Abstract
Background
While systemic anticoagulation is most widely used in haemodialysis (HD), contraindications to its use might occur in particular settings. The Solacea™ haemodialyser with an asymmetric triacetate membrane claims improved biocompatibility and has already shown promising results when used in combination with only half dose of anticoagulation. To quantify the performance of the Solacea™ when further decreasing anticoagulation to zero, fibre blocking was assessed by micro-computed tomography (micro-CT).
Methods
Ten maintenance HD patients underwent six dialysis sessions at midweek using a Solacea™ 19H dialyser, consecutively in pre-dilution haemodiafiltration (pre-HDF), HD and post-dilution HDF (post-HDF). After the first three sessions with only a quarter of their regular anticoagulation dose (one-quarter), the last three sessions were performed without anticoagulation (zero). Dialyser fibre blocking was quantified in the dialyser outlet potting using a 3D micro-CT scanning technique post-dialysis.
Results
Even in case of reduced (one-quarter) anticoagulation, the relative number of open fibres post-dialysis was almost optimal, i.e. 0.96 (0.87–0.99) with pre-HDF, 0.99 (0.97–0.99) with HD and 0.97 (0.92–0.99) with post-HDF. Fibre patency was mildly decreased for pre-HDF and HD when anticoagulation was decreased from one-quarter to zero, i.e. to 0.76 (0.61–0.85) with pre-HDF (P = 0.004) and to 0.80 (0.77–0.89) with HD (P = 0.013). Comparing the results for zero anticoagulation, post-HDF [i.e. 0.94 (0.82–0.97)] performed as well as HD and pre-HDF.
Conclusions
The Solacea™ dialyser provides promising results for use in conditions where systemic anticoagulation is contraindicated. Post-HDF, although inducing haemoconcentration in the dialyser, is equally effective for fibre patency in case of zero anticoagulation as pre-HDF and HD when using Solacea™.
Keywords: anticoagulation, arteriovenous fistula, biocompatibility, chronic haemodialysis, haemodialysis
INTRODUCTION
During haemodialysis (HD), coagulation is activated when blood comes into contact with the extracorporeal circuit (ECC). While systemic anticoagulation with heparin is most widely used, contraindications to its use might occur in specific settings. But, heparin-free HD, such as regional citrate anticoagulation or periodic saline flushes to rinse the ECC, are time-consuming techniques and might have undesired side effects such as alkalization or fluid overload.
Literature seems to provide conflicting results on the impact of dilution on anticoagulation. It was already suggested in the 1990s that pre-dilution haemodiafiltration (pre-HDF) could be an alternative to saline flushes as it also results in continuous rinsing of the circuit but without fluid overload [1]. Accordingly, pre-HDF is increasingly being used in conditions where anticoagulation is contraindicated [2]. However, some data indicate that saline flushes and convective techniques may promote rather than antagonize coagulation [3, 4].
Membranes used for online HDF treatment must have high permeability while ideally avoiding albumin leakage. Synthetic membranes such as polysulfone and polyethersulfone generally satisfy these requirements, but might also be associated with hypersensitivity reactions, ascribed to additives like polyvinylpyrrolidone to enhance the membrane’s hydrophilicity. Evidence suggests that the asymmetric triacetate (ATA™) membrane, manufactured without hydrophilization agents, has a lower risk of hypersensitivity, and induces less decrease in platelets (Plts) as indication of an excellent biocompatibility [5]. The Solacea™ haemodialyser incorporates such an asymmetric triacetate membrane, and is reported to have good biocompatibility, and high permeability and filtration performance. In a head to head comparison with half-dose of anticoagulation, virtually no signs of fibre blocking could be observed when using the Solacea™ dialyser, while clotting was much more present in a polysulfone dialyser [6].
The aim of this prospective cross-over study was to objectively quantify the performance of the Solacea™ dialyser when reducing anticoagulation to zero. To investigate the impact of haemodilution under these circumstances of pro-coagulation, tests were executed in three different dialysis modes, i.e. HD, pre-HDF and post-dilution HDF (post-HDF). Fibre blocking was objectively assessed by 3D micro-computed tomography (micro-CT).
MATERIALS AND METHODS
Patients
This single-centre cross-over study included 10 consecutive stable chronic HD patients (mean ± SD age 58.6 ± 17.0 years; nine male). Patients were eligible when they had experienced stable dialysis sessions during the last 4 weeks, and had no known coagulation disorder, active inflammation or malignancy. Power analysis was based on data from a previously performed cross-over study in patients dialysed with two different types of dialyser [6]. Using the relative number of patent fibres as primary outcome, power was 69% (α = 0.05) including only six patients.
Double-needle vascular access was achieved through a native arteriovenous fistula (n = 8) or a well-functioning double-lumen tunnelled central venous catheter, either Haemostar® 14.5 F (n = 1) (Bard, Salt Lake City, UT, USA) or Palindrome™ 14.5F (n = 1) (Medtronic, Minneapolis, MN, USA). Regular treatment of these patients was post-HDF with FX800 dialyser (n = 9) (Fresenius Medical Care, Bad Homburg, Germany) and pre-HDF with Evodial 1.3 (n = 1) (Baxter, Deerfield, IL, USA).
The protocol adhered to the Declaration of Helsinki, and was approved by the institutional research committee (Ethical Committee, Ghent University Hospital, EC 2017/1459—B670201734230, March 2018), and was registered as part of a larger study in www.ClinicalTrials.gov (NCT03820401). Written informed consent was obtained from all included patients.
Dialysis and anticoagulation
In the study protocol, each patient was dialysed for 240 min in six different regimens, using three different dialysis modes and two different anticoagulation schemes. All study sessions were performed at midweek with the ATA™ Solacea™ 19H dialyser (Nipro, Osaka, Japan). The three performed dialysis modes were HD, and pre- and post-HDF. Patients received their regular brand of low molecular weight heparin (LMWH) anticoagulation (Tinzaparin, Leo Pharma, Belgium) at the beginning of the dialysis session at only a quarter of their regular dose (one-quarter anticoagulation) or dialysis was performed without any anticoagulation (zero anticoagulation). For safety reasons, we first performed the three sessions at one-quarter anticoagulation in the order of pre-HDF, HD and post-HDF, before the three sessions with zero anticoagulation. According to protocol, it was planned that in case a test session had to be terminated prematurely, the following test session would not be executed in that patient, and a complete blocking would be registered for that patient for that session.
All test sessions were performed on a 5008 dialysis machine (Fresenius Medical Care, Bad Homburg, Germany) with blood flow at 300 mL/min and dialysate flow at 500 mL/min. In pre- and post-HDF, substitution flow was set at, respectively, 50 and 25% of blood flow (i.e. 150 and 75 mL/min). Ultrafiltration rates were set according to the patient’s inter-dialytic weight gain and clinical status.
Each experimental session was preceded by two wash-in sessions with the same type of dialyser to be used in the experimental dialysis at midweek, but always with full regular anticoagulation dose. Each patient served as his/her own control.
Micro-CT scanning and coagulation quantification
To quantify fibre blocking after 4-h dialysis, dialysers were scanned with a reference non-invasive micro-CT scanning technique [7]. In brief, at the end of the dialysis session, a standard rinsing procedure of the haemodialyser was performed with exact 300 mL rinsing solution. Next, the haemodialyser was dried for 4 h using continuous mild positive pressure ventilation, simultaneously in blood and dialysate compartment. Dialyser fibre blocking was visualised in the dialyser outlet potting using a 3D CT scanning technique on micrometre resolution, as previously described [7].
For this study, three different thresholds were used to define the surface area of an open fibre: i.e. 50, 70 and 90% of the cross-section of a non-used fibre. Comparing the number of non-blocked fibres in the tested dialyser with the total number of fibres as measured in three non-used dialyser samples, provided an objective estimate of the percentage of fibre blocking.
Statistical analysis
Statistical analyses were performed using SPSS version 26 (SPSS Inc., Chicago, IL, USA). Continuous variables were summarised as mean ± SD , and median value with interquartile range (IQR). Normality was checked with Shapiro–Wilk test. To compare different related variables, non-parametric Friedman tests for repeated measures were performed with Wilcoxon post hoc test (non-normal distribution).
RESULTS
Relevant demographic and clinical data of the patient population at baseline are summarised in Table 1. There were no patient dropouts during the experimental period, all flow settings were maintained according to the protocol and no adverse events were recorded. Table 2 shows the dialysis durations and the ultrafiltration rates in the six test sessions. Since none of the test sessions had to be terminated prematurely due to fibre clotting in the dialyser, all patients completed each of the six arms of the study protocol. One session was terminated early at 210 min due to a machine problem, but without any sign of fibre clotting as could be concluded from the dialyser scan post-dialysis.
Table 1.
Demographic and clinical data of the patient population at baseline
| Gender (male/female) | 9/1 |
| Age, years, mean ± SD | 58.6 ± 17.0 |
| Body weight, kg, mean ± SD | 71.4 ± 11.1 |
| Dialysis vintage, months, median (25pct–75pct) | 24.3 (18.8–35.1) |
| Renal disease | IgA nephropathy (n = 2); renal cell carcinoma (n = 2); diabetic nephropathy (n = 1); nephroangiosclerosis (n = 1); focal segmental glomerulosclerosis (n = 1); retroperitoneal fibrosis (n = 1); pauci- immune crescentic glomerulonephritis (n = 1); Alport (n = 1) |
| Regular anticoagulation dose | Tinzaparin 4500 (n = 5); tinzaparin 3500 (n = 5) |
| Plt inhibitors | Acetylsalicylic acid 80 mg (n = 5) |
| Hb, g/dL, mean ± SD | 11.3 ± 0.5 |
| Plts count, 10³/µL, mean ± SD | 242 ± 124 |
| aPTT (s), mean ± SD | 40.0 ± 7.4 |
| INR (−), mean ± SD | 1.0 ± 0.1 |
| CRP, mg/L, median (25pct–75pct) | 4.1 (3.0–4.5) |
IgA, immunoglobulin A; Hb, haemoglobin; aPTT, activated partial thromboplastin time; INR, international normalised ratio; CRP, C-reactive protein.
Table 2.
Characteristics of the dialysis sessions in the different experimental settings
| Dialysis mode | Dialysis duration (min) | VUF (mL) |
|---|---|---|
| Pre-HDF_1/4 | 237 ± 10a | 2264 ± 561 |
| HD_1/4 | 240 ± 1 | 2389 ± 623 |
| Post-HDF_1/4 | 239 ± 4 | 2254 ± 775 |
| Pre-HDF_0 | 241 ± 2 | 2385 ± 670 |
| HD_0 | 240 ± 0 | 2496 ± 628 |
| Post-HDF_0 | 242 ± 3 | 2341 ± 665 |
Values are presented as mean ± SD. VUF, ultrafiltration volume over the session; pre-HDF_1/4, pre-HDF with one-quarter anticoagulation; HD_1/4, HD with one-quarter anticoagulation; post-HDF_1/4, post-HDF with one-quarter anticoagulation; pre-HDF_0, pre-HDF with zero anticoagulation; HD_0, HD with zero anticoagulation; post-HDF_0, post-HDF with zero anticoagulation.
One session was terminated at 210 min due to a machine problem without coagulation problems.
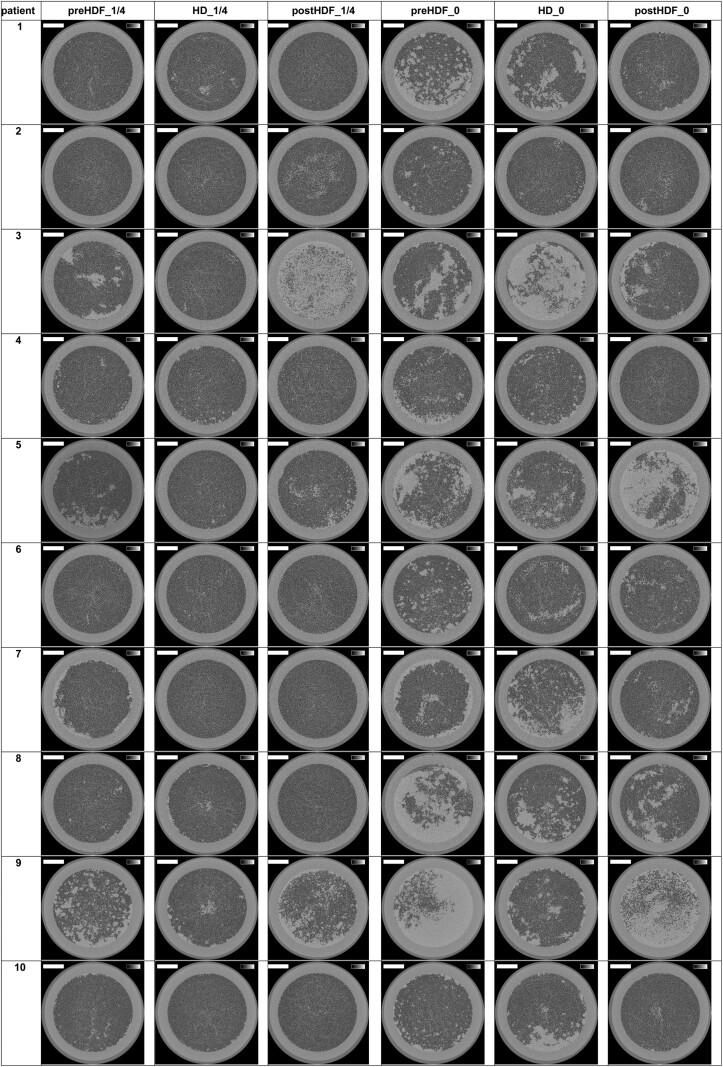
The reconstructed images of the cross-sections halfway the outlet potting are presented in Figure 1 for the 10 patients and the six experimental dialysis sessions. The lumens of open fibres are visualised as black dots.
FIGURE 1:
Cross-sections halfway the potting in 10 patients and six tested settings. The greyscale range is from 0 to 0.5 cm−1 and the scale bar denotes 10 mm.
The number of open fibres in the three non-used Solacea™ dialyser reference samples was very consistent, being 12 087 ± 4.
The relative number of open fibres in the six tested dialysis scenarios for the thresholds of 50, 70 and 90% open fibre area are presented in Table 3. For the threshold of fibres being open for 70%, the median relative number of open fibres was 0.96 (pre-HDF), 0.99 (HD) and 0.97 (post-HDF) with one-quarter anticoagulation, while this was 0.76 (pre-HDF), 0.80 (HD) and 0.94 (post-HDF) with zero anticoagulation. Testing for repeated measures showed a significant difference within the six strategies (Friedman P < 0.001). Only for pre-HDF and HD, the relative number of patent fibres was lower with zero anticoagulation as compared with one-quarter anticoagulation, irrespective of the considered threshold for counting open fibres (P = 0.004, respectively, 0.013 for 70% threshold). No difference was seen in post-HDF between zero and one-quarter anticoagulation. The anticoagulation dose (P < 0.001) but not the dialysis mode (P = 0.116) influenced the number of open fibres (threshold 70% open area).
Table 3.
Percentage of open fibres in the Solacea™ dialyser in the six tested dialysis scenarios for the thresholds of 50, 70 and 90% open fibre area
| % | 50% open area | 70% open area | 90% open area |
|---|---|---|---|
| Friedman P-value | <0.001 | <0.001 | <0.001 |
| Pre-HDF_1/4 | 96 (87–99) | 96 (87–99) | 91 (81–92) |
| HD_1/4 | 99 (97–99) | 99 (97–99) | 87 (86–91) |
| Post-HDF_1/4 | 97 (92–100) | 97 (92–99) | 88 (81–91) |
| Pre-HDF_0 | 76 (61–85)* | 76 (61–85)* | 69 (56–79)* |
| HD_0 | 81 (77–90)* | 80 (77–89)* | 72 (64–79)* |
| Post-HDF_0 | 94 (82–98) | 94 (82–97) | 86 (73–88) |
Pre-HDF_1/4, pre-HDF with one-quarter anticoagulation; HD_1/4, HD with one-quarter anticoagulation; post-HDF_1/4, post-HDF with one-quarter anticoagulation; pre-HDF_0, pre-HDF with zero anticoagulation; HD_0, HD with zero anticoagulation; post-HDF_0, post-HDF with zero anticoagulation.
Data are presented as median (25pct–75pct).
P < 0.05 versus one-quarter anticoagulation.
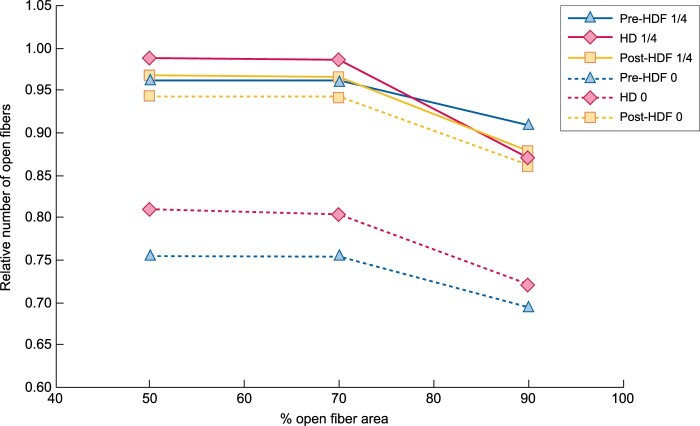
Figure 2 shows the relative number of fibres considered as open according to the three different thresholds (%) of individual fibre area free of clotting. Only a small drop (7–11%) can be seen when the fibre counting criterion shifts from 70 to 90% open fibre area, indicating that the fibres of Solacea™ are resistant to even small degrees of fibre blocking during dialysis.
FIGURE 2:
Relative number of fibres considered as open according to different decision criteria of % of fibre area free of clotting. Pre-HDF 1/4, pre-HDF with one-quarter anticoagulation; HD 1/4, HD with one-quarter anticoagulation; post-HDF 1/4, post-HDF with one-quarter anticoagulation; pre-HDF 0, pre-HDF with zero anticoagulation; HD 0, HD with zero anticoagulation; post-HDF 0, post-HDF with zero anticoagulation.
DISCUSSION
This cross-over study investigated the performance of the Solacea™ dialyser with respect to fibre blocking in three different dialysis modes, i.e. pre-HDF, HD and post-HDF, and using either one-quarter or zero anticoagulation. Our main findings are that first, Solacea™ performs excellently in avoiding clotting as expressed by the relative number of open fibres at the end of the dialysis session with only one-quarter of anticoagulation; secondly, with zero anticoagulation, fibre blocking was more prominent but still rather limited and no sessions had to be terminated prematurely; and thirdly, the dialysis mode (pre-HDF versus HD versus post-HDF) did not influence the number of open fibres.
To avoid premature termination of the dialysis session and the potential loss of patient’s blood due to clotting in the ECC, many dialysis centres have the strategy to administer generous amounts of anticoagulants. However, in conditions of active bleeding or a substantial bleeding risk, systemic anticoagulation might be contraindicated and strategies applying regional anticoagulation or even no anticoagulation should be used.
In the latter case, it is important to use biocompatible ECCs and dialysers since activation of the coagulation cascade is influenced by bio-incompatibility [8]. When using the Solacea™ dialyser without anticoagulation, we observed a substantial reduction in percentage of open fibres in only 3/10 patients, while all sessions could be completed as planned, i.e. after 240 min. Hence, our present findings confirm the advantageous biocompatible characteristics of the Solacea™ ATA™ membrane, which makes this dialyser to be recommended for use in settings when no systemic anticoagulation can be used.
This strategy seems even preferable above local anticoagulation using citrate or calcium zero dialysate since these techniques are quite labour intensive [9], and small single-centre studies showed varying success [10, 11]. Also, the use of dialysers with a heparin-coated membrane can be questionable, although their use is meanwhile established in clinical practice based on the results of observational and uncontrolled studies [12, 13]. Controlled studies, however, reported medium to high failure rates [2, 14]. In line with these reports, a more recent cross-over study also failed to support the use of dialysers with heparin-coated membranes in the setting of 4-h heparin-free HD [15].
Periodic saline flushes can be performed when anticoagulation is contraindicated. However, this is a laborious technique, which also can result in fluid overload in the patient [16, 17]. As an alternative, pre-HDF was then suggested and increasingly used [1, 2]. This strategy allows continuous rinsing of the circuit without the risk of fluid overload. We summarize the literature on this topic in Table 4. While a non-randomized study showed promising results for pre-HDF versus standard HD [21], different studies, however, found more clotting with saline flushes [4], as well as with pre-HDF compared with standard HD [3, 27]. For pre-HDF as compared with HD with a heparin-coated membrane, Laville et al. found more clotting with pre-HDF [2], while Brunot et al. found comparable results, ascribing the observed clotting to preceding surgery or lower blood flows (<250 mL/min) [24].
Table 4.
Narrative literature overview of studies dealing with the effect of online dilution on coagulation during dialysis a
| References | Country | Study type | Sample size patients | Dialysis | Anticoagulation | Clotting evaluation | Result (low < high clotting) |
|---|---|---|---|---|---|---|---|
| Klingel et al. [3] | Germany |
Randomized cross-over Single centre |
10 chronic HD | APS 900: HF-HD; pre-HF Qs200; pre-HDF Qs200 | LMWH: 50 U/kg BW; 1200 IU + 400 IU/h | + aXa, TAT and D-dimer, C5a |
HD < HF, HDF Increased coagulation with HF and HDF (higher TAT and D-dimer) |
| Davies et al. [18] | Australia | Randomized cross-over | 31 ICU | Pre-CVVH Qs 35 mL/kg/h; pre-CVVHDF Qs 600 mL/h |
Continuous heparin 8–10 IU/kg/h (aPTT = 40–55 s) |
Circuit life—visual inspection + pressure rise | CVVHDF < CVVH |
| Gritters-van den Oever et al. [19] | Netherlands |
Randomized prospective Single centre |
19 chronic HD | F8HPS LF HD; FX80 post-HDF Qs target >100 |
LMWH 50 IU/kg BW |
+ CD62p, PF4 and BTG |
HD < post-HDF → more Plt activation with post-HDF |
| Stefansson et al. [20] | Sweden | Randomized cross-over | 20 chronic HD | LF HD: Polyflux 17 L; post-HDF: polyflux 21S | LMWH ∼5000 IU (cf. intradialytic clotting) | NR | 39% more LMWH in post-HDF |
| Masakane et al. [21] | Japan |
Non-randomized cross-over Single centre |
9 chronic HD | PES membrane: super HF HD; pre-HDF; post-HDF | NR |
+ Biocompatibility + symptoms: e.g. itchiness, fatigue etc. |
Pre-HDF < HD |
| Laville et al. [2] | France, Canada, Belgium, Spain, UK, Poland, Netherlands | Randomized Multicentre | 231 chronic HD + AKI + high bleeding risk | Heparin-grafted; control: saline flushes or pre-HDF | Zero |
+ Circuit occlusion + need for extra saline flushes + premature termination |
Success rate = 1) 68.5% (heparin grafted) 2) 50% (control) |
| Frascà et al. [22] | Italy |
Non-randomized cross-over Multicentre |
44 chronic HD | Post-HDF: Polyflux; Evodial; Evodial |
(1) priming UFH + 1000 IU/h UFH (2) 1000 IU UFH at 0 h + 2h (3) LMWH 0.3/0.4 at 0 h |
+ Visual inspection + aPTT, aXa and TAT + Hb, Plts, Crea and Kt/V |
Dialysis strategy (1) < (3) < (2) → Massive clotting in dialysers: 0.8%; 10%; 1% |
| Smith et al. [23] | UK |
Randomized cross-over Muticentre |
100 chronic HD | FX80/FX100: HD; post-HDF Qs ∼20.6 L | LMWH ∼2750 IU (cf. intradialytic clotting) |
+ ↑ Venous pressure + clotting circuit |
HD < post-HD for same antico amount |
| Brunot et al. [24] | France |
Non-randomized Prospective Single centre |
179 chronic HD + high bleeding risk | HD: heparin-coated Nephral400; pre-HDF: FX800 | Zero |
+ Session failure + efficiency |
HD = pre-HDF But: QB < 250 + recent surgery ∼ ECC thrombosis |
| Tangvoraphonkchai et al. [25] | UK |
Randomized cross-over Single centre |
10 chronic HD |
Post-HDF Qs ∼18 L 219 min: FX100; Solacea™ 21H |
LMWH 2000 IU |
+ Visual inspection + TF, VIIIc, TAT, fibrinogen and D-dimer + PF4, µparticles, P selectin, CD40 + E selectin, cVCAM-1 and sICAM-1 |
+ No macroscopic clotting in dialyser headers + clotting in <10% venous chambers + no TAT increase + no difference between membranes |
| Knehtl et al. [26] | Slovenia |
Non-randomized cross-over Single centre |
22 chronic HD | Synthetic: HF HD; post-HDF | LMWH ∼ BW |
+ PFA + Hb, Plts, Hct and RBC |
HD < post-HDF → Plts count and function less favourable in post-HDF |
| Krummel et al. [27] | France |
Randomized Prospective Single centre |
155 chronic HD and AKI + high bleeding risk | FX100: HD; pre-HDF Qs50 | Zero |
+ Premature stop + D-dimer |
HD < pre-HDF → more premature termination with pre-HDF |
| Fazendeiro Matos et al. [28] | Portugal |
Retrospective Multicentre |
2829 chronic HD |
FX600 Cordiax HDF Qs ∼24 L |
UFH 40–50 IU/kg BW + 10 IU/kg/h |
+ Visual inspection + spKt/V + substitution volume |
Visual inspection ∼ UFH |
Studies were selected based on the following search criteria within PubMed: dilution AND coagulation AND dialysis, dilution AND clotting AND dialysis, HDF AND coagulation. Studies were selected based on title and abstract when they reported results of clinical studies comparing different conditions of dilution during dialysis, regardless of design and publication year.
AKI, acute kidney injury; aPTT, activated partial thromboplastin time; aXa, anti-factor Xa activity; BTG, β-thromboglobulin; BW, body weight; CVVH, continuous veno-venous hemofiltration; CVVHDF, continuous venovenous haemodiafiltration; CD62p, Plt surface marker; Crea, serum creatinine; Hct, haematocrit; HF: high flux; ICU, intensive care unit; NR, not reported; PES, polyethersulfone; PFA, Plt function analyser; PF4, Plt factor 4; Qs, substitution flow (mL/min); RBC, red blood cell count; sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, vascular cell adhesion molecule 1; TAT, human thrombin–anti-thrombin III complex; TF, tissue factor; UFH, unfractionated heparin.
The theoretical rationale to perform pre-HDF to avoid clotting is the belief that the infusion is rinsing the membrane continuously, avoiding adhesion of cells and proteins involved in blood coagulation [29]. Besides the already described hypercoagulability of haemodilution as with saline flushes [30, 31], the enhanced convective flux through the membrane with pre-HDF might also promote coagulation by the increased protein and cell adhesion on the membrane. Furthermore, pre-dilution also increases blood flow in the fibre and, with it, wall shear stress [27, 32]. Higher shear implies more diffusion from Plts towards the membrane, where they preferentially bind to von Willebrand factor (vWF) when the wall shear rate exceeds 630/s [33]. Further increasing shear rate can even make the vWF change from a lobular shape into a string, increasing tremendously the number of Plt binding sites [32]. In the Solacea™ dialyser, containing 12 087 fibres of 200 µm diameter, the threshold of 630/s wall shear rate is already surpassed at a blood flow of 360 mL/min onwards in HD mode; when the number of fibres is, however, decreased due to clotting to a number ˂10 105, this already happens at a blood flow rate of 300 mL/min in standard HD. As shear stress is further enhanced during haemofiltration, it can be postulated that current dialyser designs and dialysis protocols favour activation of coagulation by this pathway, and even more in pre-HDF, where filtration rates are highest. This mechanism might also explain why coagulation during dialysis is a non-linear phenomenon, but rather follows an exponential pattern.
To avoid haemodilution and high shear rates, also post-HDF has been investigated as a potential alternative, but several studies concluded that this strategy also resulted in more clotting problems than standard HD [19, 20, 23, 26] (Table 4). In this study, however, only anticoagulation dose but not HDF versus HD influenced the number of clotted fibres.
The small patient number (n = 10) could be considered a limitation of the study, but this allowed us to have each patient as his/her own control over the six experimental regimens. Furthermore, a post hoc power calculation revealed a power of 0.877 (one-way analysis of variance (ANOVA), Power and Sample Size, SAS Inc., Cary, NC, USA).
Our study has several strengths. First, we were using the same type of dialyser in all three dialysis strategies, and the same amount of coagulant (either one-quarter of the normal dose or zero), administered always in an identical manner (i.e. single bolus at the dialysis start). This allowed us to make a direct comparison among dilution strategies. It is likely that other studies show seemingly contradictory results as they used different combinations of dialysers, anticoagulation strategies and degrees of dilution in different arms, making direct analysis of the singled out effect of dilution cumbersome (Table 4). Secondly, while all studies summarised in Table 4 were making conclusions about coagulation based on simple visual inspection, premature termination of the session and/or unspecific coagulation parameters, we used a very sensitive technique to measure coagulation based on the objective counting of the number of blocked fibres [7].
In conclusion, the Solacea™ membrane performs very well even in conditions where systemic anticoagulation is prohibited and thus no single anticoagulant can be applied. We did not find evidence to support that pre-dilution has a beneficial impact on coagulation in such a setting.
ACKNOWLEDGEMENTS
The authors are indebted to Sofie Vermeiren for her assistance in the fibre counting process, and to the dialysis nurses, Kelly Rokegem, Sabien Inion, Elsie De Man and Isabelle Dewettinck, for their help during the clinical study.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1.Wamsiedler R, Polaschegg HD, Tattersall JE. Heparin-free dialysis with an on-line hemodiafiltration system. Artif Organs 2008; 17: 948–950 [DOI] [PubMed] [Google Scholar]
- 2.Laville M, Dorval M, Fort Ros J et al. Results of the HepZero study comparing heparin-grafted membrane and standard care show that heparin-grafted dialyzer is safe and easy to use for heparin-free dialysis. Kidney Int 2014; 86: 1260–1267 [DOI] [PubMed] [Google Scholar]
- 3.Klingel R, Schaefer M, Schwarting A et al. Comparative analysis of procoagulatory activity of haemodialysis, haemofiltration and haemodiafiltration with a polysulfone membrane (APS) and with different modes of enoxaparin anticoagulation. Nephrol Dial Transplant 2004; 19: 164–170 [DOI] [PubMed] [Google Scholar]
- 4.Sagedal S, Hartmann A, Osnes K et al. Intermittent saline flushes during haemodialysis do not alleviate coagulation and clot formation in stable patients receiving reduced doses of dalteparin. Nephrol Dial Transplant 2006; 21: 444–449 [DOI] [PubMed] [Google Scholar]
- 5.Sunohara T, Masuda T. Fundamental characteristics of the newly developed ATA membrane dialyzer. Contrib Nephrol 2017; 189: 215–221 [DOI] [PubMed] [Google Scholar]
- 6.Vanommeslaeghe F, De Somer F, Josipovic I et al. Evaluation with micro-CT of different anticoagulation strategies during hemodialysis in patients with thrombocytopenia: a randomized crossover study. Artif Organs 2019; 43: 756–763 [DOI] [PubMed] [Google Scholar]
- 7.Vanommeslaeghe F, Van Biesen W, Dierick M et al. Micro-computed tomography for the quantification of blocked fibers in hemodialyzers. Sci Rep 2018; 8: 2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Sanctis LB, Stefoni S, Cianciolo G et al. Effect of different dialysis membranes on platelet function. A tool for biocompatibility evaluation. Int J Artif Organs 1996; 19: 404–410 [PubMed] [Google Scholar]
- 9.Apsner R, Buchmayer H, Gruber D et al. Citrate for long-term hemodialysis: prospective study of 1,009 consecutive high-flux treatments in 59 patients. Am J Kidney Dis 2005; 45: 557–564 [DOI] [PubMed] [Google Scholar]
- 10.Evenepoel P, Dejagere T, Verhamme P et al. Heparin-coated polyacrylonitrile membrane versus regional citrate anticoagulation: a prospective randomized study of 2 anticoagulation strategies in patients at risk of bleeding. Am J Kidney Dis 2007; 49: 642–649 [DOI] [PubMed] [Google Scholar]
- 11.Francois K, Wissing KM, Jacobs R et al. Avoidance of systemic anticoagulation during intermittent haemodialysis with heparin-grafted polyacrilonitrile membrane and citrate-enriched dialysate: a retrospective cohort study. BMC Nephrol 2014; 15: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chanard J, Lavaud S, Paris B et al. Assessment of heparin binding to the AN69 ST hemodialysis membrane: I. Preclinical studies. ASAIO J 2005; 51: 342–347 [DOI] [PubMed] [Google Scholar]
- 13.Lavaud S, Paris B, Maheut H et al. Assessment of the heparin-binding AN69 ST hemodialysis membrane: II. Clinical studies without heparin administration. ASAIO J 2005; 51: 348–351 [DOI] [PubMed] [Google Scholar]
- 14.Islam MS, Hassan ZA, Chalmin F et al. Vitamin E-coated and heparin-coated dialyzer membranes for heparin-free hemodialysis: a multicenter, randomized, crossover trial. Am J Kidney Dis 2016; 68: 752–762 [DOI] [PubMed] [Google Scholar]
- 15.Vanommeslaeghe F, De Somer F, Josipovic I et al. Evaluation of different dialyzers and the impact of predialysis albumin priming in intermittent hemodialysis with reduced anticoagulation. Kidney Int Rep 2019; 4: 1538–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agresti J, Conroy JD, Olshan A et al. Heparin-free hemodialysis with Cuprophan hollow fiber dialyzers by a frequent saline flush, high blood flow technique. Trans Am Soc Artif Intern Organs 1985; 31: 590–594 [PubMed] [Google Scholar]
- 17.Schwab SJ, Onorato JJ, Sharar LR et al. Hemodialysis without anticoagulation. One-year prospective trial in hospitalized patients at risk for bleeding. Am J Med 1987; 83: 405–410 [DOI] [PubMed] [Google Scholar]
- 18.Davies HT, Leslie G, Pereira SM et al. A randomized comparative crossover study to assess the affect on circuit life of varying pre-dilution volume associated with CVVH and CVVHDF. Int J Artif Organs 2008; 31: 221–227 [DOI] [PubMed] [Google Scholar]
- 19.Gritters-van den Oever M, Grooteman MP, Bartels PC et al. Post-dilution haemodiafiltration and low-flux haemodialysis have dissimilar effects on platelets: a side study of CONTRAST. Nephrol Dial Transplant 2009; 24: 3461–3468 [DOI] [PubMed] [Google Scholar]
- 20.Stefansson BV, Abramson M, Nilsson U et al. Hemodiafiltration improves plasma 25-hepcidin levels: a prospective, randomized, blinded, cross-over study comparing hemodialysis and hemodiafiltration. Nephron Extra 2012; 2: 55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masakane I, Esashi S, Igarashi H. Biocompatibility of predilution on-line hemodiafiltration. Blood Purif 2013; 35: 34–38 [DOI] [PubMed] [Google Scholar]
- 22.Frasca GM, Sagripanti S, D’Arezzo M et al. Post-dilution hemodiafiltration with a heparin-grafted polyacrylonitrile membrane. Ther Apher Dial 2015; 19: 154–161 [DOI] [PubMed] [Google Scholar]
- 23.Smith JR, Zimmer N, Bell E et al. A randomized, single-blind, crossover trial of recovery time in high-flux hemodialysis and hemodiafiltration. Am J Kidney Dis 2017; 69: 762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunot V, Serre JE, Mourad G et al. Heparin-free renal replacement therapy for chronic hemodialyzed patients at high risk for bleeding: a comparison of on-line predilution hemodiafiltration with conventional hemodialysis. Hemodial Int 2018; 22: 463–473 [DOI] [PubMed] [Google Scholar]
- 25.Tangvoraphonkchai K, Riddell A, Davenport A. Platelet activation and clotting cascade activation by dialyzers designed for high volume online hemodiafiltration. Hemodial Int 2018; 22: 192–200 [DOI] [PubMed] [Google Scholar]
- 26.Knehtl M, Jakopin E, Dvorsak B et al. The effect of high-flux hemodialysis and post-dilution hemodiafiltration on platelet closure time in patients with end stage renal disease. Hemodial Int 2019; 23: 319–324 [DOI] [PubMed] [Google Scholar]
- 27.Krummel T, Cellot E, Thiery A et al. Hemodialysis without anticoagulation: less clotting in conventional hemodialysis than in predilution hemodiafiltration. Hemodial Int 2019; 23: 426–432 [DOI] [PubMed] [Google Scholar]
- 28.Fazendeiro Matos J, Pinto B, Felix C et al. Does subjective assessment of dialyzer appearance reflect dialyzer performance in online hemodiafiltration? Hemodial Int 2020; 24: 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucchi L, Ligabue G, Marietta M et al. Activation of coagulation during hemodialysis: effect of blood lines alone and whole extracorporeal circuit. Artif Organs 2006; 30: 106–110 [DOI] [PubMed] [Google Scholar]
- 30.Ng KF, Lam CC, Chan LC. In vivo effect of haemodilution with saline on coagulation: a randomized controlled trial. Br J Anaesth 2002; 88: 475–480 [DOI] [PubMed] [Google Scholar]
- 31.Ruttmann TG, James MF, Aronson I. In vivo investigation into the effects of haemodilution with hydroxyethyl starch (200/0.5) and normal saline on coagulation. Br J Anaesth 1998; 80: 612–616 [DOI] [PubMed] [Google Scholar]
- 32.Casa LD, Deaton DH, Ku DN. Role of high shear rate in thrombosis. J Vasc Surg 2015; 61: 1068–1080 [DOI] [PubMed] [Google Scholar]
- 33.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 1996; 84: 289–297 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.