Abstract
Background
Beclomethasone dipropionate (BDP) and budesonide (BUD) are commonly prescribed inhaled corticosteroids for the treatment of asthma. Fluticasone propionate (FP) is newer agent with greater potency in in‐vitro assays.
Objectives
To compare the efficacy and safety of Fluticasone to Beclomethasone or Budesonide in the treatment of chronic asthma.
Search methods
We searched the Cochrane Airways Group trial register (January 2007) and reference lists of articles. We contacted trialists and pharmaceutical companies for additional studies and searched abstracts of major respiratory society meetings (1997 to 2006).
Selection criteria
Randomised trials in children and adults comparing Fluticasone to either Beclomethasone or Budesonide in the treatment of chronic asthma.
Data collection and analysis
Two reviewers independently assessed articles for inclusion and methodological quality. One reviewer extracted data. Quantitative analyses were undertaken using RevMan analyses 1.0.1.
Main results
Seventy‐one studies (14,602 participants) representing 74 randomised comparisons met the inclusion criteria. Methodological quality was fair. Dose ratio 1:2: FP produced a significantly greater end of treatment FEV1 (0.04 litres (95% CI 0 to 0.07 litres), end of treatment and change in morning PEF, but not change in FEV1 or evening PEF. This applied to all drug doses, age groups, and delivery devices. No difference between FP and BDP/BUD were seen for trial withdrawals. FP led to fewer symptoms and less rescue medication use. When given at half the dose of BDP/BUD, FP led to a greater likelihood of pharyngitis. There was no difference in the likelihood of oral candidiasis. Plasma cortisol and 24 hour urinary cortisol was measured frequently but data presentation was limited. Dose ratio 1:1: FP produced a statistically significant difference in morning PEF, evening PEF, and FEV1 over BDP or BUD. The effects on exacerbations were mixed. There were no significant differences incidence of hoarseness, pharyngitis, candidiasis, or cough.
Authors' conclusions
Fluticasone given at half the daily dose of beclomethasone or budesonide leads to small improvements in measures of airway calibre, but it appears to have a higher risk of causing sore throat and when given at the same daily dose leads to increased hoarseness. There are concerns about adrenal suppression with Fluticasone given to children at doses greater than 400 mcg/day, but the randomised trials included in this review did not provide sufficient data to address this issue.
Plain language summary
Fluticasone versus beclomethasone or budesonide for chronic asthma in adults and children
This review compares the effectiveness of three inhaled steroids. Fluticasone (FP) was compared with either beclomethasone (BDP) or budesonide (BUD) for treating people with chronic asthma. When FP was given to children or adults at approximately half the daily dose of either BDP or BUD, it appeared to be at least as effective as the other two drugs in improving airway opening. There was not enough information available to draw conclusions concerning the effect of these drugs on symptoms, or the risk of an acute asthma exacerbation. When given at the same dose as BDP or BUD, FP treated participants had slightly better lung function. However, at the same dose FP was also associated with increased hoarseness, although it did not lead to increased incidences of other side‐effects associated with steroids such as oral thrush or sore throat.
Background
Inhaled corticosteroids (ICS) have become the mainstay of therapy for chronic asthma, and are recommended for use in recent guidelines for all patients except those with mild, intermittent symptoms (GINA 1995; BTS 2003; NHLBI 1997). Fluticasone propionate (FP) is the most recently licensed ICS for the treatment of asthma in children and adults, and joins a stable of older agents including beclomethasone dipropionate (BDP) and budesonide (BUD).
With an expanding range of drugs to choose from it is important to establish their relative efficacy and safety. All ICS's share close chemical and structural similarities. However, pharmacodynamic differences could lead to differences in their clinical effects. Potency, a measure of the microgram dose of drug required to produce a standard response, is greater for FP than either BDP or BUD. When assessed in the in‐vitro skin blanching test FP is twice as potent as BDP (Phillipps 1990), and 25% more potent than BUD (Kelly 1998). Similar rank order potencies have been shown for other assays of anti‐inflammatory activity including inhibition of basophil histamine release, eosinophil viability and expression of vascular cell adhesion molecule‐1 in cultured bronchial epithelial cells (Stellato 1999). FP also exhibits considerably greater glucocorticoid receptor (GR) binding affinity when compared to BDP and BUD (Kelly 1998). These factors could lead to greater clinical potency of FP, i.e. a lower microgram dose required to produce equal or better asthma control compared to BDP or BUD due action at sites in the lung. However, potential advantages in this respect are only important if they are not achieved at the expense of increased systemic drug activity. Increased potency at active sites in the lung may be offset when FP becomes systemically available. Higher potency at the site of the pulmonary GR will be mirrored by higher potency at the site of systemic GR's. This factor, associated with the longer elimination half life (Thorsson 1997) and higher lipophilicity (Kelly 1998) of FP compared to the older inhaled steroids should lead to longer tissue retention times, and could lead to enhanced endogenous glucocorticoid suppression and increased exogenous steroid related side effects. Single dose and repeated dose short term studies (<one week) have shown that FP leads to significantly greater reductions in morning plasma cortisol and overnight urinary cortisol excretion when compared to BUD at equal nominal daily dose (Clark 1996a; Clark 1996b; Clark 1997; Lipworth 1997).
A previous systematic review has assessed the relative efficacy of FP versus BDP and BUD (Barnes 1998). However, this review has a number of weaknesses including the lack of a well‐documented search strategy, clearly defined inclusion criteria or an assessment of methodological quality. In addition, a number of trials have become available since this review was published.
Relative efficacy/side effect ratios need to be considered when the performance of ICS's is compared in clinical practice. The objectives of this review will be to compare efficacy and safety outcomes in studies that have compared FP to either BDP or BUD in the treatment of chronic asthma. This is the second substantive update of fluticasone in comparison with beclomethasone or budesonide (Adams 2004; Adams 2005a).
Objectives
To compare the efficacy and safety of FP to BDP or BUD in the treatment of chronic asthma.
Methods
Criteria for considering studies for this review
Types of studies
We considered only prospective, randomised controlled trials. Parallel group and crossover studies were eligible, in both children and adults. We excluded studies assessing infants (under the age of two years). A diagnosis of chronic asthma was necessary. We did not consider studies concerned with the treatment of acute asthma exacerbations. Studies could be in primary care, hospital outpatient or an institutional care setting.
Types of participants
We reviewed studies in children and/or adults. We excluded studies assessing infants (i.e. under the age of two years). Patients needed to have a diagnosis of chronic asthma, we did not consider studies concerning acute asthma. We considered studies conducted in primary care, hospital outpatient and institutional care.
Types of interventions
FP delivered by mouth inhalation versus either BDP or BUD. We considered any dose of FP compared to any dose of either BDP or BUD, but the nominal daily dose of each inhaled steroid had to be stated. We calculated the nominal daily dose as the actuator dose multiplied by the number of doses administered per day. Treatment periods had to be one week or longer. Delivery devices allowed including metered dose inhalers (MDI) with or without spacer/chamber and dry powder inhalers (DPI's). We specifically excluded trials using nebulisers. For the December 2003 update we only included studies that compared FP with BDP/BUD with identical propellants. Studies that randomised participants to a stable versus step‐down treatment protocol were excluded. Studies assessing the effects of step‐down therapy were included where down‐titration was attempted in both treatment groups.
Comparisons between FP and HFA‐BDP are considered in a separate review (Lasserson 2006).
Types of outcome measures
Primary outcomes
Measures of airway calibre: FEV1, diary and clinic PEF, diurnal PEF variability
Secondary outcomes
Symptoms
Rescue bronchodilator use
Health status/health related quality of life (HRQOL)
Rates of asthma exacerbation leading to primary care physician visits, emergency room visits, hospital admission and days lost from work/school
Safety assessment: hypothalamo‐pituitary adrenal (HPA) function markers (plasma and urinary cortisol measures), oropharyngeal side effects, skin bruising
Search methods for identification of studies
There were no language restrictions to the search. Searches were current as of January 2007.
Electronic searches
The Cochrane Airways Group Specialised Register of asthma trials was searched using the following terms:
steroid* OR glucocorticoid* OR corticosteroid* OR beclomethasone OR budesonide OR fluticasone OR triamcinolone OR flunisolide OR Becotide OR Becloforte OR Pulmicort OR Flixotide
Searching other resources
Reference lists of all included studies and relevant narrative reviews were searched for additional RCTs
The UK headquarters of Glaxo Wellcome (manufacturers of Becotide, Becloforte and Flixotide) and the Swedish headquarters of Astra Zeneca (manufacturers of Pulmicort) were asked if they were aware of further missed trials.
Authors of studies were asked if they were aware of further missed trials.
The British Journal of Clinical Research and the European Journal of Clinical Research (journals not electronically indexed on MEDLINE or EMBASE) were hand‐searched.
Proceedings of the British Thoracic Society (1997 to 2004), European Respiratory Society (1997 to 2004) and the American Thoracic Society (1997 to 2004) were searched for relevant trials.
We searched web sites listing details of unpublished data or unpublished trials (www.clinicalstudyresults.org; www.ctr.gsk.co.uk; www.fda.gov)
Data collection and analysis
Selection of studies
Two authors (NPA and TJL) independently made the decision to exclude studies prior to full paper retrieval. In cases of disagreement, we retrieved the full text article. Papers retrieved in full text and likely to be included were assessed independently by the same authors, we resolved disagreement regarding eligibility by consensus.
Data extraction and management
One reviewer (NPA) extracted data for each outcome from the published results of included trials. In the case of continuous outcomes (such as FEV1), only data from the last time point can be evaluated. We extracted data from graphical plots when presented in this form; attempt was made to verify such data by contacting authors. It should be noted that we displayed continuous outcome data using negative figures, as the sign convention built into the software interprets smaller numbers as favourable (as is the case for dichotomous outcomes such as asthma exacerbation rates). This ensures that results favouring FP are consistently displayed to the left of the zero effect line in MetaView and results favouring BDP/BUD are consistently displayed to the right.
Assessment of risk of bias in included studies
We assessed the risk of bias for each included study according to recommendations described in the Cochrane Handbook. We have assessed the risk of bias for the generation and concealment of allocation schedules for the eligible studies, and blinding of treatment preparations. We have judged the degree of bias for each domain to be of high risk (No), low risk (Yes) or unclear risk (Unclear). Our previous approach is described in Appendix 1.
Dealing with missing data
We wrote to trialists (by mail, fax and/or electronic mail) to request absent or incompletely reported outcome data. We made an attempt to send requests to correct current addresses by searching MEDLINE, EMBASE and hospital World Wide Web (WWW) sites for up‐to‐date contact details.
In order to minimise the influence of publication bias on the continuous variables in our analyses, we have imputed data where it is otherwise not available. Where means and a P value were published we have calculated the variance of the study based on the P value. Where data are reported without SDs, we have imputed an average SD for the FP and BDP/BUD groups based upon those reported for the other data sets in the analysis, provided that there are at least three other trials. We report the data from estimates where the greatest number of estimates were available (i.e. where values have been imputed or estimated). We have retained analyses of data where SDs were originally available. These data are reported separately but we have retained both sets of analyses. Details of the outcomes with estimates/imputations are in Table 1.
1. Methods of imputations and estimates.
| Outcome | MD/GIV | Study | Method |
| 01:07 (FEV1 (litres)) | GIV | Lundback 1993 | Average SD based on the other studies. Published means |
| 01:08 (Change in FEV1 (litres) compared to baseline) | MD | Gustaffsson 1993 | Average SD based on the other studies. Published means |
| 01:12 (Mean change in am PEF) | GIV | Lundback 1993 | Average SD based on the other studies. Published means |
| 01:12 (Mean change in am PEF) | GIV | Langdon 1994b | Estimated from graph; published P value. |
| 01:12 (Mean change in am PEF) | GIV | Steinmetz 1996 | Average SD based on the other studies. Published means |
| 01:12 (Mean change in am PEF) | GIV | Gustaffsson 1993 | Estimated from published P value. Published mean changes. |
| 01:15 (Change in evening PEFR (L/min) compared to baseline) | GIV | Lundback 1993 | Average SD based on the other studies. Published means |
| 02:03 (FEV1 ‐ litres) | GIV | FLIT37; Agertoft 1997a | Average SD based on the other studies. Published means |
| 02:04 (Change in FEV1 (litres)) | GIV | Basran 1997 | Published means. Published P value |
| 02:10 (Morning PEF L/min) | GIV | FLIP01 | Published means. Published P value |
| 02:11 (Evening PEF L/min) | GIV | FLIP01 | Published means. Published P value |
| 02:12 (Change in morning PEFR compared to baseline (L/min)) | GIV | Basran 1997 | Published means. Published P value |
| 02:13 (Change in evening PEF compared with baseline) | GIV | Basran 1997 | Published means. Published P value |
| 02:25 (Change in daytime symptoms) | GIV | Basran 1997 | Published means. Published P value |
| 02:27 (Change in nocturnal symptoms) | GIV | Basran 1997 | Published means. Published P value |
| 02:30 (Change in rescue medication usage (daytime)) | GIV | Basran 1997 | Published means. Published P value |
| 02:32 (Change in rescue medication usage (nighttime) | GIV | Basran 1997 | Published means. Published P value |
Assessment of heterogeneity
We measured heterogeneity of effect size across pooled studies using the I square test.
Data synthesis
We calculated a weighted treatment effect across trials using RevMan 4.2. For continuous outcomes, we calculated a mean difference (MD), standardised mean difference (SMD) using generic inverse variance (GIV) where appropriate. As the GIV function has become available prior to the publication of this review, we have converted data from previous MD analyses in order to combine in with data from new studies reported as mean differences with 95% confidence intervals. Where previous effect estimates were calculated with two means and two SDs for the treatment groups, we have taken the mean difference and 95% CIs for MDs and converted the estimate to a mean difference and SEM. Where data have been available as mean differences with a 95% CI, we have calculated a SEM (standard error of the mean) for the mean difference and entered this in RevMan. We have retained all data initially analysed as MDs.
For dichotomous outcomes, we calculated Peto Odds Ratio's (OR). Pooled treatments effects are expressed with their 95% confidence intervals (95% CI). A fixed effect model was used throughout. A number of a priori conditions were established regarding the comparisons made:
Studies were distinguished as those in which participants were: (a) not treated with regular oral corticosteroid (OCS); (b) dependent upon regular OCS treatment prior to study. Trials involving OCS‐dependent participants in which the efficacy of ICS was being assessed may have had an 'OCS down‐titration' design using reduction in the use of oral steroid as an outcome measure, whilst maintaining a given level of asthma control. However, studies in which patients were not treated with regular OCS are more likely to have a design aimed at detecting improvements in asthma control. It is inappropriate to combine trials with these different designs and objectives.
The results of parallel and crossover trials were not pooled.
Studies comparing FP versus BDP or BUD at a nominal daily dose ratio of 1:2 were not pooled with those making comparisons at equal nominal daily dose.
Subgroup analysis and investigation of heterogeneity
The particular inhaled corticosteroid that was being ICS compared with FP (i.e. BDP or BUD), patient age (children or adult), delivery device (identical or different devices used for FP and BDP/BUD) and asthma severity were our a priori subgroups.
Results
Description of studies
Results of the search
See Table 2 for a full description of the search history, and Table 3 for archived details of 'What's New'.
2. Search History.
| Issue of CLIB | Time frame of search | Search results |
| Issue 1, 2002 | All years' searches to March 1999 | ': (1) INITIAL ELECTRONIC SEARCH: 6494 citations retrieved, 2162 unique citations (details in Table 04). The 19 trials identified from the FP Register were an identical set to the 13 studies from the BDP Register and the six studies from the BUD Register. In other words a total of 19 unique trials were included as a result of searching all three Registers.(2) OTHER SOURCES: Thirteen studies (Berend 2001; Bisca 1997; Dal Negro 1997; Steinmetz 1997; de Benedictis 2001; Johansson 1998; Joubert 1998; Lundback 1997; Murray 1998; Ringdal 2000; Hughes 1999a; Kemmerich 1999; Melaranci 1999) were identified as a result of searching respiratory society meeting proceedings. Four studies (Langdon 1994a; Langdon 1994b; Connolly 1995; Basran 1997) were identified as a result of hand‐searching the British Journal of Clinical Research and the European Journal of Clinical Research. Six studies (Ferguson 1999, Hughes 1999b, Malo 1999, Pickering 1996, Raphael 1999a/Raphael 1999b, Rao 1999) were identified by GSK. A total of 43 studies were therefore included in the initial version of the review. Agreement between the two independent assessments of study quality were as follows: Randomised? kappa = 1 Double blind? kappa = 0.9 Description of withdrawals kappa = 0.7 Method of randomisation kappa = 0.4 Method of double blinding kappa = 0.9 |
| Issue 2, 2004 | January 1999‐January 2002 | Four of the nine studies that were previously awaiting assessment met the inclusion criteria (Aubier 2001; Derom 1999; Heinig 1999; Nong 2001). We excluded three (Fairfax 2001; Kannisto 2000; Karakoc 2001), and two are awaiting translation (Chlumsky 1998; Dong 1999). Two published versions of studies previously included as abstracts were also identified (Ringdal 2000; Raphael 1999a). We included a further two studies from recent electronic searches (Harrison 2001; Nielsen 2000). Six new studies are included in this update. One study identified from searches is awaiting full translation (Yang 1999). Additional data for Heinig 1999 were made available by GSK. In December 2003 a total of 49 studies met the inclusion criteria for the review. |
| Issue 2, 2005 | January 2002‐January 2004 | Twelve new studies met the inclusion criteria. In January 2004 a total of 57 studies met the inclusion criteria of the review. |
| Issue 3, 2006 | January 2005‐2006 | 15 new studies met the inclusion criteria. In January 2006 a total 73 studies met the inclusion criteria of the review. |
3. What's New History.
| January 2004 (Issue 2, 2004) | This review now reflects published and unpublished evidence up to January 2004. In this update there are 7 new studies (Dose ratio 1:1: Currie 2002; Kuna 2003*; Dose ratio 1:2: Backman 2001*; Ige 2002; Kannisto 2002; Majer‐Teboul 2001; Szefler 2002). This gives a total of 57 trials included in the review. This review now incorporates data from fully published articles from two trials previously included as abstracts: Berend 1998 (now Berend 2001); de Benedictis 1998 (now de Benedictis 2001*). Both trials contribute data to the dose ratio 1:2. Following correspondence with GSK there are data from an unpublished trial previously included as an abstract: Lundback 1997*; dose ratio: 1:1. Impact upon findings Dose ratio 1:2. The confidence intervals tightened around mean differences for lung function variables, exacerbations and side‐effects with the addition of new data. In certain instances new data also led to significant heterogeneity which we have explored. Dose ratio 1:1. There is now evidence that FP is more effective than BDP/BUD on certain measures of lung function most notably FEV1, FVC and PEF. However, the effects on exacerbations have become more equivocal with the addition of new data and the level of heterogeneity in this outcome may reflect the combination of data from studies with varying baseline risks. *Denotes unpublished data made available for this review. |
| January 2005 (Issue 2, 2005) | This review now reflects published and unpublished evidence up to January 2005. There are two new trials in this update (Ferguson 2002; Molimard 2005). Furthermore, the reviewers have attempted to incorporate estimates from studies previously unable to contribute data due to inadequate data analysis in prior versions of RevMan. We have also excluded one study comparing HFA‐BDP with FP (Aubier 2001) as this is now subject to a different systematic review. The new data increased the statistical power of many of the analyses with regard to lung function and data which were measured on continuous scales (i.e. where average scores are achieved). The new evidence does not alter the conclusions of the review. |
Included studies
Seventy‐four group comparisons (71 trials reported via 134 references; 14,602 participants) met the inclusion criteria of this review. The details below refer to the 71 publications (three of which had more than one comparison, or reported more than one trial: FLIP01; FLIP01a; Raphael 1999a; Raphael 1999b; Wolfe 2000 SABA; Wolfe 2000 ICS).
Populations
Thirty‐seven trials were multi‐centre studies. These were conducted largely in Europe (Belgium, Denmark, Greece, Hungary, Italy, Norway, The Netherlands, UK and Poland). In nine of these studies, participants were also recruited from other areas of the world (Australia, Canada, Indonesia, South Africa and New Zealand). Two multi centre studies (Murray 1998, Raphael 1999a) were conducted in the USA and one in Australia (Berend 2001). Three single centre studies were conducted in India (Parakh 2004; Prasad 2004; Vedanthan 2004). One single centre study was conducted in Nigeria (Ige 2002). The remaining single centre studies were conducted in either Australia, Canada or countries of Europe.
Four studies recruited participants exclusively from primary care in the UK (Langdon 1994a, Langdon 1994b; Connolly 1995; Basran 1997). Rao 1999 recruited participants from both primary and secondary care. All other studies were conducted in a secondary care/hospital outpatient clinic setting. 14 studies were in children; all remaining studies were conducted in adolescents and adults.
Study design
Fifty‐nine studies employed a parallel group design, 14 studies were of crossover design. Only four crossover studies (Bootsma 1995; Agertoft 1997; Wolthers 1997; Ringdal 2000) employed an inhaled steroid‐free washout period between treatment periods. In one study the design was not clear (Majer‐Teboul 2001).
Thirteen studies had treatment periods of a month or less, we extracted data from one study at a 5 week cut‐off for stable steroid treatment (Kuna 2003). Forty‐seven studies had treatment periods of between 6 weeks to 5 months. Three studies (Pauwels 1998; Heinig 1999; Berend 2001) had a treatment period of six months, whilst seven studies (Egan 1999; Fabbri 1993; Lorentzen 1996; de Benedictis 2001; Lundback 1997; Hughes 1999b; Rao 1999) had treatment periods of 12 months or longer.
Interventions
In 33 studies, participants were randomised to receive FP or BDP. In 37 studies participants received FP or BUD. In two studies (Berend 2001; Vedanthan 2004) participants were randomised to one of two parallel treatment arms: either FP, or BDP/BUD.
In the majority of studies, participants received the ICS to which they had been randomised at a constant dose throughout the treatment period. In 38 studies participants were randomised to receive FP and either BDP or BUD in a nominal daily dose ratio of 1:2. In 22 studies, FP and BDP or BUD were administered at an equal nominal daily dose ratio i.e. 1:1. In two studies there were multiple dose ratio comparisons (Dahl 1993; FLIP01; FLIP01a). In one study the dose ratio was unclear (Vedanthan 2004).
Agertoft 1997a used a dose down‐titration design. Participants were randomised to either FP or BUD. Over the course of the treatment period, the ICS dose was down‐titrated to the minimum required to maintain asthma control at a pre‐defined level. The main outcome measures in this study were the number of dose reduction steps and the minimal daily dose of ICS required to maintain acceptable control.
Delivery device
A tabulated summary of the devices used in each study is provided in Table 4.
4. Delivery devices used.
| Study ID | Comparison | FP device | BDP/BUD device |
| Barnes 1993 | FP versus BDP | MDI | MDI |
| Bootsma 1995 | FP versus BDP | MDI | MDI |
| Dahl 1993 | FP versus BDP | MDI | MDI |
| Lorentzen 1996 | FP versus BDP | MDI | MDI |
| Malo 1999 | FP versus BDP | MDI | MDI |
| Raphael 1999a/Raphael 1999b | FP versus BDP | MDI | MDI |
| Boe 1994 | FP versus BDP | DISKHALER | DISKHALER |
| de Benedictis 2001 | FP versus BDP | DISKHALER | DISKHALER |
| Wolthers 1997 | FP versus BDP | DISKHALER | DISKHALER |
| Fitzgerald 1998 | FP versus BDP | MDI+SPACER | MDI + SPACER |
| Gustafsson 1993 | FP versus BDP | MDI+SPACER | MDI + SPACER |
| Melaranci 1999 | FP versus BDP | MDI+SPACER | MDI + SPACER |
| Rao 1999 | FP versus BDP | MDI+SPACER | MDI + SPACER |
| Yiallouros 1997 | FP versus BDP | MDI+SPACER | MDI + SPACER |
| Fabbri 1993 | FP versus BDP | MDI+/‐ SPACER | MDI +/‐ SPACER |
| Leblanc 1994 | FP versus BDP | MDI+/‐ SPACER | MDI +/‐ SPACER |
| Pauwels 1998 | FP versus BDP | MDI+/‐ SPACER | MDI +/‐ SPACER |
| Lundback 1993 | FP versus BDP | MDI AND DISKHALER (2 treatment arms) | MDI |
| Currie 2002 | FP versus BDP | MDI | MDI |
| Ige 2002 | FP versus BDP | MDI | MDI |
| Majer‐Teboul | FP versus BDP | UNCLEAR | UNCLEAR |
| Wolfe 2000 BDP | FP versus BDP | DISKHALER | DISKHALER |
| Wolfe 2000 ICS | FP versus BDP | DISKHALER | DISKHALER |
| FLIP01/a | FP versus BDP | MDI | MDI |
| FLIT37 | FP versus BDP | MDI | MDI |
| FLTB3013 | FP versus BDP | DISKHALER | DISKHALER |
| FLUTI/AH89/J89 | FP versus BDP | MDI | MDI |
| Egan 1999 | FP versus BDP | MDI + SPACER | MDI + SPACER |
| Prasad 2004 | FP versus BDP | MDI | MDI |
| Agertoft 1997a | FP versus BUD | DISKHALER | TURBOHALER |
| Agertoft 1997b | FP versus BUD | DISKHALER | TURBOHALER |
| Basran 1997 | FP versus BUD | DISKHALER | TURBOHALER |
| Connolly 1995 | FP versus BUD | DISKHALER | TURBOHALER |
| Derom 1999 | FP versus BUD | DISKHALER | TURBOHALER |
| Harrison 2001 | FP versus BUD | ACCUHALER | TURBOHALER |
| Heinig 1999 | FP versus BUD | DISKHALER | TURBOHALER |
| Hoekx 1996 | FP versus BUD | DISKHALER | TURBOHALER |
| Langdon 1994a | FP versus BUD | DISKHALER | TURBOHALER |
| Lundback 1998 | FP versus BUD | DISKHALER | TURBOHALER |
| Ringdal 1996 | FP versus BUD | DISKHALER | TURBOHALER |
| Ferguson 1999 | FP versus BUD | ACCUHALER | TURBOHALER |
| Kemmerich 1999a | FP versus BUD | ACCUHALER | TURBOHALER |
| Pickering 1996 | FP versus BUD | ACCUHALER | TURBOHALER |
| Nielsen 2000 | FP versus BUD | DISKHALER | TURBOHALER |
| Ringdal 1998 | FP versus BUD | ACCUHALER | TURBOHALER |
| Williams 1997 | FP versus BUD | ACCUHALER | TURBOHALER |
| Joubert 1998 | FP versus BUD | MDI | TURBOHALER |
| Steinmetz 1997 | FP versus BUD | MDI | TURBOHALER |
| Langdon 1994b | FP versus BUD | MDI | MDI |
| Hughes 1999b | FP versus BUD | MDI + SPACER | MDI + SPACER |
| Ayres 1995 | FP versus BUD | MDI +/‐ SPACER | MDI +/‐ SPACER |
| Bisca 1997 | FP versus BDP | NOT STATED | NOT STATED |
| Murray 1998 | FP versus BDP | NOT STATED | NOT STATED |
| Nong 2001 | FP versus BDP | NOT STATED | NOT STATED |
| Dal Negro 1997 | FP versus BUD | NOT STATED | NOT STATED |
| Hughes 1999a | FP versus BUD | NOT STATED | NOT STATED |
| Johansson 1998 | FP versus BUD | NOT STATED | NOT STATED |
| Berend 2001 | FP versus BDP/BUD | MDI + SPACER | MDI + SPACER OR TURBOHALER |
| Backman 2001 | FP versus BUD | DISKUS | TURBOHALER |
| Kannisto 2002 | FP versus BUD | DISKUS | TURBOHALER |
| Vedanthan 2004 | FP versus BDP/BUD | NOT STATED | NOT STATED |
| Parakh 2004 | FP versus BDP/BUD | MDI | MDI |
| Kuna 2003 | FP versus BUD | DISKUS | TURBOHALER |
| Ferguson 2002 | FP versus BUD | DISKUS | TURBOHALER |
| Stallberg 2007 | FP versus BUD | MDI | MDI |
| SD‐004‐0377 | FP versus BUD | DISKUS | DISKHALER |
Aerosol metered dose inhalers (MDIs) and a variety of dry powder inhalers (DPIs) were used. In 29 studies, the same delivery device was used for both FP and BDP/BUD treatment groups. This was mainly the case in studies that compared FP to BDP (26 studies). In only three studies of those that compared FP to BUD was the same delivery device used for both treatment arms (Ayres 1995; Langdon 1994b, Hughes 1999b). In the remainder of studies where the delivery device was stated, different devices were used for each ICS treatment arm. In the majority of studies that compared FP to BUD, FP treated participants used either the Diskhaler or Accuhaler DPI whilst BUD was administered using the Turbohaler/Turbuhaler. Inhaler device was unclear in two studies (FLPB0145; Vedanthan 2004). We have included data where available, and pooled with that of other studies. There may be differences in the relative efficacy of CFC and HFA driven FP, which may explain heterogeneity on certain outcomes. With the phasing out of CFC inhalers, it is likely that future studies will be conducted with CFC‐free inhaler devices.
In five studies (Egan 1999; Fabbri 1993; Leblanc 1994; Ayres 1995; Pauwels 1998) all participants received treatment using a metered dose inhaler, but were given the option of using a large volume spacer, provided use remained constant throughout the study.
In seven studies (Bisca 1997; Dal Negro 1997; Johansson 1998; Murray 1998; Hughes 1999a; Nong 2001; Vedanthan 2004) the delivery device used was not stated. All of these studies with the exception of Nong 2001 were published in abstract form only and methodological details were limited.
Prior treatment with corticosteroids
A single study (Lundback 1993) recruited oral steroid dependent asthmatics with the objective of assessing the relative prednisolone sparing effect of FP and BUD. One study (Ayres 1995) recruited severe asthmatics, a proportion of whom were receiving maintenance oral corticosteroids at the time of enrolment, but no attempt was made to taper prednisolone use during the trial. In all other studies, the participants were not receiving regular oral corticosteroids at the time of enrolment; indeed this was usually a specific exclusion criterion. In the majority of these studies (74%) however, some or all participants were using a regular inhaled corticosteroid at enrolment (see Table 5). In all cases, this was stopped at the point of randomisation when study medications were started.
5. Approximation of asthma severity.
| Study ID | IC FEV1 (% pred) | BL FEV1 | BL symptoms | Oral steroids at BL | ICS at BL | Author opinion | Approx severity |
| BL= baseline IC= inclusion criterion OCS= oral corticosteroid ICS= inhaled corticosteroid | |||||||
| Acun 2005 | Not stated | Not stated | Symptomatic twice weekly; night symptoms >/=1 night per week | No | Not stated | Moderate | Moderate |
| Agertoft 1997a | Not stated | 92 to 94 | Not stated | No | Yes: requiring BUD 400 to 800 mcg/d | Moderate | Moderate |
| Agertoft 1997 | Not stated | Not stated | No | No | Not stated | Mild | Mild |
| Ayres 1995 | Not stated | Morning PEFR 73 to 7 | Need for 2 or more doses beta2 agonist on 2 out of 7 days of run in period | Proportion of patients using OCS (<10 mg/d) | Yes: BDP 1 to 2 mg/d or BUD 0.8 to 1.6 mg/d | Moderate to severe | Moderate to severe |
| Backman 2001 | 50‐90% | FP: 2.44 (SD 0.77); BUD: 2.46 (SD 0.76); FEV1 % predicted: FP: 75 (SD 12); BUD: 75 (SD 15); | Not stated | No | Yes ‐ requirement for between 400 and 1200mcg/day of BDP, flunisolide or BUD. | Moderate‐severe | Moderate‐severe |
| Basran 1997 | >40 | 80 to 82 | Night‐time awakening on 2 or more out of 7 during run‐in | No | Yes: 400 to 800 mcg/d ICS | Mild to moderate | Mild to moderate |
| Barnes 1993 | Not stated | 57‐61 | Not stated | Not: Pts who had used OCS >4 times in previous 6mo excluded | Yes: FP:1000mcg | Severe | Severe |
| Berend 2001 | No details | No details | Symptoms on at least 2 days/week, night‐time symtoms on at least 2 nights/week | No | Yes: BDP or BUD 1750 mcg/d or greater | Severe | Severe |
| Bisca 1997 | No details | 48.8 to 52.3 | No details | No | No details | Moderate to severe | Moderate to severe |
| Boe 1994 | <80 | Not stated | On at least 4 out of 7 days of run‐in period | No | Yes: BDP or BUD 400‐2000 mcg/d | Not stated | Moderate to severe |
| Bootsma 1995 | 50 or greater | 75 to 85 | On at least 4 out of 7 days of run‐in period | No | Yes: mean daily dose of ICS 790 mcg/d | Not stated | Moderate to severe |
| Connolly 1995 | >50 | Not stated | Symptoms on at least 2 out of last 10 days | No | Some: BDP or BUD 200 mcg/d or less | Mild | Mild |
| Currie 2002 | FEV1 >70% predicted | FEV1 % predicted: 88.5 (SEM 2.5) | Not stated | No | Yes ‐ 13/20 participants on ICS (485 mcg/d) | Mild‐moderate | Mild to moderate |
| Dahl 1993 | Not stated | 73 to 75 | Daytime wheezing or night‐time symptoms on at least 4 days of 7 day run‐in period or PEFR variability 20% or greater | No | Yes: BDP 1000 mcg/d or less | Moderate | Moderate |
| Dal Negro 1997 | No details | No details | Asymptomatic | No | No details | Mild | Mild |
| de Benedictis 2001 | Unclear | Unpublished data (FP: N = 103, 1.46 L; BDP: N = 105, 1.49 L) | Patients had to have persistent asthma | No | Requirement of FP 100‐200 mcg/d, or BDP 200‐500 mcg/d at least previous 8 weeks, at constant dose for at least 4 weeks before run‐in period | Not stated | Moderate |
| Derom 1999 | No details | FEV1: 2.95 (SD 0.83) (FEV1 % predicted: 80.0 (SD 21.4)) | No exacerbation 4 wks before inclusion | No: Pts who had used OCS 6 months prior to study entry were excluded | No: Pts who had used ICS < 4 weeks prior to study were excluded | Mild | Mild |
| Egan 1999 | No details | 2.9‐3.1 L | Not reported | No | Yes: 1000‐2000mcg/d (BDP equivalent) | Moderate‐severe | Moderate to severe |
| Fabbri 1993 | Not stated | Morning PEFR 73 to 74 | Symptoms on at least 4 out of 7 days of run‐in period | No | Yes: BDP or BUD 1000 mcg/d or greater | Moderate to severe | Moderate to severe |
| Ferguson 1999 | Not stated | Not stated | Symptoms on at least 4 out of 7 days of run‐in period | No | Yes: BDP or BUD 400 to 800 mcg/d or FP 200 to 400 mcg/d | Moderate to severe | Moderate to severe |
| Ferguson 2006 | Not stated | 90.2%/92.3% | Not reported | No | No | Not stated | Mild |
| Fitzgerald 1998 | Not stated | 86 | Not stated | No | Yes: BDP or BUD 1‐2 mg/d | Severe | Severe |
| FLPB0145 | Not stated | Not stated | Deterioration over 5‐7 days pre‐randomisation | No | Yes BDP or Flunisolide 400‐1000mcg/d | Severe | Severe |
| FLTB3013 | Not stated | 1.83 | Symptomatic on 4 of 7 days | No | Yes: BDP 200‐500mcg/d (or equivalent) | Not stated | Moderate |
| FLIP01/FLIP01a | Not stated | Not stated | Not stated | No | No | Moderate | Mild to moderate |
| FLIT37 | Not stated | 1.32‐1.28 | Not stated | No | Yes: BDP or BUD 1‐2 mg/d | Severe | Severe |
| FLUTI/AH89/J89 | Not stated | 1.96‐2.01 | Symptoms on at least 4 out of 7 days of run‐in period | No | Yes:BDP 600‐1000mcg/d | Moderate to severe | Moderate to severe |
| Geppe 204 | Not stated | Not stated | Not stated | Not stated | Yes ‐ uncontrolled on ICS | Moderate to severe | Unclear |
| Gustafsson 1993 | Not stated | Morning PEFR 100 | If using BDP < 400 mcg/d: symptoms on at least 3 out of 7 days of run‐in period | No | Yes: BDP up to 400 mcg/d | Moderate | Moderate |
| Harrison 2001 | FEV1 <75% predicted | FEV1: FP group: 1.86 (SD 0.71), BUD group: 2.12 (SD 0.67) | Daily symptoms requiring a SABA despite high dose ICS (BDP: 1000‐2000mcg/day; FP: 500‐1000mcg/day | No | Yes: BDP: 1000‐2000mcg/day; FP: 500‐1000mcg/day | Not stated | Moderate |
| Heinig 1999 | change in FEV1 >15% in 15 minutes following administration of salbutamol 400/800mcg | FP group: 2.1 (SD 0.8), BUD group: 2.2 (SD 0.9); FVC: FP group: 3.2 (SD 1.1), BUD group: 3.3 (SD 1.1) | Pts must be 'symptomatic' at baseline | OCS for exacerbations: FP: 21%; BUD: 20% | Yes: Requirement for or response to inhaled BDP or BUD 1500 ‐ 2000mcg daily or FP: 750‐1000 mcg daily | Severe | Moderate to severe |
| Hoekx 1996 | Not stated | Morning PEFR 97 to 98 | Symptoms on at least 4 out of 7 days of run‐in period | No | Yes: ICS 200 to 400 mcg/d | Mild to moderate | Mild to moderate |
| Hughes 1999a | >70 | FEV1 86 to 88 | No details | No | Some using low dose ICS, no further details | Mild | Mild |
| Hughes 1999b | >30 | Not stated | Not stated | No | Yes: BDP or BUD 1500‐2000 mcg/d | Moderate to severe | Moderate to severe |
| Ige 2002 | >/=60% predicted | FEV1 (L/min): BDP: 2.23 (SD 0.36); FP: 2.21 (SD 0.52); FEV1 % pred: BDP: 76.8 (SD 8.55); FP: 83.5 (SD 13.37) | Total daytime asthma score of 10 (>/=10) in last seven days of screening period | No | Not clear | Mild‐moderate | Mild‐moderate |
| Johansson 1998 | No details | No details | Symptomatic despite ICS 400 mcg/d, no further details | No | Yes: BDP or BUD 400 mcg/d | Mild to moderate | Mild to moderate |
| Joubert 1998 | No details | FEV1 75 | Symptomatic despite ICS | No | Yes: mean daily dose ICS 825 mcg/d | Not stated | Moderate to severe |
| Kannisto 2002 | Not used | Mean FEV1 % pred: FP: 92 (SD 11); BUD: 92 (SD 15) | All participants suffered from symptoms presumptive of asthma (prolonged cough, wheeze during exercise, respiratory infections) | No | Not reported | Not stated | MIld‐moderate |
| Kemmerich 1999 | No details | No details | No details | No | No details | Mild to moderate | Mild to moderate |
| Kuna 2003 | FEV1 50‐90% predicted | Mean FEV1 % predicted: BUD: 79.4; FP: 79.4 | At interview participants expected to provide evidence of lack of asthma control; >/=1 asthma‐related nocturnal awakening during previous two weeks; use of rescue medication on >/=5 occasions during previous week, or asthma symptoms on >/=7 days during previous 2 weeks | No | Yes: ICS dose (median): BUD: 800; FP: 800 | Mild‐moderate | Moderate |
| Langdon 1994a | > 50 | Not stated | Symptoms on at least 4 out of 12 days of run‐in period | No | Yes: majority using BDP or BUD, up to 600 mcg/d | Not stated | Mild to moderate |
| Langdon 1994b | >50 | Not stated | Symptoms on at least 4 out of 12 days of run‐in period | No | Yes: majority using an ICS, no further details | Mild to moderate | Mild to moderate |
| Leblanc 1994 | Not stated | FEV1 71 to 73 | Symptoms on at least 4 out of 14 days of run‐in period | No | Some using BDP or BUD (no more than 400 mcg/d) | Mild to moderate | Mild to moderate |
| Lorentzen 1996 | Not stated | Not stated | Not stated | No | Yes: BDP or BUD 1 to 2 mg/d | Severe | Severe |
| Lundback 1997 | No details | No details | No details | Yes: prednisolone 5 mg/d or greater | Yes: ICS 800 mcg/d or greater | Severe | Severe |
| Lundback 1993 | Not stated | Not stated | Symptoms on 4 out of 14 days of run‐in period for patients using ICS 400‐600 mcg/d | No | Yes: ICS 400 to 1000 mcg/d | Moderate | Moderate |
| Majer Teboul 2001 | Not stated | 94.2 % predicted | Not stated | No | No. ICS in 3 months prior to study entry was exlcusion criterion | Mild | Mild |
| Malo 1999 | Not stated | FEV1 75.8 | Not stated | No | Yes: BDP or BUD 800 to 2000 mcg/d | Moderate to severe | Moderate to severe |
| Melaranci 1999 | No details | No details | No details | No | No details | Moderate | Moderate |
| Molimard 2005 | No details | 76‐9% predicted | 2 puffs SABA/day | No | Yes: inadequate control of symptoms at </=500mcg/d FP | Moderate‐severe | Moderate‐severe |
| Murray 1998 | No details | FEV1 range 45 to 80 | No details | No | Yes: BDP or TA 8 puffs/d or greater | Moderate | Moderate |
| Nielsen 2000 | >/=60% predicted | FEV1 (% pred): BUD: 81.2 (sd 8.04); FP: 83.4 (sd 11.49) | Pts were clinically stable at baseline (sparse symptoms during daytime and no night wakenings) | No | Yes: FP: 200‐500mcg/d or BUD 400 to 1000mcg/d | Not stated | Moderate |
| Nong 2001 | Not stated | Not stated | Pts were bothered by daytime and nocturnal symptoms more than once per week | No | Yes: 200‐400mcg (not stated which steroid) | Unclear | Unclear |
| O'Reilly 2001 | Not stated | Not stated | Symptomatic asthma | Not clear | Not clear | Not clear | Not clear |
| Parakh 2004 | Not stated | 1.5 to 1.8 L | History of dyspnoea and wheeze | No | Not clear (requirement for treatment all year round) | Not stated | Mild to moderate |
| Pauwels 1998 | >40 | FEV1 78 to 80 | Not stated | No | Yes: BDP or BUD 800 to 2000 mcg/d | Moderate to severe | Moderate to severe |
| Philips 2004 | >60% predicted | Not stated | Not stated | No | No | Mild | Mild |
| Prasad 2004 | <80% predicted | Not stated | night symptoms on 1/7 days; day symptoms on 3/7 days | No | No | Not stated | Mild to moderate |
| Rao 1999 | Not stated | FEV1 79 to 91 | Symptoms on at least 2 out of 7 days of run‐in period | No | No | Moderate | Moderate |
| Raphael 1999a/Raphael 1999b | 45 to 65 | FEV1 64.7 to 65.7 | > 8 puffs/week beta 2 agonist or diurnal variability in PEFR > 20% during run‐in if FEV > 65‐80 (% predicted) | No | Yes: BDP or TA 8 to 12 puffs/d | Mild/moderate and severe | Mild/moderate and severe |
| Ringdal 1996 | 45 to 90 | Not stated | Symptoms on at least 4 out of 7 days of run‐in period | No | Yes: BDP or BUD 400 to 2400 mcg/d | Moderate to severe | Moderate to severe |
| Ringdal 2000 | No details | No details | Symptoms and need to rescue beta2 agonist during 2 week run‐in | No | Yes: BDP or BUD 1500 to 1600 mcg/d | Moderate | Moderate to severe |
| SD‐004‐0377 | Not reported | Not reported | Well controlled | No | Yes (200 to 500mcg bid via MDI) | Not stated | Moderate |
| Stallberg 2007 | >70% post SABA | Not stated | Not reported | No | No | Not stated | Mild to moderate |
| Steinmetz 1997 | 50 to 80 | Not stated | Symptoms on at least 4 out of 14 days of run‐in period | No | No | Moderate | Moderate |
| Subbarao 2005 | >70% | Not stated | Stable asthma | No | No | Mild | Mild |
| Szefler 2002 | FEV1 55‐85% | FEV1 (L): FP: 3.04 (0.75); BDP: 3.01 (0.63); FEV1 % predicted: FP: 75.07 (SD 11.16); BDP: 73.33 (11.08) | median weekly average symptom scores: FP: 0.26 ; BDP: 0.35 | No | Not clear | Not stated | Mild‐moderate |
| Vedanthan 2004 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Williams 1997 | Not stated | Not stated | "Symptoms indicating a clinical requirement for ICS" if not already using one, no further details | No | Some: BDP or BUD 400 mcg/d or less, FP 200 mcg/d or less | Not stated | Mild to moderate |
| Wolthers 1997 | Not stated | Not stated | Not stated | No | No | Mild | Mild |
| Wolfe 2000 BD | 50‐80 | 69 | During 2 week run‐in: 0 days with >/= 12 puffs of SABA prn | No | No | Not stated | Mild‐moderate |
| Wolfe 2000 ICS | 50‐80 | 70 | During 2 week run‐in: 0 days with >/= 12 puffs of SABA prn | No | Yes ‐ for 3 months prior to study entry | Not stated | Moderate |
| Yiallouros 1997 | Not stated | Not stated | Not stated | No | Yes: BDP or BUD 400 to 909 mcg/m2/d | Severe | Severe |
Asthma severity
A summary of studies according to baseline FEV1 (% predicted), symptom frequency and prior use of oral/inhaled corticosteroids is given in Table 5. Using these features, along with the investigator's opinion an overall approximation of severity has been made for each study based on current GINA 1995 and NHLBI 1997 criteria. This is summarised as follows:
Mild: eight studies (Connolly 1995; Agertoft 1997; Dal Negro 1997; Ferguson 2006; Wolthers 1997; Derom 1999; Hughes 1999a; Majer‐Teboul 2001)
Mild to moderate: 18 studies (Currie 2002; Ige 2002; Kannisto 2002; Langdon 1994a; Langdon 1994b; Leblanc 1994; Hoekx 1996; Basran 1997; Williams 1997; Johansson 1998; Kemmerich 1999; Szefler 2002; Wolfe 2000 SABA; Ställberg 2007; FLIP01/FLIP01a; Parakh 2004; Prasad 2004)
Moderate: 15 studies (Dahl 1993; de Benedictis 2001; Gustafsson 1993; Kuna 2003; Lundback 1993; Agertoft 1997a; Steinmetz 1997; Murray 1998, Melaranci 1999; Rao 1999; Nielsen 2000; Harrison 2001; Wolfe 2000 ICS; FLTB3013; SD‐004‐0377)
Moderate to severe: 17 studies (Backman 2001; Egan 1999; Fabbri 1993; Boe 1994; Ayres 1995; Bootsma 1995; Molimard 2005; Ringdal 1996; Bisca 1997; Joubert 1998; Pauwels 1998; Ringdal 2000; Ferguson 1999; Heinig 1999; Malo 1999; Hughes 1999b; FLUTI/AH89/J78)
Severe: eight studies (Barnes 1993; Lorentzen 1996; Berend 2001; Yiallouros 1997; Fitzgerald 1998; Lundback 1997; FLIT37; FLPB0145)
Mild to severe: two studies (Raphael 1999a; Raphael 1999b)
Unclear: four studies (Geppe 2004; Nong 2001; Vedanthan 2004; SD‐004‐0377)
Outcomes assessed
A wide range of outcome measures was reported. All have been considered, except the following that were specified a priori as not being within the scope this review. These included: growth assessment (Agertoft 1997, Wolthers 1997; Ferguson 1999, de Benedictis 2001); biochemical markers of bone turnover (Ayres 1995; Bootsma 1995; Harrison 2001; Hoekx 1996; Berend 2001; Wolthers 1997; Pauwels 1998; Malo 1999); bone densitometry (Egan 1999; Pauwels 1998; Rao 1999). A significant amount of data reported by trials could not be included in the meta‐analysis, because it was not presented in a suitable form. This is listed in Table 6. Authors were asked for this data, but were either unable or unwilling to provide it. One study (Bootsma 1995) reported the effects of treatment on peripheral blood cell immunophenotype profiles. This outcome has not been considered, as inflammatory cell profiles are not a recognised clinical efficacy measure at the current time.
6. Outcome data not included in the meta‐analysis.
| Study ID | Jadad score | Missing data |
| Agertoft 1997a | 5 | FEV1 FVC FEF25‐75 % fall in FEV1 after 6 minute treadmill exercise test % fall in FEF 25 to 75 after 6 minute exercise treadmill test Morning PEFR. Evening PEFR Daytime asthma symptom score Night‐time asthma symptom score Rescue beta2 agonist use (puffs/d) 24 hour urinary free cortisol No SD values available for above outcomes |
| Agertoft 1997b | 4 | Morning PEFR Evening PEFR 24 hour urinary cortisol excretion No SD values available for above outcomes Daily asthma symptoms score Daily beta2 agonist use No numerical data available |
| Ayres 1995 | 3 | Symptom free days and nights; Rescue beta2 agonist free days and nights Daytime and night‐time symptom scores Above outcomes analysed by investigators using non‐parametric tests Change in FEV1 compared to baseline Change in FVC compared to baseline Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Change in diurnal variability in PEFR compared to baseline Change in clinic PEFR compared to baseline No SD values available for above outcomes Morning plasma cortisol Data log transformed and reported using geometric means by investigators: log transformed values not available |
| Barnes 1993 | 4 | Morning PEFR Evening PEFR Diurnal variability in PEFR daytime salbutamol use night‐time salbutamol use No standard deviation values for above outcomes |
| Basran 1997 | 3 | FEV1 FVC Diurnal variation in PEFR Daytime asthma symptom score Night‐time asthma symptom score Daytime beta2 agonist use Night‐time beta2 agonist use No SD values available for above outcomes |
| Berend 1997 | 1 | FEV1 FVC Clinic PEFR No numerical data available for above outcomes Change in Asthma Quality of Life Questionnaire domain scores compared to baseline Change in SF‐36 questionnaire domain scores compared to baseline SD values not available for above outcomes |
| Bisca 1997 | 1 | FEV1 (% predicted) Clinic PEFR (% predicted) Airway resistance (% predicted) MEF50 (% predicted) Numbers randomised to each treatment group not stated FVC Dairy card morning PEFR Diary card evening PEFR Symptom score Rescue beta2 agonist use No data presented for above outcomes |
| Bootsma 1995 | 4 | Histamine BHR log 10 PC20 FEV1 UNDW BHR log 10 PC20 FEV1 No SD values available for above outcome |
| Connolly 1995 | 2 | Change in diurnal variation in PEFR compared to baseline % symptom free days % symptom free nights % rescue beta2 agonist free days % rescue beta2 agonist free nights Physician assessed level of overall asthma control Patient assessed level of overall asthma control Morning plasma cortisol Non‐parametric tests used by investigators to examine treatment differences for above outcomes |
| Dahl 1993 | 4 | Morning plasma cortisol Plasma cortisol 30 min post 250 mcg ACTH Diurnal variation in PEFR Daily beta2 agonist use (puffs/day) No SD values available for above outcomes |
| dal Negro 1997 | 2 | Methacholine BHR (PD20 FEV1) Log transformed data not available |
| Derom 1999 | 3 | PEF; FEV1. No data on these outcomes were reported |
| Fabbri 1993 | 4 | Morning PEFR Evening PEFR Morning plasma cortisol Plasma cortisol post ACTH No SD values available for above outcomes |
| Ferguson 1999 | 3 | Evening PEFR Change in daytime symptom score compared to baseline Change in night‐time symptom score compared to baseline Daytime rescue beta2 agonist use No SD values available for above outcomes Morning plasma cortisol Log transformed values not available |
| Fitzgerald 1998 | 4 | Morning PEFR Evening PEFR 24 hour urinary free cortisol Plasma ACTH 8 am plasma cortisol Plasma 1 hour post synathsen (0.5mcg/1.73m2 body surface area) No SD values available for above outcomes Patient assessed efficacy scale Physician assessed efficacy scale Daytime asthma symptom scores ‐ no data presented Night‐time asthma symptom scores ‐ no data presented |
| Geppe 2004 | 1 | Unpublished conference abstract ‐ no data available |
| Gustafsson 1993 | 4 | FEV1 (% predicted) Change in FEV1 (% predicted) compared to baseline Clinic PEFR (% predicted) Change in clinic PEFR (% predicted) compared to baseline Morning PEFR (% predicted) Change in morning PEFR (% predicted) compared to baseline Evening PEFR (% predicted) Change in evening PEFR (% predicted) compared to baseline Diurnal variation in PEFR % symptom free days/nights % beta2 agonist free days Morning plasma cortisol No SD values available for above outcomes |
| Harrison 2001 | 4 | Symptom score; ß‐agonist use. Medians were reported and thus unsuitable for meta‐analysis. TCM data were reported as not significantly different |
| Hoekx 1996 | 4 | Daytime asthma symptom score % symptom free days % symptom free nights Days missed from work/school Parent completed, patient‐centred assessment of physical and social activity Above outcomes analysed by investigators using non‐parametric statistics Morning plasma cortisol Data log transformed and reported using geometric means FEV1 Clinic PEFR No numerical data available for above outcomes |
| Hughes 1999a | 1 | Rescue free days (No use of beta2 agonist, oral steroid use or physician visit) Rescue beta2 agonist use (puffs/day) Data for above outcomes reported using medians with interquartile range |
| Hughes 1999b | 3 | Change in urinary free cortisol level compared to baseline Change in plasma cortisol level (time not specified) compared to baseline No SD values available for above outcomes |
| Johansson 1998 | 2 | Change in morning PEFR compared to baseline No SD values available for above outcomes Asthma symptom score Daily use of beta2 agonists No data presented for above outcomes |
| Joubert 1998 | 2 | Change in morning PEFR compared to baseline Daily beta2 agonist use Asthma symptom score 24 hour area under curve serum cortisol No numerical data presented for above outcomes |
| Kemmerich 1999 | 1 | Morning PEFR Rescue beta2 agonist free days No data presented for above outcomes |
| Langdon 1994a | 3 | Diurnal variation in PEFR % symptom free days % symptom free nights Rescue beta2 agonist use Above outcomes analysed by investigators using non‐parametric tests Morning PEFR Morning plasma cortisol No SD values available for this outcome |
| Langdon 1994b | 2 | Daily asthma symptom score Daytime rescue beta2 agonist use Night‐time rescue beta2 agonist use Patient assessed degree of asthma control Above outcomes analysed by investigators using non‐parametric test Morning plasma cortisol No SD values available for this outcome |
| Leblanc 1994 | 4 | FEV1 FEV1 (% predicted) FVC (% predicted) FVC Morning PEFR Evening PEFR % symptom free days % symptom free nights % beta2 agonist free days Morning plasma cortisol Plasma cortisol 30min post ACTH No SD values available for above outcomes |
| Lorentzen 1996 | 5 | Morning plasma cortisol (log transformed) FEV1 FVC Clinic PEFR No SD values for above outcomes |
| Lundback 1993 | 3 | Morning PEFR Evening PEFR Diurnal variation in PEFR Morning plasma cortisol Plasma cortisol post ACTH No SD values available for above outcomes |
| Melaranci 1999 | 2 | Morning PEFR Evening PEFR Wheeze score Cough score No numerical data available |
| Nielsen 2000 | 4 | 24 hour urinary cortisol excretion; Methacholine BHR (PD 20). Data expressed for these outcomes as geometric means |
| Nong 2001 | 3 | FEV1 PEF L/min; No SEMs or SDs were available for these outcomes |
| Pauwels 1998 | 3 | FEV1 (% predicted) FVC No SD values available for above outcomes |
| Rao 1999 | 4 | FEF25‐75 Post exercise fall in FEV1 Histamine BHR (log 10 PC20 FEV1) Daily asthma symptom score No SD values for above outcomes |
| Ringdal 1996 | 4 | Daytime symptom score Night‐time symptom score % symptom free days % symptom free nights % rescue beta2 agonist free days % rescue beta2 agonist free nights Above outcomes analysed by investigators using non‐parametric tests Morning plasma cortisol Data log transformed and reported using geometric means |
| Williams 1997 | 3 | Diurnal variation in PEFR No SD values available for this outcome % symptom free days and nights (No. of patients) % rescue beta2 agonist free days and nights (No. of patients) Above outcomes analysed by investigators using non‐parametric statistics |
| Yiallouros 1997 | 3 | Morning PEFR: no SD values available Evening PEFR: no numerical data available % cough free days: effect size expressed as median % wheeze free days % symptom free activity days % cough free nights Daytime beta2 agonist use Night‐time beta2 agonist use No numerical data presented for above outcomes |
| de Benedictis | 5 | % cough free days % cough free nights % wheeze free days % wheeze free nights % days without shortness of breath % nights without shortness of breath % days without rescue medication % nights without rescue medication All data were reported as medians |
| Szefler 2002 | 2 | Symptoms Data were reported as medians |
| SD‐004‐0377 | 3 | Lung function PEF Asthma exacerbations Symptoms Data not reported |
Excluded studies
Risk of bias in included studies
Confirmation of study design was available from GSK who sponsored a number of studies (Appendix 2), or from some of the study authors directly (Bootsma 1995; Ige 2002; Rao 1999; Szefler 2002). Based upon our stated methods for assessing risk of study bias, we present an overview of our judgements for three domains relating to study level bias in Figure 1 for the included studies.
1.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
The majority of large studies which contribute the majority of evidence to our review, were characterised by satisfactory allocation procedures. Not all of the studies attempted to blind investigators and participants, with single blind or open label designs used in 20 studies, and unclear methods used in six studies available as conference abstracts.
Effects of interventions
We have presented results expressing differences where FP is the treatment intervention, and BDP or BUD the control intervention.
Categorisation of studies based on these features resulted in the generation of five main comparison groups:
Group 1. Parallel group studies in non‐OCS treated asthmatics, comparing FP to BDP/BUD at a 1:2 nominal daily dose ratio.
Group 2. Parallel group studies in non‐OCS treated patients comparing FP to BDP/BUD at 1:1 nominal daily dose ratio.
Group 3. Parallel group studies in oral steroid treated asthmatics comparing FP to BDP or BUD at a 1:2 nominal daily dose ratio.
Group 4. Crossover studies in non‐OCS treated patients comparing FP to BDP/BUD at 1:2 nominal daily dose ratio.
Group 5. Crossover studies in non‐OCS treated asthmatics, comparing FP to BDP/ BUD at a 1:1 nominal daily dose ratio.
We did not find any crossover studies in OCS treated participants that met the inclusion criteria. Results of meta‐analysis of studies with the characteristics defined by Groups 1 and 2 are shown in Comparisons 01 and 02. Few studies had the characteristics defined by Groups 4 and 5. These findings have been entered in the review, but there is no narrative discussion of the pooled results from these studies.
We considered the single dose reduction design study (Agertoft 1997a) separately and its results are discussed below. We undertook sensitivity analyses based on methodological quality and the data were re‐analysed using only studies of higher quality (Jadad score three to five). Subgroup analyses (as outlined under Methods) were planned. However, this could only be undertaken for some of the outcomes reported in the parallel group studies in non‐OCS treated participants that compared FP to BDP/BUD at a nominal daily dose ratios of 1:1 and 1:2.
NON‐ORAL STEROID TREATED ASTHMATICS
FP VERSUS BDP/BUD AT 1:2 NOMINAL DAILY DOSE RATIO
EFFICACY MEASURES
FEV1
Absolute: Fixed effect: 0.04 litres (95% CI 0 to 0.07 litres); random effects: 0.06 (‐0.01 to 0.13), 17 studies, N = 3640. See Figure 2. There was a high degree of heterogeneity (64.5 %), which disappeared when Ige 2002 was removed from the analysis. This resulted in a significant difference of 0.04 litres (95% CI 0 to 0.08). Although the Ige 2002 study reported an effect that seemed out of step with the other studies, we could not find a particularly convincing reason why this was so. Imbalances between treatment groups at baseline (duration of asthma symptoms: FP 5.2 years versus BDP 10.9 years) possibly influenced by the inappropriate method of randomisation (odd and even numbers) coupled with the small sample size (N = 20), could make the effect estimate more prone to error than those of other trials. Removing open label and single‐blind studies gave a non‐significant difference (0.02 (95% CI ‐0.02 to 0.06).
2.

Graph of end of treatment and change from baseline data for FEV1 (dose ratio 1:2).
Change from baseline: MD 0.01 litres (95% CI ‐0.02 to 0.04 litres, 12 studies, N = 2635). See Figure 2. There was a moderate level of heterogeneity (I square: 30.9%) and a Random Effects model widened the non‐significant result. The Raphael data sets are drawn from the same study and so more likely show similar responses to treatment. Furthermore, they also required evidence of sub‐optimal control according to particular criteria (see Included Studies). Selecting these patients could have affected the response to treatment, although this observation is post hoc.
Absolute values (predicted): MD 0.42% (95% CI ‐1.02 to 1.86), seven studies, N = 1444.
Change from baseline (predicted): MD ‐0.39 % (95% CI ‐1.65 to 0.86), six studies, N = 1306.
FVC
Absolute values: MD: 0.05 litres (95% CI 0 to 0.1), nine studies, N = 1854.
Change from baseline: MD: 0 litres (95% CI ‐0.05 to 0.06), five studies, N = 1129.
Absolute values (predicted): MD: 1.28 % (95% CI ‐0.84 to 3.4), two studies, N = 391.
Morning PEF
Absolute values: 5.89 L/min (95% CI 0.96 to 10.82 L/min, 12 studies N = 2955). There was a moderate degree of statistical heterogeneity (I square 36.9%). A sensitivity analysis with random effects modelling gave a non‐significant result (MD: 5.78 L/min (95% CI ‐1.48 to 13.04).
Change from baseline: MD: 7.42 L/min (95% CI 4.97 to 9.87), 17 studies, N = 4179.
Absolute values (predicted): MD 0.66% (95% CI ‐0.36 to 1.67), four studies, N = 988.
Change from baseline (predicted): MD 1.28% (95% CI 0.14 to 2.43), two studies, N = 765.
Evening PEF
Absolute values: MD 1.53 L/min (95% CI ‐3.78 to 6.84), 10 studies N = 2656.
Change from baseline: MD: 2.59 L/min (‐0.36 to 5.55), 10 studies, N = 2186.
Absolute values (predicted): MD: ‐0.06 % (95% CI ‐1.25 to 1.13), four studies, N = 988.
Change from baseline (predicted): MD ‐0.71 % (95% CI ‐2.16 to 0.75), two studies, N = 765.
Clinic PEF
Absolute values: MD: (17.04 L/min (95% CI ‐0.99 to 35.07), twelve studies N = 2545. Due to the considerable level of heterogeneity (I square 81.6%), we have retained the random effects model which gave a non‐significant result.
Change from baseline: 4.16 L/min (95% CI ‐2.11 to 10.44), six studies, N = 1979.
Absolute values (predicted): 2% (95% CI ‐0.18 to 4.18), three studies, N = 630.
Change from baseline (predicted): 0.31% (95% CI ‐1.30 to 1.92), three studies, N = 998.
Diurnal variability in PEF was reported in five studies whose Jadad scores were three or greater. However full outcome data (with endpoint mean and standard deviation values) were only available for one study (Ringdal 1996). Consistent effects were not seen for this outcome. In two studies (Barnes 1993; Ringdal 1996) significant reductions in diurnal variability favouring FP over BDP/BUD were seen, in others (Gustafsson 1993; Lundback 1993; Williams 1997) no difference between treatment groups was apparent.
Symptoms
Asthma symptoms were reported frequently. However, the use of different scales across the studies limited the possibilities for combining results to derive an authoritative pooled treatment effect.
Change in symptom scores: SMD: ‐0.19 (95% CI ‐0.31 to ‐0.07) six studies, N = 1035.
Absolute percentage of symptom free days: MD 4.9% (95% CI ‐1 to 11), two studies, N = 699.
Change in percentage of symptom free days: MD 6.43% (95% CI 0.47 to 12.39), two studies, N = 399.
Change in number of awakenings per night: MD: 0.01 (95% CI ‐0.04 to 0.06), two studies, N = 282.
A number of individual studies with a Jadad quality score of three or greater (Gustafsson 1993; Lundback 1993; Williams 1997) reported the percentage of symptom free days and nights. Meta‐analysis was not possible because the data were either not in a form suitable for analysis, and/or the original studies analysed their data using non‐parametric tests. In all studies, no significant differences between FP and BDP/BUD treatment groups were reported for these outcomes.
Three studies (Ringdal 1996; Ferguson 1999; Rao 1999) reported the change in daytime and night‐time symptoms scores compared to baseline. Complete numerical data were not available. No significant difference between FP and BDP/BUD treatment groups was apparent for either of these measures in any study.
Quality of life
Two studies reported change in different questionnaires: AQLQ (Berend 2001) and the Asthma Control questionnaire (Molimard 2005). There were some discrepant findings, and this could reflect the worse severity of patients recruited to the Berend 2001 study, which resulted in a significant result, as they may have had more impaired quality of life at baseline. Additional studies measuring these outcome would help to determine the impact of these drugs on these groups of patients.
Rescue beta‐2 agonist use
Change in percentage of rescue medication free days: MD 6.89% (95% CI 0.32 to 13.46), two studies, N = 399. The number of participants experiencing rescue beta‐2 agonist free days and nights was reported in six studies (Barnes 1993; Gustafsson 1993; Lundback 1993; Leblanc 1994; Ringdal 1996; Williams 1997), however a number of factors precluded pooling of data. In each study, no significant differences were reported between FP and BDP/BUD treatment groups.
Change in rescue medication usage (puffs/day): MD ‐0.35 puffs (95% CI ‐0.63 to ‐0.07), four studies, N = 763.
Two studies (Basran 1997; Ferguson 1999) reported daytime and night‐time beta‐2 agonist use scores. Again, complete numerical data were not presented. No significant differences between FP and BDP/BUD treatment groups were seen in either study. Nielsen 2000 reported no difference between FP and BUD in terms of the change in day usage of beta‐2 agonists. Kannisto 2002 did not detect a significant difference when data were reported as absolute scores.
Asthma exacerbations
Withdrawal due to asthma exacerbation: Peto OR 0.77 (95% CI 0.54 to 1.1), 11 studies N = 2824. The criteria for withdrawal varied between studies. In one study (Raphael 1999a; Raphael 1999b) the criteria were based upon a pre‐defined deterioration in FEV1 or PEF of 20% compared to baseline. Clinical need for oral corticosteroid was also used, either one course (Dahl 1993) or two courses (Ferguson 1999). In one study (Lundback 1993) deterioration in asthma prompting any change in asthma medication was the criterion for withdrawal. In six studies (Barnes 1993; Gustafsson 1993; Langdon 1994a, Langdon 1994b, Leblanc 1994; Connolly 1995) withdrawal criteria were not stated. There was no heterogeneity between the studies.
Participants with an exacerbation: Peto OR 0.74 (95% CI 0.53 to 1.03), four studies N = 1213.
Withdrawal due to lack of efficacy: Peto OR 0.6 (95% CI 0.33 to 1.07), seven studies, N = 1781.
EFFICACY: other measures
A number of outcome measures were reported by individual studies with a Jadad score of three or greater. A single parallel group study (Rao 1999) reported post exercise challenge drop in FEV1 and histamine BHR (PC20 FEV1). However, FP and BDP treatment groups were not compared directly. Langdon 1994b reported participant and investigator assessment of treatment success, no difference between FP and BUD treatment groups was observed.
Safety measures
Oropharyngeal side effects
Sore throat/pharyngitis: Peto OR 1.45 (95% CI 1.1 to 1.92), 15 studies, N = 3488.
Hoarseness: Peto OR 0.89 (95% CI 0.47 to 1.67), nine studies, N = 2056.
Dysphonia: Peto OR 1.3 (95% CI 0.74 to 2.28), nine studies, N = 2364.
Oral candidiasis: Peto OR 1.06 (95% CI 0.62 to 1.82), eight studies, N = 2180. Heterogeneity was present when studies were pooled (I square: 46.1%).
Upper respiratory tract symptoms: Peto OR 0.91 (95% CI 0.52 to 1.56), four studies, N = 873.
Safety ‐ other safety measures
Any adverse event: Peto OR 1.01 (95% CI 0.85 to 1.21), nine studies, N = 2364.
Withdrawals due to adverse event: Peto OR 0.99 (95% CI 0.68 to 1.44), eight studies, N = 3016.
Headache: Peto OR 1.01 (95% CI 0.74 to 1.37), ten studies, N = 2249.
Cough: Peto OR 1.20 (95% CI 0.82 to 1.75), nine studies, N = 1981.
Hypothalamo‐adrenal‐pituitary (HPA) function
Morning plasma cortisol
Morning plasma cortisol levels were reported in a number of studies. However only two studies (Berend 2001; Rao 1999) reported data suitable for inclusion in the meta‐analysis. These studies were pooled. No significant difference between FP and BDP/BUD treated groups was apparent: MD 12 nmol/L (95% CI ‐38 to 62 nmol/L).
Six further studies reported morning plasma cortisol levels. Attempts were made to obtain data from authors, but were unsuccessful. Five studies (Dahl 1993; Gustafsson 1993; Lundback 1993; Ferguson 1999; Hughes 1999b) found no significant differences between treatment groups, whilst three studies (Leblanc 1994; Lorentzen 1996; Ringdal 1996) found small, statistically significant differences between FP and BDP/BUD treatment groups that favoured FP. Because results could not be combined in a pooled analysis it was not possible to determine whether treatment responses across trials were determined by such factors as differences in doses compared, age of patients, delivery device, length of treatment or asthma severity.
HPA function: other measures 24 hour urinary free cortisol levels were reported in a single parallel group study (Hughes 1999b), and four crossover studies (Wolthers 1997; Yiallouros 1997; Fitzgerald 1998; Malo 1999). In only one study (Wolthers 1997) was a difference in response to treatments seen, with a small but significant difference in urinary cortisol levels in favour of FP compared to BDP. In all other studies, no significant difference between FP and BDP/BUD treatment groups was apparent. Because only one study (Malo 1999) presented data suitable for inclusion in the meta‐analysis, it was not possible to calculate an overall pooled treatment effect for the crossover studies.
A single parallel group study (Dahl 1993) and two crossover studies (Fitzgerald 1998; Malo 1999) reported plasma cortisol levels following a short synthetic ACTH (cosyntropin) test. Data was not in a format that allowed a pooled estimate to be calculated. No difference between FP and BDP/BUD treatment groups was apparent in any study. Nielsen 2000 reported results as geometric means and found a significant effect in favour of FP (P<0.01). This study compared increasing doses of the steroids assessed and the effects of stable doses of FP or BUD are difficult to establish from this study.
FP VERSUS BDP OR BUD AT 1:1 NOMINAL DAILY DOSE RATIO
FEV1
Absolute values: MD: 0.04 litres (95% CI 0.01 to 0.07), 11 studies, N = 2154. See Figure 3. Removing open label/single‐blind studies gave an identical result to the pooled, but the I square statistic went from 11% to 0%.
3.

Graph of end of treatment and change from baseline data for FEV1 (dose ratio 1:1).
Change in FEV1: MD: 0.01 litres (95% CI ‐0.03 to 0.05), five studies, N = 1019. See Figure 3.
Morning PEF
Absolute values: MD: 8.21 L/min (95% CI 5.1 to 11.31), 12 studies, N = 2389
Change from baseline: MD: 6.13 L/min (95% CI 1.49 to 10.77), seven studies, N = 1330.
Absolute values (predicted): 2.09% (95% CI 0.91 to 3.28), three studies, N = 747. There was a moderate level of heterogeneity for this outcome (I square 37.9%). Random Effects modelling also gave a significant effect (2.16% (95% CI 0.64 to 3.68).
Evening PEF
Absolute values: MD: 8.76 L/min (95% CI 2.90 to 14.62), five studies, N = 966.
Change from baseline: MD: L/min 8.57 L/min (95% CI 0 to 17.13), four studies, N = 646.
Absolute values (predicted): MD: 1.13% (95% CI 0.02 to 2.23), three studies, N = 745.
Clinic PEF
Absolute values: MD: 8.88 L/min (95% CI 4.40 to 13.35), nine studies, N = 2054.
Absolute values (predicted): MD: 2.08 % (95% CI 0.22 to 3.94), four studies, N = 817.
FVC
Absolute values: MD: 0.05 litres (95% CI 0.00 to 0.10), six studies, N = 1315.
Absolute values (predicted): MD: 1.90 % (95% CI ‐0.26 to 4.05), three studies, N = 589.
Exacerbations
Requirement for medication other than beta‐agonist: Peto OR 0.77 (95% CI 0.59 to 0.99), four studies, N = 1146. There was a moderate degree of heterogeneity observed in this analysis (I square 43.7%), and when a Random Effects model was applied there was no significant difference (Random Effects OR: 0.70 (95% CI 0.45, 1.09). This may have been due to differing definitions of an exacerbation, due to differing treatment periods with attendant variation in the degrees of exposure, or due to varying baseline risk in the studies. All participants in these studies were treated with inhaled steroids prior to study entry and showed some evidence of poor control, but there was subtle variation as to how these patients were selected for study entry. For example in Heinig 1999 trial, 20% of baseline participants had taken OCS treatment for exacerbations more than 4 weeks before study entry, whereas in the Fabbri 1993 study participants were excluded if they had required steroid treatment for exacerbations on three occasions in the previous six months.
One or more exacerbations: Peto OR 0.99 (95% CI 0.73 to 1.33), three studies, N = 1054.
Withdrawal due to an exacerbation: Peto OR 0.72 (95% CI 0.38 to 1.35), five studies, N = 978 No heterogeneity was observed even though the criteria for withdrawal varied. In one study (Dahl 1993) participants were withdrawn if they required a single course of rescue oral prednisolone due to a clinical exacerbation. In one study (Fabbri 1993) participants were withdrawn if they failed to improve following a course of oral prednisolone. In two studies (Hoekx 1996; Basran 1997) withdrawal criteria were simply 'worsening asthma' or withdrawal due to 'asthma related events' respectively, no further definition was given. Harrison 2001 did not define exacerbations.
Symptoms
Symptom frequency and rescue beta‐2 agonist use were often reported in these studies. However, different measures were used so only limited pooled treatment effects could be calculated.
Absolute values (proportion of symptom free days): MD 5.54% (95% CI ‐0.68 to 11.76), two studies, N = 571.
Absolute values (daytime symptoms): SMD: ‐0.10 (95% CI ‐0.34 to 0.13), two studies, N = 285.
Change from baseline (daytime symptoms): SMD ‐0.03 (95% CI ‐0.11 to 0.06), three studies, N = 534.
Change from baseline (nocturnal symptoms): SMD ‐0.03 (95% CI ‐0.15 to 0.09), three studies, N = 537.
Rescue beta‐2 agonist usage
Change from baseline (day use): ‐0.04 puffs/day (95% CI ‐0.12 to 0.04), two studies, N = 368.
Change from baseline (night use): ‐0.03 puffs/day (95% CI ‐0.13 to 0.08), two studies, N = 368.
Safety
Oropharyngeal side‐effects
Oral candidiasis: Peto OR 0.99 (95% CI 0.62 to 1.59), six studies, N = 1462.
Hoarseness: Peto OR 1.47 (95% CI 0.71 to 3.04), four studies, N = 748.
Sore throat/pharyngitis: Peto OR 1.12 (95% CI 0.77 to 1.63), six studies, N = 1407.
Cough: Peto OR 0.95 (95% CI 0.60 to 1.49), five studies, N = 1273.
Increased asthma symptoms: Peto OR 0.74 (95% CI 0.52 to 1.04), three studies, N = 933.
Safety ‐ other measures
Any adverse event: odds ratio 0.94; 95% confidence interval 0.75 to 1.18 Weight increase: Peto OR 7.01 (95% CI 0.97 to 50.49), two studies, N = 332.
Headache: Peto odds ratio 1.05 (95% CI 0.69 to 1.6), six studies, N = 1234.
HPA function
Five studies assessed aspects of HPA function. Data were not in a form suitable for inclusion in a meta‐analysis. No difference between FP and BDP/BUD treatment groups were apparent for morning plasma cortisol (Fabbri 1993; Hoekx 1996), plasma cortisol following a short synthetic ACTH stimulation test (Dahl 1993; Fabbri 1993) or 24 hour urinary free cortisol levels (Fabbri 1993). Boe 1994 and Aubier 2001 reported change in morning plasma cortisol and ACTH levels compared to baseline. A significant difference was observed (SMD ‐1.53 (95% CI: ‐2.99 to ‐0.07), although there was a high degree of heterogeneity between the two studies. We have presented only the pooled estimate derived from Random Effects modelling. Whilst there was a significant difference in favour of BDP treated participants was observed in terms of reduction in morning plasma cortisol levels in Boe 1994, there was no difference in ACTH levels was seen (FP ‐10.6 ng/L versus BDP 02.0 ng/L, P = 0.06).
Dose down‐titration design
A single study (Agertoft 1997a) met the inclusion criteria for this review. In this carefully conducted study, 219 children with moderately severe asthma first entered an evaluation period during which time their usual daily dose of BUD administered via MDI+Nebuhaler spacer was titrated to the point at which deterioration in control was considered to have occurred. Criteria for loss of control were specified a priori, and were based around daytime, night‐time and exercise‐related symptoms, rescue beta‐2 agonist use or clinical deterioration as judged by investigators. Participants requiring BUD at a daily dose of 400 or 800 mcg/d were then randomised to receive half the nominal daily baseline dose of either FP or BUD i.e. 200 or 400 mcg/d. FP was administered using a Diskhaler DPI, BUD using a Turbohaler DPI. During the first five weeks of the study participants received treatment at the dose to which they were randomised. Following this, dose reduction was attempted. Using pre‐defined criteria for acceptable control (based around symptoms, rescue beta‐2 agonist use, morning PEF and post exercise fall in FEV1/FEF 25‐75 compared to baseline) a 50% dose reduction was attempted at five weekly intervals. Doses were reduced until either control deteriorated, or a daily dose of 100 mcg was reached. The primary outcome measures were the mean number of dose reductions steps achievable, and the minimal effective dose of ICS. No significant difference was apparent between FP and BUD treatment groups either in terms of dose reduction steps (FP 1.65 versus BUD 1.59) or in terms of the minimal effective dose of ICS (FP 180 mcg/d versus BUD 188 mcg/d).
ORAL STEROID TREATED ASTHMATICS
(1) Oral steroid sparing design
A single parallel group study (Lundback 1997) assessed the relative oral steroid sparing effects of FP and BUD in oral steroid dependent asthma. This study has only been published in abstract form. 74 adult asthmatics requiring treatment with at least 5 mg/d of oral prednisolone and 800 mcg/d of inhaled steroid were randomised to treatment with either FP 1500 mcg/d via the Diskhaler DPI or BUD 1600 mcg/d via the Turbohaler DPI. Few methodological details were available, including whether or not a run‐in period to establish the minimal effective dose of prednisolone was employed, or whether pre‐defined criteria for oral steroid dose reduction were used. A significantly greater number of patients (37 out of 40) were able to discontinue oral prednisolone with FP compared to BUD treated participants (25 out of 34), although no difference between treatment groups was apparent in terms of the daily oral prednisolone dose.
(2) Non‐oral steroid sparing design
A single parallel group study (Ayres 1995) of fair methodological quality (Jadad score three) was conducted in adult asthmatic participants with moderate to severe asthma. Eleven per cent of patients were also receiving oral prednisolone (< 10 mg/d) at the time of enrolment. Participants were randomised to receive FP 1000 mcg/d, FP 2000 mcg/d or BUD 1600 mcg/d over a six week treatment period. They used a MDI delivery device, and were given the option of using a large volume spacer. No attempt was made to taper oral steroid use in participants treated with prednisolone.
A number of outcome measures were reported (Table 7). Both daily doses of FP led to significant improvements in a number of measures when compared to BUD. No significant differences between FP 1000 mcg/d and BUD 1600 mcg/d were apparent in terms of daytime and night‐time symptoms although FP 2000 mcg/d led to a significantly greater reduction in daytime and night‐time symptom scores and use of night‐time rescue beta‐2 agonist. No significant differences were seen between either daily dose of FP and BUD in terms of oropharyngeal side effects, although FP 2000 mcg/d led to a significantly greater reduction in morning plasma cortisol level compared to BUD 1600 mcg/d.
7. Non‐oral steroid sparing design: Comparison of FP and BUD.
| FP 1000 vs. BUD 1600 | FP 2000 vs. BUD 1600 | |
| Morning PEF (L/min) | 9 (95% CI 2 to 17) | 13 (95% CI 6 to 21) |
| Dirurnal variation in PEF (L/min) | 6 (95% CI 1 to 11) | 6 (95% CI 1 to 11) |
| FEV1 (litres) | 0.10 (95% CI 0.02 to 0.18) | 0.17 (95% CI 0.8 to 0.25) |
SENSITIVITY AND SUBGROUP ANALYSES
Sensitivity analyses based on study quality excluded two studies (Connolly 1995; Berend 2001). This did not lead to a significant difference in the size or direction of effect for any of the pooled estimates to which these studies contributed.
Subgroup analyses were undertaken for FEV1 outcomes reported in the parallel group studies in non‐oral steroid treated asthmatics that compared FP to BDP/BUD at a nominal daily dose ratio of 1:2 and 1:1. These were done with the purpose of : (a) identifying potential groups who appeared to show differences in response to treatment and (b) exploring sources of heterogeneity.
FEV1 measures were significantly better in FP treated participants compared to BDP/BUD treated participants. These studies included children and adults (treated with both BDP and BUD), participants with asthma of mild to moderate/severe disease, delivery devices that were the same in the two treatment groups (e.g. FP and BDP/BUD via MDI) or different between groups (e.g. FP via Diskhaler or Accuhaler and BUD via Turbohaler). No heterogeneity was found in the pooled treatment effect and subgroup analyses did not identify any groups that showed a clear difference in response to treatment. However there did appear to be a trend for higher dose range comparisons (FP 500 versus BDP/BUD 1200 mcg/d, FP 800 versus BDP/BUD 1600 mcg/d) to show a greater difference in favour of FP, when Ige 2002 was removed from the analysis. The studies that contributed to these subgroup analyses (Ringdal 1996; Steinmetz 1997) were also conducted in participants with moderate/moderately severe asthma. Although such factors may be important in determining response, this conclusion can only be speculative. No difference in the likelihood of withdrawal was seen when comparing FP to BDP/BUD. Subgroup analyses did not identify any potential groups of participants or studies defined by specific characteristics in which significant treatment differences were apparent.
Discussion
This review has assessed the relative efficacy and safety of fluticasone propionate when compared to beclomethasone and budesonide in the treatment of chronic asthma in moderately severe, symptomatic patients from 64 studies (13,634 participants). At a nominal dose ratio of 1:2 we have demonstrated that FP is at least as effective as BDP on clinical outcomes such as FEV1 and exacerbations, but could be more effective than BDP/BUD on morning peak flow. At a dose ratio of 1:1, we now have evidence that FP is marginally superior to BDP on certain measures of lung function such as am/pm PEF, FVC and FEV1. However, we noted that change from baseline outcomes for lung function and the effect of FP on exacerbations gave more equivocal findings.
Airway calibre
The largest group of participants assessed were those in which FP was compared to either BDP or BUD at a 1:2 nominal daily dose ratio, in non‐oral steroid treated asthmatics. In the studies of this type that used a parallel group design, FP resulted either in significantly higher measurements of airway calibre compared with BDP/BUD, or in results which strongly indicated that there was little in the way of clinically meaningful difference between dose ratios. Children and adults, with asthma ranging in severity from mild to severe, treated with both BDP and BUD were included. Generally, there was little heterogeneity present when the studies were pooled, suggesting that the observed treatment effects apply to all patients represented by the studies. Subgroup analyses suggested the possibility that effects in favour of FP may be greater in patients with more severe disease treated at higher dose comparisons. Some support for this hypothesis comes from the single, large parallel study that assessed the relative efficacy of FP versus BUD in adults with severe asthma, some of whom were oral steroid treated at the time of enrolment (Ayres 1995). In this study, FP 1000 mcg/d resulted in significantly greater improvements in FEV1 and morning PEF compared to BUD 1600 mcg/d. These results suggest that clinically relevant differences in effect are unlikely to be seen when FP and BDP/BUD are used at a 1:2 daily dose ratio. A logical extension of this relationship would be for even greater benefits favouring FP to be seen when given at equal nominal daily dose to BDP or BUD. However, no significant difference between the inhaled steroids were seen when data from trials comparing agents at a 1:1 dose ratio reporting change from baseline values for the same outcomes. The apparent paradox between the outcomes for the different dose ratio comparisons may be due to a number of factors. The greater number of trials and the greater statistical power of the pooled estimate of absolute score outcomes could explain the difference between these findings.
Few crossover studies assessed airways function in a way that could be analysed. No difference between FP and BDP/BUD was apparent, but few studies could be pooled. Although all studies were of high methodological quality, washout periods between treatments were not always used and the possibility of carryover effects that masked true treatment differences could not be excluded. In summary, the results of the parallel group study analysis are more reliable.
Symptoms and beta‐2 agonist use
It is difficult to draw conclusions concerning the relative efficacy of FP and BDP/BUD in reducing symptoms and rescue beta‐2 agonist use. Few numerical outcome data were available, different scales of measurement were used and the possibilities for pooling results across studies were limited. However, no individual studies (either of parallel group design or crossover design) demonstrated a significant difference between FP and BDP/BUD at a 1:2 dose ratio or at a 1:1 dose ratio. For the few instances in which data could be pooled where two studies reported symptoms measured using the same metric scale, no overall difference between treatment groups was seen.
Asthma exacerbations
A number of parallel group studies reported trial withdrawal due to an asthma exacerbation. Although varying criteria for withdrawal were used, the underlying nature of the outcome assessed (worsening asthma control) was the same. It was considered appropriate therefore to pool these studies. No difference between FP and BDP/BUD at a 1:2 nominal daily dose ratio was apparent and there was no heterogeneity, suggesting a consistent response across studies independent of the criteria used to define withdrawal. The likelihood of withdrawal due to asthma exacerbation cannot be considered equivalent to asthma exacerbations leading to emergency dept or GP attendance, but may be a reasonable surrogate for these.
In summary, data from trials that compared FP to BDP/BUD at a 1:2 nominal daily dose ratio suggest that FP and the older corticosteroids cannot be considered equivalent with respect to clinical efficacy. The greater improvements in FEV1 and PEF when administered at half the nominal daily dose of BDP/BUD suggest that the higher potency of FP demonstrated in laboratory studies is reflected in certain outcomes relevant to clinical practice. Against this, no studies found significant differences in terms of symptoms, rescue beta‐2 agonist use or asthma exacerbations. Although this cannot be taken to mean that FP at half daily dose is equivalent to BDP/BUD at full dose, it does suggest that clinically relevant differences in effect are unlikely to be seen when FP and BDP/BUD are used at a 1:2 daily dose ratio. Previous reviews have examined the dose response relationships for BDP, BUD and FP (Adams 1999, Adams 2000, Adams 2005). Although each ICS is effective in improving airway function compared to placebo, no dose response effect for daytime symptom scores, rescue beta‐2 agonist use or withdrawal due to lack of efficacy was apparent for any of these agents over wide dose ranges in non‐oral steroid treated asthmatics. In the light of this fact it should not be surprising that differences between FP and BDP/BUD were not seen in the studies included in this review, irrespective of dose ratios compared.
In a previous review, we have shown that FP exhibits a dose response effect for FEV1 and morning PEF (Adams 2005). Larger daily doses lead to greater improvements compared to lower doses. A logical expectation would be for even greater benefits favouring FP to be seen when given at equal nominal daily dose to BDP or BUD. This has not been clearly demonstrated in the current review. However, fewer studies made such a comparison and an overall pooled analysis across studies was not possible. Less weight should therefore be given to these findings, and they do not undermine the more powerful analysis that was possible for the studies that compared FP to BDP/BUD at a 1:2 dose ratio. The findings for the parallel group studies that compared FP to BDP/BUD at the 1:2 dose ratio also appear to be inconsistent with the single dose reduction study (Agertoft 1997a). In that study, acceptable asthma control was maintained with mean daily doses of FP and BUD that were not significantly different. Given the results from the FP versus BDP/BUD 1:2 ratio analysis, one would expect to see lower doses of FP necessary to maintain control. A possible explanation for the discrepancy is that in Agertoft 1997a a minimum daily dose threshold of 100 mcg was set at which no further dose reductions were attempted. Although the investigators speculate that deterioration in control was likely if doses had been reduced below this threshold, this was not directly tested, so it is not possible to exclude the possibility that further dose reduction steps could have been achieved. This could have masked a difference in the dose of FP and BUD required to achieve equivalent asthma control.
Health Status
Three studies (Pauwels 1998; Berend 2001; Molimard 2005) reported health status measured using an asthma‐specific instrument. In two of these studies of different designs, significantly better scores were seen for patients receiving FP compared to BDP. Further studies are required to confirm the effects observed in these studies, and would also help to establish the consistency of these effects in milder asthma, where the impact of disease on daily living may not be as profound.
Oral steroid‐sparing effect
Little trial evidence exists regarding the relative oral steroid‐sparing efficacy of FP and BDP or BUD. Only one included study (Lundback 1997) addressed this question. This study remains unpublished and data have been made available regarding this trial by GSK. Important design characteristics of such trials should include (a) a prolonged run‐in period to allow patients to have their prednisolone dose weaned to the minimum required to maintain asthma control prior to randomisation;(b) prednisolone dose reduction should be undertaken according to pre‐defined criteria based around acceptable asthma control. Only in this manner can unbiased estimations be made of the relative efficacy of different interventions. Unpublished information revealed that oral steroid dose was in fact increased to achieve maximum control and stability at baseline. Therefore, although Lundback 1997found that FP 1500 mcg/d allowed significantly more patients to discontinue oral prednisolone when compared to BUD 1600 mcg/d. Interpretation of these results therefore requires caution, as they could reflect the fact that participants received a higher than necessary dose of oral steroids.
Adrenal Function
Accurate assessment of the relative effects of FP versus BDP/BUD on markers of adrenal function were difficult to make using the data available for this review. When comparisons of FP to BDP/BUD at a nominal daily dose ratio of 1:2 were made, a meta‐analysis of the few parallel group studies (Berend 2001; Rao 1999) and crossover studies (Bootsma 1995; Pauwels 1998; Malo 1999) did not find a significant differences between treatment groups. The few individual studies that assessed this outcome when FP and BDP/BUD were compared at equal nominal doses did not find any difference. It was not possible to calculate a pooled treatment effect across studies because numerical data were reported incompletely. This also meant that assessment of the relative influence of FP versus BDP/BUD across the a range inhaled corticosteroid doses (e.g. 100 FP versus 200 BDP/BUD, 500 FP versus 1000 BDP/BUD, 1000 FP versus 2000 BDP/BUD) could not be done. Single dose and short term (< one week) studies have demonstrated that FP results in a significantly greater suppression of plasma cortisol than BUD when compared at equal nominal daily dose over a range of 400 to 2000 mcg/d (Clark 1996a, Clark 1996b, Wilson 1998). A recent meta‐regression analysis (Lipworth 1999) that included these studies also concluded that FP is significantly more potent that BUD in suppressing morning plasma cortisol, in other words that FP leads to greater suppression of levels than BUD when given at equal daily dose. This effect was more pronounced at higher nominal daily doses. The findings of this review are consistent with that analysis. The absence of differences between FP and BDP/BUD when compared at a dose ratio of 1:2 are consistent with a higher potency of FP in terms of cortisol suppression. The absence of significant differences between FP and BDP/BUD in the individual studies that assessed the drugs at a 1:1 nominal daily dose ratio may be explained by issues related to the timing of cortisol measurements. In all of the longer term studies included in this review, cortisol measurements were made within a two hour time window between 8 am and 10.00 am. In the short term studies that were not eligible for inclusion, cortisol measurements were taken at a strictly standardised time of either 7.30 or 8.00 am. Estimations over a time frame of 2 hours have been shown to be substantially less sensitive in detecting change compared to those taken at closely controlled times, with up to three fold differences in levels occurring between 8.00 am and 10.00 am in healthy volunteers (Lonnebo 1996). The greater variability introduced into estimations made over a two hour time frame may have masked any true differences between FP and BDP/BUD in terms of their effects on morning cortisol levels. Recent concern about the link between high dose FP and adrenal suppression in children has been raised, with data suggesting that adrenal suppression can occur in children prescribed more than 400 mcg/day of the drug (Paton 2006). Twenty‐four hour urinary cortisol, a more sensitive assay of basal adrenocortical activity than morning plasma cortisol, was not reported frequently. This measurement should not be subject to the same difficulties in interpretation as plasma cortisol. The majority of studies (both parallel group and crossover), that assessed FP versus BDP/BUD at either a 1:1 or 1:2 nominal daily dose ratio did not find significant differences between FP and the other two drugs. A recent meta‐regression analysis of a different group of short term studies (Lipworth 1999) suggested that FP is more potent than BDP/BUD in suppressing 24 hour urinary cortisol levels. The findings of the individual studies included here are not consistent with this. This difference may be a real phenomenon, however, these relatively small trials may not have had sufficient power to detect true differences. Since we were unable to calculate an overall pooled treatment effect due to limitations in the presentation of the primary trial results, this remains uncertain.
In summary, this review does not permit firm conclusions to be drawn with regard to the relative effect of FP versus BDP/BUD on morning plasma cortisol or 24 hour urinary cortisol levels in randomised trials with treatment periods of one week or longer.
Oropharyngeal side‐effects
When compared to BDP/BUD at a nominal daily dose ratio of 1:1, FP results in a significantly greater likelihood of hoarseness, although no difference was apparent when FP and BDP/BUD were compared at a dose ratio of 1:2. This suggests that the greater potency of FP, reflected in a number of measures of efficacy, also leads to greater likelihood of this pharmacologically predictable side effect when FP is given at an equal nominal dose compared to either BDP or BUD.
No difference between FP and BDP/BUD in terms of the likelihood of oral candidiasis was seen when given at either a 1:2 or a 1:1 nominal daily dose ratio, suggesting a greater margin of safety exists when considering this outcome.
FP was significantly more likely to result in sore throat when compared to BDP/BUD given in a nominal dose ratio of 1:2, but there was no difference between FP and BDP/BUD when given at a dose ratio of 1:1. This discrepancy is difficult to account for. A larger number of studies assessed the 1:2 dose ratio comparison and a more powerful meta‐analysis was possible. Whilst differences in analytical power may account for this discrepancy, this suggestion can only be speculative, especially since there is significant heterogeneity between the 1:2 dose ratio studies. Overall the results provide a sufficient level of evidence to suggest that FP is more likely to lead to sore throat when used in clinical practice when compared to BDP/BUD.
Methodological considerations
A number of included studies have been presented at respiratory society meetings, and published in abstract form only. At the time of writing, these had not been published as full journal papers, and include the following: Pickering 1996; Bisca 1997; Dal Negro 1997; Joubert 1998; Murray 1998; Hughes 1999a; Kemmerich 1999; Melaranci 1999. Limited details were available concerning the characteristics of the participants enrolled and trial methodology. Clinically relevant outcomes were reported, but numerical data were rarely presented. Grading of these studies using the Jadad scoring system has generally resulted in low scores (1 or 2). However, this is likely to be an underestimation of their true quality and is probably a reflection of the limited information presented in meeting abstracts. Previous versions of this review did not incorporate findings from certain studies which may have biased summary estimates. Table 6 indicates that some data from studies missing from the original weighted mean differences in spite of requests. GIV has led to greater flexibility in the analysis of data from published and unpublished sources, and we have been able to incorporate some of the data previously missing from the analysis. Several of these calculations pertain to non‐significant data which were under‐reported in published papers. Whilst our analyses go some way to addressing publication bias, they do not replace analyses of raw data. The outcomes where data have been imputed or estimated do not suggest that there was a significant impact on the original summary estimates.
A comprehensive search for relevant studies was undertaken. It is possible that additional studies were missed, or were not retrieved because they have not been published in any of the sources examined. Extensive searching of the GSK trials register (www.ctr.gsk.co.uk), who market Flixotide and have sponsored many of the studies included, identified considerable amounts of unpublished data.
Summary
The results of this review suggest that FP, when used at half the nominal daily dose of BDP or BUD leads to greater improvements in FEV1, morning PEF and evening PEF in non‐oral steroid treated asthmatics. This appears to be the case for both children and adults, with disease ranging in severity from mild to severe, irrespective of the delivery devices used for each drug. These differences are likely to be a reflection of the higher potency exhibited by FP in laboratory assays of anti‐inflammatory activity. There appears to be little if any difference between FP and BDP/BUD with regard to their effects on symptoms, rescue beta‐2 agonist requirement or the likelihood of exacerbations, when given at any nominal daily dose greater than FP 200 mcg/d and BDP/BUD 400 mcg/d. This conclusion needs to be viewed with some caution, since a pooled treatment effect across studies was not easily calculated. However, it is consistent with the lack of dose response seen for these outcomes demonstrated in other reviews of inhaled corticosteroids (Adams 1999; Adams 2000; Adams 2005). Too few studies have reported functional health status to draw clear conclusions regarding the relative efficacy of FP and BDP/BUD. However, two fair quality studies have reported the results of a validated, disease‐specific instrument that did find improvements in favour of FP when compared to BDP at a 1:2 nominal daily dose ratio. Further studies are needed to confirm the findings of a single study that FP at 1000 mcg/d is more effective than BUD in allowing a larger proportion of oral steroid dependent patients to stop treatment without deterioration in asthma control. The clinical significance of the small improvements that can be expected in FEV1 and morning PEF when patients are treated with FP is hard to assess. If this were accompanied by a lower risk of systemic side effects, in terms of interference with HPA function, there would be a case for recommending FP as a first line anti‐inflammatory corticosteroid in non‐oral steroid asthmatics. However, the trial evidence concerning the relative effects of FP versus BDP/BUD at any nominal daily dose comparison on morning plasma cortisol levels or 24 hour urinary cortisol excretion is difficult to interpret. An overall therapeutic ratio (i.e. advantageous efficacy effects versus systemic side effects) cannot be constructed for each ICS. A clear recommendation for FP over the older agents cannot be made on the basis of every outcome from this review, but in the most severe patients FP may confer an advantage over BDP or BUD. This, however, would need to be judged on an individual patient basis.
Authors' conclusions
Implications for practice.
Current asthma management guidelines (GINA 2002; BTS 2003; NHLBI 1997) recommend inhaled corticosteroids for all asthmatics except those with mild, intermittent symptoms. Prior assumptions that FP given at equal nominal daily dose to BDP and BUD results in greater clinical efficacy now seem justified by the assembled evidence. The results of this review lend support to the recommendation that when initiating the use of an inhaled steroid, FP can be given at half the daily dose compared to either BDP or BUD without clinically significant differences in asthma control being encountered, and may even lead to small additional improvements in certain measures, including FEV1 and diary card PEF. However, it is not possible to assess whether this is associated with a reduced risk of HPA function disturbance. The additional recommendation that individual patients have dose titrated to the minimum required to maintain control and thereby minimise any potential (unknown) risk of long‐term systemic side effects seems sensible.
Implications for research.
The development of a standardised, validated scoring system for the assessment of symptoms is needed in order that better comparisons across studies can be made.
Assessment of health status, using disease‐specific instruments, may be a more sensitive tool for detecting variations in efficacy, when comparing inhaled steroids. This has rarely been reported, and future studies need to include this measure.
Hospital and GP attendance rates, due to asthma exacerbation have not been reported. Future studies comparing inhaled steroids need to consider such outcomes, which will be important in health economic evaluations.
A complete picture of the relative value of different inhaled steroids can only be gained if efficacy/systemic side effect ratios can be constructed for each agent. Disturbance of HPA function is one aspect of systemic activity. Complete reporting of data for overnight and 24‐hour urinary cortisol measures, and more precise measurements of morning cortisol levels in primary studies would improve the accuracy of future updates of this review, and allow a better understanding of these issues.
The advent of CFC‐free propellants in inhaler devices may enhance the potency of the steroids they deliver, but this effect may be restricted to those preparations which were previously less potent than FP. Given the message of this review with regards to relative efficacy, establishing equivalence for the new generation propellants is a key research priority for future studies. We recommend that studies should be conducted which assess similar dose ratios to those examined in this review, in order to determine to what extent HFA‐BDP and FP may be deemed equally effective.
What's new
| Date | Event | Description |
|---|---|---|
| 9 September 2009 | Amended | Minor technical amendment (sequence of outcomes re‐ordered) |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 25 July 2008 | Amended | Converted to new review format. |
| 3 August 2007 | New citation required and conclusions have changed | New studies 17 randomised comparisons from 16 studies: Acun 2005; Egan 1999; Wolfe 2000 BD; Wolfe 2000 ICS; FLUTI/AH89/J78; FLIT37; FLTB3013; FLIP01; FLIP01a; FLPB0145; Geppe 2004; Parakh 2004; Prasad 2004; Ställberg 2007; Subbarao 2005; SD‐004‐0377; Vednanthan 2004. Of these seven studies were unpublished, two were unpublished comparisons from studies published and reported elsewhere, and the remainder were published studies. Additional data Unpublished data for studies previously included in this review: Dahl 1993; Langdon 1994; Lorentzen 1996; Lundback 1993; Gustafsson 1993; Fabbri 1993; Barnes 1993; Hoekx 1996; LeBlanc 1994; Yialloros 1997; Wolthers 1993; Ferguson 1999; Ferguson 2006. One study had been previously excluded from the review due to the absence of outcome data pertaining to relevant outcome. Unpublished data for this study indicated that relevant outcomes had been recorded and this study is now included in the review (Egan 1999). Impact on findings Dose ratio 1:2 Effects in FEV1 expressed as both change and end of treatment values indicate that FP is marginally superior to BDP or BUD at half the dose. Dose ratio 1:1 The inclusion of unpublished data on hoarseness has now rendered this outcome not significantly more likely in FP or BDP/BUD treated participants. Previous text in What's new section archived in Table 07 |
Acknowledgements
We would like to thank the support staff of the Cochrane Airways Group who have provided exceptional assistance in helping us to publish and revise this review, namely: Karen Blackhall, Liz Arnold, Bettina Reuben, Phillippa Mills, Sarah Tracey, Veronica Stewart, Anna Bara and Jane Dennis for assistance in the electronic search and retrieval of papers, and Steve Milan for statistical support. We are indebted to Karen Richardson and Julia Earnshaw who coordinated efforts of behalf of GSK to search for additional studies, and provided valuable information regarding a number of included trials that were sponsored by the company. We would like to thank Dr G.P. Bootsma, Dr R.K. Gregson, Dr Olusoji Ige, Dr Tonya King, Dr Violaine Giraud and Dr Piotr Kuna who were kind enough to provide additional information concerning their trials. We acknowledge the efforts of Dr Janine Bestall to prior versions of this review.
Appendices
Appendix 1. Archive of previous methods used to assess study quality
We (NPA, JB and TJL) independently assessed the methodological quality of the included studies. We were blinded to names of trialists, institution and funding sources. We scored the studies using two measures. Firstly according to the Cochrane approach:
Grade A: adequate allocation concealment Grade B: unclear allocation concealment Grade C: clearly inadequate concealment
Secondly, using a five point scoring instrument developed by Jadad 1996:
a) Was the study described as randomised? (yes = 1 no = 0) b) Was the study described as double blind? (yes = 1 no = 0) c) Was there a description of withdrawals and dropouts? (yes = 1 no = 0) d) Was the method of randomisation well described and appropriate? (yes = 1 no = 0) e) Was the method of double blinding well described and appropriate? (yes = 1 no = 0) Deduct 1 point if method of randomisation or blinding inappropriate
We measured inter‐rater agreement using the kappa statistic and resolved disagreement by consensus.
Appendix 2. GSK randomisation procedures
The procedures for randomising GSK sponsored studies has been detailed in correspondence between Richard Follows and TL, the details of which are given below:
The randomisation software is a computer‐generated, centralised programme (RandAll). After verification that the randomisation sequence is suitable for the study design (crossover, block or stratification), Clinical Supplies then package the treatments according to the randomisation list generated. Concealment of allocation is maintained by a third party, since the sites phone in and are allocated treatments on that basis. Alternatively a third party may dispense the drug at the sites. Unblinding of data for interim analyses can only be done through RandAll, and are restricted so that only those reviewing the data are unblinded to treatment group allocation.
Data and analyses
Comparison 1. FP versus BDP or BUD, parallel group studies: dose ratio 1:2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 5 | 1053 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐3.11, 2.05] |
| 2 FEV1 (% predicted) | 7 | 1444 | % (Fixed, 95% CI) | 0.42 [‐1.02, 1.86] |
| 3 Change in FEV1 (% predicted) | 4 | 537 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐2.69, 1.45] |
| 4 Change in FEV1 (% predicted) | 6 | 1306 | % (Fixed, 95% CI) | ‐0.39 [‐1.65, 0.86] |
| 5 FEV1 (litres) | 12 | 2418 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.04, 0.16] |
| 6 FEV1 (litres) | 15 | 2899 | Litres (Fixed, 95% CI) | 0.05 [0.01, 0.09] |
| 7 FEV1 (litres) ‐ (imputed/estimated values) | 17 | 3640 | Litres (Fixed, 95% CI) | 0.04 [0.00, 0.07] |
| 8 Change in FEV1 (litres) compared to baseline | 9 | 1661 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [0.00, 0.09] |
| 9 Change in FEV1 (litres) compared to baseline (imputed/estimated values) | 12 | 2635 | Litres (Fixed, 95% CI) | 0.01 [‐0.02, 0.04] |
| 10 Morning PEF (L/min) | 10 | 2643 | Mean Difference (IV, Fixed, 95% CI) | 9.03 [1.47, 16.60] |
| 11 Morning PEF (L/min) | 12 | 2955 | L/min (Fixed, 95% CI) | 5.89 [0.96, 10.82] |
| 12 Morning PEF (% predicted) | 4 | 988 | % (Fixed, 95% CI) | 0.66 [‐0.36, 1.67] |
| 13 Change in morning PEF (L/min) | 12 | 2506 | Mean Difference (IV, Fixed, 95% CI) | 7.57 [4.57, 10.58] |
| 14 Change in morning PEF (with imputed/estimated values) | 17 | 4179 | L/min (Fixed, 95% CI) | 7.42 [4.97, 9.87] |
| 15 Change in morning PEF % predicted | 2 | 765 | % (Fixed, 95% CI) | 1.28 [0.14, 2.43] |
| 16 Evening PEFR (L/min) | 10 | 2656 | L/min (Fixed, 95% CI) | 1.53 [‐3.78, 6.84] |
| 17 Evening PEFR (L/min) | 8 | 2257 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [‐2.34, 14.73] |
| 18 Evening PEF % predicted | 4 | 988 | % (Fixed, 95% CI) | ‐0.06 [‐1.25, 1.13] |
| 19 Change in evening PEFR (L/min) (imputed values) | 10 | 2186 | L/min (Fixed, 95% CI) | 2.59 [‐0.36, 5.55] |
| 20 Change in evening PEFR (L/min) (imputed values) | 8 | 1491 | Mean Difference (IV, Fixed, 95% CI) | 0.85 [‐2.92, 4.63] |
| 21 Change in evening PEFR (L/min) | 7 | 1158 | Mean Difference (IV, Fixed, 95% CI) | 2.03 [‐2.28, 6.34] |
| 22 Change in evening PEF % predicted | 2 | 765 | % (Fixed, 95% CI) | ‐0.71 [‐2.16, 0.75] |
| 23 Percentage of symptom free nights | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 24 Clinic PEFR (L/min) | 8 | 1701 | Mean Difference (IV, Fixed, 95% CI) | 19.68 [9.18, 30.18] |
| 25 Clinic PEFR (L/min) | 12 | 2545 | L/min (Random, 95% CI) | 17.04 [‐0.99, 35.07] |
| 26 Change in Clinic PEF | 3 | 469 | Mean Difference (IV, Fixed, 95% CI) | 6.36 [‐4.73, 17.44] |
| 27 Change in Clinic PEF | 6 | 1317 | L/min (Fixed, 95% CI) | 4.16 [‐2.11, 10.44] |
| 28 Clinic PEF % predicted | 3 | 630 | % (Fixed, 95% CI) | 2.00 [‐0.18, 4.18] |
| 29 Daily PEFR (% predicted) | 2 | 774 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐1.15, 3.95] |
| 30 Change in clinic PEF % predicted | 3 | 998 | % (Fixed, 95% CI) | 0.31 [‐1.30, 1.92] |
| 31 Change in clinic PEF % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 32 FVC (litres) | 9 | 1854 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 33 Change in FVC (litres) compared to baseline | 5 | 1129 | Litres (Fixed, 95% CI) | 0.00 [‐0.05, 0.06] |
| 34 Change in FVC (litres) compared to baseline | 3 | 538 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.02, 0.18] |
| 35 FVC % predicted | 2 | 391 | % (Fixed, 95% CI) | 1.28 [‐0.84, 3.40] |
| 36 Change in FVC % predicted | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 37 Change in FEF 25‐75 (L/second) compared to baseline | 2 | 399 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.03, 0.29] |
| 38 PC 20 (doubling doses) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 39 Change in Asthma Quality of Life Questionnaire | 1 | AQLQ (Fixed, 95% CI) | Totals not selected | |
| 40 Change in Asthma Control Questionnaire | 1 | ACQ (Fixed, 95% CI) | Totals not selected | |
| 41 Symptom score am | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 42 Symptom score pm | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 43 Symptom score am (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 44 Symptom score pm (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 45 Percentage of symptom free days | 3 | 952 | Mean Difference (IV, Fixed, 95% CI) | 4.90 [‐0.74, 10.54] |
| 46 Change in percentage of symptom free days | 5 | 1251 | Mean Difference (IV, Fixed, 95% CI) | 6.43 [0.47, 12.39] |
| 47 Change in daily asthma symptom score compared to baseline | 6 | 1035 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.31, ‐0.07] |
| 48 Change in percentage of symptom free nights | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 49 Rescue medication usage (puffs/day) | 2 | 134 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.50, 0.47] |
| 50 Change from baseline in percentage of rescue medication free days | 3 | 539 | Mean Difference (IV, Fixed, 95% CI) | 6.89 [0.32, 13.46] |
| 51 Change in daily use of rescue beta2 agonist (puffs/day) compared to baseline | 4 | 763 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.63, ‐0.07] |
| 52 Day use of ß‐agonist (change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 53 Night use of ß‐agonists (change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 54 Change in number of awakenings/night | 2 | 282 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 55 No change or reduction in daytime rescue beta2 agonist use (% of patients) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 56 One or more exacerbations (No. of patients) | 4 | 1213 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.53, 1.03] |
| 57 Withdrawal due to asthma exacerbation (No. of patients) | 11 | 2824 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.54, 1.10] |
| 58 Oropharyngeal candidiasis (No. of patients) | 14 | 3331 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.69, 1.87] |
| 59 Sore throat/pharyngitis (No. of patients) | 15 | 3488 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.45 [1.10, 1.92] |
| 60 Dysphonia | 2 | 588 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.74, 2.28] |
| 61 Hoarseness (No. of patients) | 10 | 2321 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.47, 1.67] |
| 62 Cough | 10 | 2214 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.14 [0.81, 1.61] |
| 63 Rhinitis | 9 | 1903 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.76, 1.77] |
| 64 Headache | 12 | 2750 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.75, 1.36] |
| 65 Morning plasma cortisol (nmol/L) | 3 | 145 | Mean Difference (IV, Fixed, 95% CI) | 11.73 [‐38.37, 61.83] |
| 66 URI symptoms | 4 | 873 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.52, 1.56] |
| 67 Any adverse event | 13 | 3241 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.92, 1.24] |
| 68 Withdrawals due to lack of efficacy | 11 | 2747 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.33, 1.07] |
| 69 Withdrawal due to adverse events | 13 | 3233 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.69, 1.47] |
| 70 FEV1 (litres) ‐ (sensitivity analysis) | 17 | Litres (Fixed, 95% CI) | Subtotals only | |
| 70.1 Blinded studies | 11 | 2712 | Litres (Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| 70.2 Single‐blind/open label studies | 6 | 928 | Litres (Fixed, 95% CI) | 0.17 [0.06, 0.27] |
1.1. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 1 FEV1 (% predicted).
1.2. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 2 FEV1 (% predicted).
1.3. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 3 Change in FEV1 (% predicted).
1.4. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 4 Change in FEV1 (% predicted).
1.5. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 5 FEV1 (litres).
1.6. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 6 FEV1 (litres).
1.7. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 7 FEV1 (litres) ‐ (imputed/estimated values).
1.8. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 8 Change in FEV1 (litres) compared to baseline.
1.9. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 9 Change in FEV1 (litres) compared to baseline (imputed/estimated values).
1.10. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 10 Morning PEF (L/min).
1.11. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 11 Morning PEF (L/min).
1.12. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 12 Morning PEF (% predicted).
1.13. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 13 Change in morning PEF (L/min).
1.14. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 14 Change in morning PEF (with imputed/estimated values).
1.15. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 15 Change in morning PEF % predicted.
1.16. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 16 Evening PEFR (L/min).
1.17. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 17 Evening PEFR (L/min).
1.18. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 18 Evening PEF % predicted.
1.19. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 19 Change in evening PEFR (L/min) (imputed values).
1.20. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 20 Change in evening PEFR (L/min) (imputed values).
1.21. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 21 Change in evening PEFR (L/min).
1.22. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 22 Change in evening PEF % predicted.
1.23. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 23 Percentage of symptom free nights.
1.24. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 24 Clinic PEFR (L/min).
1.25. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 25 Clinic PEFR (L/min).
1.26. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 26 Change in Clinic PEF.
1.27. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 27 Change in Clinic PEF.
1.28. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 28 Clinic PEF % predicted.
1.29. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 29 Daily PEFR (% predicted).
1.30. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 30 Change in clinic PEF % predicted.
1.31. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 31 Change in clinic PEF % predicted.
1.32. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 32 FVC (litres).
1.33. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 33 Change in FVC (litres) compared to baseline.
1.34. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 34 Change in FVC (litres) compared to baseline.
1.35. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 35 FVC % predicted.
1.36. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 36 Change in FVC % predicted.
1.37. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 37 Change in FEF 25‐75 (L/second) compared to baseline.
1.38. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 38 PC 20 (doubling doses).
1.39. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 39 Change in Asthma Quality of Life Questionnaire.
1.40. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 40 Change in Asthma Control Questionnaire.
1.41. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 41 Symptom score am.
1.42. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 42 Symptom score pm.
1.43. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 43 Symptom score am (change from baseline).
1.44. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 44 Symptom score pm (change from baseline).
1.45. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 45 Percentage of symptom free days.
1.46. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 46 Change in percentage of symptom free days.
1.47. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 47 Change in daily asthma symptom score compared to baseline.
1.48. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 48 Change in percentage of symptom free nights.
1.49. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 49 Rescue medication usage (puffs/day).
1.50. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 50 Change from baseline in percentage of rescue medication free days.
1.51. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 51 Change in daily use of rescue beta2 agonist (puffs/day) compared to baseline.
1.52. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 52 Day use of ß‐agonist (change from baseline).
1.53. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 53 Night use of ß‐agonists (change from baseline).
1.54. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 54 Change in number of awakenings/night.
1.55. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 55 No change or reduction in daytime rescue beta2 agonist use (% of patients).
1.56. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 56 One or more exacerbations (No. of patients).
1.57. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 57 Withdrawal due to asthma exacerbation (No. of patients).
1.58. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 58 Oropharyngeal candidiasis (No. of patients).
1.59. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 59 Sore throat/pharyngitis (No. of patients).
1.60. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 60 Dysphonia.
1.61. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 61 Hoarseness (No. of patients).
1.62. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 62 Cough.
1.63. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 63 Rhinitis.
1.64. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 64 Headache.
1.65. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 65 Morning plasma cortisol (nmol/L).
1.66. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 66 URI symptoms.
1.67. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 67 Any adverse event.
1.68. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 68 Withdrawals due to lack of efficacy.
1.69. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 69 Withdrawal due to adverse events.
1.70. Analysis.

Comparison 1 FP versus BDP or BUD, parallel group studies: dose ratio 1:2, Outcome 70 FEV1 (litres) ‐ (sensitivity analysis).
Comparison 2. FP versus BDP or BUD, parallel studies: dose ratio 1:1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 5 | 968 | % (Fixed, 95% CI) | 1.52 [‐0.02, 3.07] |
| 2 FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 FEV1 (litres) | 6 | 1084 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [0.00, 0.14] |
| 4 FEV1 (litres ‐ imputed estimates) | 11 | 2154 | Litres (Fixed, 95% CI) | 0.04 [0.01, 0.07] |
| 5 Change in FEV1 (litres) | 5 | 1019 | Litres (Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 6 Change in FEV1 % predicted | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 FVC | 6 | 1315 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 8 FVC (litres) | 3 | 652 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.01, 0.16] |
| 9 Change in FVC (litres) | 2 | 308 | Litres (Fixed, 95% CI) | ‐0.02 [‐0.12, 0.07] |
| 10 Change in FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 FVC % predicted | 3 | 589 | % (Fixed, 95% CI) | 1.90 [‐0.26, 4.05] |
| 12 Morning PEFR (L/min) (imputed/estimated values) | 12 | 2389 | L/min (Fixed, 95% CI) | 8.21 [5.10, 11.31] |
| 13 Evening PEFR (L/min) (imputed/estimated values) | 11 | 2332 | L/min (Fixed, 95% CI) | 7.17 [4.26, 10.08] |
| 14 Morning PEFR (L/min) | 6 | 1011 | Mean Difference (IV, Fixed, 95% CI) | 8.62 [2.60, 14.65] |
| 15 Morning PEF (% predicted) | 3 | 747 | % (Fixed, 95% CI) | 2.09 [0.91, 3.28] |
| 16 Change in morning PEFR compared to baseline (L/min) | 7 | 1330 | Litres (Fixed, 95% CI) | 6.13 [1.49, 10.77] |
| 17 Change in morning PEFR compared to baseline (L/min) | 5 | 901 | Mean Difference (IV, Fixed, 95% CI) | 4.04 [‐3.64, 11.72] |
| 18 Evening PEFR (L/min) | 5 | 966 | Mean Difference (IV, Fixed, 95% CI) | 8.76 [2.90, 14.62] |
| 19 Evening PEF % predicted | 3 | 745 | % (Fixed, 95% CI) | 1.13 [0.02, 2.23] |
| 20 Change in evening PEF compared with baseline | 5 | 860 | L/min (Fixed, 95% CI) | 6.77 [1.69, 11.85] |
| 21 Clinic PEFR (L/min) | 5 | 1237 | Mean Difference (IV, Fixed, 95% CI) | 9.80 [3.62, 15.99] |
| 22 Clinic PEFR (L/min) | 9 | 2054 | L/min (Fixed, 95% CI) | 8.88 [4.40, 13.35] |
| 23 Clinic PEF % predicted | 4 | 817 | % (Fixed, 95% CI) | 2.08 [0.22, 3.94] |
| 24 Requirement for medication in addition to beta‐agonist | 4 | 1146 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.53, 0.99] |
| 25 Withdrawal due to asthma exacerbation (No. of patients) | 5 | 978 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.38, 1.35] |
| 26 One or more exacerbations (No. of patients) | 3 | 1054 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.73, 1.33] |
| 27 Number of participants experiencing symptom‐free days | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 28 Number of participants experiencing symptom‐free nights | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 29 Percentage of symptom free days | 2 | 571 | Mean Difference (IV, Fixed, 95% CI) | 5.54 [‐0.68, 11.76] |
| 30 Change in % symptom free days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 31 Percentage of symptom free nights | 1 | % (Fixed, 95% CI) | Totals not selected | |
| 32 Change in % symptom free nights | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 33 Change in total symptom scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 34 Daytime asthma symptom score | 2 | 285 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.34, 0.13] |
| 35 Night‐time asthma symptom score | 2 | 289 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.04, 0.43] |
| 36 Change in daytime symptoms | 2 | 363 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.10, 0.08] |
| 37 Change in daytime symptoms | 3 | 534 | symptoms (Fixed, 95% CI) | ‐0.03 [‐0.11, 0.06] |
| 38 Change in nocturnal symptoms | 2 | 366 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.18, 0.23] |
| 39 Change in nocturnal symptoms | 3 | 537 | symptoms (Fixed, 95% CI) | ‐0.03 [‐0.15, 0.09] |
| 40 Rescue medication usage (puffs/day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 41 Change in rescue medication usage (puffs/d) | 2 | 319 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.03, ‐0.35] |
| 42 Change in rescue medication usage (daytime) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 43 Change in rescue medication usage (daytime) | 2 | 368 | Puffs/day (Fixed, 95% CI) | ‐0.04 [‐0.12, 0.04] |
| 44 Change in rescue medication usage (nighttime) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 45 Change in rescue medication usage (nighttime) | 2 | 368 | Puffs/day (Fixed, 95% CI) | ‐0.03 [‐0.13, 0.08] |
| 46 Change in % rescue free days | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 47 Daytime rescue beta2 agonist use (puffs/daytime) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 48 Night‐time rescue beta2 agonist use (puffs/night) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 49 Number of participants experiencing rescue beta2 agonist free days | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 50 Hoarseness (No. of patients) | 5 | 1016 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.71, 3.04] |
| 51 Oropharyngeal Candidiasis (No. of patients) | 5 | 1016 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.11 [0.65, 1.91] |
| 52 Sore throat/pharyngitis (No. of patients) | 6 | 1407 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.77, 1.63] |
| 53 Cough | 6 | 1443 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.62, 1.47] |
| 54 Headache | 6 | 1234 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.69, 1.60] |
| 55 Increased asthma symptoms | 3 | 933 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.52, 1.04] |
| 56 Dizziness | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 57 Mouth ulceration | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 58 Change in morning plasma cortisol (nmol/L) compared to baseline | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 59 Pruritis | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 60 Stomatitis | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 61 Weight increase | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 62 Tonsillitis | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 63 Unpleasant taste | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 64 AUC serum cortisol nmol 1 1ST ARM | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 65 Any adverse event | 8 | 1507 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.68, 1.07] |
| 66 Withdrawals due to lack of efficacy | 6 | 1395 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.39] |
| 67 Withdrawal due to adverse events | 7 | 1711 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.56, 1.38] |
| 68 FEV1 (sensitivity analysis) | 11 | Litres (Fixed, 95% CI) | Subtotals only | |
| 68.1 Double‐blind studies | 10 | 1960 | Litres (Fixed, 95% CI) | 0.04 [0.01, 0.07] |
| 68.2 Single‐blind/open label studies | 1 | 194 | Litres (Fixed, 95% CI) | ‐0.16 [‐0.39, 0.07] |
2.1. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 1 FEV1 (% predicted).
2.2. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 2 FEV1 (% predicted).
2.3. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 3 FEV1 (litres).
2.4. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 4 FEV1 (litres ‐ imputed estimates).
2.5. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 5 Change in FEV1 (litres).
2.6. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 6 Change in FEV1 % predicted.
2.7. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 7 FVC.
2.8. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 8 FVC (litres).
2.9. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 9 Change in FVC (litres).
2.10. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 10 Change in FVC (L).
2.11. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 11 FVC % predicted.
2.12. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 12 Morning PEFR (L/min) (imputed/estimated values).
2.13. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 13 Evening PEFR (L/min) (imputed/estimated values).
2.14. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 14 Morning PEFR (L/min).
2.15. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 15 Morning PEF (% predicted).
2.16. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 16 Change in morning PEFR compared to baseline (L/min).
2.17. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 17 Change in morning PEFR compared to baseline (L/min).
2.18. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 18 Evening PEFR (L/min).
2.19. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 19 Evening PEF % predicted.
2.20. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 20 Change in evening PEF compared with baseline.
2.21. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 21 Clinic PEFR (L/min).
2.22. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 22 Clinic PEFR (L/min).
2.23. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 23 Clinic PEF % predicted.
2.24. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 24 Requirement for medication in addition to beta‐agonist.
2.25. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 25 Withdrawal due to asthma exacerbation (No. of patients).
2.26. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 26 One or more exacerbations (No. of patients).
2.27. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 27 Number of participants experiencing symptom‐free days.
2.28. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 28 Number of participants experiencing symptom‐free nights.
2.29. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 29 Percentage of symptom free days.
2.30. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 30 Change in % symptom free days.
2.31. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 31 Percentage of symptom free nights.
2.32. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 32 Change in % symptom free nights.
2.33. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 33 Change in total symptom scores.
2.34. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 34 Daytime asthma symptom score.
2.35. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 35 Night‐time asthma symptom score.
2.36. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 36 Change in daytime symptoms.
2.37. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 37 Change in daytime symptoms.
2.38. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 38 Change in nocturnal symptoms.
2.39. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 39 Change in nocturnal symptoms.
2.40. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 40 Rescue medication usage (puffs/day).
2.41. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 41 Change in rescue medication usage (puffs/d).
2.42. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 42 Change in rescue medication usage (daytime).
2.43. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 43 Change in rescue medication usage (daytime).
2.44. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 44 Change in rescue medication usage (nighttime).
2.45. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 45 Change in rescue medication usage (nighttime).
2.46. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 46 Change in % rescue free days.
2.47. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 47 Daytime rescue beta2 agonist use (puffs/daytime).
2.48. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 48 Night‐time rescue beta2 agonist use (puffs/night).
2.49. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 49 Number of participants experiencing rescue beta2 agonist free days.
2.50. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 50 Hoarseness (No. of patients).
2.51. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 51 Oropharyngeal Candidiasis (No. of patients).
2.52. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 52 Sore throat/pharyngitis (No. of patients).
2.53. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 53 Cough.
2.54. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 54 Headache.
2.55. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 55 Increased asthma symptoms.
2.58. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 58 Change in morning plasma cortisol (nmol/L) compared to baseline.
2.61. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 61 Weight increase.
2.62. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 62 Tonsillitis.
2.64. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 64 AUC serum cortisol nmol 1 1ST ARM.
2.65. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 65 Any adverse event.
2.66. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 66 Withdrawals due to lack of efficacy.
2.67. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 67 Withdrawal due to adverse events.
2.68. Analysis.

Comparison 2 FP versus BDP or BUD, parallel studies: dose ratio 1:1, Outcome 68 FEV1 (sensitivity analysis).
Comparison 3. FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 | 17 | 3640 | Litres (Fixed, 95% CI) | 0.04 [0.00, 0.07] |
| 1.1 BDP | 11 | 1943 | Litres (Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| 1.2 BUD | 6 | 1697 | Litres (Fixed, 95% CI) | 0.09 [0.02, 0.15] |
| 2 Change in FEV1 compared to baseline | 12 | 2635 | L/min (Fixed, 95% CI) | 0.01 [‐0.02, 0.05] |
| 2.1 BDP | 7 | 1490 | L/min (Fixed, 95% CI) | 0.04 [‐0.01, 0.08] |
| 2.2 BUD | 5 | 1145 | L/min (Fixed, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 3 FEV1 predicted | 7 | 1444 | % (Fixed, 95% CI) | 0.42 [‐1.02, 1.86] |
| 3.1 BDP | 6 | 1189 | % (Fixed, 95% CI) | 0.63 [‐0.91, 2.16] |
| 3.2 BUD | 1 | 255 | % (Fixed, 95% CI) | ‐1.0 [‐5.06, 3.06] |
| 4 Change in predicted FEV1 | 6 | 1306 | % (Fixed, 95% CI) | ‐0.39 [‐1.65, 0.86] |
| 4.1 BDP | 4 | 992 | % (Fixed, 95% CI) | ‐0.18 [‐1.63, 1.28] |
| 4.2 BUD | 2 | 314 | % (Fixed, 95% CI) | ‐1.04 [‐3.54, 1.47] |
| 5 FVC | 9 | 1854 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 5.1 BDP | 5 | 871 | Litres (Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| 5.2 BUD | 4 | 983 | Litres (Fixed, 95% CI) | 0.12 [0.03, 0.20] |
| 6 FVC % predicted | 2 | 391 | % (Fixed, 95% CI) | 1.28 [‐0.84, 3.40] |
| 6.1 BDP | 2 | 391 | % (Fixed, 95% CI) | 1.28 [‐0.84, 3.40] |
| 6.2 BUD | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Mean morning PEF | 12 | 2955 | L/min (Fixed, 95% CI) | 5.89 [0.96, 10.82] |
| 7.1 BDP | 5 | 944 | L/min (Fixed, 95% CI) | 0.37 [‐6.07, 6.82] |
| 7.2 BUD | 7 | 2011 | L/min (Fixed, 95% CI) | 13.66 [6.01, 21.32] |
| 8 Mean change in am PEF | 17 | 4144 | L/min (Fixed, 95% CI) | 7.79 [5.28, 10.29] |
| 8.1 BDP | 6 | 1395 | L/min (Fixed, 95% CI) | 7.47 [2.99, 11.96] |
| 8.2 BUD | 10 | 2629 | L/min (Fixed, 95% CI) | 7.74 [4.65, 10.83] |
| 8.3 Either | 1 | 120 | L/min (Fixed, 95% CI) | 12.14 [‐2.23, 26.51] |
| 9 Evening PEF | 10 | 2656 | L/min (Fixed, 95% CI) | 1.53 [‐3.78, 6.84] |
| 9.1 BDP | 5 | 943 | L/min (Fixed, 95% CI) | ‐2.00 [‐8.30, 4.29] |
| 9.2 BUD | 5 | 1713 | L/min (Fixed, 95% CI) | 10.17 [0.32, 20.02] |
| 10 Mean change in evening PEFR compared to baseline | 10 | 2186 | L/min (Fixed, 95% CI) | 2.59 [‐0.36, 5.55] |
| 10.1 BDP | 6 | 1415 | L/min (Fixed, 95% CI) | 2.82 [‐0.96, 6.60] |
| 10.2 BUD | 4 | 771 | L/min (Fixed, 95% CI) | 2.24 [‐2.49, 6.97] |
| 11 Clinic PEF | 12 | 2545 | L/min (Random, 95% CI) | 17.04 [‐0.99, 35.07] |
| 11.1 BDP | 7 | 1276 | L/min (Random, 95% CI) | 22.68 [‐10.02, 55.39] |
| 11.2 BUD | 5 | 1269 | L/min (Random, 95% CI) | 13.08 [2.21, 23.95] |
| 12 Change in Clinic PEF | 6 | 1322 | L/min (Fixed, 95% CI) | 5.10 [‐1.18, 11.38] |
| 12.1 BDP | 3 | 853 | L/min (Fixed, 95% CI) | 4.51 [‐3.11, 12.13] |
| 12.2 BUD | 3 | 469 | L/min (Fixed, 95% CI) | 6.36 [‐4.73, 17.44] |
| 13 Clinic PEF % predicted | 3 | 630 | % (Fixed, 95% CI) | 2.00 [‐0.18, 4.18] |
| 13.1 BDP | 3 | 630 | % (Fixed, 95% CI) | 2.00 [‐0.18, 4.18] |
| 13.2 BUD | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 1 FEV1.
3.2. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 2 Change in FEV1 compared to baseline.
3.3. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 3 FEV1 predicted.
3.4. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 4 Change in predicted FEV1.
3.5. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 5 FVC.
3.6. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 6 FVC % predicted.
3.7. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 7 Mean morning PEF.
3.8. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 8 Mean change in am PEF.
3.9. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 9 Evening PEF.
3.10. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 10 Mean change in evening PEFR compared to baseline.
3.11. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 11 Clinic PEF.
3.12. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 12 Change in Clinic PEF.
3.13. Analysis.

Comparison 3 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by drug, Outcome 13 Clinic PEF % predicted.
Comparison 4. FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 | 10 | 1957 | Litres (Fixed, 95% CI) | 0.04 [0.01, 0.07] |
| 1.1 BDP | 7 | 1413 | Litres (Fixed, 95% CI) | 0.05 [0.02, 0.09] |
| 1.2 BUD | 3 | 544 | Litres (Fixed, 95% CI) | 0.02 [‐0.04, 0.07] |
| 2 Change in FEV1 | 5 | 1019 | Litres (Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2.1 BDP | 2 | 437 | Litres (Fixed, 95% CI) | 0.01 [‐0.09, 0.10] |
| 2.2 BUD | 3 | 582 | Litres (Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 3 FEV1 predicted | 4 | 771 | % (Fixed, 95% CI) | 1.76 [‐0.10, 3.63] |
| 3.1 BDP | 3 | 590 | % (Fixed, 95% CI) | 1.99 [‐0.13, 4.11] |
| 3.2 BUD | 1 | 181 | % (Fixed, 95% CI) | 1.0 [‐2.92, 4.92] |
| 4 FVC | 6 | 1315 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 4.1 BDP | 5 | 983 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 4.2 BUD | 1 | 332 | Litres (Fixed, 95% CI) | 0.1 [‐0.14, 0.34] |
| 5 Morning PEFR | 11 | 2192 | L/min (Fixed, 95% CI) | 8.15 [5.04, 11.27] |
| 5.1 BDP | 6 | 1413 | L/min (Fixed, 95% CI) | 9.05 [5.08, 13.02] |
| 5.2 BUD | 5 | 779 | L/min (Fixed, 95% CI) | 6.71 [1.69, 11.74] |
| 6 Evening PEFR | 10 | 2135 | L/min (Fixed, 95% CI) | 6.92 [3.97, 9.88] |
| 6.1 BDP | 6 | 1372 | L/min (Fixed, 95% CI) | 8.52 [4.61, 12.43] |
| 6.2 BUD | 4 | 763 | L/min (Fixed, 95% CI) | 4.81 [0.30, 9.32] |
| 7 Morning PEF predicted | 3 | 747 | % (Fixed, 95% CI) | 2.09 [0.91, 3.28] |
| 7.1 BDP | 2 | 521 | % (Fixed, 95% CI) | 1.57 [0.09, 3.06] |
| 7.2 BUD | 1 | 226 | % (Fixed, 95% CI) | 3.0 [1.04, 4.96] |
| 8 Change in morning PEFR compared to baseline | 4 | 696 | Litres (Fixed, 95% CI) | 3.65 [‐4.72, 12.01] |
| 8.1 BDP | 2 | 328 | Litres (Fixed, 95% CI) | 1.48 [‐11.01, 13.96] |
| 8.2 BUD | 2 | 368 | Litres (Fixed, 95% CI) | 5.42 [‐5.85, 16.68] |
| 9 Evening PEF % predicted | 3 | 745 | % (Fixed, 95% CI) | 1.13 [0.02, 2.23] |
| 9.1 BDP | 2 | 519 | % (Fixed, 95% CI) | 0.72 [‐0.61, 2.06] |
| 9.2 BUD | 1 | 226 | % (Fixed, 95% CI) | 2.0 [0.04, 3.96] |
| 10 Change in evening PEF compared with baseline | 3 | 484 | L/min (Fixed, 95% CI) | 7.87 [‐2.35, 18.10] |
| 10.1 BDP | 1 | 116 | L/min (Fixed, 95% CI) | 4.1 [‐30.96, 39.16] |
| 10.2 BUD | 2 | 368 | L/min (Fixed, 95% CI) | 8.23 [‐2.46, 18.91] |
| 11 Clinic PEFR | 9 | 2054 | L/min (Fixed, 95% CI) | 8.88 [4.40, 13.35] |
| 11.1 BDP | 7 | 1511 | L/min (Fixed, 95% CI) | 10.38 [5.24, 15.51] |
| 11.2 BUD | 2 | 543 | L/min (Fixed, 95% CI) | 4.17 [‐4.93, 13.26] |
| 12 Clinic PEF % predicted | 4 | 817 | % (Fixed, 95% CI) | 2.08 [0.22, 3.94] |
| 12.1 BDP | 3 | 606 | % (Fixed, 95% CI) | 2.10 [‐0.02, 4.22] |
| 12.2 BUD | 1 | 211 | % (Fixed, 95% CI) | 2.0 [‐1.92, 5.92] |
4.1. Analysis.

Comparison 4 FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug, Outcome 1 FEV1.
4.2. Analysis.

Comparison 4 FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug, Outcome 2 Change in FEV1.
4.3. Analysis.

Comparison 4 FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug, Outcome 3 FEV1 predicted.
4.4. Analysis.

Comparison 4 FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug, Outcome 4 FVC.
4.5. Analysis.

Comparison 4 FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug, Outcome 5 Morning PEFR.
4.6. Analysis.

Comparison 4 FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug, Outcome 6 Evening PEFR.
4.7. Analysis.

Comparison 4 FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug, Outcome 7 Morning PEF predicted.
4.8. Analysis.

Comparison 4 FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug, Outcome 8 Change in morning PEFR compared to baseline.
4.9. Analysis.

Comparison 4 FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug, Outcome 9 Evening PEF % predicted.
4.10. Analysis.

Comparison 4 FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug, Outcome 10 Change in evening PEF compared with baseline.
4.11. Analysis.

Comparison 4 FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug, Outcome 11 Clinic PEFR.
4.12. Analysis.

Comparison 4 FP versus BDP or BUD, parallel studies: dose ratio 1:1 subgroup by drug, Outcome 12 Clinic PEF % predicted.
Comparison 5. FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 | 17 | 3640 | Litres (Fixed, 95% CI) | 0.04 [0.01, 0.08] |
| 1.1 Children | 2 | 679 | Litres (Fixed, 95% CI) | ‐0.00 [‐0.08, 0.07] |
| 1.2 Adults | 15 | 2961 | Litres (Fixed, 95% CI) | 0.06 [0.02, 0.10] |
| 2 Change in FEV1 compared to baseline | 12 | 2635 | L/min (Fixed, 95% CI) | 0.01 [‐0.02, 0.05] |
| 2.1 Children | 2 | 616 | L/min (Fixed, 95% CI) | ‐0.04 [‐0.10, 0.02] |
| 2.2 Adults | 10 | 2019 | L/min (Fixed, 95% CI) | 0.04 [0.00, 0.08] |
| 3 FEV1 predicted | 7 | 1444 | % (Fixed, 95% CI) | 0.42 [‐1.02, 1.86] |
| 3.1 Children | 2 | 406 | % (Fixed, 95% CI) | 0.10 [‐2.82, 3.03] |
| 3.2 Adults | 5 | 1038 | % (Fixed, 95% CI) | 0.52 [‐1.13, 2.18] |
| 4 Change in FEV1 predicted | 6 | 1306 | % (Fixed, 95% CI) | ‐0.39 [‐1.65, 0.86] |
| 4.1 Children | 2 | 457 | % (Fixed, 95% CI) | ‐0.78 [‐3.18, 1.63] |
| 4.2 Adults | 4 | 849 | % (Fixed, 95% CI) | ‐0.25 [‐1.72, 1.23] |
| 5 FVC | 9 | 1854 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 5.1 Children | 1 | 296 | Litres (Fixed, 95% CI) | 0.09 [‐0.04, 0.22] |
| 5.2 Adults | 8 | 1558 | Litres (Fixed, 95% CI) | 0.04 [‐0.01, 0.10] |
| 6 FVC % predicted | 2 | 391 | % (Fixed, 95% CI) | 1.28 [‐0.84, 3.40] |
| 6.1 Children | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 2 | 391 | % (Fixed, 95% CI) | 1.28 [‐0.84, 3.40] |
| 7 Mean morning PEFR | 12 | 2955 | L/min (Fixed, 95% CI) | 5.89 [0.96, 10.82] |
| 7.1 Children | 1 | 308 | L/min (Fixed, 95% CI) | 12.0 [‐5.57, 29.57] |
| 7.2 Adults | 11 | 2647 | L/min (Fixed, 95% CI) | 5.36 [0.23, 10.50] |
| 8 Mean change in am PEFR | 17 | 4144 | L/min (Fixed, 95% CI) | 7.79 [5.28, 10.29] |
| 8.1 Children | 3 | 889 | L/min (Fixed, 95% CI) | 7.14 [2.69, 11.59] |
| 8.2 Adults | 14 | 3255 | L/min (Fixed, 95% CI) | 8.09 [5.06, 11.12] |
| 9 Mean evening PEFR | 10 | 2656 | L/min (Fixed, 95% CI) | 1.53 [‐3.78, 6.84] |
| 9.1 Children | 1 | 308 | L/min (Fixed, 95% CI) | 12.0 [‐11.12, 35.12] |
| 9.2 Adults | 9 | 2348 | L/min (Fixed, 95% CI) | 0.95 [‐4.50, 6.40] |
| 10 Change in evening PEF compared to baseline | 10 | 2186 | L/min (Fixed, 95% CI) | 2.59 [‐0.36, 5.55] |
| 10.1 Children | 1 | 397 | L/min (Fixed, 95% CI) | 4.0 [0.00, 10.00] |
| 10.2 Adults | 9 | 1789 | L/min (Fixed, 95% CI) | 2.15 [‐1.24, 5.54] |
| 11 Clinic PEF | 12 | 2545 | L/min (Random, 95% CI) | 17.04 [‐0.99, 35.07] |
| 11.1 Children | 2 | 705 | L/min (Random, 95% CI) | 16.0 [‐1.09, 33.09] |
| 11.2 Adults | 10 | 1840 | L/min (Random, 95% CI) | 17.44 [‐3.52, 38.39] |
| 12 Change in Clinic PEFR | 6 | 1322 | L/min (Fixed, 95% CI) | 5.10 [‐1.18, 11.38] |
| 12.1 Children | 1 | 397 | L/min (Fixed, 95% CI) | ‐1.0 [‐8.00, 8.00] |
| 12.2 Adults | 5 | 925 | L/min (Fixed, 95% CI) | 10.90 [2.13, 19.66] |
| 13 Clinic PEF % predicted | 3 | 630 | % (Fixed, 95% CI) | 2.00 [‐0.18, 4.18] |
| 13.1 Children | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Adults | 3 | 630 | % (Fixed, 95% CI) | 2.00 [‐0.18, 4.18] |
5.1. Analysis.

Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 1 FEV1.
5.2. Analysis.

Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 2 Change in FEV1 compared to baseline.
5.3. Analysis.

Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 3 FEV1 predicted.
5.4. Analysis.
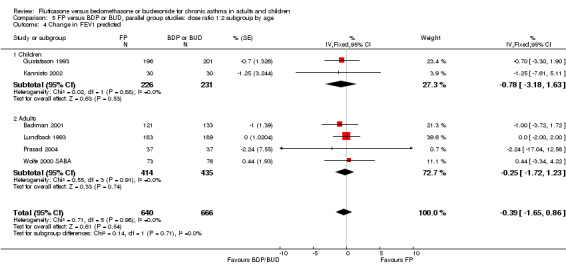
Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 4 Change in FEV1 predicted.
5.5. Analysis.

Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 5 FVC.
5.6. Analysis.

Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 6 FVC % predicted.
5.7. Analysis.

Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 7 Mean morning PEFR.
5.8. Analysis.

Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 8 Mean change in am PEFR.
5.9. Analysis.

Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 9 Mean evening PEFR.
5.10. Analysis.
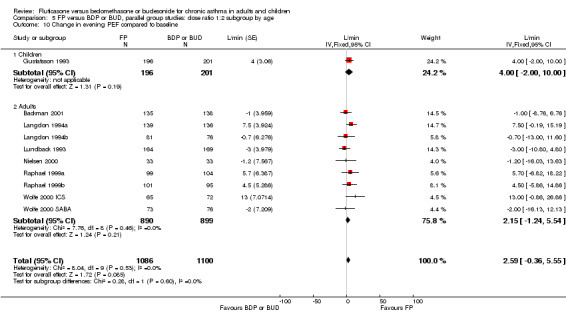
Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 10 Change in evening PEF compared to baseline.
5.11. Analysis.

Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 11 Clinic PEF.
5.12. Analysis.

Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 12 Change in Clinic PEFR.
5.13. Analysis.

Comparison 5 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by age, Outcome 13 Clinic PEF % predicted.
Comparison 6. FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 | 10 | 1957 | Litres (Fixed, 95% CI) | 0.04 [0.01, 0.07] |
| 1.1 Children | 3 | 694 | Litres (Fixed, 95% CI) | 0.04 [0.00, 0.08] |
| 1.2 Adults | 7 | 1263 | Litres (Fixed, 95% CI) | 0.04 [‐0.01, 0.10] |
| 2 Change in FEV1 | 5 | 1019 | Litres (Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2.1 Children | 1 | 214 | Litres (Fixed, 95% CI) | 0.02 [‐0.03, 0.07] |
| 2.2 Adults | 4 | 805 | Litres (Fixed, 95% CI) | ‐0.01 [‐0.07, 0.06] |
| 3 FEV1 predicted | 4 | 771 | % (Fixed, 95% CI) | 1.76 [‐0.10, 3.63] |
| 3.1 Children | 1 | 181 | % (Fixed, 95% CI) | 1.0 [‐2.92, 4.92] |
| 3.2 Adults | 3 | 590 | % (Fixed, 95% CI) | 1.99 [‐0.13, 4.11] |
| 4 FVC | 6 | 1315 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 4.1 Adults | 4 | 795 | Litres (Fixed, 95% CI) | 0.08 [‐0.01, 0.17] |
| 4.2 Children | 2 | 520 | Litres (Fixed, 95% CI) | 0.04 [‐0.02, 0.09] |
| 5 Morning PEFR | 11 | 2192 | L/min (Fixed, 95% CI) | 8.15 [5.04, 11.27] |
| 5.1 Adults | 6 | 1260 | L/min (Fixed, 95% CI) | 10.34 [4.49, 16.18] |
| 5.2 Children | 4 | 904 | L/min (Fixed, 95% CI) | 6.90 [3.20, 10.59] |
| 5.3 Unclear | 1 | 28 | L/min (Fixed, 95% CI) | 52.0 [12.45, 91.55] |
| 6 Change in morning PEFR compared to baseline | 5 | 858 | Litres (Fixed, 95% CI) | 4.60 [‐2.91, 12.12] |
| 6.1 Adults | 4 | 696 | Litres (Fixed, 95% CI) | 3.65 [‐4.72, 12.01] |
| 6.2 Children | 1 | 162 | Litres (Fixed, 95% CI) | 8.6 [‐8.53, 25.73] |
| 7 Morning PEF predicted | 3 | 747 | % (Fixed, 95% CI) | 2.09 [0.91, 3.28] |
| 7.1 Children | 1 | 226 | % (Fixed, 95% CI) | 3.0 [1.04, 4.96] |
| 7.2 Adults | 2 | 521 | % (Fixed, 95% CI) | 1.57 [0.09, 3.06] |
| 8 Evening PEFR | 10 | 2135 | L/min (Fixed, 95% CI) | 6.92 [3.97, 9.88] |
| 8.1 Adults | 6 | 1222 | L/min (Fixed, 95% CI) | 7.31 [1.50, 13.13] |
| 8.2 Children | 3 | 884 | L/min (Fixed, 95% CI) | 6.46 [3.01, 9.91] |
| 8.3 Unclear | 1 | 29 | L/min (Fixed, 95% CI) | 46.0 [8.30, 83.70] |
| 9 Change in evening PEF compared with baseline | 4 | 646 | L/min (Fixed, 95% CI) | 8.57 [0.00, 17.13] |
| 9.1 Adults | 3 | 484 | L/min (Fixed, 95% CI) | 7.87 [‐2.35, 18.10] |
| 9.2 Children | 1 | 162 | L/min (Fixed, 95% CI) | 10.2 [‐5.50, 25.90] |
| 10 Evening PEF % predicted | 3 | 745 | % (Fixed, 95% CI) | 1.13 [0.02, 2.23] |
| 10.1 BDP | 2 | 519 | % (Fixed, 95% CI) | 0.72 [‐0.61, 2.06] |
| 10.2 BUD | 1 | 226 | % (Fixed, 95% CI) | 2.0 [0.04, 3.96] |
| 11 Clinic PEFR | 9 | 2054 | L/min (Fixed, 95% CI) | 8.88 [4.40, 13.35] |
| 11.1 Adults | 6 | 1217 | L/min (Fixed, 95% CI) | 8.30 [0.43, 16.17] |
| 11.2 Children | 3 | 837 | L/min (Fixed, 95% CI) | 9.15 [3.72, 14.58] |
| 12 Clinic PEF predicted | 4 | 817 | % (Fixed, 95% CI) | 2.08 [0.22, 3.94] |
| 12.1 Adults | 3 | 606 | % (Fixed, 95% CI) | 2.10 [‐0.02, 4.22] |
| 12.2 Children | 1 | 211 | % (Fixed, 95% CI) | 2.0 [‐1.92, 5.92] |
6.1. Analysis.

Comparison 6 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age, Outcome 1 FEV1.
6.2. Analysis.

Comparison 6 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age, Outcome 2 Change in FEV1.
6.3. Analysis.

Comparison 6 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age, Outcome 3 FEV1 predicted.
6.4. Analysis.

Comparison 6 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age, Outcome 4 FVC.
6.5. Analysis.

Comparison 6 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age, Outcome 5 Morning PEFR.
6.6. Analysis.

Comparison 6 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age, Outcome 6 Change in morning PEFR compared to baseline.
6.7. Analysis.

Comparison 6 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age, Outcome 7 Morning PEF predicted.
6.8. Analysis.

Comparison 6 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age, Outcome 8 Evening PEFR.
6.9. Analysis.

Comparison 6 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age, Outcome 9 Change in evening PEF compared with baseline.
6.10. Analysis.

Comparison 6 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age, Outcome 10 Evening PEF % predicted.
6.11. Analysis.

Comparison 6 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age, Outcome 11 Clinic PEFR.
6.12. Analysis.

Comparison 6 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by age, Outcome 12 Clinic PEF predicted.
Comparison 7. FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 | 17 | 3640 | Litres (Fixed, 95% CI) | 0.04 [0.01, 0.08] |
| 1.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 1 | 25 | Litres (Fixed, 95% CI) | 0.12 [‐0.39, 0.63] |
| 1.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 7 | 1344 | Litres (Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 1.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 2 | 317 | Litres (Fixed, 95% CI) | 0.08 [‐0.03, 0.19] |
| 1.4 FP 500 mcg/d v BDP/BUD 800‐1200 mcg/d | 3 | 1063 | Litres (Fixed, 95% CI) | 0.09 [‐0.01, 0.19] |
| 1.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 1 | 518 | Litres (Fixed, 95% CI) | 0.11 [‐0.02, 0.24] |
| 1.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 3 | 373 | Litres (Fixed, 95% CI) | 0.05 [‐0.04, 0.13] |
| 2 Change in FEV1 compared to baseline | 12 | 2635 | L/min (Fixed, 95% CI) | 0.01 [‐0.02, 0.05] |
| 2.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 7 | 1308 | L/min (Fixed, 95% CI) | ‐0.01 [‐0.06, 0.03] |
| 2.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 1 | 196 | L/min (Fixed, 95% CI) | 0.15 [0.01, 0.29] |
| 2.4 FP 500 mcg/d v BDP/BUD 800‐1200 mcg/d | 3 | 830 | L/min (Fixed, 95% CI) | 0.02 [‐0.03, 0.08] |
| 2.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 FP 1000 mcg/d v BDP/BUD 1600 mcg/d | 1 | 301 | L/min (Fixed, 95% CI) | 0.07 [‐0.04, 0.18] |
| 2.7 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 FEV1 predicted | 7 | 1444 | % (Fixed, 95% CI) | 0.42 [‐1.02, 1.86] |
| 3.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 5 | 1053 | % (Fixed, 95% CI) | 0.39 [‐1.28, 2.05] |
| 3.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 FP 500 mcg/d v BDP/BUD 1200 mcg/d | 1 | 255 | % (Fixed, 95% CI) | ‐1.0 [‐5.06, 3.06] |
| 3.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 1 | 136 | % (Fixed, 95% CI) | 2.0 [‐2.00, 6.00] |
| 4 Change in predicted FEV1 | 6 | 1306 | % (Fixed, 95% CI) | ‐0.39 [‐1.65, 0.86] |
| 4.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 3 | 620 | % (Fixed, 95% CI) | ‐0.37 [‐2.49, 1.75] |
| 4.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 FP 500 mcg/d v BDP/BUD 800‐1200 mcg/d | 3 | 686 | % (Fixed, 95% CI) | ‐0.40 [‐1.97, 1.16] |
| 4.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 FVC | 9 | 1854 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 5.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 3 | 637 | Litres (Fixed, 95% CI) | 0.02 [‐0.05, 0.09] |
| 5.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 2 | 317 | Litres (Fixed, 95% CI) | 0.09 [‐0.04, 0.22] |
| 5.4 FP 500 mcg/d v BDP/BUD 800‐1200 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 1 | 527 | Litres (Fixed, 95% CI) | 0.15 [0.02, 0.28] |
| 5.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 3 | 373 | Litres (Fixed, 95% CI) | 0.01 [‐0.09, 0.12] |
| 6 FVC predicted | 2 | 391 | % (Fixed, 95% CI) | 1.28 [‐0.84, 3.40] |
| 6.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 1 | 255 | % (Fixed, 95% CI) | 1.0 [‐1.50, 3.50] |
| 6.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 FP 500 mcg/d v BDP/BUD 1200 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 1 | 136 | % (Fixed, 95% CI) | 2.0 [0.00, 6.00] |
| 7 Mean morning PEFR | 12 | 2955 | Litres (Fixed, 95% CI) | 5.89 [0.96, 10.82] |
| 7.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 5 | 954 | Litres (Fixed, 95% CI) | 2.34 [‐4.75, 9.42] |
| 7.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 2 | 577 | Litres (Fixed, 95% CI) | 16.51 [5.30, 27.71] |
| 7.4 FP 500 mcg/d v BDP/BUD 1000‐1200 mcg/d | 2 | 630 | Litres (Fixed, 95% CI) | 9.52 [‐5.32, 24.36] |
| 7.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 1 | 527 | Litres (Fixed, 95% CI) | 21.3 [2.97, 39.63] |
| 7.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 2 | 267 | Litres (Fixed, 95% CI) | ‐7.34 [‐20.52, 5.83] |
| 8 Change in am PEF compared to baseline | 17 | 4144 | L/min (Fixed, 95% CI) | 7.79 [5.28, 10.29] |
| 8.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 8 | 1688 | L/min (Fixed, 95% CI) | 7.77 [4.04, 11.49] |
| 8.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 2 | 471 | L/min (Fixed, 95% CI) | 13.85 [5.52, 22.17] |
| 8.4 FP 500 mcg/d v BDP/BUD 800‐1200 mcg/d | 5 | 1347 | L/min (Fixed, 95% CI) | 4.89 [0.06, 9.71] |
| 8.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 1 | 518 | L/min (Fixed, 95% CI) | 8.5 [2.18, 14.82] |
| 8.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.7 FP half usual maintenance dose of BDP/BUD | 1 | 120 | L/min (Fixed, 95% CI) | 12.14 [‐2.23, 26.51] |
| 9 Evening PEFR | 10 | 2656 | L/min (Fixed, 95% CI) | 1.53 [‐3.78, 6.84] |
| 9.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 5 | 956 | L/min (Fixed, 95% CI) | 0.78 [‐6.34, 7.90] |
| 9.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 1 | 308 | L/min (Fixed, 95% CI) | 12.0 [‐11.12, 35.12] |
| 9.4 FP 500 mcg/d v BDP/BUD 1200 mcg/d | 2 | 730 | L/min (Fixed, 95% CI) | 5.92 [‐9.04, 20.88] |
| 9.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 1 | 518 | L/min (Fixed, 95% CI) | 17.7 [‐0.38, 35.78] |
| 9.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 1 | 144 | L/min (Fixed, 95% CI) | ‐10.0 [‐22.50, 2.50] |
| 10 Change in evening PEFR compared to baseline | 10 | 2186 | L/min (Fixed, 95% CI) | 2.59 [‐0.36, 5.55] |
| 10.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 6 | 1109 | L/min (Fixed, 95% CI) | 3.51 [‐0.73, 7.76] |
| 10.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 2 | 471 | L/min (Fixed, 95% CI) | 6.43 [0.26, 12.61] |
| 10.4 FP 500 mcg/d v BDP/BUD 1200 mcg/d | 2 | 606 | L/min (Fixed, 95% CI) | ‐1.99 [‐7.50, 3.51] |
| 10.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Clinic PEFR | 12 | 2545 | L/min (Random, 95% CI) | 17.04 [‐0.99, 35.07] |
| 11.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | L/min (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 5 | 1060 | L/min (Random, 95% CI) | 40.43 [‐1.68, 82.55] |
| 11.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 2 | 338 | L/min (Random, 95% CI) | 16.33 [‐0.48, 33.14] |
| 11.4 FP 500 mcg/d v BDP/BUD 1200 mcg/d | 1 | 256 | L/min (Random, 95% CI) | ‐7.00 [‐33.99, 19.99] |
| 11.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 1 | 518 | L/min (Random, 95% CI) | 20.2 [1.29, 39.11] |
| 11.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 3 | 373 | L/min (Random, 95% CI) | ‐7.28 [‐49.34, 34.78] |
| 12 Change in Clinic PEFR | 6 | 1322 | L/min (Fixed, 95% CI) | 5.10 [‐1.18, 11.38] |
| 12.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 4 | 674 | L/min (Fixed, 95% CI) | 0.79 [‐7.31, 8.90] |
| 12.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.4 FP 500 mcg/d v BDP/BUD 1200 mcg/d | 2 | 648 | L/min (Fixed, 95% CI) | 11.57 [1.64, 21.50] |
| 12.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Clinic PEF % predicted | 3 | 630 | % (Fixed, 95% CI) | 2.00 [‐0.18, 4.18] |
| 13.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 2 | 495 | % (Fixed, 95% CI) | 1.39 [‐0.95, 3.73] |
| 13.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.4 FP 500 mcg/d v BDP/BUD 1200 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 1 | 135 | % (Fixed, 95% CI) | 6.0 [0.00, 12.00] |
7.1. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 1 FEV1.
7.2. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 2 Change in FEV1 compared to baseline.
7.3. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 3 FEV1 predicted.
7.4. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 4 Change in predicted FEV1.
7.5. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 5 FVC.
7.6. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 6 FVC predicted.
7.7. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 7 Mean morning PEFR.
7.8. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 8 Change in am PEF compared to baseline.
7.9. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 9 Evening PEFR.
7.10. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 10 Change in evening PEFR compared to baseline.
7.11. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 11 Clinic PEFR.
7.12. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 12 Change in Clinic PEFR.
7.13. Analysis.

Comparison 7 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by dose range, Outcome 13 Clinic PEF % predicted.
Comparison 8. FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 | 10 | 1957 | Litres (Fixed, 95% CI) | 0.04 [0.01, 0.07] |
| 1.1 FP 100 mcg/d v BDP/BUD 100 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 FP 200 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 FP 4‐500 mcg/d v BDP/BUD 4‐500 mcg/d | 4 | 936 | Litres (Fixed, 95% CI) | 0.04 [0.00, 0.07] |
| 1.4 FP 8‐1000 mcg/d v BDP/BUD 8‐1000 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 FP 1000‐2000mcg/d v BDP/BUD 1000‐2000mcg/d | 6 | 1021 | Litres (Fixed, 95% CI) | 0.06 [‐0.02, 0.13] |
| 2 Change in FEV1 | 5 | 1019 | Litres (Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2.1 FP 100 mcg/d v BDP/BUD 100 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 FP 200 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 FP 4‐500 mcg/d v BDP/BUD 4‐500 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 FP 800‐999mcg/d v BDP/BUD 800‐999mcg/d | 1 | 197 | Litres (Fixed, 95% CI) | 0.02 [‐0.09, 0.13] |
| 2.5 FP 1000‐2000mcg/d v BDP/BUD 1000‐2000mcg/d | 2 | 437 | Litres (Fixed, 95% CI) | 0.01 [‐0.09, 0.10] |
| 2.6 1:1 dose ratio based upon pre‐study ICS steroid use | 2 | 385 | Litres (Fixed, 95% CI) | 0.01 [‐0.04, 0.06] |
| 3 FEV1 predicted | 4 | 771 | % (Fixed, 95% CI) | 1.76 [‐0.10, 3.63] |
| 3.1 FP 100 mcg/d v BDP/BUD 100 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 FP 200 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 FP 4‐500 mcg/d v BDP/BUD 4‐500 mcg/d | 2 | 423 | % (Fixed, 95% CI) | 0.97 [‐2.28, 4.22] |
| 3.4 FP 800‐999mcg/d v BDP/BUD 800‐999mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 FP 1000‐2000mcg/d v BDP/BUD 1000‐2000mcg/d | 2 | 348 | % (Fixed, 95% CI) | 2.15 [‐0.12, 4.43] |
| 3.6 1:1 dose ratio based upon pre‐study ICS steroid use | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 FVC | 6 | 1315 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 4.1 FP 100 mcg/d v BDP/BUD 100 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 FP 200 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 FP 4‐500 mcg/d v BDP/BUD 4‐500 mcg/d | 2 | 520 | Litres (Fixed, 95% CI) | 0.04 [‐0.02, 0.09] |
| 4.4 FP 800‐999mcg/d v BDP/BUD 800‐999mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 FP 1000‐2000mcg/d v BDP/BUD 1000‐2000mcg/d | 4 | 795 | Litres (Fixed, 95% CI) | 0.08 [‐0.01, 0.17] |
| 4.6 1:1 dose ratio based upon pre‐study ICS steroid use | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Morning PEFR | 11 | 2192 | L/min (Fixed, 95% CI) | 8.15 [5.04, 11.27] |
| 5.1 FP 100 mcg/d v BDP/BUD 100 mcg/d | 1 | 28 | L/min (Fixed, 95% CI) | 52.0 [12.45, 91.55] |
| 5.2 FP 200 mcg/d v BDP/BUD 200 mcg/d | 1 | 16 | L/min (Fixed, 95% CI) | ‐1.66 [‐59.81, 56.49] |
| 5.3 FP 4‐500 mcg/d v BDP/BUD 4‐500 mcg/d | 4 | 1146 | L/min (Fixed, 95% CI) | 7.18 [3.51, 10.85] |
| 5.4 FP 800‐999mcg/d v BDP/BUD 800‐999mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 FP 1000‐2000mcg/d v BDP/BUD 1000‐2000mcg/d | 4 | 831 | L/min (Fixed, 95% CI) | 10.00 [3.91, 16.09] |
| 5.6 1:1 dose ratio based upon pre‐study ICS steroid use | 1 | 171 | L/min (Fixed, 95% CI) | 4.8 [‐29.57, 39.17] |
| 6 Change in morning PEFR compared to baseline | 5 | 858 | Litres (Fixed, 95% CI) | 4.60 [‐2.91, 12.12] |
| 6.1 FP 100 mcg/d v BDP/BUD 100 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 FP 200 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 FP 4‐500 mcg/d v BDP/BUD 4‐500 mcg/d | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 FP 800‐999mcg/d v BDP/BUD 800‐999mcg/d | 1 | 197 | Litres (Fixed, 95% CI) | 4.17 [‐7.65, 15.99] |
| 6.5 FP 1000‐2000mcg/d v BDP/BUD 1000‐2000mcg/d | 3 | 490 | Litres (Fixed, 95% CI) | 3.95 [‐6.14, 14.04] |
| 6.6 1:1 dose ratio based upon pre‐study ICS steroid use | 1 | 171 | Litres (Fixed, 95% CI) | 17.8 [‐19.42, 55.02] |
| 7 Morning PEF predicted | 3 | 747 | % (Fixed, 95% CI) | 2.09 [0.91, 3.28] |
| 7.1 BDP | 2 | 521 | % (Fixed, 95% CI) | 1.57 [0.09, 3.06] |
| 7.2 BUD | 1 | 226 | % (Fixed, 95% CI) | 3.0 [1.04, 4.96] |
| 8 Evening PEFR | 10 | 2135 | L/min (Fixed, 95% CI) | 6.92 [3.97, 9.88] |
| 8.1 FP 100 mcg/d v BDP/BUD 100 mcg/d | 1 | 29 | L/min (Fixed, 95% CI) | 46.0 [8.30, 83.70] |
| 8.2 FP 200 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 FP 4‐500 mcg/d v BDP/BUD 4‐500 mcg/d | 4 | 1140 | L/min (Fixed, 95% CI) | 6.69 [3.27, 10.10] |
| 8.4 FP 800‐999mcg/d v BDP/BUD 800‐999mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 FP 1000‐2000mcg/d v BDP/BUD 1000‐2000mcg/d | 4 | 795 | L/min (Fixed, 95% CI) | 6.71 [0.66, 12.77] |
| 8.6 1:1 dose ratio based upon pre‐study ICS steroid use | 1 | 171 | L/min (Fixed, 95% CI) | 5.20 [‐29.18, 39.58] |
| 9 Change in evening PEF compared with baseline | 4 | 646 | L/min (Fixed, 95% CI) | 8.57 [0.00, 17.13] |
| 9.1 FP 100 mcg/d v BDP/BUD 100 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 FP 200 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 FP 4‐500 mcg/d v BDP/BUD 4‐500 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 FP 800‐999mcg/d v BDP/BUD 800‐999mcg/d | 1 | 197 | L/min (Fixed, 95% CI) | 8.14 [‐2.82, 19.10] |
| 9.5 FP 1000‐2000mcg/d v BDP/BUD 1000‐2000mcg/d | 2 | 278 | L/min (Fixed, 95% CI) | 9.18 [‐5.15, 23.51] |
| 9.6 1:1 dose ratio based upon pre‐study ICS steroid use | 1 | 171 | L/min (Fixed, 95% CI) | 9.9 [‐38.67, 58.47] |
| 10 Evening PEF % predicted | 3 | 745 | % (Fixed, 95% CI) | 1.13 [0.02, 2.23] |
| 10.1 BDP | 2 | 519 | % (Fixed, 95% CI) | 0.72 [‐0.61, 2.06] |
| 10.2 BUD | 1 | 226 | % (Fixed, 95% CI) | 2.0 [0.04, 3.96] |
| 11 Clinic PEFR | 9 | 2054 | L/min (Fixed, 95% CI) | 8.88 [4.40, 13.35] |
| 11.1 FP 100 mcg/d v BDP/BUD 100 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 FP 200 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 FP 4‐500 mcg/d v BDP/BUD 4‐500 mcg/d | 4 | 1077 | L/min (Fixed, 95% CI) | 8.74 [3.67, 13.80] |
| 11.4 FP 800‐999mcg/d v BDP/BUD 800‐999mcg/d | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.5 FP 1000‐2000mcg/d v BDP/BUD 1000‐2000mcg/d | 5 | 977 | L/min (Fixed, 95% CI) | 9.36 [‐0.15, 18.88] |
| 11.6 1:1 dose ratio based upon pre‐study ICS steroid use | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Clinic PEF % predicted | 4 | 817 | % (Fixed, 95% CI) | 2.08 [0.22, 3.94] |
| 12.1 FP 100 mcg/d v BDP/BUD 100 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 FP 200 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 FP 4‐500 mcg/d v BDP/BUD 4‐500 mcg/d | 2 | 451 | % (Fixed, 95% CI) | 1.88 [‐0.58, 4.33] |
| 12.4 FP 800‐999mcg/d v BDP/BUD 800‐999mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 FP 1000‐2000mcg/d v BDP/BUD 1000‐2000mcg/d | 2 | 366 | % (Fixed, 95% CI) | 2.35 [‐0.51, 5.22] |
| 12.6 1:1 dose ratio based upon pre‐study ICS steroid use | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.

Comparison 8 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range, Outcome 1 FEV1.
8.2. Analysis.

Comparison 8 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range, Outcome 2 Change in FEV1.
8.3. Analysis.

Comparison 8 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range, Outcome 3 FEV1 predicted.
8.4. Analysis.
Comparison 8 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range, Outcome 4 FVC.
8.5. Analysis.

Comparison 8 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range, Outcome 5 Morning PEFR.
8.6. Analysis.

Comparison 8 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range, Outcome 6 Change in morning PEFR compared to baseline.
8.7. Analysis.

Comparison 8 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range, Outcome 7 Morning PEF predicted.
8.8. Analysis.

Comparison 8 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range, Outcome 8 Evening PEFR.
8.9. Analysis.

Comparison 8 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range, Outcome 9 Change in evening PEF compared with baseline.
8.10. Analysis.

Comparison 8 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range, Outcome 10 Evening PEF % predicted.
8.11. Analysis.

Comparison 8 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range, Outcome 11 Clinic PEFR.
8.12. Analysis.

Comparison 8 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by dose range, Outcome 12 Clinic PEF % predicted.
Comparison 9. FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 | 17 | 3640 | Litres (Fixed, 95% CI) | 0.04 [0.01, 0.08] |
| 1.1 Mild | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Mild to moderate | 6 | 627 | Litres (Fixed, 95% CI) | 0.08 [0.01, 0.16] |
| 1.3 Moderate | 6 | 1826 | Litres (Fixed, 95% CI) | ‐0.00 [‐0.05, 0.05] |
| 1.4 Moderate to severe | 3 | 838 | Litres (Fixed, 95% CI) | 0.09 [0.00, 0.17] |
| 1.5 Severe | 2 | 349 | Litres (Fixed, 95% CI) | 0.05 [‐0.03, 0.14] |
| 1.6 Unclear | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Change in FEV1 compared to baseline | 12 | 2635 | Litres (Fixed, 95% CI) | 0.01 [‐0.02, 0.05] |
| 2.1 Mild | 1 | 233 | Litres (Fixed, 95% CI) | ‐0.05 [‐0.12, 0.02] |
| 2.2 Mild to moderate | 3 | 506 | Litres (Fixed, 95% CI) | 0.03 [‐0.05, 0.10] |
| 2.3 Moderate | 4 | 1132 | Litres (Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 2.4 Moderate to severe | 1 | 301 | Litres (Fixed, 95% CI) | 0.07 [‐0.04, 0.18] |
| 2.5 Severe | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Mild to severe | 2 | 399 | Litres (Fixed, 95% CI) | 0.14 [0.04, 0.23] |
| 2.7 Unclear | 1 | 64 | Litres (Fixed, 95% CI) | ‐0.14 [‐0.53, 0.25] |
| 3 FEV1 predicted | 7 | 1444 | % (Fixed, 95% CI) | 0.42 [‐1.02, 1.86] |
| 3.1 Mild | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Mild to moderate | 2 | 404 | % (Fixed, 95% CI) | 0.94 [‐1.21, 3.10] |
| 3.3 Moderate | 4 | 904 | % (Fixed, 95% CI) | ‐0.61 [‐2.82, 1.60] |
| 3.4 Moderate to severe | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Severe | 1 | 136 | % (Fixed, 95% CI) | 2.0 [‐2.00, 6.00] |
| 4 Change in predicted FEV1 | 6 | 1306 | % (Fixed, 95% CI) | ‐0.39 [‐1.65, 0.86] |
| 4.1 Mild | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Mild to moderate | 3 | 283 | % (Fixed, 95% CI) | ‐0.10 [‐3.28, 3.07] |
| 4.3 Moderate | 3 | 1023 | % (Fixed, 95% CI) | ‐0.45 [‐1.82, 0.92] |
| 4.4 Moderate to severe | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Severe | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 FVC | 9 | 1854 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 5.1 Mild | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Mild to moderate | 3 | 415 | Litres (Fixed, 95% CI) | 0.05 [‐0.05, 0.15] |
| 5.3 Moderate | 1 | 243 | Litres (Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 5.4 Moderate to severe | 3 | 847 | Litres (Fixed, 95% CI) | 0.11 [0.02, 0.20] |
| 5.5 Severe | 2 | 349 | Litres (Fixed, 95% CI) | 0.02 [‐0.08, 0.13] |
| 6 FVC predicted | 2 | 391 | % (Fixed, 95% CI) | 1.28 [‐0.84, 3.40] |
| 6.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 1 | 255 | % (Fixed, 95% CI) | 1.0 [‐1.50, 3.50] |
| 6.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 FP 500 mcg/d v BDP/BUD 1200 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 1 | 136 | % (Fixed, 95% CI) | 2.0 [0.00, 6.00] |
| 7 Mean morning PEFR | 12 | 2955 | L/min (Fixed, 95% CI) | 5.89 [0.96, 10.82] |
| 7.1 Mild | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Mild to moderate | 4 | 829 | L/min (Fixed, 95% CI) | 7.51 [0.78, 14.23] |
| 7.3 Moderate | 4 | 1024 | L/min (Fixed, 95% CI) | 2.31 [‐9.59, 14.21] |
| 7.4 Moderate to severe | 2 | 835 | L/min (Fixed, 95% CI) | 16.45 [3.77, 29.14] |
| 7.5 Severe | 2 | 267 | L/min (Fixed, 95% CI) | ‐7.34 [‐20.52, 5.83] |
| 7.6 Unclear | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Mean change in am PEFR | 17 | 4144 | Litres (Fixed, 95% CI) | 7.79 [5.28, 10.29] |
| 8.1 Mild | 2 | 389 | Litres (Fixed, 95% CI) | 6.09 [‐1.81, 14.00] |
| 8.2 Mild to moderate | 5 | 1073 | Litres (Fixed, 95% CI) | 8.31 [3.53, 13.09] |
| 8.3 Moderate | 6 | 1645 | Litres (Fixed, 95% CI) | 5.34 [1.12, 9.56] |
| 8.4 Moderate to severe | 1 | 518 | Litres (Fixed, 95% CI) | 8.5 [2.18, 14.82] |
| 8.5 Severe | 1 | 120 | Litres (Fixed, 95% CI) | 12.14 [‐2.23, 26.51] |
| 8.6 Mild to severe | 2 | 399 | Litres (Fixed, 95% CI) | 15.37 [6.79, 23.95] |
| 8.7 Unclear | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Evening PEFR | 10 | 2656 | L/min (Fixed, 95% CI) | 1.53 [‐3.78, 6.84] |
| 9.1 Mild | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Mild to moderate | 3 | 561 | L/min (Fixed, 95% CI) | 2.50 [‐5.07, 10.08] |
| 9.3 Moderate | 4 | 1125 | L/min (Fixed, 95% CI) | ‐0.27 [‐12.44, 11.89] |
| 9.4 Moderate to severe | 2 | 826 | L/min (Fixed, 95% CI) | 15.54 [1.29, 29.78] |
| 9.5 Severe | 1 | 144 | L/min (Fixed, 95% CI) | ‐10.0 [‐22.50, 2.50] |
| 9.6 Mild to severe | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.7 Unclear | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Mean change in evening PEFR compared to baseline | 10 | 2186 | L/min (Fixed, 95% CI) | 2.59 [‐0.36, 5.55] |
| 10.1 Mild | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Mild to moderate | 3 | 581 | L/min (Fixed, 95% CI) | 3.93 [‐1.99, 9.85] |
| 10.3 Moderate | 5 | 1206 | L/min (Fixed, 95% CI) | 1.52 [‐2.24, 5.29] |
| 10.4 Moderate to severe | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Severe | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 Mild to severe | 2 | 399 | L/min (Fixed, 95% CI) | 4.99 [‐2.99, 12.97] |
| 10.7 Unclear | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Clinic PEF | 12 | 2545 | L/min (Random, 95% CI) | 17.04 [‐0.99, 35.07] |
| 11.1 Mild | 0 | 0 | L/min (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Mild to moderate | 4 | 453 | L/min (Random, 95% CI) | 50.85 [‐17.03, 118.74] |
| 11.3 Moderate | 3 | 884 | L/min (Random, 95% CI) | 3.24 [‐9.18, 15.67] |
| 11.4 Moderate to severe | 3 | 859 | L/min (Random, 95% CI) | 11.83 [‐11.64, 35.30] |
| 11.5 Severe | 2 | 349 | L/min (Random, 95% CI) | 5.98 [‐33.17, 45.13] |
| 12 Change in Clinic PEF | 6 | 1322 | L/min (Fixed, 95% CI) | 5.10 [‐1.18, 11.38] |
| 12.1 Mild | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Mild to moderate | 2 | 213 | L/min (Fixed, 95% CI) | 9.00 [‐10.03, 28.04] |
| 12.3 Moderate | 3 | 1045 | L/min (Fixed, 95% CI) | 4.67 [‐2.00, 11.33] |
| 12.4 Moderate to severe | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 Severe | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.6 Unclear | 1 | 64 | L/min (Fixed, 95% CI) | ‐3.48 [‐98.54, 91.58] |
| 13 Clinic PEF % predicted | 3 | 630 | % (Fixed, 95% CI) | 2.00 [‐0.18, 4.18] |
| 13.1 Mild | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Mild to moderate | 1 | 255 | % (Fixed, 95% CI) | 2.0 [‐1.50, 5.50] |
| 13.3 Moderate | 1 | 240 | % (Fixed, 95% CI) | 0.9 [‐2.25, 4.05] |
| 13.4 Moderate to severe | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 Severe | 1 | 135 | % (Fixed, 95% CI) | 6.0 [0.00, 12.00] |
| 13.6 Unclear | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 1 FEV1.
9.2. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 2 Change in FEV1 compared to baseline.
9.3. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 3 FEV1 predicted.
9.4. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 4 Change in predicted FEV1.
9.5. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 5 FVC.
9.6. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 6 FVC predicted.
9.7. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 7 Mean morning PEFR.
9.8. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 8 Mean change in am PEFR.
9.9. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 9 Evening PEFR.
9.10. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 10 Mean change in evening PEFR compared to baseline.
9.11. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 11 Clinic PEF.
9.12. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 12 Change in Clinic PEF.
9.13. Analysis.

Comparison 9 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by asthma severity, Outcome 13 Clinic PEF % predicted.
Comparison 10. FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 | 10 | 1957 | Litres (Fixed, 95% CI) | 0.04 [0.01, 0.07] |
| 1.1 Mild | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Mild to moderate | 1 | 181 | Litres (Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| 1.3 Moderate | 4 | 786 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.09] |
| 1.4 Moderate to severe | 4 | 896 | Litres (Fixed, 95% CI) | 0.07 [‐0.01, 0.14] |
| 1.5 Severe | 1 | 94 | Litres (Fixed, 95% CI) | 0.01 [‐0.26, 0.28] |
| 1.6 Unclear | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Change in FEV1 | 5 | 1019 | Litres (Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2.1 Mild | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Mild to moderate | 1 | 171 | Litres (Fixed, 95% CI) | ‐0.1 [‐0.26, 0.06] |
| 2.3 Moderate | 2 | 411 | Litres (Fixed, 95% CI) | 0.02 [‐0.03, 0.07] |
| 2.4 Moderate to severe | 2 | 437 | Litres (Fixed, 95% CI) | 0.01 [‐0.09, 0.10] |
| 2.5 Severe | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Unclear | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 FEV1 predicted | 4 | 771 | % (Fixed, 95% CI) | 1.76 [‐0.10, 3.63] |
| 3.1 Mild | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Mild to moderate | 1 | 181 | % (Fixed, 95% CI) | 1.0 [‐2.92, 4.92] |
| 3.3 Moderate | 1 | 242 | % (Fixed, 95% CI) | 0.9 [‐4.90, 6.70] |
| 3.4 Moderate to severe | 1 | 254 | % (Fixed, 95% CI) | 3.0 [0.00, 6.00] |
| 3.5 Severe | 1 | 94 | % (Fixed, 95% CI) | 1.0 [‐2.50, 4.50] |
| 3.6 Unclear | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 FVC | 6 | 1315 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 4.1 Mild | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Mild to moderate | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Moderate | 2 | 520 | Litres (Fixed, 95% CI) | 0.04 [‐0.02, 0.09] |
| 4.4 Moderate to severe | 3 | 701 | Litres (Fixed, 95% CI) | 0.12 [0.02, 0.23] |
| 4.5 Severe | 1 | 94 | Litres (Fixed, 95% CI) | ‐0.06 [‐0.24, 0.12] |
| 4.6 Unclear | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Sub‐category | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Morning PEFR | 11 | 2192 | L/min (Fixed, 95% CI) | 8.15 [5.04, 11.27] |
| 5.1 Mild | 1 | 16 | L/min (Fixed, 95% CI) | ‐1.66 [‐59.81, 56.49] |
| 5.2 Mild to moderate | 3 | 428 | L/min (Fixed, 95% CI) | 8.07 [1.76, 14.37] |
| 5.3 Moderate | 4 | 948 | L/min (Fixed, 95% CI) | 7.16 [2.72, 11.59] |
| 5.4 Moderate to severe | 3 | 800 | L/min (Fixed, 95% CI) | 10.23 [4.12, 16.35] |
| 5.5 Severe | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Unclear | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Change in morning PEFR compared to baseline | 5 | 858 | Litres (Fixed, 95% CI) | 4.60 [‐2.91, 12.12] |
| 6.1 Mild | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Mild to moderate | 1 | 171 | Litres (Fixed, 95% CI) | 17.8 [‐19.42, 55.02] |
| 6.3 Moderate | 1 | 197 | Litres (Fixed, 95% CI) | 4.17 [‐7.65, 15.99] |
| 6.4 Moderate to severe | 2 | 328 | Litres (Fixed, 95% CI) | 1.48 [‐11.01, 13.96] |
| 6.5 Severe | 1 | 162 | Litres (Fixed, 95% CI) | 8.6 [‐8.53, 25.73] |
| 6.6 Unclear | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Morning PEF predicted | 3 | 747 | % (Fixed, 95% CI) | 2.09 [0.91, 3.28] |
| 7.1 Mild | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Mild to moderate | 1 | 226 | % (Fixed, 95% CI) | 3.0 [1.04, 4.96] |
| 7.3 Moderate | 1 | 258 | % (Fixed, 95% CI) | 0.8 [‐1.04, 2.64] |
| 7.4 Moderate to severe | 1 | 263 | % (Fixed, 95% CI) | 3.0 [0.50, 5.50] |
| 7.5 Severe | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.6 Unclear | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Evening PEFR | 10 | 2135 | L/min (Fixed, 95% CI) | 6.92 [3.97, 9.88] |
| 8.1 Mild | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Mild to moderate | 3 | 429 | L/min (Fixed, 95% CI) | 5.84 [0.46, 11.21] |
| 8.3 Moderate | 4 | 942 | L/min (Fixed, 95% CI) | 7.64 [3.29, 11.99] |
| 8.4 Moderate to severe | 3 | 764 | L/min (Fixed, 95% CI) | 6.92 [0.84, 13.00] |
| 8.5 Severe | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 Unclear | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Change in evening PEF compared with baseline | 4 | 646 | L/min (Fixed, 95% CI) | 8.57 [0.00, 17.13] |
| 9.1 Mild | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Mild to moderate | 1 | 171 | L/min (Fixed, 95% CI) | 9.9 [‐38.67, 58.47] |
| 9.3 Moderate | 1 | 197 | L/min (Fixed, 95% CI) | 8.14 [‐2.82, 19.10] |
| 9.4 Moderate to severe | 1 | 116 | L/min (Fixed, 95% CI) | 4.1 [‐30.96, 39.16] |
| 9.5 Severe | 1 | 162 | L/min (Fixed, 95% CI) | 10.2 [‐5.50, 25.90] |
| 9.6 Unclear | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Evening PEF predicted | 3 | 745 | % (Fixed, 95% CI) | 1.13 [0.02, 2.23] |
| 10.1 BDP | 2 | 519 | % (Fixed, 95% CI) | 0.72 [‐0.61, 2.06] |
| 10.2 BUD | 1 | 226 | % (Fixed, 95% CI) | 2.0 [0.04, 3.96] |
| 11 Clinic PEF | 9 | 2054 | L/min (Fixed, 95% CI) | 8.88 [4.40, 13.35] |
| 11.1 Mild | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Mild to moderate | 1 | 211 | L/min (Fixed, 95% CI) | 5.0 [‐4.80, 14.80] |
| 11.3 Moderate | 3 | 866 | L/min (Fixed, 95% CI) | 10.10 [4.18, 16.01] |
| 11.4 Moderate to severe | 4 | 866 | L/min (Fixed, 95% CI) | 12.81 [2.14, 23.49] |
| 11.5 Severe | 1 | 111 | L/min (Fixed, 95% CI) | ‐4.0 [‐23.00, 17.00] |
| 11.6 Unclear | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Clinic PEF predicted | 4 | 817 | % (Fixed, 95% CI) | 2.08 [0.22, 3.94] |
| 12.1 Mild | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Mild to moderate | 1 | 211 | % (Fixed, 95% CI) | 2.0 [‐1.92, 5.92] |
| 12.3 Moderate | 1 | 240 | % (Fixed, 95% CI) | 1.8 [‐1.35, 4.95] |
| 12.4 Moderate to severe | 1 | 255 | % (Fixed, 95% CI) | 4.0 [0.50, 7.50] |
| 12.5 Severe | 1 | 111 | % (Fixed, 95% CI) | ‐1.0 [‐4.00, 4.00] |
| 12.6 Unclear | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.

Comparison 10 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity, Outcome 1 FEV1.
10.2. Analysis.

Comparison 10 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity, Outcome 2 Change in FEV1.
10.3. Analysis.

Comparison 10 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity, Outcome 3 FEV1 predicted.
10.4. Analysis.

Comparison 10 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity, Outcome 4 FVC.
10.5. Analysis.

Comparison 10 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity, Outcome 5 Morning PEFR.
10.6. Analysis.

Comparison 10 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity, Outcome 6 Change in morning PEFR compared to baseline.
10.7. Analysis.

Comparison 10 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity, Outcome 7 Morning PEF predicted.
10.8. Analysis.

Comparison 10 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity, Outcome 8 Evening PEFR.
10.9. Analysis.

Comparison 10 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity, Outcome 9 Change in evening PEF compared with baseline.
10.10. Analysis.

Comparison 10 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity, Outcome 10 Evening PEF predicted.
10.11. Analysis.

Comparison 10 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity, Outcome 11 Clinic PEF.
10.12. Analysis.

Comparison 10 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by asthma severity, Outcome 12 Clinic PEF predicted.
Comparison 11. FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 | 17 | 3640 | Litres (Fixed, 95% CI) | 0.04 [0.01, 0.08] |
| 1.1 Same device for both interventions | 13 | 2121 | Litres (Fixed, 95% CI) | 0.03 [‐0.01, 0.07] |
| 1.2 Different device for each intervention | 4 | 1519 | Litres (Fixed, 95% CI) | 0.09 [0.02, 0.16] |
| 2 Change in FEV1 compared to baseline | 12 | 2635 | L/min (Fixed, 95% CI) | 0.01 [‐0.02, 0.05] |
| 2.1 Same device for both interventions | 7 | 1565 | L/min (Fixed, 95% CI) | 0.03 [‐0.01, 0.08] |
| 2.2 Different device for each intervention | 4 | 1006 | L/min (Fixed, 95% CI) | ‐0.00 [‐0.05, 0.05] |
| 2.3 Unclear | 1 | 64 | L/min (Fixed, 95% CI) | ‐0.14 [‐0.53, 0.25] |
| 3 FEV1 predicted | 7 | 1444 | % (Fixed, 95% CI) | 0.42 [‐1.02, 1.86] |
| 3.1 Same device for both interventions | 6 | 1189 | % (Fixed, 95% CI) | 0.63 [‐0.91, 2.16] |
| 3.2 Different device for each intervention | 1 | 255 | % (Fixed, 95% CI) | ‐1.0 [‐5.06, 3.06] |
| 4 Change in predicted FEV1 | 6 | 1306 | % (Fixed, 95% CI) | ‐0.39 [‐1.65, 0.86] |
| 4.1 Same device for both interventions | 4 | 992 | % (Fixed, 95% CI) | ‐0.18 [‐1.63, 1.28] |
| 4.2 Different device for each intervention | 2 | 314 | % (Fixed, 95% CI) | ‐1.04 [‐3.54, 1.47] |
| 5 FVC | 9 | 1854 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 5.1 Same device for both interventions | 6 | 892 | Litres (Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| 5.2 Different device for each intervention | 3 | 962 | Litres (Fixed, 95% CI) | 0.12 [0.03, 0.20] |
| 6 FVC predicted | 2 | 391 | % (Fixed, 95% CI) | 1.28 [‐0.84, 3.40] |
| 6.1 FP 100 mcg/d v BDP/BUD 200 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 FP 200 mcg/d v BDP/BUD 4‐500 mcg/d | 1 | 255 | % (Fixed, 95% CI) | 1.0 [‐1.50, 3.50] |
| 6.3 FP 400 mcg/d v BDP/BUD 800 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 FP 500 mcg/d v BDP/BUD 1200 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 FP 800 mcg/d v BDP/BUD 1600 mcg/d | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 FP 1000 mcg/d v BDP/BUD 2000 mcg/d | 1 | 136 | % (Fixed, 95% CI) | 2.0 [0.00, 6.00] |
| 7 Mean morning PEF | 12 | 2955 | Litres (Fixed, 95% CI) | 5.89 [0.96, 10.82] |
| 7.1 Same device for both interventions | 6 | 1101 | Litres (Fixed, 95% CI) | 0.43 [‐5.89, 6.75] |
| 7.2 Different device for each intervention | 6 | 1854 | Litres (Fixed, 95% CI) | 14.34 [6.47, 22.21] |
| 8 Mean change in am PEF | 17 | 4144 | Litres (Fixed, 95% CI) | 7.79 [5.28, 10.29] |
| 8.1 Same device for both interventions | 8 | 1618 | Litres (Fixed, 95% CI) | 6.59 [2.35, 10.83] |
| 8.2 Different device for each intervention | 9 | 2526 | Litres (Fixed, 95% CI) | 8.43 [5.33, 11.54] |
| 9 Evening PEFR | 10 | 2656 | L/min (Fixed, 95% CI) | 1.53 [‐3.78, 6.84] |
| 9.1 Same device for both interventions | 6 | 1100 | L/min (Fixed, 95% CI) | ‐1.86 [‐8.05, 4.32] |
| 9.2 Different device for each intervention | 4 | 1556 | L/min (Fixed, 95% CI) | 10.97 [0.65, 21.28] |
| 10 Mean change in evening PEF compared to baseline | 10 | 2186 | L/min (Fixed, 95% CI) | 2.59 [‐0.36, 5.55] |
| 10.1 Same device for both interventions | 8 | 1638 | L/min (Fixed, 95% CI) | 2.31 [‐1.20, 5.82] |
| 10.2 Different device for each intervention | 2 | 548 | L/min (Fixed, 95% CI) | 3.29 [‐2.17, 8.75] |
| 11 Clinic PEF | 12 | 2545 | L/min (Random, 95% CI) | 17.04 [‐0.99, 35.07] |
| 11.1 Same device for both interventions | 8 | 1297 | L/min (Random, 95% CI) | 22.92 [‐8.13, 53.97] |
| 11.2 Different device for each intervention | 4 | 1248 | L/min (Random, 95% CI) | 12.90 [1.95, 23.85] |
| 12 Change in Clinic PEF | 6 | 1322 | L/min (Fixed, 95% CI) | 5.10 [‐1.18, 11.38] |
| 12.1 Same device for both interventions | 3 | 863 | L/min (Fixed, 95% CI) | 4.46 [‐3.11, 12.02] |
| 12.2 Different device for each intervention | 2 | 395 | L/min (Fixed, 95% CI) | 6.67 [‐4.65, 18.00] |
| 12.3 Unclear | 1 | 64 | L/min (Fixed, 95% CI) | ‐3.48 [‐98.54, 91.58] |
| 13 Clinic PEF % predicted | 3 | 630 | % (Fixed, 95% CI) | 2.00 [‐0.18, 4.18] |
| 13.1 Same device for both interventions | 3 | 630 | % (Fixed, 95% CI) | 2.00 [‐0.18, 4.18] |
| 13.2 Different device for each intervention | 0 | 0 | % (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
11.1. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 1 FEV1.
11.2. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 2 Change in FEV1 compared to baseline.
11.3. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 3 FEV1 predicted.
11.4. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 4 Change in predicted FEV1.
11.5. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 5 FVC.
11.6. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 6 FVC predicted.
11.7. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 7 Mean morning PEF.
11.8. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 8 Mean change in am PEF.
11.9. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 9 Evening PEFR.
11.10. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 10 Mean change in evening PEF compared to baseline.
11.11. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 11 Clinic PEF.
11.12. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 12 Change in Clinic PEF.
11.13. Analysis.

Comparison 11 FP versus BDP or BUD, parallel group studies: dose ratio 1:2 subgroup by delivery device, Outcome 13 Clinic PEF % predicted.
Comparison 12. FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 | 10 | 1957 | Litres (Fixed, 95% CI) | 0.04 [0.01, 0.07] |
| 1.1 Same device for both interventions | 7 | 1413 | Litres (Fixed, 95% CI) | 0.05 [0.02, 0.09] |
| 1.2 Different device for each intervention | 3 | 544 | Litres (Fixed, 95% CI) | 0.02 [‐0.04, 0.07] |
| 2 Change in FEV1 | 5 | 1019 | Litres (Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 2.1 Same device for both interventions | 2 | 437 | Litres (Fixed, 95% CI) | 0.01 [‐0.09, 0.10] |
| 2.2 Different device for each intervention | 3 | 582 | Litres (Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 3 FEV1 predicted | 4 | 771 | % (Fixed, 95% CI) | 1.76 [‐0.10, 3.63] |
| 3.1 Same device for both interventions | 3 | 590 | % (Fixed, 95% CI) | 1.99 [‐0.13, 4.11] |
| 3.2 Different device for each intervention | 1 | 181 | % (Fixed, 95% CI) | 1.0 [‐2.92, 4.92] |
| 4 FVC | 6 | 1315 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 4.1 Same device for both interventions | 5 | 983 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.10] |
| 4.2 Different device for each intervention | 1 | 332 | Litres (Fixed, 95% CI) | 0.1 [‐0.14, 0.34] |
| 5 Morning PEF | 11 | 2192 | L/min (Fixed, 95% CI) | 8.15 [5.04, 11.27] |
| 5.1 Same device for both interventions | 6 | 1413 | L/min (Fixed, 95% CI) | 9.05 [5.08, 13.02] |
| 5.2 Different device for each intervention | 5 | 779 | L/min (Fixed, 95% CI) | 6.71 [1.69, 11.74] |
| 6 Change in morning PEFR compared to baseline | 5 | 858 | Litres (Fixed, 95% CI) | 4.60 [‐2.91, 12.12] |
| 6.1 Same device for both interventions | 2 | 328 | Litres (Fixed, 95% CI) | 1.48 [‐11.01, 13.96] |
| 6.2 Different device for each intervention | 2 | 368 | Litres (Fixed, 95% CI) | 5.42 [‐5.85, 16.68] |
| 6.3 Unclear | 1 | 162 | Litres (Fixed, 95% CI) | 8.6 [‐8.53, 25.73] |
| 7 Morning PEF predicted | 3 | 747 | % (Fixed, 95% CI) | 2.09 [0.91, 3.28] |
| 7.1 Same device for both interventions | 2 | 521 | % (Fixed, 95% CI) | 1.57 [0.09, 3.06] |
| 7.2 Different device for each intervention | 1 | 226 | % (Fixed, 95% CI) | 3.0 [1.04, 4.96] |
| 8 Evening PEF | 10 | 2135 | L/min (Fixed, 95% CI) | 6.92 [3.97, 9.88] |
| 8.1 Same device for both interventions | 6 | 1372 | L/min (Fixed, 95% CI) | 8.52 [4.61, 12.43] |
| 8.2 Different device for each intervention | 4 | 763 | L/min (Fixed, 95% CI) | 4.81 [0.30, 9.32] |
| 9 Change in evening PEF compared with baseline | 4 | 646 | L/min (Fixed, 95% CI) | 8.57 [0.00, 17.13] |
| 9.1 Same device for both interventions | 1 | 116 | L/min (Fixed, 95% CI) | 4.1 [‐30.96, 39.16] |
| 9.2 Different device for each intervention | 2 | 368 | L/min (Fixed, 95% CI) | 8.23 [‐2.46, 18.91] |
| 9.3 Unclear | 1 | 162 | L/min (Fixed, 95% CI) | 10.2 [‐5.50, 25.90] |
| 10 Evening PEF predicted | 3 | 745 | % (Fixed, 95% CI) | 1.13 [0.02, 2.23] |
| 10.1 Same device for both interventions | 2 | 519 | % (Fixed, 95% CI) | 0.72 [‐0.61, 2.06] |
| 10.2 Different device for each intervention | 1 | 226 | % (Fixed, 95% CI) | 2.0 [0.04, 3.96] |
| 11 Clinic PEFR (L/min) | 9 | 2054 | L/min (Fixed, 95% CI) | 8.88 [4.40, 13.35] |
| 11.1 Same device for both interventions | 7 | 1511 | L/min (Fixed, 95% CI) | 10.38 [5.24, 15.51] |
| 11.2 Different device for each intervention | 2 | 543 | L/min (Fixed, 95% CI) | 4.17 [‐4.93, 13.26] |
| 12 Clinic PEF % predicted | 4 | 817 | % (Fixed, 95% CI) | 2.08 [0.22, 3.94] |
| 12.1 Same device for both interventions | 3 | 606 | % (Fixed, 95% CI) | 2.10 [‐0.02, 4.22] |
| 12.2 Different device for each intervention | 1 | 211 | % (Fixed, 95% CI) | 2.0 [‐1.92, 5.92] |
12.1. Analysis.
Comparison 12 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device, Outcome 1 FEV1.
12.2. Analysis.

Comparison 12 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device, Outcome 2 Change in FEV1.
12.3. Analysis.

Comparison 12 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device, Outcome 3 FEV1 predicted.
12.4. Analysis.

Comparison 12 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device, Outcome 4 FVC.
12.5. Analysis.

Comparison 12 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device, Outcome 5 Morning PEF.
12.6. Analysis.

Comparison 12 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device, Outcome 6 Change in morning PEFR compared to baseline.
12.7. Analysis.

Comparison 12 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device, Outcome 7 Morning PEF predicted.
12.8. Analysis.

Comparison 12 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device, Outcome 8 Evening PEF.
12.9. Analysis.

Comparison 12 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device, Outcome 9 Change in evening PEF compared with baseline.
12.10. Analysis.

Comparison 12 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device, Outcome 10 Evening PEF predicted.
12.11. Analysis.

Comparison 12 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device, Outcome 11 Clinic PEFR (L/min).
12.12. Analysis.

Comparison 12 FP versus BDP or BUD, parallel group studies: dose ratio 1:1 subgroup by delivery device, Outcome 12 Clinic PEF % predicted.
Comparison 13. FP versus BDP or BUD, crossover studies: dose ratio 1:1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 % reduction from baseline in cortisol AUC0‐20 compared to baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Exacerbation | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Nocturnal urinary cortisol | 2 | 84 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.21, 0.65] |
| 4 PEF | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
13.1. Analysis.

Comparison 13 FP versus BDP or BUD, crossover studies: dose ratio 1:1, Outcome 1 % reduction from baseline in cortisol AUC0‐20 compared to baseline.
13.2. Analysis.

Comparison 13 FP versus BDP or BUD, crossover studies: dose ratio 1:1, Outcome 2 Exacerbation.
13.3. Analysis.

Comparison 13 FP versus BDP or BUD, crossover studies: dose ratio 1:1, Outcome 3 Nocturnal urinary cortisol.
13.4. Analysis.

Comparison 13 FP versus BDP or BUD, crossover studies: dose ratio 1:1, Outcome 4 PEF.
13.5. Analysis.

Comparison 13 FP versus BDP or BUD, crossover studies: dose ratio 1:1, Outcome 5 FEV1 (% predicted).
Comparison 14. FP versus BDP or BUD, crossover studies: dose ratio 1:2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 FEV1 (litres) | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.23, 0.26] |
| 3 FVC (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Morning PEFR (L/min) | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | 3.02 [‐28.28, 34.31] |
| 5 Evening PEFR (L/min) | 2 | 76 | Mean Difference (IV, Fixed, 95% CI) | 5.86 [‐23.58, 35.31] |
| 6 Daytime breathlessness score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Night‐time breathlessness score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Percentage of symptom free days | 2 | 618 | Mean Difference (IV, Fixed, 95% CI) | ‐1.33 [‐7.62, 4.97] |
| 9 Percentage of symptom free nights | 2 | 618 | Mean Difference (IV, Fixed, 95% CI) | 1.39 [‐3.59, 6.38] |
| 10 One or more night‐time awaking due to asthma symptoms (No. of patients) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 11 Symptoms on wakening in the morning (No. of patients) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 12 Percentage of rescue beta2 agonist free days (No. of patients) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Morning plasma cortisol (nmol/L) | 3 | 782 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐27.70, 27.02] |
| 14 Plasma cortisol 30 min post 25U cosyntropin (nmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15 24 hour urinary free cortisol (nmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
14.1. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 1 FEV1 (% predicted).
14.2. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 2 FEV1 (litres).
14.3. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 3 FVC (% predicted).
14.4. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 4 Morning PEFR (L/min).
14.5. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 5 Evening PEFR (L/min).
14.6. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 6 Daytime breathlessness score.
14.7. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 7 Night‐time breathlessness score.
14.8. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 8 Percentage of symptom free days.
14.9. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 9 Percentage of symptom free nights.
14.10. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 10 One or more night‐time awaking due to asthma symptoms (No. of patients).
14.11. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 11 Symptoms on wakening in the morning (No. of patients).
14.12. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 12 Percentage of rescue beta2 agonist free days (No. of patients).
14.13. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 13 Morning plasma cortisol (nmol/L).
14.14. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 14 Plasma cortisol 30 min post 25U cosyntropin (nmol/L).
14.15. Analysis.

Comparison 14 FP versus BDP or BUD, crossover studies: dose ratio 1:2, Outcome 15 24 hour urinary free cortisol (nmol/L).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acun 2005.
| Methods | Setting: Hospital OPD in Turkey Design: parallel group trial Length of intervention period: 52 weeks Randomisation: yes; method not described Masking: open label Excluded: stated (none) Withdrawals: not stated Baseline characteristics: comparable between groups Jadad score: 1 | |
| Participants | 100 children: 51M 49F Age range: 4‐11.5 years Inclusion criteria: symptomatic 2 x week; recent diagnosis of moderate persistent asthma; nocturnal symptoms >1 night per week; within 10th‐90th centile | |
| Interventions | FP: 125 BID (250mcg/d)
BUD: 200 BID (400mcg/d) Inhaler device: MDI |
|
| Outcomes | Growth rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | Open label study |
Agertoft 1997.
| Methods | Setting: Denmark, paediatric outpatient clinic Design: crossover, 2 week washout Length of intervention period: 2 weeks Randomisation: yes, computer generated random sequence with balanced blocks Masking: double‐blind Excluded: stated (none) Withdrawals: stated (one child from low dose group due to sore throat) Baseline characteristics: comparable between groups Jadad score: 5 | |
| Participants | 48 children: 27M 21F Age range: 6 to 12 years Inclusion criteria: Pre‐pubertal children 'Mild' asthma requiring treatment with as needed beta2 agonists only Exclusion criteria: Inhaled or oral steroid use in last 2 months | |
| Interventions | Group 1
FP: 200mcg/d via Diskhaler DPI BUD: 200 mcg/d via Turbuhaler DPI Group 2 FP: 400mcg/d via Diskhaler DPI BUD: 400 mcg/d via Turbuhaler DPI |
|
| Outcomes | FEV1 Morning PEFR Evening PEFR Daily asthma symptom score Daily use of beta2agonist 24 hour urinary cortisol excretion Growth by lower leg knemometry | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random sequence with balanced blocks |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Double dummy design |
Agertoft 1997a.
| Methods | Setting: Denmark, paediatric outpatient clinic Design: parallel group, dose down‐titration design Length of intervention period: variable (see notes) Randomisation: yes, computer generated sequence Allocation concealment: unclear Masking: double blind (double dummy) Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 219 children: 136M 83F Age range: 5 to 16 years Inclusion criteria: Children with asthma aged 5‐16 years Requiring BUD 400 or 800 mcg/d via MDI+Nebuhaler spacer for asthma control at end of evaluation period Able to use Turbuhaler/Diskhaler delivery devices Exclusion criteria: Systemic corticosteroids/theophylline/cromoglycate use in last month Asthma exacerbation in last 2 months Respiratory tract infection in last month Poor compliance | |
| Interventions | FP: half (100 or 200) mcg/d baseline dose BUD via Diskhaler DPI BUD: half (100 or 200) mcg/d baseline dose BUD via Turbuhaler DPI Patients treated for 5 weeks on above regimen. Dose reduction was then attempted at five weekly intervals. A priori defined criteria for deterioration in asthma and acceptable asthma control were applied. Patients with acceptable control had their daily dose of ICS reduced by 50% until deterioration in control occurred or ICS dose of 100 mcg/d was achieved. |
|
| Outcomes | Following dose down‐titration period: Dose reduction steps from baseline Minimal effective ICS dose (mcg/d) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Double blind (double dummy) |
Ayres 1995.
| Methods | Setting: multicentre study worldwide, hospital outpatient clinics Design: parallel group Length of intervention period: 6 weeks Randomisation: computer generated random sequence Allocation concealment: yes (central coding by pharmaceutical company sponsor) Masking: double blind Excluded: stated Withdrawals: adverse event rates reported, unclear if any led to patient withdrawal Baseline characteristics: comparable between groups Jadad score: 3 | |
| Participants | 862 adults enrolled, 671randomised Age range: 18 to 70 years Inclusion criteria: Adults with a clinical history of severe asthma Requiring BDP 1‐2 mg/d or BUD 0.8‐1.6 mg/d BUD for asthma control During run‐in period: Asthma symptom scores of 1 or more on 4 out of last 7 days and either: 1. At least 15% reversibility FEV1 post beta2 agonist or: 2. Diurnal variation in PEFR 15% or greater on 4 out of last 7 days or: 3. Need for 2 or more doses beta2 agonist each of last 7 days with either a). % predicted FEV1 80% or greater b) Mean morning PEFR 80% or greater in last 7 days Exclusion criteria: Alteration of normal asthma medication during run‐in period Hospital admission due to asthma exacerbation in last month Systemic corticosteroids > 10mg daily Suspected of being steroid hypersensitive Concomitant disease likely to complicate evaluation of drug Current smokers | |
| Interventions | FP:
1. 125 mcg 4 puffs 2xdaily (1000 mcg/d)
2. 250 mcg 4 puffs 2xdaily (2000 mcg/d) BUD: 200 mcg 4 puffs 2xdaily (1600 mcg/d) Delivery device: MDI +/‐ spacer |
|
| Outcomes | Outcomes expressed as a change compared to baseline: FEV1 FVC Clinic PEFR Morning PEFR Evening PEFR Diurnal variation in PEFR Symptom free days Symptom free nights Daytime symptom score Night‐time symptom score Rescue beta2 agonist free days Asthma exacerbations Morning plasma cortisol Biochemical markers of bone turnover |
|
| Notes | Details concerning randomisation method provided by Glaxo Wellcome Patients were given the option of using spacer device |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random sequence |
| Allocation concealment? | Low risk | Central coding by pharmaceutical company sponsor |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Backman 2001.
| Methods | Setting: multicentre study (nine countries) Design: parallel group trial Length of intervention period: 4 weeks Randomisation: PACT Allocation concealment: adequate Masking: Open label Excluded: Yes (post‐run in) Withdrawals: Stated Jadad score: 3 | |
| Participants | 277 randomised (FP: 137; BUD: 140). Mean age (range): FP: 46.3 (19‐81); BUD: 44.3 (18‐87); Baseline FEV1 (L): FP: 2.44 (SD 0.77); BUD: 2.46 (SD 0.76); FEV1 % predicted: FP: 75 (SD 12); BUD: 75 (SD 15); +ve atopic (%): FP: 61; BUD: 62; Duration of asthma (N): <1‐5 years: FP: 24; BUD: 36; >5‐10 years: FP 28; BUD: 23; >10 years: FP: 85; BUD: 81
Inclusion criteria: >18 years of age; documented history of reversible airways obstruction; FEV1 (% predicted) between 50‐90%; stable phase of disease; requirement for between 400 and 1200mcg/day of BDP, flunisolide or BUD. Exclusion criteria: Use of Diskus or Tubruhaler in previous 6 months; no treatment for airways disease in previous 4 weeks. |
|
| Interventions | FP 500 (via Diskus) versus BUD1200 (via Turbuhaler). 2‐week baseline period where PEF (am and pm); symptoms and rescue medication usage were measured |
|
| Outcomes | FEV1; am PEF; pm PEF; serum cortisol; adverse events; device handling | |
| Notes | Details concerning randomisation method provided by Glaxo Wellcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | High risk | Open label design |
Barnes 1993.
| Methods | Setting: multicentre study Europe, hospital outpatient clinic Design: parallel group Length of intervention period: 6 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes (coded, sealed envelopes) Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 154 adults randomised. FEV1: 2.95 (SD 0.83) (FEV1 % predicted: 80.0 (SD 21.4)); Mean FVC: 4.42 L (SD 0.94). 8 females. Inclusion criteria: either sex; 18‐60 years of age; ATS defined asthma; = 40% predicted value; Either post‐BD increase in FEV1 of at least 200ml or = 12% of baseline, OR dirunal variation of PEF = 15% on at least 2 days/week during run‐in. Exclusion criteria: Exacerbation 4 weeks before inclusion; use of oral steroids within 6 months ; use of ICS/other systemic steroids within 4 weeks |
|
| Interventions | FP: 250 mcg 2 puffs 2xdaily (1000 mcg/d) BDP: 250 mcg 4 puffs 2xdaily (2000 mcg/d) Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) FVC (% predicted) PEFR (% predicted) Morning PEFR Evening PEFR Diurnal variation in PEFR % symptom‐free days % symptom‐free nights % beta2‐agonist free days % beta‐2 agonist nights Daytime beta2 agonist use (puffs) Night‐time beta2 agonist use (puffs) Oral candidiasis Upper respiratory tract infection Oropharyngeal side effects Plasma cortisol (sample time during day not specified) | |
| Notes | Details concerning randomisation method provided by Glaxo Wellcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Basran 1997.
| Methods | Setting: multicentre study UK, primary care and hospital outpatient clinics Design: parallel group Length of intervention period: 8 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes (central coded schedule prepared by pharmaceutical company sponsors) Masking: open, no blinding Excluded: stated Withdrawals: stated Jadad score: 3 | |
| Participants | 229 adults enrolled, 176 randomised Age range: 18 to 60 years Inclusion criteria: FEV1 > 40 (% predicted) Current ICS therapy 400 of 800 mcg/d During 2 week run‐in period one or more of the following: 1) 20% or greater diurnal PEFR variability 2) 7 or more puffs beta2 agonist daily for 7 consecutive days 3) night time awakening 2 out of 7 nights Exclusion criteria: Respiratory tract infection in last month Serious concurrent disease | |
| Interventions | FP: half pre‐study nominal daily dose (200 or 400 mcg/d) via Diskhaler DPI BUD: half pre‐study nominal daily dose (200 or 400 mcg/d) via Turbohaler DPI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Diurnal variation in PEFR Daytime asthma symptom score Night‐time asthma symptom score Daytime beta2 agonist use Night‐time beta2 agonist use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | Central coded schedule prepared by pharmaceutical company sponsors |
| Blinding? All outcomes | High risk | Open label |
Berend 2001.
| Methods | Setting: multicentre study Australia, hospital outpatient clinics Design: parallel group Length of intervention period: 6 months Randomisation: yes, method not stated Allocation concealment: unclear Masking: open, non‐blinded Excluded: not stated Withdrawals: not stated Baseline characteristics: comparable Jadad score: 1 | |
| Participants | 133 adults: 64M 69F Age range: 20 to 78 years Inclusion criteria: Adults requiring BDP or BUD > 1750 mcg/d for asthma control One or more of following: 190ml or greater FEV1 reversibility after inhaled beta2 agonist Asthma symptoms on 2 or more days/week for 2 weeks in last month Night‐time symptoms 2 or more nights/week Diurnal PEFR variability 15% or greater Exclusion criteria: Regular oral steroid therapy Significant co‐existent disease | |
| Interventions | FP: half usual maintenance dose (mcg/d) via MDI + spacer BDP or BUD: usual maintenance dose via MDI+ spacer or Turbuhaler DPI |
|
| Outcomes | FEV1 FVC Morning PEFR Clinic PEFR HRQOL: Change in Asthma Quality of Life Questionnaire domain scores compared to baseline HRQOL: Change in Short‐form 36 questionnaire domain scores compared to baseline Morning plasma cortisol Urine cortisol:creatinine ratio (1 hour post waking sample) Asthma exacerbations Local oral side‐effects Extent and ease of skin bruising Biochemical markers of bone turnover | |
| Notes | Study in abstract form until 2001, full published version available for 2004 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | Open label |
Bisca 1997.
| Methods | Setting: Romania, hospital outpatient clinic Design: parallel group Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: not stated Excluded: not stated Withdrawals: not stated Jadad score: 1 | |
| Participants | 48 children
Age range: 4 to 18 years
Inclusion criteria:
Children with asthma, no further details
Exclusion criteria:
Not stated Baseline asthma control FEV1 (% predicted): Mean FP group 52.3, mean BDP group 48.8 |
|
| Interventions | FP: 200 mcg/d BDP: 400 mcg/d Delivery device: not stated |
|
| Outcomes | FEV1 (% predicted) FVC Clinic PEFR (% predicted) MEF50 (% predicted) Airways resistance Morning PEFR Evening PEFR Asthma symptoms score Rescue beta2 agonist use | |
| Notes | Study in abstract form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Boe 1994.
| Methods | Setting: multicentre study Norway, hospital outpatient clinic Design: parallel group Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 134 adults: 80M 54F Age range: 20 to 75 years Inclusion criteria: Patients > 18 years with a clinical diagnosis of asthma Receiving 400 ‐ 2000 mcg/d of BDP or BUD for at least 4 weeks After run‐in period at least 2 of following: 1. FEV1 < 80 (% predicted) 2. Mean morning PEFR during last 7 days < 80 (% predicted) 3. 20% or greater diurnal variability in PEFR on at least 4 of last 7 days 4. Asthma symptoms on at least 4 of last 7 days Exclusion criteria: Oral corticosteroids with in the last 4 weeks | |
| Interventions | FP: 500 mcg 2 actuations 2xdaily (2000 mcg/d) BDP: 400 mcg 2 actuations 2xdaily (1600 mcg daily) Delivery device: Diskhaler DPI |
|
| Outcomes | Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Daytime symptom score Night‐time symptom score Beta2 agonist use daytime (puffs) Beta2 agonist use night‐time (puffs) Clinic PEFR FEV1 FVC Change in morning serum cortisol compared to baseline Change in serum ACTH compared to baseline Oral candidiasis Oropharyngeal side effects | |
| Notes | No reply from author to clarify details of randomisation procedure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Bootsma 1995.
| Methods | Setting: The Netherlands, hospital outpatient clinic Design: crossover, 3 week washout Length of intervention period: 3 weeks Randomisation: yes, by computer generated sequence Allocation concealment: yes (central coding by pharmaceutical company sponsors) Masking: double blind Excluded: stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 21 adults: 10M 11F Mean age 30.2 years Inclusion criteria: Adults with asthma (ATS criteria 1987) During run‐in period: 4 or more symptomatic days during at least 7 days of last 2 weeks FEV1 at least 50 (% predicted) Histamine BHR (PC20 FEV1) < 4mg/ml Exclusion criteria: Subjects with seasonal allergy Use of systemic steroids in last 6 months Lower respiratory tract infection in last 6 weeks | |
| Interventions | FP: 125 mcg 3 puffs 2xdaily (750 mcg/d) BDP: 250 mcg 3 puffs 2xdaily (1500 mcg/d) Delivery device: MDI |
|
| Outcomes | FEV1 Morning PEFR Evening PEFR Diurnal variability PEFR (%) Daytime breathlessness score Night‐time breathlessness score Daily use of rescue beta2 agonist (puffs/day) Histamine BHR (PC20 FEV1) UNDW BHR (PC20 FEV1) Morning serum cortisol Serum and urinary markers of bone turnover Blood inflammatory cell profiles Blood eosinophil count Serum ECP | |
| Notes | Author provided details of randomisation method and data for morning plasma cortisol measurements Carryover effects were tested for and excluded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | Central coding by pharmaceutical company sponsors |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Connolly 1995.
| Methods | Setting: multicentre study UK, primary care Design: parallel group Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: open, no blinding Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 2 | |
| Participants | 283 adults enrolled, 190 randomised: 43M 39F Age range: 18‐70 years Inclusion criteria: Adult asthmatic patients Receiving BDP or BUD 200 mcg/d or less During 2 week run‐in period: Symptom score 1 or greater on 10 successive days FEV1 > 50 (% predicted) Able to use delivery devices Exclusion criteria: Current treatment with OCS or > 6 courses in last year Change in asthma therapy in last 6 weeks Serious coexistent illness | |
| Interventions | FP: 100 mcg 1 actuation 2xdaily (200 mcg/d) via Diskhaler DPI BUD: 200 mcg 1 actuation 2xdaily (400 mcg/d)via Tubuhaler DPI |
|
| Outcomes | Change in morning PEFR compared to baseline Change in diurnal variation in PEFR compared to baseline % symptom free days % symptom free nights % rescue beta2 agonist free days % rescue beta2 agonist free nights Physician assessed level of overall asthma control Patient assessed level of overall asthma control Morning plasma cortisol | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | Open label |
Currie 2002.
| Methods | Setting: single centre study Design: crossover study Length of intervention period: 2 x 6 week treatment arms Randomisation: Not described. Allocation concealment: Not described Masking: double blind Excluded: Not stated Withdrawals: Stated (non‐ITT) Jadad score: 3 | |
| Participants | N=20. (M/F: 8/12); Mean age: 38 (SEM 4); Mean ICS usage: 485mcg (78) for 13 participants, remaining 5 used SABA prn; FEV1 % predicted: 88.5 (SEM 2.5); am PEF (L/min): 443 (SEM 19); OUCC (nmol/mmol): 6.59 (SEM 0.7); Methacholine PD20 (mcg): 96.6 (SEM 22.1) Inclusion criteria: FEV1 >70% predicted, PD20 <500mcg; SABA only or constant ICS dose up to 1200mcg Exclusion criteria: history URTI; prior OCS usage | |
| Interventions | FP500 versus BDP500. Inhaler device: pMDI. | |
| Outcomes | FEV1 (% predicted); am PEF (L/min); OUCC (nmol/mmol); exhaled tidal NO (ppb); methacholine PD20; overnight urinary cortisol/creatinine | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Dahl 1993.
| Methods | Setting: world‐wide multicentre study, hospital outpatient clinic Design: parallel group Length of intervention period: 4 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes (central coding by pharmaceutical company sponsors) Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 825 adults: 297M 528F Age range: 17 to 74 years Inclusion criteria: Adults with moderately severe chronic asthma requiring BDP 1000 mcg/d or less. During run‐in period: Daytime or night‐time symptoms during at least 4 days or: Diurnal variation in PEFR of 20% or more Exclusion criteria: Systemic steroids within the last month Serious concurrent disease | |
| Interventions | FP:
1. 100 mcg/d
2. 200 mcg/d
3. 400 mcg/d
4. 800 mcg/d BDP: 400 mcg/d Delivery device: MDI |
|
| Outcomes | Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Morning PEFR Evening PEFR Diurnal variation in PEFR FEV1 (% predicted) FVC Clinic PEFR (% predicted) % symptom free days Rescue beta2 agonist use (puffs/day) Plasma cortisol Plasma cortisol 30 mins after 250 mcg ACTH Incidence of oral candidiasis Incidence of oropharyngeal side effects | |
| Notes | Details of randomisation method and data provided by Glaxo Wellcome Unpublished data available from www.ctr.gsk.co.uk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Dal Negro 1997.
| Methods | Setting: Italy, hospital outpatient clinic Design: crossover, 6 week 'washout' when patients treated with BDP Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind (double dummy) Excluded: not stated Withdrawals: not stated Jadad score: 2 | |
| Participants | 8 adults: 4M 4F Age range: 19 to 39 years Inclusion criteria: Adult asthmatics No further details Exclusion criteria: Smokers Baseline asthma control FEV1 (% predicted): > 80 Symptom frequency: asymptomatic | |
| Interventions | FP: 250 mcg 2xdaily (500 mcg/d) BUD: 400mcg 2xdaily (800 mcg/d) Delivery device: not stated |
|
| Outcomes | Methacholine BHR (PD20 FEV1) | |
| Notes | Study in abstract form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Double blind (double dummy) |
de Benedictis 2001.
| Methods | Setting: Multicentre trial Design: parallel group Length of intervention period: 52 weeks Randomisation: PACT (computer package for GSK) Allocation concealment: Adequate Masking: double‐blind Excluded: Yes (post run‐in) Withdrawals: Stated Jadad score: 5 | |
| Participants | 343 children randomised. Mean age: FP: 7.6 (SD 1.7); BDP: 7.6 (SD 2.0). No baseline data given for lung function, current treatment or symptoms. Inclusion criteria: Boys aged 4‐11, girls aged 4‐9 with sexual maturity Tanner rating stage 1; requirement of FP 100‐200 mcg/d, or BDP 200‐500 mcg/d at least previous 8 weeks, at constant dose for at least 4 weeks before run‐in period Exclusion criteria: Intermittent asthma; disoders that could affect growth; OCS/parnteral CS rx; admission to hospital in last 4 weeks before run‐in phase with respiratory disease. | |
| Interventions | FP: 200 mcg 2xdaily (400 mcg/d) BDP: 200 mcg 2xdaily (400 mcg/d) Delivery device: Diskhaler DPI |
|
| Outcomes | PEF (am and pm); FEV1; symptoms; rescue medication; growth | |
| Notes | Unpublished data supplied by GSK 140904 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Derom 1999.
| Methods | Setting: single centre Design: crossover Length of intervention period: 5 x 1 week treatment arms Randomisation: Not stated Allocation concealment: Not described. Masking: Double‐blind, double dummy Excluded: Not stated Withdrawals: Not stated Jadad score: 3 | |
| Participants | 23 adults randomised. FEV1: 2.95 (SD 0.83) (FEV1 % predicted: 80.0 (SD 21.4)); Mean FVC: 4.42 L (SD 0.94). 8 females. Inclusion criteria: either sex; 18‐60 years of age; ATS defined asthma; = 40% predicted value; Either post‐BD increase in FEV1 of at least 200ml or = 12% of baseline, OR dirunal variation of PEF = 15% on at least 2 days/week during run‐in. Exclusion criteria: Exacerbation 4 weeks before inclusion; use of oral steroids within 6 months ; use of ICS/other systemic steroids within 4 weeks |
|
| Interventions | FP 400mcg; FP 2000mcg; BUD: 400mcg; BUD 1600mcg; Placebo administered over 1 week. FP via Diskhaler; BUD versus Turbohaler Concomitant therapy: IP, xanthines, sodium cromoglycate permitted provided doses kept at constant level 4 weeks prior to inclusion |
|
| Outcomes | FEV1 PEFR Serum cortisol White blood cell count Neutrophils Basophils | |
| Notes | Data reported for effects after 24hours @ 1 week. Nominal dose ratio: 1:1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Double‐blind, double dummy |
Egan 1999.
| Methods | Setting: single centre (UK) Design: parallel group Length of intervention period: 104 weeks Randomisation: Not stated Allocation concealment: Not described. Masking: Double‐blind Excluded: Not stated Withdrawals: Stated Jadad score: 3 | |
| Participants | 36 adults randomised. FEV1: 2.9‐3.1 L. 17 females. Inclusion criteria: either sex; 18‐50 years of age; BDP/BUD treatment 1‐2000mcg/d. Exclusion criteria: No changes to treatment in 8 weeks prior to study entry; OCS treatment in 8 weeks prior to study entry |
|
| Interventions | 1) FP 1000mcg/d 2) BDP 2000mcg/d Inhaler device: MDI plus spacer |
|
| Outcomes | FEV1 PEFR Serum cortisol White blood cell count Bone densitometry Withdrawals | |
| Notes | Nominal dose ratio: 1:2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Unclear risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Fabbri 1993.
| Methods | Setting: multicentre study Europe and Australia, hospital outpatient clinic Design: parallel group Length of intervention period: 12 months Randomisation: yes, computer generated sequence Allocation concealment: yes (central coding by pharmaceutical company) Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 274 adults: 155M 119F Age range: 17 to 80 years Inclusion criteria: Patients with symptomatic moderate to severe asthma Receiving BDP or BUD 1000 mcg/d or greater During run‐in period at least 2 of following: 1. Mean morning PEFR on last 7 days 70 (% predicted) or less 2. 15% or greater reversibility in FEV1 after inhaled beat2 agonist 3. Diurnal variability in PEFR 20% or greater in 4 out last 7 days 4. Asthma symptoms on 4 or more days out last 7 days Exclusion criteria: Requirement for treatment with 2000 or more mcg daily BDP/BUD or systemic steroids within last month or on more than 3 occasions in the last 6 months Hypersensitivity to inhaled corticosteroids Severe co‐existent disease | |
| Interventions | FP: 750 mcg 2xdaily (1500 mcg/d) BDP: 750 mcg 2xdaily (1500 mcg/d) Delivery device: MDI +/‐ spacer |
|
| Outcomes | Change in FEV1 compared to baseline Change in clinic PEFR compared to baseline Morning PEFR Evening PEFR % symptom free days % symptom free nights % beta2 agonist free days Asthma exacerbations Withdrawal due to asthma exacerbation (No. of patients) Morning plasma cortisol 24 hour urinary free cortisol Plasma cortisol 30 and 60 mins post 250 mcg tetracosactin Oral Candidisasis Oropharyngeal side effects | |
| Notes | Details of randomisation method provided by Glaxo Wellcome Unpublished data available from www.ctr.gsk.co.uk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Ferguson 1999.
| Methods | Setting: multicentre study (Canada, Denmark, Indonesia, South Africa, The Netherlands), paediatric outpatient clinics Design: parallel group Length of intervention period: 20 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind (double dummy) Excluded: stated Withdrawals: stated Baseline characteristics: comparable age, height, duration of asthma Jadad score: 3 | |
| Participants | 442 children enrolled, 333 randomised: 223M 110F Mean (SD) age: 8.2 (2) years FP group, 7.9 (2) years BUD group Inclusion criteria: Children with moderate to severe asthma Requiring BDP or BUD 400‐800 mcg/d or FP 200‐400 mcg/d for asthma control for one month or longer Ability to use inhaler device and complete diary card with parental assistance During 7 run‐in period: daily symptom score of 1 or greater on 4 consecutive days, and mean morning PEFR < 85 (% predicted) over last 4 days or 15% or greater reversibility in PEFR after inhaled beta2 agonist Exclusion criteria: Patients with seasonal or exercise‐induced symptoms only Hospital admission due to asthma in last month Previous systemic corticosteroid use Co‐existent serious illness | |
| Interventions | FP: 200 mcg 2xdaily (400 mcg/d) via Diskus/Accuhaler DPI BUD: 400 mcg 2xdaily (800 mcg/d) via Turbuhaler DPI |
|
| Outcomes | Morning PEFR Change in daytime symptom score compared to baseline Change in night‐time symptom score compared to baseline Daytime rescue beta2 agonist use Night‐time rescue beta2 agonist use Change in height compared to baseline Change in morning plasma cortisol compared to baseline Asthma exacerbations Oro‐pharyngeal side effects Height assessment | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Double blind (double dummy) |
Ferguson 2006.
| Methods | Setting: Multicentre trial Design: parallel group Length of intervention period: 52 weeks Randomisation: Not reported Allocation concealment: Uncleear Masking: double‐blind; double dummy Excluded: Yes (post run‐in) Withdrawals: Not stated Jadad score: 2 | |
| Participants | 233 children (age range: 6‐9 years) Mean age: 7.3 years; mean FEV1: FP: 90.2%; BUD: 92.3% Inclusion criteria: FEV1 >/=60%; prepubescent children aged between 6‐9 years; persistent asthma. No ICS during run‐in | |
| Interventions | FP200 (Diskus) versus BUD400 (Turbuhaler) (6 month run‐in) | |
| Outcomes | Growth velocity; mean change in am PEF; mean change in FEV1 | |
| Notes | Unpublished conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Double‐dummy design |
Fitzgerald 1998.
| Methods | Setting: Australia, paediatric outpatient clinic Design: crossover, no washout Length of intervention period: 12 weeks Randomisation: yes, computer generated sequence Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: female preponderance in BDP‐FP sequence group, otherwise comparable Jadad score: 4 | |
| Participants | 34 children randomised, 30 completed study: 19M 11F Age range: 5‐15 years Inclusion criteria: School aged children with persistent severe asthma defined as requiring 1000‐2000 mcg/d of BDP or BUD to control asthma symptoms Exclusion criteria: None stated | |
| Interventions | FP: 375 mcg 2xdaily (750 mcg/d) BDP: 750 mcg 2xdaily (1500 mcg/d) Delivery device: MDI+large volume spacer |
|
| Outcomes | Morning PEF Evening PEF Daytime asthma symptom score Night‐time asthma symptom score 24 hour urinary free cortisol Plasma ACTH 8 am plasma cortisol Plasma 1 hour post Synacthen (0.5mcg/1.73m2 body surface area) Patient assessed efficacy scale Physician assessed efficacy scale No. of asthma exacerbations No. of asthma exacerbations requiring oral steroids | |
| Notes | No reply from author to clarify details of randomisation method | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FLIP01.
| Methods | Setting: multicentre study in Belgium, Netherlands, Germany, Switzerland Design: parallel group Length of intervention period: 5 weeks Randomisation: yes, method unclear Allocation concealment: unclear Masking: double‐blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 110 participants ‐ age not reported Inclusion criteria: Moderate asthma (no steroids) | |
| Interventions | i) FP 50mcg/d ii) FP 100mcg/d iii) FP 200mcg/d iv) BDP 100mcg/d v) BDP 200mcg/d Inhaler device: MDI |
|
| Outcomes | Withdrawals; am & pm PEF; symptoms; adverse events | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FLIP01a.
| Methods | As Above | |
| Participants | As above | |
| Interventions | As above | |
| Outcomes | As above | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | As for FLIP01 |
| Allocation concealment? | Low risk | As for FLIP01 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FLIT37.
| Methods | Setting: multicentre study in Netherlands, New Zealand and UK Design: parallel group Length of intervention period: 4 weeks Randomisation: yes, method unclear Allocation concealment: unclear Masking: double‐blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 111 adults (over 65 years). Median age: 69‐70 years.
Inclusion criteria: >65 years; established history of chronic asthma; treatment with high dose ICS (1000‐2000mcg/d BDP or BUD); at end of run‐in, subjects required to have FEV1 </=75% predicted; reversibility of FEV1 at least 15% post inhalation of SABA. Exclusion criteria: change in prophylactic medication; hospitalised due to respiratory disease; OCS on 4 + occasions in 6 months prior to study entry; seasonally allergy; serious co‐morbidity |
|
| Interventions | FP: 750mcg 2xdaily (1500mcg/d) BDP: 750mcg 2xdaily (1500mcg/d) Delivery device: MDI |
|
| Outcomes | Morning PEF Evening PEF Clinic PEF Symptoms FEV1 (L) FVC (L) Withdrawal Adverse events | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FLPB0145.
| Methods | Setting: multicentre study in France Design: parallel group Length of intervention period: 4 weeks Randomisation: yes, method unclear Allocation concealment: unclear Masking: double‐blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 190 children (6‐16 years). Mean age: 11 years Inclusion criteria: 6‐16 years; severe asthma (definition according to NHLBI criteria); treated for 4 weeks prior to study with 400mcg/d ‐ 1mg BDP or flunisolide plus BD; deterioration of asthma during 5‐7 day observation period; treatment with systemic steroids 4 weeks prior to randomisation. | |
| Interventions | FP: 1000mcg/d BUD: 1000mcg/d Inhaler device: unclear |
|
| Outcomes | Withdrawals (n) Change in FEV1 % predicted (mean) Change in am PEF L/min Change in pm PEF L/min Change in medication usage (puffs/d) Adverse events | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Double‐blind, double dummy |
FLTB3013.
| Methods | Setting: multicentre study in Australia, Czech Republic, Austria, Netherlands, Germany, Norway, Portugal, South Africa Design: parallel group Length of intervention period: 12 weeks Randomisation: yes, method unclear Allocation concealment: unclear Masking: double‐blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 316 children (4‐16 years). Mean age: 9 years Inclusion criteria: 4‐16 years; history of chronic asthma resulting in cough/bronchoconstriction; requirement of 200‐500mcg/d BDP; On 4 of last 4 days of run‐in: PEF </=85% predicted; symptoms; Diurnal variation >/=20%; use of >4 doses of SABA | |
| Interventions | i) FP400mcg/d ii) BDP400mcg/d Inhaler device: Diskhaler |
|
| Outcomes | Withdrawals; clinic, am & pm PEF; symptoms; FEV1; FVC; FEF; Exacerbations; Adverse events | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
FLUTI/AH89/J78.
| Methods | Setting: multicentre study in UK Design: parallel group Length of intervention period: 12 weeks Randomisation: yes, method unclear Allocation concealment: unclear Masking: open label Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 2 | |
| Participants | 197 adults. Mean age: 51‐52.
Inclusion criteria: >18 years; 600‐1000mcg/d; Two of following criteria:
i) am PEF <70% predicted in last 7 days of baseline; ii) >/=15% reversibility within 12 weeks prior to start of study; iii) diurnal variation >/=20% on at least 4/last 7 days; asthma score >/=1 on at least 4/last 7 days. Exclusion criteria: maintenance OCS/parental administration of CS; pregnant women, lactation or likely to become pregnant during course of study |
|
| Interventions | FP: 500mcg 2xdaily (1000mcg/d) BUD: 600mcg 2xdaily (1200mcg/d) Delivery device: MDI |
|
| Outcomes | Morning PEF; Evening PEF; Clinic PEF; Symptoms; FEV1 (L); FVC (L) Withdrawal; Assessment of asthma control; QoL (unspecified scale); Adverse events | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Geppe 2004.
| Methods | Setting: multicentre study in Russia Design: parallel group Length of intervention period: 12 weeks Randomisation: yes, method unclear Allocation concealment: unclear Masking: open label Excluded: not stated Withdrawals: stated Baseline characteristics: Not stated Jadad score: 1 | |
| Participants | 205 children. Age range: 4‐10 years. Inclusion criteria: moderate‐severe asthma previously uncontrolled on ICS. | |
| Interventions | FP 100mcg BID (200mcg/d) BDP 200mcg (400mcg/d) FP/SAL combination (not of interest to this review) |
|
| Outcomes | Symptom free days; % days with SABA use; morning PEF; adverse events | |
| Notes | Unpublished conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | Open label |
Gustafsson 1993.
| Methods | Setting: multicentre study Europe and Canada, paediatric outpatient clinic Design: parallel group Length of intervention period: 6 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes (central coding by pharmaceutical company sponsors) Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 398 children: 225M 173F Age range: 4 to 19 years Inclusion criteria: Children with a clinical diagnosis of asthma Asthma controlled using BDP 400 mcg/d or demonstrating inadequate control on less than 400 mcg/d such that during run‐in period: Night‐time symptoms or 1 or more days out of 7 or: Daytime symptoms on 3 or more days out of 7 or: PEFR 80 (% predicted) or less 3 or more days out of 7 or: 15% or greater reversibility FEV1 after inhaled beta2 agonist Exclusion criteria: Use of oral steroids within previous month More than 3 courses of oral steroids within previous 3 months Respiratory tract infection within previous 2 weeks | |
| Interventions | FP: 100 mcg 2xdaily (200 mcg/d) BDP: 200 mcg 2xdaily (400 mcg/d) Delivery device: MDI+ large volume spacer |
|
| Outcomes | FEV1 (% predicted); Change in FEV1 (% predicted) compared to baseline Clinic PEFR (% predicted); Change in clinic PEFR (% predicted) compared to baseline; Morning PEFR (% predicted); Change in morning PEFR (% predicted) compared to baseline; Evening PEFR (% predicted); Change in evening PEFR (% predicted) compared to baseline; Diurnal variation in PEFR; % symptom free days; % symptom free nights; % beta2 agonist free days; adverse events | |
| Notes | Details of randomisation method provided by Glaxo Wellcome Unpublished data available from www.ctr.gsk.co.uk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Harrison 2001.
| Methods | Setting: Single centre Design: Parallel group Length of intervention period: 4 weeks Randomisation: Computer generated random numbers Allocation concealment: Not described. Masking: Double‐blind, double‐dummy Excluded: Not described Withdrawals: Stated (ITT population) Jadad score: 4 | |
| Participants | 31 adults randomised. FP: 16 (11F), BUD: 15 (10F). FEV1: FP group: 1.86 (SD 0.71), BUD group: 2.12 (SD 0.67); am PEF (L/min): FP group: 317 (SD 105), BUD group: 348 (92); pm PEF (L/min): FP group: 332 (SD 100), BUD: 357 (SD 89); Daily symptom score: FP group: 2.25 (1 to 4), BUD group: 2 (1 to 6); Daily ß‐agonist use (puffs/day): FP group: 6 (2.5‐12), BUD group: 5 (2 to 12). Inclusion criteria: 16‐50 years of age; current non‐smokers with smoking history of <10 pack years; Stable asthma; FEV1 <75% predicted; daily symptoms requiring a SABA despite high dose ICS (BDP: 1000‐2000mcg/day; FP: 500‐1000mcg/day; diurnal variation in PEF 15% on two days during run‐in period or 15% reversibility to 200mcg inhaled salbutamol Exclusion criteria: Change in asthma medication in 8 weeks prior to study; OCS/topical/nasal steroid treatment; leukotriene agents. Asthma subgroup inclusion criteria: Stable asthma; FEV1 <75% predicted; daily symptoms requiring a SABA despite high dose ICS (BDP: 1000‐2000mcg/day; FP: 500‐1000mcg/day; diurnal variation in PEF 15% on two days during run‐in period or 15% reversibility to 200mcg inhaled salbutamol. Exclusion criteria: Change in asthma medication in 8 weeks prior to study. |
|
| Interventions | FP: 1500 mcg/day versus BUD: 1600mcg/day. FP via Accuhaler; BUD via Turbuhaler | |
| Outcomes | Am PEF Pm PEF PD20 FEV1 Symptoms ß‐agonist use Urinary total cortisol Serum osteocalcin Serum cortisol | |
| Notes | Asthmatic participants' data reported separately from the healthy participants data. Nominal dose ratio: 1:1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random numbers |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Double‐blind, double‐dummy |
Heinig 1999.
| Methods | Setting: Multicentre study (47 centres in N America and Europe) Design: Parallel group trial Length of intervention period: 24 weeks Randomisation: Not stated Allocation concealment: Unclear Masking: Double blind, double dummy Excluded: Yes (post run‐in) Withdrawals: Stated (ITT) Jadad score: 3 | |
| Participants | 548 adults enrolled. 395 randomised. FP: 198; BUD: 197 (199M). 59 current smokers; FEV1: FP group: 2.1 (SD 0.8), BUD group: 2.2 (SD 0.9); FVC: FP group: 3.2 (SD 1.1), BUD group: 3.3 (SD 1.1); Concurrent medication: FP group: 39%, BUD group: 34%; OCS for exacerbations: FP group: 21%, BUD group: 20% Inclusion criteria: Symptomatic patients; 18‐75 years; documented history of reversible airways disease (change in FEV1 >15% in 15 minutes following administration of salbutamol 400/800mcg; requirement for or response to inhaled BDP or BUD 1500 ‐ 2000mcg daily or FP: 750‐1000 mcg daily Exclusion criteria: Serious uncontrolled systemic disease (bone disease) at start of run‐in; patients who had required OCS or treatment with research medication within 1 month of study; pregnant/lactating women |
|
| Interventions | FP 2000mcg versus BUD 2000mcg daily. Participants given placebo inhalers of other device to maintain blinding. BUD given via Turburhaler, FP given via Diskhaler. | |
| Outcomes | FEV1 PEF Symptoms Exacerbations Rescue medication usage Adverse events | |
| Notes | Nominal dose ratio: 1:1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Descirbed as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Double‐dummy design |
Hoekx 1996.
| Methods | Setting: multicentre study Europe, hospital outpatient clinics Design: parallel group Length of intervention period: 8 weeks Randomisation: yes, computer generated random sequence (PACT programme) Allocation concealment: yes (central coding by pharmaceutical company sponsors) Masking: double blind Excluded: stated Withdrawals: stated, 8 patients in total, 3 due to failure to meet inclusion criteria. 5 others (2 in FP group, 3 in BUD group) due to 'asthma and related events'. Baseline characteristics: comparable between groups Jadad score: 4 | |
| Participants | 285 children enrolled, 229 randomised: 156M 73F Age range: 4‐13 years Inclusion criteria: Mild to moderate asthma receiving ICS 200‐400 mcg/d During run‐in period 2 of 4 criteria: Daytime or night‐time symptoms on at least 4 of 7 consecutive days One or more awakenings due to asthma symptoms on one or more night/early morning PEFR (% predicted) 75 or less on 4 of 7 days 15% or greater reversibility in FEV1 following beta2 agonist Exclusion criteria: Systemic steroids within last 3 months Unable to use delivery devices or peak flow meter Known or suspected corticosteroid hypersensitivity Seasonal allergy Infection or concurrent disease considered likely to affect asthma Any investigational drug within previous month | |
| Interventions | FP: 100 mcg 2 actuations 2xdaily (400 mcg/d) via Diskhaler DPI BUD: 200 mcg 1 actuation 2xdaily (400 mcg/d) via Turbuhaler DPI |
|
| Outcomes | FEV1 Clinic PEFR Morning PEFR Evening PEFR Daytime asthma symptom score % symptom free days % symptom free nights Days missed from school (patients) Days missed from work (parents) Parent completed, patient‐centred assessment of physical and social activity Morning serum cortisol Biochemical markers of bone turnover | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Double‐dummy |
Hughes 1999a.
| Methods | Setting: USA, primary/secondary care unclear Design: parallel group Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: open, no blinding Excluded: not stated Withdrawals: not stated Jadad score: 1 | |
| Participants | 71 subjects
Age range: not stated
Inclusion criteria:
Patients with asthma controlled on low dose ICS (dose range not specified)
FEV1 > 70 (% predicted)
12% or greater reversibility in FEV1 after inhaled beta2 agonist
Exclusion criteria:
Not stated Baseline asthma control Mean FEV1 85.8‐87.6 (% predicted) in each treatment group Symptom frequency: not stated |
|
| Interventions | FP: 88 mcg 2xdaily (176 mcg/d) BUD: 200 mcg 2xdaily (400 mcg/d) Delivery device: not stated |
|
| Outcomes | Rescue free days (No use of beta2 agonist, oral steroid use or physician visit) Rescue beta2 agonist use (puffs/day) | |
| Notes | Study in abstract form only A montelukast treatment arm was assessed: results not considered in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | Open label design |
Hughes 1999b.
| Methods | Setting: UK, hospital outpatient clinic Design: parallel group Length of intervention period: 12 months Randomisation: yes, stratified randomisation (using minimisation routine) Allocation concealment: unclear Masking: open, non‐blinded Excluded: stated Withdrawals: stated Baseline characteristics: comparable age, sex distribution, ICS use between treatment groups Jadad score: 3 | |
| Participants | 62 adults enrolled, 59 randomised: 36M 23F Age range: 21‐70 years Inclusion criteria: Asthmatic patients receiving BDP or BUD 1500‐2000 mcg/d FEV1 > 30 (% predicted) Exclusion criteria: Oral corticosteroid use in last 3 months 3 or more courses of oral steroids in last 12 months HRT therapy, fractures, arthritis, metabolic bone disease | |
| Interventions | FP: 500 mcg 2 x daily (1000 mcg/d) BUD: 800 mcg 2 x daily (1600 mcg/d) Delivery device: MDI+spacer |
|
| Outcomes | Bone mineral density assessment Biochemical markers of bone turnover Change in urinary free cortisol level compared to baseline Change in plasma cortisol level (time not specified) compared to baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Stratified randomisation (using minimisation routine) |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | Open, non‐blinded |
Ige 2002.
| Methods | Setting: Single centre (Nigeria) Design: Parallel group Length of intervention period: 8 weeks Randomisation: Random numbers table (odd: FP; even: BDP following correspondence with trialist) Allocation concealment: Unclear Masking: open label Excluded: yes Withdrawals: Stated Jadad score: 1 | |
| Participants | 30 adults enrolled. 20 randomised BDP: 10; FP: 10). Duration of asthma (years): BDP: 10.9 (SD 6.62); FP: 5.22 (SD 4.55); Clinic PEF (L/min): BDP: 343 (SD 57.13); FP: 356 (SD 88.58); Clinic FEV1 (L/min): BDP: 2.23 (SD 0.36); FP: 2.21 (SD 0.52); FEV1 % pred: BDP: 76.8 (SD 8.55); FP: 83.5 (SD 13.37); % Reversibility: BDP: 20.91 (SD 5.54); FP: 2.71 (SD 8.57); Nocturnal awakenings/week: BDP: 8.7 (SD 2.5); FP: 7.5 (SD 2.12); AM asthma: BDP: 5.7 (SD 1.16); FP: 5.9 (SD 1.1); Daytime asthma: BDP: 7 (SD 0); FP: 7 (SD 0); Ventolin usage: BDP: 38 (SD 7.72); FP: 37.5 (SD 6.13) Inclusion criteria: confirmed diagnosis of asthma; FEV1 >/=60% predicted; reversibility of 15% increase in FEV1/PEF after SABA (400mcg) in week of screening; Total daytime asthma score of 10 (>/=10) in last seven days of screening period; ability to comply with trial regimen, use PEF meter & complete diary card Exclusion criteria: Exacerbation/RTI in month prior to study entry; treatment with oral/parenteral steroids in month prior to entry | |
| Interventions | 1 week screening period, followed by randomisation to either BDP200 twice daily (total dose 400mcg) or FP110 twice daily (total dose 220mcg) via MDI. | |
| Outcomes | FEV1; PEF; Symptoms; Rescue medication usage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random numbers table |
| Allocation concealment? | High risk | Even and odd numbering determined treatment group allocation |
| Blinding? All outcomes | High risk | Open label |
Johansson 1998.
| Methods | Setting: Sweden, primary/secondary care unclear Design: parallel group Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: no demographic data presented Jadad score: 2 | |
| Participants | 219 subjects Age range: not stated Inclusion criteria: Asthmatic patients symptomatic despite treatment with BDP or BUD 400 mcg/d Exclusion criteria: Not stated | |
| Interventions | FP: 200 mcg/d (once daily) BUD: 400 mcg/d (once daily) Delivery device: not stated |
|
| Outcomes | Change in morning PEFR compared to baseline Asthma symptom score Daily use of beta2 agonists | |
| Notes | Study in abstract form only Study also included a treatment arm with FP 100 mcg 2xdaily: results not considered |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Joubert 1998.
| Methods | Setting: multicentre study South Africa, Greece, Spain, Hungary Primary/secondary care unclear Design: parallel group Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: no demographic data presented Jadad score: 2 | |
| Participants | 258 adults Age range: not stated Inclusion criteria: Adult asthmatics with symptoms despite treatment with ICS (mean dose 825 mcg/d) Exclusion criteria: Not stated | |
| Interventions | FP: 375 mcg 2xdaily (750 mcg/d) via MDI BUD: 400 mcg 2xdaily (800 mcg/d) via Turbuhaler DPI |
|
| Outcomes | Change in morning PEFR compared to baseline Daily beta2 agonist use Asthma symptom score 24 hour area under curve serum cortisol | |
| Notes | Study in abstract form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Kannisto 2002.
| Methods | Setting: Single centre (Finland) Design: Parallel group Length of intervention period: 16 weeks Randomisation: Not reported Allocation concealment: Unclear Masking: Open label Excluded: Not stated Withdrawals: Stated Jadad score: 2 | |
| Participants | 60 children randomised (75 recruited in total, 15 also recruited to CROM which was not considered in this review). Mean FEV1 % pred: FP: 92 (SD 11); BUD: 92 (SD 15) . Age range: 5.5‐14.7 years. Atopy (>/=1 +ve skin prick test) present in 77% children. All children suffered from symptoms presumptive of asthma (prolonged cough, wheeze during exercise, respiratory infections). Inclusion criteria: Positive reaction to one of 3 diagnostic tests: 1) free running test (leading to fall in FEV1/Wright PEF of >/=15%, 2) BDT if FEV1 increased by >/=10% or PEF increased by >/=15%; 3) PEF >/= 15% variability on at least 4 days during 2‐week follow‐up period Exclusion criteria: not reported | |
| Interventions | FP: 500mcg/day versus BUD: 800mcg/day for first 2 months. Dosage halved for second 2 months of the study. Inhaler device: BUD: Turbohaler, FP: Diskus. | |
| Outcomes | FEV1 (change from baseline as %); rescue medication usage: doses/week; changes in height, N with fall in FEV1 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | Open label design |
Kemmerich 1999.
| Methods | Setting: multicentre study Germany, primary/secondary care setting unclear Design: crossover, no washout Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: not stated Excluded: not stated Withdrawals: not stated Baseline characteristics: no demographic data presented Jadad score: 1 | |
| Participants | 100 subjects Age range: not stated Inclusion criteria: Asthmatic patients with mild disease (not further defined) No further details Exclusion criteria: Not stated | |
| Interventions | FP: 200 mcg/d via Diskus/Accuhaler DPI BUD: 400 mcg/d via Turbuhaler DPI |
|
| Outcomes | Morning PEFR Rescue beta2 agonist free days | |
| Notes | Study in abstract form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Kuna 2003.
| Methods | Setting: Multicentre study in Western Europe, Eastern Europe and South Africa Design: parallel group Length of intervention period: 5 weeks Randomisation: computer‐generated block randomisation Allocation concealment: sealed envelopes Masking: double‐blind, double‐dummy Excluded: Yes (post run‐in) Withdrawals: Stated (ITT) Jadad score: 5 | |
| Participants | 272 adults enrolled. 197 randomised. BUD: 96; FP: 101. Gender (F/M) BUD: 63/33; FP: 59/42; Median asthma duration: BUD: 10.7 years; FP: 10.6 years; ICS dose (median): BUD: 800; FP: 800; Mean FEV1 % predicted: BUD: 79.4; FP: 79.4; Mean am PEF: BUD: 390; FP: 402 Inclusion criteria: 18‐65 years; ATS diagnosis of asthma; FEV1 50‐90% predicted; FEV1 reversibility </=12% post SABA; ICS treatment for >/=1 year for asthma; Constant dose between 8‐1600mcg/d BID for 6 weeks prior to enrolment; at interview participants expected to provide evidence of lack of asthma control; >/=1 asthma‐related nocturnal awakening during previous two weeks; use of rescue medication on >/=5 occasions during previous week, or asthma symptoms on >/=7 days during previous 2 weeks Exclusion criteria: Asthma exacerbation/RTI within 4 weeks prior to enrolment, pregnancyother relevant pulmonary cardiovascular/other diseases likely to affect study results |
|
| Interventions | FP800 versus BUD800. Dosage stepped down over study. Data only used for initial 5 weeks when dose stable. Run‐in of 4‐6 weeks on high dose BDP (1000mcg BID). Delivery devices: Turbohaler & Diskhaler | |
| Outcomes | Time to withdrawal; am PEF; FEV1; tolerability | |
| Notes | P values published for 5 week change | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated block randomisation |
| Allocation concealment? | Low risk | Concealment: sealed envelopes |
| Blinding? All outcomes | Low risk | Double‐blind, double‐dummy |
Langdon 1994a.
| Methods | Setting: multicentre study UK, primary care Design: parallel group Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: open, no blinding Excluded: stated Withdrawals: stated Baseline characteristics: comparable age, asthma duration, baseline ICS use between treatment groups Jadad score: 2 | |
| Participants | 397 adults enrolled, 281 randomised Age range: 18 to 70 years Inclusion criteria: Adult asthmatic patients Treatment with BDP or BUD 600 mcg/d or less FEV1 > 50 (% predicted) During 2 week run‐in period: Diurnal variation PEFR 15% or greater on 4 successive days and/or daytime asthma symptoms on 4 successive days 15% or greater reversibility in FEV1 after inhaled beta2 agonist Exclusion criteria: Current or recent treatment with oral corticosteroids Change in asthma therapy in last 6 weeks Respiratory tract infection in last 6 weeks | |
| Interventions | FP: 200 mcg 2xdaily (400 mcg/d) via Diskhaler DPI BUD: 400 mcg 2xdaily (800 mcg/d) via Turbuhaler DPI |
|
| Outcomes | Morning PEFR Evening PEFR Diurnal variation in PEFR Daily asthma symptom score Daytime rescue beta2 agonist use Night‐time rescue beta2 agonist use Patient assessed degree of asthma control Physicican assessed success of treatment Morning plasma cortisol | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | Open label |
Langdon 1994b.
| Methods | Setting: multicentre study UK, primary care Design: parallel group Length of intervention period: 8 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes (central centre coding of randomisation schedules) Masking: open, unblinded Excluded: stated Withdrawals: stated Baseline characteristics: comparable age, asthma duration and severity between treatment groups Jadad score: 3 | |
| Participants | 214 adults enrolled, 157 randomised: 83M 74F Mean (SD) age: 48 (15) years FP group, 46 (17) years BUD group Inclusion criteria: Adult asthmatics 16 years of age or over 15% or greater reversibility in FEV1 after inhaled beta2 agonist Receiving ICS for asthma control FEV1 >50 (% predicted) During 2 week run‐in: asthma symptoms on an least 4 of last 12 days Exclusion criteria: Current use of OCS, > 6 courses OCS in last 12 months or course OCS in last 6 weeks Change in asthma therapy in last 6 weeks Respiratory tract infection in last 4 weeks | |
| Interventions | FP: 50 mcg 2 puffs 2xdaily (200 mcg/d) BUD: 200 mcg 1 puff 2xdaily (400 mcg/d) Delivery device: MDI |
|
| Outcomes | FEV1 FVC Clinic PEFR Morning PEFR Evening PEFR Daily asthma symptom score Daytime rescue beta2 agonist use Night‐time rescue beta2 agonist use Morning plasma cortisol Patient assessed degree of asthma control Physicican assessed success of treatment | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | High risk | Open label |
Leblanc 1994.
| Methods | Setting: multicentre study Europe and Canada, hospital outpatient clinic Design: parallel group Length of intervention period: 4 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes (central coding by pharmaceutical company sponsors) Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 261 adults Age range: 18 to 80 years Inclusion criteria: Patients with mild to moderate asthma During run‐in period: Variability in PEFR > 20% PEFR bronchodilator response > 15% Asthma symptoms on at least 4 days or nights of the run‐in period Exclusion criteria: Requirement of > 400 mcg BDP or BUD on a regular basis in preceding month Oral steroids within preceding month Severe concurrent disease | |
| Interventions | FP: 100 mcg 2xdaily (200 mcg/d) BDP: 200 mcg 2xdaily (400 mcg/d) Delivery device: MDI +/‐ spacer |
|
| Outcomes | FEV1 FEV1 (% predicted) FVC (% predicted) FVC Morning PEFR Evening PEFR % symptom free days % symptom free nights % beta2 agonist free days Morning plasma cortisol Oral candidiasis Oropharyngeal side effects | |
| Notes | Details of randomisation method provided by Glaxo Wellcome Unpublished data available from www.ctr.gsk.co.uk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Lorentzen 1996.
| Methods | Setting: multicentre study Europe, hospital outpatient clinic Design: parallel group Length of intervention period: 12 months Randomisation: yes, computer generated sequence Allocation concealment: yes (central coding by pharmaceutical company sponsors) Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 5 | |
| Participants | 213 patients randomised 104 male 109 female Age range: 18 to 77 years Inclusion criteria: Clinical history of severe chronic asthma Requiring and responding to inhaled beta2 agonists and high doses of ICS No change in regular asthma medication for at least one month Exclusion criteria: Recent hospital admission due to asthma Systemic corticosteroids or respiratory tract infection within last month Hypersensitivity to corticosteroids Pregancy Inability to use aersol MDI | |
| Interventions | FP: 250 mcg 2 puffs 2xdaily (1000 mcg/d) BDP: 250mcg 4 puffs 2xdaily (2000 mcg/d) Delivery device: MDI |
|
| Outcomes | Morning plasma cortisol FEV1 FVC Clinic PEFR Oral Candidiasis Oropharyngeal side effects Asthma exacerbations (No. of patients) | |
| Notes | Details of randomisation method provided by Glaxo Wellcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Lundback 1993.
| Methods | Setting: multicentre study Europe and Canada, hospital outpatient clinic Design: parallel group Length of intervention period: 6 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes (central coding by pharmaceutical company) Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 585 adults: 306M 279F Age range: 18 to 90 years Inclusion criteria: Clinical diagnosis of moderately severe asthma Using 400‐1000 mcg/d ICS During run‐in period: Patients on 400‐600 mcg ICS daily: 4 or more days of asthma symptoms, or at least 15% reversibility in FEV1 following beta2 agonist Patients on 600‐1000 mcg ICS daily: stable symptoms during run‐in period Exclusion criteria: Change in prophylactic treatment in last month Hospital admission due to respiratory disease in month preceding study Systemic steroids within preceding month 3 or more courses of systemic steroids in 6 months Concurrent serious illness | |
| Interventions | FP:
1. 500 mcg/d via MDI
2. 500 mcg/d via Diskhaler DPI BDP: 1000 mcg/d via MDI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Diurnal variation PEFR % patients with no change/improvement in daytime symptom score % patients with no change/improvement in night‐time symptom score % patients with same/reduced daytime requirement for beta2 agonists % patients with same/reduced night‐time requirement for beta2 agonists Morning plasma cortisol Oral Candidiasis Oropharyngeal side effects | |
| Notes | Details of randomisation method and standard deviation values for outcomes not reported in original paper provided by Glaxo Wellcome Randomised study followed by an open 46 week treatment period: results not considered in this review Unpublished data available from www.ctr.gsk.co.uk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Double‐dummy |
Lundback 1997.
| Methods | Setting: Sweden, hospital outpatient clinic Design: parallel group Length of intervention period: 12 months Randomisation: yes, PACT. Allocation concealment: unclear Masking: Open label trial Excluded: no details Withdrawals: no complete details Baseline characteristics: no details Jadad score: 2 | |
| Participants | 74 participants Age range: not stated Inclusion criteria: Diagnosis of "chronic severe asthma" Treatment with prednisolone 5mg/d and ICS 800 mcg/d or greater Exclusion criteria: Not stated | |
| Interventions | FP: 750 mcg 2xdaily (1500 mcg/d) via Diskhaler DPI BUD: 800 mcg 2xdaily (1600 mcg/d) via Turbuhaler DPI |
|
| Outcomes | Discontinuation of oral prednisolone (% of patients) Oral prednisolone consumption over treatment period FEV1 Morning PEFR Asthma symptom score Health status (scale not stated) | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | High risk | Open label |
Majer‐Teboul 2001.
| Methods | Setting: Not stated Design: Not stated Length of intervention period: 8 days Randomisation: not stated Allocation concealment: not stated Masking: double‐blind Excluded: not stated Withdrawals: not stated Jadad score: 2 | |
| Participants | 36 randomised. Participants described as having mild asthma (mean age 27.4 (SD 9.3)). FEV1 (% predicted) 94.2 (SD 14.7). All participants were treated with prn SABA. Exclusion criteria: ICS treatment for 3 months prior to the study | |
| Interventions | FP 500 mcg/day versus BDP 1000mcg/day versus placebo. Study duration: 8 days. Inhaler device not clear. | |
| Outcomes | Nitric oxide | |
| Notes | Unpublished conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Malo 1999.
| Methods | Setting: Canada, hospital outpatient clinic Design: crossover, no washout Length of intervention period: 4 months Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 69 adults: 30M 39F Mean (SD) age: 48.4 (14.5) years Inclusion criteria: Adults with asthma (ATS criteria) Moderate to severe disease (no definition) Stable for 3 months or longer (no definition) Requiring BDP or BUD 800‐2000 mcg/d for asthma control 15% or greater variability in FEV1 over preceding 2 years Exclusion criteria: Regular oral steroid use Any oral steroid use within last 3 months Use of aspirin, non‐steroidals or anticoagulants, history of bleeding disorders, smokers | |
| Interventions | 1. FP at nominal daily dose half normal mcg dose of BDP 2. BDP usual nominal maintenance dose Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) FVC (% predicted) Diary card assessed skin bruising severity score Diary card assessed skin bruising frequency score Clinic assessed skin bruising score One or more night‐time awakening due to symptoms (No. of patients) Symptoms on wakening in morning (No. of patients) 24 hour urinary cortisol Plasma cortisol Plasma cortisol post synthetic ACTH Urinary calcium Urinary phosphorous Serum osteocalcin Serum bone specific alkaline phosphatase | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Melaranci 1999.
| Methods | Setting: Italy, paediatric outpatient clinic Design: parallel group Length of intervention period: 15 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: no details Jadad score: 2 | |
| Participants | 143 children Age range: 3 to 11 years Inclusion criteria: Children with asthma (not otherwise defined) Exclusion criteria: Not stated | |
| Interventions | FP: 100 mcg 2xdaily (200 mcg/d) BDP: 100 mcg 2xdaily (200 mcg/d) Delivery device: MDI + Babyhaler spacer |
|
| Outcomes | Morning PEFR Evening PEFR Wheeze score Cough score | |
| Notes | Study in abstract form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Molimard 2005.
| Methods | Setting: France, outpatient clinic Design: parallel group Length of intervention period: 12 weeks Randomisation: yes, via remote call centre Allocation concealment: adequate Masking: open label study Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 460 adults ; Age range: 18‐77. Mean FEV1: 76.7‐79.3%
Inclusion criteria: 18‐60 years; moderate to severe asthma; not controlled on FP <500mcg/d; nocturnal discomfort during previous 5 days/asthma requiring 2 puffs/d beta‐agonist in last 7 days Exclusion criteria: COPD; upper/lower RTI in previous month; exacerbation of asthma leading to hospitalisation; systemic steroids in 4 weeks prior to inclusion. |
|
| Interventions | 1) FP: 1000mcg/d 2) BUD: 1600mcg/d 3) QVAR: 800mcg/d | |
| Outcomes | FEV1; AQLQ; Rescue medication usage; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Low risk | Remote telephone system |
| Blinding? All outcomes | High risk | Open label |
Murray 1998.
| Methods | Setting: multicentre study USA, hospital outpatient clinics Design: parallel group Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: not stated Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: no demographic data presented Jadad score: 2 | |
| Participants | 790 adults Age range: not stated Inclusion criteria: Adult asthmatics using BDP or TA 8 puffs/d or greater FEV1 45‐80 (% predicted) Exclusion criteria: Not stated | |
| Interventions | FP:
1. 88 mcg 2xdaily (176 mcg/d)
2. 220 mcg 2xdaily (440 mcg/d) BDP: 1. 168 mcg 2xdaily (336 mcg/d) 2. 336 mcg 2xdaily (672 mcg/d) Delivery device: not stated |
|
| Outcomes | FEV1 Morning PEFR Daily beta2 agonist use (puffs/d) Asthma symptom score Withdrawal due to lack of efficacy | |
| Notes | Study in abstract form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Unclear risk | Information not available |
Nielsen 2000.
| Methods | Setting: Single centre (Denmark) Design: Parallel group Length of intervention period: 2 weeks Randomisation: Computer generated programme off site Allocation concealment: Adequate Masking: Double blind Excluded: Not stated Withdrawals: Stated (ITT) Jadad score: 4 | |
| Participants | 66 adults randomised (BUD: 33; FP: 33) M: 37; F: 29. Mean asthma duration yr: BUD: 17 (sd 11.49); FP: 19 (sd 17.23) +ve skin prick test: 24/33; FEV1 (% pred): BUD: 81.2 (sd 8.04); FP: 83.4 (sd 11.49); blood eosinophils 10(9)/L BUD: 0.30 (sd 0.23) FP: 0.20 (sd 0.11); ICS dose: BUD: 745 (sd 229.78); FP 685 (sd 241.27) Inclusion criteria: Asthma diagnosed according to guidelines (history of episodic wheeze cough and /pr dyspnoea reversible to BD; ICS for at least 1mo prior to study entry (FP: 200‐500mcg; BDP/BUD: 400‐1000mcg ‐ dosage fixed for at least 1 mo prior to study entry); BHR: 20% (PD20) </= 800mcg methacholine bromide Exclusion criteria: Systemic steroids 2 mo prior to study entry; URTI within 6 w |
|
| Interventions | Run‐in phase of 2‐4 weeks, during which participants received 200 mcg BUD daily. After this, participants were randomised to receive 250mcg FP or 400mcg BUD b.i.d.. Two weeks later they received double the dose and again two weeks later. Inhaler device: FP: Diskhaler; BUD: Turbuhaler |
|
| Outcomes | PEF (change) Symptoms (change) PD20 Plasma cortisol Eosinophils Beta2 agonist use (change) Adverse events | |
| Notes | Data extracted and entered for 2 weeks due to the varying dosage of steroids administered subsequently. Nominal dose ratio: 1:2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated programme |
| Allocation concealment? | Low risk | Third party |
| Blinding? All outcomes | Low risk | Double dummy |
Nong 2001.
| Methods | Setting: Single centre (Korea) Design: Parallel group Length of intervention period: 8 weeks Randomisation: Not stated Allocation concealment: Unclear Masking: open label Excluded: Not stated Withdrawals: Stated (non‐ITT) Jadad score: 2 | |
| Participants | 77 children randomised. Family history of atopy: FP group: 22, BDP group: 11; Allergic rhinitis: FP group: 17, BDP group: 8; Atopic eczema/dermatitis: FP group: 14, BDP group: 8; Duration of asthma: <1 year FP group: 1 BDP group: 0, 1‐5 years FP group: 21, BDP group: 10, 6‐10 years: FP group: 17, BDP Group: 8; >10 years: FP Group: 2, BDP Group: 1. Participants taking: Inhaled ß‐agonists: FP Group: 39, BDP Group: 16; Oral ß‐agonists: FP Group: 5, BDP: 3; Theophylline: FP Group: 7, BDP Group: 5; Ketotifen: FP Group: 11, BDP Group: 6; Sodium cromoglycate: FP Group: 25, BDP Group: 10; ICS: FP Group: 36, BDP Group: 16 Inclusion criteria: Children aged 4 ‐ 14; 'moderate to severe asthma' (no criteria given); bothered by daytime symptoms more than once per week Exclusion criteria: Changes in medication 1 month prior to study entry |
|
| Interventions | Inhaled FP 200mcgvia MDI versus inhaled BDP 400mcg via MDI. Duration: 8 weeks (plus 2 weeks run‐in and 2 weeks post‐treatment) | |
| Outcomes | FEV1 PEF Symptoms Eosinophil count Adverse events | |
| Notes | High attrition rate in this study (FP: n = 10; BDP: n = 7). Nominal dose ratio: 1:2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | Open label study |
Parakh 2004.
| Methods | Setting: India, outpatient clinic Design: parallel group Length of intervention period: 12 weeks Randomisation: unclear Masking: single‐blind Excluded: unclear Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 42 adults randomised; Age range: 15 to 45 years. Inclusion criteria: Adults with a clinical history of dyspnoea and wheeze, requiring therapy for most of the year. Exclusion criteria: Smoking history; evidence of COPD; steroid‐dependent asthma | |
| Interventions | Group A: FP50 4 puffs BID (400mcg/d); Group B: BUD200 2 puffs BID (800mcg/d); Group C: BDP 200 2 puffs BID (800mcg/d) Inhaler device: MDI |
|
| Outcomes | Symptoms; FEV1; PEF; FVC; withdrawals | |
| Notes | Described as single‐blind, although presentation of FP and BDP/BUD regimens different | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | Single blind study |
Pauwels 1998.
| Methods | Setting: multicentre study Belgium, hospital outpatient clinic Design: crossover, no washout Length of intervention period: 6 months Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 340 adults: 196M 144F Age range: 18 to 75 years Inclusion criteria: Moderate to severe asthma Requiring 800‐2000 mcg/d BDP or BUD for asthma control 15% or greater reversibility in FEV1 after inhaled beta2 agonist FEV1 > 40 (% predicted) Exclusion criteria: > 6 weeks of oral corticosteroids within last year Oral steroids, hospitalisation due to asthma or respiratory tract infection in last month Use of any drugs affecting bone metabolism | |
| Interventions | Patients randomised to receive FP or BDP in a nominal daily dose ratio of 1:2
Dose was individualised depending on pre‐study daily ICS dose: Pre‐study: BDP 1000 /BUD 800 mcg/d Randomised to: BDP 1000 or FP 500 mcg/d Pre‐study: BDP 1500/ BUD 1200 mcg/d Randomised to: BDP1500 or FP 750 mcg/d Pre‐study: BDP 2000/ BUD 1600 mcg/d Randomised to: BDP 2000 or FP 1000 mcg/d |
|
| Outcomes | FEV1 (% predicted) FVC % symptom free days % symptom free nights % rescue salbutamol free days Hyland Living With Asthma Questionnaire (LWAQ) Serum cortisol Serum osteocalcin Serum calcium Urinary hydroxyproline Bone mineral density by dual photon x‐ray absorptiometry (DEXA) Withdrawal due to asthma (No. of patients) Oropharyngeal side effects | |
| Notes | No reply from author to clarify details of randomisation method Patients had the choice of using either an MDI or an MDI with large volume spacer, provided they continued to use the same device throughout the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Phillips 2004.
| Methods | Setting: single‐centre study, UK Design: crossover Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 18 adults with mild asthma. Age range: 18‐65 years Inclusion criteria: diagnosis and symptoms consistent with asthma Exclusion criteria: inhaled steroids within 4 weeks of study entry; recent exacerbation | |
| Interventions | FP 750mcg BID (1500mcg/d) BUD 800mcg BID (1600mcg/d) Placebo Inhaler device: DPI |
|
| Outcomes | PD20; FEV1; am PEF | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Prasad 2004.
| Methods | Setting: India, outpatient clinic; Design: parallel group; Length of intervention period: 12 weeks; Randomisation: alternate allocation (inadequate); Masking: double‐blind; Excluded: unclear; Withdrawals: stated; Baseline characteristics: comparable; Jadad score: 2 | |
| Participants | 74 adults; age range: 12‐60. Inclusion criteria: asthma on the basis of clinical history and PFT; FEV1 <80% predicted (response to beta‐agonist <15%); night symptoms on 1/7 days; day symptoms on 3/7 days; Exclusion criteria: Patients taking oral/inhaled steroids for 1 month prior to study entry; smokers & unstable asthma | |
| Interventions | FP 50 2 puffs BID (200mcg/d) BDP 100 2 puffs BID (400mcg/d) Inhaler device: MDI |
|
| Outcomes | FEV1; PEF; FEV1/FVC ratio; symptoms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Alternate allocation |
| Allocation concealment? | High risk | Not adequate |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Rao 1999.
| Methods | Setting: UK, paediatric outpatient and primary care Design: parallel group Length of intervention period: 20 months Randomisation: yes, computer generated sequence Allocation concealment: yes (central coding by pharmaceutical company sponsors) Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 23 children: 15M 8F Age range: 5 to 10 years Inclusion criteria: Pre‐pubertal children with moderately severe asthma defined by episodic breathlessness and wheeze with symptom relief following bronchodilator and symptoms during run‐in period Exclusion criteria: Previous regular use of inhaled corticosteroid Systemic corticosteroid in last 2 weeks Respiratory tract infection in last 2 weeks | |
| Interventions | FP: 100 mcg 2xdaily (200 mcg/d) BDP: 200 mcg 2xdaily (400 mcg/d) Delivery device: MDI + spacer |
|
| Outcomes | Morning plasma cortisol FEV1 (% predicted) FEF25‐75 Post exercise fall in FEV1 Histamine BHR (log 10 PC20 FEV1) Daily asthma symptom score Bone mineral density by dual energy x‐ray absorptiometry Serum and urine markers of bone turnover Height assessment | |
| Notes | Details of randomisation method provided by authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | Central coding by pharmaceutical company sponsors) |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Raphael 1999a.
| Methods | Setting: multicentre study USA, primary care and hospital outpatient clinics Design: parallel group Length of intervention period: 12 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes (central coding by pharmaceutical company sponsors) Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 5 | |
| Participants | 399 adolescents and adults: 167M 232F Age range: 12 to 83 years Inclusion criteria: 12 years of age or older with established diagnosis of asthma (no further details) At end of run‐in period: FEV1 of 45‐65 (% predicted), or if FEV1 65‐80 (% predicted) additional evidence of sub‐optimal control (> 8 puffs rescue beta2 agonist/week, diurnal PEFR variability > 20%, any night‐time wakening due to asthma symptoms requiring beta2 agonist) 12% or greater increase in FEV1 after inhaled beta2 agonist Regular treatment with BDP or TA 8‐12 puffs/day for one month or longer Exclusion criteria: Use of systemic steroids, leukotriene modifiers, sodium cromoglycate or nedocromil within last month Smokers Asthma exacerbation during run‐in period | |
| Interventions | FP 44 mcg 2 puffs 2xdaily (176 mcg/d) BDP: 42 mcg 4 puffs 2xdaily (336 mcg/d) Delivery device: MDI |
|
| Outcomes | Outcomes expressed as a change compared to baseline: FEV1 FEF25‐75 FVC Morning PEFR Evening PEFR Rescue beta2 agonist use Daily asthma symptom score % days with no rescue beta2 agonist use % days with no symptoms Asthma exacerbations Oropharyngeal side effects Oropharyngeal Candidiasis |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated sequence |
| Allocation concealment? | Low risk | Central coding by pharmaceutical company sponsors |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Raphael 1999b.
| Methods | See Raphael 1999a | |
| Participants | See Raphael 1999a | |
| Interventions | FP: 110 mcg 2 puffs 2xdaily (440 mcg/d) 4. BDP 42 mcg 8 puffs 2xdaily (672 mcg/d) Delivery device: MDI |
|
| Outcomes | See Raphael 1999a | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | As for Raphael 1999a |
| Allocation concealment? | Low risk | As for Raphael 1999a |
| Blinding? All outcomes | Low risk | As for Raphael 1999a |
Ringdal 1996.
| Methods | Setting: multicentre study New Zealand and Scandinavia, hospital outpatient clinics Design: parallel group Length of intervention period: 12 weeks Randomisation: yes, computer generated random sequence (PACT programme) Allocation concealment: yes (central coding by pharmaceutical company sponsors) Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable between groups Jadad score: 4 | |
| Participants | 518 adults: 279M 239F Age range: 18 to 75 years Inclusion criteria: Clinical history of reversible airways obstruction Requirement for regular inhaled corticosteroids at constant dosage for at least 4 weeks FEV1 (% predicted) 45 to 90 During run‐in period: Mean morning PEFR 90% or less of the response obtained following inhaled salbutamol at start of study, over last 7 days Requiring 2 or more doses beta2 agonist, or 2 or greater asthma symptom score on at least 4 of last 7 days Exclusion criteria: Oral steroids within last month Respiratory tract infection or admission to hospital with respiratory disease within the last month Severe concomitant disease, pregnancy or lactation | |
| Interventions | FP: 800 mcg/d via Diskhaler DPI BUD: 1600 mcg/d via Turbuhaler DPI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Diurnal variation in PEFR Daily PEFR (% predicted) Clinic PEFR Daytime symptom score Night‐time symptom score % symptom free days % symptom free nights % rescue beta2 agonist free days % rescue beta2 agonist free nights Morning plasma cortisol | |
| Notes | Study in abstract form Carryover effects were tested for and excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random sequence (PACT programme) |
| Allocation concealment? | Low risk | Central coding by pharmaceutical company sponsors |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Ringdal 2000.
| Methods | Setting: Sweden, primary/secondary care setting unclear Design: crossover, 2 week washout Length of intervention period: 2 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind (double dummy) Excluded: not stated Withdrawals: 3 dropouts, reasons not stated Baseline characteristics: Jadad score: 3 | |
| Participants | 48 adults randomised, 45 completed study: 31M 14F Mean age: 50 years Inclusion criteria: Adult asthmatics treated with BDP or BUD 1500 to 1600 mcg/d No further details Exclusion criteria: Not stated | |
| Interventions | FP: 750 mcg 2xdaily (1500 mcg/d) via Diskus/Accuhaler DPI BUD: 800 mcg 2xdaily (1600 mcg/d) via Turbuhaler DPI |
|
| Outcomes | Morning serum cortisol (AUC 0830‐1030) 12 hour night time urinary cortisol | |
| Notes | Carryover effects were tested for and excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Double dummy |
SD‐004‐0377.
| Methods | Setting: Unclear Design: Parallel group Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind (double dummy) Excluded: not stated Withdrawals: Not reported Baseline characteristics: Not reported Jadad score: 3 | |
| Participants | 549 adults (35 years) 168M
PEF: 380L/min, 0.08puffs/d rescue medication Entry criteria: well controlled on 200‐500mcg BDP equivalent bid via MDI; 18‐65 years of age; FEV1/PEF >/=85% predicted; Exclusion criteria: recent hospitalisation with asthma; previous use of Diskus/Diskhaler; COPD |
|
| Interventions | 1. FP 200mcg/d 2. BUD 200mcg/d Inhaler device: FP: Diskus; BUD: Diskhaler |
|
| Outcomes | Time to loss of control Asthma symptoms PEF Withdrawal due to deterioration of asthma | |
| Notes | Unpublished study abstract available from http://www.astrazenecaclinicaltrials.com | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Doubel dummy |
Steinmetz 1997.
| Methods | Setting: multicentre study Germany, hospital outpatient clinics Design: parallel group Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: open, no blinding Excluded: stated Withdrawals: not stated Baseline characteristics: stated that patients in each group were of comparable age, asthma duration, baseline FEV1/PEFR but no tabulated demographic data presented Jadad score: 1 | |
| Participants | 497 adults recruited, 457 randomised: 224M 233F Age range: 18‐74 years Inclusion criteria: Patients with asthma Documented asthma symptoms and/or 15% or greater diurnal variability in diary card recorded PEFR on at least 4 days of 2 week run‐in period FEV1 or morning PEFR 50‐80 (% predicted) Exclusion criteria: Use of any corticosteroid within last 3 weeks Current use of sodium cromoglycate, nedocromil or ketotifen Respiratory tract infection within last 2 weeks | |
| Interventions | FP: 125 mcg 2 puffs 2xdaily (500 mcg/d) via MDI BUD: 200 mcg 3 actuations 2xdaily (1200 mcg/d) via Turbuhaler DPI |
|
| Outcomes | FEV1 Morning PEFR Evening PEFR % symptom free days Evaluation of treatment as ''very effective'' (No. of physicians) Oropharyngeal side‐effects | |
| Notes | Study in abstract form only Carryover effects were tested for and excluded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | Open label |
Ställberg 2007.
| Methods | Setting: multicentre study in Europe, hospital outpatient clinic Design: parallel group Length of intervention period: 44 weeks Randomisation: yes, centralised block randomisation Allocation concealment: not clear Masking: double blind (identical inhaler devices) Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 5 | |
| Participants | 229 adults (39 years) 100M
FEV1 90% predicted; 2.5 rescue puffs/d Entry criteria: FEV1 >70% predicted post SABA; >16 years of age; symptoms in last 2 months; Exclusion criteria: recent use of OCS; cromoglycate, LABA, antileukotrienes, ACE‐inhibitors or beta‐blockers. |
|
| Interventions | Stepped down dosing regimens:
FP250 bid (weeks 1‐12)/100 bid (12‐20)/50 bid (20‐28)/50 od (28‐36) BUD400 bid (weeks 1‐12)/200 bid (12‐20)/100 bid (20‐28)/100 od (28‐36) |
|
| Outcomes | Potency ratio (lowest effective doses) Exacerbation of asthma | |
| Notes | Unpublished data available from http://www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Subburao 2005.
| Methods | Setting: single centre Design: crossover study Length of intervention period: 1 week (3 week washout) Randomisation: yes, method unclear Allocation concealment: unclear Masking: double‐blind, double dummy Excluded: not stated Withdrawals: Stated Jadad score: 3 | |
| Participants | 26 adults with mild asthma. Age range: 20‐52 Stable asthma Inclusion criteria: mild asthma (beta‐agonist less than twice weekly) Exclusion criteria: recent exacerbation | |
| Interventions | FP200mcg BID (400mcg/d) BUD200mcg BID (400mcg/d) Placebo Inhaler device: DPI |
|
| Outcomes | EAR; LAR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Double dummy |
Szefler 2002.
| Methods | Setting: multicentre (N America) Design: parallel group Length of intervention period: 6 weeks Randomisation: randomisation occurred at each centre Allocation concealment: unclear Masking: open label Excluded: not stated Withdrawals: Stated Jadad score: 2 | |
| Participants | 30 adults randomised (FP: 15; BDP: 15). FEV1 (L): FP: 3.04 (0.75); BDP: 3.01 (0.63); FEV1 % predicted: FP: 75.07 (SD 11.16); BDP: 73.33 (11.08); median weekly average symptom scores: FP: 0.26 ; BDP: 0.35; median weekly average of rescue medication: FP: 1.83; BDP: 1.63 Inclusion criteria: 18‐55 years; FEV1 55‐85%; >/=12% reversibility post SABA; improvement >/=200mL in FEV1, methacholine PC20 </=8 mg/mL; fall in FEV1 post exercise of >/=12%; am plasma value >/=5mcg/dL; smoking history of <10 pack years Exclusion criteria: Smoking in previous year; topical/inhaled steroid treatment for any condition in previous 6 months, systemic steroids in previous 12 months | |
| Interventions | Serially increased doses of CFC FP or CFC BDP. Doses of FP/BDP taken at initial 6 weeks (FP: 44mcg BID ‐ 88mcg/d; BDP: 84mcg BID ‐ 168mcg/d). Inhaler device: MDI. | |
| Outcomes | Cortisol; FEV1 (L); methacholine PC 20; exhaled nitric oxide; exercise max absolute fall in FEV1; exercise fall in AUC; sputum eosinophils + 0.2 (%), neutrophils (%), eosinophilic cationic protein; symptoms; rescue medication usage | |
| Notes | Efficacy data only taken for first 6 weeks. Trialists responded with information on allocation concealment & blinding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Randomisation occurred at each centre |
| Blinding? All outcomes | High risk | Open label |
Vedanthan 2004.
| Methods | Setting: single centre (India) Design: parallel group Length of intervention period: 12 weeks Randomisation: random numbers table Allocation concealment: unclear Masking: open label Excluded: not stated Withdrawals: not stated Jadad score: 1 | |
| Participants | N unclear Inclusion criteria: Adults participants with GINA stage II, III or IV |
|
| Interventions | FP versus BDP or BUD at equivalent doses. Details of dosing regimen, number of puffs and inhaler device unclear | |
| Outcomes | Quality of Life (SGRQ) | |
| Notes | Unpublished conference abstract. Nominal dose ratio: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random numbers table |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | High risk | Open label |
Williams 1997.
| Methods | Setting: multicentre study UK, hospital outpatient clinic Design: parallel group Length of intervention period: 4 weeks Randomisation: yes, computer generated random sequence Allocation concealment: yes (central coding by pharmaceutical company sponsors) Masking: non‐blinded Excluded: stated Withdrawals: stated, 9 in FP group 11 BUD group, exact reasons unclear, but some related to asthma exacerbations Baseline characteristics: comparable between groups Jadad score: 3 | |
| Participants | 338 subjects enrolled, 323 randomised Age range: 4 to 11 years Inclusion criteria: Children with a clinical diagnosis of asthma Receiving or had symptoms indicating a clinical requirement for, inhaled corticosteroids at a daily dose of 400 mcg BUD or BDP, or 200 mcg FP. Able to use delivery devices and peak flow meter Exclusion criteria: Any medication via the Turbohaler within the last 12 months or previous use of Accuhaler | |
| Interventions | FP: 100 mcg 2xdaily (200 mcg/d) via Accuhaler DPI BUD: 200 mcg 2xdaily (400 mcg/d) via Turbohaler DPI |
|
| Outcomes | Change in morning PEFR compared to baseline Diurnal variation in PEFR Symptom free nights (No. of patients) Symptom free days (No. of patients) Rescue beta2 agonist free days (No. of patients) Rescue beta2 agonist free nights (No. of patients) | |
| Notes | Details of randomisation method and data for morning PEFR provided by Glaxo Wellcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random sequence |
| Allocation concealment? | Low risk | Central coding by pharmaceutical company sponsors |
| Blinding? All outcomes | High risk | Open label |
Wolfe 2000 ICS.
| Methods | Setting: multicentre study, USA
Design: parallel group
Length of intervention period: 12 weeks
Randomisation: yes (not stated)
Allocation concealment: not stated
Design: parallel group
Masking: double blind, double dummy
Excluded: stated
Withdrawals: stated Jadad score: 4 |
|
| Participants | 358 adults and adolescents screened. 199 randomisedData not available for BDP participants (not published in original trial). Inclusion criteria: ICS >/=8 puffs day of BDP (42mcg/puff) or TAA (100mcg/puff) for three months; asthma stability defined as 0 days with >/= 12 puffs of SABA prn; </=4 mornings when PEF decreased by >/= 20% from previous pm's PEF; </=2 nights with awakenings caused by asthma requiring inhaled SABA; FEV1 between 50 & 80% predicted & +/‐15% screening value; adequate compliance during run‐in. Exclusion criteria: Exclusion criteria: pregnancy/lactation; use of methotrexate/gold; use inhaled cromolyn; use of oral, intranasal or parenteral steroids within 4 weeks of study start; significant concomitant illness; immunotherapy requiring change in dose within 12 weeks; concurrent use of prescription/over the counter medication that may affect course of asthma/interact with sympathomimetic amines. Use of loratadine/intranasal cromolyn for allergic rhinitis permitted if treatment started before screening visit and continued without change |
|
| Interventions | FP100BID versus BDP168BID (336mcg/d) Inhaler device: Diskus Run‐in period: 2 weeks (ICS dosage regimen maintained). |
|
| Outcomes | FEV1; am PEF; pm PEF; symptoms; rescue medication usage; nocturnal awakenings; withdrawals; adverse events | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Unclear risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Double dummy |
Wolfe 2000 SABA.
| Methods | Setting: multicentre study, USA
Design: parallel group
Length of intervention period: 12 weeks
Randomisation: yes (not stated)
Allocation concealment: not stated
Design: parallel group
Masking: double blind, double dummy
Excluded: stated
Withdrawals: stated Jadad score: 4 |
|
| Participants | 390 adults and adolescents screened. 253 randomised. Data available for BDP participants (not published in original trial). Inclusion criteria: >/=12 years old; diagnosis of asthma based upon ATS criteria; requirement for pharmacotherapy for 6 months; BD ‐ study: no steroid treatment for 1 month prior to study entry; FEV1 50‐80% predicted; reversibility >/=15% Exclusion criteria: pregnancy/lactation; use of methotrexate/gold; use inhaled cromolyn; use of oral, intranasal or parenteral steroids within 4 weeks of study start; significant concomitant illness; immunotherapy requiring change in dose within 12 weeks; concurrent use of prescription/over the counter medication that may affect course of asthma/interact with sympathomimetic amines. Use of loratadine/intranasal cromolyn for allergic rhinitis permitted if treatment started before screening visit and continued without change |
|
| Interventions | FP100BID versus BDP168BID (336mcg/d). Inhaler device: Diskus. Study duration: 12 weeks, preceded by run‐in period ‐ placebo device for 2 weeks; prn SABA, SAL and XANTH continued (if SAL and XANTH used pre‐run‐in) |
|
| Outcomes | FEV1; am PEF; pm PEF; symptoms; rescue medication usage; nocturnal awakenings; withdrawals; adverse events | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Double dummy |
Wolthers 1997.
| Methods | Setting: Denmark, hospital outpatient clinic Design: crossover, 2 week washout Length of intervention period: 2 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 19 children: 15M 4F Age range: 7 to 14 years Inclusion criteria: Children with mild asthma requiring treatment with beta2 agonists only Exclusion criteria: Treatment with either inhaled or oral corticosteroids in the preceding 2 months | |
| Interventions | 1.FP 100 mcg 1 actuation 2xdaily (200 mcg/d) 2. BDP 200 mcg 1 actuation 2xdaily (400 mcg/d 3. BDP 400 mcg 1 actuation 2xdaily (800 mcg/d) Delivery device: Diskhaler DPI |
|
| Outcomes | FEV1 Morning PEFR Evening PEFR % symptom free days % symptom free nights Urinary free cortisol/creatinine ratio Growth by lower leg knemometry Serum markers of bone and collagen turnover | |
| Notes | Unpublished data available from www.ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
Yiallouros 1997.
| Methods | Setting: UK, paediatric outpatient clinic Design: crossover, no washout Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: authors state well matched for sex ratio, height, duration of asthma, history of atopy Jadad score: 3 | |
| Participants | 34 children: 23M 11F Age range: 5 to 13.1 years Inclusion criteria: Children with severe but clinically stable chronic asthma Established on either inhaled BDP or BUD for at least 3 months prior to study Exclusion criteria: Oral steroids in previous 3 months Oral steroids for 10 or more consecutive days or for 20 days in total in previous year Co‐existing respiratory disease Respiratory tract infection in previous 2 weeks Seasonal pattern to asthma | |
| Interventions | FP: half daily mcg dose of pre study ICS via MDI+spacer BDP: dose equal to pre‐study ICS via MDI+spacer |
|
| Outcomes | 24 hour urinary cortisol Morning PEFR Evening PEFR % cough free days % wheeze free days % symptom free activity days % cough free nights Daytime beta2 agonist use Night‐time beta2 agonist use | |
| Notes | No reply from author to clarify details of randomisation method Unpublished data available from www.ctr.gsk.co.uk |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | See Appendix 2 |
| Allocation concealment? | Low risk | See Appendix 2 |
| Blinding? All outcomes | Low risk | Identical inhaler devices |
ACTH: adrenocorticotropic hormone; BDP: beclomethasone dipropionate; BHR: bronchial hyperresponsiveness; BID: twice daily; BUD: budesonide; DPI: dry powder inhaler; ECP: eosinophil cationic protein; FEF25‐75: forced expiratory flow at 25 to 75% of FVC; FEV1: forced expired volume in one second; FP: fluticasone propionate; FVC: forced vital capacity; HRQOL: health‐related quality of life; ICS: inhaled corticosteroid; mcg/d: micrograms per day; ITT: intention‐to‐treat; MDI: metered dose inhaler; OCS: oral corticosteroid; PC20 FEV1: provocative concentration of inhalant required to produce a 20% fall in FEV1; PD20 FEV1: provocative dose of inhalant required to produce a 20% fall in FEV1; PEFR: peak expiratory flow rate; SABA: short‐acting beta agonist; TA: triamcinolone acetonide; UNDW: ultrasonically nebulised distilled water; EAR: early phase allergen response; LAR: late phase allergen response
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aubier 2001 | Copmparison of HFA‐BDP with FP |
| Barnes 1999a | Health economic analysis |
| Barnes 1999b | Health economic analysis |
| Clark 1996a | Treatment period less than one week |
| Clark 1996b | Treatment period less than one week |
| Clark 1997 | Treatment periods of less than one week |
| Dorinsky 2004 | Wrong comparison |
| Ericsson 1999 | Health economic analysis |
| Fairfax 2001 | FP and BDP administered with different propellants. |
| Fowler 2002 | Comparison of different propellants |
| Harmanci 2001 | Unclear if RCT |
| Harrison 1999 | Not RCT |
| Hawkins 2003 | Step‐down versus stable regimen |
| Kannisto 2000 | Subjects randomised to the FP arm of this study received a varibable dose over the treatment period |
| Karakoc 2001 | FP not administered in comparison. FP administered after BDP/BUD |
| Lipworth 1997 | Study < 1 week duration |
| Lofdahl 1999 | Study conducted in asthmatic and healthy volunteers, < 1 week duration |
| Medici 2000 | Only outcomes assessed concerned with bone density and biochemical markers of bone turnover |
| O'Reilly 1998 | Nebuliser delivery device employed |
| Price 1998 | Health economic analysis |
| Primhak 1999 | Knemometry and biochemical markers of bone turnover only assessed. |
| Spelman 1999 | Health economic analysis |
| Stempel 1999 | Health economic analysis |
| Thorsson 1999 | Healthy volunteers assessed |
| Thwaites 1997 | Health economic analysis |
| Tsoi 1998 | Daily dose of FP unclear |
| Wilson 1998 | Crossover study with intervention periods of 4 days |
Contributions of authors
Nick Adams retrieved papers identified by electronic search, handsearched additional sources for relevant studies, assessed trials for methodological quality, contacted authors to clarify details of trial design and/or request missing data, extracted data from included trials and wrote text of review.
Toby Lasserson (December 2003‐January 2005 updates): assessed new papers, extracted study characteristics, data and undertook quality assessment, assessed studies for baseline severity, contacted authors for data, entered data, amended Results section with new findings.
Paul Jones provided editorial support.
Chris Cates provided editorial, statistical and clinical input for the 2007 update of this review.
Contribution of previous author: Janine Bestall retrieved papers identified by search, assessed trials for methodological quality, contacted authors for clarification or trial details and/or request missing data.
Sources of support
Internal sources
NHS Cochrane Grant scheme, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Acun 2005 {published data only}
- Acun C, Tomac N, Ermis B, Onk G. Effects of inhaled corticosteroids on growth in asthmatic children: a comparison of fluticasone propionate with budesonide. Allergy & Asthma Proceedings 2005;26(3):204‐6. [PubMed] [Google Scholar]
Agertoft 1997 {published data only}
- Agertoft L, Pedersen S. Bone, growth and collagen markers in children treated with fluticasone propionate and budesonide. European Respiratory Journal 1996;9(Suppl 23):295s. [DOI] [PubMed] [Google Scholar]
- Agertoft L, Pedersen S. Short‐term knemometry and urine cortisol excretion in children treated with fluticasone propionate and budesonide: a dose response study. European Respiratory Journal 1997;10:1507‐12. [DOI] [PubMed] [Google Scholar]
- FLIS02. A single‐centre, placebo‐controlled, randomised, double‐blind, double‐dummy, cross‐over study to investigate longitudinal growth using knemometry in children with mild asthma during treatment with budesonide (200µg/ day and 400µg/ day) and fluticasone propionate (200µg/ day and 400µg/ day) as dry powders. http://ctr.gsk.co.uk 2005. [Google Scholar]
Agertoft 1997a {published data only}
- Agertoft L, Pedersen S. A randomized, double‐blind dose reduction study to compare the minimal effective dose of budesonide Turbuhaler and fluticasone propionate Diskhaler. Journal of Allergy & Clinical Immunology 1997;99:773‐80. [DOI] [PubMed] [Google Scholar]
Ayres 1995 {published data only}
- Ayres JG, Bateman ED, Lundback B, Harris TAJ. High dose fluticasone propionate, 1 mg daily, versus fluticasone propionate, 2 mg daily, or budesonide, 1.6 mg daily, in patients with chronic severe asthma. European Respiratory Journal 1995;8:579‐86. [PubMed] [Google Scholar]
Backman 2001 {published and unpublished data}
- Backman R, Baumgarten C, Sharma RK. Fluticasone propionate via Diskus inhaler at half the microgram dose of budesonide via Turbuhaler inhaler. Clinical Drug Investigation 2001;11:735‐43. [Google Scholar]
- Backman R, Pickering CAC, Baumgarten C, Huskisson SC. A comparison of fluticasone propionate via Diskus (Accuhaler) inhaler and budesonide via Turbuhaler inhaler in adult asthmatics. Journal of Allergy & Clinical Immunology. 1997; Vol. 249:249.
- Pickering CAC, Backman R, Baumgarten C, Huskisson SC. Fluticasone propionate 250 mcg bd compared to budesonide 600 mcg bd in adult asthmatics. European Respiratory Journal 1996;23(Supp 9):79. [Google Scholar]
Barnes 1993 {published and unpublished data}
- Barnes NC, Marone G, Maria GU, Visser S, Utama I, Payne SL. A comparison of fluticasone propionate, 1 mg daily, with beclomethasone dipropionate, 2 mg daily, in the treatment of severe asthma. International Study Group. European Respiratory Journal 1993;6:877‐85. [PubMed] [Google Scholar]
- FLIT35. http://ctr.gsk.co.uk 2005.
Basran 1997 {published data only}
- Basran G, Campbell M, Knox A, Scott R, Smith R, Vernon J, Wade A. An open study comparing equal doses of budesonide via Turbohaler with fluticasone propionate via Diskhaler in the treatment of adult asthmatic patients. European Journal of Clinical Research 1997;9:185‐97. [Google Scholar]
Berend 2001 {published and unpublished data}
- Berend N. A six month comparison of the efficacy of high dose fluticasone propionate (FP) with beclomethasone dipropionate (BDP) and budesonide (BUD) in adults with severe asthma. European Respiratory Journal 1997;10 Suppl (25):105. [Google Scholar]
- Berend N, Kellett B, Kent N, Sly PD. Improved safety with equivalent asthma control in adults with chronic severe asthma on high‐dose fluticasone propionate. Respirology 2001;6(3):237‐46. [DOI] [PubMed] [Google Scholar]
- Gibson P, Rutherford C, Price M, Lindsay P. Comparison of the quality of life differences in severe asthma after treatment with beclomethasone dipropionate or budesonide and fluticasone propionate at approximately half the microgram dose. European Respiratory Journal. 1998; Vol. 12, issue Suppl 28:35.
- Jenkins C. High dose inhaled steroids and skin bruising. European Respiratory Journal 1998;12(Suppl 28):435. [Google Scholar]
- Rutherford C, Mills R, Gibson PG, Price MJ, Keech M. Improvement in health‐related quality of life with fluticasone propionate compared with budesonide or beclomethasone dipropionate in adults with severe asthma. Respirology 2003;8(3):371‐5. [DOI] [PubMed] [Google Scholar]
Bisca 1997 {published data only}
- Bisca N. Comparison of fluticasone propionate with beclomethasone dipropionate in moderate to severe childhood asthma. European Respiratory Journal. 1997; Vol. 10:219.
Boe 1994 {published data only}
- Boe J, Bakke P, Rodolen T, Skovlund E, Gulsvik A. High‐dose inhaled steroids in asthmatics: moderate efficacy gain and suppression of the hypothalamic‐pituitary‐adrenal (HPA) axis. European Respiratory Journal 1994;7(12):2179‐84. [DOI] [PubMed] [Google Scholar]
Bootsma 1995 {published data only}
- Bootsma GP, Dekhuijzen PN, Festen J, Mulder PG, Swinkels LM, Herwaarden CL. Fluticasone propionate does not influence bone metabolism in contrast to beclomethasone dipropionate. American Journal of Respiratory & Critical Care Medicine 1996;153:924‐30. [DOI] [PubMed] [Google Scholar]
- Bootsma GP, Dekhuijzen PN, Festen J, Mulder PG, Herwaarden CL. Comparison of fluticasone propionate and beclomethasone dipropionate on direct and indirect measurements of bronchial hyperresponsiveness in patients with stable asthma. Thorax 1995;50:1044‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootsma GP, Koenderman L, Dekhuijzen PN, Festen J, Lammers JW, Herwaarden CL. Effects of fluticasone propionate and beclomethasone dipropionate on parameters of inflammation in peripheral blood of patients with asthma. Allergy 1998;53:653‐61. [DOI] [PubMed] [Google Scholar]
Connolly 1995 {published data only}
- Connolly A. A comparison of fluticasone propionate 100 mcg twice daily with budesonide 200 mcg twice daily via their respective powder devices in the treatment of mild asthma. European Journal of Clinical Research 1995;7:15‐29. [Google Scholar]
Currie 2002 {published data only}
- Currie GP, Fowler S, Wilson A, Sims EJ, Orr L, Lipworth BJ. Airway systemic ratio of hydrofluoroalkane fluticasone and beclomethasone in asthmatics. Journal of Allergy, Asthma and Immunolgy 2002;109(Suppl 1):Abstract No: 769. [Google Scholar]
- Currie GP, Fowler SJ, Wilson AM, Sims EJ, Orr LC, Lipworth BJ. Airway and systemic effects of hydrofluoroalkane fluticasone and beclomethasone in patients with asthma. Thorax 2002;57(10):865‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahl 1993 {published and unpublished data}
- Dahl R, Lundback B, Malo JL, Mazza JA, Nieminen MM, Saarelainen P, Barnacle H. A dose‐ranging study of fluticasone propionate in adult patients with moderate asthma. International Study Group. Chest 1993;104:1352‐8. [DOI] [PubMed] [Google Scholar]
- FLIT05. www.clinicalstudyresults.org.
Dal Negro 1997 {published data only}
- Dal Negro R, Micheletto C, Turco P, Pomari C. Fluticasone propionate 500 mcg, budesonide 800 mcg, and beclomethasone dip. 1000 mcg: different protection degrees against the methacholine‐induced bronchoconstriction. American Journal of Respiratory & Critical Care Medicine. 1997; Vol. 155:A153.
- Micheletto C, Pomari C, dal Negro R. Efficacy of fluticasone prop. (FTP) and beclomethasone dipr. (BDP) on PD20 FEV1and PD20 PtcO2 to methacholine (MCH) in mild asthmatics [abstract]. European Respiratory Journal 1996;9(Suppl 23):79s. [Google Scholar]
de Benedictis 2001 {published data only}
- FLTB3015. www.clinicalstudyresults.org 2005.
- Benedictis FM, Medley HV, Williams L. Long‐term study to compare safety and efficacy of fluticasone propionate (FP) with beclomethasone dipropionate (BDP) in asthmatic children. European Respiratory Journal. 1998; Vol. 12 Suppl (28):142.
- Benedictis FM, Teper A, Green RJ, Boner AL, Williams L, Medley H, et al. Effects of 2 inhaled corticosteroids on growth. Archives of Pediatrics & Adolescent Medicine 2001;155(11):1248‐54. [DOI] [PubMed] [Google Scholar]
Derom 1999 {published data only}
- Derom E, Schoor J, Verhaeghe W, Vincken W, Pauwels R. Systemic effects of inhaled fluticasone propionate and budesonide in adult patients with asthma. American Journal of Respiratory & Critical Care Medicine 1999;160(1):157‐61. [DOI] [PubMed] [Google Scholar]
Egan 1999 {published and unpublished data}
- Adams JE, Egan J, et al. Effects on bone mineral density of inhaled corticosteroids (fluticasone propionate and beclomethasone dipropionate: a two year double‐blind randomised study [abstract]. Osteoporos‐Int 1996;6(Suppl 1):287. [Google Scholar]
- Egan J, Kalra S, Adams JE, Eastell R, Maden C, Woodcock A. A two‐year double‐blind randomised study comparing the effects of fluticasone propionate 1000 mcg/day and beclomethasone dipropionate 2000 mcg/day on bone mineral density and bone metabolism in asthma patients. European Respiratory Journal 1996;9(Suppl 23):52S. [PubMed] [Google Scholar]
- Egan JJ, Maden C, Kalra S, Adams JE, Eastell R, Woodcock AA. A randomized, double‐blind study comparing the effects of beclomethasone and fluticasone on bone density over two years. European Respiratory Journal 1999;13(6):1267‐75. [PubMed] [Google Scholar]
- FLTB3036/FLIP56. www.clinicalstudyresults.org 2006.
Fabbri 1993 {published and unpublished data}
- FLIT26. A multicentre, double‐blind, parallel‐group study to compare the long‐term efficacy and safety of fluticasone propionate 750µg bd (1500µg daily) with beclomethasone dipropionate 750µg bd (1500µg daily) in patients with severe asthma. http://ctr.gsk.co.uk 2005.
- Fabbri L, Burge PS, Croonenborgh L, Warlies F, Weeke B, Ciaccia A, Parker C. Comparison of fluticasone propionate with beclomethasone dipropionate in moderate to severe asthma treated for one year. Thorax 1993;48:817‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne SL, Thwaites RMA, Collins N. Estimation from clinical trial data of direct costs associated with asthma exacerbations treated in hospital. European Respiratory Journal 1997;10 Suppl (28):106. [Google Scholar]
Ferguson 1999 {published data only}
- FLIQ46. http://ctr.gsk.co.uk 2005. [Google Scholar]
- Ferguson AC, Spier S, Manjra A, Versteegh FG, Mark S, Zhang P. Efficacy and safety of high‐dose inhaled steroids in children with asthma: a comparison of fluticasone propionate with budesonide. Journal of Pediatrics 1999;134(4):422‐7. [DOI] [PubMed] [Google Scholar]
- Manjra AI, Versteegh FGA, Mehra S, Zhang P, Mark S. Clinical equivalence of fluticasone propionate (FP) 400 mcg daily via the Diskus inhaler and budesonide (B) 800 mcg daily via the Turbuhaler in asthmatic children. European Respiratory Journal. 1998; Vol. 12:87.
Ferguson 2006 {published and unpublished data}
- FMS40001. http:ctr.gsk.co.uk 2005.
- Ferguson AC, Bever HP, Teper AM, Lasytsya O, Goldfrad CH, Whitehead PJ. A comparison of the relative growth velocities with budesonide and fluticasone propionate in children with asthma. Respiratory Medicine 2006;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ferguson AC, Bever HP, Teper AM, Lasytsya O, Whitehead PJ. Randomised, double‐blind, double‐dummy, parallel‐group comparison of the effect of fluticasone propionate (FP) 100µg bd and budesonide (BUD) 200µg bd on childhood growth: protocol and baseline characteristic. European Respiratory Journal 2001;18(Supp 33):289s. [Google Scholar]
- Ferguson AC, Bever HP, Teper AM, Lasytsya OI, Whitehead PJ. Fluticasone propionate 100µg bd (FP100) has significantly less effect than budesonide 200µg bd (BUD200) on childhood growth over 1 year of treatment in asthmatics. European Respiratory Journal 2002;20(Suppl 38):219s. [Google Scholar]
- Ferguson AC, Bever HP, Teper AM, Lasytsya OI, Whitehead PJ. Significantly reduced growth velocity over 1 year with budesonide 200µg bd (BUD200) compared to fluticasone propionate 100µg bd (FP100) in children with asthma. American Thoracic Society 99th International Conference. 2003:A117 [Poster D63].
Fitzgerald 1998 {published data only}
- Fitzgerald D, Asperen P, Mellis C, Honner M, Smith L, Ambler G. Fluticasone propionate 750 micrograms/day versus beclomethasone dipropionate 1500 micrograms/day: comparison of efficacy and adrenal function in paediatric asthma. Thorax 1998;53(8):656‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asperen P, Fitzgerald D, Mellis C, Honner M, Smith L, Ambler G. Adrenal function in asthmatic children treated with fluticasone propionate (FP) 750MCG/day and beclomethasone dipropionate (BDP) 1500MCG/day [abstract]. European Respiratory Journal 1996;9(Suppl 23):115s. [Google Scholar]
- Asperen P, Fitzgerald D, Mellis C, Honner M, Smith L, Ambler G. Fluticasone propionate (FP) 750 mcg/day is as effective as beclomethasone dipropionate (BDP) 1500 mcg/day in paediatric asthma [abstract]. European Respiratory Journal 1996;9(Suppl 23):114s. [Google Scholar]
FLIP01 {published data only}
- FLIP01. Inhaled fluticasone propionate for the treatment of moderately severe asthma in patients not already taking inhaled steroids. A dose‐ranging study and comparison with inhaled beclomethasone dipropionate. http://ctr.gsk.co.uk 2005. [Google Scholar]
FLIP01a {published data only}
- FLIP01. Inhaled fluticasone propionate for the treatment of moderately severe asthma in patients not already taking inhaled steroids. A dose‐ranging study and comparison with inhaled beclomethasone dipropionate. http://ctr.gsk.co.uk 2005. [Google Scholar]
FLIT37 {unpublished data only}
- FLIT37. A multicentre, randomised, double‐blind, parallel‐group study to compare the safety and efficacy of fluticasone propionate 750µg bd (1500µg/d) with that of beclomethasone dipropionate 750µg bd (1500µg/d) in geriatric patients with chronic asthma. http://ctr.gsk.co.uk 2005. [Google Scholar]
FLPB0145 {unpublished data only}
- FLPB1045. Comparison of fluticasone powder 1mg/day to budesonide powder 1mg/day in deterioration of severe asthma in children: a double‐blind, randomised, multicentre study with parallel groups. http:ctr.gsk.co.uk 2005. [Google Scholar]
FLTB3013 {published data only}
- FLTB3013. A multi‐centre, randomised, double‐blind, parallel‐group clinical trial to compare the safety and efficacy of the dry powder formulation of fluticasone propionate 400µg/day administered for 12 weeks to paediatric patients with chronic asthma. http://ctr.gsk.co.uk 2005. [Google Scholar]
FLUTI/AH89/J78 {unpublished data only}
- FLUTI/AH89/J789. A phase III, multi‐centre, open‐label, parallel group study to compare the efficacy and tolerability of fluticasone propionate 500 micrograms bd with budesonide 600 micrograms bd administered via a metered dose inhaler in adult patients with moderate to severe symptomatic asthma. http://ctr.gsk.co.uk 2005. [Google Scholar]
Geppe 2004 {published data only}
- Geppe NA, Karpushkina AV, Kolossova NG, Yarovaya EB. The effects of fluticasone propionate /salmeterol 50/100ug bid in children with asthma versus beclometasone propionate 200ug BD fluticasone propionate 100ug bid dry powder inhalers [Abstract]. European Respiratory Journal 2004;24(Suppl 48):378s. [Google Scholar]
Gustafsson 1993 {published data only}
- FLIT40. http://ctr.gsk.co.uk. [Google Scholar]
- Gustafsson P, Tsanakas J, Gold M, Primhak R, Radford M, Gillies E. Comparison of the efficacy and safety of inhaled fluticasone propionate 200 micrograms/day with inhaled beclomethasone dipropionate 400 micrograms/day in mild and moderate asthma. Archives of Disease in Childhood 1993;69:206‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 2001 {published data only}
- Harrison TW, Wisniewski A, Honour J, Tattersfield AE. Comparison of the systemic effects of fluticasone propionate and budesonide given by dry powder inhaler in healthy and asthmatic subjects. Thorax 2001;56(3):186‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heinig 1999 {published and unpublished data}
- Heinig JH, Boulet LP, Croonenborghs L, Mollers MJ. The effect of high‐dose fluticasone propionate and budesonide on lung function and asthma exacerbations in patients with severe asthma. Respiratory Medicine 1999;93(9):613‐20. [DOI] [PubMed] [Google Scholar]
Hoekx 1996 {published data only}
- FLIP58. A multi‐centre, randomised, double‐blind, double‐dummy, parallel‐group study to compare the efficacy and safety of fluticasone propionate 400mg daily with budesonide 400mg daily in children with asthma. http://ctr.gsk.co.uk 2005.
- Hoekx JC, Hedlin G, Pedersen W, Sorva R, Hollingworth K, Efthimiou J. Fluticasone propionate compared with budesonide: a double‐blind trial in asthmatic children using powder devices at a dosage of 400 microg x day(‐1). European Respiratory Journal 1996;9:2263‐72. [DOI] [PubMed] [Google Scholar]
Hughes 1999a {published data only}
- Hughes GL, Edelman J, Turpin J, Liss C, Weeks K, Schweiger D, et al. Randomized, open‐label pilot study comparing the effects of montelukast sodium tablets, fluticasone aerosol inhaler and budesonide dry powder inhaler on asthma control in mild asthmatics. American Journal of Respiratory & Critical Care Medicine 1999;159(3 part 2):A641. [Google Scholar]
Hughes 1999b {published data only}
- Hughes JA, Conry BG, Male SM, Eastell R. One year prospective open study of the effect of high dose inhaled steroids, fluticasone propionate, and budesonide on bone markers and bone mineral density. Thorax 1999;54(3):223‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ige 2002 {published data only}
- Ige OM, Sogaolou OM. The clinical efficacy of fluticasone propionate compared with beclamethasone dipropionate in patients with mild to moderate bronchial asthma at the University College Hospital, Ibadan, Nigeria. West African Journal of Medicine 2002;21(4):297‐301. [DOI] [PubMed] [Google Scholar]
Johansson 1998 {published data only}
- Johansson LO. A comparison of once‐daily of fluticasone propionate (FP) 200 mcg and budesonide (BUD) 400 mcg twice‐daily of fluticasone (FP) 100 mcg. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A404. [Google Scholar]
- Johansson LO. A comparison of once‐daily regimen of fluticasone propionate (FP) 200 mcg and budesonide (BUD) 400 mcg and twice‐daily regimen of fluticasone propionate (FP) 100 mcg. European Respiratory Journal. 1998; Vol. 12 Suppl (28):38.
Joubert 1998 {published data only}
- Joubert J, Boszormenyi G, Sanchis J, Siafakas N. A comparison of the efficacy and systemic activity of budesonide and fluticasone propionate in asthmatic patients. European Respiratory Journal. 1998; Vol. 12:37.
Kannisto 2002 {published data only}
- Kannisto S, Voutilainen R, Remes K, Korppi M. Efficacy and safety of inhaled steroid and cromone treatment in school‐age children: A randomized pragmatic pilot study. Pediatric Allergy & Immunology 2002;13(1):24‐30. [DOI] [PubMed] [Google Scholar]
Kemmerich 1999 {published data only}
- Kemmerich B, Bruckner OJ, Petro W. Superiority of fluticasone powder from the Diskus over budesonide from the Turbuhaler in mild and moderate asthma. American Journal of Respiratory and Critical Care Medicine 1999;159(3 part 2):A627. [Google Scholar]
Kuna 2003 {published and unpublished data}
- Kuna P. A randomized, double‐blind, double‐dummy, parallel‐group, multicenter, dose‐reduction trial of the minimal effective doses of budesonide and fluticasone dry‐powder inhalers in adults with mild to moderate asthma. Clinical Therapeutics 2003;25(8):2182‐97. [DOI] [PubMed] [Google Scholar]
- Kuna P. Same minimal effective dose of budesonide Turbuhaler and fluticasone Diskus/Accuhaler in adult asthmatics. European Respiratory Journal 2001;18(Suppl 3):158s. [Google Scholar]
Langdon 1994a {published data only}
- Booth PC, Capsey LJ, Langdon CG, Wells NEJ. A comparison of the cost‐effectiveness of alternative prophylactic therapies in the treatment of adult asthma. British Journal of Medical Economics 1995;8:65‐72. [Google Scholar]
- Langdon CG, Capsey LJ. Fluticasone propionate and budesonide in adult asthmatics: a comparison using dry‐powder inhaler devices. British Journal of Clinical Research 1994;5:85‐99. [Google Scholar]
Langdon 1994b {published and unpublished data}
- FLUTI/AH89/F079. A phase III, multicentre, open‐label, parallel‐group study to compare the efficacy and tolerability of fluticasone propionate 100 micrograms bd with budesonide 200 micrograms bd administered via a metered‐dose inhaler in adult patients with mild to moderate asthma. http://ctr.gsk.co.uk 2005. [Google Scholar]
- Langdon CG, Thompson J. A multicentre study to compare the efficacy and safety of inhaled fluticasone propionate and budesonide via metered‐dose inhalers in adults with mild‐to‐moderate asthma. British Journal of Clinical Research 1994;5:73‐84. [Google Scholar]
Leblanc 1994 {published and unpublished data}
- FLIT14. A multicentre, double‐blind, randomised, four‐week, parallel‐group comparison of fluticasone propionate 100µg bd (200µg/day) with beclomethasone dipropionate 200µg bd (400µg/day) in adult patients with mild to moderate asthma. http://ctr.gsk.co.uk 2005.
- FLIT14 pt 2. A multicentre, open 12‐month study to assess the tolerance of fluticasone propionate administered via the pressurised inhaler at doses between 100µg bd (200µg/day) and 500µg bd (1000µg/day) in adult patients with mild to moderate asthma. http://ctr.gsk.co.uk 2005.
- Leblanc P, Mink S, Keistinen T, Saarelainen PA, Ringdal N, Payne SL. A comparison of fluticasone propionate 200 micrograms/day with beclomethasone dipropionate 400 micrograms/day in adult asthma. Allergy 1994;49:380‐5. [DOI] [PubMed] [Google Scholar]
Lorentzen 1996 {published and unpublished data}
- FMS40230. http://www.clinicalstudyresults.org 2005. [Google Scholar]
- Lorentzen KA, Helmond JL, Bauer K, Langaker KE, Bonifazi F, Harris TA. Fluticasone propionate 1 mg daily and beclomethasone dipropionate 2 mg daily: a comparison over 1 yr. Respiratory Medicine 1996;90:609‐17. [DOI] [PubMed] [Google Scholar]
Lundback 1993 {published data only}
- FLIT 72 (Long‐Term Follow‐Up). A multicentre, randomised, double‐blind, parallel group, six‐week study to compare the efficacy and safety of fluticasone propionate 500mcg daily via the metered dose inhaler and via the Diskhaler inhaler with beclomethasone dipropionate 1000mcg daily via the metered dose inhaler followed by a 46‐week assessment of the tolerance to fluticasone propionate 500mcg daily via the metered dose inhaler and beclomethasone dipropionate 1000mcg daily via the metered dose inhaler in patients with chronic asthma (results of the 46 week safety study). http://ctr.gsk.co.uk 2005. [Google Scholar]
- FLIT72. A multicentre, randomised, double‐blind, parallel group, six‐week study to compare the efficacy and safety of fluticasone propionate 500µg daily via the pressurised inhaler and via the diskhaler™ Inhaler with beclomethasone dipropionate 1000µg daily via the pressurised inhaler in patients with moderately severe chronic asthma. http://ctr.gsk.co.uk. [Google Scholar]
- Kesten S. Inhaled steroids in the long‐term treatment of asthma: a double‐blind, randomised, parallel group comparison of fluticasone propionate 500 µg daily and beclomethasone dipropionate 1000 µg daily over 12 months [abstract]. European Respiratory Journal 1993;6(Suppl 17):257s. [Google Scholar]
- Lundback B. A double‐blind randomised study to compare the efficacy and safety of fluticasone propionate (FP) 500 ug daily via a metered dose inhaler (MDI) with FP 500 ug daily via a diskhaler and beclomethasone dipropionate (BDP) 1000 ug daily via MDI in adult patients with chronic asthma. European Respiratory Journal 1992;5(Suppl 15):367s. [Google Scholar]
- Lundback B, Alexander M, Day J, Hebert J, Holzer R, Uffelen R, et al. Evaluation of fluticasone propionate (500 micrograms day‐1) administered either as dry powder via a Diskhaler inhaler or pressurized inhaler and compared with beclomethasone dipropionate (1000 micrograms day‐1) administered by pressurized inhaler. Respiratory Medicine 1993;87:609‐20. [DOI] [PubMed] [Google Scholar]
Lundback 1997 {unpublished data only}
- Lundback B, Bosh T, Brazell C. Quality of life improvements are greater in patients with severe asthma receiving inhaled fluticasone propionate 750mcg/d via dishkaler compared with budesonide 800mcg/d via turbuhaler. Amercian Thoracic Society conference abstract CD 1997. [Google Scholar]
- Lundback B, Sandstrom T, Ekstrom T, Hermansson BA, Alton M, Tunsater A. Comparison of the oral corticosteroid sparing effect of inhaled fluticasone propionate (FP) 750 mcg bd via the Diskhaler with budesonide (BUD) 800 mcg bd via the Turbuhaler in patients with chronic severe asthma. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A456. [Google Scholar]
- Lundback B, Sandstrom T, Garrett R, Zhang P, Bosh T. Comparison of the oral corticosteroid sparing effect of inhaled fluticasone propionate (FP) 750 µg via the Diskhaler with budesonide (BUD) 800 µg bd via the Turbuhaler in patients with chronic severe asthma [abstract]. European Respiratory Journal 1997;10(Suppl 25):172s. [Google Scholar]
Majer‐Teboul 2001 {unpublished data only}
- Majer‐Teboul C, Chemali‐Hudry J, Texereau J, Granier C, Dinh‐Xuan AT. Similar anti‐inflammatory effects of flixotide™ 500mug/day vs becotide™ 1000mug/day on exhaled NO after 7‐days treatment in asthma.. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23; www.abstracts2view.com 2001. [Google Scholar]
Malo 1999 {published data only}
- Malo JL, Cartier A, Ghezzo H, Mark S, Brown J, Laviolette M, Boulet LP. Skin bruising, adrenal function and markers of bone metabolism in asthmatics using inhaled beclomethasone and fluticasone. European Respiratory Journal 1999;13(5):993‐8. [DOI] [PubMed] [Google Scholar]
- Malo JL, Cartier A, Laviolette M, Boulet LP. Skin bruising adrenal function and bone metabolism in asthmatic subjects on inhaled beclomethasone and fluticasone [abstract]. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A406. [Google Scholar]
Melaranci 1999 {published data only}
- Melaranci C. Fluticasone propionate versus beclomethasone dipropionate in pediatric patients with moderate asthma. American Journal of Respiratory & Critical Care Medicine 1999;159(3 part 2):A631. [Google Scholar]
Molimard 2005 {published data only}
- Giraud V, Martinat Y, Molimard M. Improvement of asthma control: comparative study with beclomethasone extra fine aerosol, fluticasone or budesonide [Abstract]. American Thoracic Society 100th International Conference, May 21‐26. 2004:A37; Poster J121.
- Molimard M, Martinat Y, Rogeaux Y, Moyse D, Pello JY, Giraud V. Improvement of asthma control with beclomethasone extrafine aerosol compared to fluticasone and budesonide. Respiratory Medicine 2005;99(6):770‐8. [DOI] [PubMed] [Google Scholar]
Murray 1998 {published data only}
- Murray JJ, Friedman B, Chervinsky P, Fogarty C, Baker JW, Rogenes PR, et al. Fluticasone propionate (FP) is more effective than higher doses of beclomethasone dipropionate (BDP) in patients with moderate asthma. American Journal of Respiratory & Critical Care Medicine. 1998; Vol. 157:A407.
Nielsen 2000 {published data only}
- FLIQ50. http://ctr.gsk.co.uk 2006. [Google Scholar]
- Nielsen LP, Dahl R. Comparison of the relative potencies of fluticasone propionate and budesonide upon bronchial hyperresponsiveness and HPA axis function in asthmatic patients. Thorax 1999;54(Suppl 3):A31. [Google Scholar]
- Nielsen LP, Dahl R. Therapeutic ratio of inhaled corticosteroids in adult asthma: A dose‐range comparison between fluticasone propionate and budesonide, measuring their effect on bronchial hyperresponsiveness and adrenal cortex function. American Journal of Respiratory & Critical Care Medicine 2000;162(6):2053‐7. [DOI] [PubMed] [Google Scholar]
Nong 2001 {published data only}
- Nong BR, Huang YF, Hsieh KS, Huang YY, Huang CF, Chuang SL, et al. A comparison of clinical use of fluticasone propionate and beclomethasone dipropionate in pediatric asthma. Kao‐Hsiung i Hsueh Ko Hsueh Tsa Chih [Kaohsiung Journal of Medical Sciences] 2001;17(6):302‐11. [PubMed] [Google Scholar]
Parakh 2004 {published data only}
- Parakh U, Gupta K, Sharma S, Gaur SN. A comparative evaluation of the efficacy of inhaled beclomethasone dipropionate, budesonide and fluticasone propionate in the management of bronchial asthma. Indian Journal of Allergy Asthma and Immunology 2004;18(1):33‐8. [Google Scholar]
Pauwels 1998 {published data only}
- Pauwels RA, Yernault JC, Demedts MG, Geusens P. Safety and efficacy of fluticasone and beclomethasone in moderate to severe asthma. Belgian Multicenter Study Group. American Journal of Respiratory & Critical Care Medicine 1998;157:827‐32. [DOI] [PubMed] [Google Scholar]
Phillips 2004 {published data only}
- Phillips K, Oborne J, Lewis S, Harrison TW, Tattersfield AE. Time course of action of two inhaled corticosteroids, fluticasone propionate and budesonide. Thorax 2004;59(1):26‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prasad 2004 {published data only}
- Prasad R, Bandhu M, Kant S, Pandey US, Ahuja RC, Agarwal CG. A comparative study on efficacy of inhaled fluticasone propionate and beclomethasone dipropionate in patients of bronchial asthma. Indian Journal of Allergy, Asthma & Immunology 2004;18(2):73‐7. [Google Scholar]
Rao 1999 {published data only}
- Gregson RK, Rao R, Murrills AJ, Taylor PA, Warner JO. Effect of inhaled corticosteroids on bone mineral density in childhood asthma: comparison of fluticasone propionate with beclomethasone dipropionate. Osteoporosis International 1998;8(5):418‐22. [DOI] [PubMed] [Google Scholar]
- Rao R, Gregson RK, Jones AC, Miles EA, Campbell MJ, Warner JO. Systemic effects of inhaled corticosteroids on growth and bone turnover in childhood asthma: a comparison of fluticasone with beclomethasone. European Respiratory Journal 1999;13:87‐94. [DOI] [PubMed] [Google Scholar]
- Rao R, Gregson RK, Jones AC, Taylor PA, Murrills AJ, Warner JO. Effects of inhaled corticosteroids on growth and bone turnover in childhood asthma: comparison of fluticasone propionate and beclomethasone dipropionate. European Respiratory Journal 1997;10(Suppl 25):24s. [Google Scholar]
Raphael 1999a {published data only}
- Raphael GD, Lanier RQ, Baker J, Edwards L, Rickard K, Lincourt WR. A comparison of multiple doses of fluticasone propionate and beclomethasone dipropionate in subjects with persistent asthma. Journal of Allergy & Clinical Immunology 1999;103(5 Pt 1):796‐803. [DOI] [PubMed] [Google Scholar]
Raphael 1999b {published data only}
- Raphael GD, Lanier RQ, Baker J, Edwards L, Rickard K, Lincourt WR. A comparison of multiple doses of fluticasone propionate and beclomethasone dipropionate in subjects with persistent asthma. Journal of Allergy & Clinical Immunology 1999;103(5 Pt 1):796‐803. [DOI] [PubMed] [Google Scholar]
Ringdal 1996 {published data only}
- Ringdal N, Swinburn P, Backman R, Plaschke P, Sips AP, Kjaersgaard P, et al. A blinded comparison of fluticasone propionate with budesonide via powder devices in adult patients with moderate‐to‐severe asthma: a clinical evaluation. Mediators of Inflammation 1996;5:382‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ringdal 2000 {published data only}
- Ringdal B, Lundback M, Alton M, Rak S, Eivindson A, Bratten G, et al. Comparison of the effect on HPA‐axis of inhaled fluticasone propionate (FP) 1500 mcg/day via Diskus and budesonide (BUD) 1600 mcg/day via Turbuhaler in adult asthmatic patients. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A406. [Google Scholar]
- Ringdal N, Lundback B, Alton M, Rak S, Evindson A, Bratten G, et al. Comparison of the effect on HPA‐Axis of inhaled fluticasone propionate (FP) 1500 mcg/day via Diskus and budesonide (BUD) 1600 mcg/day via Turbuhaler in adult asthmatic patients. European Respiratory Journal 1998;12(Suppl 28):37. [Google Scholar]
- Ringdal N, Lundbäck B, Alton M, Rak S, Eivindson A, Bratten G, et al. Comparable effects of inhaled fluticasone propionate and budesonide in the HPA‐axis in adult asthmatic patients. Respiratory Medicine 2000;94:482‐9. [DOI] [PubMed] [Google Scholar]
SD‐004‐0377 {unpublished data only}
- SD 004 0377. Once daily administration of 200 µg budesonide Turbuhaler® and 200 µg fluticasone propionate via Diskus® inhaler in stable asthmatics(SD‐004‐0377). http://www.astrazenecaclinicaltrials.com/Article/515450.aspx 2006. [Google Scholar]
Steinmetz 1997 {published data only}
- Steinmetz KO. Comparative efficacy and safety of fluticasone propionate MDI versus budesonide powder inhalation in the treatment of moderate asthma [Vergleich der Wirksamkeit und Verträglichkeit von fluticasonpropionat‐dosieraerosol und budesonid‐pulverinhalat bei mittelschwerem asthma]. Atemwegs Und Lungenkrankheiten 1997;23(12):730‐5. [Google Scholar]
- Steinmetz KO, Trautmann M. Efficacy of fluticasone propionate (0.5mg daily) via MDI and budesonide (1.2 mg daily) via Turbuhaler in the treatment of steroid‐naïve asthmatics. American Journal of Respiratory & Critical Care Medicine 1996;153(4 part 2):A338. [Google Scholar]
- Steinmetz KO, Volmer T, Trautmann M, Kielhorn A. Cost effectiveness of fluticasone and budesonide in patients with moderate asthma. Clinical Drug Investigation 1998;16(2):117‐23. [DOI] [PubMed] [Google Scholar]
Ställberg 2007 {published and unpublished data}
- FAS40017. A double blind, randomised, parallel group study with Flixotide Diskus (250microg) bd and Pulmicort Turbohaler (400microg) bd to compare lowest effective maintenance doses followed by an open health economic evaluation. http://ctr.gsk.co.uk 2005.
- Stallberg B, Pilman E, Skoogh BE, Hermansson BA, Vardcentral T. The potency ratio fluticasone propionate/budesonide [Abstract]. European Respiratory Journal 2003;22(Suppl 45):P445. [Google Scholar]
- Ställberg B, Pilman E, Skoogh BE, Hermansson BA. Potency ratio fluticasone propionate (Flixotide Diskus)/budesonide (Pulmicort Turbuhaler). Respiratory Medicine 2007;101(3):610‐5. [DOI] [PubMed] [Google Scholar]
Subburao 2005 {published data only}
- Subbarao P, Dorman SC, Rerecich T, Watson RM, Gauvreau GM, O'Byrne PM. Protection by budesonide and fluticasone on allergen‐induced airway responses after discontinuation of therapy. Journal of Allergy and Clinical Immunology 2005;115(4):745‐50. [DOI] [PubMed] [Google Scholar]
- Subbarao P, Watson RM, Rerecich T, O'Byrne PM. Protective effects of budesonide and fluticasone on allergen‐induced airway hyperresponsiveness and inflammation 12 hours after discontinuation of therapy [abstract]. American Thoracic Society 99th International Conference. 2003; Vol. D094 Poster 614.
Szefler 2002 {published data only}
- Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. Journal of Allergy & Clinical Immunology 2002;109(3):410‐8. [DOI] [PubMed] [Google Scholar]
Vedanthan 2004 {published data only}
- Vedanthan PK, Palaksha S, Partasarathi G, Thomas S, Jayaraj BS, Mahesh PA. Comparison of quality of life (QOL) assessment in patients with asthma on equipotent doses of fluticasone and beclomethasone inhalers [Abstract]. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004:C94 Poster 622.
Williams 1997 {published data only}
- Williams J, Richards KA. Ease of handling and clinical efficacy of fluticasone propionate Accuhaler/Diskus inhaler compared with the Turbohaler inhaler in paediatric patients. British Journal of Clinical Practice 1997;51:147‐53. [PubMed] [Google Scholar]
Wolfe 2000 ICS {unpublished data only}
- FLTA2004. http://ctr.gsk.co.uk. [Google Scholar]
- Stanford R, Wightman D, Lincourt W, Edwards L, Crim C. Patient satisfaction with fluticasone propionate 250µg once daily. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A769. [Google Scholar]
- Wolfe J, Rooklin A, Grady J, Munk ZM, Stevens A, Prillaman B, et al. Comparison of once‐ and twice‐daily dosing of fluticasone propionate 200 micrograms per day administered by diskus device in patients with asthma treated with or without inhaled corticosteroids. Journal of Allergy and Clinical Immunology 2000;105(6 Pt 1):1153‐61. [DOI] [PubMed] [Google Scholar]
Wolfe 2000 SABA {unpublished data only}
- FLTA2003. http://ctr.gsk.co.uk. [Google Scholar]
- Stanford R, Wightman D, Lincourt W, Edwards L, Crim C. Patient satisfaction with fluticasone propionate 250µg once daily. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A769. [Google Scholar]
- Wolfe J, Rooklin A, Grady J, Munk ZM, Stevens A, Prillaman B, et al. Comparison of once‐ and twice‐daily dosing of fluticasone propionate 200 micrograms per day administered by diskus device in patients with asthma treated with or without inhaled corticosteroids. Journal of Allergy and Clinical Immunology 2000;105(6 Pt 1):1153‐61. [DOI] [PubMed] [Google Scholar]
Wolthers 1997 {published data only}
- FLIP51. http://ctr.gsk.co.uk 2005. [Google Scholar]
- Wolthers OD, Hansen M, Juul A, Nielsen HK, Pedersen S. Knemometry, urine cortisol excretion, and measures of insulin‐like growth factor axis and collagen turnover in children treated with inhaled glucocorticosteroids. Pediatric Research 1997;41:44‐50. [DOI] [PubMed] [Google Scholar]
- Wolthers OD, Pedersen S. Short‐term growth during treatment with inhaled fluticasone propionate and beclomethasone dipropionate. Archives of Disease in Childhood 1993;68:673‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yiallouros 1997 {published data only}
- FLIT81. http://ctr.gsk.co.uk 2005. [Google Scholar]
- Yiallouros PK, Milner AD, Conway E, Honour JW. Adrenal function and high dose inhaled corticosteroids for asthma. Archives of Disease in Childhood 1997;76:405‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aubier 2001 {published data only}
- Aubier M, Wettenger R, Gans SJ. Efficacy of HFA‐beclomethasone dipropionate extra‐fine aerosol (800 microg day(‐1)) versus HFA‐fluticasone propionate (1000 microg day(‐1)) in patients with asthma. Respiratory Medicine 2001;95(3):212‐20. [DOI] [PubMed] [Google Scholar]
- O'Reilly JF, Stephenson PS, Evans KE. Cost‐effectiveness of fluticasone compared with beclomethasone extrafine aerosol. European Respiratory Journal 2001;18(Supp 33):49s. [Google Scholar]
Barnes 1999a {published data only}
- Barnes NC, Price MJ, Thwaites RMA. Impact of disease severity on the cost‐effectiveness of fluticasone propionate and budesonide in asthma. European Respiratory Journal 1999;14 Suppl (30):371. [Google Scholar]
Barnes 1999b {published data only}
- Barnes NC, Thwaites RM, Price MJ. The cost‐effectiveness of inhaled fluticasone propionate and budesonide in the treatment of asthma in adults and children. Respiratory Medicine 1999;93(6):402‐7. [DOI] [PubMed] [Google Scholar]
Clark 1996a {published data only}
- Clark DJ, Clark RA, Lipworth BJ. Adrenal suppression with inhaled budesonide and fluticasone propionate given by large volume spacer to asthmatic children. Thorax 1996;51:941‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 1996b {published data only}
- Clark DJ, Grove A, Cargill RI, Lipworth BJ. Comparative adrenal suppression with inhaled budesonide and fluticasone propionate in adult asthmatic patients. Thorax 1996;51:262‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 1997 {published data only}
- Clark DJ, Lipworth BJ. Adrenal suppression with chronic dosing of fluticasone propionate compared with budesonide in adult asthmatic patients. Thorax 1997;52:55‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dorinsky 2004 {published data only}
- Dorinsky P, Emmett A, Sutton L. Reduced risk for asthma exacerbations in pediatric patients receiving salmeterol plus inhaled corticosteroids (ICS) vs ICS alone [Abstract]. European Respiratory Journal 2004;24(Suppl 48):308s. [Google Scholar]
- Dorinsky P, Kerwin E, Schoaf L, Ellsworth A, Housse K. Effectiveness and safety of fluticasone propionate /salmeterol 250/50mcg administered once daily to patients with persistent asthma [Abstract]. European Respiratory Journal 2004;24(Suppl 48):309s. [Google Scholar]
- Dorinsky P, Yancey S, Reilly D, Stauffer J, Edwards L, Sutton L. Control of airway inflammation is maintained in asthma patients following a reduction in ICS dose with fluticasone propionate /salmeterol (FSC) compared with higher dose fluticasone propionate (FP) alone [Abstract]. European Respiratory Journal 2004;24(Suppl 48):308s. [Google Scholar]
Ericsson 1999 {published data only}
- Ericsson A, Leuenberger P, Perruchoud D, Cheung C, Grassi C. Budesonide Turbohaler gave lower health care costs than fluticasone propionate. European Respiratory Journal 1999;14 Suppl (30):508. [Google Scholar]
Fairfax 2001 {published data only}
- Fairfax A, Hall I, Spelman R. A randomized, double‐blind comparison of beclomethasone dipropionate extra fine aerosol and fluticasone propionate. Annals of Allergy, Asthma, & Immunology 2001;86(5):575‐82. [DOI] [PubMed] [Google Scholar]
Fowler 2002 {published data only}
- Fowler SJ, Orr LC, Sims EJ, Wilson AM, Currie GP, McFarlane L, et al. Therapeutic ratio of hydrofluoroalkane and chlorofluorocarbon formulations of fluticasone propionate. Chest 2002;122(2):618‐23. [DOI] [PubMed] [Google Scholar]
Harmanci 2001 {published data only}
- Harmanci E, Colak O, Metintas M, Alatas O, Yurdasiper A. Fluticasone propionate and budesonide do not influence bone metabolism in the long term treatment of asthma. Allergologia et Immunopathologia 2001;29(1):22‐7. [DOI] [PubMed] [Google Scholar]
- Harmanci E, Colak O, Ozdemir N, Alatas O, Isik R. Fluticasone propionate and budesonide does not influence bone metabolism in the long term treatment of asthma. European Respiratory Journal. 1998; Vol. 12 Suppl (28):434.
Harrison 1999 {published data only}
- Harrison TW, Wisniewski J, Honour JW, Tattersfield AE. Systemic activity of inhaled fluticasone propionate and budesonide in subjects with and without asthma. European Respiratory Journal. 1999; Vol. 14 Suppl (30):466.
Hawkins 2003 {published data only}
- Hawkins G, McMahon AD, Twaddle S, Wood SF, Ford I, Thomson NC. Stepping down inhaled corticosteroids in asthma: randomised controlled trial. British Medical Journal 2003;326(7399):1115‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See S, Rubin S. Tapering inhaled steroids effective for chronic asthma. Journal of Family Practice 2003;52(10):748‐51. [PubMed] [Google Scholar]
Kannisto 2000 {published data only}
- Kannisto S, Korppi M, Remes K, Voutilainen R. Adrenal suppression, evaluated by a low dose adrenocorticotropin test, and growth in asthmatic children treated with inhaled steroids. Journal of Clinical Endocrinology & Metabolism 2000;85(2):652‐7. [DOI] [PubMed] [Google Scholar]
Karakoc 2001 {published data only}
- Karakoc F, Karadag B, Kut A, Ersu R, Bakac S, Cebeci D, et al. A comparison of the efficacy and safety of a half dose of fluticasone propionate with beclomethasone dipropionate and budesonide in childhood asthma. Journal of Asthma 2001;38(3):229‐37. [DOI] [PubMed] [Google Scholar]
Lipworth 1997 {published data only}
- Lipworth BJ, Clark DJ, McFarlane LC. Adrenocortical activity with repeated twice daily dosing of fluticasone propionate and budesonide given via a large volume spacer to asthmatic school children. Thorax 1997;52:686‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipworth BJ, Wilson DJ, Clark DJ. Adrenal activity with chronic dosing of fluticasone propionate (FP) and budesonide (BUD) in asthmatic children. American Journal of Respiratory & Critical Care Medicine. 1997; Vol. 155:A267.
Lofdahl 1999 {published data only}
- Lofdahl CG, Thorsson L. Systemic availability of inhaled fluticasone propionate and budesonide in asthmatic patients and healthy subjects. European Respiratory Journal. 1999; Vol. 14 Suppl (30):466.
Medici 2000 {published data only}
- Medici TC, Grebski E, Häcki M, Rüegsegger P, Maden C, Efthimiou J. Effect of one year treatment with inhaled fluticasone propionate or beclomethasone dipropionate on bone density and bone metabolism: a randomised parallel group study in adult asthmatic subjects. Thorax 2000;55:375‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici TG, Grebski E, Hacki M, Ruegsegger P, Maden C, Efthimiou J. One‐year treatment with inhaled fluticasone propionate or beclomethasone dipropionate does not affect bone density and bone metabolism. A randomised, parallel group study in adult asthmatics. American Journal of Respiratory & Critical Care Medicine. 1998; Vol. 157:A407. [DOI] [PMC free article] [PubMed]
O'Reilly 1998 {published data only}
- O'Reilly JF, Weir DC, Banham S, Basran GS, Boyd G, Patel KR. Is high‐dose fluticasone propionate via a metered‐dose inhaler and Volumatic as efficacious as nebulized budesonide in adult asthmatics?. Respiratory Medicine 1998;92(1):111‐7. [DOI] [PubMed] [Google Scholar]
Price 1998 {published data only}
- Price DB, Appleby JL. Fluticasone propionate: an audit of outcomes and cost‐effectiveness in primary care. Respiratory Medicine 1998;92:351‐3. [DOI] [PubMed] [Google Scholar]
Primhak 1999 {published data only}
- Primhak RA, Smith CM, Powell CVE. A comparison of the systemic effects of high dose inhaled steroids in asthmatic children. American Journal of Respiratory & Critical Care Medicine 1999;159(3 part 2):A909. [Google Scholar]
Spelman 1999 {published data only}
- Spelman R, Hughes J, Price M. The cost effectiveness of inhaled fluticasone propionate compared with budesonide in adults and children with asthma in Ireland. American Journal of Respiratory & Critical Care Medicine 1999;159(3 part 2):A762. [Google Scholar]
Stempel 1999 {published data only}
- Stempel D, Stanford R, Thwaites R, Price M. The costs and effectiveness of inhaled fluticasone propionate and budesonide in the management of asthma. American Journal of Respiratory & Critical Care Medicine 1999;159(3 part 2):A630. [Google Scholar]
Thorsson 1999 {published data only}
- Thorsson l, Edsbacker S. Lung uptake of budesonide via dry powder inhaler was greater than that of fluticasone propionate via dry powder inhaler or PMDI. European Respiratory Journal. 1999; Vol. 14 Suppl (30):62S.
Thwaites 1997 {published data only}
Tsoi 1998 {published data only}
- Tsoi A, Gafurov M, Shor O. The maintenance treatment of asthmatic patients with comparable doses of different inhaled corticosteroids (IC). European Respiratory Journal. 1998; Vol. 12:63.
Wilson 1998 {published data only}
- Wilson AM, Clark DJ, Devlin MM, McFarlane LC, Lipworth BJ. Adrenocortical activity with repeated administration of one‐daily inhaled fluticasone propionate and budesonide in asthmatic adults. European Journal of Clinical Pharmacology 1998;53(5):317‐20. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chlumsky 1998 {published data only}
- Chlumsky J. Comparison of the clinical effect of fluticasone propionate and beclomethasone dipropionate in patients with bronchial asthma [Srovnani klinickeho ucinku flutikazon propionatu s beklometazon dipropionatem u pacientu s bronchialnim astmatem]. Prakticky Lekar 1998;78(4):186‐9. [Google Scholar]
Djordevic 2000 {unpublished data only}
- Djordevic DV, Zivkovic DJS, Stankovic IJ, Pejcic TA, Rancic MR, Ristic LM, et al. A comparison of fluticasone propionate 250mcg/day with beclomethasone dipropionate 500mcg/day in adult asthma. European Respiratory Journal 2000;16(Suppl 31):281s. [Google Scholar]
Dong 1999 {published data only}
- Dong KY, Young SK, Chul MA, Won KK, Chang J, Sung KK, et al. Fluticasone propionate and beclomethasone dipropionate in asthmatic patients. [Korean]. Tuberculosis & Respiratory Diseases 1999;47(5):629‐41. [Google Scholar]
Thomas 2005 {published data only}
- Thomas S, Parthasarathi G, Palaksha S, Jayaraj BS, Gowda HB, Mahesh PA. Quality of life assessment in asthmatic patients receiving fluticasone compare with equipotent doses of beclomethasone or budesonide. Lung India 2005;22(3):86‐91. [Google Scholar]
Yang 1999 {published data only}
- Yang DK, Kim YS, Ahn CM, Ko WK, Chang J, et al. Fluticasone propionate beclomethasone dipropionate. Tuberculosis and Respiratory Disease 1999;47(5):629‐41. [Google Scholar]
Additional references
Adams 1999
- Adams N, Bestall J, Jones PW. Beclomethasone at different doses for chronic asthma. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD002879] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2000
- Adams NP, Bestall JB, Jones PW. Inhaled budesonide at different doses for chronic asthma. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2005
- Adams NP, Bestall JC, Jones PW, Lasserson TJ, Griffiths B, Cates CJ. Fluticasone at different doses for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Barnes 1998
- Barnes NC, Hallett C, Harris TAJ. Clinical experience with fluticasone propionate in asthma: a meta‐analysis of efficacy and systemic activity compared with budesonide and beclomethasone dipropionate at half the microgram dose or less. Respiratory Medicine 1998;92:95‐104. [DOI] [PubMed] [Google Scholar]
BTS 2003
- British Thoracic Society. British Guidelines on Asthma Management. Thorax 2003;58(Supplement 1). [Google Scholar]
Cochrane Handbook
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. Available from www.cochrane‐handbook.org.. The Cochrane Collaboration, 2008. [Google Scholar]
GINA 1995
- National Asthma Education and Prevention Program. Global initiative for asthma management and prevention NHBLI/WHO workshop report. National Institute of Health, Bethseda, MD 1995, issue NIH Publication No. 95‐3659.
GINA 2002
- NHLBI/WHO Workshop. Global Initiative on Asthma (GINA): Management and Prevention. www.ginasthma.com. Second Edition. Washington: NIH Publication, 2002. [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Kelly 1998
- Kelly HW. Establishing a therapeutic index for the inhaled corticosteroids: part I. Pharmacokinetic/pharmacodynamic comparison of the inhaled corticosteroids. Journal of Allergy & Clinical Immunology 1998;102:36‐51. [DOI] [PubMed] [Google Scholar]
Lasserson 2006
- Lasserson TJ, Cates CJ, Jones A‐B, Steele EH, White J. Fluticasone versus HFA‐beclomethasone dipropionate for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005309.pub3] [DOI] [PubMed] [Google Scholar]
Lipworth 1999
- Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy . A systematic review and meta‐analysis. Archives of Internal Medicine 1999;159:941‐55. [DOI] [PubMed] [Google Scholar]
Lonnebo 1996
- Lonnebo A, Grahnen A, Jansson B, Bruden RM Ling‐Andersson A. An assessment of the systemic effects of single repeated doses of inhaled fluticasone propionate, and inhaled budesonide in healthy volunteers. European Journal of Clinical Pharmacology 1996;49:459‐63. [DOI] [PubMed] [Google Scholar]
NHLBI 1997
- National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Managment of Asthma, Expert Panel Report No. 2. Bethesda MD: NIH/National Heart, Lung and Blood Institute 1997, issue NIH Publication No. 97‐4051.
Paton 2006
- Paton J, Jardine E, McNeill E, Beaton S, Galloway P, Young D, et al. Adrenal responses to low dose synthetic ACTH (Synacthen) in children receiving high dose inhaled fluticasone. Archives of Disease in Childhood 2006;91(10):808‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillipps 1990
- Phillipps GH. Structure‐activity relationships of topically active steroids: the selection of fluticasone propionate. Respiratory Medicine 1990;84 Suppl (A):19‐23. [DOI] [PubMed] [Google Scholar]
Stellato 1999
- Stellato C, Atsuta J, Bickel CA, Schleimer RP. An in vitro comparison of commonly used topical glucocorticoid preparations. Journal of Allergy & Clinical Immunology 1999;104:623‐9. [DOI] [PubMed] [Google Scholar]
Thorsson 1997
- Thorsson L, Dahlstrom K, Edsbacker S, Kallen A, Paulson J, Wiren JE. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in healthy subjects. British Journal of Clinical Pharmacology 1997;43(2):155‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Adams 2004
- Adams N, Bestall JM, Lasserson TJ, Jones PW. Inhaled fluticasone versus inhaled beclomethasone or inhaled budesonide for chronic asthma.. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI] [PubMed] [Google Scholar]
Adams 2005a
- Adams N, Bestall JM, Lasserson TJ, Jones PW. Inhaled fluticasone versus inhaled beclomethasone or inhaled budesonide for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI] [PubMed] [Google Scholar]


