Abstract
Aims and Objectives
Quarantine during the COVID‐19 pandemic resulted in longer‐term sedentary behaviours and mental health problems. Our study aimed to evaluate the impact of the Otago exercise programme (OEP) on physical function and mental health among elderly with cognitive frailty during COVID‐19.
Background
Lockdowns and restrictions during the COVID‐19 pandemic result in longer‐term sedentary behaviours related disease and mental problem. Older people with cognitive frailty are more vulnerable to be influenced. Timely intervention may achieve better outcomes, OEP exercise was designed as a balance and muscle‐strengthening programme for elderly people.
Design
A parallel‐group, assessor‐blinded randomised controlled trial was performed according to CONSORT guidelines.
Methods
This study was conducted from July 2020 to October 2020 among 62 elderly people with cognitive frailty from a nursing home. Participants were randomly divided into an OEP group (n = 31) or a control group (n = 31). Both groups received sleep‐ and diet‐related health education. The OEP group also received a 12‐week group exercise programme. The Five Times Sit to Stand Test (FTSST), Berg Balance Scale (BBS), and Timed Up and Go Test (TUGT) were used to assess physical function. The Geriatric Depression Scale‐15 (GDS‐15) and the 12‐Item Short Form Health Survey Mental Component Summary (SF‐12 MCS) were used to assess mental health. Outcomes were measured at 6 and 12 weeks.
Results
Physical function and mental health were similar in the two groups at baseline. At 12 weeks, the OEP group (difference in change from baseline: FTSST, −2.78; TUGT, −3.73; BBS, 2.17; GDS‐15, −0.72; SF‐12 MCS, 2.58; all p < .001) exhibited significantly greater improvements than the control group (difference in change from baseline: FTSST, 1.55; TUGT, 1.66; BBS, −0.10; GDS‐15, 1.07; SF‐12 MCS, −5.95; all p < .001).
Conclusion
Our findings showed the OEP group had better physical function and mental health outcomes than the control group. OEP can be used to improve the physical and mental function among elderly people with cognitive frailty during the COVID‐19 pandemic.
Relevance to clinical practice
Otago exercise program intervention programmes should be implemented to improve physical function for cognitive frailty elderly to reduce the harm of longer‐term sedentary behaviours, and to ruduce depression symptom and improve mental health, particularly during COVID‐19 pandemic period.
Keywords: advanced nursing practice, health promotion, mental health, older people, quality of life, randomised controlled trial
What does this paper contribute to the wider global community?
Lockdowns and restrictions during the COVID‐19 pandemic result in longer‐term sedentary behaviours related disease and mental problem.
The study found that OEP group has significant improvement on lower extremity strength, balance and functional mobility, and mental health for elderly with cognitive frailty.
OEP can be used as a rehabilitation strategy for cognitive frailty elderly during COVID‐19 pandemic period.
1. INTRODUCTION
The Johns Hopkins University Center for Systems Science and Engineering reported that as at 28 January 2021, COVID‐19 had infected more than 100 million people and accounted for 2 million deaths globally (https://www.eficiens.com/coronavirus‐statistics/). Elderly people living in nursing homes are at higher risk for COVID‐19 because of old age, immunocompromised status and chronic disease (Davidson & Szanton, 2020; Szczerbińska, 2020). High risk of death occurs in older adults (Lloyd‐Sherlock et al., 2020). It is reported that 5,050 people had contracted COVID‐19 and 132 persons were infected in 16 care homes in Hong Kong China (Chow, 2021). Given this epidemiological phenomenon, the Government of China enforced lockdowns and restrictions across the mainland (Chinese Center of Disease Control & Prevention, 2021) to reduce infection. Although this was one of the best options available to combat the virus, it also resulted in lower physical function associated with longer‐term sedentary behaviours related to frailty and disabilities, as well as poor mental health outcomes (e.g. depression and cognitive decline) (Brooks et al., 2020; Pitkälä, 2020). Because isolation and quarantine limited outdoor exercise, several studies encouraged physical exercise to increase the function of the immune system and various organ systems (Brooks et al., 2020; Pitkälä, 2020). The number of confirmed COVID‐19 cases in China dropped to 293 in May 2020, and the Chinese Government issued guidelines on normal prevention and control for COVID‐19, which had moved from an emergency state to a normal state (http://www.nhc.gov.cn/). It was also indicated that residents in nursing homes should take personal protection measures. Nursing homes in Hunan, China, therefore partially opened for visits, meaning that rehabilitation care for this population could resume.
1.1. Background
Cognitive frailty is considered a heterogeneous clinical manifestation characterised by the simultaneous presence of both physical frailty and cognitive impairment, but excluding Alzheimer's dementia or other dementias (Kelaiditi et al., 2013). According to the different types of cognitive impairment, cognitive frailty can be classified as potentially reversible and reversible.
Reversible cognitive impairment is indicated by subjective cognitive decline or positive fluid and imaging biomarkers of amyloid‐beta accumulation and neurodegeneration. The evaluation of reversible cognitive frailty is subjective and may place a burden on an individual. Therefore, our study focused on the potentially reversible type of cognitive frailty. Potentially reversible cognitive impairment is considered mild cognitive impairment (MCI) (Ruan et al., 2015).
MCI is widely regarded as an intermediate stage of cognitive impairment, which occurs between the changes seen in normal cognitive ageing and those associated with dementia (Vega & Newhouse, 2014). As a syndrome, MCI is defined as subjective and objective decline in cognition and function greater than expected for an individual's age and education level, although they do not meet the criteria for a diagnosis of dementia (Petersen, 2004).
The prevalence of cognitive frailty has been reported as 0.72% ~ 50.1% based on different assessment tools and individuals (Feng et al., 2017; Merchant et al., 2017; Solfrizzi et al., 2017), and that in nursing homes has been reported as 26.2% (Yan & Jing, 2018). Longitudinal studies found that cognitive frailty was a risk factor for poor quality of life, disability, dementia and even death (Arai et al., 2018). Fortunately, cognitive frailty is reversible, timely intervention may achieve better outcomes, and it has become the main target for primary prevention (Lee et al., 2018).
Recent research on ageing has focused on interventions for frailty with cognitive decline, such as pharmaceuticals or nutraceuticals (Ruan et al., 2018), oxygen‐ozone treatment (Scassellati et al., 2020) and exercise (Casas‐Herrero et al., 2019). The Aging‐ONDUAL‐TASK study showed that multicomponent exercise with simultaneous cognitive training was effective for physical, cognitive and emotional variables linked to frailty (Rezola‐Pardo et al., 2019). A systematic review that investigated frail older people in residential care, the community and nursing homes demonstrated that most studies provided evidence that structured exercise training (long duration, performed three times per week) had a positive impact on frail elderly people and may be used for frailty management (Theou et al., 2011). A conceptual review of physical activity for functional and cognitive impairment suggested that exercise was the only intervention found to consistently improve important components of frailty, including sarcopenia, physical function, cognitive condition and depression (Landi et al., 2010). Although many previous studies focused on frailty, few studies explored interventions for older people with cognitive frailty, which is a particularly vulnerable group, especially during crises such as COVID‐19.
A previous study (Yoon et al., 2018) showed that resistance exercise training was an effective strategy for cognitive frailty, although the exercise parameters were unclear. The Otago exercise programme (OEP) was originally designed as a home‐based, supervised, progressive balance and muscle‐strengthening programme for elderly people (Campbell et al., 1997). Benefits of the OEP include reducing falls by improving balance, strength and cognitive function (Almarzouki et al., 2020; Liu‐Ambrose et al., 2008). However, no studies have examined the effect of OEP on those with cognitive frailty. Therefore, this study aimed to assess effect of 12 weeks of OEP among elderly with cognitive frailty living in nursing homes.
1.2. Aim and hypotheses
This study aimed to assess the effect of 12 weeks group OEP among older adults with cognitive frailty who was living in nursing homes during the COVID‐19 pandemic in China. We hypothesised that: (1) there would be significant differences in physical and mental function among participants who received the OEP intervention after 12 weeks; and (2) the level of physical function and mental health would be higher in the intervention group after 12 weeks than in the control group.
2. METHODS
2.1. Research design
A parallel‐group, assessor‐blind randomised controlled trial was conducted in a nursing home for 12 weeks from July 2020 to October 2020 during the COVID‐19 pandemic. This randomised trial followed the Consolidated Standards of Reporting Trials (CONSORT) 2010 guidelines (see File S1). Randomisation using a random numbers table was used to divide participants into an experimental (OEP) group or a control group. Both groups received health education for 30 min at least once a month. In addition to health education, participants in the OEP group received 12‐week group OEP training for 30 min per session, three sessions per week. A flow diagram of the study is shown in Figure 1.
FIGURE 1.
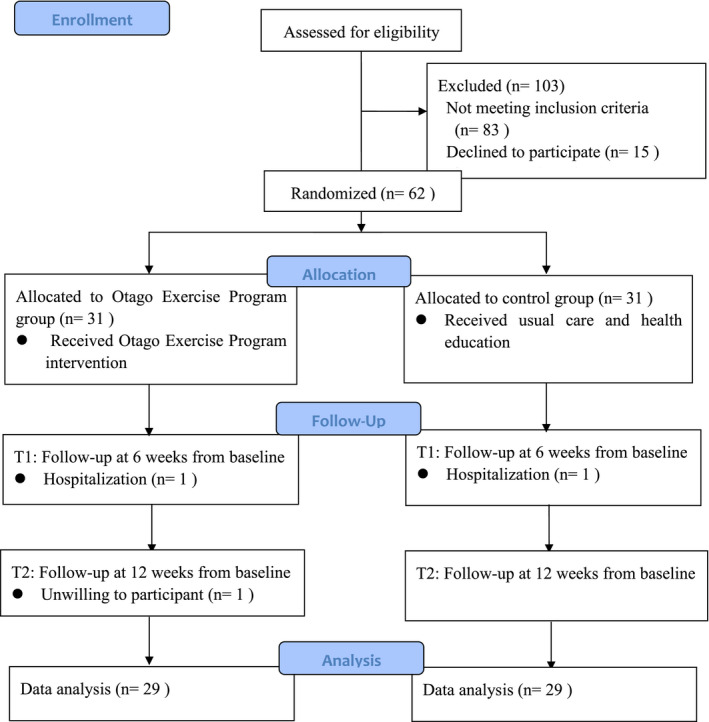
CONSORT flow diagram for the data collection procedure
2.2. Assessment of cognitive frailty
Cognitive frailty refers to a clinical state of the co‐occurrence of physical frailty (defined by Fried's criteria), with MCI according to the Ruan criteria (Ruan et al., 2015). Physical frailty can be classified using five domains: unintentional weight loss, which is defined as involuntary weight loss over 4.5 kg or 5% of body weight in the previous year; exhaustion, which is indicated by two self‐reported questions (e.g. ‘In the last week, how often I felt everything I did was an effort or I could not get going’) drawn from the Center for Epidemiological Studies Depression scale, with answers of more than 3 days considered as exhaustion; low physical activity, which was defined by <270 kcal/week for females or <383 kcal/week for males according to the Minnesota Questionnaire Assessment Scale (Taylor et al., 1978); weak muscle strength as evaluated by hand strength, which was measured by using electronic hand dynamometer (Zhongshan Camry Electronic Co. Ltd, Guangdong, China) and, finally, slowness was evaluated by gait speed, which was identified by measuring the time needed to walk 4.6 m as quickly as possible. Weak muscle strength and slowness cut‐off points were based on sex‐ and body mass index‐specific cut‐off points and sex‐ and height‐specific cut‐off points, respectively (Wu et al., 2014). Participants were classified as frail (presence of three or more criteria), pre‐frail (presence one or two criteria) or robust (no criteria).
Cognitive function was measured with the Beijing version of the Montreal Cognitive Assessment (MOCA‐BJ) scale. The test covers seven domains that detect cognitive impairment: visualisation/executive functions (maximum = 5), naming (maximum = 3), attention (maximum = 3), language facilities (maximum = 3), delayed memory (maximum = 5), abstraction (maximum = 2) and orientation (maximum = 6) (Yu et al., 2012). The total MOCA‐BJ score ranges from 0 to 30 points, and scores between 19 and 25 indicate MCI.
2.3. Participants
Participants were recruited between June and July 2020 from a nursing home in Changsha. In total, 62 participants with cognitive frailty aged 75–92 years (average, 84.34 years) were recruited for this study at baseline. The inclusion criteria were as follows: (a) aged ≥75 years; (b) Fried score ≥1; (c) MoCA‐BJ score of 19–25; (d) able to perform OEP as determined by a physiotherapist; (e) resident in the nursing home for more than 3 months; (f) literate; and (g) willing to participate. Physical frailty was classified as pre‐frail (score 1–2) or frail (score 3–5) based on the Fried criteria (Fried et al., 2001). Exclusion criteria were as follows: (a) suffering severe diseases, such as paralysis, severe heart disease, or fractures; and (b) participating in another clinical exercise study.
The sample size was calculated using Gpower 3.1.9.2 software for repeated measures analysis of variance (ANOVA). We assumed the correlation among repeated measures was 0.2. Therefore, 62 participants were required to achieve a medium effect size (0.25) at a power of 80% and a significance level of 0.05.
2.4. Interventions
Both study groups received an active intervention for 12 weeks. Participants in the control group received 30 min of health education at least once a month covering exercise knowledge. The contents of this education included the benefit of exercise, recommendations for physical activity for the elderly, and how to exercise scientifically, based on a Chinese exercise book for older people. In addition, we provided sleep‐ and diet‐related information. Control group participants were also asked to maintain their regular activity habits and requested not to seek another intervention exercise programme during the study period.
In addition to receiving the same health education as the control group, the OEP group received 12 weeks of group OEP training with a frequency of three sessions a week at 30 min per session. The OEP consisted of 5 min warm‐up, 10 min resistance training and 15 min balance exercise. Each movement had different levels, and each level had different requirements. The warm‐up included head and neck exercise, body warm‐up, and ankle warm‐up. Sandbags weighing about 0.5 kg were used to increase ankle cuff weight for lower extremity strength resistance training, which comprised knee extensors, knee flexors and hip abductors (repeated as they could or 10 times), ankle plantarflexors and ankle dorsiflexors (10 repetitions, using a handrail or not). The balance exercises comprised knee bends (four levels), backwards walking (two levels), ‘8’ shape walking (two levels), sideways walking (two levels), tandem stand (two levels), heel‐toe walking (two levels), one‐leg standing (three levels), heel walking (two levels), toe walking (two levels), backwards heel‐toe walking (one level), sit‐to‐stand exercises (four levels) and stair walking (Liu‐Ambrose et al., 2008). Physiotherapists guided participants to increase or decrease the exercise level according to an exercise level table; if the participant completed a lower level, they could progress to the next level. More detail is available from the Otago Medical School website (www.acc.co.nz/otagoexerciseprogramme).
The OEP was guided by a physiotherapist who had clinical experience with elderly people, and was led by nurses. Participants randomised to the OEP group were first evaluated by the physiotherapist to ensure that all exercises were completed at their function level. To reduce bias and for better exercise guidance, OEP exercises were conducted in a quiet room on Monday, Wednesday and Friday. Exercise safety was ensured by the supervising nurses. In addition, each participant received individual exercise safety education from the nurses, including wearing suitable shoes, drinking enough water during the movements, eating food before exercise to avoid hypoglycaemia and not exercising without protection in case of falls. In addition, participants' vital signs were monitored before and after training. As well as monitoring participants' temperature, pulse oximetry was used to detect respiratory problems, and a sphygmomanometer was used to monitor blood pressure. If the blood oxygen was lower than 90% or blood pressure was lower than 90/60 mmHg or higher than 140/90 mmHg, that participant was required to stop the exercise and a doctor called for treatment. After the training session, an exercise diary for each participant was recorded by the supervising nurses, which detailed the exercise time and frequency and any events.
The conditions of two groups were matched as closely as possible to control for confounding variables. To reduce bias, the health education knowledge in both groups was performed by the same nurses.
2.5. Prevention of COVID‐19 during implementation
Measures were taken to prevent and control the spread of COVID‐19 in the nursing home during the study implementation. First, we adopted closed management, with participants advised not to leave the nursing home, and voluntary service and social practice visits also suspended. The body surface temperatures of participants and research workers were monitored before every OEP session, and they were also required to undergo COVID‐19 nucleic acid detection. People were allowed to enter the exercise session if they had a temperature ≤37.0℃ and negative nucleic acid detection results. During the exercise session, staff encouraged participants to keep at least 1 metre from each other and wear masks during activities. To ensure participants were informed about the situation, COVID‐19‐related knowledge and news were broadcast on a television. After each exercise session, the training room was sterilised.
2.6. Randomisation and blinding
These participants were randomly divided into two equal‐sized groups using a random number table (31 participants in each group). Although the participants and physiotherapist were unable to be blinded, the outcome evaluation and data analysis assessor remained blinded throughout the study.
2.7. Outcome measures
2.7.1. Physical function
Physical function comprises lower limb strength, balance and functional mobility. The Five Times Sit to Stand Test (FTSST) was used to assess lower limb strength and dynamic balance (Goldberg et al., 2012). Participants were asked to sit in an armless straight‐back chair (46 cm height). They were instructed to complete sit‐to‐stand once with arms folded across their chests (Duncan et al., 2011). Those who accomplished this on the first attempt were guided to complete five repetitions of this sit‐to‐stand as quickly as possible. The test began with the signal ‘start’ and stopped after completion of five movements; the duration was recorded by stopwatch. The Berg Balance Scale (BBS) was used to evaluate balance. Participants were required to accomplish 14 tasks related to static and dynamic standing balance, ability to sit, stand up and transfer. The maximum BBS score is 56, with each item scored from 0 to 4 (0 indicates the lowest balance performance and 4 indicates the highest). The final score is the sum of the points for each task. The BBS is a simple and safe tool and takes around 15 min to complete (Qutubuddin et al., 2005). The intra‐rater reliability and inter‐rater reliability for the BBS were reported to be high, with pooled estimates of 0.98 and 0.97, respectively (Downs et al., 2013). The Timed Up and Go Test (TUGT) was used to measure function mobility in dynamic activities (Podsiadlo & Richardson, 1991). Participants were required to sit in a chair with feet flat on the floor and backs pressed against the seat rest for preparation. We assessed the time they took to stand up from the chair, walk a distance of 3 metres at safe walking speed in a linear path, turn around, walk back to the chair and sit down (Sebastião et al., 2016).
2.7.2. Mental health
The mental health variables were the Geriatric Depression Scale (GDS‐15) and 12‐Item Short Form Health Survey Mental Component Summary (SF‐12 MCS) scores. A Chinese‐language version of the GDS‐15 was used to measure the depressive mood (Mui, 1996). This scale has 15 items requiring a ‘yes’ or ‘no’ response. The total score ranges from 0 to 15, with scores of 5 or above considered to indicate depression symptoms (Jeon et al., 2014). The Cronbach's alpha for the Chinese GDS‐15 was 0.793, and the retest reliability was 0.728 (Tang, 2013). This scale has been extensively used in China (Quail et al., 2020; Zhao et al., 2019). The SF‐12 is used to evaluate the health‐related quality of life and covers eight subscales: general health, physical function, role‐physical, bodily pain, vitality (VT), social functioning (SF), role‐emotional (RE) and mental health (MH) (Ware et al., 1996). The SF‐12 MCS score is the mental dimension of the SF‐12 and is calculated from the SF, RE, MH and VT scores. The Cronbach's alpha was 0.910, and the split‐half reliability coefficient was 0.812 in Chinese community‐dwelling elderly people (Shou et al., 2016).
2.8. Data collection and procedure
Participants were recruited from a nursing home in Changsha. Outcome assessors received training to ensure standardisation of the data collection procedure and instruction on administering scales and tools. After providing consent, participants in both groups were asked to report information about demographic (age, sex, marital status, education level religion, smoking and drinking) and clinical characteristics (disease and drug, insomnia, history of falls and use of mobility aids) factors at baseline. Outcomes (physical function, cognitive function and depression) were measured before the intervention and at 6 and 12 weeks after the intervention started. Assessors introduced the questionnaire information to participants in a face‐to‐face interview and provided help as needed for those who had difficulty completing the scales.
2.9. Ethical considerations
The study was approved by the Ethics Committee of Xiangya Nursing School of Central South University (No. E202042). Informed consent was obtained from all participants before the study started. Participants were informed about their right to withdraw from the exercise programme at any time and assured of the confidentiality of data collection and management. The participants in the control group were advised that they could receive the same intervention after this study finished if they wished. This study was registered with the Chinese Clinical Trial Registry (ChiCTR2000039592).
2.10. Data analysis
All data analyses were performed using SPSS version 18.0. Participants' baseline demographic and questionnaire data were summarised using descriptive statistics. Mean ±standard deviation was used to describe continuous variables and number (percentage) for categorical variables. Two‐sample t tests, Kruskal–Wallis tests, and chi‐square tests were used to compare differences between the OEP and control groups. Two‐way ANOVA with repeated measurement (group × time) was used to compare the intervention effects over time on measured outcomes. The changes in mean difference scores for outcome variables were compared using two‐sample t tests.
2.11. Validity and reliability
To enhance the validity and reliability of the data, privacy and a quiet environment were chosen for data collection in addition to the use of validated instruments. Data integrity was inspected carefully after each participant completed the questionnaire. We also performed double entry of the data to ensure accuracy before conducting the statistical analyses.
3. RESULTS
A total of 165 nursing home residents were initially recruited, although 103 residents were excluded: 83 did not meet the inclusion criteria, 15 refused to participate, and five were excluded for other reasons. All participants were screened with a face‐to‐face interview and provided written informed consent. Finally, 62 participants were included in this study and coded. At baseline, the 62 participants that met the inclusion criteria were randomised to the control group (n = 31) or the OEP group (n = 31). Two participants withdrew from this study at 6 weeks because of hospitalisation, and one was unwilling to participate at 12 weeks. Finally, the control group included 30 participants, and the OEP group included 29 participants. Figure 1 shows the flow diagram of the study progress.
The mean age of the OEP group was 84.75 ± 5.41 years and that of the control group was 84.59 ± 4.21 years, which were comparable. Most participants in both groups were female (control group 60% vs. OEP group 82.8%) and married (control group 73.3% vs. OEP group 93.1%). There were no statistically significant differences at baseline in sociodemographic and clinical characteristics between the two groups (see Table 1). Table 2 summarises the baseline characteristics for physical function and mental health in the two groups. There were no significant differences between the two groups in baseline scores for the FTSST, TUGT, and BBS, depression, and SF‐12 MCS.
TABLE 1.
Baseline characteristics by study groups
| Control group (n = 30) | OEP group (n = 29) | χ 2 | p | |
|---|---|---|---|---|
| Age (year) | ||||
| Mean (SD) | 84.75 (5.41) | 84.59 (4.21) | −0.150 a | .988 |
| Sex, n (%) | ||||
| Male | 12 (40) | 5 (17.2) | 3.724 | .054 |
| Female | 18 (60) | 24 (82.8) | ||
| BMI (kg/m2) | ||||
| Mean (SD) | 22.91 (4.31) | 23.71 (3.31) | −0.795 a | .430 |
| Marriage status, n (%) | ||||
| Widowed | 8 (26.7) | 2 (6.9) | 2.810 b | .094 |
| Married | 22 (73.3) | 27 (93.1) | ||
| Education level, n (%) | ||||
| Primary school or below | 12 (40) | 7 (24.1) | 4.515 | .211 |
| Junior high school | 3 (10) | 8 (27.6) | ||
| Senior high school | 7 (23.3) | 9 (31) | ||
| College degree or above | 8 (26.7) | 5 (17.2) | ||
| Religion, n (%) | 6 (20) | 6 (20.7) | 0.004 | .948 |
| Comorbidity, n (%) | ||||
| Hypertenson | 19 (63.3) | 21 (72.4) | 0.557 | .456 |
| Heart disease | 12 (40) | 15 (51.7) | 0.817 | .366 |
| Cataracts | 3 (10) | 8 (27.6) | 3.007 | .083 |
| Insomnia, n (%) | 13 (43.3) | 11 (37.9) | 0.178 | .673 |
| Using walking aid, n (%) | 11 (36.7) | 11 (37.9) | 0.010 | .920 |
| Physical frailty (score) | ||||
| Mean (SD) | 2.23 (1.04) | 2.23 (0.98) | 0.131 a | .895 |
| MoCA (scores) | ||||
| Mean (SD) | 21.67 (2.30) | 21.21 (2.77) | 0.694 a | .491 |
Abbreviations: BMI, Body mass index; MoCA, Montreal Cognitive Assessment.
Independent t test.
Fisher's exact test
TABLE 2.
Baseline outcome variables of participants in the control and OEP groups
|
Control group (n = 30) Mean (SD) |
OEP group (n = 29) Mean (SD) |
t | p | |
|---|---|---|---|---|
| FTSST (time, second) | 17.46 (6.47) | 16.20 (4.74) | 0.915 | .364 |
| TUGT (time, second) | 17.69 (6.66) | 16.80 (6.63) | 0.310 | .758 |
| Berg balance scale (scores) | 43.80 (5.74) | 45.55 (6.57) | −1.092 | .280 |
| GDS‐15 (scores) | 3.90 (2.28) | 3.97 (2.49) | −0.106 | .916 |
| SF‐12 MCS (scores) | 53.55 (9.74) | 50.55 (9.27) | 1.211 | .231 |
Abbreviations: FTSST, Five Times Sit to Stand Test; GDS‐15, Geriatric Depression Scale‐15; SF‐12 MCS, Short Form 12‐item health survey Mental Component Summary; TUGT, Timed Up and Go Test.
3.1. Effect of the intervention
The effects of the OEP intervention on physical function and mental health outcomes are shown in Table 3. For physical function, there were significant main effects on FTSST (p < .05), TUGT (p < .05) and BBS (p < .05) scores, which indicated different physical function effects in the two groups. Significant interaction effects were observed for FTSST (p < .001), TUGT (p < .001) and BBS (p < .001) scores. There were significant differences (p < .05) in the two groups in changes in FTSST, TUGT and BBS scores at 6 and 12 weeks compared with baseline (Table 4). The difference in the two groups for the FTSST change from baseline at 6 weeks was 0.39 (control group) and −1.28 (OEP group), and from baseline at 12 weeks was 1.55 (control group) and −2.78 (OEP group). That for TUGT change from baseline at 6 weeks was 0.62 (control group) and −1.63 (OEP group), and at 12 weeks was 1.66 (control group) and −1.63 (OEP group). The change in BBS scores at 6 weeks was −0.27 (control group) and 2.17 (OEP group), and at 12 weeks was −0.10 (control group) and 4.31 (OEP group).
TABLE 3.
Comparison of mean scores of outcome variables between two groups
| Variables | Baseline | 6 weeks | 12 weeks | p for groups × time interaction | p for groups | p for time |
|---|---|---|---|---|---|---|
| FTSST (time, second) | ||||||
|
Control group Mean (SD) |
17.46 (6.47) | 17.85 (6.16) | 19.01 (6.15) | .000* | .030* | .065 |
|
OEP group Mean (SD) |
16.20 (4.74) | 14.92 (4.47) | 13.41 (4.12) | |||
| TUGT (time, second) | ||||||
|
Control group Mean (SD) |
17.70 (6.66) | 17.25 (6.60) | 19.44 (6.57) | .000* | .037* | .019* |
| OEP group Mean (SD) | 16.80 (6.63) | 15.17 (6.25) | 13.08 (4.65) | |||
| Berg balance scale scores | ||||||
|
Control group Mean (SD) |
43.80 (1.13) | 43.67 (1.12) | 43.83 (1.09) | .000* | .013* | .000* |
|
OEP group Mean (SD) |
45.55 (1.14) | 47.72 (1.14) | 49.86 (1.11) | |||
| GDS‐15 (scores) | ||||||
|
Control group Mean (SD) |
3.90 (2.28) | 4.17 (2.35) | 4.97 (2.71) | .000* | .175 | .010* |
|
OEP group Mean (SD) |
3.97 (2.49) | 3.38 (2.19) | 3.24 (2.10) | |||
| SF‐12 MCS (scores) | ||||||
|
Control group Mean (SD) |
52.70 (8.94) | 49.87 (8.20) | 46.75 (9.22) | .000* | .381 | .033* |
|
OEP group Mean (SD) |
49.98 (8.67) | 52.24 (8.06) | 52.56 (8.05) | |||
Abbreviations: CI, Confidence interval; FTSST, Five Times Sit to Stand Test; GDS‐15, Geriatric Depression Scale‐15; MD, mean difference; SD, Standard deviation; SF‐12 MCS, Short Form 12‐item health survey Mental Component Summary;TUGT, Timed Up and Go Test.
p < .05.
TABLE 4.
The change of outcome variables during 12 weeks between two groups
| Variables | Control group | OEP group | t | p |
|---|---|---|---|---|
| FTSST (time, second) | ||||
| Baseline | 17.46 (6.47) | 16.20 (4.74) | 0.915 | .364 |
| Change at 6 weeks | 0.39 (2.24) | −1.28 (1.66) | 3.266 | .002 |
| Change at 12 weeks | 1.55 (1.40) | −2.78 (2.00) | 9.676 | .000 |
| TUGT (time, second) | ||||
| Baseline | 17.69 (6.66) | 16.80 (6.63) | 0.310 | .758 |
| Change at 6 weeks | 0.62 (0.87) | −1.63 (2.11) | 5.407 | .000 |
| Change at 12 weeks | 1.66 (2.00) | −3.73 (3.61) | 7.082 | .000 |
| Berg balance scale (scores) | ||||
| Baseline | 43.80 (5.74) | 45.55 (6.57) | −1.092 | .280 |
| Change at 6 weeks | −0.27 (2.35) | 2.17 (2.17) | −4.138 | .000 |
| Change at 12 weeks | −0.10 (2.55) | 4.31 (3.41) | −5.635 | .000 |
| GDS‐15 (scores) | ||||
| Baseline | 3.90 (2.28) | 3.97 (2.49) | −0.106 | .916 |
| Change at 6 weeks | 0.27 (1.11) | −0.59 (1.09) | 2.979 | .004 |
| Change at 12 weeks | 1.07 (1.72) | −0.72 (1.16) | 4.669 | .000 |
| SF‐12 MCS (scores) | ||||
| Baseline | 53.55 (9.74) | 50.55 (9.27) | 1.211 | .231 |
| Change at 6 weeks | −2.83 (5.42) | 2.25 (5.90) | −3.452 | .001 |
| Change at 12 weeks | −5.95 (7.24) | 2.58 (6.26) | −4.835 | .000 |
Abbreviations: FTSST, Five Times Sit to Stand Test; GDS‐15, Geriatric Depression Scale‐15; MD, mean difference; SF‐12 MCS, Short Form 12‐item health survey Mental Component Summary;TUGT, Timed Up and Go Test.
For mental health, the significant time effect (p < .001) and interaction effect between the intervention groups and time (p < .001) indicated the group effects on change in GDS‐15 and SF‐12 MCS scores from baseline differed significantly over time (see Table 3). There were significant differences (p < .05) between the two groups in the changes in GDS‐15 and SF‐12 MCS scores at 6 and 12 weeks compared with baseline (Table 4). The differences in the two groups for GDS‐15 change from baseline at 6 weeks was 0.27 (control group) and −0.59 (OEP group) and at 12 weeks was 1.07 (control group) and −0.72 (OEP group). The SF‐12 MCS at 6 weeks was −2.83 (control group) and 2.25 (OEP group), and at 12 weeks was −5.95 (control group) and 2.58 (OEP group).
The interaction diagram for FTSST, TUGT, BBS, GDS‐15 and SF‐12 MCS scores from baseline to follow‐up (6 and 12 weeks) between the OEP and control groups was depicted in figures (2, 3, 4, 5, 6).
FIGURE 2.
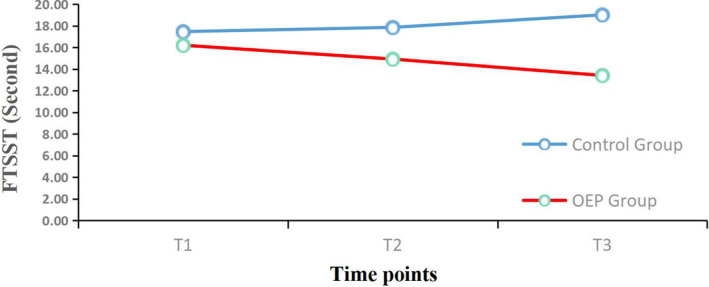
Mean changes from baseline for the Five Times Sit to Stand Test. (FTSST) over the 12‐week period. OEP: Otago exercise program; T1 = Before intervention; T2 = 6 weeks follow‐up; T3 = 12 weeks follow‐up
FIGURE 3.
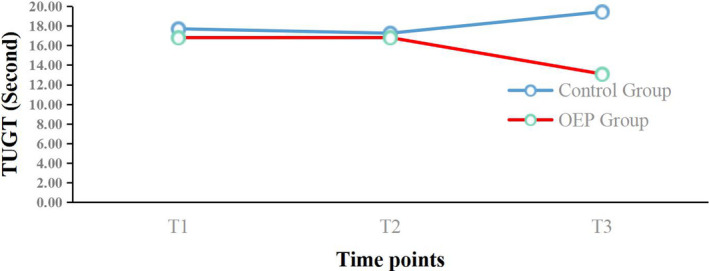
Mean changes from baseline for the Timed Up and Go Test (TUGT) over the 12‐week period. OEP: Otago exercise program; T1 = Before intervention; T2 = 6 weeks follow‐up; T3 = 12 weeks follow‐up
FIGURE 4.
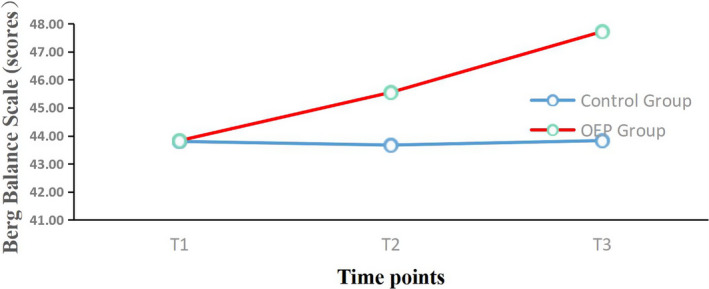
Mean changes from baseline for the Berg Balance Scale (BBS) over the. 12‐week period. OEP: Otago exercise program; T1 = Before intervention; T2 = 6 weeks follow‐up; T3 = 12 weeks follow‐up
FIGURE 5.
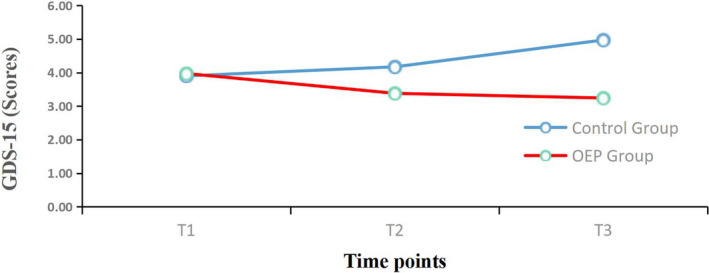
Mean changes from baseline for the Geriatric Depression Scale‐15. (GDS‐15) over the 12‐week period. OEP: Otago exercise program; T1 = Before intervention; T2 = 6 weeks follow‐up; T3 = 12 weeks follow‐up
FIGURE 6.
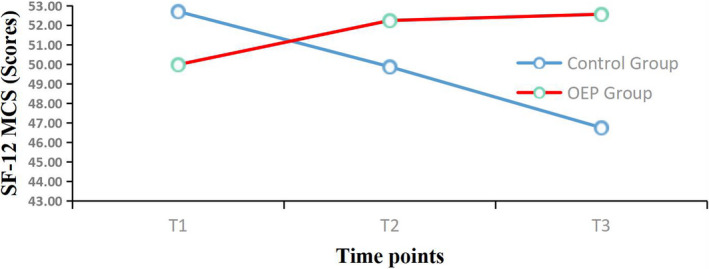
Mean changes from baseline for the Short Form 12‐item Health Survey Mental Component Summary (SF‐12 MCS) over the 12‐week period. OEP: Otago exercise program; T1 = Before intervention; T2 = 6 weeks follow‐up; T3 = 12 weeks follow‐up
4. DISCUSSION
People with advanced age and cognitive frailty are more vulnerable to COVID‐19. Our study aimed to determine the effects of 12 weeks of OEP training on physical function and mental health outcomes among elderly people with cognitive frailty. The results showed that OEP was an effective way to improve functional mobility and mental health outcomes in this population. Our results support a recent study that reported physical exercise was an effective therapy for both mental and physical health among elderly people during the COVID‐19 pandemic (Jiménez‐Pavón et al., 2020). Previous studies (Bray et al., 2016; Cadore et al., 2013) suggested the suitable frequency of multicomponent exercise was 2–3 times per week for 30–45 min, which was consistent with the exercise parameters in our study. However, another study (Smith et al., 2003) suggested a duration of exercise of 5 months or longer may contribute to better outcomes. Therefore, further studies are needed to explore the optimal dose of OEP for elderly people with cognitive frailty.
To comprehensively evaluate physical function, the FTSST was used to evaluate lower extremity strength, and the TUGT and BBS were used to evaluate functional mobility and balance, respectively. We found significant improvements in FTSST, TUGT, and BBS scores following the OEP training compared with the control group after 12 weeks. Although the OEP offers an effective method for improving physical function in older people, it is the first time that this programme has been used among those with cognitive frailty during the COVID‐19 pandemic. A previous study (Olanrewaju et al., 2020) found that a longer sedentary time was associated with a thinner medial temporal lobe, abnormal cerebral blood flow, increased white matter hyperintensities and decreased brain‐derived neurotrophic factor levels. These changes were related to physical frailty and cognitive function. The reason for the positive change in physical function shown in this study may be that regular exercise minimised the effects of a sedentary lifestyle during the COVID‐19 pandemic.
For lower limb muscle strength, the OEP movement includes weight‐bearing exercises (e.g. weight‐bearing knee abduction and flexion, weight‐bearing hip abduction). Strength training can stimulate the body and increase the demand for protein, leading to increased myoprotein synthesis and muscle oxygen consumption, which may increase muscle content and improve lower limb muscle strength (Lord et al., 1996). A recent study (Leem et al., 2019) used an electronic muscle strength meter to measure the effects of OEP exercise on muscle strength, and found positive effects. From a biomechanical perspective, a decreased muscle contraction function of the lower extremities in elderly people can also cause a decline in muscle strength (Hewston et al., 2020). The ‘pad tip’ and ‘tiptoe’ movements in the OEP have positive effects on stretching the lower extremity muscles, which may help to slow the progress of muscle strength decline. Therefore, we recommended OEP training as a daily life activity for elderly people to increase lower limb muscle strength. However, the weight of the sandbag used in our study was consistent at 0.5 kg; further studies need to gradually increase the lower limb load to better train lower limb muscle strength.
Functional mobility and balance are affected by physiological ageing and degeneration of proprioception, and the vestibular system occurs as one grows older (Yoo et al., 2013). Our study showed that the OEP can improve the balance function. The programme contains 12 balance movements, which are helpful in training the brain and coordination of muscles and nerves, improving the body's proprioceptive ability, training the visual vestibular function and regaining balance when moving unconsciously (Benavent‐Caballeret al., 2016; Ries et al., 2015). In addition, a study that investigated the effect of video‐supported group‐based OEP on community‐dwelling older adults that was performed for 4 months found the mean difference in BBS score between the intervention and control groups was 3.5 points, whereas that in this study was 4.3 points (Benavent‐Caballer et al., 2016; Ries et al., 2015). This suggested that face‐to‐face group OEP may have a better effect on improving balance than video‐supported group‐based OEP.
We found that group OEP reduced participants' depression scores and improved their mental health‐related quality of life. A previous study (Kyrdalen et al., 2014) showed that the group OEP had a medium effect size (0.54) for mental health‐related quality of life among fall‐prone older people. However, that study did not explore the effects of group OEP on elderly people with cognitive frailty. Our study reinforces previous findings in this field.
Social isolation may lead to depression and mental health problems (Santini et al., 2020). Depression may increase levels of inflammatory cytokines such as interleukin 6, tumour necrosis factor α and C‐reactive protein (Ruan et al., 2017). These inflammatory factors can cause decline in muscle density and skeletal muscle mass, which may bring about cognitive frailty. Several studies have reported that regular exercise can reduce the level of chronic inflammatory cytokines and tumour necrosis factors related to depressive symptoms (Paolucci et al., 2018). In addition, constructs such as self‐concept and self‐esteem promote mental health and reduce the negative influence of social isolation (Folkins & Sime, 1981; Maugeri et al., 2020). As a regular and systematic physical activity, the OEP may promote mental health through reducing chronic inflammatory reactions and promoting self‐concept. In addition, each OEP exercise is simple and has moderate intensity, which creates a relaxed and comfortable exercise atmosphere for elderly people, which may help to relieve the depression caused by excessive excitement in the cerebral cortex. The OEP can effectively improve muscle strength and balance ability among elderly people, and alleviate the symptoms of depression, thereby promoting improvement in health‐related quality of life.
4.1. Limitations
This study showed that the OEP was effective for improving physical function and mental health among elderly people with cognitive frailty. However, it also had several limitations. The small sample size and short intervention period may be considered limitations. Our study only showed the short‐term effect of OEP among older people with cognitive frailty, and the data are therefore only representative of a short‐term effect. Studies including a large number of participants and longer‐term intervention are needed to build on our findings. Because of the restrictions on personnel during the COVID‐19 outbreak, we could only conduct the OEP with the intervention group. This means it is difficult to separate the beneficial effects of the programme from a beneficial Hawthorne effect.
4.2. Future research
Our research indicated that it is hard for those aged over 85 years to perform ‘8’ shape walking and sit‐to‐stand exercises. Therefore, personalised exercise programmes should be targeted at people over 85 years. As previously described, the exercise parameters in this study were 3 sessions per week for 30 min per session; these parameters could be adjusted. We are also planning further research on the effect of the OEP on physical and cognitive frailty among older people with cognitive frailty. We will conduct a study to compare the effects of the OEP with an alternative exercise programme, such as Taiji or Baduanjin.
5. CONCLUSION
In conclusion, our findings indicate that the OEP group had better outcomes with respect to physical and mental function. We recommend the OEP can be used to improve the physical and mental function among older people with cognitive frailty during the COVID‐19 pandemic and similar lockdown/quarantine situations.
6. RELEVANCE TO CLINICAL PRACTICE
Relevance to clinical practice Otago exercise program intervention programmes should be implemented to improve physical function for cognitive frailty elderly to reduce the harm of longer‐term sedentary behaviours, and to ruduce depression symptom and improve mental health, particularly during COVID‐19 pandemic period.
CONFLICT OF INTEREST
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
AUTHOR CONTRIBUTIONS
Xi Chen, MD, RN, Nurse Practitioner, involved in conception and design, drafting the manuscript. Liping ZHAO, Professor, PhD, RN, Jianliang CHEN, MD, Associate Professor, Jinnan OU, MD, Associate Professor and Youshuo LIU, PhD,Professor, involved in revising it critically for important intellectual content. Zhiming ZHOU, MD, Professor, Dongli WEI, BSN, RN, Associate Professor and Xiaomei YANG, BSN, Associate Professor involved in acquisition of data, analysis and interpretation of data. Hua ZHANG, BSN, Associate Professor involved in conception and design. Yan LI, MD, Associate Professor involved in conception and design. Jin HUANG, PhD, RN, Professor, involved in funding and Revising it critically for important intellectual content.
ETHICAL APPROVAL
Ethical approval was obtained from the Ethics Committee of the Xiangya Nursing school, Central South University (No. E202042). The study has been registered at Chinese Clinical Trial Registry (ChiCTR2000039592).
Supporting information
File S1
ACKNOWLEDGEMENTS
We thank Changsha NO.1 Social Welfare Institution and Geriatric Rehabilitation Hospital of Changsha, and all those participants for help. Appreciation is extended to all the researchers for their work on this study. ‘We thank staff at Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for critically reviewing a draft of this manuscript’.
Chen, X. , Zhao, L. , Liu, Y. , Zhou, Z. , Zhang, H. , Wei, D. , Chen, J. , Li, Y. , Ou, J. , Huang, J. , Yang, X. , & Ma, C. (2021). Otago exercise programme for physical function and mental health among older adults with cognitive frailty during COVID‐19: A randomised controlled trial. Journal of Clinical Nursing, 00, 1–14. 10.1111/jocn.15964
Funding information
The study is supported by National Natural Science Foundation of China (Grant No.81770833, 81974223, 82071593), Health and Family Planning Commission of Hunan Province, China (Grant No.B2019153), Natural Science Foundation of Hunan Province (Grant No.2020SK3003), and Health commission Foundation Hunan Province of and the Nursing Research Project of the Second Xiangya Hospital, Central South University (2019‐HLKY‐32)
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Almarzouki, R. , Bains, G. , Lohman, E. , Bradley, B. , Nelson, T. , Alqabbani, S. , Alonazi, A. , & Daher, N. (2020). Improved balance in middle‐aged adults after 8 weeks of a modified version of Otago Exercise Program: A randomized controlled trial. PLoS ONE, 15(7), e235734. 10.1371/journal.pone.0235734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai, H. , Satake, S. , & Kozaki, K. (2018). Cognitive frailty in geriatrics. Clinics in Geriatric Medicine, 34(4), 667–675. 10.1016/j.cger.2018.06.011 [DOI] [PubMed] [Google Scholar]
- Benavent‐Caballer, V. , Rosado‐Calatayud, P. , Segura‐Ortí, E. , Amer‐Cuenca, J. J. , & Lisón J. F. (2016). The effectiveness of a video‐supported group‐based Otago exercise programme on physical performance in community‐dwelling older adults: a preliminary study. Physiotherapy, 102(3), 280–286. 10.1016/j.physio.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Bray, N. W. , Smart, R. R. , Jakobi, J. M. , & Jones, G. R. (2016). Exercise prescription to reverse frailty. Applied Physiology, Nutrition and Metabolism, 41(10), 1112–1116. 10.1139/apnm-2016-0226 [DOI] [PubMed] [Google Scholar]
- Brooks, S. K. , Webster, R. K. , Smith, L. E. , Woodland, L. , Wessely, S. , Greenberg, N. , & Rubin, G. J. (2020). The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet, 395(10227), 912–920. 10.1016/S0140-6736(20)30460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadore, E. L. , Rodríguez‐Mañas, L. , Sinclair, A. , & Izquierdo, M. (2013). Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Research, 16(2), 105–114. 10.1089/rej.2012.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, A. J. , Robertson, M. C. , Gardner, M. M. , Norton, R. N. , Tilyard, M. W. , & Buchner, D. M. (1997). Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ, 315(7115), 1065–1069. 10.1136/bmj.315.7115.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas‐Herrero, A. , Anton‐Rodrigo, I. , Zambom‐Ferraresi, F. , Sáez de Asteasu, M. L. , Martinez‐Velilla, N. , Elexpuru‐Estomba, J. , Marin‐Epelde, I. , Ramon‐Espinoza, F. , Petidier‐Torregrosa, R. , Sanchez‐Sanchez, J. L. , Ibañez, B. , & Izquierdo, M. (2019). Effect of a multicomponent exercise programme (VIVIFRAIL) on functional capacity in frail community elders with cognitive decline: study protocol for a randomized multicentre control trial. Trials, 20(1), 362. 10.1186/s13063-019-3426-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Center of Disease Control and Prevention (2021). Guideline for prevention and control of 2019 novel coronavirus pneumonia in special settings (Ⅱ) ‐‐nursing home 2021/1/29, 2021. Retrieved from http://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_2275/202002/t20200204_212201.html [Google Scholar]
- Chow, L. (2021). Care homes and COVID‐19 in Hong Kong: how the lessons from SARS were used to good effect. Age and Ageing, 50(1), 21–24. 10.1093/ageing/afaa234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, P. M. , & Szanton, S. L. (2020). Nursing homes and COVID‐19: We can and should do better. Journal of Clinical Nursing, 29(15–16), 2758–2759. 10.1111/jocn.15297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs, S. , Marquez, J. , & Chiarelli, P. (2013). The Berg Balance Scale has high intra‐ and inter‐rater reliability but absolute reliability varies across the scale: A systematic review. Journal of Physiotherapy, 59(2), 93–99. 10.1016/S1836-9553(13)70161-9 [DOI] [PubMed] [Google Scholar]
- Duncan, R. P. , Leddy, A. L. , & Earhart, G. M. (2011). Five times sit‐to‐stand test performance in Parkinson's disease. Archives of Physical Medicine and Rehabilitation, 92(9), 1431–1436. 10.1016/j.apmr.2011.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, L. , Zin Nyunt, M. S. , Gao, Q. , Feng, L. , Yap, K. B. , & Ng, T.‐P. (2017). Cognitive frailty and adverse health outcomes: findings from the Singapore longitudinal ageing studies (SLAS). Journal of the American Medical Directors Association, 18(3), 252–258. 10.1016/j.jamda.2016.09.015 [DOI] [PubMed] [Google Scholar]
- Folkins, C. H. , & Sime, W. E. (1981). Physical fitness training and mental health. American Psychologist, 36(4), 373–389. 10.1037//0003-066x.36.4.373 [DOI] [PubMed] [Google Scholar]
- Fried, L. P. , Tangen, C. M. , Walston, J. , Newman, A. B. , Hirsch, C. , Gottdiener, J. , Seeman, T. , Tracy, R. , Kop, W. J. , Burke, G. , & McBurnie, M. A. (2001). Frailty in older adults: Evidence for a phenotype. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 56(3), M146–M156. 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- Goldberg, A. , Chavis, M. , Watkins, J. , & Wilson, T. (2012). The five‐times‐sit‐to‐stand test: validity, reliability and detectable change in older females. Aging Clinical and Experimental Research, 24(4), 339–344. 10.1007/BF03325265 [DOI] [PubMed] [Google Scholar]
- Hewston, P. , Kennedy, C. C. , Borhan, S. , Merom, D. , Santaguida, P. , Ioannidis, G. , Marr, S. , Santesso, N. , Thabane, L. , Bray, S. , & Papaioannou, A. (2020). Effects of dance on cognitive function in older adults: A systematic review and meta‐analysis. Age and Ageing, 50(4), 1084–1092. 10.1093/ageing/afaa270 [DOI] [PubMed] [Google Scholar]
- Jeon, S. Y. , Han, S. J. , Jeong, J. H. , & Fregni, F. (2014). Effect of exercise on balance in persons with mild cognitive impairment. NeuroRehabilitation, 35(2), 271–278. 10.3233/NRE-141120 [DOI] [PubMed] [Google Scholar]
- Jiménez‐Pavón, D. , Carbonell‐Baeza, A. , & Lavie, C. J. (2020). Physical exercise as therapy to fight against the mental and physical consequences of COVID‐19 quarantine: Special focus in older people. Progress in Cardiovascular Diseases, 63(3), 386–388. 10.1016/j.pcad.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelaiditi, E. , Cesari, M. , Canevelli, M. , Abellan van Kan, G. , Ousset, P.‐J. , Gillette‐Guyonnet, S. , Ritz, P. , Duveau, F. , Soto, M. E. , Provencher, V. , Nourhashemi, F. , Salva, A. , Robert, P. , Andrieu, S. , Rolland, Y. , Touchon, J. , Fitten, J. L. , & Vellas, B. (2013). Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. The Journal of Nutrition, Health & Aging, 17(9), 726–734. 10.1007/s12603-013-0367-2 [DOI] [PubMed] [Google Scholar]
- Kyrdalen, I. L. , Moen, K. , Røysland, A. S. , & Helbostad, J. L. (2014). The Otago Exercise Program performed as group training versus home training in fall‐prone older people: a randomized controlled Trial. Physiotherapy Research International, 19(2), 108–116. 10.1002/pri.1571 [DOI] [PubMed] [Google Scholar]
- Landi, F. , Abbatecola, A. M. , Provinciali, M. , Corsonello, A. , Bustacchini, S. , Manigrasso, L. , Cherubini, A. , Bernabei, R. , & Lattanzio, F. (2010). Moving against frailty: Does physical activity matter? Biogerontology, 11(5), 537–545. 10.1007/s10522-010-9296-1 [DOI] [PubMed] [Google Scholar]
- Lee, W. J. , Peng, L. N. , Liang, C. K. , Loh, C. H. , & Chen, L. K. (2018). Cognitive frailty predicting all‐cause mortality among community‐living older adults in Taiwan: A 4‐year nationwide population‐based cohort study. PLoS ONE, 13(7), e200447. 10.1371/journal.pone.0200447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem, S. H. , Kim, J. H. , & Lee, B. H. (2019). Effects of Otago exercise combined with action observation training on balance and gait in the old people. Journal of Exercise Rehabilitation, 15(6), 848–854. 10.12965/jer.1938720.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu‐Ambrose, T. , Donaldson, M. G. , Ahamed, Y. , Graf, P. , Cook, W. L. , Close, J. , Lord, S. R. , & Khan, K. M. (2008). Otago home‐based strength and balance retraining improves executive functioning in older fallers: a randomized controlled trial. Journal of the American Geriatrics Society, 56(10), 1821–1830. 10.1111/j.1532-5415.2008.01931.x [DOI] [PubMed] [Google Scholar]
- Lloyd‐Sherlock, P. , Ebrahim, S. , Geffen L., & McKee M. (2020). Bearing the brunt of covid‐19: older people in low and middle income countries. BMJ, m1052. 10.1136/bmj.m1052 [DOI] [PubMed] [Google Scholar]
- Lord, S. R. , Lloyd, D. G. , & Keung li, S. (1996). Sensori‐motor function, gait patterns and falls in community‐dwelling women. Age and Ageing, 25(4), 292–299. 10.1093/ageing/25.4.292 [DOI] [PubMed] [Google Scholar]
- Maugeri, G. , Castrogiovanni, P. , Battaglia, G. , Pippi, R. , D'Agata, V. , Palma, A. , Di Rosa, M. , & Musumeci, G. (2020). The impact of physical activity on psychological health during Covid‐19 pandemic in Italy. Heliyon, 6(6), e4315. 10.1016/j.heliyon.2020.e04315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant, R. A. , Chen, M. Z. , Tan, L. W. L. , Lim, M. Y. D. , Ho, H. K. , & van Dam, R. M. (2017). Singapore healthy older people everyday (HOPE) study: prevalence of frailty and associated factors in older adults. Journal of the American Medical Directors Association, 18(8), 734–739. 10.1016/j.jamda.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Mui, A. C. (1996). Geriatric Depression Scale as a community screening instrument for elderly Chinese immigrants. International Psychogeriatrics, 8(3), 445–458. 10.1017/S1041610296002803 [DOI] [PubMed] [Google Scholar]
- Olanrewaju, O. , Stockwell, S. , Stubbs, B. , & Smith, L. (2020). Sedentary behaviours, cognitive function, and possible mechanisms in older adults: A systematic review. Aging Clinical and Experimental Research, 32(6), 969–984. 10.1007/s40520-019-01457-3 [DOI] [PubMed] [Google Scholar]
- Paolucci, E. M. , Loukov, D. , Bowdish, D. , & Heisz, J. J. (2018). Exercise reduces depression and inflammation but intensity matters. Biological Psychology, 133, 79–84. 10.1016/j.biopsycho.2018.01.015 [DOI] [PubMed] [Google Scholar]
- Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256(3), 183–194. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Pitkälä, K. H. (2020). COVID‐19 has hit nursing homes hard. European Geriatric Medicine, 11, 1–3, 10.1007/s41999-020-00411-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsiadlo, D. , & Richardson, S. (1991). The timed "Up & Go": a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society, 39(2), 142–148. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- Quail, Z. , Carter, M. M. , Wei, A. , & Li, X. (2020). Management of cognitive decline in Alzheimer's disease using a non‐pharmacological intervention program: A case report. Medicine (Baltimore), 99(21), e20128. 10.1097/MD.0000000000020128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qutubuddin, A. A. , Pegg, P. O. , Cifu, D. X. , Brown, R. , McNamee, S. , & Carne, W. (2005). Validating the Berg Balance Scale for patients with Parkinson's disease: A key to rehabilitation evaluation. Archives of Physical Medicine and Rehabilitation, 86(4), 789–792. 10.1016/j.apmr.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Ries, J. D. , Hutson, J. , Maralit, L. A. , & Brown M. B. (2015). Group balance training specifically designed for individuals with alzheimer disease. Journal of Geriatric Physical Therapy, 38(4), 183–193. 10.1519/jpt.0000000000000030 [DOI] [PubMed] [Google Scholar]
- Rezola‐Pardo, C. , Arrieta, H. , Gil, S. M. , Yanguas, J. J. , Iturburu, M. , Irazusta, J. , Sanz, B. , & Rodriguez‐Larrad, A. (2019). A randomized controlled trial protocol to test the efficacy of a dual‐task multicomponent exercise program in the attenuation of frailty in long‐term nursing home residents: Aging‐ON(DUAL‐TASK) study. BMC Geriatrics, 19(1), 6. 10.1186/s12877-018-1020-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan, Q. , D'Onofrio, G. , Wu, T. , Greco, A. , Sancarlo, D. , & Yu, Z. (2017). Sexual dimorphism of frailty and cognitive impairment: Potential underlying mechanisms (Review). Molecular Medicine Reports, 16(3), 3023–3033. 10.3892/mmr.2017.6988 [DOI] [PubMed] [Google Scholar]
- Ruan, Q. , Ruan, J. , Zhang, W. , Qian, F. , & Yu, Z. (2018). Targeting NAD(+) degradation: The therapeutic potential of flavonoids for Alzheimer's disease and cognitive frailty. Pharmacological Research, 128, 345–358. 10.1016/j.phrs.2017.08.010 [DOI] [PubMed] [Google Scholar]
- Ruan, Q. , Yu, Z. , Chen, M. A. , Bao, Z. , Li, J. , & He, W. (2015). Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Research Reviews, 20, 1–10. 10.1016/j.arr.2014.12.004 [DOI] [PubMed] [Google Scholar]
- Santini, Z. I. , Jose, P. E. , York Cornwell, E. , Koyanagi, A. I. , Nielsen, L. , Hinrichsen, C. , Meilstrup, C. , Madsen, K. R. , & Koushede, V. (2020). Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): A longitudinal mediation analysis. Lancet Public Health, 5(1), e62–e70. 10.1016/S2468-2667(19)30230-0 [DOI] [PubMed] [Google Scholar]
- Scassellati, C. , Ciani, M. , Galoforo, A. C. , Zanardini, R. , Bonvicini, C. , & Geroldi, C. (2020). Molecular mechanisms in cognitive frailty: Potential therapeutic targets for oxygen‐ozone treatment. Mechanisms of Ageing and Development, 186, 111210. 10.1016/j.mad.2020.111210 [DOI] [PubMed] [Google Scholar]
- Sebastião, E. , Sandroff, B. M. , Learmonth, Y. C. , & Motl, R. W. (2016). Validity of the timed up and go test as a measure of functional mobility in persons with multiple sclerosis. Archives of Physical Medicine and Rehabilitation, 97(7), 1072–1077. 10.1016/j.apmr.2015.12.031 [DOI] [PubMed] [Google Scholar]
- Shou, J. , Ren, L. , Wang, H. , Yan, F. , Cao, X. , Wang, H. , Wang, Z. , Zhu, S. , & Liu, Y. (2016). Reliability and validity of 12‐item short‐form health survey (SF‐12) for the health status of Chinese community elderly population in Xujiahui district of Shanghai. Aging Clinical and Experimental Research, 28(2), 339–346. 10.1007/s40520-015-0401-9 [DOI] [PubMed] [Google Scholar]
- Smith, K. , Winegard, K. , Hicks, A. L. , & McCartney, N. (2003). Two years of resistance training in older men and women: the effects of three years of detraining on the retention of dynamic strength. Canadian Journal of Applied Physiology, 28(3), 462–474. 10.1139/h03-034 [DOI] [PubMed] [Google Scholar]
- Solfrizzi, V. , Scafato, E. , Lozupone, M. , Seripa, D. , Giannini, M. , Sardone, R. , Bonfiglio, C. , Abbrescia, D. I. , Galluzzo, L. , Gandin, C. , Baldereschi, M. , Di Carlo, A. , Inzitari, D. , Daniele, A. , Sabbà, C. , Logroscino, G. , Panza, F. , Scafato, E. , Farchi, G. , … Carbonin, P. (2017). Additive role of a potentially reversible cognitive frailty model and inflammatory state on the risk of disability: The Italian longitudinal study on aging. American Journal of Geriatric Psychiatry, 25(11), 1236–1248. 10.1016/j.jagp.2017.05.018 [DOI] [PubMed] [Google Scholar]
- Szczerbińska, K. (2020). Could we have done better with COVID‐19 in nursing homes? European Geriatric Medicine, 11(4), 639–643. 10.1007/s41999-020-00362-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, D. (2013). Application of short form geriatric depression scale (GDS‐15) in Chinese elderly. Chinese Journal of Clinical Psychology, 3(21), 402–405. [Google Scholar]
- Taylor, H. L. , Jacobs, D. R. , Schucker, B. , Knudsen, J. , Leon, A. S. , & Debacker, G. (1978). A questionnaire for the assessment of leisure time physical activities. Journal of Chronic Diseases, 31(12), 741–755. 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- Theou, O. , Stathokostas, L. , Roland, K. P. , Jakobi, J. M. , Patterson, C. , Vandervoort, A. A. , & Jones, G. R. (2011). The effectiveness of exercise interventions for the management of frailty: A systematic review. Journal of Aging Research, 2011, 569194. 10.4061/2011/569194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega, J. N. , & Newhouse, P. A. (2014). Mild cognitive impairment: diagnosis, longitudinal course, and emerging treatments. Current Psychiatry Reports, 16(10), 490. 10.1007/s11920-014-0490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware, J. J. , Kosinski, M. , & Keller, S. D. (1996). A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care, 34(3), 220–233. 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- Wu, I. C. , Lin, C. C. , Hsiung, C. A. , Wang, C. Y. , Wu, C. H. , Chan, D. C. , Li, T. C. , Lin, W. Y. , Huang, K. C. , Chen, C. Y. , & Hsu, C. C. (2014). Epidemiology of sarcopenia among community‐dwelling older adults in Taiwan: a pooled analysis for a broader adoption of sarcopenia assessments. Geriatrics Gerontology International, 14(Suppl 1), 52–60. 10.1111/ggi.12193 [DOI] [PubMed] [Google Scholar]
- Yan, Z. , & Jing, C. (2018). Prevalence status and risk factors of mild cognitive impairment of the elderly with frailty in pension facilities. Practice Geriatric, 10(32), 996–998. 10.3969/j.issn.1003-9198.2018.10.027 [DOI] [Google Scholar]
- Yoo, H. N. , Chung, E. , & Lee, B. H. (2013). The effects of augmented reality‐based otago exercise on balance, gait, and falls efficacy of elderly women. Journal of Physical Therapy Science, 25(7), 797–801. 10.1589/jpts.25.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, D. H. , Lee, J. Y. , & Song, W. (2018). Effects of resistance exercise training on cognitive function and physical performance in cognitive frailty: A randomized controlled trial. The Journal of Nutrition, Health & Aging, 22(8), 944–951. 10.1007/s12603-018-1090-9 [DOI] [PubMed] [Google Scholar]
- Yu, J. , Li, J. , & Huang, X. (2012). The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: A community‐based study. BMC Psychiatry, 12, 156. 10.1186/1471-244X-12-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , He, J. , Yi, J. , & Yao, S. (2019). Factor structure and measurement invariance across gender groups of the 15‐item geriatric depression scale among Chinese elders. Frontiers in Psychology, 10, 1360. 10.3389/fpsyg.2019.01360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


