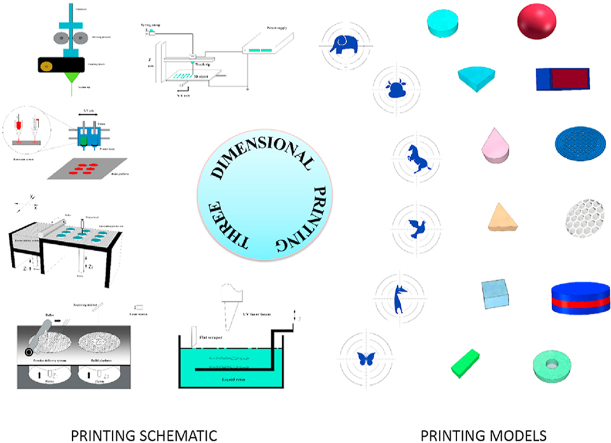
Abstract
Three-dimensional printing is a technology that prints the products layer-by-layer, in which materials are deposited according to the digital model designed by computer aided design (CAD) software. This technology has competitive advantages regarding product design complexity, product personalization, and on-demand manufacturing. The emergence of 3D technology provides innovative strategies and new ways to develop novel drug delivery systems. This review summarizes the application of 3D printing technologies in the pharmaceutical field, with an emphasis on the advantages of 3D printing technologies for achieving rapid drug delivery, personalized drug delivery, compound drug delivery and customized drug delivery. In addition, this article illustrates the limitations and challenges of 3D printing technologies in the field of pharmaceutical formulation development.
KEY WORDS: Three-dimensional printing technology, Drug delivery system, Pharmaceutical, Personalized medicine, Additive manufacturing, On-demand manufacturing, Advantages, Limitations and challenges
Graphical abstract
This review summarizes the application of 3D printing technologies in the pharmaceutical field,with an emphasis on the advantages of 3D printing technologies for achieving rapid drug delivery, personalized drug delivery, compound drug delivery and customized drug delivery.
1. Introduction
Three-dimensional printing (3DP) technology is a burgeoning fabrication approach that emerged in the 1980s1. In general, 3D printing technology involves four main parts: digital model design and development, digital slicing, G code filed conversion, and 3D printer manufacture2. This technology has competitive advantages in complex structure design and personalized drug delivery system manufacturing in comparison with traditional manufacturing processes. In recent decades, 3D printing technologies have developed rapidly and have had profound applications in the fields of aerospace, mechanical manufacturing, construction and biomedical engineering3. However, the application started relatively late in the pharmaceutical field. In July 2015, a levetiracetam tablet (Spritam®) prepared by 3D printing technology was approved by the U.S. Food and Drug Administration (FDA), which indicated the recognition of 3D printing technology in the pharmaceutical field4. Since then, a new chapter in drug delivery system enlargement by 3D printing technology has begun5. An increasing number of pharmaceutical researchers are focusing their attention on three-dimensional printing preparations and developing a series of personalized preparations. The number of published articles related to 3D printing preparations has also been increasing year by year6, including many original studies7, 8, 9 and review articles10, 11, 12, 13, which provide a very good reference for subsequent in-depth research. However, because 3D printing technology is an emerging technology, its related information is updated and iterated quickly, so the relevant research progress on 3D printing technology also needs to be updated and supplemented in time. This review summarizes the commonly used types of 3D printing technologies and their most representative and latest applications in the pharmaceutical field with an emphasis on opportunities in drug delivery systems and the major challenges of this technology.
2. 3D printing technologies
In recent years, various printing technologies have been applied in the rapid-printing field. The stability of medicines and the accuracy of dosage are the key considerations in the selection of printing equipment and technology in drug delivery system manufacturing10. The printing technologies extensively developed in the pharmaceutical field primarily include the following five categories: extrusion molding printing technology (EMP)14, drop on powder printing technology (DOP)15; selective laser sintering technology (SLS)16, stereolithography technology (SLA)17 and electrohydrodynamic 3D printing technology (EHD)18. The 3D printing technologies primarily differ in the various layers of material that are formed and assembled to produce the desired dosage form Table 115, 16, 17,19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34. Summarizes the main advantages and drawbacks associated with each technology.
Table 1.
Summary of various dosage forms prepared by 3D printing technologies.
| 3D technology | Parameter | Material | Advantage | Limitation | Application in drug delivery system | Ref. |
|---|---|---|---|---|---|---|
| FDM |
|
|
|
|
|
19, 20, 21 |
| SSE |
|
|
|
|
|
22, 23, 24 |
| DOP |
|
|
|
|
|
15,25, 26, 27 |
| SLS |
|
|
|
|
|
16,28,29 |
| SLA |
|
|
|
|
|
17,30,31 |
| EHD |
|
|
|
|
|
32, 33, 34 |
CA, cellulose acetate; DOP, drop on powder printing; EHD, electrohydrodynamic 3D printing; FDM, Fused deposition modeling technology; GelMA, gelatin methacryloyl; HPC, hydroxyl propyl cellulose; HPMC, hydroxyl propyl methylcellulose; HPMCAS, hydroxyl propyl methyl cellulose acetate succinate; MCC, microcrystalline cellulose; PCL, polycaprolactone; PE, polyethylene; PEG, polyethylene glycol; PEGDA, poly(ethylene glycol) diacrylate; PEG-DMA, poly(ethylene glycol) dimethacrylate; PEO, poly(ethylene oxide); pHEMA, poly(2-hydroxyethyl methacrylate); PLA, polylactic acid; PLGA, polylactide-co-glycoside; PPF/DEF, poly(propylene fumarate)/diethyl fumarate; PVA, polyvinyl alcohol; PVA-PEG, polyvinyl alcohol-polyethylene glycol; PVP, povidone; SLA, stereolithography; SLS, selective laser sintering; SSE, semisolid extrusion molding technology; TCP, tricalcium phosphate.
2.1. Extrusion molding printing technology (EMP)
Extrusion molding printing technology is one of the most frequently used technologies. Formulation scientists are paying considerable attention to this technology. Extrusion molding printing technology can be primarily divided into fused deposition modeling (FDM) and semisolid extrusion molding technology (SSE) according to the different molding materials.
2.1.1. FDM technology
In FDM technology, drug-loaded polymers are heated to a critical state, making them a semifluid state, and then extruded from the printing nozzle according to the model parameters35. The material solidifies on the printing platform, and the desired product can be obtained. The main parameters involved in this technology include the extrusion temperature, extrusion speed, print head movement speed, nozzle diameter, layer thickness, and product filled percentage. The instruments used in this technology are relatively cheap, and the operation process is simple. Products printed by this technology have better mechanical properties and exhibit more flexible product design capabilities, especially for the design of complex pharmaceutical preparations, such as core‒shell structures and compartment structures36. The key step in the development of FDM is the preparation of drug-loaded filaments. Filament materials usually include polylactic acid (PLA)21,37, polylactide-coglycoside (PLGA)37, polyvinyl alcohol (PVA)38, polycaprolactone (PCL)39,40 and other cellulose derivatives41. Drug-loaded filaments are most commonly obtained by placing the filament materials in saturated methanol or ethanol solvent containing the drug and then drying them thoroughly (the passive soaking method). However, the drug load of this approach is relatively low, and as a consequence, hot melt extrusion (HME) with a single screw or twin screw is used to facilitate the preparation of filaments. In 2017, Zhang et al.42 discussed the potential of combining HME technology with FDM technology for tablet preparation, and Verstraete et al.43 successfully developed TPU-based dosage forms loaded with 60% (w/w) crystalline drug. These studies have greatly improved and confirmed the drug loading capacity of FDM technology and are of great significance to the application of FDM in solid dosage form development. Moreover, the process that includes the drug loading filament preparation can be omitted if a 3D printer was developed to be compatible with the polymer and the drug in their original form as a raw material44. This compatibility will greatly promote the application of FDM technology in the field of pharmaceutical preparations. However, another drawback of FDM technology is the case in which printing involves a high-temperature heating process (usually over 150 °C). The operating temperature is relatively high, and it is not suitable for thermally unstable drugs, such as 4-aminosalicylic acid, which degrades at approximately 210 °C45. Several modifications to FDM have been applied to reduce the thermal stress of the printing process; for example, Kollamaram et al.46 used a low-melting point povidone to prepare drug-loaded filaments. The results showed that filaments with lower melting points can reduce the printing process temperature to as low as 90 °C. Other investigations have further decreased the printing temperature by using water in the filament preparation step as a temporary plasticizer47 or by replacing filaments with softer extruded polymer strands as low as 54 °C48. However, the printing process still requires the hot-melt extrusion process to prepare the drug-loaded filaments before printing, and needs to operate the fabrication process of tablets with thermal treatment. With the exploration of low-temperature carrier materials and the development of multinozzle printers and color printers, FDM technology will be further developed and become the most prevalent technology of drug delivery system production in the pharmaceutical field.
2.1.2. Semisolid extrusion molding technology (SSE)
Semisolid extrusion 3D printing technology (SSE), also known as pressure-assisted microsyringe extrusion technology (PAM)49, extrudes the paste evenly via a syringe-based print head under pressure or screw gear rotation and deposits layer by layer on the platform for printing according to the modeling software. The diameter of the print head connected to the syringe ranges from 0.35 to 0.85 mm, and the pressure required during the printing process is in the range of 0.4–3.8 bar. Compared with other printing technologies, SSE possesses advantages regarding the printing materials and the printing process. Because there is no heating process for the materials, SSE is suitable for thermosensitive drugs, such as guaifenesin50, where the risk of degradation can be avoided. Moreover, a variety of pharmaceutical ingredients are available for the fabrication of the starting materials. Unlike the FDM printing process, SSE uses the pressure of screw gear rotation directly to extrude the semisolid materials into the printer head without deformation; therefore, the properties of starting materials as the semisolid formulation play an important role in the SSE 3D printing process. In particular, the printability, extrudability and shape retention ability of printing materials are important evaluation indices. According to previous studies, the starting materials should express shear-thinning behavior and maintain a yield stress of less than 4000 Pa and a loss factor (tanδ = G”/G’) between 0.2 and 0.751,52. However, its disadvantage is that the printing process requires the use of organic solvents to prepare the paste, which may cause the problem of residual organic solvents in the tablets53. In addition, this novel printing method needs to overcome many obstacles, such as the use of heavy machinery (hot extruder motor components), which usually requires sufficient torque for extrusion to balance an effective extrusion process54.
2.2. Drop on powder printing (DOP)
DOP uses droplets ejected from the print head to glue the powder particles in a deposited powder on the platform55. The production process starts with layers of powder, and each layer of powder is evenly spread on the build platform by a roller. Following the specified pattern designed in the computer, the print head ejects droplets containing binders, such as PVP K30 and hydroxyl propyl methylcellulose (HPMC), or active pharmaceutical ingredients onto the powder bed at an accurate speed. After printing one layer, the platform is lowered one layer along the vertical axis, and then the new powder layer from the feeding chamber is spread above the previous layer. This process is repeated until the dosage forms are finished. Postprocessing involves the elimination of residual solvent and the recovery of unprocessed powder, which supports the holistic structure.
Based on the different working mechanisms, the often used drop-on-demand print head includes two main types, termed piezoelectric and thermal56. Compared with the piezoelectric print head, the thermal print head is cheaper to fabricate but allows fewer choices of solvents with high vapor pressure, which makes the solvent evaporate more easily. The thermal print head uses a heater to evaporate a small amount of fluid by heating the temperature to 200–300 °C so that the formed bubbles push the ejection of droplets32. However, less than 0.5% of the liquid in the print head experiences this high temperature for a few microseconds57, and it has been proven that no substantial degradation of proteins (human growth hormone and insulin) occurs for the thermal print head27. Focus comes to the piezoelectric print head, and the piezoelectric crystals are charged under a voltage causing the deformation of the liquid which forces the droplets out of a nozzle. Therefore, the piezoelectric print head is more popular for various choices of materials.
Many important parameters are in the preparation process, such as the diameter of the nozzle, the print head moving speed, the droplet spacing, the line spacing, the layer thickness, the velocity and frequency of the droplets, and the distance between the print head and the spread powder56,58,59. The desired physical properties and drug release behaviors can be realized by modifying these parameters. The flowability of printing ink primarily depends on its physical properties, such as the surface tension, concentration and viscosity60. Hence, these properties of printing ink can be modified by dissolving appropriate amounts of active pharmaceutical ingredients (APIs) or binders, even without substances, to formulate homemade ink compatible with a certain ink cartridge25,61. Theoretically, the printing ink can be filled for various APIs or pharmaceutical grade binders, such as PVP, HPMC, HPC, CMC-Na and PEO62. Therefore, investigations of the effects of different conventional pharmaceutical grade excipients on the physical properties of printed dosage forms via DOP are valuable for providing more information for further research63. With respect to solidification mechanisms, DOP is similar to wet granulation used in tablet preparation64. The adhesive can help to form solid bridges by crystallizing dissolved particles with solvent evaporation. Because object integrity is entirely dependent on this weak force instead of mechanical compression force, dosage forms are easily fabricated with micron-scale interconnected pores, which has considerable advantages in preparing orally disintegrating tablets26,65. At present, the defects of the product printed from DOP are primarily manifested in two aspects: low resolution and high fragility. Therefore, in-depth research, exploration and development of printing technology and its instruments are necessary.
2.3. Selective laser sintering (SLS)
Similar to DOP, SLS is also a powder-based processing technique that uses a CO2 laser beam instead of liquid binders to sinter the selected regions of powders in each layer with high precision. SLS is a promising technology that offers a high resolution with a 30–60 μm, solvent-free, single-step method for drug delivery. SLS uses a laser to sinter the powder without liquid binders, saving the time of solvent evaporation. The temperature in the process chamber is generally kept between 40 and 50 °C, lower than the melting point of the raw material throughout the printing process, and the powder should be slowly cooled after printing to avoid stress and curl distortions66,67. In addition, filling the process chamber with inert gas, generally nitrogen, is necessary to protect the materials from oxidation. The powder particles range in size from 58 to 180 μm, and a layer thickness between 0.1 and 0.3 mm obtains a better sintering result29,68. Moreover, SLS can accomplish the printing process all at once, unlike FDM, whose first step generates long filaments consisting of APIs and thermoplastic polymers via hot melted extrusion69. The thermoplastic polymers commonly used in SLS mainly include polycarbonates (PC), polyamides (PA), polylactide (PLLA), polylactic acid (PLA), poly (ether-ether-ketone) (PEEK), polycaprolactone (PCL), polyethylene (HDPE), polymethylmethacrylate (PMMA), polyurethane (PU) and polyvinyl alcohol (PVA)70. However, compared with some of the other 3D printing technologies, the ease of breaking down drugs and excipients with a high energy laser is widely recognized. Thus, SLS did not received sufficient attention in the field of pharmaceuticals until the feasibility of this technology was verified in recent years. SLS is currently mainly exploited in tissue engineering of medicine and drug delivery system manufacturing28,71, 72, 73, 74. In 2017, Fina et al.16 managed to prepare paracetamol tablets using Kollicoat IR and Eudragit L100–55, with immediate and modified release characteristics respectively. No drug degradation was observed, and the mechanical properties of the tablets met the demands of the US pharmacopeia. Recently, Hamed et al.75 developed amorphous lopinavir (LPV) printlets using SLS printing technology and understood the effect of formulations and printing process parameters on physicochemicals. These results demonstrated the feasibility of SLS for manufacturing drug delivery systems. With the exploration of thermoplastic polymers and other absorbent excipients, SLS can provide more possibilities for 3D printing technology.
2.4. Stereolithography (SLA)
SLA was the first commercially available technology and was the origin of solid-free fabrication invented by Charles et al.76 in 1986. The printing criteria of SLA are based on the selective photopolymerization of liquid photosensitive resins using ultraviolet laser sources77,78. In the beginning, a thin layer of resin liquid with drug and photoinitiator is scanned point by point to polymerize. The next layer firmly adheres to the basic layer as the depth of curing is slightly larger than the one-layer thickness, resulting in polymerization between unreacted groups and resins in two adjacent layers. This process repeats until the objects are achieved. Post-processing, which aims to eliminate the toxicity of resin as well as improve mechanical strength, is important for removing redundant resin and photoinitiator.
In terms of resolution, SLA is the best 3D printing technology (20 μm compared with 50–200 μm for other fabrication technologies), so it has great advantages in modeling precise structures30. SLA is mostly applied in producing oral solid dosages79,80, microneedle patches81,82 and hydrogels83. Low demand for the chemical structure and properties of drugs or excipients is another advantage of SLA. If drugs and excipients are miscible with the resin, they can be incorporated into it because they will be trapped in it when it is polymerized and cross linked84.
Apart from the few options for photosensitive resin in the pharmaceutical field, it remains a major challenge that SLA printers can only use a specific resin formulation during one printing process unless the previous formulation is replaced with a new formulation when the printing is paused. Therefore, it is complicated to fabricate dosage forms with different formulations. In addition, over the past few years, although some photocrosslinkable polymers, such as PEGDA31 and GelMA have been developed85, FDA-approved photosensitive polymers remain limited86.
2.5. Electrohydrodynamic 3D printing (EHD)
Electrohydrodynamic 3D printing (EHD) is an emerging 3D printing technology that can pattern fibrous materials via digitally-controlled deposition of materials (layer-by-layer) to create well-ordered free-form geometries. The main parts of this working system include a high voltage power supply, a high precision X–Y–Z moving stage along with the controller, one or more syringe pumps and a fine nozzle print head87. This technology provides an ambient environment suitable for most drugs and polymers, especially thermally stable drugs. To date, a wide range of materials with viscosities ranging from 1 to 10,000 mPa s have been processed to prepare pharmaceutical drug carriers based on this technology88, such as polyvinyl alcohol (PVA), cellulose acetate (CA), polycaprolactone (PCL) and poly (ethylene oxide) (PEO)89. In addition, EHD 3D printing has the advantage of a higher controllable resolution90. EHD 3D printing enables micro to nanoscale fiber engineering and alignment and enables digital control of the deposition of materials to fabricate customized geometries and well-ordered complex structures, such as core‒shell91, dual-core graphene composite matrices33, film patches34, customized cylindrical capsules88, wounding dressings92 and Janus fibers93, using concentrated organic/polymer solutions. In short, EHD, as an emerging tool, offers a powerful approach for the evolving field of small-scale pharmaceutical technologies for tailoring medicines to individual patient needs by printing a vast array of predefined amounts of therapeutics arranged in a specific pattern on a porous film32. It is envisaged that the EHD 3D printing approach can be flexible and can meet the dosage form requirements needed at various anatomical locations for individual drug delivery and tailored active release.
3. Advantages and opportunities
3D printing is an emerging technology with substantial potential. It is anticipated that 3D printing technology will offer a promising method for drug delivery system manufacture with distinctive and personalized characteristics94. This versatility may lead to a shift in the production model of pharmaceutical preparation95,96. This section focuses on the competitive advantages of 3D printing technology in the pharmaceutical field.
3.1. Preparation of orally disintegrating formulations
In recent years, with the increasing advantages of orally disintegrating tablets (ODTs), the development of ODTs has received more attention. ODT dosage forms are generally manufactured by direct compression, wet granulation and compression, freeze-drying, hot melt extrusion, etc97. However, the porosity of the tablets fabricated by these methods is inadequate, which affects the rate of disintegration. Therefore, more proportions of functional materials should be mixed to achieve rapid disintegration and obtain proper compressibility in the preparation process, so that it is more suitable for small-dose drugs. The preparation principle of 3D printing technology is layer-by-layer instead of compression, so the formulation exhibits a higher porosity and faster disintegration rate. Consequently, through the screening of prescriptions and designing of CAD software, the printed products could show excellent mechanical properties, which implies the advantages of preparing oral disintegrating tablets by 3D printing technology.
As early as 2009, Yu et al.98 selected paracetamol as a model drug and prepared ODT devices containing loose powder using DOP based on CAD models. The inner regions were established by dropping the binder onto selected regions. In vitro tests revealed that all the tablets disintegrated rapidly, and the average disintegration and wetting times were 23.4 and 67.6 s, respectively. These results confirmed that printing technology offers a new strategy for the manufacture of ODT drug delivery devices. The printed tablet Spritam® exhibited quantitative release while disintegrating rapidly in seconds99,100 (Fig. 1). The printed ODT dosage form not only has the characteristics of rapid dissolution, decomposition, melting and splitting but can also reduce the dysphagia of patients. In addition, the dosage form offers the possibility of easy production and industrialization. Since then, numerous investigations have focused on 3D printed ODT dosage forms. Lin et al.101 developed Suxiao Jiuxin orally disintegrating tablets by combining DOP and traditional Chinese medicine. The results showed that the 3D printed Chinese medicine not only improves the stability of borneol and solves the problem of drug degradation in the traditional process, but also effectively alleviates the limitation of swallowing caused by excessive single doses. Recently, Allahham et al.102 explored the feasibility of applying SLS 3D printing to producing oral disintegrating printlets containing ondansetron. Ondansetron was first incorporated into drug–cyclodextrin complexes and then combined with the filler mannitol. The in vitro dissolution of printed tablets exhibited fast disintegration and released more than 90% of the drug in 5 min, which was comparable with that of the commercial product.
Figure 1.
Comparison of the dissolution rate of Aprecia ZipDose tablet and OTC fast-melting drug. Available from: http://news.bioon.com/article/6671853.html.
In general, 3D printing technology has unique advantages in preparing oral disintegrating dosage forms. It can not only solve the problem of dysphagia among patients, especially elderly individuals and children, but also improve the speed of the drug's effect. This design concept provides further inspiration for the production of orally disintegrating dosage forms in the future.
3.2. Preparation of compound formulations
With the development of certain diseases, it is difficult for traditional drugs to achieve effective prevention and treatment. In 2001, the World Health Organization (WHO) first proposed the use of fixed-dose combinations (FDCs) to control cardiovascular diseases (CVDs)103. Nicholas and Malcolm (2003) introduced the theory of the “polypills” to prevent disease in the British Medical Journal (BMJ)104; subsequently, the fixed compound formulation (Caduet®) produced by Pfizer Pharmaceuticals was launched in China, and research on “polypill” preparations has gradually become popular. However, the complexity of the preparation process, high cost and time consumption are major obstacles to traditional technologies in the production of “polypills”.
In contrast, 3D printing technology offers advantages in preparing compound formulations, e.g., simple processes, great flexibility, high repeatability, and accurate drug loading. The emergence of 3D printing technology overcomes the technical limitations of traditional barriers and lays a foundation for new technology of pharmaceutical preparations. At present, numerous studies are engaged in the preparation of compound formulations based on 3D printing technology.
Khaled et al.105 pioneered the manufacturing of a defined-release compound tablet capable of releasing three drugs through two release mechanisms (osmotic release and diffusion through the shell and gel layers, respectively). This work indicated the possibility of using printing technology to develop compound formulations. In the same year, Khaled et al.106 also used SSE 3D printing technology to construct a “polypill” involving five active ingredients and showing personalized drug release behavior. The patient only needs to take one tablet to achieve the desired therapeutic effect, which greatly improves the patient's compliance. In 2019, Robles-Martinez et al.107 successfully modified a commercial SLA 3D printer, fabricated a bespoke and tailored polypills containing six active ingredients. The printed objects showed great mechanical properties, and the different material inclusions enabled distinct drug release profiles of the six active ingredients within dissolution tests.
These studies on compound formulations indicate that printing technology possesses great prospects for multimaterial production because of its capability for spatially separated material conformations. This concept could revolutionize the pharmaceutical industry, enabling the manufacture of personalized drug delivery systems on demand. Therefore, 3D printing technology can be adapted to prepare compound formulations with various structures containing multiple active ingredients and products with specific pharmacokinetic properties. The development and application of 3D printed compound formulations can not only solve the problem of drug incompatibility, but also help to achieve individualized medication for patients and greatly improve the compliance of patients, especially those with various chronic diseases.
3.3. Preparation of high drug loading formulations
3D printing technology shows significant advantages in improving the drug load capability of the preparation. In the pharmaceutical field, many drugs are in large doses. In the process of using the traditional preparation method, not only the compression performance of each component needs to be considered but also a variety of excipients need to be added. Therefore, the final prepared tablet can only load 30%–40% of the active ingredients, resulting in a large tablet size, which may cause difficulty swallowing and reduce patient compliance. The emergence of a printing approach provides a promising strategy for the preparation of dosage forms with high drug loading characteristics.
The first printed preparation, Spritam®, prepared by Aprecia, is available in four doses, 250, 500, 750 and 1000 mg. The loading capacity of the printed tablets can reach 50%–80%, which greatly reduces the size of the tablets and decreases the swallowing difficulty of patients. Subsequently, Khaled et al.108 selected paracetamol as a model drug and PVP K25 and NaCCS as pharmaceutical excipients and successfully prepared a high-load immediate-release paracetamol oral tablet based on SSE 3D printing technology. The results showed that the maximum drug loading reached 80% and the in vitro release reached more than 90% in 10 min. This experiment fully proves that the import of printing approaches into the field of drug delivery systems can avoid the challenges of the physical form of paracetamol exposed to the traditional compression molding process. Recently, Cui et al.109 selected levetiracetam as the model drug, and successfully fabricated a high-loaded fast-release formulation of levetiracetam based on SSE technology. The results showed that the maximum drug loading was as high as 96%, which greatly reduced the proportion of pharmaceutical excipients in the tablets, thereby decreasing the volume of the formulation.
In general, because of its unique preparation principle, 3D printing technology can greatly reduce the proportion of excipients added to the formulation and manufacture high-drug loading tablets, which reduces the difficulty of preparing high-dose drugs to a certain extent.
3.4. Preparation of tailored dose formulation
Personalized medication refers to the formulations of safe, reasonable, and effective drug treatment programs based on careful consideration of each patient's gender, age, body weight, genetic factors, physiological and other conditions. The response of patients to doses varies greatly. If so many people use a limited dose, it will inevitably lead to each group of patients receiving a dose higher or lower than the required dose, resulting in adverse reactions or insufficient treatment levels110. In particular, personalization for geriatric or pediatric patients is urgently needed. Solving the personalized dose issue, such as by crushing the tablet into halves or quarters or opening the capsule, has been considered an option to adjust the dose for each person. However, these strategies not only fail to offer accurate and precise controls of the dose, but also often require a pharmaceutical technician for their implementation, which takes more time and money22. Three-dimension printing exhibits great potential as a fabrication method that will shift the paradigm of the production of customized drug dosage systems.
3D printing technology is highly flexible and modifies the size of the tablets in response to patient needs with simplicity compared to traditional manufacturing technologies111. This ability is promising for modifying the dose by manipulating the scale of the printed dosage forms via software. This approach is particularly important for formulations of pediatric doses that often require a wide range of doses. Researchers have applied various types of 3D printers to manufacture drug dosage systems. Skowyra et al.112 successfully fabricated prednisolone-loaded PVA filaments with an FDM 3D printer. The results demonstrated the feasibility of controlling the weight and drug dose of the tablets by adjusting the model volume (R2 = 0.9983). When producing formulations with theoretical drug doses of 2, 3, 4, 5, 7.5 and 10 mg, a strong relationship (R2 = 0.9905) between the theoretical and printed doses was gained with an accuracy range of 88.7%–107%. In the same year, this team developed a series of different theophylline preparations (60, 120, 200, 250, and 300 mg) and HCT-EM double-layer tablets in a similar way113,114. The results showed that the printing doses were also highly accurate. Hereafter, Arafat et al.115 explored the feasibility and accuracy of using FDM printing technology to prepare microdose formulations115. The filaments loaded with sodium warfarin were melted by HME and then further printed using an FDM 3D printer to manufacture capsular-ovoid shaped tablets loaded at 200 or 400 μg. The results showed that the range of dose accuracy was 91.5%–102.4%, and the coefficient of determination between the target and achieved dose was 0.9931. This study demonstrated that producing dosage forms with the desired dose of warfarin is feasible through volume modification even with a minute dose of 500 μg. Tian et al.116 assessed the accuracy of DOP technology in fabricating individualized doses of warfarin tablets. Three dosage levels of 1, 2 and 3 mg were manufactured and the quality evaluation results showed that the relative errors were very low. The experimental results showed that DOP technology is also an important approach to preparing individualized medicine.
In 2019, our group explored and constructed a dose regulation system based on SSE 3D printing technology117. Three patterns were intended for adjusting the volumes to control the tablet weight and print customized individualized dosing. A greatly linear correlation between the weight and the volume was obtained. The dose accuracy was in the range of 103.3%–96.2%, with a variation coefficient ranging from 0.6% to 3.2%. Recently, Yan et al.23 developed an individual drug delivery system for small-scale pharmacies. The orodispersible films (ODFs) of cube designs with target doses of 1.25, 2.5 and 5 mg were fabricated by SSE 3D printing and a good linear relationship between the theoretical model volume and drug content was proven (R2 = 0.999). In conclusion, this study showed the possibility and high application of SSE micro 3D printing in fabricating precise doses and customized drug delivery systems.
Compared with traditional pharmaceutical technologies, the printing approach is more conducive to the personalization of medication because the adjustment of digital design is much simpler and more effective than the transformation of equipment. 3D printing technology can regulate the dose by simply changing the size or filling rate of the tablets, which can skillfully solve various problems caused by the traditional divided dose method and bring more convenience to medical staffs and patients118. 3D printing technology is a novel approach to the production and control of drug doses. It provides a highly adjustable, reasonably priced and smallest-sized digital control platform for the production of customized medicines for patients.
3.5. Preparation of special and customized geometric shapes
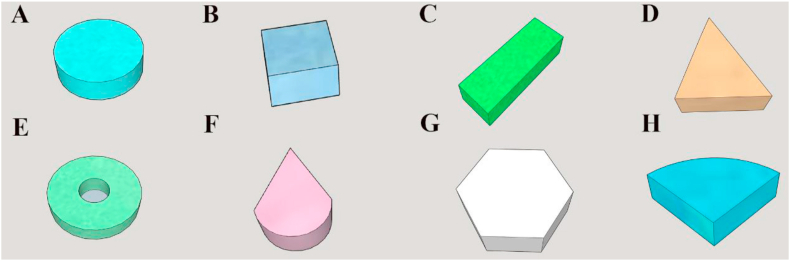
Although traditional preparation technologies can manufacture many different colors of medicines, the capability for production of special tablet shapes remains limited. 3D printing technology has more flexibility and precise spatial positioning capabilities and can be applied to a series of special geometric shapes, as descripted in Fig. 2119.
Figure 2.
3D representation of the prepared printlets (A) disc; (B) cube; (C) cuboid; (D) triangle; (E) torus; (F) cone; (G) pentagon; (H) sector.
3.5.1. Improving drug release by regulating geometric shapes
Goyanes et al.120 used a printing method to create a variety of difficult-to-manufacture special-shaped tablets that are not easy to achieve by traditional methods and investigated the relationship with drug dissolution. Five different geometries were printed, and the in vitro dissolution showed that when the surface area of the 3D tablets remained unchanged, the tablets with pyramidal shapes exhibited the fastest release rate, followed by the toroidal, cubic, spherical, and finally cylindrical tablets. Fina et al.121 used an SLS 3D printer to produce tablets with cylindrical, gyroid lattice and bilayer shapes presenting customized release behavior based on four pharmaceutical-grade polymers Eudragit L100-55, PEO, Eudragit RL and EC. The results revealed that the gyroid lattice structure can change the drug release of all four polymers. In addition, the different shapes of the tablet affect not only the dissolution of the drug in vitro but also its operation in the body, and the tetrahedral tablet can stay in the stomach for a longer time after swelling.
The above examples fully prove the feasibility of using 3D printing technology to define drug release, eliminating the step for changing the composition of the prescription. By printing these geometric shapes, the release of the drug can be customized. This approach can actually customize the drug release behavior for the patient by simply regulating the 3D model.
3.5.2. Improving patient compliance by regulating geometric shapes
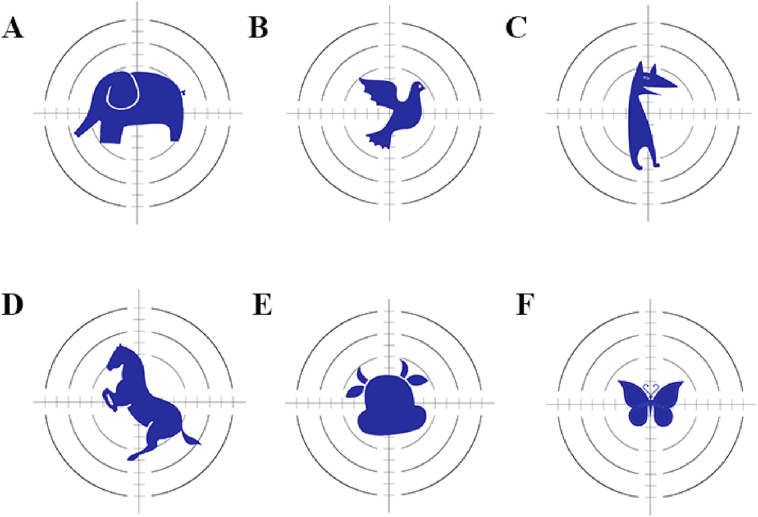
3D printing technology provides the advantage of personalizing the design of dosage forms. For the pediatric population, a more attractive product can result in higher patient compliance and treatment adherence. The application of printing methods in the pharmaceutical field has substantially improved the convenience and diversity of the production of pediatric formulations122. 3D printing technology can accurately adjust the geometric shapes of the tablet and print different cartoon animal tablets according to the children's favorite shape123 (Fig. 3). Recently, pediatric-friendly chocolate-based chewable tablets were fabricated based on extrusion-based 3D printing technology. The tablets were designed into six shapes resembling simple structures of cartoon characters124. The combination of the customization offered by 3D printing technology and the palatability of the natural product chocolate results in an attractive dosage form for the pediatric population. Hence, with the continuous transformation and upgrading of color printers, the color 3D printer can realize the preparation of medicines with specific colors more effectively and quickly, thereby greatly improving the compliance of clinical pediatric patients with medication119.
Figure 3.
Pediatric-friendly dosage forms with different animal cartoon shapes (A) elephant; (B) pigeon; (C) dog; (D) horse; (E) taurean; (F) butterfly.
3.5.3. Improving the therapeutic effect by regulating geometric shapes
The flexibility of the printing approach in geometric shapes also encourages the preparation of implants and transdermal formulations. The implantable drug delivery system is a sterile, solid, controlled-release formulation prepared for cavity, tissue or subcutaneous implantation125. Therefore, to better fit the cavity and the injured part, pharmaceutical preparations usually need to be processed into various shapes. Compared with traditional processes, 3D printing technology can accurately control the geometric shapes, internal structure and surface area that control the dissolution kinetics through precise adjustment of the microstructure126. For example, Sarkar et al.8 designed tricalcium phosphate (TCP) scaffolds with predesigned pores using 3D printing technology. The results showed that the interconnected porosity and biodegradability of 3DP TCP ceramics allowed controlled release kinetics of genistein, daidzein and glycitein in acidic and physiological buffer media for 16 days, which could be used to better simulate the clinical microenvironment. From this perspective, 3D printing allows the creation of a wide variety of patient-specific implants with complex porous architectures and mechanical strength compatible with that of cancellous bone. In addition, PLA/PCL-based progesterone contraceptive vaginal rings by Fu et al.127 were developed using HME-coupled FDM printing technology and were designed into various shapes (“O”, “Y” or “M”) that are more beneficial for the clinical application to patients. The “O”-shaped vaginal ring showed a higher drug release rate than those of “Y” and “M” due to its shape and S/A value, and all the rings exhibited sustained release of progesterone for more than seven days. These special formulations not only allow a matching of the patient's administration site to the greatest extent but also substantially reduce or eliminate the burst effect and exhibit a more personalized release feature than those of conventional implant manufacturing methods128.
The transdermal drug delivery system (TDDS) refers to the dosage form that is administered on the skin surface so that the drug passes through the skin at a constant rate or near a constant rate, enters the systemic circulation and produces a systemic or local therapeutic effect. It not only achieves topical administration but also avoids the hepatic first pass effect and continuously controls the rate of administration. 3D printing technology has substantial advantages for transdermal drug delivery, especially in the field of transdermal patches and microneedles129,130. Goyanes et al.131 combined 3D scanning and printing methods to prepare anti-acne transdermal patches. The 3D scanning method was first adopted to develop a 3D nose model suitable for personalized morphology, and then the nose-shaped mask was printed based on the 3D model using FDM and SLA technologies. The results showed that the SLA method can prepare 3D printed devices (nose-shape) with higher resolution and higher drug loading than those of FDM, with no degradation. Recently, Chaudhari et al.132 also designed and fabricated medicated skin patches incorporated with quercetin-PVP using FDM based on the HME technique. The in vivo profile of an optimized patch was studied in rats, showing a prolonged Tmax, lowered Cmax, and reduced fluctuations in plasma concentrations. All data demonstrated the feasibility of using 3D printing technology to develop medicated skin patches to overcome the current delivery challenges.
In the preparation of microneedles, Economidou et al.133 used biocompatible resins based on SLA to manufacture pyramidal and spare microneedle (MN) designs for transdermal insulin delivery. During the printing process, MN tips were covered with thin coating films composed of insulin and drug carriers. Compared with metal arrays, 3D-printed MNs showed better piercing ability. In vivo studies revealed a fast drug release with lower glucose levels completed within 60 min and sustained hypoglycemic effects for over 4 h compared to those of subcutaneous injections. With respect to the microneedle system, Wu et al.134 also successfully fabricated a microneedle patch system by applying extrusion-based 3D printing and the post stretching method, and demonstrated the transdermal drug delivery of the microneedle for the treatment of type 1 diabetes. The printed microneedle patches exhibited a complete structure and showed sufficient mechanical strength to penetrate the skin of mice. These 3D-printed microneedle systems enabled a convenient, minimally invasive platform for drug delivery, and indicated the great potential of 3D printing as a novel technology for the production of microneedle patches for transdermal drug delivery in other diseases135.
3.6. Preparation of personalized and innovative oral delivery devices
Traditional manufacturing technology has advantages in mass production and cost, but it obviously cannot adjust the microstructure and spatial distribution, and further improvement remains necessary. The dosage forms prepared in the traditional way primarily achieve the regulation of drug distribution and drug release through the selection of excipients and the coating method, limiting the design of dosage forms with personalized structures. The emergence of printing methods has greatly promoted the application and design of dosage forms for digitization. The flexible design and improvement of the model can produce more preparations with personalized structures and customized release mechanisms, which create new opportunities for drug delivery systems136,137. Therefore, an increasing number of studies have begun to focus on researching drug delivery systems with personalized and innovative structures to obtain better clinical treatment effects through structural adjustment and control.
3.6.1. Honeycomb structure
Kyobula et al.138 selected fenofibrate as a model drug and beeswax as a drug carrier and successfully fabricated 3D printed tablets with honeycomb structures using DOP technology, as shown in Fig. 4. Changing the cell size to adjust the distribution of the honeycomb structure, thereby changing the surface area, can finally achieve adjustment of the drug release curve without changing the formulation. Experimental and predicted drug factors were compared and found, and other parameters need to be considered in the process of designing the dosage form, such as the honeycomb geometry and material wettability, which can better control the drug release behavior. This study showed that DOP technology has great potential for printing tablets with highly personalized structures, which will further expand the design feasibility of drug delivery systems.
Figure 4.
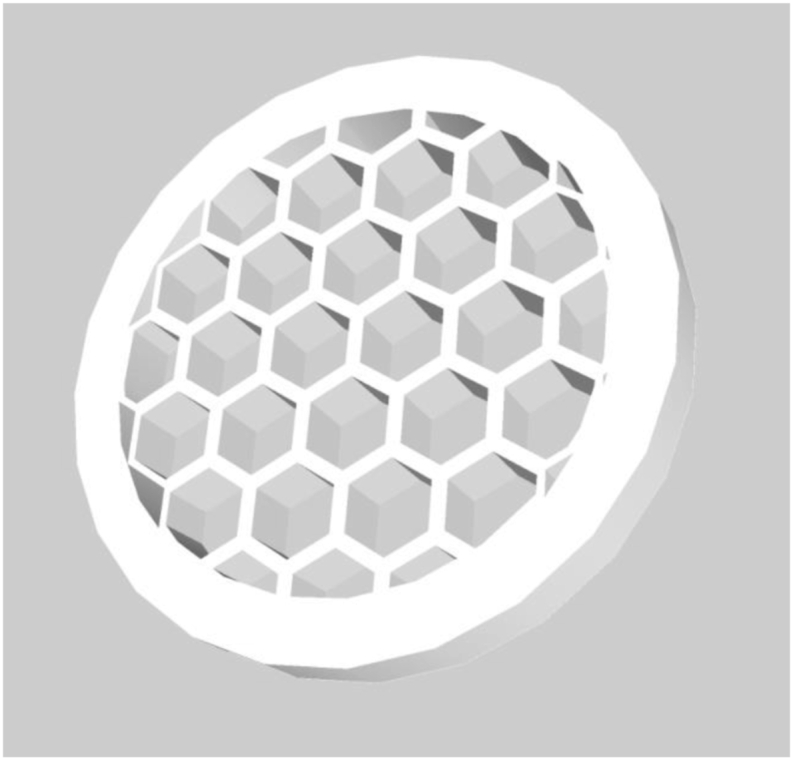
Rendered images of honeycomb-like structure design.
3.6.2. Caplet structure
Sadia et al.139 attempted to design and prepare an innovative structure of caplets with perforated channels using FDM 3D printing technology to accelerate drug release. The caplet-shaped perforation width was defined as 0.2, 0.4, 0.6, 0.8, and 1.0 mm, and its length was variable. In vitro drug release profiles showed that when the widths of the channel were greater than 0.6 mm, the drug release was measured up to the rapid release standard stipulated by the USP. Moreover, in the case of identical specific surface area ratios, the shorter multichannel (8.6 mm) was more effective than the long channel (18.2 mm) in accelerating drug release. This phenomenon may be related to the faster fragmentation and the reduction of the flow resistance of the channel when they are dissolved. The design of these channels could be adapted for stents or implants to promote drug release from tablets.
3.6.3. Core‒shell structure
Li et al.140 chose polyvinyl alcohol (PVA) as the drug-loading filament and glipizide as the model drug, and used FDM 3D printing technology to develop and print the dual chamber (Fig. 5). By rationally arranging the drug concentration distribution in the tablets, the drug release behavior was successfully modified. The drug dissolution results demonstrated that the radial dimensions of the inner and outer layers of the tablet exhibit a substantial effect on the dissolution profile. The model can accurately control the thickness of the inner and outer layers, and the drug content between the layers can effectively regulate the release behavior of the chip package and achieve the effect of precisely controlled release. Recently, Reddy Dumpa et al.141 developed core‒shell gastroretentive floating pulsatile drug delivery systems by applying FDM 3D printing. The dissolution study showed that all core‒shell tablets floated without any lag time and exhibited good floating behavior. In addition, core‒shell platforms have also been investigated based on various printing technologies to improve the treatment effect, such as a core‒shell multidrug platform constructed to improve gastrointestinal tract microbial health7 or liquid-core‒shell microparticles developed to advance cell/tissue engineering, diagnostics and drug delivery142. Thus, 3D printing provides an effective strategy for the production of complex customized drug delivery systems for individual pharmacotherapy.
Figure 5.
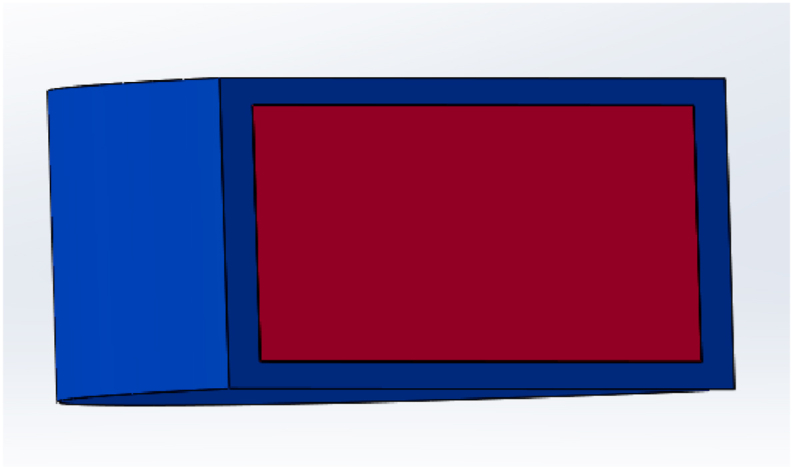
Rendered images of core‒shell structure design.
3.6.4. Lattice internal structure
Li et al.143 explored the feasibility of using SSE 3D printing as an innovative manufacturing method for the fabrication of gastrofloating tablets. A tablet with an innovative low-density lattice structure was prepared to extend the residence time in the stomach (Fig. 6). The dissolution profiles indicated that without the addition of foaming agents, the prepared tablets could maintain a floating state for more than 8 h, which increased the drug release effect while prolonging the gastric retention time. Moreover, Chai et al.144, Wen et al.145, Li et al.146 and Giri et al.69 also used various 3D printing technologies to design and print gastrofloating tablets with internal hollows or low-densities, respectively. These studies demonstrate that 3D printing technology can be adapted to prepare gastrofloating tablets over a long time (more than 8 h). These studies also prove the convenience of printing controlled-release targeted formulations based on 3D printing technology and provide a technical foundation for the industrial production of these dosage forms.
Figure 6.
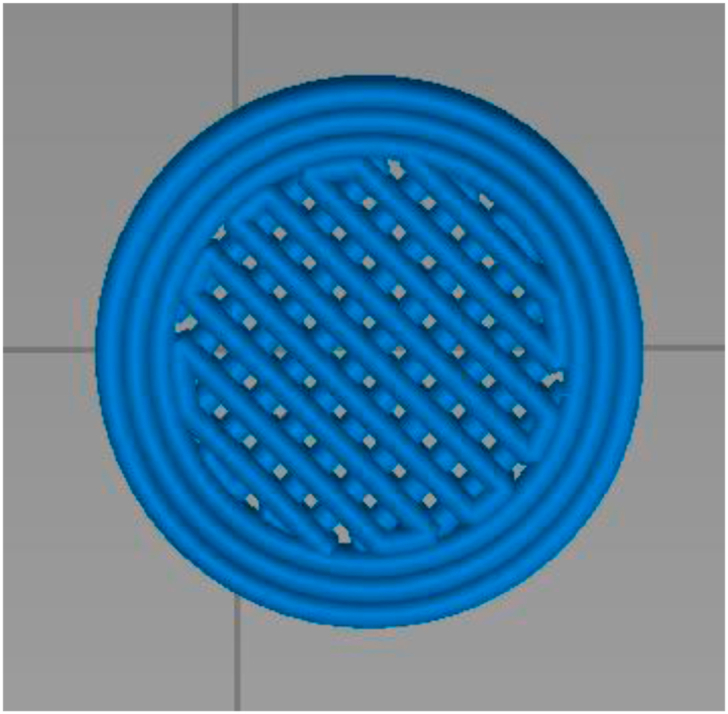
Rendered images of lattice internal structure design.
3.6.5. Sandwich structure
In 2007, Huang et al.147 designed and printed a sandwich-type gentamicin implants using DOP (Fig. 7). The dissolution behavior revealed that the cumulative release amount of the sandwich structure implants on day 5 could be controlled below 45% (the burst release amount of common framework structure implants on day 5 was 70%). The results confirmed that the structure of the sandwich can reduce the surface area of the release, which can substantially inhibit the burst release amount, prolong the effective release time of the drug, and finally achieve the purpose of long-term sustained release of the implant.
Figure 7.
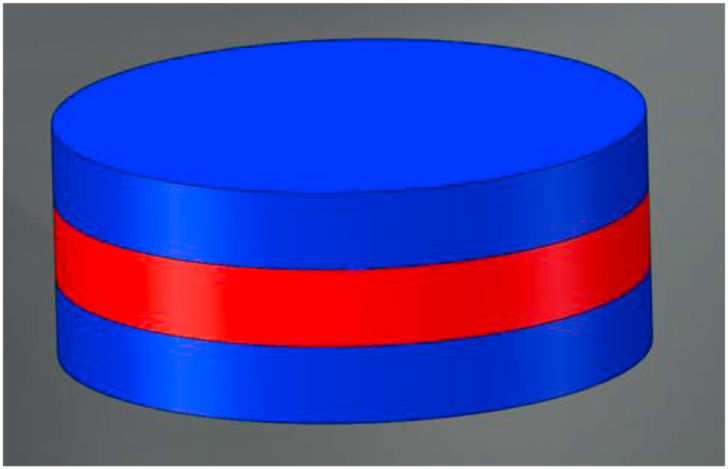
Rendered images of sandwich structure design.
3.6.6. Concept devices
Recently, many works have also focused on in-depth exploration of the preparation of concept devices of complex structures using 3D printing technology. These design concepts showcase a powerful and economical approach of digital design to provide healthcare staff with a highly adjustable “polypill” solution, to accommodate the increasing number of patients who receive multiple and complex dosing regimens. For example, Pereira et al.148 developed a highly modular multicompartmental capsule platform of complex structure using FDM printing aligned with hot-filling syringes. The platform was designed in two capsule skeletons (concentric format and parallel format) with four separate compartments. Drug release is controlled through digital manipulation of shell thickness in the concentric format or the size of the rate-limiting pores in the parallel format. Target drug release profiles are achieved with variable orders and configurations, hence confirming the modular nature with capacity to accommodate therapeutics of different properties. Overall, the successful fabrication of 3D printed concept devices provides new ideas for the design and printing of dosage forms with customized structures and drug release behavior.
The purpose of these studies is not to prove which structure is the most suitable but to demonstrate the freedom and flexibility of changing drug release behavior by personalizing the structure of the tablets. 3D printing methods have developed rapidly, and the emergence of various types poses challenges to traditional approaches. Their versatility, speed, cost and potential for tailored manufacturing will compete with many traditional manufacturing technologies149. The development of 3D printing technology is expected to provide a novel manufacturing approach for the design of personalized preparations, suitable for advanced dosage forms.
4. Main challenges
Despite the advantages of 3D printing technology, many technical difficulties and obstacles urgently need to be overcame to promote wider application of DDSs150,151. These sections summarize the current shortcomings involved in the exploration of excipients, the development of printing software and instruments, the optimization of the preparation's mechanical properties, and the situation of the relevant regulatory landscape.
4.1. Excipients
In the preparation process, all types of 3D printing technologies have certain requirements on the properties of excipients due to their unique printing principles. For FDM technology, the heating and melting steps are involved in the printing process, so it is important to select a suitable drug carrier. The carrier excipient that has been reported most frequently is PVA, but its melting temperature is relatively high, which is not suitable for thermally unstable drugs, such as 4-ASA45 or levetiracetam109. In recent years, an increasing number of researchers have attempted to combine HME technology with 3D printing technology19,152 or low-temperature 3D printing technology153, using PVP, HPMC, Kollidon, talc and triethyl citrate as excipients to prepare low-temperature printed filaments to solve the problem of drug degradation46 and improve drug loading43. For SLA and SLS technologies, the excipients are limited to photopolymers and laser sinterable materials, and these polymers are not included under the FDA's generally recognized as safe (GRAS) list. To date, only a few excipients have been used for printing, most of which are expensive, toxic, smelly, and need to be protected from light to avoid premature polymerization. In addition, safety manufacturing is expected for drug preparation. For DOP and SSE, one of the substantial related advantages is the feasibility of applying these technologies to various active drugs and excipients, such as epoxy resins, cheese, hydrogels, and chocolate. Even so, both technologies would relate organic solvents. In DOP technology, organic solvents are chosen as printing inks. In SSE technology, the possible addition of organic solvents is primarily adapted to prepare a soft paste. Therefore, the residual solvents in some of the final 3D printed tablets are the major limitation. According to ICH guidelines Q3C (R5), there are certain acceptance limits for the solvents, and therefore, the choice of solvents is limited, and there is a minimum tolerable residual amount for each solvent. Thus, to solve this limitation, multidisciplinary research needs to be strengthened, such as by developing new types of 3D printers154.
In general, the excipients available for 3D printing technology are relatively limited in comparison with those of traditional pharmaceutical processes. Especially for special dosage forms of individual administration, selecting the printing excipients may be necessary. In addition, many of the materials applied in the printing process are nonpharmaceutical grade, and the compatibility and toxic side effects severely hinder the application of these materials in pharmaceutical formulations. Therefore, to promote the wider application of printing approaches in the pharmaceutical field, the investigation of nontoxic, biodegradable, biocompatible, and physicochemically stable excipients need to be accelerated.
4.2. Printing software and instrument
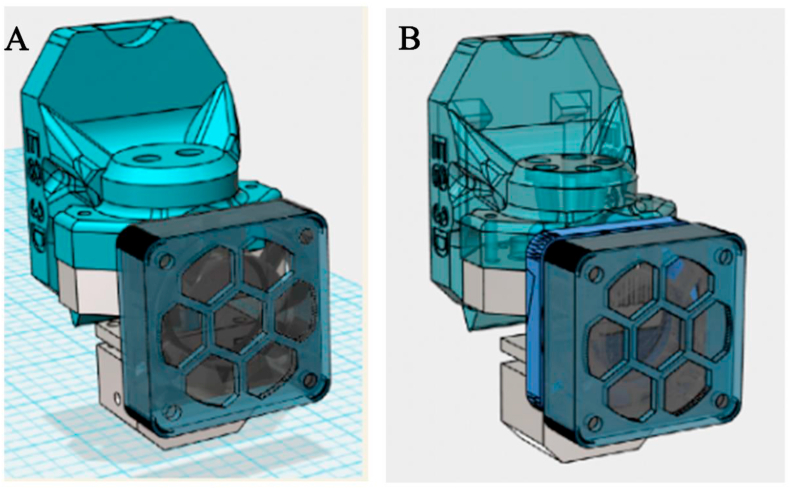
The 3D printing process primarily includes four main steps: modeling, slicing, printing, and postprocessing. In the printing process, the computer is first used to establish a printing model and slice it to set the printing path of each layer, and then the formulation with a complex structure is fabricated according to the prefabricated model. Therefore, as the complexity of the required structure increases, the modeling and slicing software need to be constantly updated to meet higher printing standards. However, it is understood that few software programs and models are dedicated to 3D printing technology. For DOP technology, the print head must be stopped and restarted multiple times, which is very demanding for the stability of the print head56. In addition, the clogging of 3D printing nozzles, the migration and leakage of binders, and the difference in powder feed have reduced the completion rate of printing and affected the performance of printing formulations155. Belonging to extrusion molding technology, multiple print nozzles have been developed and updated for suitability with FDM and SSE technologies, as shown in Fig. 8. However, while achieving diversified formulas, the positioning of the double nozzles may be inaccurate, which severely affects the products’ properties, such as content uniformity, hardness, and friability. Therefore, the mechanical equipment, operating procedures, driving control system, and key components of 3D printers urgently need to be further optimized and upgraded. In addition, the recovery and disposal of excess powders in the printing process of SLS, SLA, and DOP must be considered in production, and potential occupational health hazards should also be considered156.
Figure 8.
(A) The two-in-one printed nozzle (B) five-in-one printed nozzle (Available from: https://user.qzone.qq.com/492892870/main. Reproduced with permission from this picture painter).
In general, the technical hurdles of 3D printers for the preparation of pharmaceutical formulations continue to restrict the development of 3D printing technology; furthermore, the 3D printers used in medicine do not meet the manufacturing practice (GMP) standard, and thus the process and products must be validated to ensure that they are safe for human health. Nonetheless, the development and use of 3D printers is expected to continue.
4.3. Mechanical properties
The mechanical properties of the dosage forms are considered a quality control parameter to ensure that the prepared tablets are reproducible and suitable for postprocessing. In the 3D printing process, because of its unique printing principle, different polymers or powders are stacked on top of each other, resulting in a rough surface and relatively insufficient mechanical strength of the products157. Factors such as the viscosity of the adhesive, surface tension, and fineness of the nozzle affect the performance of the products158. In addition, postprinting processes such as drying methods, drying time and drying temperature may also affect the appearance and quality of the products. These are extremely important for 3D printing technologies based on DOP, FDM and SSE. In terms of DOP, although Spritam® produced using this technology has a high porosity that gives this formulation a competitive advantage over other fast-disintegrating tablets, its poor mechanical resistance remains an important issue (<40 N). Therefore, it is essential to ameliorate the mechanical behavior of products by optimizing printing equipment, such as computer control programs, refining of adhesive nozzles, and optimizing printing process parameters.
4.4. Regulatory landscape
The 3D printing method and its numerous underlying mechanisms for revolutionizing dosage form application and development have attracted the focus of regulatory authorities; at the same time, it has raised many questions regarding the supervision and quality evaluation of 3D-printed products before and after marketing53. In 2017, the FDA proposed its final guidance on technical considerations for the regulation of 3D-printed medical devices, focusing on the design, manufacture and use of the device159. The guidelines provide preliminary regulatory requirements for 3D-printed products for medical applications. Nevertheless, these terms may not be extended to all 3D-printed medical devices because a separate assessment of safety and effectiveness might be required, especially for products that match the patient6. Although the FDA authorized the first printed tablets, no regulations or guidelines regarding 3D-printed medicines are currently available. The development of printing approaches in the pharmaceutical field will also bring problems, such as how to investigate the key parameters of various printing technologies, how to evaluate the performance of 3D-printed medicines and study the in vitro and in vivo release of preparations, how to control the quality of 3D-printed formulations. Fortunately, the FDA's Office of Testing and Research is currently conducting discussions to find solutions to these problems160. In addition, an increasing number of experts are advancing research in this area. With the improvement of relevant regulatory regulations, the development of printing technology in pharmaceutical formulations could make a leap from theoretical exploration to practical solutions.
5. Future perspectives
3D printing preparation technology and traditional preparation technology are complementary in a certain manner. Traditional preparation technology has gradually matured after a long period of practice and has unique advantages in industrialization. On the other hand, as an emerging technology, 3D printing can realize the precise shaping of a variety of materials and can overcome the issues of traditional preparation technology in many aspects. The development of printing methods in pharmaceutical formulations not only broadens the application range of 3D printing technology, but also provides new methods for pharmaceutical investigation and fosters the development of personalized drug delivery. It increases the precision and complexity of pharmaceutical formulations, and provides basic technical support for compound medicines. Although 3D printing technology still has many technical and regulatory challenges, these problems will presumably be solved in the future. In conclusion, 3D printing technology will bring new opportunities and hopes for the development of medicines, and accelerate the arrival of personalized and intelligent drug delivery.
Acknowledgments
We wish to acknowledge that this work was supported by the National Science and Technology Major Project which belongs to “The research on the key technology of 3D printing techniques in the field of pharmaceutical preparation” (No. 2017ZX09201-003-011, China). We also acknowledge that this work was also supported by the China Pharmaceutical Association-Yiling Biomedical Innovation Project (China). In addition, we are also grateful for financial and instrumental support from Jingxin Pharmaceutical Co., Ltd. (Zhejiang, China).
Author contributions
Mengsuo Cui and Hao Pan wrote the manuscript. Yupei Su and Dongyang Fang drew the picture involved in this manuscript. Sen Qiao revised the format of this manuscript. Weisan Pan and Pingtian Ding revised the manuscript and provide quality control. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Pingtian Ding, Email: dingpingtian@qq.com.
Weisan Pan, Email: ppwwss@163.com.
References
- 1.Jakus A.E., Geisendorfer N.R., Lewis P.L., Shah R.N. 3D-printing porosity: a new approach to creating elevated porosity materials and structures. Acta Biomater. 2018;72:94–109. doi: 10.1016/j.actbio.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Zeeshan F., Madheswaran T., Pandey M., Gorain B. Three-dimensional (3-D) printing technology exploited for the fabrication of drug delivery systems. Curr Pharmaceut Des. 2018;24:5019–5028. doi: 10.2174/1381612825666190101111525. [DOI] [PubMed] [Google Scholar]
- 3.Pandey M., Choudhury H., Fern J.L.C., Kee A.T.K., Kou J., Jing J.L.J. 3D printing for oral drug delivery: a new tool to customize drug delivery. Drug Deliv Transl Res. 2020;10:986–1001. doi: 10.1007/s13346-020-00737-0. [DOI] [PubMed] [Google Scholar]
- 4.Drugs.com. FDA approves spritam (levetiracetam) as the first 3D printed drug product. Available from: https://www.drugs.com/newdrugs/fda-approves-spritam-levetiracetam-first-3d-printedproduct-4240.html. [Assessed on 16 August 2016].
- 5.Durga Prasad Reddy R., Sharma V. Additive manufacturing in drug delivery applications: a review. Int J Pharm. 2020;589:119820. doi: 10.1016/j.ijpharm.2020.119820. [DOI] [PubMed] [Google Scholar]
- 6.Gioumouxouzis C.I., Karavasili C., Fatouros D.G. Recent advances in pharmaceutical dosage forms and devices using additive manufacturing technologies. Drug Discov Today. 2019;24:636–643. doi: 10.1016/j.drudis.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Zhu L.F., Chen X., Ahmad Z., Peng Y., Chang M.W. A core–shell multi-drug platform to improve gastrointestinal tract microbial health using 3D printing. Biofabrication. 2020;12 doi: 10.1088/1758-5090/ab782c. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar N., Bose S. Controlled release of soy isoflavones from multifunctional 3D printed bone tissue engineering scaffolds. Acta Biomater. 2020;114:407–420. doi: 10.1016/j.actbio.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Aita I., Rahman J., Breitkreutz J., Quodbach J. 3D-Printing with precise layer-wise dose adjustments for paediatric use via pressure-assisted microsyringe printing. Eur J Pharm Biopharm. 2020;157:59–65. doi: 10.1016/j.ejpb.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Tan Y.J.N., Yong W.P., Kochhar J.S., Khanolkar J., Yao X., Sun Y. On-demand fully customizable drug tablets via 3D printing technology for personalized medicine. J Control Release. 2020;322:42–52. doi: 10.1016/j.jconrel.2020.02.046. [DOI] [PubMed] [Google Scholar]
- 11.Norman J., Madurawe R.D., Moore C.M., Khan M.A., Khairuzzaman A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev. 2017;108:39–50. doi: 10.1016/j.addr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Li X., Liu B., Pei B., Chen J., Zhou D., Peng J. Inkjet bioprinting of biomaterials. Chem Rev. 2020;120:10793–10833. doi: 10.1021/acs.chemrev.0c00008. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X., Li H., Huang L., Zhang M., Fan W., Cui L. 3D printing promotes the development of drugs. Biomed Pharmacother. 2020;131:110644. doi: 10.1016/j.biopha.2020.110644. [DOI] [PubMed] [Google Scholar]
- 14.Xu P., Li J., Meda A., Osei-Yeboah F., Peterson M.L., Repka M. Development of a quantitative method to evaluate the printability of filaments for fused deposition modeling 3D printing. Int J Pharm. 2020;588:119760. doi: 10.1016/j.ijpharm.2020.119760. [DOI] [PubMed] [Google Scholar]
- 15.Daly R., Harrington T.S., Martin G.D., Hutchings I.M. Inkjet printing for pharmaceutics—a review of research and manufacturing. Int J Pharm. 2015;494:554–567. doi: 10.1016/j.ijpharm.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Fina F., Goyanes A., Gaisford S., Basit A.W. Selective laser sintering (SLS) 3D printing of medicines. Int J Pharm. 2017;529:285–293. doi: 10.1016/j.ijpharm.2017.06.082. [DOI] [PubMed] [Google Scholar]
- 17.Wang J., Goyanes A., Gaisford S., Basit A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int J Pharm. 2016;503:207–212. doi: 10.1016/j.ijpharm.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Wu S., Li J.S., Mai J., Chang M.W. Three-dimensional electrohydrodynamic printing and spinning of flexible composite structures for oral multidrug forms. ACS Appl Mater Interfaces. 2018;10:24876–24885. doi: 10.1021/acsami.8b08880. [DOI] [PubMed] [Google Scholar]
- 19.Cailleaux S., Sanchez-Ballester N.M., Gueche Y.A., Bataille B., Soulairol I. Fused deposition modeling (FDM), the new asset for the production of tailored medicines. J Control Release. 2020;330:821–841. doi: 10.1016/j.jconrel.2020.10.056. [DOI] [PubMed] [Google Scholar]
- 20.Melocchi A., Uboldi M., Cerea M., Foppoli A., Maroni A., Moutaharrik S. A graphical review on the escalation of fused deposition modeling (FDM) 3D printing in the pharmaceutical field. J Pharm Sci. 2020;109:2943–2957. doi: 10.1016/j.xphs.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Melocchi A., Parietti F., Loreti G., Maroni A., Gazzaniga A., Zema L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J Drug Deliv Sci Technol. 2015;30:360–367. [Google Scholar]
- 22.Yu I., Chen R.K. A Feasibility study of an extrusion-based fabrication process for personalized drugs. J Pers Med. 2020;10:16. doi: 10.3390/jpm10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan T.T., Lv Z.F., Tian P., Lin M.M., Lin W., Huang S.Y. Semi-solid extrusion 3D printing ODFs: an individual drug delivery system for small scale pharmacy. Drug Dev Ind Pharm. 2020;46:531–538. doi: 10.1080/03639045.2020.1734018. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y., Qin H., Acevedo N.C., Shi X. Development of methylcellulose-based sustained-release dosage by semisolid extrusion additive manufacturing in drug delivery system. J Biomed Mater Res B Appl Biomater. 2020;109:257–268. doi: 10.1002/jbm.b.34697. [DOI] [PubMed] [Google Scholar]
- 25.Infanger S., Haemmerli A., Iliev S., Baier A., Stoyanov E., Quodbach J. Powder bed 3D-printing of highly loaded drug delivery devices with hydroxypropyl cellulose as solid binder. Int J Pharm. 2019;555:198–206. doi: 10.1016/j.ijpharm.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 26.Shi K., Tan D.K., Nokhodchi A., Maniruzzaman M. Drop-on-powder 3D printing of tablets with an anti-cancer drug, 5-fluorouracil. Pharmaceutics. 2019;11:150. doi: 10.3390/pharmaceutics11040150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephen G.M.A.S., Nora C., Kim C., Dion ABSc, Michael JWDSc. Aerosolization of protein solutions using thermal inkjet technology. J Aerosol Med. 2002;15:351–357. doi: 10.1089/089426802760292717. [DOI] [PubMed] [Google Scholar]
- 28.Tolochko N.M.S., Laoui T. Selective laser sintering of single- and two-component metal powders. Rapid Prototyp J. 2003;9:68–78. [Google Scholar]
- 29.Kruth J.P.W.X., Laoui T., Froyen L. Lasers and materials in selective laser sintering. Assem Autom. 2003;23:357–371. [Google Scholar]
- 30.Melchels F.P.W., Feijen J., Grijpma D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31:6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 31.Vehse M.P.S., Sternberg K. Drug delivery from poly(ethylene glycol) diacrylate scaffolds produced by DLC based micro-stereolithography. Macromol Symp. 2014;346:43–47. [Google Scholar]
- 32.Elele E., Shen Y., Susarla R., Khusid B., Keyvan G., Michniak-Kohn B. Electrodeless electrohydrodynamic drop-on-demand encapsulation of drugs into porous polymer films for fabrication of personalized dosage units. J Pharm Sci. 2012;101:2523–2533. doi: 10.1002/jps.23165. [DOI] [PubMed] [Google Scholar]
- 33.Wang B., Chen X., Ahmad Z., Huang J., Chang M.W. 3D electrohydrodynamic printing of highly aligned dual-core graphene composite matrices. Carbon. 2019;153:285–297. [Google Scholar]
- 34.Wang J.C., Zheng H.X., Chang M.W., Ahmad Z., Li J.S. Preparation of active 3D film patches via aligned fiber electrohydrodynamic (EHD) printing. Sci Rep. 2017;7:43924. doi: 10.1038/srep43924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thakkar R., Pillai A.R., Zhang J., Zhang Y., Kulkarni V., Maniruzzaman M. Novel on-demand 3-dimensional (3-D) printed tablets using fill density as an effective release-controlling tool. Polymers. 2020;12:1872. doi: 10.3390/polym12091872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Awad A., Trenfield S.J., Gaisford S., Basit A.W. 3D printed medicines: a new branch of digital healthcare. Int J Pharm. 2018;548:586–596. doi: 10.1016/j.ijpharm.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Water J.J., Bohr A., Boetker J., Aho J., Sandler N., Nielsen H.M. Three-dimensional printing of drug-eluting implants: preparation of an antimicrobial polylactide feedstock material. J Pharm Sci. 2015;104:1099–1107. doi: 10.1002/jps.24305. [DOI] [PubMed] [Google Scholar]
- 38.Goyanes A., Buanz A.B., Basit A.W., Gaisford S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int J Pharm. 2014;476:88–92. doi: 10.1016/j.ijpharm.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 39.Khorasani M., Edinger M., Raijada D., Botker J., Aho J., Rantanen J. Near-infrared chemical imaging (NIR-CI) of 3D printed pharmaceuticals. Int J Pharm. 2016;515:324–330. doi: 10.1016/j.ijpharm.2016.09.075. [DOI] [PubMed] [Google Scholar]
- 40.Beck R.C.R., Chaves P.S., Goyanes A., Vukosavljevic B., Buanz A., Windbergs M. 3D printed tablets loaded with polymeric nanocapsules: an innovative approach to produce customized drug delivery systems. Int J Pharm. 2017;528:268–279. doi: 10.1016/j.ijpharm.2017.05.074. [DOI] [PubMed] [Google Scholar]
- 41.Melocchi A., Parietti F., Maroni A., Foppoli A., Gazzaniga A., Zema L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int J Pharm. 2016;509:255–263. doi: 10.1016/j.ijpharm.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J., Feng X., Patil H., Tiwari R.V., Repka M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int J Pharm. 2017;519:186–197. doi: 10.1016/j.ijpharm.2016.12.049. [DOI] [PubMed] [Google Scholar]
- 43.Verstraete G., Samaro A., Grymonpre W., Vanhoorne V., Van Snick B., Boone M.N. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int J Pharm. 2018;536:318–325. doi: 10.1016/j.ijpharm.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Shaqour B., Reigada I., Gorecka Z., Choinska E., Verleije B., Beyers K. 3D-printed drug delivery systems: the effects of drug incorporation methods on their release and antibacterial efficiency. Materials. 2020;13:3364. doi: 10.3390/ma13153364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goyanes A., Buanz A.B., Hatton G.B., Gaisford S., Basit A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur J Pharm Biopharm. 2015;89:157–162. doi: 10.1016/j.ejpb.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Kollamaram G., Croker D.M., Walker G.M., Goyanes A., Basit A.W., Gaisford S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int J Pharm. 2018;545:144–152. doi: 10.1016/j.ijpharm.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 47.Kempin W., Domsta V., Grathoff G., Brecht I., Semmling B., Tillmann S. Immediate release 3D-printed tablets produced via fused deposition modeling of a thermo-sensitive drug. Pharm Res (N Y) 2018;35:124. doi: 10.1007/s11095-018-2405-6. [DOI] [PubMed] [Google Scholar]
- 48.Pereira B.C., Isreb A., Forbes R.T., Dores F., Habashy R., Petit J.B. ‘Temporary plasticiser': a novel solution to fabricate 3D printed patient-centred cardiovascular 'Polypill' architectures. Eur J Pharm Biopharm. 2019;135:94–103. doi: 10.1016/j.ejpb.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 49.El Aita I., Breitkreutz J., Quodbach J. On-demand manufacturing of immediate release levetiracetam tablets using pressure-assisted microsyringe printing. Eur J Pharm Biopharm. 2019;134:29–36. doi: 10.1016/j.ejpb.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Khaled S.A., Burley J.C., Alexander M.R., Roberts C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int J Pharm. 2014;461:105–111. doi: 10.1016/j.ijpharm.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Khaled S.A., Alexander M.R., Irvine D.J., Woldman R.D., Wallace M.J., Sharpe S. Extrusion 3D printing of paracetamol tablets from a single formulation with tunable release profiles through control of tablet geometry. AAPS PharmSciTech. 2018;19:3403–3413. doi: 10.1208/s12249-018-1107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng Y.L., Qin H.T., Acevedo N.C., Jiang X.P., Shi X.L. 3D printing of extended-release tablets of theophylline using hydroxypropyl methylcellulose (HPMC) hydrogels. Int J Pharm. 2020;591:119983. doi: 10.1016/j.ijpharm.2020.119983. [DOI] [PubMed] [Google Scholar]
- 53.Zema L., Melocchi A., Maroni A., Gazzaniga A. Three-dimensional printing of medicinal products and the challenge of personalized therapy. J Pharm Sci. 2017;106:1697–1705. doi: 10.1016/j.xphs.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 54.Dores F., Kuzminska M., Soares C., Bohus M., Shervington L A., Habashy R. Temperature and solvent facilitated extrusion based 3D printing for pharmaceuticals. Eur J Pharm Sci. 2020;152:105430. doi: 10.1016/j.ejps.2020.105430. [DOI] [PubMed] [Google Scholar]
- 55.Yuan S.Q., Shen F., Chua C.K., Zhou K. Polymeric composites for powder-based additive manufacturing: materials and applications. Prog Polym Sci. 2019;91:141–168. [Google Scholar]
- 56.Yu D.G., Zhu L.M., Branford-White C.J., Yang X.L. Three-dimensional printing in pharmaceutics: promises and problems. J Pharma Sci. 2008;97:3666–3690. doi: 10.1002/jps.21284. [DOI] [PubMed] [Google Scholar]
- 57.Melendez P.A., Kane K.M., Ashvar C.S., Albrecht M., Smith P.A. Thermal inkjet application in the preparation of oral dosage forms: dispensing of prednisolone solutions and polymorphic characterization by solid-state spectroscopic techniques. J Pharm Sci. 2008;97:2619–2636. doi: 10.1002/jps.21189. [DOI] [PubMed] [Google Scholar]
- 58.Vithani K., Goyanes A., Jannin V., Basit A.W., Gaisford S., Boyd B.J. An overview of 3D printing technologies for soft materials and potential opportunities for lipid-based drug delivery systems. Pharm Res (N Y) 2018;36:1. doi: 10.1007/s11095-018-2531-1. [DOI] [PubMed] [Google Scholar]
- 59.Wang C.C., Tejwani Motwani M.R., Roach W.J., Kay J.L., Yoo J., Surprenant H.L. Development of near zero-order release dosage forms using three-dimensional printing (3-DP) technology. Drug Dev Ind Pharm. 2006;32:367–376. doi: 10.1080/03639040500519300. [DOI] [PubMed] [Google Scholar]
- 60.Kolakovic R., Viitala T., Ihalainen P., Genina N., Peltonen J., Sandler N. Printing technologies in fabrication of drug delivery systems. Expet Opin Drug Deliv. 2013;10:1711–1723. doi: 10.1517/17425247.2013.859134. [DOI] [PubMed] [Google Scholar]
- 61.Buanz A.B., Saunders M.H., Basit A.W., Gaisford S. Preparation of personalized-dose salbutamol sulphate oral films with thermal ink-jet printing. Pharm Res (N Y) 2011;28:2386–2392. doi: 10.1007/s11095-011-0450-5. [DOI] [PubMed] [Google Scholar]
- 62.Wilts E.M., Ma D., Bai Y., Williams C.B., Long T.E. Comparison of linear and 4-arm star poly(vinylpyrrolidone) for aqueous binder jetting additive manufacturing of personalized dosage tablets. ACS Appl Mater Interfaces. 2019;11:23938–23947. doi: 10.1021/acsami.9b08116. [DOI] [PubMed] [Google Scholar]
- 63.Tian P., Yang F., Yu L.P., Lin M.M., Lin W., Lin Q.F. Applications of excipients in the field of 3D printed pharmaceuticals. Drug Dev Ind Pharm. 2019;45:905–913. doi: 10.1080/03639045.2019.1576723. [DOI] [PubMed] [Google Scholar]
- 64.Michael E.A., Kevin M.G.T. 4th ed. the Bristish Library; London: 2013. Aultons pharmaceutics: the design and manufacture of medicines. [Google Scholar]
- 65.Trenfield S.J., Awad A., Madla C.M., Hatton G.B., Firth J., Goyanes A. Shaping the future: recent advances of 3D printing in drug delivery and healthcare. Expet Opin Drug Deliv. 2019;16:1081–1094. doi: 10.1080/17425247.2019.1660318. [DOI] [PubMed] [Google Scholar]
- 66.Jamroz W., Szafraniec J., Kurek M., Jachowicz R. 3D Printing in pharmaceutical and medical applications-recent achievements and challenges. Pharm Res (N Y) 2018;35:176. doi: 10.1007/s11095-018-2454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ligon S.C., Liska R., Stampfl J., Gurr M., Mulhaupt R. Polymers for 3D printing and customized additive manufacturing. Chem Rev. 2017;117:10212–10290. doi: 10.1021/acs.chemrev.7b00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leong K.F., Chua C.K., Gui W.S., Verani Building porous biopolymeric microstructures for controlled drug delivery devices using selective laser sintering. Int J Adv Manuf Technol. 2006;31:483–489. [Google Scholar]
- 69.Giri B.R., Song E.S., Kwon J., Lee J.H., Park J.B., Kim D.W. Fabrication of intragastric floating, controlled release 3D printed theophylline tablets using hot-melt extrusion and fused deposition modeling. Pharmaceutics. 2020;12:77. doi: 10.3390/pharmaceutics12010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Awad A., Fina F., Goyanes A., Gaisford S., Basit A.W. 3D printing: principles and pharmaceutical applications of selective laser sintering. Int J Pharm. 2020;586:119594. doi: 10.1016/j.ijpharm.2020.119594. [DOI] [PubMed] [Google Scholar]
- 71.Williams J.M., Adewunmi A., Schek R.M., Flanagan C.L., Krebsbach P.H., Feinberg S.E. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 72.Shi Y.S., Pan T., Zhu W., Yan C.Z., Xia Z.D. Artificial bone scaffolds of coral imitation prepared by selective laser sintering. J Mech Behav Biomed Mater. 2020;104:103664. doi: 10.1016/j.jmbbm.2020.103664. [DOI] [PubMed] [Google Scholar]
- 73.Shuai C.J., Gao C.D., Nie Y., Hu H.L., Zhou Y., Peng S.P. Structure and properties of nano-hydroxypatite scaffolds for bone tissue engineering with a selective laser sintering system. Nanotechnology. 2011;22:285703. doi: 10.1088/0957-4484/22/28/285703. [DOI] [PubMed] [Google Scholar]
- 74.Bertrand P., Bayle F., Combe C., Goeuriot P., Smurov I. Ceramic components manufacturing by selective laser sintering. Appl Surf Sci. 2007;254:989–992. [Google Scholar]
- 75.Hamed R., Mohamed E.M., Rahman Z., Khan M.A. 3D-printing of lopinavir printlets by selective laser sintering and quantification of crystalline fraction by XRPD-chemometric models. Int J Pharm. 2020;592:120059. doi: 10.1016/j.ijpharm.2020.120059. [DOI] [PubMed] [Google Scholar]
- 76.Charles WH, inventor; UVP, Inc., assignee. Apparatus for production of three-dimensional objects by stereolithography. United States patent US No. 4575330. 1986 Mar 11.
- 77.Voet V.S.D., Strating T., Schnelting G.H.M., Dijkstra P., Tietema M., Xu J. Biobased acrylate photocurable resin formulation for stereolithography 3D printing. ACS Omega. 2018;3:1403–1408. doi: 10.1021/acsomega.7b01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Awad A., Trenfield S.J., Goyanes A., Gaisford S., Basit A.W. Reshaping drug development using 3D printing. Drug Discov Today. 2018;23:1547–1555. doi: 10.1016/j.drudis.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 79.Healy A.V., Fuenmayor E., Doran P., Geever L.M., Higginbotham C.L., Lyons J.G. Additive manufacturing of personalized pharmaceutical dosage forms via stereolithography. Pharmaceutics. 2019;11:645. doi: 10.3390/pharmaceutics11120645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez P.R., Goyanes A., Basit A.W., Gaisford S. Influence of geometry on the drug release profiles of stereolithographic (SLA) 3D-printed tablets. AAPS PharmSciTech. 2018;19:3355–3361. doi: 10.1208/s12249-018-1075-3. [DOI] [PubMed] [Google Scholar]
- 81.Economidou S.N., Lamprou D.A., Douroumis D. 3D printing applications for transdermal drug delivery. Int J Pharm. 2018;544:415–424. doi: 10.1016/j.ijpharm.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 82.Yeung C., Chen S., King B., Lin H., King K., Akhtar F. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics. 2019;13 doi: 10.1063/1.5127778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang P., Berry D., Moran A., He F., Tam T., Chen L. Controlled growth factor release in 3D-printed hydrogels. Adv Healthc Mater. 2019;9:1900977. doi: 10.1002/adhm.201900977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martinez P.R., Goyanes A., Basit A.W., Gaisford S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int J Pharm. 2017;532:313–317. doi: 10.1016/j.ijpharm.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 85.Wang Z., Kumar H., Tian Z., Jin X., Holzman J.F., Menard F. Visible light photoinitiation of cell-adhesive gelatin methacryloyl hydrogels for stereolithography 3D bioprinting. ACS Appl Mater Interfaces. 2018;10:26859–26869. doi: 10.1021/acsami.8b06607. [DOI] [PubMed] [Google Scholar]
- 86.Goole J., Amighi K. 3D printing in pharmaceutics: a new tool for designing customized drug delivery systems. Int J Pharm. 2016;499:376–394. doi: 10.1016/j.ijpharm.2015.12.071. [DOI] [PubMed] [Google Scholar]
- 87.Wang B.L., Wu S.T., Ahmad Z., Li J.S., Chang M.W. Co-printing of vertical axis aligned micron-scaled filaments via simultaneous dual needle electrohydrodynamic printing. Eur Polym J. 2018;104:81–89. [Google Scholar]
- 88.Li X.F., Zhang C.C., Wu S.T., Chen X., Mai J., Chang M.W. Precision printing of customized cylindrical capsules with multifunctional layers for oral drug delivery. ACS Appl Mater Interfaces. 2019;11:39179–39191. doi: 10.1021/acsami.9b13568. [DOI] [PubMed] [Google Scholar]
- 89.Wu S., Ahmad Z., Li J.S., Chang M.W. Fabrication of flexible composite drug films via foldable linkages using electrohydrodynamic printing. Mater Sci Eng C Mater Biol Appl. 2020;108:110393. doi: 10.1016/j.msec.2019.110393. [DOI] [PubMed] [Google Scholar]
- 90.Yao Z.C., Wang J.C., Ahmad Z., Li J.S., Chang M.W. Fabrication of patterned three-dimensional micron scaled core-sheath architectures for drug patches. Mater Sci Eng C Mater Biol Appl. 2019;97:776–783. doi: 10.1016/j.msec.2018.12.110. [DOI] [PubMed] [Google Scholar]
- 91.Wang B., Chen X., Ahmad Z., Huang J., Chang M.W. Engineering on-demand magnetic core-shell composite wound dressing matrices via electrohydrodynamic micro-scale printing. Adv Eng Mater. 2019;21:1900699. [Google Scholar]
- 92.Muwaffak Z., Goyanes A., Clark V., Basit A.W., Hilton S.T., Gaisford S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int J Pharm. 2017;527:161–170. doi: 10.1016/j.ijpharm.2017.04.077. [DOI] [PubMed] [Google Scholar]
- 93.Yao Z.C., Wang J.C., Wang B.L., Ahmad Z., Li J.S., Chang M.W. A novel approach for tailored medicines: direct writing of Janus fibers. J Drug Deliv Sci Technol. 2019;50:372–379. [Google Scholar]
- 94.Souto E.B., Campos J.C., Filho S.C., Teixeira M.C., Martins-Gomes C., Zielinska A. 3D printing in the design of pharmaceutical dosage forms. Pharmaceut Dev Technol. 2019;24:1044–1053. doi: 10.1080/10837450.2019.1630426. [DOI] [PubMed] [Google Scholar]
- 95.Aguilar A.D.L., Linares V., Casas M., Caraballo I. 3D printed drug delivery systems based on natural products. Pharmaceutics. 2020;12:620. doi: 10.3390/pharmaceutics12070620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beg S., Almalki W.H., Malik A., Farhan M., Aatif M., Rahman z. 3D printing for drug delivery and biomedical applications. Drug Discov Today. 2020;25:1668–1681. doi: 10.1016/j.drudis.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 97.Comoglu T., Dilek Ozyilmaz E. Orally disintegrating tablets and orally disintegrating mini tablets–novel dosage forms for pediatric use. Pharmaceut Dev Technol. 2019;24:902–914. doi: 10.1080/10837450.2019.1615090. [DOI] [PubMed] [Google Scholar]
- 98.Yu D.G., Shen X.X., Branford-White C., Zhu L.M., White K., Yang X.L. Novel oral fast-disintegrating drug delivery devices with predefined inner structure fabricated by three-dimensional printing. J Pharm Pharmacol. 2009;61:323–329. doi: 10.1211/jpp/61.03.0006. [DOI] [PubMed] [Google Scholar]
- 99.Jacob J, Coyle N, West TG, Monkhouse DC, Surprenant HL, Jain NB, inventors; Aprecia Pharmaceuticals Company, assignee. Rapid disperse dosage form containing levetiracetam. United States patent US No. 9,339,489,B2. 2016 May 17.
- 100.The first 3D printing drugs were approved by the FDA. 2015. http://news.bioon.com/article/6671853.html Available from:
- 101.Lin Q.F., Yang F., Fan K.Y. Study on the preparation of SuXiao JiuXin orally disintegrating tablets by 3D printing. J Guangdong Pharm Univ. 2016;32:1–4. [Google Scholar]
- 102.Allahham N., Fina F., Marcuta C., Kraschew L., Mohr W., Gaisford S. Selective laser sintering 3D printing of orally disintegrating printlets containing ondansetron. Pharmaceutics. 2020;12:110. doi: 10.3390/pharmaceutics12020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wise J. Polypill holds promise for people with chronic disease. Bull World Health Organ. 2005;83:885–887. [PMC free article] [PubMed] [Google Scholar]
- 104.Wald N.J., Law M.R. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326:1419–1423. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khaled S.A., Burley J.C., Alexander M.R., Yang J., Roberts C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int J Pharm. 2015;494:643–650. doi: 10.1016/j.ijpharm.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 106.Khaled S.A., Burley J.C., Alexander M.R., Yang J., Roberts C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015;217:308–314. doi: 10.1016/j.jconrel.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 107.Robles-Martinez P., Xu X., Trenfield S.J., Awad A., Goyanes A., Telford R. 3D Printing of a multi-layered polypill containing six drugs using a novel stereolithographic method. Pharmaceutics. 2019;11:274. doi: 10.3390/pharmaceutics11060274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khaled S.A., Alexander M.R., Wildman R.D., Wallace M.J., Sharpe S., Yoo J. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int J Pharm. 2018;538:223–230. doi: 10.1016/j.ijpharm.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 109.Cui M.S., Pan H., Fang D.Y., Qiao S., Wang S., Pan W.S. Fabrication of high drug loading levetiracetam tablets using semi-solid extrusion 3D printing. J Drug Deliv Sci Technol. 2020;57:1016–1033. [Google Scholar]
- 110.Herrada-Manchon H., Rodriguez-Gonzalez D., Alejandro Fernandez M., Sune-Pou M., Perez-Lozano P., Garcia-Montoya E. 3D printed gummies: personalized drug dosage in a safe and appealing way. Int J Pharm. 2020;587:119687. doi: 10.1016/j.ijpharm.2020.119687. [DOI] [PubMed] [Google Scholar]
- 111.Alomari M., Mohamed F.H., Basit A.W., Gaisford S. Personalised dosing: printing a dose of one's own medicine. Int J Pharm. 2015;494:568–577. doi: 10.1016/j.ijpharm.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 112.Skowyra J., Pietrzak K., Alhnan M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci. 2015;68:11–17. doi: 10.1016/j.ejps.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 113.Pietrzak K., Isreb A., Alhnan M.A. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur J Pharm Biopharm. 2015;96:380–387. doi: 10.1016/j.ejpb.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 114.Sadia M., Isreb A., Abbadi I., Isreb M., Aziz D., Selo A. From ‘fixed dose combinations' to ‘a dynamic dose combiner': 3D printed bilayer antihypertensive tablets. Eur J Pharm Sci. 2018;123:484–494. doi: 10.1016/j.ejps.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 115.Arafat B., Qinna N., Cieszynska M., Forbes R.T., Alhnan M.A. Tailored on demand anti-coagulant dosing: an in vitro and in vivo evaluation of 3D printed purpose-designed oral dosage forms. Eur J Pharm Biopharm. 2018;128:282–289. doi: 10.1016/j.ejpb.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 116.Tian P., Yang F., Xu Y., Lin M.M., Yu L.P., Lin W. Oral disintegrating patient-tailored tablets of warfarin sodium produced by 3D printing. Drug Dev Ind Pharm. 2018;44:1918–1923. doi: 10.1080/03639045.2018.1503291. [DOI] [PubMed] [Google Scholar]
- 117.Cui M.S., Li Y.N., Wang S., Chai Y.Y., Lou J.T., Chen F. Exploration and preparation of a dose-flexible regulation system for levetiracetam tablets via novel semi-solid extrusion 3D printing. J Pharm Sci. 2019;108:977–986. doi: 10.1016/j.xphs.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 118.Trenfield S.J., Goyanes A., Telford R., Wilsdon D., Rowland M., Gaisford S. 3D printed drug products: non-destructive dose verification using a rapid point-and-shoot approach. Int J Pharm. 2018;549:283–292. doi: 10.1016/j.ijpharm.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 119.Goyanes A., Scarpa M., Kamlow M., Gaisford S., Basit A.W., Orlu M. Patient acceptability of 3D printed medicines. Int J Pharm. 2017;530:71–78. doi: 10.1016/j.ijpharm.2017.07.064. [DOI] [PubMed] [Google Scholar]
- 120.Goyanes A., Robles Martinez P., Buanz A., Basit A.W., Gaisford S. Effect of geometry on drug release from 3D printed tablets. Int J Pharm. 2015;494:657–663. doi: 10.1016/j.ijpharm.2015.04.069. [DOI] [PubMed] [Google Scholar]
- 121.Fina F., Goyanes A., Madla C.M., Award A., Trenfield S.J., Kuek J.M. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int J Pharm. 2018;547:44–52. doi: 10.1016/j.ijpharm.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 122.Preis M., Öblom H. 3D-printed drugs for children—are we ready yet?. AAPS PharmSciTech. 2017;18:303–308. doi: 10.1208/s12249-016-0704-y. [DOI] [PubMed] [Google Scholar]
- 123.Wang X.T., Zhou J.X., Yang W., Pang J.L., Zhang W.F., Chen G. Warpage optimization and influence factors analysis of 3D printing personalized JJY tablets. Drug Dev Ind Pharm. 2020;46:388–394. doi: 10.1080/03639045.2020.1724129. [DOI] [PubMed] [Google Scholar]
- 124.Karavasili C., Gkaragkounis A., Moschakis T., Ritzoulis C., Fatouros D.G. Pediatric-friendly chocolate-based dosage forms for the oral administration of both hydrophilic and lipophilic drugs fabricated with extrusion-based 3D printing. Eur J Pharm Sci. 2020;147:105291. doi: 10.1016/j.ejps.2020.105291. [DOI] [PubMed] [Google Scholar]
- 125.Notario-Perez F., Cazorla-Luna R., Martin-Illana A., Galante J., Ruiz-Caro R., Das Neves J. Design, fabrication and characterisation of drug-loaded vaginal films: state-of-the-art. J Control Release. 2020;327:477–499. doi: 10.1016/j.jconrel.2020.08.032. [DOI] [PubMed] [Google Scholar]
- 126.Wang H., Deng Z.W., Chen J., Qi X., Pang L.B., Lin B.C. A novel vehicle-like drug delivery 3D printing scaffold and its applications for a rat femoral bone repairing in vitro and in vivo. Int J Biol Sci. 2020;16:1821–1832. doi: 10.7150/ijbs.37552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fu J.H., Yu X., Jin Y.G. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int J Pharm. 2018;539:75–82. doi: 10.1016/j.ijpharm.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 128.Limongi T., Susa F., Allione M., di Fabrizio E. Drug delivery applications of three-dimensional printed (3DP) mesoporous scaffolds. Pharmaceutics. 2020;12:851. doi: 10.3390/pharmaceutics12090851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jacob S., Nair A.B., Patel V., Shah J. 3D printing technologies: recent development and emerging applications in various drug delivery systems. AAPS PharmSciTech. 2020;21:220. doi: 10.1208/s12249-020-01771-4. [DOI] [PubMed] [Google Scholar]
- 130.Lim S.H., Kathuria H., Amir M.H.B., Zhang X., Duong H.T.T., Paul H. High resolution photopolymer for 3D printing of personalised microneedle for transdermal delivery of anti-wrinkle small peptide. J Control Release. 2020;329:907–918. doi: 10.1016/j.jconrel.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 131.Goyanes A., Det-Amornrat U., Wang J., Basit A.W., Gaisford S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J Control Release. 2016;234:41–48. doi: 10.1016/j.jconrel.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 132.Chaudhari V.S., Malakar T.K., Murty U.S., Banerjee S. Extruded filaments derived 3D printed medicated skin patch to mitigate destructive pulmonary tuberculosis: design to delivery. Expet Opin Drug Deliv. 2020:1–13. doi: 10.1080/17425247.2021.1845648. [DOI] [PubMed] [Google Scholar]
- 133.Economidou S.N., Pere C.P.P., Reid A., Uddin M.J., Windmill J.F.C., Lamprou D.A. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater Sci Eng C Mater Biol Appl. 2019;102:743–755. doi: 10.1016/j.msec.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 134.Wu M.X., Zhang Y.J., Huang H., li J.W., Liu H.Y., Guo Z.Y. Assisted 3D printing of microneedle patches for minimally invasive glucose control in diabetes. Mater Sci Eng C. 2020;117:111299. doi: 10.1016/j.msec.2020.111299. [DOI] [PubMed] [Google Scholar]
- 135.Cordeiro A.S., Tekko I.A., Jomaa M.H., Vora L., McAlister E., Volpe-Zanutto F. Two-photon polymerisation 3D printing of microneedle array templates with versatile designs: application in the development of polymeric drug delivery systems. Pharm Res (N Y) 2020;37:174. doi: 10.1007/s11095-020-02887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang J.X., Yang W.W., Vo A.Q., Feng X., Ye X.F., Kim D.W. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: structure and drug release correlation. Carbohydr Polym. 2017;177:49–57. doi: 10.1016/j.carbpol.2017.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cui M.S., Yang Y.N., Jia D.Y., Li P.F., Li Q.J., Chen F. Effect of novel internal structures on printability and drug release behavior of 3D printed tablets. J Drug Deliv Sci Technol. 2019;49:14–23. [Google Scholar]
- 138.Kyobula M., Adedeji A., Alexander M.R., Saleh E., Wildman R., Ashcroft J. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J Control Release. 2017;261:207–215. doi: 10.1016/j.jconrel.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 139.Sadia M., Arafat B., Ahmed W., Forbes R.T., Alhnan M.A. Channelled tablets: an innovative approach to accelerating drug release from 3D printed tablets. J Control Release. 2018;269:355–363. doi: 10.1016/j.jconrel.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 140.Li Q.J., Wen H.Y., Jia D.Y., Guan X.Y., Pan H., Yang Y. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. Int J Pharm. 2017;525:5–11. doi: 10.1016/j.ijpharm.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 141.Reddy Dumpa N., Bandari S., Michael A.R. Novel gastroprotective floating pulsatile drug delivery system produced via hot-melt extrusion and fused deposition modeling 3D printing. Pharmaceutics. 2020;12:52. doi: 10.3390/pharmaceutics12010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Acevedo R., Reataino M.A., Yu D.Y., Stephen W.H., Flank S., Sochol R.D. 3D nanoprinted liquid-core-shell microparticles. J Microelectromech Syst. 2020:1–6. [Google Scholar]
- 143.Li Q.J., Guan X.Y., Cui M.S., Zhu Z.H., Chen K., Wen H.Y. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int J Pharm. 2018;535:325–332. doi: 10.1016/j.ijpharm.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 144.Chai X.Y., Chai H.Y., Wang X.Y., Yang J.J., Li J., Zhao Y. Fused deposition modeling (FDM) 3D printed tablets for intragastric floating delivery of domperidone. Sci Rep. 2017;7:2829. doi: 10.1038/s41598-017-03097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wen H.Y., He B.S., Wang H.Y., Chen F., Li P.F., Cui M.S. Structure-based gastro-retentive and controlled-release drug delivery with novel 3D printing. AAPS PharmSciTech. 2019;20:68. doi: 10.1208/s12249-018-1237-3. [DOI] [PubMed] [Google Scholar]
- 146.Li P.F., Zhang S.M., Sun W.L., Cui M.S., Wen H.Y., Li Qj. Flexibility of 3D extruded printing for a novel controlled-release pueraria gastric floating tablet: design of internal structure. AAPS PharmSciTech. 2019;20:236. doi: 10.1208/s12249-019-1455-3. [DOI] [PubMed] [Google Scholar]
- 147.Huang W.D. Implantable and controlled drug delivery device fabricated by three dimensional printing and its releasing character in vitro. Chin J New Drugs. 2008;18:1989–1994. [Google Scholar]
- 148.Pereira B.C., Isreb A., Isreb M., Forbes R.T., Oga E.F., Alhnan M.A. Additive manufacturing of a point-of-care "Polypill:" Fabrication of concept capsules of complex geometry with bespoke release against cardiovascular disease. Adv Healthc Mater. 2020;9:2000236. doi: 10.1002/adhm.202000236. [DOI] [PubMed] [Google Scholar]
- 149.Kocak E., Yildiz A., Acarturk F. Three dimensional bioprinting technology: applications in pharmaceutical and biomedical area. Colloids Surf B Biointerfaces. 2020;197:111396. doi: 10.1016/j.colsurfb.2020.111396. [DOI] [PubMed] [Google Scholar]
- 150.Okafor-Muo O.L., Hassanin H., Kayyali R., ElShaer A. 3D printing of solid oral dosage forms: numerous challenges with unique opportunities. J Pharm Sci. 2020;109:3535–3550. doi: 10.1016/j.xphs.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 151.Wallis M., Al-Dulimi Z., Tan D.K., Maniruzzaman M., Nokhodchi A. 3D printing for enhanced drug delivery: current state-of-the-art and challenges. Drug Dev Ind Pharm. 2020;46:1385–1401. doi: 10.1080/03639045.2020.1801714. [DOI] [PubMed] [Google Scholar]
- 152.Pereira G.G., Figueiredo S., Fernandes A.I., Pinto J.F. Polymer selection for hot-melt extrusion coupled to fused deposition modelling in pharmaceutics. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12090795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Naseri E., Butler H., MacNevin W., Ahmed M., Ahmadi A. Low-temperature solvent-based 3D printing of PLGA: a parametric printability study. Drug Dev Ind Pharm. 2020;46:173–178. doi: 10.1080/03639045.2019.1711389. [DOI] [PubMed] [Google Scholar]
- 154.Annaji M., Ramesh S., Poudel I., Govindarajulu M., Arnold R.D., Dhanasekaran M. Application of extrusion-based 3D printed dosage forms in the treatment of chronic diseases. J Pharm Sci. 2020;109:3551–3568. doi: 10.1016/j.xphs.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 155.Huang S.H., Liu P., Mokasdar A., Hou L. Additive manufacturing and its societal impact: a literature review. Int J Adv Manuf Technol. 2012;67:1191–1203. [Google Scholar]
- 156.Pham D.T., Gault R.S. A comparison of rapid prototyping technologies. Int J Mach Tool Manufact. 1998;38:1257–1287. [Google Scholar]
- 157.Zheng F., Huang S.W. Advances in study on three-dimensional printing in pharmaceutics. CHM. 2016;8:121–125. [Google Scholar]
- 158.Gaylo CM, Pryor TJ, inventors; Therics, Inc., assignee. Apparatus, systems and methods for use in three-dimensional printing. United States patent US No. 6986654. 2006 Jan 17.
- 159.Technical considerations for additive manufactured medical devices-guidance for industry and food and drug administration staff. 2017. https://wwwfdagov/downloads/MedicalDevices/DeviceRegulation and Guidance/GuidanceDocuments/UCM499809pdf Available from: [Google Scholar]
- 160.CDER researchers explore the promise and potential of 3D printed pharmaceuticals. 2017. Available from: https://wwwfederalregistergov/documents/2017/09/29/2017-20861/advancement-of-emerging-technology-applications-for-pharmaceutical-innovaton-and-modernization. [Google Scholar]