Abstract
Radiation therapy (RT) is an essential component of effective cancer care and is used across nearly all cancer types. The delivery of RT is becoming more precise through rapid advances in both computing and imaging. The direct integration of magnetic resonance imag-ing (MRI) with linear accelerators represents an exciting development with the potential todramatically impact cancer research and treatment. These impacts extend beyond improved imaging and dose deposition. Real-time MRI-guided RT is actively transforming the work flows and capabilities of virtually every aspect of RT. It has the opportunity to change entirely the delivery methods and response assessments of numerous malignancies. This review intends to approach the topic of MRI-based RT guidance from a vendor neutral and international perspective. It also aims to provide an introduction to this topic targeted towards oncologists without a speciality focus in RT. Speciality implications, areas for physician education and research opportunities are identified as they are associated with MRI-guided RT. The uniquely disruptive implications of MRI-guided RT are discussed and placed in context. We further aim to describe and outline important future changes to the speciality of radiation oncology that will occur with MRI-guided RT. The impacts on RT caused by MRI guidance include target identification, RT planning, quality assurance, treatment delivery, training, clinical workflow, tumour response assessment and treatment scheduling. In addition, entirely novel research areas that may be enabled by MRI guidance are identified for future investigation.
1. Introduction
Clinical evidence supports the use of radiation therapy (RT) in 50% of all cancer patients. With global cancer cases to reach 24.9M by 2035, further advances in RT are important to improve cancer outcomes and to minimise side effects [1]. Image-guided RT (IGRT) has represented an important advance in RT for well over a decade [2]. IGRT has been widely adopted by the radiation oncology community and is used in the majority of radiation treatments [3,4]. Radiation oncologists are amply trained in the acquisition and interpretation of computed tomography (CT) images used for IGRT. Contemporary IGRT allows for increasingly precise target localisation along with tumour and normal structure position verification in three dimensions [2].
Magnetic resonance imaging (MRI) offers superior soft tissue discrimination, increased sensitivity for tumour detection and dynamic biological and functional data about tumours and normal tissues. MRI has been used for well over a decade to help define and direct RT volumes in many cancer sites. MRI’s role in RT has largely remained limited to the initial radiation planning stages, that is, before treatment begins. MRI devices have recently merged with radiation delivery devices (linear accelerators) to form an entirely novel RT paradigm categorised as MR-guided RT (or ‘MRgRT’). MRgRT possesses the ability to acquire an MRI immediately before, during and after the patient is treated with RT, all with the patient in the same treatment position. In this review, we summarise the current approaches to MRgRT and highlight its implications and opportunities on oncology at large.
2. Methods
An experienced cohort of radiation oncologists and medical physicists were assembled, bringing together users of the two commercially available MRgRT systems. Each author had intimate familiarity with the logistics and technical challenges associated with MRgRT in the two currently available commercial units. A literature search was conducted via PubMed using the key words ‘MR/MRI-Guided RT (766 items reviewed)’, ‘MR Linac (248 items reviewed)’, ‘ViewRay (54 items reviewed) ‘, and search results were curated. We restricted our search to English-language articles published from 1995 to 2019 and those that focused on single unit MRgRT devices. Abstract only publications were excluded. Research articles focused on combining MR images with RT treatment were also excluded as they were extensive in nature and considered beyond the scope of this review. The literature search was restricted solely to series focused on the combination MR-linear accelerator-based devices; articles examining in-room MR solutions or ‘MR on rails’ were also excluded.
3. Discussion and observations
The current state of MRgRT is rapidly evolving and expanding. This technology is positioned to dramatically impact the treatment of thousands of cancer patients annually. It will also rapidly introduce entirely novel research questions and opportunities. MR guidance has some similarities, but also crucial differences, to other recent technological developments in the delivery of RT. Fig. 1 presents a general overview of workflow differences introduced with MR guidance.
Fig. 1.
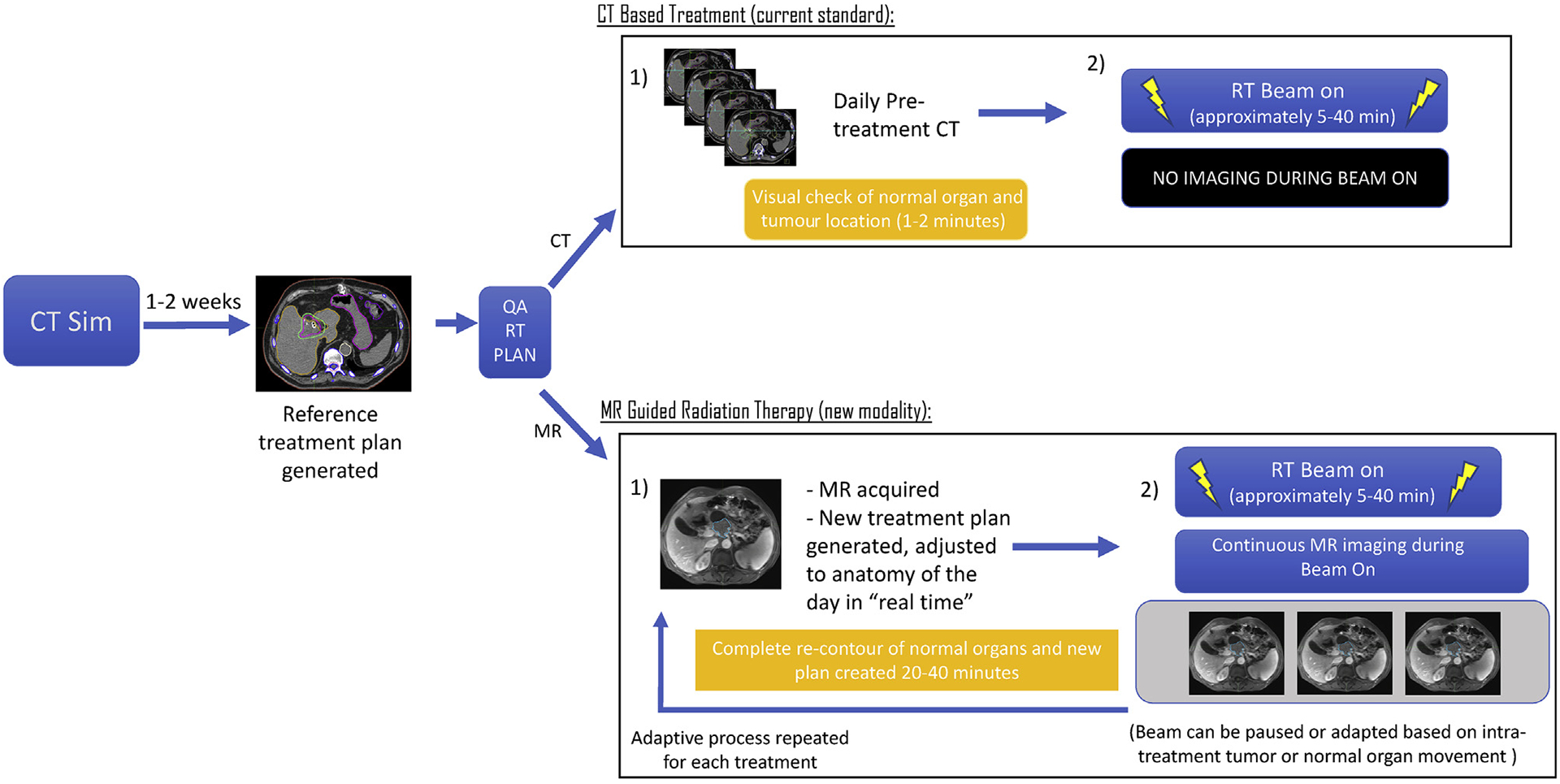
Differences between CT and MR-based RT. RT, radiation therapy; CT, computed tomography; MR, magnetic resonance.
RT has undergone several transformations over the past fifteen years. The routine use of CT simulation required a primary knowledge expansion on the part of radiation oncologists who had to familiarise themselves with cross-sectional and three dimensional anatomy as depicted on CT-based imaging. Since the introduction of CT-based planning, other major technological advances in the form of intensity-modulated RT (IMRT) and proton therapy have been introduced. The introduction of IMRT represented a considerable improvement in radiation dose sculpting and normal tissue avoidance. Protons and heavy ions represent further gains in dosimetric conformality. MRgRT is unique in its contribution of extremely high quality imaging at the time of treatment. To help illustrate the impact of MRgRT, we have divided the discussion into several categories outlining the current and anticipated changes to the discipline of RT following the introduction of MRgRT.
3.1. Differences between MRgRT and other technological advances in RT
While IMRT and proton therapy have resulted in important improvements in radiation dose deposition, neither has addressed two central problems facing RT. The specific challenges that remain inadequately addressed include (1) high doses of radiation delivered to normal organs in very close proximity to the treated tumour (which is often unavoidable secondary to the need for a planning target volume [PTV] expansion to account for daily set up uncertainties) and (2) personalisation of RT via active monitoring of biologic tumour response. Total dose deposition and conformality of dose have been an understandable focus of radiation oncology technological advancement for decades. This has motivated the development of IMRT, IGRT and proton therapy. Important to realise is that radiotherapy planning involves delivering dose not only to the tumour volume but also to a rim of surrounding normal tissue to take into account systematic and random errors such as calibration uncertainty and organ motion. While the high-dose conformality with IMRT and proton therapy have enabled dose escalation, the radiation oncology community has seen that dose escalation alone may fail to improve outcomes [5]. This might be limited by the need for a PTV expanding into some critical local structures. Indeed, CT-based strategies for dose escalation are often compromised by the large uncertainty of tumour and normal structures with low soft-tissue contrast on CT images. Moreover, current CT-based methods suffer from an incapability of monitoring tumour and normal structure movement during radiation delivery. MR guidance directly addresses and improves upon these issues. The ability to determine the location of the tumour and adjacent normal organ/tissue boundaries, together with the ability to account for intratreatment and intertreatment motion, will reduce radiation dose to normal organs, thereby widening the therapeutic window. A summary of major technologic developments in radiation oncology and their potential contributions to clinical oncology research can be seen in Table 1.
Table 1.
Technological advances in RT.
| Technological development | Changes to RT | Clinical trials enabled By technological development | Limitations of technological development |
|---|---|---|---|
| CT-guided intensity-modulated RT (IMRT) [30] |
|
|
|
| Proton therapy |
|
|
|
| MR-guided intensity-modulated RT |
|
|
|
MR, magnetic resonance; DWI, diffusion weighted image; NTCP, normal tissue complication probability.
3.2. Existing technological implementations of MRgRT
At the time of writing this article, there are two commercially available MRgRT technologies. These devices are manufactured by ViewRay (ViewRay Technologies Inc, Oakwood Village, Ohio) and Elekta (Elekta AB, Stockholm, Sweden). There are also at least two devices that are in active development: one is by an Australian-based development group [6] and the second is the Aurora-RT system (MagnetTx Oncology Solutions, Edmonton, Alberta, Canada) [7]. These devices are gaining rapid and wide market traction (eg. 36 Elekta Unity systems have been sold and approximately 51 from ViewRay). Key differences between devices are presented in Table 2. The discussion will focus on the two commercially available devices, namely the ViewRay MRIdian and Elekta Unity MR Linac systems. To date, ViewRay has produced two different systems consisting of their first device, a split 0.35 T magnetic resonance scanner with a ring gantry and 3 multileaf collimator-equipped 60Co heads (no longer in production) followed by their second device, capable of 6 MV photon production combined, again, with a 0.35 T MR [8]. The ViewRay MRI-cobalt device has been US Food and Drug Administration (FDA) approved since May 22, 2012, and Viewray MRIdian linear accelerator has been approved since February 24, 2017 and the Elekta Unity system received FDA approval on December 5th, 2018. The Elekta Unity system is a 1.5 T MR produced by Philips combined with a 7 MV linear accelerator produced by Elekta [9,10]. There have been over 5000 patients treated with these systems, with multiple published series describing the initial clinical experience and case mix [12,23,34–48]. Details regarding each of these systems are presented in Table 2.
Table 2.
Types of MR Linac technologies.
| Commercial name | Manufacture | MRI field strength | Bore size | Beam strength | Clinical outcome publicationsa |
|---|---|---|---|---|---|
| Commercially available | |||||
| ViewRay [8] | ViewRay Technologies Inc, | 0.35 T | 70 cm | Co-60 source | [12,23,34–48] |
| - Co-60 | Oakwood Village, Ohio | 6 MV | |||
| - Linac | |||||
| Elekta | Elekta AB, Stockholm, Sweden | 1.5 T | 70 cm | 7 MV | [24,50,51] |
| Unity [49,50] | |||||
| In development | |||||
| Australian MRI Linac System [6] | Australian MRI-Linac Program | 1 T | 82 cm | 6 MV | N/A |
| Aurora-RT System [52] | MagnetTx, Edmonton, Alberta, Canada | 0.6 T | 60 cm | 6 MV | N/A |
MV, megavoltage; cm, centimetres; Co-60, Cobalt-60.
Clinical outcome publications involved the treatment of patients with reported outcomes.
3.3. Implications of MR guidance on radiation treatment volumes
As mentioned previously, at the core of RT is the PTV [11]. This PTV margin is needed to account for interfractional and intrafractional variability of setup of the tumour and normal structures. Typically ranging from 3 mm to 15 mm, the PTV structure extends considerably into adjacent normal organs or tissues that do not contain tumour. The need to incorporate a PTV margin with the associated exposure of adjacent normal structures currently limits the dose and fractionation schemes to what is tolerated by normal tissues rather than what is required to achieve tumour control. A fundamental change that will likely be seen with MR guidance is that using MRI immediately before and during a treatment delivery will enable accurate delineation and monitoring of tumour and normal structures at every treatment. This will result in much smaller irradiated volumes. For example, the elimination of PTV margins in the treatment of breast cancer has resulted in a 52% reduction in the PTV volume, which likely has important implications for cosmetic outcomes [12]. This will result in lower doses of radiation to normal structures very close to the tumour. Fig. 2 visually illustrates differences, and potential benefits, of smaller PTV expansions.
Fig. 2.
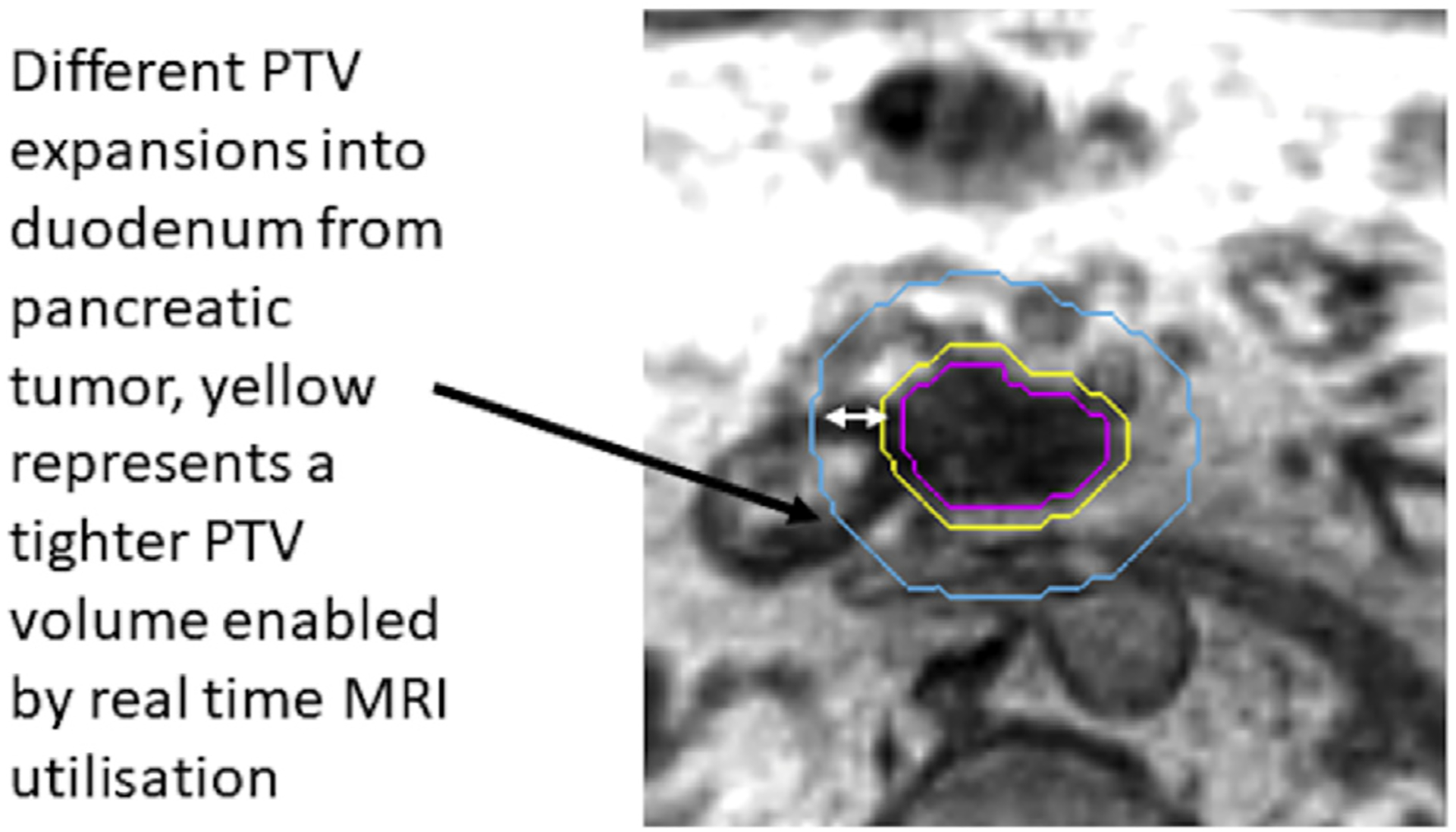
Examples of differences in expansions potentially enabled with real-time MR guidance. MRI, magnetic resonance imaging; PTV, planning target volume.
While exciting and promising, this change also has important implications on physician and other radiation caregiver time. Such an MRI guidance strategy requires a radiation oncologist, medical physicist and radiation therapist to spend additional time at the machine to adapt treatment delivery on a daily basis, as tumour and normal organ changes are detected [13]. Fig. 1 highlights some of these key differences in workflow that may be presented with MRgRT. Radiation oncologists, medical physicists and radiation therapists will have to adapt their schedules to account for the additional time necessary to monitor the tumour and normal organs now seen continuously with MRgRT.
3.4. Training and education in MRgRT for radiation oncologists
Routine use of MRI will require ‘up-skilling’ radiation oncology professionals to acquire an enhanced MRI knowledge base and skill set. Although MR has benefits at the time of simulation, it is not routinely used across the world. To optimise response evaluation, regional organ definitions and data provided by various MRI sequences, these sequences will need to be understood by radiation oncologists. Radiation oncology societies will need to work with MR societies to develop educational programs to ensure radiation oncologists, medical physicists, dosimetrists and radiation therapists/radiographers are adequately trained in MR utilisation, assessment and safety. In addition, the images acquired during the process of radiation treatment planning and delivery may be of value to other specialities, particularly in medical systems in which MR resources are scarce. An infrastructure through which treatment images could be easily shared with oncology colleagues may also be necessary for radiation oncology departments to consider.
3.5. Reimbursement associated with MR guidance
Additional physician and medical physicist time and effort will be a component of the MRgRT workflow. This includes, but is not limited to, time spent at the treatment machine in delineating tumour and normal structures, adapting treatment plans based on the daily MRI, observing real-time tumour and normal structure changes and evaluating treatment response from functional imaging [14]. Professional society government/payer relation groups involved with developing reimbursement codes may need to consider if additional billing codes are needed (and if they are justified) to account for this type of treatment delivery. Until such reimbursement codes are clarified, guidance as to the use of existing codes will be necessary to appropriately account for the time, effort and risk involved in the delivery of MRgRT. It is imperative that radiation oncology research groups collect data to establish the value of these added efforts through high-quality, peer-reviewed, prospective research by showing measurable clinical improvements in cancer specific outcomes and/or reduction of toxicity as compared to existing radiotherapy delivery technologies. To justify this additional time, effort, and cost, value must be proven. For this purpose, efforts are underway through two active research consortia to collect such data to prove the value-added of these technologies [15,16].
3.6. Unique challenges associated with MR-guided RT
RT deposits radiation dose via secondary charged particles, primarily electrons. The exposure of these electrons to a strong magnetic field changes the manner in which radiation dose is deposited. One well described effect, entitled the electron return effect, presents such an example of this challenge [17]. This effect is the result of electrons moving in a circular pattern in the presence of a magnetic field, as opposed to a more linear path in the absence of a magnetic field. This effect results in a complicated radiation dose effect, particularly at tissue–air interfaces. Fortunately, advanced treatment planning software can model this dose effect, and this can be accounted for in the process of RT planning [18]. It should also be considered that dose calculation in the presence of B-fields is a novel and important challenge. MR images are also subject to geometric distortion which can alter the appearance of normal anatomy or the target. Such distortion must be carefully considered during a treatment course [19,20]. The impact of immobilising patients in a confined space for extended lengths of time for image acquisition and treatment delivery must also be carefully considered. Claustrophobia is a common concern, and the impact on patient-reported quality of life has been examined; in total, approximately 5% of patients seem to have found this treatment unacceptably long [21]. Moreover, robust quality assurance methods of radiation treatment plans may require an entirely novel approach, given the influence of the magnetic field [22]. There is also a necessity for compromises in the functionality of both the MRI device, along with the radiation delivery device when combining these units given the technical complexity of combining these devices. Such compromises may lead to longer treatment times. During this treatment time, there may be an increase need to account for the movement of normal organs. In addition, the process of online adaptive replanning will introduce entirely novel challenges, with regard to physician time and workflow. Finally, MRgRT will present unique challenges with increasing need for improvements in mechanism for automation, archiving imaging, deformable image registration, exquisite Rad Onc attention to ferrous materials and dose accumulation using MRI.
3.7. Future directions and routine MR-based tumour response monitoring
The current clinical experiences of the MRgRT systems have been focused on daily plan changes based on geometric changes in the organs at risk [23,24]. These have formed the basis of multiple ongoing prospective studies, especially in pancreatic cancer (NCT01972919, NCT02283372, NCT03621644). However, the collection and application of these MRI-based data may enable the delivery of the most effective dose to individual tumour biology rather than the highest dose of radiation that can be delivered to a given tumour based on histology, stage and location. Reduction in the PTV and improvements in tumour targeting will represent important, if linear, steps forward towards better and more conformal treatments for patients treated with MRgRT. While they are critically important, such innovation by itself would not represent a true transformation of RT. The ability to routinely acquire daily, functional MRI and subsequently act on those data, directly in the treatment setting, presents an entirely new paradigm for the speciality. Imaging-based biomarkers will represent the future of cancer treatment delivery, and numerous candidate biomarkers have been extensively discussed and published [25]. Radiation oncology is well positioned to embrace this shift, and MR guidance is optimally suited to enable response-based, personalised RT. Indeed, the future of a biological, image-guided adaptive RT (BIGART) is very exciting. There are multiple established, MR-based response metrics in patients undergoing treatment for cancer. A summary of a variety of these metrics can be seen in Table 3. Most of these have been examined in patients being treated with chemotherapy, and some have been examined in patients undergoing RT (Table 3). To date, the routine acquisition of diagnostic quality MRIs during a course of RT has been prohibitively expensive and inconvenient for patients. Time on an MRI scanner is a highly limited resource at nearly all hospitals. The introduction of the MR Linac, with the capability to acquire many MRI sequences routinely during daily treatment, introduces a novel method of data collection. Rapidly, centres will start observing changes on MRI that occur in both the tumour and normal tissues during a treatment course. These changes, uniquely seen on MRI, can then be tested as early biomarkers correlated with cancer-specific outcomes. Contrast agents during treatment may also hold promise for investigation [26]. The potential for early changes in MR (e.g. diffusion-weighted imaging) to be correlated with long-term outcomes has been shown in multiple tumours during a course of treatment with radiation, summarised in Table 3. These findings need robust prospective validation, which routine use of MRI guided RT may provide. In addition to the use of current functional MR sequences, the MR Linac workflow allows for quantitative feature extraction using radiomic approaches to develop imaging-based biomarkers. This concept of daily response assessment may introduce an entirely novel dose prescription method into RT. Specifically, rather than treating to a prespecified ‘historic’ dose, a patient’s treatment dose could be determined by an imaging biomarker goal. One could envision targeting a specific apparent diffusion coefficient level (for example) as the goal for a patient’s treatment. This would represent a distinct paradigm change from the historic method of prescribing dose founded on a population basis. The future of dose prescription in RT could become a biologically adaptive, imaging biomarker–driven dose for a specific patient. Moreover, MR-based functional imaging could represent radiosensitive or radioresistant subvolumes of disease that may benefit from differential dosing strategies. A visual representation of this potential ‘threshold’ based dosing strategy is seen in Fig. 3. Consortium-based collaboration, validation and qualification will be absolutely critical to validate the clinical significance of the imaging metrics that are acquired during a course of radiation. The implications of a shift of this nature are extremely promising for patients. One of the most frequently asked patient questions during a radiation treatment course, ‘is this working?’, could be answered by the radiation oncologist with a much greater degree of confidence. Table 3 summarises the potential imaging-based response characteristics. It becomes feasible to visualise a future of RT driven by tumour-specific imaging signatures of treatment response. This could enable personalisation of RT dosing strategies or the potential for routine, functional adaptive dose-painting strategies [27].
Table 3.
Potential imaging biomarkers of treatment response to be acquired using an MR Linac.
| Candidate imaging biomarkers | Candidate imaging metric | Tumours site with diagnostic significance | Example clinical series showing clinical significance of imaging changes during radiation therapy |
|---|---|---|---|
| Diffusion-weighted imaging [53] |
|
|
|
| IVIM [61] |
|
|
|
| DCE-MRI [69],a |
|
|
|
| Relaxometry [75] |
|
|
N/A |
| CEST [76] |
|
|
IVIM, intravoxel incoherent motion; MRI, magnetic resonance imaging; DCE, dynamic contrast-enhanced; CEST, chemical exchange saturation transfer; ADC, apparent diffusion coefficient; BOLD, blood oxygen level dependent; IAUC, initial area under the curve.
Limited by the need for exogenous contrast agents.
Fig. 3.
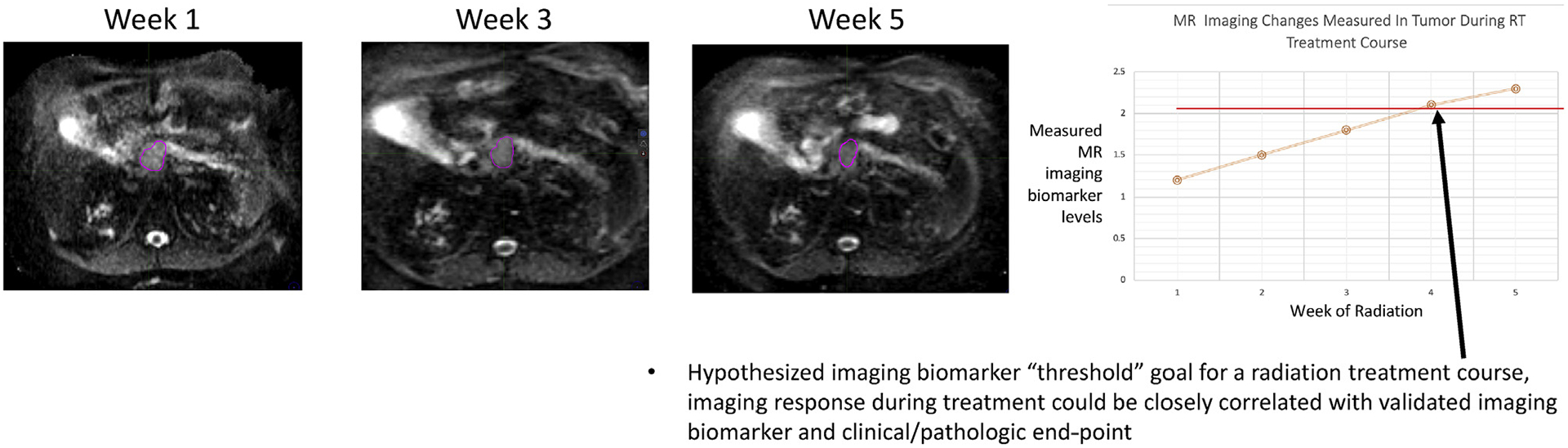
Hypothesised novel method of radiation dosing with routine MR guidance. RT, RT; MR, magnetic resonance.
4. Conclusions
MR-guided RT is an exciting and rapidly advancing area of cancer research, accelerating with both computational and hardware advances. MR guidance is positioned to transform many aspects of RT as we currently know them. Even more novel integration of MRI into treatment delivery devices is also under development, such as MR-guided proton therapy [28,29]. Oncology research teams should prepare for innovative clinical trials involving personalisation and adaption of RT to a level that has simply not been seen thus far within the speciality of radiation oncology.
Conflict of interest statement
David A. Jaffray receives royalties for image-guidance technologies from Elekta. He has held multiple research and educational grants from Elekta, Varian, Philips, and Raysearch over the past 10 years. He holds patents in the domain of image-guided radiotherapy. B.W. Raaymakers reports receiving institutional research support from Elekta AB, Stockholm, Sweden and an honorarium for educational presentations at Elekta AB meetings. J.J.W. Lagendijk reports receiving institutional research support from Elekta AB, Stockholm, Sweden, and an honorarium for educational presentations at Elekta AB meetings. Kevin J. Harrington acknowledges research funding from ICR/RM NIHR Biomedical Research Centre and CRUK ART-NET Network Accelerator Award. Arjun Sahgal has been an advisor/consultant with Abbvie, Merck, Roche, Varian (Medical Advisory Group) and Elekta (Gamma Knife Icon); has been an ex officio Board Member to International Stereotactic Radiosurgery Society (ISRS); received an honorarium for past educational seminars with Elekta AB, Accuray Inc, Varian (CNS Teaching Faculty), BrainLAB and Medtronic Kyphon; received research grant with Elekta AB and received travel accommodations/expenses from Elekta, Varian and BrainLAB. Dr. Sahgal also belongs to the Elekta MR Linac Research Consortium, Elekta Spine, Oligometastases and Linac Based SRS Consortia. Parag Parikh received research funding from Varian and Viewray and has stock ownership of Nuvaira Inc. Cliff Robinson receieved grant funding from Varian, Elekta and Merck; was a consultant in Varian; was a principal investigator of PACIFIC-4 and a consultant in Astra-Zeneca; was a consultant in EMD Serono; was a speaker of ViewRay, received travel expenses from Siemens; was a consultant and had stock ownership in Radialogica. Chris Schultz receieved institutional research support from Elekta AB, Siemens Healthineers, Philips Healthcare and Accuray. William A. Hall received institutional research support from Elekta AB, Siemens Healthineers, Philips Healthcare and Accuray. Percy Lee has been an advisor/consultant with Viewray, AstraZeneca, BrainLab and Varian Inc. He has received an honorarium for past educational seminars with Viewray, AstraZeneca, BrainLab and Varian Inc. He has received research grants from Viewray; clinical trials supporting grants from AstraZeneca Inc. and educational meeting unrestricted supporting grants from BrainLab, Elekta, Varian and Viewray, Inc. His travel accommodations/expenses have been paid by Viewray, AstraZeneca and Varian Inc. Dr. Lee also belongs to the Viewray MR Linac Research Consortium (C2T2). X. Allen Li received research funding from Elekta AB, Siemens Healthineers and Accuray and an honorarium for past educational seminars with Elekta and Accuray. Laura Dawson received licencing agreement for deformable image registration software from Raysearch. Funds are paid to his institution then distributed. Michael Bassetti received travel funding from Viewray Inc and research funding from Merck and AstraZeneca and clinical trials supporting grants EMD Serono. Dr Bassetti is also a participant in the Viewray MR Linac Research Consortium (C2T2). Beth Erickson received institutional research support from Elekta AB, Siemens Healthineers, Philips Healthcare and Accuray. Clifton Dave Fuller is a Sabin Family Foundation Fellow. Dr. Fuller receives funding and salary support from the National Institutes of Health (NIH), including the National Institute of Dental and Craniofacial Research Award (1R01DE025248-01/R56DE025248-01); a National Science Foundation (NSF), Division of Mathematical Sciences, Joint NIH/NSF Initiative on Quantitative Approaches to Biomedical Big Data (QuBBD) Grant (NSF 1557679); the NIH Big Data to Knowledge (BD2K) Program of the National Cancer Institute (NCI) Early Stage Development of Technologies in Biomedical Computing, Informatics, and Big Data Science Award (1R01CA214825-01); NCI Early Phase Clinical Trials in Imaging and Image-Guided Interventions Program (1R01CA218148-01); an NIH/NCI Cancer Center Support Grant (CCSG) Pilot Research Program Award from the UT MD Anderson CCSG Radiation Oncology and Cancer Imaging Program (P30CA016672) and an NIH/NCI Head and Neck Specialised Programs of Research Excellence (SPORE) Developmental Research Program Award (P50 CA097007-10). Dr. Fuller has received direct industry grant support and travel funding from Elekta AB. Marcel Verheij acknowledges research funding from the Dutch Cancer Society, Elekta AB, Roche and Astra-Zeneca. All other authors declare no conflicts of interest.
References
- [1].Atun R, Jaffray DA, Barton MB, Bray F, Baumann M, Vikram B, et al. Expanding global access to radiotherapy. Lancet Oncol 2015;16(10):1153–86. [DOI] [PubMed] [Google Scholar]
- [2].Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol 2012;9(12):688–99. [DOI] [PubMed] [Google Scholar]
- [3].Jaffray DA, Carlone MC, Milosevic MF, Breen SL, Stanescu T, Rink A, et al. A facility for magnetic resonance-guided radiation therapy. Semin Radiat Oncol 2014;24(3):193–5. [DOI] [PubMed] [Google Scholar]
- [4].Simpson DR, Lawson JD, Nath SK, Rose BS, Mundt AJ, Mell LK. A survey of image-guided radiation therapy use in the United States. Cancer 2010;116(16):3953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16(2):187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Keall PJ, Barton M, Crozier S, Australian Mri-Linac Program icfIIICCCLHSUUoNQSWS, Wollongong. The Australian magnetic resonance imaging-linac program. Semin Radiat Oncol 2014;24(3):203–6. [DOI] [PubMed] [Google Scholar]
- [7].Fallone BG, Murray B, Rathee S, Stanescu T, Steciw S, Vidakovic S, et al. First MR images obtained during megavoltage photon irradiation from a prototype integrated linac-MR system. Med Phys 2009;36(6):2084–8. [DOI] [PubMed] [Google Scholar]
- [8].Mutic S, Dempsey JF. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol 2014;24(3):196–9. [DOI] [PubMed] [Google Scholar]
- [9].Lagendijk JJ, Raaymakers BW, Raaijmakers AJ, Overweg J, Brown KJ, Kerkhof EM, et al. MRI/linac integration. Radiother Oncol 2008;86(1):25–9. [DOI] [PubMed] [Google Scholar]
- [10].Lagendijk JJ, Raaymakers BW, van Vulpen M. The magnetic resonance imaging-linac system. Semin Radiat Oncol 2014;24(3):207–9. [DOI] [PubMed] [Google Scholar]
- [11].Fischer-Valuck BW, Henke L, Green O, Kashani R, Acharya S, Bradley JD, et al. Two-and-a-half-year clinical experience with the world’s first magnetic resonance image guided radiation therapy system. Adv Radiat Oncol 2017;2(3):485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Acharya S, Fischer-Valuck BW, Kashani R, Parikh P, Yang D, Zhao T, et al. Online magnetic resonance image guided adaptive radiation therapy: first clinical applications. Int J Radiat Oncol Biol Phys 2016;94(2):394–403. [DOI] [PubMed] [Google Scholar]
- [13].Boldrini L, Cusumano D, Chiloiro G, Casa C, Masciocchi C, Lenkowicz J, et al. Delta radiomics for rectal cancer response prediction with hybrid 0.35 T magnetic resonance-guided radiotherapy (MRgRT): a hypothesis-generating study for an innovative personalized medicine approach. Radiol Med 2018February;124(2):145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Thomas DH, Santhanam A, Kishan AU, Cao M, Lamb J, Min Y, et al. Initial clinical observations of intra- and interfractional motion variation in MR-guided lung SBRT. Br J Radiol 2018;91(1083):20170522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park JM, Shin KH, Kim JI, Park SY, Jeon SH, Choi N, et al. Air-electron stream interactions during magnetic resonance IGRT: skin irradiation outside the treatment field during accelerated partial breast irradiation. Strahlenther Onkol 2018;194(1):50–9. [DOI] [PubMed] [Google Scholar]
- [16].Chen AM, Cao M, Hsu S, Lamb J, Mikaeilian A, Yang Y, et al. Magnetic resonance imaging guided reirradiation of recurrent and second primary head and neck cancer. Adv Radiat Oncol 2017;2(2):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Raghavan G, Kishan AU, Cao M, Chen AM. Anatomic and dosimetric changes in patients with head and neck cancer treated with an integrated MRI-tri-(60)Co teletherapy device. Br J Radiol 2016;89(1067):20160624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yang Y, Cao M, Sheng K, Gao Y, Chen A, Kamrava M, et al. Longitudinal diffusion MRI for treatment response assessment: preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system. Med Phys 2016;43(3):1369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Padgett KR, Simpson GN, Llorente R, Samuels MA, Dogan N. Feasibility of adaptive MR-guided stereotactic body radiotherapy (SBRT) of lung tumors. Cureus 2018;10(4):e2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Acharya S, Fischer-Valuck BW, Mazur TR, Curcuru A, Sona K, Kashani R, et al. Magnetic resonance image guided radiation therapy for external beam accelerated partial-breast irradiation: evaluation of delivered dose and intrafractional cavity motion. Int J Radiat Oncol Biol Phys 2016;96(4):785–92. [DOI] [PubMed] [Google Scholar]
- [21].Henke L, Kashani R, Yang D, Zhao T, Green O, Olsen L, et al. Simulated online adaptive magnetic resonance-guided stereotactic body radiation therapy for the treatment of oligometastatic disease of the abdomen and central thorax: characterization of potential advantages. Int J Radiat Oncol Biol Phys 2016;96(5):1078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Henke L, Kashani R, Robinson C, Curcuru A, DeWees T, Bradley J, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol 2018;126(3):519–26. [DOI] [PubMed] [Google Scholar]
- [23].Palacios MA, Bohoudi O, Bruynzeel AME, van Sorsen de Koste JR, Cobussen P, Slotman BJ, et al. Role of daily plan adaptation in mr-guided stereotactic ablative radiation therapy for adrenal metastases. Int J Radiat Oncol Biol Phys 2018;102(2):426–33. [DOI] [PubMed] [Google Scholar]
- [24].van Sornsen de Koste JR, Palacios MA, Bruynzeel AME, Slotman BJ, Senan S, Lagerwaard FJ. MR-guided gated stereotactic radiation therapy delivery for lung, adrenal, and pancreatic tumors: a geometric analysis. Int J Radiat Oncol Biol Phys 2018;102(4):858–66. [DOI] [PubMed] [Google Scholar]
- [25].Mehta S, Gajjar SR, Padgett KR, Asher D, Stoyanova R, Ford JC, et al. Daily tracking of glioblastoma resection cavity, cerebral edema, and tumor volume with mri-guided radiation therapy. Cureus 2018;10(3):e2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].El-Bared N, Portelance L, Spieler BO, Kwon D, Padgett KR, Brown KM, et al. Dosimetric benefits and practical pitfalls of daily online adaptive MRI-guided stereotactic radiation therapy for pancreatic cancer. Pract Radiat Oncol 2019January;9(1):e46–54. [DOI] [PubMed] [Google Scholar]
- [27].Rudra S, Jiang N, Rosenberg SA, Olsen JR, Roach MC, Wan L, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med 2019;8(5):2123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Burnet NG, Thomas SJ, Burton KE, Jefferies SJ. Defining the tumour and target volumes for radiotherapy. Cancer Imaging 2004;4(2):153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tyran M, Jiang N, Cao M, Raldow A, Lamb JM, Low D, et al. Retrospective evaluation of decision-making for pancreatic stereotactic MR-guided adaptive radiotherapy. Radiother Oncol 2018;129(2):319–25. [DOI] [PubMed] [Google Scholar]
- [30].Kontaxis C, Bol GH, Lagendijk JJ, Raaymakers BW. A new methodology for inter- and intrafraction plan adaptation for the MR-linac. Phys Med Biol 2015;60(19):7485–97. [DOI] [PubMed] [Google Scholar]
- [31].Kerkmeijer LG, Fuller CD, Verkooijen HM, Verheij M, Choudhury A, Harrington KJ, et al. The MRI-linear accelerator consortium: evidence-based clinical introduction of an innovation in radiation oncology connecting researchers, methodology, data collection, quality assurance, and technical development. Front Oncol 2016;6:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].C2T2 Consortium [Web Page]. November10, 2018. Available from: https://viewray.com/clinical-trials/#.
- [33].Raaijmakers AJ, Raaymakers BW, Lagendijk JJ. Integrating a MRI scanner with a 6 MV radiotherapy accelerator: dose increase at tissue-air interfaces in a lateral magnetic field due to returning electrons. Phys Med Biol 2005;50(7):1363–76. [DOI] [PubMed] [Google Scholar]
- [34].Chen X, Prior P, Chen GP, Schultz CJ, Li XA. Technical note: dose effects of 1.5 T transverse magnetic field on tissue interfaces in MRI-guided radiotherapy. Med Phys 2016;43(8):4797. [DOI] [PubMed] [Google Scholar]
- [35].Weygand J, Fuller CD, Ibbott GS, Mohamed AS, Ding Y, Yang J, et al. Spatial precision in magnetic resonance imaging-guided radiation therapy: the role of geometric distortion. Int J Radiat Oncol Biol Phys 2016;95(4):1304–16. [DOI] [PubMed] [Google Scholar]
- [36].Emmerich J, Laun FB, Pfaffenberger A, Schilling R, Denoix M, Maier F, et al. Technical note: on the size of susceptibility-induced MR image distortions in prostate and cervix in the context of MR-guided radiation therapy. Med Phys 2018;45(4):1586–93. [DOI] [PubMed] [Google Scholar]
- [37].Tetar S, Bruynzeel A, Bakker R, Jeulink M, Slotman BJ, Oei S, et al. Patient-reported outcome measurements on the tolerance of magnetic resonance imaging-guided radiation therapy. Cureus 2018;10(2):e2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hackett SL, van Asselen B, Wolthaus JW, Kok JG, Woodings SJ, Lagendijk JJ, et al. Consequences of air around an ionization chamber: are existing solid phantoms suitable for reference dosimetry on an MR-linac? Med Phys 2016;43(7):3961. [DOI] [PubMed] [Google Scholar]
- [39].Werensteijn-Honingh AM, Kroon PS, Winkel D, Aalbers EM, van Asselen B, Bol GH, et al. Feasibility of stereotactic radiotherapy using a 1.5T MR-linac: multi-fraction treatment of pelvic lymph node oligometastases. Radiother Oncol 2019;134:50–4. [DOI] [PubMed] [Google Scholar]
- [40].O’Connor JP, Aboagye EO, Adams JE, Aerts HJ, Barrington SF, Beer AJ, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol 2017;14(3):169–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wojcieszynski AP, Rosenberg SA, Brower JV, Hullett CR, Geurts MW, Labby ZE, et al. Gadoxetate for direct tumor therapy and tracking with real-time MRI-guided stereotactic body radiation therapy of the liver. Radiother Oncol 2016;118(2):416–8. [DOI] [PubMed] [Google Scholar]
- [42].van der Heide UA, Houweling AC, Groenendaal G, Beets-Tan RG, Lambin P. Functional MRI for radiotherapy dose painting. Magn Reson Imaging 2012;30(9):1216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schellhammer S, Karsch L, Smeets J, L’Abbate C, Henrotin S, Van der Kraaij E, et al. First in-beam MR scanner for image-guided proton therapy: beam alignment and magnetic field effects. Radiother Oncol. 127(Supplement 1):S318–S319. [Google Scholar]
- [44].Raaymakers BW, Raaijmakers AJ, Lagendijk JJ. Feasibility of MRI guided proton therapy: magnetic field dose effects. Phys Med Biol 2008;53(20):5615–22. [DOI] [PubMed] [Google Scholar]
- [45].Bortfeld T IMRT: a review and preview. Phys Med Biol 2006;51(13):R363–79. [DOI] [PubMed] [Google Scholar]
- [46].Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12(2):127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liao Z, Lee JJ, Komaki R, Gomez DR, O’Reilly MS, Fossella FV, et al. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non-small-cell lung cancer. J Clin Oncol 2018;36(18):1813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sio TT, Lin HK, Shi Q, Gunn GB, Cleeland CS, Lee JJ, et al. Intensity modulated proton therapy versus intensity modulated photon radiation therapy for oropharyngeal cancer: first comparative results of patient-reported outcomes. Int J Radiat Oncol Biol Phys 2016;95(4):1107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Raaymakers BW, Lagendijk JJ, Overweg J, Kok JG, Raaijmakers AJ, Kerkhof EM, et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol 2009;54(12):N229–37. [DOI] [PubMed] [Google Scholar]
- [50].Raaymakers BW, Jurgenliemk-Schulz IM, Bol GH, Glitzner M, Kotte A, van Asselen B, et al. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol 2017;62(23):L41–50. [DOI] [PubMed] [Google Scholar]
- [51].Winkel D, Bol GH, Kiekebosch IH, Van Asselen B, Kroon PS, Jurgenliemk-Schulz IM, et al. Evaluation of online plan adaptation strategies for the 1.5T MR-linac based on “First-In-Man” Treatments. Cureus 2018;10(4):e2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fallone BG. The rotating biplanar linac-magnetic resonance imaging system. Semin Radiat Oncol 2014;24(3):200–2. [DOI] [PubMed] [Google Scholar]
- [53].Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009;11(2):102–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shaverdian N, Yang Y, Hu P, Hart S, Sheng K, Lamb J, et al. Feasibility evaluation of diffusion-weighted imaging using an integrated MRI-radiotherapy system for response assessment to neoadjuvant therapy in rectal cancer. Br J Radiol 2017;90(1071):20160739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sun YS, Zhang XP, Tang L, Ji JF, Gu J, Cai Y, et al. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology 2010;254(1):170–8. [DOI] [PubMed] [Google Scholar]
- [56].Hamstra DA, Galban CJ, Meyer CR, Johnson TD, Sundgren PC, Tsien C, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol 2008;26(20):3387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dalah E, Erickson B, Oshima K, Schott D, Hall WA, Paulson E, et al. Correlation of ADC with pathological treatment response for radiation therapy of pancreatic cancer. Transl Oncol 2018;11(2):391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ho JC, Allen PK, Bhosale PR, Rauch GM, Fuller CD, Mohamed AS, et al. Diffusion-weighted magnetic resonance imaging as a predictor of outcome in cervical cancer after chemoradiation. Int J Radiat Oncol Biol Phys 2017;97(3):546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Park SY, Kim CK, Park BK, Park W, Park HC, Han DH, et al. Early changes in apparent diffusion coefficient from diffusion-weighted MR imaging during radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2012;83(2):749–55. [DOI] [PubMed] [Google Scholar]
- [60].Eccles CL, Haider EA, Haider MA, Fung S, Lockwood G, Dawson LA. Change in diffusion weighted MRI during liver cancer radiotherapy: preliminary observations. Acta Oncol 2009;48(7):1034–43. [DOI] [PubMed] [Google Scholar]
- [61].Federau C Intravoxel incoherent motion MRI as a means to measure in vivo perfusion: a review of the evidence. NMR Biomed 2017;30(11). [DOI] [PubMed] [Google Scholar]
- [62].Nougaret S, Vargas HA, Lakhman Y, Sudre R, Do RK, Bibeau F, et al. Intravoxel incoherent motion-derived histogram metrics for assessment of response after combined chemotherapy and radiation therapy in rectal cancer: initial experience and comparison between single-section and volumetric analyses. Radiology 2016;280(2):446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Paudyal R, Oh JH, Riaz N, Venigalla P, Li J, Hatzoglou V, et al. Intravoxel incoherent motion diffusion-weighted MRI during chemoradiation therapy to characterize and monitor treatment response in human papillomavirus head and neck squamous cell carcinoma. J Magn Reson Imaging 2017;45(4):1013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lu L, Li Y, Li W. The role of intravoxel incoherent motion MRI in predicting early treatment response to chemoradiation for metastatic lymph nodes in nasopharyngeal carcinoma. Adv Ther 2016;33(7):1158–68. [DOI] [PubMed] [Google Scholar]
- [65].Zhu L, Wang H, Zhu L, Meng J, Xu Y, Liu B, et al. Predictive and prognostic value of intravoxel incoherent motion (IVIM) MR imaging in patients with advanced cervical cancers undergoing concurrent chemo-radiotherapy. Sci Rep 2017;7(1):11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhu L, Zhu L, Shi H, Wang H, Yan J, Liu B, et al. Evaluating early response of cervical cancer under concurrent chemo-radiotherapy by intravoxel incoherent motion MR imaging. BMC Cancer 2016;16:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gaeta M, Benedetto C, Minutoli F, D’Angelo T, Amato E, Mazziotti S, et al. Use of diffusion-weighted, intravoxel incoherent motion, and dynamic contrast-enhanced MR imaging in the assessment of response to radiotherapy of lytic bone metastases from breast cancer. Acad Radiol 2014;21(10):1286–93. [DOI] [PubMed] [Google Scholar]
- [68].Li FP, Wang H, Hou J, Tang J, Lu Q, Wang LL, et al. Utility of intravoxel incoherent motion diffusion-weighted imaging in predicting early response to concurrent chemoradiotherapy in oesophageal squamous cell carcinoma. Clin Radiol 2018;73(8):756 e17–26. [DOI] [PubMed] [Google Scholar]
- [69].O’Connor JP, Jackson A, Parker GJ, Roberts C, Jayson GC. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol 2012;9(3):167–77. [DOI] [PubMed] [Google Scholar]
- [70].You D, Aryal M, Samuels SE, Eisbruch A, Cao Y. Temporal feature extraction from DCE-MRI to identify poorly perfused subvolumes of tumors related to outcomes of radiation therapy in head and neck cancer. Tomography 2016;2(4):341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bonekamp D, Wolf MB, Edler C, Katayama S, Schlemmer HP, Herfarth K, et al. Dynamic contrast enhanced MRI monitoring of primary proton and carbon ion irradiation of prostate cancer using a novel hypofractionated raster scan technique. Radiother Oncol 2016;120(2):313–9. [DOI] [PubMed] [Google Scholar]
- [72].Farjam R, Tsien CI, Lawrence TS, Cao Y. DCE-MRI defined subvolumes of a brain metastatic lesion by principle component analysis and fuzzy-c-means clustering for response assessment of radiation therapy. Med Phys 2014;41(1):011708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bisdas S, Smrdel U, Bajrovic FF, Surlan-Popovic K. Assessment of progression-free-survival in glioblastomas by intratreatment dynamic contrast-enhanced MRI. Clin Neuroradiol 2016;26(1):39–45. [DOI] [PubMed] [Google Scholar]
- [74].Coolens C, Driscoll B, Moseley J, Brock KK, Dawson LA. Feasibility of 4D perfusion CT imaging for the assessment of liver treatment response following SBRT and sorafenib. Adv Radiat Oncol 2016;1(3):194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gilani IA, Sepponen R. Quantitative rotating frame relaxometry methods in MRI. NMR Biomed 2016;29(6):841–61. [DOI] [PubMed] [Google Scholar]
- [76].Wu B, Warnock G, Zaiss M, Lin C, Chen M, Zhou Z, et al. An overview of CEST MRI for non-MR physicists. EJNMMI Phys 2016;3(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mehrabian H, Myrehaug S, Soliman H, Sahgal A, Stanisz GJ. Evaluation of glioblastoma response to therapy with chemical exchange saturation transfer. Int J Radiat Oncol Biol Phys 2018;101(3):713–23. [DOI] [PubMed] [Google Scholar]
- [78].Mehrabian H, Lam WW, Myrehaug S, Sahgal A, Stanisz GJ. Glioblastoma (GBM) effects on quantitative MRI of contralateral normal appearing white matter. J Neurooncol 2018;139(1):97–106. [DOI] [PubMed] [Google Scholar]
- [79].Mehrabian H, Myrehaug S, Soliman H, Sahgal A, Stanisz GJ. Quantitative magnetization transfer in monitoring glioblastoma (GBM) response to therapy. Sci Rep 2018;8(1):2475. [DOI] [PMC free article] [PubMed] [Google Scholar]


