Key Points.
Paediatric multisystem inflammatory syndrome in children has been on the rise in the second wave of the pandemic, and varied presentations posing diagnostic and treatment challenges are not surprising.
There is limited knowledge and understanding of the pathogenesis, clinical spectrum and therapeutic options in managing neonatal cases.
The disease is only evolving. Diagnostic criteria specific to neonates and more clinical trials with neonatal cohort as participants in the future might resolve these issues.
SARS‐CoV‐2 infection in children accounts for about 1–8% of cases world‐wide, most of them asymptomatic or mildly symptomatic. Neonatal infection is rare and usually asymptomatic. Since April 2020, severe manifestations were seen in children in Europe and North America, presenting as Kawasaki disease‐like illness involving multiple organs. The Centers for Disease Control and Prevention termed this multisystem inflammatory syndrome in children (MIS‐C) and developed a case definition. The World Health Organization developed a similar case definition with slight modifications. The appropriateness of this definition for neonatal scenarios is debatable. Anecdotal reports reveal that the second wave of SARS‐CoV‐2 in the Indian context has affected neonates with more severity and a wide spectrum of presentations. Neurological manifestations presenting as seizures and encephalopathy, cardiac manifestations with shock, coronary artery dilatation, arrhythmias, disseminated intravascular coagulation, renal problems and death are seen. 1 , 2 , 3 , 4
We report a case of SARS‐CoV‐2 infection in a 7‐day‐old term neonate with possible MIS‐C, presenting with features of encephalitis.
Case Report
A 7‐day‐old term newborn with normal antenatal and perinatal history presented with poor feeding and reduced activity for 1 day. The baby was exclusively breastfed. The mother tested positive for SARS‐CoV‐2 infection one day before the presentation. The baby was seen in the emergency department by a neonatal doctor. On examination, the baby was haemodynamically stable. Neurological examination revealed reduced tone, sluggish reflexes including Moro, absent suck and no bulging of the anterior fontanelle. The baby was admitted with suspected SARS‐CoV‐2 infection/late‐onset sepsis, and antibiotics (piperacillin‐tazobactam and amikacin) were initiated. One hour post‐admission, the baby developed seizures with arching, posturing, and up‐rolling of the eyes. Levetiracetam was given as a loading dose at 30 mg/kg. Other supportive treatment was given. Initial investigations showed negative septic screen, normal cerebrospinal fluid analysis, elevated D‐dimer levels (890 ng/mL), elevated serum ferritin levels (623 ng/mL) and normal procalcitonin levels. Nasopharyngeal and throat swab samples tested positive for SARS‐CoV‐2, and cerebrospinal fluid polymerase chain reaction test was negative for other neurotropic viruses (Table 1).
Table 1.
Cerebrospinal fluid sample – polymerase chain reaction result for neurotropic virus
| SI.No | Virus tested | Result |
|---|---|---|
| 1 | Cytomegalovirus | Not detected |
| 2 | Herpes simplex virus 1 and 2 | Not detected |
| 3 | Varicella zoster | Not detected |
| 4 | Epstein–Bar virus | Not detected |
| 5 | Human herpes virus 6 and 7 | Not detected |
| 6 | Human adenovirus | Not detected |
| 7 | Human parvovirus B19 | Not detected |
| 8 | Human parechovirus | Not detected |
| 9 | Human enterovirus | Not detected |
| 10 | Mumps virus | Not detected |
At 24 h of admission, the baby had a fever of 38.3 C, seizures became persistent, a maximum dose of levetiracetam was given and phenobarbitone was added as a second medication to control seizures. The baby was intubated and put on ventilator support for a brief period in view of apnoea. Antiepileptic drug dosages were optimised to control seizures. Chest radiograph was normal.
Magnetic resonance imaging of the brain with contrast done on day 3 of illness showed diffuse changes involving the periventricular white matter, external capsule and internal capsule; peripheral bilateral thalami show T2 fluid‐attenuated inversion recovery hyperintensity with diffusion restriction (Fig. 1).
Fig 1.
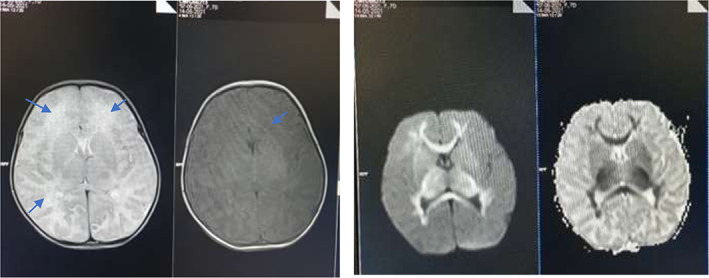
Magnetic resonance imaging findings on day 3 of illness. (a) T2W images reveal mild hyperintensity in corresponding white matter thalami, with subtle and patchy restricted diffusivity T1 images demonstrating altered signals in periventricular white matter (arrows). Myelination is seen as appropriate for age. (b) Apparent diffusion coefficient (ADC) and Diffusion‐weighted imaging (DWI) reveal extensive restricted diffusion in periventricular and deep white matter, corpus callosum, internal capsule and along the pyramidal tracts.
Dexamethasone 0.15 mg/kg/dose q12h along with remdesivir 5 mg/kg/dose loading were commenced after discussion with a paediatric neurologist. Seizures were controlled with levetiracetam and phenobarbitone.
On day 7 of illness, D‐dimer and C‐reactive protein (CRP) showed marked elevation of 5000 ng/mL and 60 mg/dL, respectively. Antibodies to SARS‐CoV‐2 (IgG/IgM) were negative. Two‐dimensional echocardiogram showed a small coronary artery aneurysm (Z‐score 3.5), and the biventricular function was preserved. Diagnosis of MIS‐C was strongly considered. Intravenous (IV) methylprednisolone 10 mg/kg/day with IV immunoglobulin 2 g/kg over 24 h and enoxaparin 1 mg/kg twice daily were given. Electroencephalogram on day 7 of illness had low‐voltage activity background and no electrographic seizures. The steroid was tapered and stopped over the next 10 days. Remdesivir was given (2.5 mg/kg/day) for a total of 5 days. Enoxaparin was continued at 1 mg/kg/day q12h. Seizures were controlled with two antiepileptic drugs; after 72 h of no seizures, phenobarbitone was stopped. Oral levetiracetam was continued.
Outcome
The baby had no further seizures, neurological examination showed normal tone and good suck and Moro reflexes; the baby was breastfeeding well. D‐dimer and CRP normalised before discharge. The baby was discharged on enoxaparin as prophylaxis for a coronary artery aneurysm. Two‐dimensional echocardiogram after 2 weeks showed normalisation of coronary artery size, hence enoxaparin was stopped.
Discussion
Paediatric SARS‐CoV‐2 infection is on the rise in the second wave of this pandemic infection, more so in the Indian subcontinent. 2 Paediatric MIS‐C has been reported across the world after the first reports from the UK in late April 2020. Various diagnostic criteria 5 have been postulated by the World Health Organization, Centers for Disease Control and the Royal College of Paediatrics and Child Health. MIS‐C mainly occurs in children older than classic Kawaski disease; whether the criteria for diagnosis can be applied to neonates is questionable. Fever may be a less reliable diagnostic criterion in neonates, particularly those born preterm. Our patient had neurological symptoms including seizures and cardiac involvement with dilated coronary arteries. Inflammatory markers were raised as expected in MIS‐C including CRP, D‐dimer and lactate dehydrogenase, with negative procalcitonin. However, these raised markers can overlap in babies who are sick and septic. 6 Our patient was positive for SARS‐CoV‐2 by PCR but had no detectable anti‐SARS‐CoV‐2 antibodies. One postulated mechanism of MIS‐C in children is that anti‐SARS‐CoV‐2 antibodies (IgG) bind to neutrophil and macrophage receptors causing activation and secretion of cytokines. In neonates whose mother was infected with SARS‐CoV‐2 and produced IgG antibodies, the antibodies could cross the placenta and cause MIS in the neonatal period. However, neonates with no detectable SARS‐CoV‐2 IgG antibody have been reported with features of MIS‐C, 3 as in our case. The diagnostic criteria do not mandate the need for anti‐SARS‐CoV‐2 antibodies. 5 Neurological presentation occurs in about 10% of neonatal cases. 3 The clinical presentation can be varied and novel as reported in case studies. 3 , 4 Our patient's echocardiogram showed a small aneurysm (Z‐score 3.5), but ventricular function was preserved, whereas ventricular function is depressed in most cases of MIS‐C. Z‐score of 2 to <2.5 is considered to be dilated and ≥2.5 to <5 is considered to be a small aneurysm. Z‐scores for coronary artery measurements and interpretation using nomograms in smaller babies again pose a big challenge. 7 , 8 Although the typical features were conspicuously absent in our patient, a high index of suspicion and supportive clinical features caused us to initiate specific treatment to treat MIS‐C. The diagnosis of neonatal MIS‐C is supported by the clinical presentation, SARS‐CoV‐2 PCR positivity, cardiac imaging, and the clinical response to steroids and immunoglobulin.
The management of neonates with possible MIS‐C is evolving. No clinical trials have yet included neonates. Clinical guidelines are mostly based on expert advice based on case reports/series. 9 , 10 Their applicability to small infants and preterm babies is debatable.
While still controversial, we believe there is accumulating evidence including our case, that neonates with SARS‐CoV‐2 infection can develop MIS. A high index of clinical suspicion is advisable, particularly when the mother has proven or suspected SARS‐CoV‐2 infection. Due to limited evidence on the applicability of current diagnostic criteria, clinicians may face clinical dilemmas regarding possible neonatal MIS. Available treatments such as IV immunoglobulin, steroids and/or heparin should be used judiciously.
Acknowledgements
We thank the parents of the infant who gave consent to publish the case report. We thank all nurses of Chinmay Mission Hospital whose constant and dedicated care helped the baby to recover.
Shivshankar Diggikar and Ranjith Nanjegowda contributed equally to this study.
Conflict of interest: None declared.
References
- 1. Jiang L, Tang K, Levin M et al. COVID‐19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020; 20: e276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dhanalakshmi K, Venkataraman A, Balasubramanian S et al. Epidemiological and clinical profile of pediatric inflammatory multisystem syndrome — Temporally associated with SARS‐CoV‐2 (PIMS‐TS) in Indian children. Indian Pediatr. 2020; 57: 1010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pawar R, Gavade V, Patil N et al. Neonatal multisystem inflammatory syndrome (MIS‐N) associated with prenatal maternal SARS‐CoV‐2: A case series. Children 2021; 8: 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kappanayil M, Balan S, Alawani S et al. Multisystem inflammatory syndrome in a neonate, temporally associated with prenatal exposure to SARS‐CoV‐2: A case report. Lancet Child Adolesc. Health. 2021; 5: 304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Multisystem Inflammatory Syndrome in Children and Adolescents with COVID‐19: Scientific Brief, 15 May 2020. Geneva: WHO; 2020. Available from: https://apps.who.int/iris/handle/10665/332095 [accessed 1 July 2021].
- 6. Iroh Tam P, Bendel C. Diagnostics for neonatal sepsis: Current approaches and future directions. Pediatr. Res. 2017; 82: 574–83. [DOI] [PubMed] [Google Scholar]
- 7. McCrindle BW, Rowley AH, Newburger JW et al. Diagnosis, treatment, and long‐term management of kawasaki disease: a scientific statement for health professionals from the american heart association. Circulation. 2017; 135: e927–e999. 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 8. Manlhiot C, Millar K, Golding F, McCrindle B. Improved classification of coronary artery abnormalities based only on coronary artery z‐scores after Kawasaki disease. Pediatr. Cardiol. 2009; 31: 242–9. [DOI] [PubMed] [Google Scholar]
- 9. Henderson L, Canna S, Friedman K et al. American College of Rheumatology Clinical Guidance for multisystem inflammatory syndrome in children associated with SARS–CoV‐2 and hyperinflammation in pediatric COVID‐19: Version 2. Arthritis Rheumatol. 2021; 73: e13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harwood R, Allin B, Jones CE, Whittaker E, Ramnarayan P, Ramanan A. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID‐19 (PIMS‐TS): Results of a national Delphi process. Lancet 2020; 5: 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


