Conflict of interest
None to declare.
Dear Editor,
The recent approval of highly effective prophylactic vaccines against COVID‐19 is a monumental step in the global fight against the ongoing SARS‐CoV‐2 pandemic. Two types of SARS‐CoV‐2 vaccines are currently used, messenger‐RNA (mRNA) vaccines and recombinant adenoviral (AdV) vector vaccines. 1 Both of them encode the production of the SARS‐CoV‐2 spike protein, which is the primary target for neutralizing antibodies. We report a case of subacute cutaneous lupus erythematosus (SCLE) that transitioned into systemic lupus erythematosus (SLE) following AdV‐vaccination with AZD1222.
A 62‐year‐old woman presented with a generalized morbilliform exanthema and new onset of fatigue and musculoskeletal pain (Fig. 1). Six months before the first visit to our department, the patient had experienced erythematosquamous papules and plaques symmetrically located in the sun‐exposed areas (chest, upper back, lower arms, and dorsal hands). Laboratory investigations found a normal blood cell count and serum chemistry, but increased titres for antinuclear antibodies (1 : 320; normal <1 : 160) and positivity for anti‐Ro/SSA(60) antibodies. All other extractable nuclear antigens were unremarkable. Further work up including chest X‐ray, abdominal ultrasound, and heart echography was unremarkable. The patient felt otherwise healthy, and there were no trigger factors such as cigarette smoking, infections, drug intake or ultraviolet‐light exposure. Based on these findings, a diagnosis of SCLE was made by her rheumatologist, and treatment with hydroxychloroquine (200 mg twice daily) was started, resulting in a significant improvement of skin lesions (leaving mild erythema at the upper back and dorsal hands).
Figure 1.
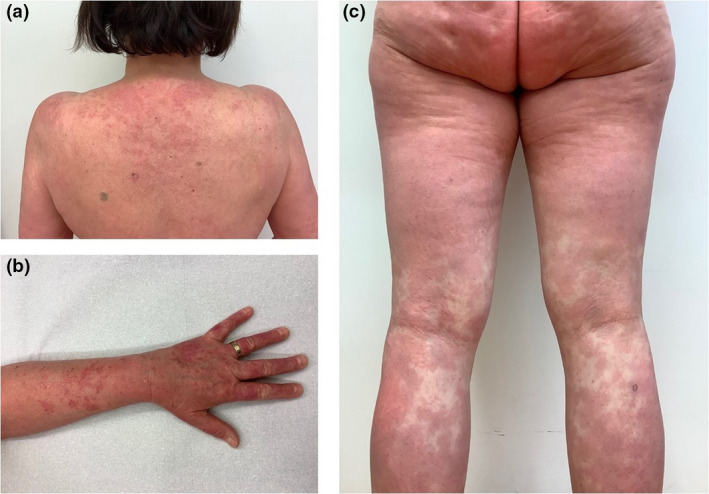
Clinical findings of the patient at the first presentation in our department. (a, b) Erythematous papules and plaques located at the upper back, lower arms and dorsal hands, characteristic for subacute cutaneous lupus erythematosus. (c) Concomitant widespread erythematous confluent macules on the buttocks and legs, characteristic for generalized acute cutaneous lupus erythematosus.
On 15 March 2021, the patient received the first dose of the anti‐COVID‐19 AdV‐vaccine AZD1222. There were no systemic or local side effects during the first days after vaccination. However, erythematous confluent macules spread out symmetrically over the entire body 10 days later, concomitantly with malaise, fatigue, and acute pain in multiple muscles and joints (Fig 1). The patient was, therefore, admitted to our hospital. A skin biopsy taken from the left lower leg revealed typical features of cutaneous lupus erythematosus (CLE; vacuolar interface dermatitis, dense dermal lymphocytic infiltrates, and strong mucin deposition) as well as a positive lupus band test (linear immunoglobulin class‐G deposits at the dermoepidermal junction; Fig. 2). Laboratory investigations at this time revealed increased anti‐double‐stranded DNA antibody levels, leukocytopenia (3.2 × 109/L, normal 4.4–11.0 × 109/L) and C3/C4‐hypocomplementemia (C3: 48 mg/dL, normal 90–170 mg/dL; C4: 3, normal 18–49 mg/dL). These findings indicate a transition of SCLE into SLE, fulfilling the current SLE classification criteria. 2 Under tapered glucocorticosteroid therapy with prednisolone 250 mg/day, the skin lesions and systemic symptoms significantly improved within 7 days.
Figure 2.
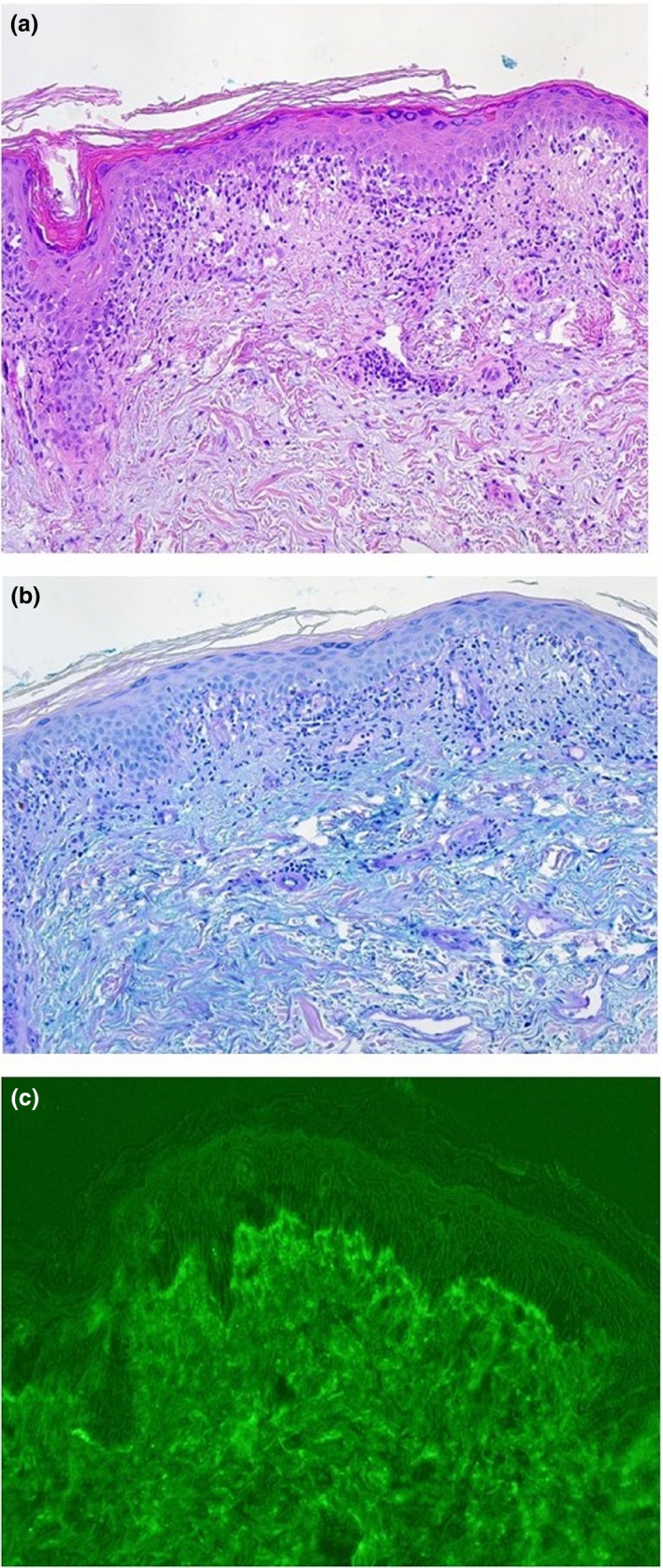
(a) Vacuolar interface dermatitis and dense dermal lymphocytic infiltrates, 4 mm punch biopsy taken from the left lower leg. (haematoxylin and eosin, original magnification ×100). (b) Strong dermal mucine deposition (alcian blue staining, original magnification x 100). (c) Linear immunoglobulin class‐G deposits at the dermoepidermal junction (called positive lupus band) from non‐UV‐exposed skin (lower legs).
So far, there is limited knowledge about the safety and efficacy of the COVID‐19 vaccines in patients with autoimmune rheumatic diseases, including cutaneous LE or SLE. 3 Nevertheless, a strong agreement exists that lupus patients should get vaccinated and might be prioritised before the general population. 3 , 4 Although rare, flares of SLE, new‐onset SLE or lupus‐like syndromes have been described following application of several vaccines, for example hepatitis‐B, HPV or influenza. 5 , 6 , 7 Interestingly, besides the production of high spike‐protein levels, both mRNA and AdV SARS‐CoV‐2‐vaccines trigger innate sensors by intrinsic adjuvant activity, resulting in the production of type I interferon (IFN) and multiple pro‐inflammatory cytokines. 1 Dysregulation of the type 1 IFN pathways plays a critical role in SLE. Moreover, type I IFN‐related genes are highly upregulated in CLE and correlate with disease activity. 8 , 9 Facing the close temporal relationship and absence of other potential trigger factors we speculate that AdV‐vaccination with AZD1222 might have caused the shift of SCLE into SLE in our patient. Interestingly, a case of Rowell’s syndrome, a particular subtype of SCLE, was recently reported after application of the mRNA‐vaccine BNT162b2, indicating that both types of SARS‐CoV‐2‐vaccines might induce or aggravate lupus‐like conditions. 10 Physicians treating patients with autoimmune rheumatic diseases should also consider SARS‐CoV‐2‐vaccination as a potential cause of unexplained disease deterioration.
Acknowledgement
Anke Bartröver, Tanja Kleinecke, and Stefanie Fittkau provided excellent technical assistance. The patient in this manuscript has given written informed consent to the publication of case details.
References
- 1. Teijaro JR, Farber DL. COVID‐19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 2021; 21: 195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aringer M, Costenbader K, Daikh D et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 3. Tang W, Askanase AD, Khalili L, Merrill JT. SARS‐CoV‐2 vaccines in patients with SLE. Lupus Sci Med. 2021; 8: e000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American College of Rheumatology (ACR) COVID‐19 Vaccine Clinical Guidance Task Force . COVID‐19 vaccine clinical guidance summary for patients with rheumatic and musculoskeletal diseases, 2021. URL https://www.rheumatology.org/Portals/0/Files/COVID‐19‐Vaccine‐Clinical‐Guidance‐Rheumatic‐Diseases‐Summary (last accessed: 9 June 2021).
- 5. Crowe SR, Merrill JT, Vista ES et al. Influenza vaccination responses in human systemic lupus erythematosus: impact of clinical and demographic features. Arthritis Rheum 2011; 63: 2396–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agmon‐Levin N, Zafrir Y, Paz Z et al. Ten cases of systemic lupus erythematosus related to hepatitis B vaccine. Lupus 2009; 18: 1192–1197. [DOI] [PubMed] [Google Scholar]
- 7. Geier DA, Geier MR. Quadrivalent human papillomavirus vaccine and autoimmune adverse events: a case‐control assessment of the vaccine adverse event reporting system (VAERS) database. Immunol Res 2017; 65: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wenzel J, Zahn S, Mikus S et al. The expression pattern of interferon‐inducible proteins reflects the characteristic histological distribution of infiltrating immune cells in different cutaneous lupus erythematosus subsets. Br J Dermatol 2007; 157: 752–727. [DOI] [PubMed] [Google Scholar]
- 9. Patel J, Borucki R, Werth VP. An update on the pathogenesis of cutaneous lupus erythematosus and its role in clinical practice. Curr Rheumatol Rep 2020; 22: 69. [DOI] [PubMed] [Google Scholar]
- 10. Gambichler T, Scholl L, Dickel H, Ocker L, Stranzenbach R. Prompt onset of Rowell's syndrome following the first BNT162b2 SARS‐CoV‐2 vaccination. J Eur Acad Dermatol Venereol 2021; 35: e415–e416. 10.1111/jdv.17225 [DOI] [PMC free article] [PubMed] [Google Scholar]


