Abstract
Purpose:
We report the intracranial efficacy of selpercatinib, a highly potent and selective RET inhibitor, approved in the United States for RET fusion-positive non–small cell lung cancers (NSCLC).
Patients and Methods:
In the global phase 1/2 LIBRETTO-001 trial (NCT03157128) in advanced RET-altered solid tumors, selpercatinib was dosed orally (160 mg twice every day) in 28-day cycles. Patients with baseline intracranial metastases had MRI/CT scans every 8 weeks for 1 year (12 weeks thereafter). In this pre-planned analysis of patients with RET fusion-positive NSCLC with baseline intracranial metastases, the primary endpoint was independently assessed intracranial objective response rate (ORR) per RECIST 1.1. Secondary endpoints included intracranial disease control rate, intracranial duration of response, and intracranial progression-free survival (PFS) independently reviewed.
Results:
Eighty patients with NSCLC had brain metastases at baseline. Patients were heavily pretreated (median = 2 systemic therapies, range = 0–10); 56% of patients received ≥1 course of intracranial radiation (14% whole brain radiotherapy, 45% stereotactic radiosurgery). Among 22 patients with measurable intracranial disease at baseline, intracranial ORR was 82% [95% confidence interval (CI), 60–95], including 23% with complete responses. Among all intracranial responders (measurable and nonmeasurable, n = 38), median duration of intracranial response was not reached (95% CI, 9.3–NE) at a median duration of follow-up of 9.5 months (IQR = 5.7, 12.0). At 12 months, 55% of intracranial responses were ongoing. In all 80 patients, median intracranial PFS was 13.7 months (95% CI, 10.9–NE) at a median duration of follow-up of 11.0 months (IQR = 7.4, 16.5). No new safety signals were revealed in patients with brain metastases compared with the full NSCLC trial population.
Conclusions:
Selpercatinib has robust and durable intracranial efficacy in patients with RET fusion-positive NSCLC.
Translational Relevance.
Brain metastases frequently occur in RET fusion-positive non–small cell lung cancers (NSCLC), with an approximate 50% lifetime prevalence reported. Intracranial metastases are a major cause of morbidity and mortality in this patient population. Thus, there is a need for novel RET-directed, targeted therapy strategies with high efficacy. Selpercatinib, a selective and potent RET inhibitor, shows compelling preliminary evidence of activity in patients with brain metastases. This phase 1/2 trial (LIBRETTO-001) evaluated the efficacy and safety of selpercatinib in patients with RET fusion-positive NSCLCs with intracranial metastases. In this study, selpercatinib was well tolerated, achieving high intracranial response rate, and prolonged intracranial duration of response and intracranial progression-free survival. Combined, these results support selpercatinib as a new standard of care therapy for the primary treatment of brain metastases for patients with RET fusion-positive NSCLC.
Introduction
The rearranged during transfection (RET) proto-oncogene encodes the RET receptor tyrosine kinase, a transmembrane glycoprotein that is involved in the development and maintenance of several tissue types (1). Activating RET alterations, such as recurrent gene fusions, lead to ligand-independent, constitutively active RET tyrosine kinase signaling that drives oncogenesis and tumor progression (2,3,4). Oncogenic RET fusions are found in 1% to 2% of non–small cell lung cancers (NSCLC; refs. 5, 6). A global multi-institutional registry of patients with RET fusion-positive NSCLC found that approximately half of these patients develop brain metastases during their lifetime (7); leptomeningeal disease has also been observed (8).
Intracranial sanctuary site metastasis is a liability shared by many other oncogene-addicted cancers, including EGFR-mutant or ALK fusion-positive NSCLCs. A major advance in the management of these tumors has been the development of brain-penetrant tyrosine-kinase inhibitors (9, 10). These agents not only prevent or delay intracranial treatment failure, but are also increasingly utilized as primary therapy for patients with brain metastases instead of localized interventions such as radiotherapy, an intervention potentially associated with long-term quality of life impairment (11).
Selpercatinib (LOXO-292), a highly potent and selective RET inhibitor, has marked and durable efficacy in patients with treatment-naïve or platinum chemotherapy-treated RET fusion-positive NSCLCs (12). On the basis of these data, selpercatinib has received approval in the United States for any line of therapy of RET fusion-positive metastatic NSCLCs, and is the first RET-selective inhibitor granted EU approval (13, 14). Given that several RET fusion-positive cancers harbor a proclivity for intracranial metastasis, selpercatinib was specifically designed to achieve levels in the central nervous system (CNS) necessary to inhibit RET. Consistently, selpercatinib demonstrated robust intracranial efficacy in orthotopically implanted RET fusion-positive tumors in mice (15).
Preclinical observations, anecdotal case reports (8, 15), and preliminary experience from a prospective clinical trial (7) suggest that selpercatinib is active in patients with brain metastases. To date, however, the true intracranial efficacy of selpercatinib in a large prospective series of RET fusion-positive NSCLCs remains unknown. To address this key evidence gap, we conducted a pre-planned analysis of selpercatinib in patents with RET fusion-positive NSCLC and brain metastases enrolled to the global phase 1/2 LIBRETTO-001 trial (NCT03157128).
Patients and Methods
Study design and treatment
LIBRETTO-001 is an ongoing, global, first-in-human, open label, phase 1/2 clinical trial (ClinicalTrials.gov NCT03157128) open at 89 investigative sites in 16 countries. A total of 31 sites from 11 countries enrolled at least one patient with a RET fusion-positive NSCLC and investigator-assessed brain metastases at baseline in the analysis dataset used here. Full details of the trial design have been published (12). Briefly, patients eligible for this pre-planned analysis were required to meet the following inclusion criteria: age ≥12 years; presence of a prospectively-identified RET fusion as determined by locally-obtained testing performed in a certified laboratory; ECOG performance status 0 to 2; adequate organ function; and a QTc interval of ≤470 milliseconds. Any number of prior therapies were permitted. Brain imaging was a requirement at baseline for all RET fusion-positive solid tumor NSCLC patients. MRI was preferred; CT with contrast was acceptable if MRI was contraindicated. Patients with known brain metastases were eligible for the trial if neurological symptoms and CNS imaging were stable and their steroid dose was stable for 14 days prior to the first dose of selpercatinib, and no CNS surgery or radiation had been performed for 28 days [14 days for stereotactic radiosurgery (SRS)] prior to dosing. All prior local treatments for CNS disease (e.g., surgery, whole brain radiation, SRS), the start and stop dates for each prior local therapy, and the specific lesions treated (if SRS and/or surgery) were recorded. For patients who had received CNS radiation prior to the trial, intracranial lesions needed to show postradiation progression to be selected as a target lesion at baseline.
This protocol adhered to the principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonization. The institutional review board of each investigative site approved the trial, and all patients provided written informed consent.
Selpercatinib doses ranged from 20 mg once a day to 240 mg twice a day for patients enrolled in the phase 1 dose escalation portion of the study. Dose escalation to dose levels determined to be safe was allowed for phase 1 patients after a minimum of one cycle of treatment. In the phase 2 portion of the study, selpercatinib was dosed orally at 160 mg twice a day in 28-day continuous cycles. Treatment continued until death, progressive disease (PD), unacceptable toxicity, or withdrawal of consent. Patients could continue selpercatinib treatment after documented progression if they were continuing to derive clinical benefit in the opinion of the investigator.
The main efficacy endpoint for the current analysis was intracranial objective response rate (ORR) by RECIST 1.1 (16) determined by an independent review committee (IRC), a pre-planned secondary endpoint for the overall LIBRETTO-001 program. The IRC was composed of expert radiologists who were blinded to investigator-determined systemic response. IRC radiologists were provided with prior or on-study radiation information and a history of all prior treatments for CNS disease. Intracranial ORR (%) was defined as the proportion of patients with a best overall intracranial response of complete response (CR) or partial response (PR) relative to the total number of patients with baseline intracranial disease. All responses were required to be confirmed by a repeat assessment performed no sooner than 28 days later. Intracranial disease control rate (DCR) was defined as the percentage of patients who had a best overall intracranial response of CR, PR, or stable disease (SD) lasting 16 weeks or more after selpercatinib initiation. Consistent with RECIST 1.1, patients with exclusively nonmeasurable intracranial disease at baseline could be classified for best overall response as CR (in the case where all nonmeasurable lesions resolved), PD, or non-CR/non-PD. Another prespecified secondary endpoint was intracranial duration of response (DoR) as determined by IRC, defined as the time from start of an intracranial response until intracranial progression or death, regardless of cause. Intracranial progression-free survival (PFS) was an exploratory endpoint defined as the time from treatment start to intracranial disease progression as assessed by IRC or death from any cause. Extracranial progression was not included in the intracranial PFS assessment. Safety was another exploratory endpoint for the population with NSCLC and intracranial metastases.
Trial assessments
Radiologic tumor assessments (MRI, preferentially; CT, with and without intravenous contrast when MRI was clinically contraindicated) were conducted at baseline for all patients with phase 2 RET fusion-positive solid tumor NSCLC. Repeat brain imaging using the same modality as at baseline was conducted for all patients with brain metastases identified by baseline imaging every 8 weeks for 1 year, and every 12 weeks thereafter. Safety was assessed according to the NCI CTCAEs (version 4.03; ref. 17).
Statistical analysis
All analyses were prespecified in the Statistical Analysis Plan. The Clopper–Pearson method was used to construct 95% confidence interval (CI) for response rates. Kaplan–Meier method was used to estimate median for intracranial DoR and PFS. Median follow up was calculated using the reverse Kaplan–Meier method, that is median follow up is calculated like the Kaplan–Meier estimate of the survival function, but with the meaning of the status indicator reversed so that the event of interest becomes the censor. SAS statistical software, version 9.2 (SAS Institute) was used to perform all analyses.
Data sharing
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an IRC identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms will be provided in a secure data sharing environment for up to 2 years per proposal. For details on submitting a request, see the Vivli website: www.vivli.org.
Results
Baseline patient characteristics and treatment
A total of 531 patients with RET fusion-positive cancers were enrolled to phase 1 or phase 2 of the trial between May 2017 and June 17, 2019, including 80 patients with RET fusion-positive NSCLC and investigator-determined baseline brain metastases (92.5% by MRI, 5% by CT, 2.5% missing) that met criteria for inclusion in the current analysis (online appendix, Supplementary Fig. S1). Among these 80 patients, 22 patients had at least one baseline measurable intracranial lesion and 58 had exclusively nonmeasurable baseline intracranial lesions.
The demographic and disease characteristics of patients with baseline brain metastases are summarized in Table 1. The median age was 62 years (range 36–86 years), and most patients had an ECOG performance status of zero or one. Consistent with previous analyses, the most common RET fusion partner was KIF5B (70% of patients). Most patients had received prior systemic therapy (91%), with a median of two prior treatments (range 0–10), including 79% of patients who were treated with platinum-based chemotherapy and 41% of patients who were treated with one or more multikinase inhibitors. Prior therapy for brain metastases included surgery in 9%, stereotactic radiosurgery in 45%, and whole brain radiotherapy (WBRT) in 14% of patients. Of the 45 patients who received prior cranial radiotherapy, 73% had completed this therapy at least 2 months prior to beginning selpercatinib treatment.
Table 1.
Demographic and disease characteristics of patients with RET fusion-positive NSCLC and intracranial disease.
| Characteristics | All patients with RET fusion-positive NSCLC and intracranial metastases (N = 80) |
|---|---|
| Age | |
| Median (range), years | 62 (36–86) |
| Sex, n (%) | |
| Female | 54 (68) |
| Male | 26 (33) |
| Race, n (%) | |
| White | 44 (55) |
| Asian | 31 (39) |
| Black or African American | 2 (3) |
| Other | 2 (3) |
| Unknown | 1 (1) |
| Smoking history, n (%) | |
| Never | 63 (79) |
| Former | 16 (20) |
| Current | 1 (1) |
| ECOG performance status, n (%) | |
| 0 | 22 (28) |
| 1 | 54 (68) |
| 2 | 4 (5) |
| NSCLC histologic subtype, n (%) | |
| Adenocarcinoma | 69 (86) |
| Large cell neuroendocrine carcinoma | 2 (3) |
| NSCLC-NOS | 8 (10) |
| Other | 1 (1) |
| RET fusion partner, n (%) | |
| KIF5B | 56 (70) |
| CCDC6 | 11 (14) |
| NCOA4 | 2 (3) |
| Other | 4 (5) |
| Unknowna | 7 (9) |
| Prior therapy, n (%) | |
| Number of prior systemic regimens | |
| 0 | 7 (9) |
| 1–2 | 43 (54) |
| 3 or more | 30 (38) |
| Median prior systemic regimen (range) | 2 (0–10) |
| Type of prior systemic therapyb | |
| Platinum chemotherapy | 63 (79) |
| Anti PD-1/PD-L1 antibody | 43 (54) |
| Multikinase inhibitor | 33 (41) |
| Taxane chemotherapy | 25 (31) |
| Other systemic therapy | 31 (39) |
| Intracranial radiotherapyb | 45 (56) |
| Whole brain radiation therapy | 11 (14) |
| Stereotactic radiosurgery | 36 (45) |
| Intracranial radiotherapy timing | |
| Completed >2 months prior to selpercatinib treatment | 33 (41) |
| Intracranial surgery | 7 (9) |
aRET fusion identified by molecular analysis with an assay unable to identify the fusion partner (e.g. FISH).
bPatients may be counted in more than one row.
At the time of data cut-off, 46 of 80 patients with NSCLC with brain metastases (58%) remained on therapy with selpercatinib; 23 of 80 patients (29%) had discontinued treatment due to PD (any PD, not limited to intracranial metastases progression; online appendix, Supplementary Table S1). After accounting for intra-patient dose escalation permitted during the phase 1 portion of the trial, 95% of patients received at least one dose of selpercatinib at the recommended phase 2 dose of 160 mg twice a day.
Selpercatinib intracranial efficacy
At the time of the data cutoff, the median duration of follow-up was 9.5 months [interquartile range (IQR) = 5.7–12.0 months]. Among the 22 patients with measurable intracranial disease at baseline, the intracranial ORR was 82% (95% CI, 60–95), including 23% with a CR and 59% with a PR (Table 2; Fig. 1). In addition, 18% of patients exhibited SD as the best response to selpercatinib. Because all the patients achieved a tumor response or disease stabilization, the intracranial disease control rate was 100%. Among the subset of eight patients with measurable disease and prior cranial radiotherapy, the intracranial ORR was 75% (six of eight patients responding; 95% CI, 35–97; online appendix, Supplementary Table S2). The intracranial ORR for patients without prior cranial radiotherapy was 86% (12 of 14 patients responding; 95% CI, 57–98).
Table 2.
Intracranial tumor response by IRC assessment in patients with RET fusion-positive NSCLC and measurable intracranial disease per RECIST 1.1.
| Patients with measurable intracranial disease (N = 22) | |
|---|---|
| Intracranial ORR, n (%) | 18 (82) |
| 95% confidence intervala | 60–95 |
| Intracranial best overall response, n (%) | |
| CR | 5 (23) |
| PR | 13 (59) |
| SD | 4 (18) |
| PD | 0 |
| Intracranial disease control rate, n (%)b | 22 (100) |
a95% CI was calculated using Clopper–Pearson method.
bIntracranial disease control rate was defined as the percentage of patients who had a best overall intracranial response of CR, PR, or SD lasting 16 weeks or more after selpercatinib initiation.
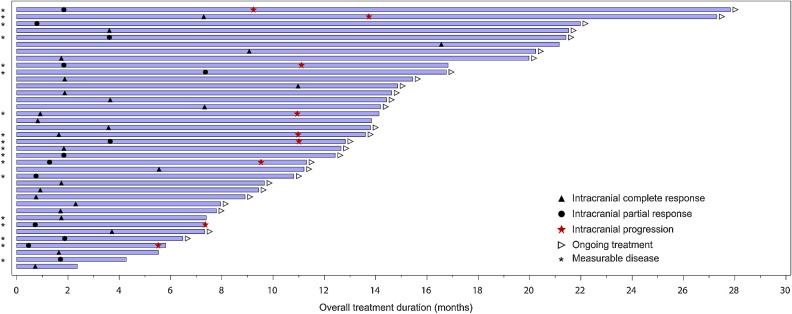
Figure 1.
Intracranial response to selpercatinib. A waterfall plot of the maximum change in intracranial tumor size is shown for the 22 patients with measurable disease at baseline. Vertical bars represent the best percent change from baseline in the sum of diameters for all intracranial target lesions, with the color of the bar representing the corresponding tumor response designation. Symbols represent prior stereotactic radiosurgery (SRS) and prior systemic therapies. Note: Because the intracranial best overall response in Table 2 is based on RECIST 1.1 requirements, including the need for a confirmatory scan, the tumor response designation does not exactly correlate with table data. MKI, multikinase inhibitor.
Among the remaining 58 patients with exclusively nonmeasurable intracranial disease at baseline, 34% (20 of 58 patients) achieved a complete intracranial response on the basis of complete resolution of all nonmeasurable lesions and 29 patients had non-CR/non-PD (CR and non-CR/non-PD corresponds to the clinical benefit rate for nonmeasurable intracranial disease). Only five patients (9%) had PD as best intracranial response (online appendix, Supplementary Table S3).
Thirty-eight patients from the 80-patient population (48%) with baseline brain metastases had an intracranial response to selpercatinib. Among this group of responders, the median intracranial DoR was not reached (95% CI, 9.3–NE; Table 3; Fig. 2A) at a median duration of follow-up of 9.5 months (IQR = 5.7–12.0). Overall, 71% were censored at the time of the analysis. At 1-year, 55% (95% CI, 32–73) of intracranial responses were ongoing. Of note, the longest intracranial response was ongoing at 21.2 months. Among all 80 patients, the median intracranial PFS was 13.7 months (Table 3; Fig. 2B), although this median estimate is unstable as only 30 patients (38%) had experienced an event at a median duration of follow-up of 11.0 months (IQR = 7.4–16.5). Time to response and response duration are displayed in Fig. 3 for all responders (n = 38).
Table 3.
Duration of intracranial tumor response and intracranial PFS by IRC assessment in patients with RET fusion-positive NSCLC and measurable and nonmeasurable intracranial disease.
| Total patients (N = 80) | |
|---|---|
| Duration of intracranial response | |
| Respondersa | 38 |
| Censored, n (%)b | 27 (71) |
| Intracranial DoR, median (months) (95% CI)c,d | NE (9.3–NE) |
| Intracranial duration of follow-up, median (months) (IQR)c | 9.5 (5.7, 12.0) |
| Intracranial DoRc,e | |
| % of patients ≥6 months (95% CI) | 91 (75–97) |
| % of patients ≥12 months (95% CI) | 55 (32–73) |
| PFS | |
| Censored, n (%)b | 50 (62.5) |
| Median, months (95% CI)c,d | 13.7 (10.9–NE) |
| Median follow-up, (months) (IQR)c | 11.0 (7.4, 16.5) |
| % progression/death-freec,e | |
| ≥6 months (95% CI) | 79 (68–87) |
| ≥12 months (95% CI) | 55 (41–67) |
Abbreviation: NE, not estimable.
aPatients with intracranial best response of CR or PR based on IRC assessments using RECIST (version 1.1).
bStatus as of the patient's last disease assessment on or before December 16, 2019.
cEstimate based on Kaplan–Meier method.
d95% CI was calculated using Brookmeyer and Crowley method.
e95% CI was calculated using Greenwood formula.
Figure 2.
Kaplan–Meier plot of (A) intracranial DoR and (B) intracranial PFS. A, The plot depicts the DoR for all responding patients with measurable or nonmeasurable intracranial metastases. B, The plot was constructed with data derived from all patients with measurable or nonmeasurable intracranial metastases treated with selpercatinib. NE, nonestimable.
Figure 3.
Duration of selpercatinib therapy. Treatment duration, time to intracranial response, and intracranial progression events are shown in this swimmer's plot for patients with measurable and nonmeasurable intracranial disease (n = 38). The complete response and PR symbols indicate the time of the first scan showing an intracranial response (that was then confirmed at a subsequent assessment).
Selpercatinib safety
Among patients with NSCLC and baseline brain metastases, selpercatinib treatment was associated with a low rate of treatment discontinuation due to adverse events judged by the investigator as possibly related to selpercatinib treatment (TRAE) (3%, 2 of 80 patients). Supplementary Table S4 summarizes total (all grade) treatment-emergent adverse events (TEAE) and TRAEs. TEAEs and TRAEs were reported at similar levels in patients with baseline intracranial disease as in all RET fusion-positive NSCLCs with and without intracranial disease (n = 253).
Among patients with intracranial disease, most TEAEs and TRAEs were low grade (Supplementary Table S5). The only TEAEs reported as grade 3/4 in >10% of patients with NSCLC and baseline brain metastases were alanine aminotransferase (ALT) increase (18%), aspartate aminotransferase (AST) increase (11%), hypertension (21%, all grade 3), and hyponatremia (11%). Grade 3/4 elevated ALT and AST and hypertension were reported at similar levels as TRAEs. No Grade 5 TRAEs were reported among the patients with NSCLC and baseline brain metastases.
Discussion
Intracranial metastases are a major cause of morbidity and mortality for patients with oncogene-addicted cancers. The results of this global, multicenter study demonstrate that selpercatinib has robust intracranial efficacy by blinded independent review of patients with RET fusion-positive NSCLCs and brain metastases. The drug achieved a high intracranial response rate and the intracranial DoR and intracranial PFS were prolonged. Moreover, selpercatinib treatment was well tolerated in this patient population, with no new safety signals identified. Taken together, these data support selpercatinib as a new standard of care for primary treatment of brain metastases for patients with RET fusion-positive NSCLC. Comprehensive molecular profiling analysis is warranted in the future to further analyze the biomarkers of intracranial response and resistance to selpercatinib.
The intracranial activity of selpercatinib in this phase 1/2 trial is broadly consistent with the intracranial activity observed with other contemporary targeted therapies for genomically-driven NSCLCs. In ALK fusion-positive lung cancers, alectinib achieved an intracranial ORR of 64%, an intracranial disease control rate of 90%, and durable disease control (median intracranial DoR of 10.8 months) among patients with measurable disease in a comparable analysis of two single-arm phase 2 trials (10). At 6 months, 58% of patients were progression/death-free. In EGFR-mutant lung cancers, osimertinib achieved an intracranial ORR of 54% and an intracranial disease control rate of 92% in a pooled analysis of two phase 2 trials (18). At 6 months, 72% of patients were intracranial progression/death-free. By comparison, selpercatinib treatment resulted in an intracranial ORR of 82% and an intracranial disease control rate of 100%, and at 6 months, 79% of patients were intracranial progression/death-free. Median intracranial DoR was not reached (95% CI, 9.3–NE). Both alectinib and osimertinib are recognized as standards of care for tyrosine kinase inhibitor-naïve patients with ALK fusion-positive and EGFR-mutant lung cancers, respectively, similar to the role of selpercatinib in RET fusion-positive lung cancers.
Selpercatinib's activity in the CNS has important implications beyond RET fusion-positive NSCLCs with brain metastases. A CR to selpercatinib in leptomeningeal disease has already been described in a patient with RET fusion-positive NSCLC (8), demonstrating the activity of the drug beyond parenchymal disease. Selpercatinib has been shown to be active against intracranial metastases in a patient with RET fusion-positive thyroid cancer (19), a patient with RET-mutant medullary thyroid cancer (20), and a pediatric patient with RET fusion-positive congenital mesoblastic nephroma (21). LIBRETTO-001 continues to enroll patients with non-lung/non-thyroid cancers that harbor RET fusions or mutations. Additional confirmation of the drug's activity in this setting will help establish the overall impact of selpercatinib on intracranial disease in patients with RET-dependent cancers of any histology in both adult and pediatric populations.
Although this prospective, pre-planned, independently-reviewed analysis has many strengths, it does have some important limitations. Patients in this cohort had received a variety of both systemic and local therapies for management of their RET fusion-positive NSCLCs. Despite this, intracranial activity was observed across various treatment subgroups. In addition, at the time of analysis, a majority of patients remained progression-free and a majority of responses were ongoing; thus, stable medians could not be estimated. Ongoing follow-up will reveal more precise estimates of intracranial response durability and PFS. Moreover, this study did not specifically address whether selpercatinib can prevent or delay intracranial progression in patients with NSCLC that begin treatment without intracranial involvement. As a phase 1/2 trial, LIBRETTO-001 did not require head and neck MRI/CT scans during treatment unless intracranial disease was identified at baseline and the trial could not address this question. Intriguingly, other tyrosine kinase inhibitors with substantial intracranial activity have already been shown to prolong the time to the acquisition of CNS metastases in fusion-positive lung cancers compared with earlier-generation kinase inhibitors with less optimal intracranial activity (22, 23). However, there is a lack of prospective data evaluating long-term outcomes of tyrosine kinase inhibitors alone compared with SRS and tyrosine kinase inhibitors in managing brain metastases.
Selpercatinib is currently being evaluated in LIBRETTO-431 (NCT04194944), an ongoing randomized, global, phase 3 study of selpercatinib versus platinum-pemetrexed with or without pembrolizumab in treatment-naïve patients with RET fusion-positive NSCLCs. This trial will allow the further characterization of selpercatinib activity in patients with NSCLC and intracranial metastases.
Authors' Disclosures
V. Subbiah reports grants from Eli Lilly/Loxo Oncology and grant and advisory board/consultant position with Eli Lilly/Loxo Oncology during the conduct of the study, as well as research grants from Roche/Genentech, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, D3, Pfizer, Multivir, Amgen, AbbVie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, NCI-CTEP, UT MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals, Novartis, Pharmamar, and Medimmune; employment with Helsinn, Incyte, QED Pharma, Daiichi-Sankyo, Signant Health, Novartis, and Medimmune; travel funds from Pharmamar, Incyte, ASCO, and ESMO; and other support from Medscape outside the submitted work. J.F. Gainor reports personal fees and other support from Bristol-Myers Squibb, Merck, Genentech/Roche, Lilly/Loxo, Blueprint, Helsinn, and Jounce; personal fees from AstraZeneca, Mirati, Amgen, iTeos, GlydeBio, Pfizer, Takeda; grants, personal fees, and other support from Novartis; and other support from Ironwood outside the submitted work. G.R. Oxnard reports personal fees from Foundation Medicine and Roche outside the submitted work. D.S.W. Tan reports grants and personal fees from Amgen, Pfizer, Novartis, and AstraZeneca and personal fees from Bayer, Boehringer Ingelheim, C4 Therapeutics, MSD, and Merck outside the submitted work. D.H. Owen reports grants from BMS, Merck, Palobiofarma, and Genentech and personal fees from themednet.org outside the submitted work. B.C. Cho reports grants from Novartis, Bayer, AstraZeneca, MOGAM Institute, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono, Dizal Pharma, MSD, AbbVie, Medpacto, GIInnovation, Eli Lilly, Blueprint Medicines, and Interpark Bio Convergence Corp; personal fees from Novartis, AstraZeneca, Boehringer-Ingelheim, Roche, BMS, Ono, Yuhan, Pfizer, Eli Lilly, Janssen, Takeda, MSD, Medpacto, Blueprint Medicines, TheraCanVac Inc., Gencurix Inc., Bridgebio Therapeutics, KANAPH Therapeutic Inc., Cyrus Therapeutics, Interpark Bio Convergence Corp. Guardant Health, and Oscotec Inc.; and other support from Interpark Bio Convergence Corp., Champions Oncology, and DAAN Biotherapeutics outside the submitted work. H.H. Loong reports personal fees from Boehringer-Ingelheim, Eli Lilly, Illumina, Novartis, Takeda, Eisai, Guardant Health, and Bayer and grants from MSD outside the submitted work. C.E. McCoach reports personal fees from AstraZeneca, Takeda, and Guardant Health; grants and personal fees from Novartis; grants from Revolution Medicines; and personal fees and other support from Genentech outside the submitted work, as well as employment with Genentech Inc. J. Weiss reports grants from Eli Lilly during the conduct of the study and personal fees from Eli Lilly outside the submitted work. L. Bazhenova reports personal fees from Johnson and Johnson, Novartis, Neuvogen, Daiichi Sankyo, Boehringer Ingelheim, BMS, Merck, and Regeneron outside the submitted work. K. Park reports other support from Eli Lilly during the conduct of the study and other support from Eli Lilly and Loxo outside the submitted work. H. Daga reports personal fees from Chugai outside the submitted work. B. Besse reports grants from 4D Pharma, AbbVie, Amgen, Aptitude Health, AstraZeneca, BeiGene, Blueprint Medicines, BMS, Boehringer Ingelheim, Celgene, Cergentis, Cristal Therapeutics, Daiichi-Sankyo, Eli Lilly, GSK, Inivata, Janssen, Onxeo, OSE Immunotherapeutics, Pfizer, Roche-Genentech, Sanofi, Takeda, and Tolero Pharmaceuticals during the conduct of the study. O. Gautschi reports other support from Lilly, Amgen, Bayer, and Novartis during the conduct of the study. C. Rolfo reports personal fees from AstraZeneca, MSD, Roche, Inivata, ArcherDX, MD Serono, BMS, Novartis, Boston Pharmaceuticals, and Pfizer outside the submitted work. E.Y. Zhu reports other support from Loxo Oncology during the conduct of the study. J.F. Kherani reports other support from Loxo Oncology at Lilly during the conduct of the study and other support from Loxo Oncology at Lilly outside the submitted work. X. Huang reports other support from Loxo Oncology during the conduct of the study. A. Drilon reports personal fees from Loxo/Lilly during the conduct of the study; personal fees from Ignyta/Genentech/Roche, Bayer, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Beigene, BergenBio, Hengrui, Exelixis, Tyra, Verastem, MORE Health, AbbVie, 14ner/Elevation, Remedica, ArcherDX, Monopteros, Novartis, EMD Serono, Melendi, GlaxoSmithKlein, Teva, Taiho, PharmaMar, and Foundation Medicine outside the submitted work. No disclosures were reported by the other authors.
Supplementary Material
Acknowledgments
Research support for the study was provided by Loxo Oncology, Inc., a wholly owned subsidiary of Eli Lilly and Company. V. Subbiah was supported by NIH grant R01CA242845; M.D. Anderson was supported by Cancer Center Support Grant (P30 CA016672). The authors thank the patients, their families, and the trial teams at the participating centers. Medical writing assistance was provided by Mary Dugan Wood, funded by Eli Lilly and Company.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 4131
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Clin Cancer Res 2021;27:4160–7
Authors' Contributions
V. Subbiah: Conceptualization, resources, data curation, software, formal analysis, validation, investigation, methodology, writing–original draft, writing–review and editing. J.F. Gainor: Resources, data curation, formal analysis, investigation, writing–review and editing. G.R. Oxnard: Resources, data curation, formal analysis, investigation, writing–review and editing. D.S.W. Tan: Conceptualization, resources, data curation, formal analysis, investigation, methodology, writing–original draft, writing–review and editing. D.H. Owen: Resources, data curation, investigation, methodology, writing–original draft, writing–review and editing. B.C. Cho: Conceptualization, resources, data curation, investigation, methodology, writing–original draft, writing–review and editing. H.H. Loong: Resources, data curation, formal analysis, investigation, writing–original draft, writing–review and editing. C.E. McCoach: Resources, data curation, investigation, writing–review and editing. J. Weiss: Resources, data curation, formal analysis, investigation, writing–review and editing. Y.J. Kim: Resources, data curation, investigation, writing–original draft, writing–review and editing. L. Bazhenova: Resources, data curation, formal analysis, investigation, writing–review and editing. K. Park: Resources, data curation, formal analysis, investigation, writing–original draft, writing–review and editing. H. Daga: Resources, data curation, investigation, writing–review and editing. B. Besse: Resources, data curation, investigation, writing–review and editing. O. Gautschi: Resources, data curation, investigation, writing–review and editing. C. Rolfo: Resources, data curation, investigation, writing–review and editing. E.Y. Zhu: Conceptualization, resources, data curation, formal analysis, investigation, methodology, writing–review and editing. J.F. Kherani: Resources, data curation, formal analysis, investigation, writing–review and editing. X. Huang: Resources, data curation, software, formal analysis, validation, investigation, writing–review and editing. S. Kang: Resources, data curation, software, formal analysis, validation, investigation, writing–review and editing. A. Drilon: Conceptualization, resources, data curation, formal analysis, investigation, methodology, writing–original draft, writing–review and editing.
References
- 1. Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer 2014;14:173–86. [DOI] [PubMed] [Google Scholar]
- 2. Subbiah V, Cote GJ. Advances in targeting RET-dependent cancers. Cancer Discov 2020;10:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drilon A, Hu ZI, Lai GGY, Tan DSW. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 2018;15:151–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Subbiah V, Yang D, Velcheti V, Drilon A, Meric-Bernstam F. State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol 2020;38:1209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsuta K, Kohno T, Yoshida A, Shimada Y, Asamura H, Furuta K, et al. RET-rearranged non-small-cell lung carcinoma: a clinicopathological and molecular analysis. Brit J Cancer 2014;110:1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang R, Hu H, Pan Y, Li Y, Ye T, Li C, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol 2012;30:4352–9. [DOI] [PubMed] [Google Scholar]
- 7. Drilon A, Lin JJ, Filleron T, Ni A, Milia J, Bergagnini I, et al. Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol 2018;13:1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo R, Schreyer M, Chang JC, Rothenberg SM, Henry D, Cotzia P, et al. Response to selective RET inhibition with LOXO-292 in a patient with RET fusion-positive lung cancer with leptomeningeal metastases. JCO Precision Oncol 2019;3:PO.19.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park S, Lee MH, Seong M, Kim ST, Kang JH, Cho BC, et al. A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol 2020;31:1397–404. [DOI] [PubMed] [Google Scholar]
- 10. Gadgeel SM, Shaw AT, Govindan R, Gandhi L, Socinski MA, Camidge DR, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non–small-cell lung cancer. J Clin Oncol 2016;34:4079–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aoyama H, Tago M, Kato N, Toyoda T, Kenjyo M, Hirota S, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys 2007;68:1388–95. [DOI] [PubMed] [Google Scholar]
- 12. Drilon A, Oxnard GR, Tan SWD, Loong HHF, Johnson M, Gainor J, et al. Efficacy of selpercatinib in RET fusion-positive non-small cell lung cancer. N Engl J Med 2020;383:813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Markham A. Selpercatinib: first approval. Drugs 2020;80:1119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Medicines Agency Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 7-10 December 2020. https://www.ema.europa.eu/en/news/meeting-highlights-committee-medicinal-products-human-use-chmp-7-10-december-2020. Accessed March 1, 2021.
- 15. Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol 2018;29:1869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), v4.0 (v4.03). https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Updated: 2010. Accessed July 9, 2020.
- 18. Goss G, Tsai CM, Shepherd FA, Ahn MJ, Bazhenova L, Crino L, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol 2018;29:687–93. [DOI] [PubMed] [Google Scholar]
- 19. Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 2020;383:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andreev-Drakhlin A, Cabanillas M, Amini B, Subbiah V. Systemic and CNS activity of selective RET inhibition with selpercatinib (LOXO-292) in a patient with RET-mutant medullary thyroid cancer with extensive CNS metastases. JCO Precis Oncol 2020;38:3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ortiz MV, Gerdemann U, Raju SG, Henry D, Smith S, Rothenberg SM, et al. Activity of the highly specific RET inhibitor selpercatinib (LOXO-292) in pediatric patients with tumors harboring RET gene alterations. JCO Precis Oncol 2020;4:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med 2017;377:829–38. [DOI] [PubMed] [Google Scholar]
- 23. Camidge DR, Kim HR, Ahn MJ, Yang JC, Han JY, Lee JS, et al. Brigatinib versus crizotinib in ALK-positive non–small-cell lung cancer. N Engl J Med 2018;379:2027–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.