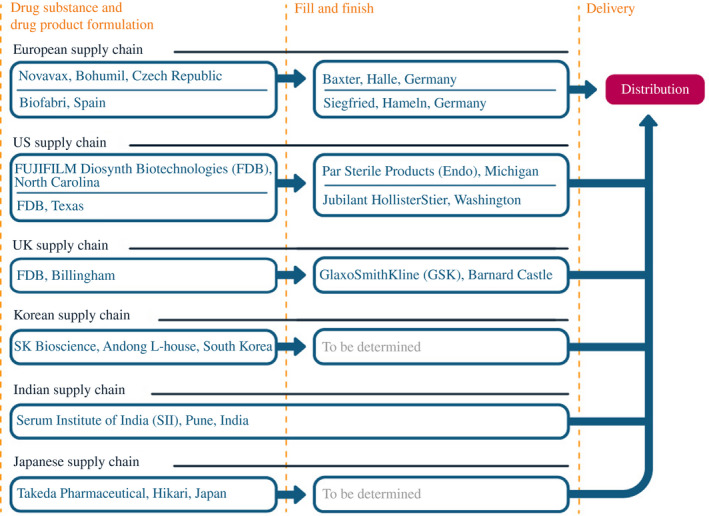
How Novavax scaled up its manufacturing network
Note: As of 30 June 2021
Source: Constructed by the authors based on firm announcements and media reports. See Table A5 in the accompanying database for timing and links to original sources
Novavax also contracted with a number of other companies to fill and finish its vaccine. Par Sterile Products (Endo) signed on in September 2020 to use its Michigan plant. Later agreements were made with Jubilant HollisterStier in Washington State, Baxter in Germany and GSK in England.
As of July 2021, however, despite some promising results from clinical trials, the Novavax vaccine remained under review by regulators. It had not yet been authorised for emergency use anywhere, despite so many facilities having made preparations to manufacture the vaccine.
3.6. CureVac
CureVac is a German biotech firm based in Tübingen that would also eventually develop an mRNA COVID‐19 vaccine candidate. Its advancements were so promising that, by March 2020, President Trump was alleged to have offered the company $1 billion for exclusive rights to its vaccine – a story confirmed by German government officials, but that CureVac denied.52 By June, regulators in Germany and Belgium authorised CureVac's candidate, CVnCoV, to begin clinical trials. As described in more detail below, CureVac also received considerable financial support to develop its COVID‐19 vaccine from Germany, the European Investment Bank, CEPI, as well as through partnerships with other pharmaceutical companies like GSK.
Like Moderna and Novavax, however, CureVac had very little pre‐pandemic manufacturing capacity of its own. Thus, beginning in November 2020, it announced partnerships with both major pharmaceutical companies as well as smaller CDMOs to create a new, pan‐European manufacturing supply chain. By 15 April 2021, CureVac was able to announce that its newly formed network of suppliers could manufacture 300 million vaccine doses by the end of 2021, expanding to up to 1 billion doses by the end of 2022.53
CureVac's mRNA drug substance was to be manufactured in four different countries at seven different plants (Figure 11). In Germany, that included CureVac's own facility in Tübingen, in addition to manufacturing sites belonging to Rentschler Biopharma in Laupheim, Celonic Group in Heidelberg, and Bayer in Wuppertal. Novartis would also manufacture the drug substance in Austria, as would GlaxoSmithKline in Belgium, and Wacker Chemie in the Netherlands. CureVac contracted with Fareva to provide fill and finish at two different sites in France. Furthermore, alongside other vaccine manufacturers in the spring of 2021, CureVac executives also complained that US use of the Defense Production Act was restricting exports and preventing access to critical inputs. Nevertheless, by May, those problems seemed fixed with the company confirming to Reuters that ‘CureVac is grateful that with the help of the EU and U.S. officials, some critical issues could be resolved’.54
FIGURE 11.
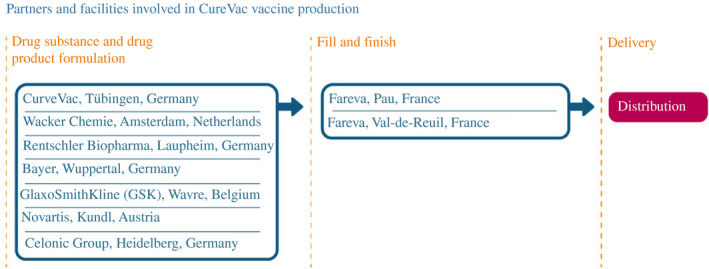
How CureVac scaled up its manufacturing network
Note: As of 30 June 2021
Source: Constructed by the authors based on firm announcements and media reports. See Table A6 in the accompanying database for timing and links to original sources
However, on June 16, CureVac reported disappointing results in its Phase 3 trial, sowing doubt as to whether its candidate would ever be authorised for use.55 Hopes for CVnCoV had been growing throughout the pandemic, especially given the emergence of viral variants that continued to kill hundreds of thousands of people worldwide as well as the public health success of the mRNA vaccines from Pfizer/BioNTech and Moderna.
A host of questions emerged from the disappointing CVnCoV results. What would and should be done with all of the manufacturing capacity tied up in the CureVac network? In July, a Novartis executive indicated company plans to manufacture its share – 50 million doses – of the CureVac vaccine by the end of 2021 anyway.56 But was that sensible, or was it better for those and other CureVac production network resources to be repurposed to manufacture another vaccine instead? Alternatively, CureVac and GSK had been partnering since February 2021 to develop ‘next‐generation’ mRNA vaccines to address emerging variants.57 Would CureVac attempt to hold onto the capacity it had already lined up, potentially to manufacture one of those future vaccines?
4. POLICY INTERVENTIONS AND VACCINE SUPPLY CHAINS DURING THE PANDEMIC
These COVID‐19 vaccines, and the timing, geography and firm‐to‐firm relationships in their manufacturing supply chains, did not emerge randomly. Neither did policy, which likely played an important role. Given the endogeneity, determining exactly how policy affected the manufacturing supply chains that arose in 2020 and 2021 – and thus how alternative policy choices might have allowed things to evolve differently – will be a challenge. This section catalogues key government initiatives that are likely to have been important.
4.1. Operation Warp Speed and the Defense Production Act in the United States
The US government announced the framework behind Operation Warp Speed (OWS) on 15 May 2020.58 It used the Department of Defense, the Department of Health and Human Services, the Biomedical Advanced Research and Development Authority (BARDA), and other agencies to create OWS to coordinate clinical trials and scale up manufacturing in advance of regulatory approval of potential vaccines. This ‘at‐risk’ approach – spending money that would be lost if a vaccine were not ultimately approved – was essential to making rapid progress. OWS also helped expedite the development of viable vaccines able to obtain authorisation from the FDA for emergency public use. Table 4 summarises the forms of support for vaccine development the US government provided.59
TABLE 4.
US federal subsidies or contracts to COVID‐19 vaccine supply chains, 11 February 2020–30 June 2021
| Company | Amount (millions of dollars) | Date | Task |
|---|---|---|---|
| Vaccine sponsors | |||
| Johnson & Johnson (Janssen) | 21 | 11 February 2020 | Support nonclinical studies and a Phase 1 clinical trial |
| 436 | 27 March 2020 | (Contract amendment) | |
| 1000 | 5 August 2020 | Demonstrate large‐scale manufacturing, 100 million doses | |
| 85 | 21 August 2020 | Unknown | |
| 454 | 13 November 2020 | Support Phase 3 clinical trial (contract amendment) | |
| 32 | 25 March 2021 | Expand Phase 2a trial for adolescent population | |
| Sanofi/GSK | 31 | 10 April 2020 | Accelerate nonclinical studies and a Phase 1 clinical trial |
| 2040 | 30 July 2020 | Conduct Phase 3 clinical trial, support manufacturing demonstration project | |
| Merck and IAVI | 38 | 15 April 2020 | Accelerate development of vaccine candidate |
| Moderna | 430 | 16 April 2020 | Accelerate development of vaccine candidate |
| 53 | 24 May 2020 | Expand manufacturing capacity | |
| 472 | 25 July 2020 | Support Phase 3 clinical trial | |
| 1530 | 11 August 2020 | Support Lonza's manufacturing of 100 million doses | |
| 1670 | 11 December 2020 | Purchase another 100 million doses | |
| 1750 | 11 February 2021 | Purchase another 100 million doses | |
| 63 | 12 March 2021 | Support Phases 2 and 3 of adolescent study and booster for adults | |
| 236 | 18 April 2021 | Support for clinical studies (cost increase) | |
| 144 | 15 June 2021 | Support Phase 2 and 3 clinical trials for children 6 months to 12 years old | |
| 3300 | 15 June 2021 | Purchase another 200 million doses | |
| Novavax | 60 | 4 June 2020 | Manufacture components for use in Phase 2 and 3 clinical trials |
| 1600 | 6 July 2020 | Demonstrate commercial‐scale manufacturing | |
| Pfizer (BioNTech) | 1950 | 21 July 2020 | Purchase 100 million doses |
| 2010 | 22 December 2020 | Purchase another 100 million doses, with option for 400 million more | |
| 2010 | 11 February 2021 | Purchase another 100 million doses | |
| AstraZeneca (Oxford) | 1600 | 28 October 2020 | Accelerate development and manufacturing to begin Phase 3 clinical trial |
| Contract manufacturers | |||
| Emergent BioSolutions | 628 | 30 May 2020 |
Contract for manufacturing, fill and finish Purchase of additional equipment for manufacturing |
| 20 | 6 August 2020 | ||
| 23 | 24 March 2021 | ||
| Fujifilm Diosynth Biotechnologies (Texas A&M University) | 265 | 24 July 2020 | Contract for manufacturing |
| 8 | 24 November 2020 | ||
| Grand River Aseptic Manufacturing (GRAM) | 161 | 6 August 2020 | Contract for fill and finish, including for Johnson & Johnson's vaccine |
| Ology Bio | 106 | 17 August 2020 | Contract for fill and finish |
| Merck | 105 | 1 March 2021 | Produce drug substance, formulate and fill vials of Johnson & Johnson's vaccine |
| Equipment and other input suppliers | |||
| SiO2 Materials Science | 143 | 5 June 2020 | Establish US‐based production for glass tubing and vials |
| Corning | 204 | 5 June 2020 | Expand capacity for glass tubing and vials |
| 57 | 23 March 2021 | ||
| Becton, Dickinson and Co. | 42 | 1 July 2020 | Expand capacity for syringes and needles |
| Retractable Technologies | 54 | 1 July 2020 | Expand capacity for syringes and needles |
| Smiths Medical | 21 | 11 July 2020 | Expand capacity for syringes and needles |
| Cytiva | 31 | 13 October 2020 | Expand capacity for cellular material, mixer bags, and bioreactors |
| ApiJect Systems | 590 a | 19 November 2020 | Expand capacity for prefilled, single‐dose injectors |
| Meissner Filtration Products | 13 | 1 April 2021 | Expand capacity for filtration products for vaccine manufacturing |
Sources: Compiled by the authors from Biomedical Advanced Research and Development Authority, 2021, BARDA's Rapidly Expanding COVID‐19 Medical Countermeasure Portfolio and BARDA's COVID‐19 Domestic Manufacturing & Infrastructure Investments; Novavax; GRAM; and US International Development Finance Corporation.
Loan to finance 75 per cent of project's capital costs.
Its first disbursements, in February–June 2020, were primarily to support nonclinical studies, then clinical studies and the small‐scale manufacturing that candidates without at‐the‐ready, in‐house production facilities needed to support those studies. The government provided funding to candidates that ultimately worked (Johnson & Johnson, Moderna); candidates that were either still in the pipeline or had been deployed outside the United States (Novavax, AstraZeneca, Sanofi/GSK); and candidates that never made it out of clinical trials (Merck and IAVI). Subsidising at risk did mean failures: ultimately, the United States spent more than $3 billion on candidates that had not been approved by the FDA as of July 2021.
In July 2020, OWS started making sizable advance purchase commitments for a portfolio of vaccine candidates, providing billions of dollars of funding at risk (the earliest data from any of the Phase 3 trials would not arrive until November). This funding allowed the companies to begin the lengthy process of setting up their supply chains, forging new commercial relationships, and establishing manufacturing facilities. It provided more than $1 billion each to Moderna, Pfizer, Johnson & Johnson, Novavax, AstraZeneca and the Sanofi/GSK candidate.
OWS also coordinated and matched contract manufacturers with vaccine sponsors to ensure that those purchase orders would be fulfilled. It made at‐risk investments with Emergent BioSolutions, GRAM and FDB in May–November 2020, as well as matching them to AstraZeneca, Johnson & Johnson, and Novavax. The funding and partnerships allowed those facilities to begin earlier than others the process of acquiring the specialised equipment, inputs, and technology necessary and to prepare for drug substance manufacturing, formulation, and fill and finish.
Despite funding and lead‐time, much of the manufacturing scale‐up in the United States did not go smoothly. Recall the Emergent facility problems with the Johnson & Johnson and AstraZeneca vaccines described earlier. Furthermore, as of July 2021, the Novavax candidate had not been authorised for use. (The ultimate test of the FDB facilities in the United States, for example, would only arise from pressures to meet large‐scale commercial demand that would not result without regulatory authorisation.)
OWS did more than simply purchase inputs these companies needed for manufacturing. It also subsidised hundreds of millions of dollars of production capacity expansion at separate firms providing those critical inputs. This funding covered capital equipment, such as bioreactors, as well as mixer bags and cellular materials from companies like Cytiva. (In April 2021, the Biden administration subsidised expansion at Meissner Filtration Products, likely in response to complaints by vaccine company CEOs about equipment shortages.)
OWS also subsidised capacity expansion for production of the glass vials, syringes, and other ancillary supplies needed for packaging the vaccine and administering the injection of doses into arms. In 2020, OWS sent funding to companies like Corning; SiO2 Materials Science; Becton, Dickinson and Co.; Retractable Technologies; and Smiths Medical in an attempt to head off concern that once the vaccines had been manufactured, holdups might arise because of shortages of complementary inputs needed for delivery.
The DPA was the second potentially important US policy initiative deployed to expand vaccine manufacturing during the pandemic.60 The US government gave priority ratings under DPA to each vaccine maker's contract in 2020. (The exception was Pfizer, which did not receive a priority rating for its initial contract in July but did for its second contract for an additional 100 million doses on December 22.) A priority‐rated contract had two primary effects. First, vaccine manufacturers had to use their US facilities to prioritise US government orders for doses over any other competing claims on their resources – forcing, for example, Moderna's US supply chain to satisfy a US government contract for 100 million doses before it could produce any other products or sell doses of its vaccines to other potential consumers, whether in the United States or abroad. Second, a priority‐rated contract allowed vaccine makers to go to their input suppliers and demand that their contract be prioritised over any other orders for those same materials.
Prioritising vaccine manufacturing likely untangled some potential input bottlenecks in the US supply chain. For example, a DPA contract forced Catalent to tell Horizon to find another facility it had reserved to fill and finish Tepezza, its thyroid eye disease drug, because the Indiana plant had been ordered to bottle COVID‐19 vaccines.61 Furthermore, the US government also reportedly embedded military logistics experts into the supply chains to help facilitate the allocation of those scarce supplies.62 This may have been a response to the highly likely event that the various vaccine manufacturers, each armed with priority‐rated contracts, all placed nearly simultaneous orders for the same equipment and raw materials with the limited number of specialised input suppliers.
While there were numerous complaints, exactly what input shortages arose and whose orders got de‐prioritised because of DPA invocation remains unknown. The policy became a lightning rod when the Biden administration began to publicise its use for unlocking bottlenecks for Pfizer in early February 2021.63 One direct problem with the DPA emerged from its lack of transparency.
Trading partners and vaccine manufacturers outside the United States reacted by accusing the US government of using DPA as an export‐restricting policy, not simply to reallocate inputs towards higher‐priority vaccine production and away from other uses. First in March and then again in April, SII's CEO publicly accused the US government of banning exports of vaccine supplies. The CEOs of Biological E., Novavax, and CureVac expressed similar concerns.64 French President Emmanuel Macron elevated the issue politically by echoing the sentiments in May.65 Given the lack of transparency involving how and when DPA was used, it was impossible to refute accusations that the effect of the policy was to restrict exports.
Rumours over DPA abuse ultimately took on a life of their own. In response to worsening conditions on the ground in India and pleas for access to inputs, on April 26 the White House announced emergency shipments of ‘[Merck] Millipore filters that would have been used to manufacture AstraZeneca vaccine that will be used to manufacture the Covishield AstraZeneca vaccine [sic] serum’.66 On June 3, the US government announced that it was removing DPA priority ratings for the vaccines from Novavax, AstraZeneca and Sanofi/GSK.67
OWS did subsidise the expansion of critical inputs. But supplies still remained scarce, and some rationing was needed. Had the US government not intervened and simply left allocation to markets, American manufacturers may have outbid foreign competitors even without OWS and DPA. And without policymakers' interventions, vaccine makers with less public health priority – because they had not been authorised by regulators, for example – might have ended up with the inputs, leaving shortages globally at plants making the (authorised) Pfizer, Moderna, Johnson & Johnson and AstraZeneca vaccines.68
4.2. The United Kingdom
The UK government also made at‐risk public investments in its domestic vaccine manufacturing supply chain during the pandemic, albeit to a lesser extent than the United States and in a somewhat different manner (Table 5). Three of the seven vaccines for which the UK government made advance purchase commitments ultimately established some domestic manufacturing facilities.69 The United Kingdom also imported Pfizer and Moderna vaccines (from the EU supply chain), ordered doses of Johnson & Johnson to be delivered late in 2021 and held options to purchase vaccines from Sanofi/GSK.
TABLE 5.
UK subsidies for vaccine supply chain
| Amount (millions of British pounds) | Company | Date | Task |
|---|---|---|---|
| Clinical trials | |||
| 65.5 | Oxford | May 2020 | Support trials |
| 18.5 | Imperial College | May 2020 | Support Phase 3 trials |
| Manufacturing for clinical trials | |||
| 31 | Oxford Biomedica | June 2020 | Support early manufacturing of the University of Oxford and Imperial College London vaccines and develop manufacturing skills |
| Vaccine sponsors (as of December 8, 2020) | |||
| 2900 (914 up front) | AstraZeneca | August 2020 | Purchase 100 million doses |
| Valneva | September 2020 | Purchase 60 million doses, investment in Livingston manufacturing facility | |
| Pfizer/BioNTech | October 2020 | Purchase 40 million doses | |
| Novavax | October 2020 | Purchase 60 million doses, FDB will manufacture at Billingham site | |
| Moderna | November 2020 | Purchase 7 million doses | |
| 800 (nonbinding) | Sanofi/GSK | July 2020 | Purchase 60 million doses |
| Johnson & Johnson | August 2020 | Purchase 30 million doses | |
| Fill and finish | |||
| 42 | Wockhardt UK | August 2020 | Reserve two fill‐and‐finish facilities for 18 months |
| Other | |||
| 127 |
Cell and Gene Therapy Catapult Manufacturing Innovation Centre |
July 2020 | Purchase the centre, support its conversion and costs from June 2021 |
| 93 |
Vaccine Manufacturing and Innovation Centre (VMIC) |
May 2020 | Accelerate VMIC completion date from summer 2022 to summer 2021 and expand its scope |
|
8.6 5 |
Centre of Process Innovation |
June 2020 March 2021 |
Develop facilities for vaccine production using mRNA‐based technology |
| 33 | Human Challenge Program | Develop new clinical trial capability to accelerate vaccine development and advance mechanistic understanding of viral controlled infection | |
Sources: Constructed by the authors from UK National Audit Office (2020), UK Department for Business, Energy and Industrial Strategy (2020) and other sources (hyperlinks provide original sources).
The UK subsidy strategy featured a two‐pronged approach. It started early, spending £84 million in May 2020 to subsidise acceleration of clinical trials for two home‐grown candidates – the one from Oxford (AstraZeneca) and another from Imperial College London. The government also gave subsidies of £31 million to manufacture the vaccine candidates for clinical trials and £42 million to reserve fill‐and‐finish capacity for 18 months at UK sites. Over the following few months, it negotiated five binding advance purchase commitments, at a cost of £2.9 billion (for 267 million doses). Of that, the United Kingdom paid out £914 million up front, much of it nonrefundable, for the companies to further develop their clinical trials and set up their supply chains at risk. Concerned that allowing the last step to take place outside of its borders could put its access to vaccine doses at risk of potential EU export control policy, in March 2021 the UK government reportedly played matchmaker between Novavax and GSK to convince the latter to use an English facility for fill and finish. The GSK announcement described an agreement between GSK, Novavax and the UK Government Vaccine Taskforce; no terms of government subsidies were mentioned.70
The second component of the UK subsidy strategy involved more than £266 million designed to enhance the long‐term ability of the United Kingdom to manufacture vaccines. It included training programmes for staff and development of new national databases to speed up registrations needed for future clinical trials.
4.3. The European Union, Germany and other countries
Other economies also subsidised vaccine manufacturers, but very differently, and mostly at much later points in the vaccine development process (Table 6).
TABLE 6.
Examples of other government subsidies to vaccine supply chains
| Company | Amount | Date | Nature of funding |
|---|---|---|---|
| European Union | |||
| Pfizer/BioNTech | €100 million | June 2020 | Debt financing to expand BioNTech manufacturing capacity |
| CureVac | €75 million | July 2020 | Loan to support vaccine development and accelerate completion of Tübingen production site |
| AstraZeneca | Unknown | August 2020 | Advance purchase agreement for 300 million doses |
| Sanofi/GSK | Unknown | September 2020 | Advance purchase agreement for 300 million doses |
| Johnson & Johnson | Unknown | October 2020 | Advance purchase agreement for 200 million doses (one‐shot regimen) |
| Pfizer/BioNTech | Unknown | November 2020 | Advance purchase agreement for 200 million doses |
| CureVac | Unknown | November 2020 | Advance purchase agreement for 225 million doses |
| Moderna | Unknown | November 2020 | Advance purchase agreement for 80 million doses |
| Germany | |||
| CureVac | €300 million | June 2020 | Government equity stake of 23 per cent |
| CureVac | €252 million | September 2020 | Grant for further development of vaccine candidate and rapid expansion of vaccine production |
| BioNTech | €375 million | September 2020 | Grant to expand vaccine development and manufacturing capabilities in Germany as well as number of participants in late‐stage clinical trials |
| Australia | |||
| CSL | Unknown | September 2020 | Funding to outfit production facilities with equipment and workforce to manufacture AstraZeneca vaccine |
| Japan | |||
| JCR Pharmaceuticals | Unknown | December 2020 | Grant to build new manufacturing facility |
| India | |||
| Serum Institute of India | $400 million | April 2021 | Grant to expand manufacturing capacity |
| Bharat Biotech | $210 million | April 2021 | Grant to expand manufacturing capacity |
| Singapore | |||
| BioNTech | Unknown | May 2021 | Construction of mRNA manufacturing facility in Singapore |
Source: Constructed by the authors. Hyperlinks provide original sources.
The European Union received considerable initial criticism for the slow pace of its vaccine rollout relative to peers and ran into disputes with AstraZeneca and the United Kingdom. Its subsidisation strategy was much different from those of the United States or the United Kingdom. It provided only €175 million in 2020 to two companies – BioNTech and CureVac – in the form of debt financing and loans to further develop their manufacturing capabilities.
Although the European Union did make advance purchase agreements with six vaccine sponsors, those relationships were established much later than they were in the United States and the United Kingdom, possibly reducing the willingness of companies to make at‐risk investments to scale up their European manufacturing capacities more quickly. (The exact terms of the agreements remain unknown, making it difficult to judge how much guaranteed funding the companies would receive if, for example, vaccines could not be delivered for reasons outside their control, including their failure to pass clinical trials.) There is no evidence that the European Union subsidised the reservation of fill‐and‐finish capacity or any of the other key inputs needed to massively scale up vaccine production in which the companies reported shortages.
European Commission President Ursula von der Leyen recognised some of the weakness in the Commission's approach. In a February 2021 interview with the Financial Times, she said, ‘The US has a strong advantage by having BARDA [the Biomedical Advanced Research and Development Authority]... this is an infrastructure Europe did not have… But Europe has to build up to be prepared for whatever comes, and also for the next possible pandemics. This is the HERA incubator’, referring to the proposed Health Emergency Preparedness and Response Authority, an EU initiative to address future pandemic preparedness.71
Elsewhere in Europe, Germany invested nearly €1 billion in 2020 in BioNTech and CureVac, the two biotechs developing mRNA vaccine candidates. In June, the German government took a 23 per cent ownership stake in CureVac; in September, it committed another €252 million to CureVac and €375 million to BioNTech to accelerate development and local manufacturing capacity.
Other major economies, including Australia and Japan, also provided subsidies, but they appeared smaller in scope, arose much later and did little to scale up the broader vaccine supply chain (see Table 6). India only subsidised major vaccine manufacturers like SII and Bharat Biotech late in the process, beginning in April 2021.
4.4. CEPI and the World Bank
Another important source of funding for vaccine supply chains came from CEPI, a global partnership between public, private, philanthropic and civil society organisations. Through November 2020, CEPI had raised $1.3 billion for vaccine research and development. The nine candidates in its ‘Wave 1’ portfolio included AstraZeneca/Oxford, Moderna and Novavax, among others (Table 7).
TABLE 7.
CEPI's financial support for COVID‐19 vaccines and supply chain
| Company | Amount | Purpose of funding |
|---|---|---|
| Candidates in Wave 1 in 2020 | ||
| Clover Biopharmaceuticals | Up to $328 million | Development of COVID‐19 vaccine candidate, preclinical studies and Phase 1 clinical trials, Phase 2 and 3 efficacy study, and initial manufacturing |
| CureVac | Up to $8.3 million | Development of COVID‐19 vaccine candidate |
| Inovio | Up to $22.5 million | Development of COVID‐19 vaccine candidate and support of Phase 1 and 2 trials in South Korea |
| Institut Pasteur | Up to $4.9 million | Development of COVID‐19 vaccine candidate |
| Moderna | Up to $1 million | Development of COVID‐19 vaccine candidate |
| Novavax | Up to $388 million | Preclinical studies, Phase 1 and 2 clinical trials, and large‐scale vaccine production |
| AstraZeneca/Oxford | Up to $384 million | Manufacture of vaccine materials required for preclinical and Phase 1 testing and support for manufacturing of 300 million doses, ring‐fenced for the COVAX Facility |
| University of Hong Kong | $620,000 | Development of COVID‐19 vaccine candidate |
| University of Queensland | Unknown | Development of COVID‐19 vaccine candidate |
| Manufacturing supply chain | ||
| Biological E. | Up to $5 million | Scale‐up of vaccine manufacturing |
| Biofabri, Spain | Unknown | Reservation of manufacturing capacity for CEPI‐designated COVID‐19 vaccines from November 2020 to May 2022 (estimated at more than 500 million doses), with option to extend or expand the reservation (October 2020) |
| GC Pharma, South Korea | Unknown | Reservation of manufacturing capacity for CEPI‐designated COVID‐19 vaccines from March 2021 to May 2022 (estimated at more than 500 million doses) with an option to extend or expand the reservation (October 2020) |
| SK bioscience, South Korea | Unknown | Reservation in August 2020 of manufacturing capacity for 2 billion doses of vaccine for COVAX by end of 2021 |
| Stevanato Group, Italy | Unknown | Purchase 100 million Type 1 Borosilicate glass vials to hold up to 2 billion doses of a vaccine |
Source: Compiled by the authors from CEPI.
CEPI's at‐risk funding approach shared some features of the OWS model, although it was smaller in scale. Like OWS, it funded promising candidates early on, helping clinical trials and manufacturing at risk. It later worked directly to reserve capacity at CDMOs in Spain and South Korea, including at SK bioscience to manufacture the Novavax vaccine upon regulatory approval.
Finally, in June 2021, the World Bank announced financial support for Aspen, the South African company providing fill and finish for the Johnson & Johnson COVID‐19 vaccine.72 The World Bank mobilised a €600 million long‐term financing package that also included contributions from development agencies in France, Germany and the United States. The agreement would refinance existing debt and help facilitate Aspen's vaccine manufacturing capacity.
5. ECONOMIC AND POLICY ANALYSIS AND OPEN QUESTIONS
Researchers and policymakers will work for years distilling the details of COVID‐19 vaccine manufacturing in order to address whether more doses could have been produced faster some other way. This section raises six economic questions for further investigation.
5.1. Were at‐risk investments sufficiently large, diverse and geographically distributed?
The fact that major economies heavily subsidised many more vaccine candidates than were ultimately deployed was unequivocally correct given the context of the pandemic, which killed millions and caused trillions of dollars in economic losses. A diverse portfolio was critical, because unpredictable real‐world problems could (and did) emerge to affect any given candidate. Some subsidised candidates (e.g. Merck and IAVI) failed entirely. Others (e.g. Novavax, CureVac, Valneva and Sanofi/GSK) may yet succeed clinically, but they will have taken much longer than Pfizer/BioNTech, Moderna, AstraZeneca, and Johnson & Johnson to obtain regulatory approval.
Even if appropriate in theory, some of the at‐risk public investments to scale up manufacturing for candidates that regulators green‐lighted (e.g. Emergent in the United States to produce AstraZeneca and Johnson & Johnson) proved problematic. Some facilities ran into quality control issues; others were slow to expand because of learning challenges or inadequate access to inputs. Government policies prevented some companies from exporting. The diversity of candidates and the global diversity of production mattered.
Pfizer and Moderna may turn out to be the success stories ex post. But if those previously untried technologies had not worked, vaccines from Johnson & Johnson, AstraZeneca and even Novavax or CureVac might have played an even more important role than they did in saving lives. Some of these less successful vaccines may be relegated to the annals of history, but they were equivalent to an insurance policy.
Evenett et al. (2021) note that the lack of at‐risk subsidies during the previous, H1N1 pandemic of 2009–2010 – and the fact that some companies were burned by making investments that could not be recouped, as governments pulled funding when that pandemic waned – may have played a role in the unwillingness of certain companies to act this time around. One lesson is that companies should not be punished for taking financial risks on society's behalf, lest those risks be discouraged the next time.
5.2. Was there excessive concentration of input suppliers and insufficient public investment upstream?
During the pandemic, vaccine manufacturers complained about a series of input shortages. Lipids, bioreactor bags, filtration pumps, filters, and other equipment and raw materials were in short supply, potentially slowing the scaling‐up of vaccine production. Some shortages were to be expected, given the surge in demand for customised inputs. But questions remain. Could alternative policies have discouraged input demand from becoming too concentrated in the same suppliers? Was there enough public investment in expanding the capacity of the company's manufacturing those key inputs?73
Pall is a key vaccine equipment supplier and part of the consortium the Oxford vaccine originators convened in early April 2020. Clive Glover, Pall's director for cell and gene therapy, described the equipment implications: ‘The need to standardize was a necessity for this project because there are more than 20 different sites manufacturing [the Oxford (AstraZeneca) vaccine], each using the 50 or so consumables required for the manufacturing process. If each site had its own customized version, there would need to be more than 1,000 parts!’.74
Standardising the equipment and production process for a vaccine across facilities would have costs and benefits. A benefit is the speed at which each new plant could scale up manufacturing of a consistent drug product, if they could access the standardised inputs. The cost is that, due to the specialised nature of some of those inputs, the standardised equipment may have been coming from a limited number of suppliers who found themselves unable to keep up with demand.
Policymakers needed transparency regarding the capacity and utilisation rates of the input providers in the vaccine supply chain to determine whether some were overburdened and needed, where feasible, to have their tasks reallocated to others. Use of DPA granted the US government some insight into the equipment and raw material providers that US vaccine manufacturers were using. Other governments did not have this insight, and the US government lacked information on the input demands of vaccine manufacturers in other countries, except when they complained about it publicly.
Another policy problem may have been too little global public investment in expanding the production capacity of those input suppliers. Although the United States, and to a lesser extent CEPI, subsidised upstream equipment and raw material providers in addition to downstream vaccine manufacturers, their efforts were likely insufficient.
One potential explanation involves the geographic concentration of the input suppliers. Suppose input providers were located primarily in the United States. Although US government subsidies may have been sufficient for the needs of domestic manufacturers and thus nationally optimal, they may have been globally suboptimal. The size of the globally optimal subsidies to US input providers may have been much larger, requiring expansion of input production capacity big enough to satisfy increases in demand also arising from vaccine manufacturers in Europe, India and elsewhere.
A second, contributing explanation may have been that input suppliers were simply located in different countries from vaccine manufacturers. In theory, given the global nature of the pandemic, governments should have incentive to subsidise input providers even if the downstream manufacturers were located in a different country. Such subsidies would address the positive externality of public health gains of resolving the pandemic affecting its population, since that population would have access to the final vaccines manufactured elsewhere through international trade. But as Bown and Bollyky (2021) explain, here the policy failure of too little input subsidies could arise due to concerns over vaccine export restrictions. Access to finished vaccines was highly uncertain during the pandemic, given the implicit and explicit export restrictions that vaccine manufacturing countries and groups of countries – including the United States, the United Kingdom, the European Union and India – all employed.
The policy ‘failures’ in these cases would be the lack of international policy coordination.75 One contributing solution would be to create a mechanism for other countries to subsidise the expansion of US (or foreign) input capacity destined for their downstream manufacturers. Cooperation may also require an explicit, publicised, enforceable agreement between the major producers not to implement export‐restricting policies and to establish some mutually acceptable way of transparently rationing inputs in short supply.
5.3. In the face of scarcity, were inputs and available capacity reallocated efficiently?
Once there were multiple viable candidates, legitimate questions arose about how to allocate and reallocate available inputs to best scale up overall production quickly. There is still much to be learned about the details of what happened. Could the process have been coordinated more efficiently across countries? Could public health and scientific evidence have played a more central role in determining which vaccines to produce where and when? What were the costs and unintended consequences of the allocation decisions that were made?
US use of the DPA reallocated orders of some raw materials and equipment towards vaccine manufacturers. It is likely that DPA was a useful way to reallocate some inputs and that, without well‐functioning secondary markets, these input reallocations may not have happened. What is not known is what orders ended up being de‐prioritised in favour of the vaccines.
There is anecdotal evidence that entire facilities were repurposed for vaccine manufacturing. In a May 2020 Reuters interview, a Pfizer executive indicated that the company planned to rely on its ‘network of around 200 outside contractors, which includes Catalent Inc., Lonza Group AG and Thermo Fisher Scientific Inc., to play a bigger role in producing some of its existing medicines’.76 There were also the examples described earlier from Catalent and IDT Biologika suddenly breaking arrangements with other pharmaceutical companies in order to use their plants to fill and finish COVID‐19 vaccines.
There were other, potentially large, resource allocation inefficiencies that may have also resulted in capacity underutilisation. Novavax, which had reserved a number of vaccine manufacturing facilities, experienced delays in getting its vaccine through clinical trials. CureVac similarly lined up capacity at a number of plants before its disappointing Phase 3 trials were revealed in June 2021. Did these efforts tie up production that could have been used to make more of one of the other vaccines authorised for use? These experiences highlight an important trade‐off – reserving capacity for one vaccine ends up taking away capacity that could have been used to manufacture another vaccine, at least over some set period of time.
A similar issue arose in India, where Biological E. reported having tremendous production capacity. It licensed technology for two foreign vaccine candidates, Johnson & Johnson and Baylor College of Medicine, in August 2020. By July 2021, neither had received authorisation for emergency use from the Indian government, potentially contributing to the slow pace of Biological E.'s manufacturing expansion, because doing so would have been at risk.77 The Johnson & Johnson vaccine had successfully completed clinical trials and been deployed in other markets, including the United States and European Union, highlighting a problem of uncoordinated global regulators.
In response to some of these concerns, in July 2021, two policy developments emerged. First, CEPI and partners in COVAX launched a ‘marketplace’ intended to match vaccine manufacturers in need of critical inputs with available supplies. The new mechanism would allow for the potential reallocation of inputs made idle by vaccine candidates that failed to gain regulatory approval. The idea was for CEPI to work confidentially to ‘identify matching offers and requests and connect potential matches, prioritising based on objective criteria including whether the manufacturer has a COVAX advance purchase agreement and WHO emergency use listing in place’ (CEPI, 2021).
Second, the WTO published a joint indicative list of critical inputs for COVID‐19 vaccines, after convening an expert technical symposium on vaccine supply chains (WTO, 2021). Trade facilitation could help eliminate bottlenecks at the border caused by product misclassifications or regulatory misunderstandings that might also inadvertently slow production.
5.4. How did learning by doing arise?
Learning by doing – or the process of becoming more productive, the more output generated – was supposedly critical for new vaccine manufacturing. Examples include the slow increase in production at AstraZeneca's plants in Belgium resulting in low initial yields for drug substance that may have contributed to its tensions with the European Commission. Johnson & Johnson and AstraZeneca lost tens of millions of doses from contamination at a Baltimore plant, highlighting the importance of quality control.
Other examples were more subtle. Even ultimate success stories, like Pfizer, admitted the need to scale back 2020 production targets in November because ‘some early batches of the raw materials failed to meet the standards’.78 In January 2021, it made the equivalent statement for its European targets: ‘As part of the normal productivity improvements to increase capacity, we must make modifications to the process and facility that will require additional regulatory approvals. [Although this will] temporarily impact shipments in late January to early February, it will provide a significant increase in doses available for patients in late February and March’.79 Pfizer's learning‐by‐doing is captured in a February interview with USA Today, in which the company indicated that it had cut the amount of time to make a batch of its COVID‐19 vaccine nearly in half—from 110 to 60 days, indicating, ‘just in the last month we've doubled output’.80
Output also increased through other means. In January, the EMA announced that six – not five – doses could be extracted from a single Pfizer vial, increasing supplies by 20 per cent.81 In February 2021, Pfizer/BioNTech learned that the vaccine was stable under less rigorous conditions and thus did not need ultra‐cold storage.82 The FDA later granted approval to weaken the conditions, implying fewer unused doses would have to be destroyed due to thawing and harder‐to‐access destinations could be more easily reached.
Moderna experienced similar learning‐by‐doing. In March 2021, the Wall Street Journal reported that ‘Moderna shortened the time it needed to inspect and package newly manufactured vials of its vaccine’.83 In April, the FDA allowed Moderna to expand its output per unit of input in three ways.84 First, it allowed it to include 11 doses, not 10, in each vial, increasing the immediate supply by 10 per cent. Second, it allowed Moderna to ship larger vials containing up to 15 doses of its vaccine, up from 10 or 11. Third, it allowed the Moderna vaccine to be kept at room temperature for 24 hours, up from the previously authorised 12 hours, which presumably meant that health care workers were forced to dispose of fewer unused doses and that more doses could arrive at hard‐to‐reach destinations.
Yet, open questions remain. Could firms have learned faster, to get more vaccine output quicker, to save even more lives? In these multi‐plant supply chains, did learning spill over to other facilities manufacturing the same vaccine? Did it spill over to plants for firms making different vaccines?
5.5. How did the modular, fragmented structure of the industry affect scaling‐up?
CDMOs ended up playing a critical role in numerous vaccine supply chains. Had the vaccines instead been produced only by large, integrated pharmaceutical companies, the outcomes would probably have been different.
Having to mix and match various smaller firms along the supply chain required more coordination and transactions costs. – Starting new relationships at arm's length was made more difficult by the pandemic, which severely curtailed travel. Relative to an integrated firm, the fragmented structure may also have made technology transfer harder, learning‐by‐doing slower and lessons learned more difficult to share.
However, fragmentation may also have had considerable benefits. One was transparency. Arm's length contractual arrangements (and press statements) yielded more information to the public and to policymakers about the progress of vaccine production. Fragmentation may also have increased competition and made it easier to reallocate resources and production capacity towards the most promising vaccine candidates. Finally, to fill and finish 500 million doses, it may have been easier to find capacity at five different contract manufacturers each able to produce 100 million doses than to a find one integrated company able to both manufacture the drug substance and fill and finish 500 million doses in house.
5.6. Did international interdependence prevent worse outcomes from arising?
Headlines throughout the vaccine rollout in early 2021 highlighted hoarding, vaccine nationalism, and implicit and explicit export restrictions. The failure to distribute vaccines based on global public health needs under the COVAX programme was a failure of first‐order importance. The explicit (and entrenched) America First approach of the Trump administration even before the pandemic made clear that the rest of the world could not rely on American exports for vaccines. That explicit stance, as well as the implicit fear that other countries would do the same thing, almost certainly contributed to many company decisions to establish parallel supply chains in different markets rather than building out additional capacity in the United States or any other single location.
Yet, there is also evidence that international interdependence played a positive role. Exports were the only way many countries would receive any vaccines at all. Given that the pandemic showed lockdowns could affect industrial production (albeit in other sectors) or even shipping, simply scaling‐up mega‐facilities in fewer countries and further reducing geographic diversification may not have resulted in more output.
Furthermore, some of the arguments about export restrictions on inputs may turn out to have been overblown. (Only data will resolve the issue.) Exports of key inputs may not have been much larger even without the use of DPA. Put differently, inputs were in short supply globally, and most of the firms willing to pay for those inputs were located in the United States or other high‐income countries where manufacturing was taking place. A bigger policy failure than export restrictions on raw materials and equipment may have been the insufficient public investment to scale up of global production of those critical inputs in the first place.
Finally, there is some evidence that trade (and interdependence) helped keep international markets open, preventing matters from getting worse. Some two‐way trade in drug substance between the United States and the European Union took place in late 2020 and early 2021, as Pfizer reportedly made shipments from the United States to Germany and Johnson & Johnson did the same from the Netherlands to the United States.
Formulating the Pfizer/BioNTech vaccine at facilities in Europe may have required lipid nanoparticles that may not have been immediately available within the EU. UK exports to Belgium and Germany, for example, increased in early 2021 (see again Figure 3). That interdependence could have been a contributing factor in the European Union's decision not to stop exports to the United Kingdom of vaccines being bottled in Europe, potentially preventing EU–UK tensions from escalating.
The free flow of vaccine inputs and vaccines between EU Member States (and Switzerland, where Lonza produced the Moderna drug substance) was critical. The positive impact of that interdependence should not be taken for granted, as it was not enough to stop France and Germany from imposing export bans on personal protective equipment leaving their borders in March 2020, even to other EU Member States with high disease caseloads, such as Italy.85 Yet, export restrictions did not imperil intra‐European supply chains for vaccines; the evidence is consistent with Moderna's vaccine going from the Lonza plant in Switzerland to fill and finish facilities in Spain and France, for example (see again Figure 5).
6. CONCLUSION
In the 8 months following authorisation of the first COVID‐19 vaccine for emergency use, the impact of the vaccines on public health and economic activity was positive but still emerging. By the end of July 2021, roughly 4 billion doses had been administered worldwide. Most required a two‐dose regimen – if that trajectory continued, close to 14 billion shots would be needed to inoculate the global population. This number does not account for the potential need for boosters, or other challenges arising from the emergence of viral variants.86
Vaccine delivery played out differently around the world (Figure 12). The United States and European Union increasingly administered the mRNA vaccines, with Pfizer/BioNTech at least initially playing a larger role than Moderna. Take‐up of the (single‐dose) Johnson & Johnson vaccine in both markets was much more limited. In the European Union, AstraZeneca/Oxford peaked in mid‐April at roughly 22 per cent of all doses administered, falling to about 15 per cent by the end of July. (The United States did not authorise AstraZeneca/Oxford for use.)
FIGURE 12.
US and EU increasingly relied on mRNA vaccines as doses ramped up worldwide
Note: EU, European Union. Other vaccines administered in the European Union include Sputnik V (Slovakia and Hungary) and Sinopharm (Hungary). As of end July 2021, China administered only domestic vaccines, such as those from Sinovac and Sinopharm. Vaccinations in India were dominated by the Serum Institute of India's production of the AstraZeneca/Oxford vaccine, and to a lesser extent the vaccine from Bharat Biotech. Vaccines administered in Africa were dominated by imports
Source: Constructed by the authors with data from European Centre for Disease Prevention and Control and Our World in Data.
Elsewhere the story was different. Albeit with larger populations, India and China had administered more total doses than the United States and European Union by the end of July (panel b). India's vaccinations were dominated by SII's local production of the AstraZeneca/Oxford vaccine.87 China administered only domestic vaccines, such as those from Sinovac and Sinopharm, as its regulators had not yet approved any developed overseas. Africa was at another extreme: only about 60 million doses had been administered in total, implying that only about 3 per cent of the continent's population had received even a first dose of any vaccine, which was dominated by imports.
Much of the story about COVID‐19 vaccinations was still unfolding, with much more analysis needed. As a first step, this paper has shown how new vaccine manufacturing supply chains emerged to produce the billions of doses delivered by Pfizer/BioNTech, Moderna, AstraZeneca/Oxford and Johnson & Johnson. Heavy government involvement – especially considerable public investment made at risk – shaped the evolution of these supply chains and the speed at which they were formed. But more information is needed – on the inputs that went in and the outputs that came out – from the dozens of production facilities in the supply chains behind those brand names.
As increasingly detailed data emerge, researchers must investigate how production was scaled up and what impact policy had in order to shed light on two critical questions. Could more vaccine doses have been manufactured more quickly some other way? Would alternative policy choices have made a difference? Answers will hopefully help prepare policymakers for the next pandemic.
APPENDIX A.
TABLE A1.
Pfizer/BioNTech supply chain
| Company | Location | Role | Key dates |
|---|---|---|---|
| BioNTech | Mainz, Germany | Reports rapid progress on COVID‐19 vaccine program | 16 March 2020 |
| Shanghai Fosun Pharmaceutical | Shanghai, China | Collaborates on strategic development and commercialisation to advance BioNTech's mRNA vaccine candidate in China | 16 March 2020 |
| Pfizer | New York, US | Partners with BioNTech in clinical development and manufacturing for markets outside China | 17 March 2020 |
| Drug substance | |||
| Pfizer | Missouri, US | Manufactures DNA plasmids | 5 May 2020 |
| Pfizer | Massachusetts, US | Manufactures mRNA from DNA | 5 May 2020 |
| Exelead | Indiana, US | Assists in manufacturing the vaccine | 27 May 2020 |
| BioNTech | Mainz, Germany | Manufactures mRNA from DNA | Unknown |
| BioNTech | Marburg, Germany | Manufactures mRNA from DNA |
17 September 2020 (acquisition) 10 February 2021 (operational) |
| Dermapharm | Brehna, Germany | Formulates mRNA active ingredients enveloped by lipids | 10 September 2020 |
| Dermapharm | Reinbek, Germany | Formulates mRNA active ingredients enveloped by lipids | 30 April 2021 |
| Shanghai Fosun Pharmaceutical | Shanghai, China | Forms joint venture with BioNTech in which BioNTech contributes license and know‐how and Fosun contributes manufacturing facility and cash | 9 May 2021 |
| BioNTech | Singapore | Establishes fully integrated mRNA manufacturing facility to be operational as early as 2023 | 10 May 2021 |
| Pfizer | Dublin, Ireland | Manufactures mRNA from DNA | 19 May 2021 |
| AGC Biologics | Heidelberg, Germany | Manufactures DNA plasmids | 7 June 2021 |
| Lipid nanoparticles | |||
| Acuitas | British Columbia, Canada | Licenses technology for lipid nanoparticles | By 1 July 2020 |
| Avanti Polar Lipids (Croda) | Alabama, US | Manufactures lipids | 10 November 2020 |
| Croda a | Snaith, UK | Manufactures lipids | Unknown |
| Polymun | Klosterneuburg, Austria | Manufactures lipids | Unknown |
| Evonik | Hanau, Germany | Manufactures lipids |
10 February 2021 (agreement) 22 April 2021 (first delivery) |
| Evonik | Dossenheim, Germany | Manufactures lipids |
10 February 2021 (agreement) 22 April 2021 (first delivery) |
| AMRI | New York, US | Manufactures lipids | 25 February 2021 |
| Merck | Darmstadt, Germany | Manufactures lipids | 5 February 2021 |
| Pfizer | Connecticut, US | Manufactures lipids | 19 February 2021 |
| Fill and finish | |||
| Pfizer | Michigan, US |
Handles formulation, fill and finish Announces plant expansion |
5 May 2020 |
| Pfizer | Kansas, US | Handles fill and finish | 19 February 2021 |
| Pfizer | Puurs, Belgium | Handles formulation, fill and finish | 5 May 2020 |
| Siegfried | Hameln, Germany | Handles fill and finish | 14 September 2020 |
| Delpharm | Saint‐Rémy, France | Handles fill and finish | 19 November 2020 |
| Sanofi | Frankfurt, Germany | Handles fill and finish | 27 January 2021 |
| Novartis | Stein, Switzerland | Handles fill and finish | 29 January 2021 |
| Thermo Fisher | Monza, Italy | Handles fill and finish | 25 March 2021 |
| Biovac Institute | Cape Town, South Africa | Handles fill and finish | 21 July 2021 |
Reported by the Telegraph.
Source: Constructed by the authors. Hyperlinks provide original sources.
TABLE A2.
Moderna supply chain
| Company/institution | Location | Role | Key dates |
|---|---|---|---|
| Moderna | Massachusetts, US | Receives funding from Coalition for Epidemic Preparedness Innovations (CEPI) to accelerate development of mRNA vaccine against the novel coronavirus | 23 January 2020 |
| National Institutes of Health | Maryland, US | Co‐invents the vaccine | 23 January 2020 |
| Moderna | Massachusetts, US | Manufactures vaccine for clinical trials | 23 January 2020 |
| Moderna | Massachusetts, US | Ships mRNA vaccine candidate for Phase 1 study | 24 February 2020 |
| Lonza | Basel, Switzerland | Participates in worldwide strategic collaboration | 1 May 2020 |
| Drug substance | |||
| Lonza | New Hampshire, US | Manufactures drug substance | 1 May 2020 |
| Lonza | Visp, Switzerland | Manufactures drug substance | 1 May 2020 |
| Rovi | Granada, Spain | Manufactures drug substance | 12 April 2021 |
| Moderna | Massachusetts, US | Renovates facility to expand manufacturing capacity | 4 May 2021 |
| Aldevron | North Dakota, US | Supplies plasmid DNA to serve as genetic template for mRNA vaccine | 24 May 2021 |
| Lonza | Geleen, Netherlands | Manufactures drug substance | 2 June 2021 |
| Lipid nanoparticles | |||
| CordenPharma |
Colorado, US Liestal, Switzerland Chenôve, France |
Manufactures lipids | 28 May 2020 |
| Fill and finish | |||
| Catalent | Indiana, US |
Handles fill and finish Extends contract |
25 June 2020 |
| Baxter | Indiana, US | Handles fill and finish | 8 March 2021 |
| Sanofi | New Jersey, US | Handles fill and finish | 26 April 2021 |
| Rovi | Madrid, Spain | Handles fill and finish | 9 July 2020 |
| Recipharm | Monts, France | Handles fill and finish | 30 December 2020 |
| Samsung Biologics | Incheon, South Korea | Handles fill and finish | 22 May 2021 |
| Thermo Fisher | North Carolina, US | Handles fill and finish | 1 June 2021 |
Source: Constructed by the authors. Hyperlinks provide original sources.
TABLE A3.
AstraZeneca/Oxford supply chain
| Company | Location | Role | Key dates |
|---|---|---|---|
| Oxford University | Oxford, UK | Identifies COVID‐19 vaccine candidate | 18 March 2020 |
| AstraZeneca | Cambridge, UK | Develops, manufactures, and distributes Oxford vaccine | 30 April 2020 |
| Drug substance | |||
| Oxford Biomedica | Oxford, UK | Signs initial clinical and commercial supply agreements | 28 May 2020 |
| Serum Institute of India (SII) | Pune, India | Signs licensing agreement to supply 1 billion doses to poor countries, with commitment to provide 400 million before end of 2020 | 4 June 2020 |
| Emergent BioSolutions | Maryland, US |
Manufactures drug substance for clinical trials Manufactures drug substance at scale |
11 June 2020 27 July 2020 |
| Catalent | Maryland, US | Manufactures drug substance | 24 August 2020 |
| Cobra Biologics UK | Keele, UK | Manufactures drug substance | 16 June 2020 |
| NovaSep (Thermo Fisher) a | Seneffe, Belgium |
Manufactures drug substance Signs multiyear contract |
15 June 2020 |
| mAbxience | Garín, Argentina | Manufactures drug substance | 17 August 2020 |
| Halix | Leiden, Netherlands | Manufactures drug substance | 8 December 2020 |
| Siam Bioscience | Bangkok, Thailand | Manufactures drug substance for Thailand and Association of Southeast Asian Nation (ASEAN) countries | 12 October 2020 |
| IDT Biologika | Dessau, Germany | Signs letter of intent for joint investment and capacity to manufacture drug substance | 10 February 2021 |
| CSL | Broadmeadows, Australia |
Manufactures drug substance |
8 November 2020 |
| JCR Pharmaceuticals | Kobe, Japan |
Collaboration agreement Manufactures drug substance at new plant |
August, 2020 b March 2021 b |
| BioKangtai | Shenzhen, China | Manufactures drug substance, formulation, fill and finish for 100 million doses | 21 August 2020 |
| Fill and finish | |||
| Symbiosis Pharmaceutical | Scotland | Handles fill and finish for clinical trials | 22 June 2020 |
| AstraZeneca | Ohio, US | Handles fill and finish | 11 June 2020 c |
| CP Pharmaceuticals (Wockhardt) | Wrexham, Wales, UK | Handles fill and finish | 3 August 2020 |
| Catalent | Anagni, Italy | Handles fill and finish | 15 June 2020 |
| Fiocruz Institute | Rio de Janeiro, Brazil | Handles fill and finish, eventually manufactures drug product | 27 June 2020 |
| Laboratorios Liomont | Mexico | Handles fill and finish for Latin American (except Brazil) | 17 August 2020 |
| CSL | Parkville, Australia | Handles fill and finish | 8 November 2020 |
| KM Biologics b | Kumamoto prefecture, Japan | Handles fill and finish | 18 December 2020 |
| Daiichi Sankyo | Japan | Handles fill and finish | 5 February 2021 |
| Insud Pharma | Azuqueca de Henares, Guadalajara, Spain | Handles fill and finish | 20 January 2021 |
| IDT Biologika | Dessau, Germany | Handles fill and finish | 10 February 2021 |
Plant taken over by Thermo Fisher in January 2021.
Reported in FiercePharma.
Jacobs Engineering announces it is retrofitting the plant.
Source: Constructed by the authors. Hyperlinks provide original sources.
TABLE A4.
Johnson & Johnson (Janssen) supply chain
| Company/institution | Location | Role | Key dates |
|---|---|---|---|
| Janssen Pharmaceutica (Johnson & Johnson) | Beerse, Belgium | Identifies COVID‐19 vaccine candidate | 30 March 2020 |
| Beth Israel Deaconess Medical Center | Massachusetts, US | Co‐invents vaccine | 30 March 2020 |
| Drug substance | |||
| Johnson & Johnson | Leiden, Netherlands | Manufactures drug substance for clinical trials | April, 2020 |
| Emergent BioSolutions | Maryland, US | Manufactures drug substance | 23 April 2020 |
| Merck | North Carolina, US | Manufactures drug substance (eventually) | 2 March 2021 |
| Biological E. | Paonta Sahib, Himachal Pradesh, India | Manufactures drug substance and drug product; purchases new plant from Akorn India | 13 August 2020 |
| Fill and finish | |||
| Catalent | Indiana, US | Handles fill and finish | 29 April 2020 |
| Catalent | Anagni, Italy |
Handles fill and finish a Adds capacity |
17 March 2021 |
| Grand River Aseptic Manufacturing (GRAM) | Michigan, US | Handles fill and finish | 25 September 2020 |
| Merck | Pennsylvania, US | Handles fill and finish | 2 March 2021 |
| Aspen Pharmacare | Gqeberha, South Africa | Handles fill and finish | 2 November 2020 |
| Reig Jofre | Barcelona, Spain | Handles fill and finish | 15 December 2020 |
| Sanofi Pasteur | Marcy l'Etoile, France | Handles fill and finish | 22 February 2021 |
| IDT Biologika | Dessau, Germany | Handles fill and finish | 15 March 2021 |
Reported by FiercePharma.
Source: Constructed by the authors. Hyperlinks provide original sources.
TABLE A5.
Novavax supply chain
| Company/institution | Location | Role | Key dates |
|---|---|---|---|
| Novavax | Maryland, US | Identifies COVID‐19 vaccine candidate | 8 April 2020 |
| Drug substance | |||
| Emergent BioSolutions | Maryland, US | Manufactures drug substance for clinical trials | 8 April 2020 |
| Novavax | Bohumil, Czech Republic | Purchases plant expected to manufacture 1 billion doses of drug substance | 27 May 2020 |
| Fujifilm Diosynth Biotechnologies (FDB) | North Carolina, US | Manufactures drug substance | 23 July 2020 |
| FDB | Texas, US | Manufactures drug substance | 27 July 2020 |
| FDB | Billingham, UK | Manufactures drug substance | 14 August 2020 |
| Takeda Pharmaceutical | Hikari, Japan |
Signs collaboration agreement Reaches final agreement for drug substance manufacturing |
|
| SK bioscience | Andong L‐house, South Korea |
Signs collaboration agreement Reaches final agreement for drug substance manufacturing |
13 August 2020 |
| Biofabri | Spain | Manufactures drug substance | 21 October 2020 |
| Serum Institute of India (SII) | Pune, India |
Signs supply and license agreement Signs amendment for drug substance manufacturing |
30 July 2020 |
| National Research Council's Biologics Manufacturing Centre | Montréal, Canada | Signs Memorandum of Understanding with government of Canada for drug substance manufacturing | 2 February 2021 |
| Adjuvant | |||
| Desert King | California, US | Procures saponin (raw material) for adjuvant from its facilities in Chile | 30 September 2020 a |
| AGC Biologics | Copenhagen, Denmark | Manufactures Matrix‐M adjuvant | 4 June 2020 |
| AGC Biologics | Washington, US | Manufactures Matrix‐M adjuvant | 10 August 2020 |
| PolyPeptide Group | California, US | Manufactures Matrix‐M adjuvant | 3 June 2020 |
| PolyPeptide Group | Malmö, Sweden | Manufactures Matrix‐M adjuvant | 3 June 2020 |
| Fill and finish | |||
| Par Sterile Products (Endo) | Michigan, US | Handles fill and finish | 25 September 2020 |
| Baxter | Halle, Germany | Handles fill and finish | 11 January 2021 |
| GSK | Barnard Castle, England, UK | Handles fill and finish | 29 March 2021 |
| Jubilant HollisterStier | Washington, US | Handles fill and finish | 31 March 2021 |
| Siegfried | Hameln, Germany | Handles fill and finish | 4 May 2021 |
Novavax SEC third quarter, 2020 10‐Q filing.
Source: Constructed by the authors. Hyperlinks provide original sources.
TABLE A6.
CureVac supply chain
| Company | Location | Task | Key dates |
|---|---|---|---|
| CureVac | Tübingen, Germany | German and Belgian regulators authorise clinical phase 1 trial for its COVID‐19 vaccine candidate, CVnCoV | 17 June 2020 |
| Drug substance | |||
| CureVac | Tübingen, Germany | Expands manufacturing facilities with funding from European Investment Bank | 6 July 2020 |
| Wacker Chemie AG | Amsterdam, Netherlands | Manufacture mRNA active substance for CVnCoV in first half of 2021. | 23 November 2020 |
| Rentschler Biopharma | Laupheim, Germany | Manufacture of mRNA active substance for CVnCoV and formulation. | 1 February 2021 |
| Bayer | Wuppertal, Germany | Manufacture 160 million doses in 2022, potentially some towards the end of 2021 | 1 February 2021 |
| GSK | Wavre, Belgium | Manufacture 100 million doses in 2021 | 3 February 2021 |
| Novartis | Kundl, Austria | Manufacture mRNA and pre‐formulated active ingredient for up to 50 million doses in 2021 and up to around 200 million doses in 2022. | 4 March 2021 |
| Celonic Group | Heidelberg, Germany | Manufacture mRNA drug substance as well as LNP formulation of the bulk drug product with more than 50 million doses in 2021. | 30 March 2021 |
| Fill and finish | |||
| Fareva |
Pau, France Val‐de‐Reuil, France |
Fill vials with the vaccine and the diluent, supporting production of millions of doses | 9 December 2020 |
Source: Constructed by the authors. Hyperlinks provide original sources.
Bown, C. P. , & Bollyky, T. J. (2022). How COVID‐19 vaccine supply chains emerged in the midst of a pandemic. The World Economy, 45, 468–522. 10.1111/twec.13183
After this paper was written but before it was published, Bollyky became a senior consultant for the Coalition for Epidemic Preparedness Innovations (CEPI).
For helpful comments and suggestions, the authors thank Chris Adams, David Greenaway (the editor), Anna Isaac, Soumaya Keynes, Jacob Kirkegaard, Sam Lowe, Jim Miller, Chris Rogers, Kadee Russ, Dave Vanness and Prashant Yadav. Hexuan Li and Yilin Wang provided outstanding research assistance. Melina Kolb, William Melancon and Oliver Ward assisted with graphics. Any errors are our own.
Funding information
None
Footnotes
For ease of exposition, this paper sometimes refers to these six as ‘vaccines’ even though, as of July 2021, those from Novavax and CureVac technically should be referred to as ‘candidates’, since neither was yet (and might not ever be) authorized by regulators for public use.
‘It'll take months – or even years – to create 7 billion doses (or possibly 14 billion, if it's a multi‐dose vaccine)’, wrote Bill Gates, co‐chair of the Bill and Melinda Gates Foundation, one of the foundational groups seeking to accelerate COVID‐19 vaccines, early in the pandemic (Bill Gates, ‘What you need to know about the COVID‐19 vaccine’, GatesNotes, 30 April 2020). Importantly, a two‐dose regimen was required for all of the vaccines described below, with the exception of Johnson & Johnson (one dose), implying the need for closer to 14 billion doses than 7 billion. The need to provide additional doses either as a booster or as a third dose in the regimen may increase the global vaccine production needs even further.
COVAX signed up to the programme most of the world's poorest countries, as well as lower‐middle‐income economies. It had trouble meeting its early goals, however, mostly because the vaccine‐manufacturing countries refused to share sufficient doses with the programme. See Bollyky and Bown (2020a, 2020b) and Bown and Bollyky (2021).
See, for example, Castillo et al. (2021), Cakmakli et al. (2021), Gagnon et al. (2021) and Hafner et al. (2020) for estimates of the economic costs of failing to scale up vaccine manufacturing. For research on advance market commitments for new vaccines, see Kremer, Levin, and Snyder (2020).
Small molecule drugs – for example aspirin or penicillin – are relatively simple and can be manufactured by chemical synthesis. In contrast, biological products such as vaccines are complex mixtures that are not easily identified or characterized.
For ease of exposition, we have included “formulation” into step 3. In reality, for some vaccines and supply chain relationships described below, the formulation phase occurs alongside the “fill and finish” process at the separate production facility of step 4.
Although not a focus here, contract research organizations (CROs) also emerged to help manage the clinical trial process and interactions with regulators, and clinical trials themselves were increasingly conducted abroad or across multiple countries. For the governance of clinical trials, see OECD (2013).
The fragmentation of the pharmaceutical industry and the rise in contract manufacturers share some similarities with the global semiconductor industry (Bown 2020). For global value chains more broadly and the pandemic, see Antràs (2020).
WHO. 2021. COVID‐19 Vaccine Tracker and Landscape, last accessed 9 July 2021.
See, for example, UK National Audit Office (2020, p. 25).
See Emma Cott, Elliot deBruyn, and Jonathan Corum, ‘How Pfizer Makes Its Covid‐19 Vaccine’, New York Times, April 28, 2021; Elizabeth Weise and Karen Weintraub, ‘A COVID‐19 Vaccine Life Cycle: From DNA to Doses’, USA Today, 7 February 2021.
A spokesperson for Croda International said, ‘We manufacture components within the UK that we ship to Pfizer facilities in multiple locations, including Belgium’. See Bill Gardner and Ben Riley‐Smith, ‘Exclusive: Pfizer Warns EU to Back Down on Covid Vaccine Threat to UK’, Telegraph, 19 March 2021.
Financial Times, ‘EU Threat to Vaccine Exports Exposes Mutual Risks to Global Supply Chain’, 18 March 2021.
Steffen Klusmann und Thomas Schulz, ‘To See People Finally Benefitting from Our Work Is Really Moving’. Interview with Özlem Türeci and Uğur Şahin, Der Spiegel, 4 January 2021.
Sharon LaFraniere and Katie Thomas, ‘Pfizer Nears Deal With Trump Administration to Provide More Vaccine Doses’, New York Times, 22 December 2020.
Peter Loftus, ‘Covid‐19 Vaccine Manufacturing in US Races Ahead’, Wall Street Journal, 21 March 2021.
CNBC, ‘Pfizer Chairman and CEO Albert Bourla Speaks with CNBC’s ‘Squawk Box’ Today,’ December 14, 2020.
White House (2021a).
Marisa Taylor and Robin Respaut, ‘Exclusive: Moderna Spars with US Scientists over COVID‐19 Vaccine Trials’, Reuters, 7 July 2020.
In Japan, Moderna would also contract with Takeda to run its clinical trials.
Reuters reported that three new production lines in Visp, Switzerland ‘costing 70 million Swiss francs ($80 million) each and due to supply a combined 300 million doses annually, are not yet producing vaccine, though the first line could become operational within days. A Lonza site in Portsmouth, New Hampshire, with 100 million doses annual capacity, began large‐scale production last year for US‐bound Moderna vaccine’ (John Miller, ‘Moderna Vaccine to Criss‐Cross Continent before Europeans Get Shots’, Reuters, January 6, 2021).
Moderna also announced that Samsung Biologics would do fill and finish in South Korea, but it did not initially indicate where the drug product would be imported from.
See Fraiser Kansteiner, ‘The Next Big COVID‐19 Bottleneck? A Shortage of Trained Vaccine Workers, Experts Say’, FiercePharma, 23 April 2021; John Miller, ‘Help Wanted: Lonza Seeks Workers to Lift Moderna Vaccine Output’, Reuters, 29 April 2021.
Rebecca Robbins and Benjamin Mueller, ‘After Admitting Mistake, AstraZeneca Faces Difficult Questions about Its Vaccine’, New York Times, 25 November 2020.
Jeffrey Gettleman, ‘Indian Billionaires Bet Big on Head Start in Coronavirus Vaccine Race,’ New York Times, 1 August 2020.
Indrani Bagchi, ‘SII Fails to Deliver, New Delhi's Vaccine Diplomacy Hits Hurdle’, Times of India, March 21, 2021.
Gavi, ‘COVAX Updates Participants on Delivery Delays for Vaccines from Serum Institute of India (SII) and AstraZeneca’, 25 March 2021; Sohini Das, ‘AstraZeneca Has Sent Us Legal Notice for Vaccine Supply Delay: Poonawalla’, Business Standard, 7 April 2021.
https://twitter.com/adarpoonawalla/status/1382978713302683653?s=20. His comments followed concerns he had raised in March. See Economic Times, ‘US Export Curbs Can Limit COVID‐19 Vaccine Production, Availability: SII CEO Adar Poonawalla’, 5 March 2021.
See also Bollyky and Bown (2021).
Stephanie Findlay, ‘India's Vaccine Shortage Will Last Months, Biggest Manufacturer Warns’, Financial Times, 2 May 2021.
Chris Hamby, ‘Biotech Company That Botched Vaccines Faces Investor Revolt’, New York Times, 6 July 2021.
The Halix plant was not approved by the EMA for EU production until 29 March 2021, even though it was part of the original Oxford consortium in April 2020. Dutch broadcaster NOS reported that the Dutch government had declined a request for a €10 million investment to expand the Halix production facility in April 2020 (Thomas Spekschoor, ‘The Netherlands Missed Out on Millions of Oxford Vaccines’, NOS, 30 March 2021).
Jenny Strasburg and Laurence Norman, ‘Behind AstraZeneca's Covid‐19 Vaccine Stumble’, Wall Street Journal, 28 January 2021.
MHRA. 2021. Conditions of Authorisation for COVID‐19 Vaccine AstraZeneca (Regulation 174). Amended 23 February 2021.
Carlo Martuscelli, Anna Isaac, Paola Tamma, Jakob Hanke Vela and Helen Collis, ‘EU Sends Italian Police to Find AstraZeneca Vaccines, Triggering Global Angst’, Politico, 24 March 2021.
Carlos Slim Foundation, ‘AstraZeneca Announces Agreement with Carlos Slim Foundation to Supply COVID‐19 Vaccine to Latin America’, 1 October 2020.
In December, the New York Times published an unflattering profile of the company (Sui‐Lee Wee and Javier C. Hernández, ‘Scandal Dogs AstraZeneca's Vaccine Partner in China’, New York Times, 7 December 2020.)
Janssen, ‘Johnson & Johnson Temporarily Pauses All Dosing in Our Janssen COVID‐19 Vaccine Candidate Clinical Trials’, Press release, 12 October 2020.
Hallie Levine, ‘From Lab to Vaccine Vial: The Historic Manufacturing Journey of Johnson & Johnson's Janssen COVID‐19 Vaccine’, Johnson & Johnson, 3 March 2021.
Sharon LaFraniere and Noah Weiland, ‘Factory Mix‐Up Ruins up to 15 Million Vaccine Doses from Johnson & Johnson’, New York Times, 31 March 2021.
On 11 June 2021, the FDA issued a memo outlining problems at the Emergent facility (Food and Drug Administration 2021).
Reuters, ‘Emergent to resume J&J COVID‐19 vaccine production at Baltimore plant’, 29 July 2021.
See ‘Aspen Statement on Manufacture and Supply of Covid‐19 Vaccines’, 14 June 2021 and ‘Aspen Confirms Release of COVID‐19 Vaccines to Johnson & Johnson for Supply to South Africa’, 26 July 2021.
Leroy Lee, ‘Biological E. Buys Akorn India to Boost Vaccine Manufacturing Capacity’, Mint, 17 August 2020.
Krishna N. Das, ‘India's Biological E. Looking to Make 600 million J&J Vaccine Shots a Year’, Reuters, 10 February 2021. In the interview, Datla indicated that Biological E. was simultaneously developing another vaccine, with Baylor College of Medicine, and was to produce 1 billion doses by the end of 2021. That vaccine was undergoing Phase 3 trials ‘soon’ only as of May (Krishna Das, ‘India's Biological E. to Begin Phase III Trial of Vaccine, Production from August’, Reuters, 7 May 2021).
Stephanie Findlay and Donato Paolo Mancini, ‘Indian Vaccine Makers Decry US Use of Wartime Powers to Protect Supplies’, Financial Times, 15 March 2021.
Swati Bharadwaj, ‘‘Made in India’ J&J vaccine May Roll Out Only in Fourth Quarter’, Times of India, 5 May 2021.
In March 2020, Novavax entered into an agreement with Emergent BioSolutions to supply vaccine product for use in its clinical trials (‘Novavax Identifies Coronavirus Vaccine Candidate; Accelerates Initiation of First‐in‐Human Trial to Mid‐May’, Press release, 8 April 2020).
See Hans von der Burchard, ‘German Firm Insists Trump Didn't Try to Buy Coronavirus Vaccine’, Politico, 17 March 2020.
‘CureVac Announces Financial Results and Business Updates for the Fourth Quarter and Full‐Year of 2020’. Press release, 15 April 2021.
Ludwig Burger, ‘EU persuades U.S. to ease COVID export restrictions for CureVac‐sources’, Reuters, 21 May 2021. See also the DPA discussion below.
Ludwig Burger, ‘CureVac fails in pivotal COVID‐19 vaccine trial with 47% efficacy’, Reuters, 17 June 2021.
Mark Terry, ‘Novartis Delivers Strong Second Quarter and 50 Million CureVac Vaccines’, BioSpace, 21 July 2021.
‘GSK and CureVac to develop next generation mRNA COVID‐19 vaccines’, Press release, 2 February 2021.
This section extends and updates analysis initially presented in Bown and Bollyky (2021). In June 2021, OWS was renamed the Countermeasures Acceleration Group (CAG) (see Nicholas Florko, ‘Operation Warp Speed—Now, the ‘CAG’— Is Here to Stay,’ STAT, 29 June 2021).
Spiro and Emanuel (2020) suggested many of the policies undertaken by OWS.
‘Horizon Therapeutics plc Announces Short‐Term TEPEZZA® (teprotumumab‐trbw) Supply Disruption Due to Government‐Mandated (Operation Warp Speed) COVID‐19 Vaccine Production’, News release, 17 December 2020.
Staff at MilliporeSigma, a key equipment supplier was ‘in near‐daily communication with “colonels and majors,” the pharmaceutical companies and their contract manufacturers to fulfill those orders’. (Riley Griffin, ‘A Cold War‐Era Law and Vaccines’, Bloomberg, 2 January 2021.)
White House (2021a).
See Section 3 for Biological E. and SII. For Novavax, see James Tapper, ‘Global Covid Vaccine Rollout Threatened by Shortage of Vital Components’, Observer, 7 April 2021. For CureVac, see Reuters, ‘Vaccine Supply Chains Disrupted by US Restrictions: CureVac Co‐Founder’, 7 April 2021.
Yahoo News, ‘French President Macron Urges US, UK to Stop Blocking Covid‐19 Vaccine Exports’ , 7 May 2021.
White House official Tim Manning sent out an explanatory tweet on April 26 denying the accusations that was then followed up with a press briefing (White House, 2021b).
White House (2021c).
Some allocation to vaccines that were not authorized inevitably occurred. Complaints by the CureVac CEO led to a US government intervention to help it access inputs in short supply by May. By June, easing the restrictions seemed wasteful, given announcement of CureVac's disappointing Phase 3 results described earlier.
As of July 2021, only one of these vaccines (AstraZeneca) had been granted emergency use; the other two (Novavax and Valneva) had not yet been cleared by regulators.
‘GSK to Support Manufacture of Novavax’ COVID‐19 Vaccine’, Press release, 29 March 2021.
See European Commission, ‘HERA Incubator: Anticipating together the threat of COVID‐19 variants’, Communication from the Commission to the European Parliament, the European Council and the Council, COM(2021) 78 final, 17 February 2021.
World Bank, ‘IFC, Proparco, DEG and DFC Support South African COVID‐19 Vaccine Maker, Aspen’, Press release, June 30.
This section draws from some of the questions first identified in Bown and Rogers (2021).
Clive Glover ‘Supporting Rapid Development with a Standardized Single‐Use Manifold and AAV Platform Process’, Pall Blog, 3 February 2021.
Bollyky and Bown (2020a) and Bown and Bollyky (2021) propose an explicit COVID‐19 Vaccine Investment and Trade Agreement (CVITA) to formalize these incentives and cooperation.
Carl O'Donnell and Michael Erman, ‘Pfizer to Outsource Some Drug Production, Focus on Coronavirus Vaccine’, Reuters, 9 May 2020.
The Indian government did grant emergency use to Sputnik V, the Russian vaccine, even though the WHO had not approved it.
Costas Paris, ‘Supply‐Chain Obstacles Led to Last Month's Cut to Pfizer's Covid‐19 Vaccine‐Rollout Target’, Wall Street Journal, 3 December 2020.
Vicky McKeever, ‘Pfizer to Temporarily Reduce Covid Vaccine Deliveries to Europe’, CNBC, 15 January 2021.
Elizabeth Weise, ‘Pfizer Expects to Cut Covid‐19 Vaccine Production Time by Close to 50% as Production Ramps Up, Efficiencies Increase’, USA Today, 7 February 2021.
A controversy then arose over who would get access to those doses, as the contracts were for doses, not vials. The European Commission initially thought it had more doses than expected, but Pfizer cut back its delivery of vials, expanding supplies elsewhere (Donato Paolo Mancini, Miles Johnson, Michael Peel, Guy Chazan and Hannah Kuchler, ‘EU and BioNTech/Pfizer Clash over Reduced Vaccine Deliveries’, Financial Times, 20 January 2021).
Hannah Kuchler and Joe Miller, ‘BioNTech/Pfizer Covid Vaccine No Longer Needs Ultra‐Cold Storage’, Financial Times, 19 February 2021.
Peter Loftus, ‘Covid‐19 Vaccine Manufacturing in US Races Ahead’, Wall Street Journal, 21 March 2021.
Peter Loftus, ‘Moderna Gets Permission to Fill Covid‐19 Vaccine Vials with More Doses’, Wall Street Journal, 2 April 2021.
See Bown (Forthcoming).
The United States and European Union dominated administered doses of the single‐dose Johnson & Johnson vaccine through July, with less than 25 million doses, or less than 1 per cent of the doses administered globally.
Covishield, the AstraZeneca vaccine being manufactured by SII, was estimated to make up roughly 75 per cent of administered doses, with Bharat Biotech's Covaxin making up most of the rest (Times of India, ‘51.6 crore vaccine doses would be made available by July 31, 35.6 crore already provided: Centre to SC’, 26 June 2021).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the working paper version of this article found at www.piie.com.
REFERENCES
- Antràs, P. (2020). De‐globalisation? Global value chains in the post‐COVID‐19 age. NBER Working Paper 281115. National Bureau of Economic Research. https://www.nber.org/papers/w28115 [Google Scholar]
- Bollyky, T. J. , & Bown, C. P. (2020a). The tragedy of vaccine nationalism: Only cooperation can end the pandemic. Foreign Affairs pp. 99(5), 96–109. [Google Scholar]
- Bollyky, T. J. , & Bown, C. P. (2020b). Vaccine nationalism will prolong the pandemic. A global problem calls for collective action. Foreign Affairs, December 29. [Google Scholar]
- Bollyky, T. J. , & Bown, C. P. (2021). The real vaccine procurement problem: Why America should make its supply chain more transparent. June 24. Foreign Affairs. [Google Scholar]
- Bown, C. P. (Forthcoming). How COVID‐19 medical supply shortages led to extraordinary trade and industrial policy. Asian Economic Policy Review. 10.1111/aepr.12359 [DOI] [Google Scholar]
- Bown, C. P. (2020). How the United States marched the semiconductor industry into its trade war with China. East Asian Economic Review, 24(4), 349–388. 10.11644/KIEP.EAER.2020.24.4.384 [DOI] [Google Scholar]
- Bown, C. P. , & Bollyky, T. J. (2021). Here's how to get billions of COVID‐19 vaccine doses to the world. PIIE Trade and Investment Policy Watch, March 18. Peterson Institute for International Economics. [Google Scholar]
- Bown, C. P. , & Rogers, C. (2021). The US did not ban exports of vaccine supplies. But more help is needed. PIIE Trade and Investment Policy Watch, June 7. Peterson Institute for International Economics. [Google Scholar]
- Cakmakli, C. , Demiralp, S. , Kalemli‐Ozcan, S. , Yesiltas, S. , & Yildirim, M. (2021). The economic case for global vaccinations: An epidemiological model with international production networks. CEPR Discussion Paper DP15710, January. Center for Economic and Policy Research. [Google Scholar]
- Castillo, J. C. , Ahuja, A. , Athey, S. , Baker, A. , Budish, E. , Chipty, T. , Glennerster, R. , Kominers, S. D. , Kremer, M. , Larson, G. , Lee, J. , Prendergast, C. , Snyder, C. M. , Tabarrok, A. , Tan, B. J. , & Więcek, W. (2021). Market design to accelerate COVID‐19 vaccine supply. Science, 371(6534), 1107–1109. 10.1126/science.abg0889 [DOI] [PubMed] [Google Scholar]
- Coalition for Epidemic Preparedness Innovations (2020). Survey of global drug substances and drug product landscape, June 29. Coalition for Epidemic Preparedness Innovations. [Google Scholar]
- Coalition for Epidemic Preparedness Innovations (2021). CEPI launches COVAX Marketplace to match buyers and sellers of critical manufacturing supplies and speed up global access to COVID‐19 vaccines through COVAX, July 15. Coalition for Epidemic Preparedness Innovations. [Google Scholar]
- Evenett, S. , Hoekman, B. , Rocha, N. , & Ruta, M. (2021). The Covid‐19 vaccine production club: Will value chains temper nationalism? Working Paper, March. World Bank. https://openknowledge.worldbank.org/handle/10986/35244 [Google Scholar]
- Food and Drug Administration (2021). Memorandum to Janssen COVID‐19 Vaccine EUA 27205. Peter Marks, Director, Center for Biologics Evaluation and Research. Subject: Assessment of Certain Janssen COVID‐19 Vaccine Batches, June 11. [Google Scholar]
- Gagnon, J. E. , Kamin, S. , & Kearns, J. (2021). Economic costs and benefits of accelerated COVID‐19 vaccinations. Policy Brief 21‐11, May. Peterson Institute for International Economics. [Google Scholar]
- Hafner, M. , Yerushalmi, E. , Fays, C. , Dufresne, E. , & Van Stolk, C. (2020). COVID‐19 and the cost of vaccine nationalism. RAND Corporation. [PMC free article] [PubMed] [Google Scholar]
- Kis, Z. , Kontoravdi, C. , Shattock, R. , & Shah, N. (2021). Resources, production scales and time required for producing RNA vaccines for the global pandemic demand. Vaccines, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer, M. , Levin, J. D. , & Snyder, C. M. (2020). Designing advance market commitments for new vaccines. NBER Working Paper 28168. National Bureau of Economic Research. https://www.nber.org/papers/w28168 [Google Scholar]
- Miller, J. (2021). Pharma outsourcing outlook for 2021: The CDMO industry scales up. January 13. DCAT Value Chains Insight. [Google Scholar]
- OECD (2013). OECD recommendation on the governance of clinical trials. OECD Publishing. [Google Scholar]
- Plotkin, S. , Robinson, J. M. , Cunningham, G. , Iqbal, R. , & Larsen, S. (2017). The complexity and cost of vaccine manufacturing: An overview. Vaccines, 35, 4064–4071. 10.1016/j.vaccine.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro, T. , & Emanuel, Z. (2020). A comprehensive COVID‐19 vaccine plan: Efficient manufacturing, financing, and distribution of a COVID‐19 vaccine. July 28. Center for American Progress. [Google Scholar]
- UK Department for Business, Energy and Industrial Strategy (2020). UK vaccine taskforce 2020 achievements and future strategy: End of year report. December. UK Department for Business, Energy and Industrial Strategy. [Google Scholar]
- UK National Audit Office (2020). Investigation into preparations for potential COVID‐19 vaccines. Report by the Comptroller and Auditor General. December 16. Department for Business, Energy & Industrial Strategy, Department of Health & Social Care, NHS England and NHS Improvement, and Public Health England. [Google Scholar]
- White House (2021a). Press briefing by White House COVID‐19 response team and public health officials. February 5. White House. [Google Scholar]
- White House (2021b). Background press call by senior administration officials on COVID‐19 in India. April 26. White House. [Google Scholar]
- White House (2021c). Press briefing by White House COVID‐19 response team and public health officials. June 3. White House. [Google Scholar]
- WTO (2021). WTO issues joint indicative list of critical inputs for COVID‐19 vaccines. WTO. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in the working paper version of this article found at www.piie.com.


