Conflicts of interest
No conflict of interest.
Funding
No funding to declare.
A 46‐year‐old Caucasian man presented with psoriasis flare‐up, which occurred a day after second dose of COVID‐19 Pfizer‐BioNTech BNT16B2b2 mRNA vaccine. The patient had been suffering from plaque psoriasis for 24 years. During the last 21 months, his psoriasis was completely clear (PASI 0 points) due to deucravacitinib treatment in the clinical trial. Before entering the clinical trial two years ago, the severity of his psoriasis was assessed as PASI 18 points. For the last 48 weeks, he has been in the open‐label phase of above‐mentioned trial receiving deucravacitinib 6 mg orally once daily. Regarding COVID‐19 vaccination, the patient received his first dose of Pfizer‐BioNTech BNT16B2b2 mRNA vaccine and experienced only pain at the site of vaccine injection lasting for 24 hours. Five days after the second dose (administered 3 weeks after the first shot), he noticed psoriatic lesions on his lower legs, which quickly spread with time extending to the whole lower extremities and trunk. He presented again with pain at the injection site accompanied with fewer up to 39°C and malaise lasting for 48 hours. On admission, one week after the disease exacerbation, physical examination revealed highly inflammatory, psoriatic plaques with gross, silver scaling localized mostly on patient’s lower legs (Fig. 1a and b). Moreover, multiple, smaller lesions were visible on patient’s back and chest (Fig. 1c). The patient did not complain of neither associated itch nor pain. The PASI score was 18.5 points.
Figure 1.
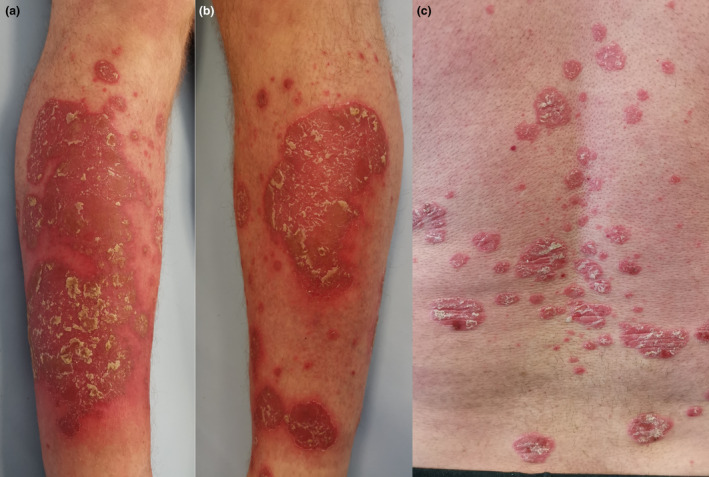
Highly inflammatory psoriatic lesions on patient’s calf (a) and lower leg (b). Multiple psoriatic plaques on patient’s back (c).
Treatment of chronic inflammatory disorders changed drastically during COVID‐19 pandemic. Problems with drugs availability and irregular consultations with dermatologist caused many patients to suffer from exacerbations.1 Moreover, the pandemic triggered insecurity about the use of novel treatment modalities.1 Yet, it is important to emphasize that psoriatic patients may present higher risk of respiratory diseases because of systemic inflammation.2 Therefore, and due to the lack of data on safety of novel, mRNA COVID‐19 vaccines, concern on its impact on patients suffering from inflammatory diseases has been raised. Vaccination, in general, is an uncommon factor triggering psoriasis flares; nevertheless, the association of vaccination with the new development or exacerbation of this skin disease has been reported.3, 4 The available reports include mostly cases of psoriasis flare‐ups after vaccination for influenza (H1N1), pneumococcal pneumonia and yellow fever.3, 4, 5 However, until now, there was no well‐described association with novel mRNA COVID‐19 vaccines. Possible cutaneous reactions after COVID‐19 vaccination were characterized recently in a registry‐based study of 414 cases by McMahon et al.6 Among others, authors described cases of local site reactions, swelling, erythema, urticaria, erythromelalgia and flares of existing dermatologic disorders. Regarding the prevalence of psoriasis flare‐ups, amid 414 cutaneous reactions, authors stated that it occurred only in two patients, which seems to be very rare. Moreover, there was no explicit description of skin lesions, nor its association with particular vaccination.6 Therefore, to the best of our knowledge, our case is the first, well‐described example of psoriatic flare‐up after COVID‐19 Pfizer‐BioNTech BNT16B2b2 mRNA vaccine. The mechanisms responsible for psoriasis exacerbation after vaccination are yet to be understood. It is possible that similarly to influenza vaccines, it may be caused by both dysregulation of immune system due to viral components and vaccine adjuvants.3 Moreover, mRNA vaccines, like BCG or diphtheria, may cause a significant increase in IL‐6 production and recruitment of Th17 cells, which play an important role in pathomechanism of psoriasis.3 Nevertheless, even though psoriasis flares are rare, because of extensive and rapid vaccination, medical professionals should pay close attention to possible adverse effects and counteract the worsening of patient’s clinical condition.
The patients in this manuscript have given written informed consent to publication of their case details.
References
- 1.Rob F, Hugo J, Tivadar Set al. Compliance, safety concerns and anxiety in patients treated with biologics for psoriasis during the COVID‐19 pandemic national lockdown: a multicenter study in the Czech Republic. J Eur Acad Dermatol Venereol 2020; 34(11): e682–e684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet. 2021; 397(10281): 1301–1315. [DOI] [PubMed] [Google Scholar]
- 3.Gunes AT, Fetil E, Akarsu S, Ozbagcivan O, Babayeva L. Possible Triggering Effect of Influenza Vaccination on Psoriasis. J Immunol Res. 2015; 2015: 258430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoneyama S, Kamiya K, Kishimoto M, Komine M, Ohtsuki M. Generalized exacerbation of psoriasis vulgaris induced by pneumococcal polysaccharide vaccine. J Dermatol. 2019; 46(11): e442–e443. [DOI] [PubMed] [Google Scholar]
- 5.de Barros MH , Avelleira JCR, Mendes KAP. Impact of yellow fever vaccine on patients with psoriasis: preliminary results. An Bras Dermatol. 2019; 94(6): 757–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahon DE, Amerson E, Rosenbach Met al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: A registry‐based study of 414 cases. J Am Acad Dermatol. 2021; 85(1): 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


