Abstract
Several cardiovascular diseases and arrhythmic disorders have been described in COVID‐19 era as likely related to SARS‐CoV‐2 infection. The prognostic relevance of bradyarrhythmias during the infection has not been yet described and no data are available about long‐term heart conduction disorders. A review of literature concerning the association between hypokinetic arrhythmias and COVID‐19 from January 2020 to February 2021 was performed. The key‐words used for the research were: “sinus node disfunction,” “sick sinus syndrome (SSS),” “sino‐atrial block,” “atrio‐ventricular block (AVB),” “bradyarrhythmias,” and “COVID‐19″ or ”SARS‐CoV‐2.″ Excluding “relative bradycardia,” a total of 38 cases of bradyarrhythmia related to SARS‐CoV‐2 infection have been described, even in very young people, requiring in many cases a definitive pacemaker implantation. Furthermore, we report a case of non‐hospitalized 47‐years old man with a SSS developed as a consequence of mild SARS‐CoV‐2 infection. While in all described cases heart conduction disorders were found at presentation of the infection or during hospitalization for COVID‐19, in our case the diagnosis of SSS was made after the resolution of the infection. Although rarely, heart conduction disorders may occur during COVID‐19 and the present case highlights that a cardiological follow up may be desirable even after the resolution of infection, especially in the presence of symptoms suggesting a possible heart involvement.
Keywords: atrio‐ventricular block, heart conduction disorders, SARS‐CoV‐2, sick sinus syndrome, sinus node dysfunction
1. INTRODUCTION
Several cardiovascular diseases such as myocardial infarction, myocarditis, pulmonary embolism, vasculitis have been described in the Coronavirus Disease 2019 (COVID‐19) era, probably related to the infection caused by Severe Acute Respiratory Syndrome Corona Virus 2 (SARS‐CoV‐2).1, 2
Among the mechanisms of cardiovascular damage, cardiac inflammation as well as the pro‐thrombotic status and the high level of oxidative stress have been proposed,3, 4 mainly as a consequence of SARS‐CoV‐2 binding to Angiotensin‐Converting‐Enzime‐2 (ACE‐2) receptor and the excess of cytokines production, the so called “cytokine storm.” This binding leads to an increased concentration of angiotensin II which exerts inflammatory and pro‐thrombotic actions.2, 5 Furthermore, cardiac cell death might also be due to virus replication as well as to the increase of pro‐inflammatory cytokines, fever, medications and hypoxemia.6 The presence of a certain degree of sustained inflammation could also have consequences on cardiac function even after the resolution of the infection, as recently observed.7 Although older age, male sex, thrombotic events, and hypoalbuminemia are well‐known risk factors for unfavorable outcomes during the course of SARS‐CoV‐2 infection, recently other arrhythmic disorders, including atrial fibrillation, have been recognized as additional markers of poor prognosis.8, 9 Ventricular arrhythmias have also been reported; however often related to drug‐induced lengthening of the QT interval. In particular, hydroxychloroquine, which has been widely used in the first wave of the ongoing pandemic, has been reported to cause arrhythmic storms.10 Less commonly, hypokinetic arrhythmias such as sick sinus syndrome (SSS), atrio‐ventricular block (AVB) and bundle branch block (BBB) have been described.11, 12, 13 However, their role as potential prognostic markers has not been defined yet, suggesting the need of additional investigations.14 We performed a review of updated literature regarding the association between hypokinetic arrhythmias and SARS‐CoV‐2 infection and also we report the case of a SSS in a previously healthy young man developing after the resolution of a mild SARS‐CoV‐2 infection.
2. METHODS
A review of current literature from January 2020 to February 2021 was performed. The studies were identified by searching electronic databases such as Pubmed and ScienceDirect. The key‐words used for the research were: “sinus node disfunction,” “SSS,” “sino‐atrial block,” “AVB,” “bradyarrhythmias,” and “COVID‐19″ or ”SARS‐CoV‐2.″ Only publications written in English and Italian languages were included in the literature research. Reference lists of all included studies were screened for potential additional studies. Data collected from each clinical case were: age, gender, respiratory failure at presentation or during hospitalization, time of worsening from clinical presentation, C‐reactive protein (CRP) value, need for endotracheal intubation, type of conduction disorder and its reversibility, comorbidity, heart failure, need of permanent pacemaker, in‐hospital follow‐up. Results were expressed as median (range) or as mean (± standard deviation) for continuous variables, as appropriate, whereas for categorical variables number and percentages were used. In Table 1 are summarized all cases reported in scientific literature. In addition, we report a clinical case which focuses on the possibility of bradyarrhythmia onset early after mild infection of SARS‐CoV‐2.
TABLE 1.
Cases of heart block related to COVID‐19 reported in literature
| Case # | Age | Sex | Com. | Hosp. | C‐RP (mg/dL) | RF | Day of clinical worsening (from first symptoms) | Endotracheal Intubation | Bradyarrhythmia | Day of bradyarrhythmia's presentation (from hospitalization) | BBB | AV interval (ms) | QRS interval (ms) | QRS morphology | Transience | HF | Pacemaker implantation | Exitus | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | M | No | Yes | 1.2 | Yes | 5 | No | 3° AVB | n.a. | No | n.a. | 110 | n.a. | Yes | No | No | No | 15 | |
| 2 | 58 | M | No | Yes | n.a. | No | 9 | No | 2:1 AVB | 1 | Yes | n.a. | 130 | LBBB | No | Yes | Yes | No | 16 | |
| 3 | 10 | M | No | Yes | 22 | No | 7 | No | 3° AVB | 3 | No | n.a. | 110 | n.a. | Yes | Yes | No | No | 17 | |
| 4 | 54 | M | No | Yes | n.a. | Yes | 13 | No | 3° AVB | 14 | Yes | n.a. | 130 | LBBB | Yes | No | No | No | 18 | |
| 5 | 82 | M | S. | Yes | 12 | Yes | 4 | Yes | 3° AVB | 1 | No | n.a. | 110 | n.a. | No | No | No | Yes | 19 | |
| 6 | 55 | M | No | Yes | 22 | Yes | 6 | Yes | 2° type 2 AVB | 6 | Yes | n.a. | 130 | RBBB | Yes | No | No | No | 19 | |
| 7 | 43 | M | No | Yes | 34 | Yes | 5 | Yes | 2° type 2 AVB | 24 | No | n.a. | 110 | n.a. | Yes | No | No | No | 19 | |
| 8 | 74 | F | DM | Yes | n.a. | Yes | 6 | Yes | 2° type 2 AVB | n.a. | Yes | n.a. | 140 | RBBB | No | No | No | Yes | 20 | |
| 9 | 71 | M | No | Yes | 3 | Yes | 3 | No | 3° AVB | 3 | Yes | n.a. | 150 | LBBB | NO | No | Yes | No | 21 | |
| 10 | 11 | M | No | Yes | 33 | Yes | 7 | Yes | 2° type 2 AVB | 4 | Yes | n.a. | 130 | LBBB | Yes | No | No | No | 22 | |
| 11 | n.a. | n.a. | n.a. | Yes | n.a. | No | n.a. | n.a. | 3° AVB | 0 | No | n.a. | n.a. | n.a. | No | n.a. | Yes | Yes | 23 | |
| 12 | n.a. | n.a. | n.a. | Yes | n.a. | No | n.a. | n.a. | 3° AVB | 0 | Yes | n.a. | n.a. | LBBB | No | n.a. | Yes | Yes | 23 | |
| 13 | n.a. | n.a. | n.a. | Yes | n.a. | Yes | n.a. | n.a. | 3° AVB | 15 | No | n.a. | n.a. | n.a. | Yes | n.a. | No | No | 23 | |
| 14 | n.a. | n.a. | n.a. | Yes | n.a. | Yes | n.a. | n.a. | 2° type 2 AVB | 0 | Yes | n.a. | n.a. | RBBB | Yes | n.a. | Yes | No | 23 | |
| 15 | n.a. | n.a. | n.a. | Yes | n.a. | Yes | n.a. | n.a. | SSS | 30 | No | n.a. | n.a. | n.a. | No | n.a. | Yes | Yes | 23 | |
| 16 | n.a. | n.a. | n.a. | Yes | n.a. | Yes | n.a. | n.a. | SSS | 29 | n.a. | n.a. | n.a. | n.a. | No | n.a. | Yes | Yes | 23 | |
| 17 | n.a. | n.a. | n.a. | Yes | n.a. | Yes | n.a. | Yes | SSS | 4 | Yes | n.a. | n.a. | RBBB | No | n.a. | Yes | Yes | 23 | |
| 18 | 34 | M | No | Yes | 10 | Yes | 5 | Yes | SSS | n.a. | No | 200 | 110 | n.a. | No | No | Yes | No | 24 | |
| 19 | 36 | M | No | Yes | n.a. | No | n.a | No | SSS | n.a. | No | 180 | 110 | n.a. | No | No | Yes | No | 25 | |
| 20 | 67 | M | No | Yes | 54 | Yes | 6 | Yes | Sinus arrest | 6 | No | 200 | 110 | n.a. | Yes | No | No | No | 26 | |
| 21 | 54 | F | No | Yes | 6.3 | Yes | n.a | No | 3° AVB | n.a. | Yes | n.a. | 120 | RBBB | Yes | No | No | No | 27 | |
| 22 | 70 | F | No | Yes | 27 | Yes | 10 | Yes | SSS | 2 | No | n.a. | 110 | n.a. | Yes | No | No | No | 28 | |
| 23 | 81 | M | OSAS | Yes | 13 | Yes | 7 | Yes | SSS | 4 | No | n.a. | 110 | n.a. | Yes | No | No | No | 28 | |
| 24 | 76 | M | DM | Yes | 6,9 | Yes | 6 | No | 3° AVB | 0 | Yes | n.a. | 130 | RBBB | Yes | n.a. | No | No | 29 | |
| 25 | 82 | M | No | Yes | n.a. | Yes | n.a. | n.a. | 3° AVB | 9 | n.a. | n.a. | n.a. | n.a. | No | n.a. | Yes | No | 29 | |
| 26 | 69 | F | H,DM,S | Yes | n.a. | Yes | 6 | No | 2° type 2 AVB | 8 | No | n.a. | 110 | n.a. | Yes | No | No | No | 30 | |
| 27 | 83 | F | H. | Yes | n.a. | No | 8 | No | SSS | 8 | No | 160 | 110 | n.a. | Yes | No | No | No | 30 | |
| 28 | 55 | F | DM | Yes | 5.7 | No | n.a. | n.a. | 3° AVB | n.a. | n.a. | n.a. | n.a. | n.a. | No | No | Yes | No | 31 | |
| 29 | 56 | F | No | Yes | 4.8 | No | n.a. | n.a. | 3° AVB | n.a. | n.a. | n.a. | n.a. | n.a. | No | No | Yes | No | 31 | |
| 30 | 67 | M | H.,DM | Yes | 8.1 | No | n.a. | n.a. | 3° AVB | n.a. | n.a. | n.a. | n.a. | n.a. | No | No | Yes | No | 31 | |
| 31 | 80 | M | H.,M.I. | Yes | 10.6 | No | n.a. | n.a. | 3° AVB | n.a. | n.a. | n.a. | n.a. | n.a. | No | No | Yes | No | 31 | |
| 32 | 45 | F | No | Yes | 39.5 | No | n.a. | n.a. | SSS | n.a. | n.a. | n.a. | n.a. | n.a. | Yes | No | No | No | 31 | |
| 33 | 55 | M | I.M. | Yes | 7.4 | No | n.a. | n.a. | 3° AVB | n.a. | n.a. | n.a. | n.a. | n.a. | No | No | Yes | No | 31 | |
| 34 | 69 | M | HF | Yes | n.a. | No | n.a. | n.a. | SSS | n.a. | n.a. | n.a. | n.a. | n.a. | Yes | Yes | No | No | 31 | |
| 35 | 53 | M | No | Yes | 5.7 | No | 4 | No | 2° type 2 AVB | 3 | No | 110 | n.a. | No | No | Yes | No | 6 | ||
| 36 | 53 | M | No | Yes | 38 | Yes | n.a. | Yes | SSS | 3 | n.a. | n.a. | n.a. | n.a. | No | No | Yes | Yes | 32 | |
| 37 | 75 | F | No | Yes | 11 | Yes | n.a. | No | 3° AVB | 0 | Yes | 140 | RBBB | No | No | Yes | Yes | 33 | ||
| 38 | 71 | F | DM | Yes | 10.9 | No | 3 | No | 3° AVB | 1 | No | n.a. | 110 | n.a. | No | No | Yes | No | 34 | |
|
|
47 | M | No | No | n.a. | No | n.a. | No | SSS | 60 | No | 160 | 110 | n.a. | No | No | No | No | n.a. |
Abbreviations: C‐RP, C‐reactive protein; Com, Comorbidities; Hosp., Hospitalization; RF, Respiratory failure at presentation; HCD, Heart conduction disorder; AVB, Atrio‐ventricular block; RBBB, Right bundle branch block; LBBB, Left bundle branch block; SSS, Sick sinus syndrome; HF, Heart failure; M.I., Previous myocardial infarction; DM, Diabetes mellitus, S., Previous stroke; H., Hypertension; Ref, Bibliographic reference; n.a., Not applicable.
aPresent case.
2.1. Case report
A 47‐year‐old man without relevant cardiovascular and medical history underwent cardiological consultation due to persistent fatigue. His recent history included SARS‐CoV‐2 infection, diagnosed after the occurrence of fever, headache, and dry cough. Since his clinical condition was good and stable, the patient did not require hospitalization and spent his quarantine at home, with disappearance of symptoms after 4 days. No medications, with the exception of paracetamol, were necessary. The patient turned negative for SARS‐CoV‐2 at nasopharyngeal swab 20 days after the first positivity.
Two months after recovery a cardiology consult was requested for fatigue. The patient was afebrile, heart rate 55 beats per min (bpm) with irregular pulse, respiratory rate 14/min, blood pressure 120/70 mmHg, oxygen saturation 98%. Physical examination and chest X‐ray were normal. Blood examinations including serum troponin, CRP, D‐Dimer, and thyroid function were normal. The 12‐leads electrocardiogram (EKG) showed a sinus bradycardia with 55 bpm, narrow QRS, normal atrio‐ventricular interval and sinus pauses of about 2 s. A 6‐min walking test showed normal oxygenation and physiological increase of heart rate. An Holter EKG showed severe sinus arrhythmia with daytime bradycardia up to 30 bpm, junctional escape rhythm with atrio‐ventricular dissociation and numerous electrical pauses with a maximum R‐R interval of 2616 ms (Figure 1 a,b,c). The patient reported only fatigue and he had never experienced syncope. A cardiac magnetic resonance imaging was performed (Philips Achieva 1.5 T, cines, T2‐weighted, perfusion and late gadolinium enhancement sequences; 0.1 mL/kg of gadobutrol 1.0 mmol/mL as contrast agent) and showed normal origin of coronary arteries, normal ejection fraction and the absence of myocardial edema or late gadolinium enhancement.
FIGURE 1.
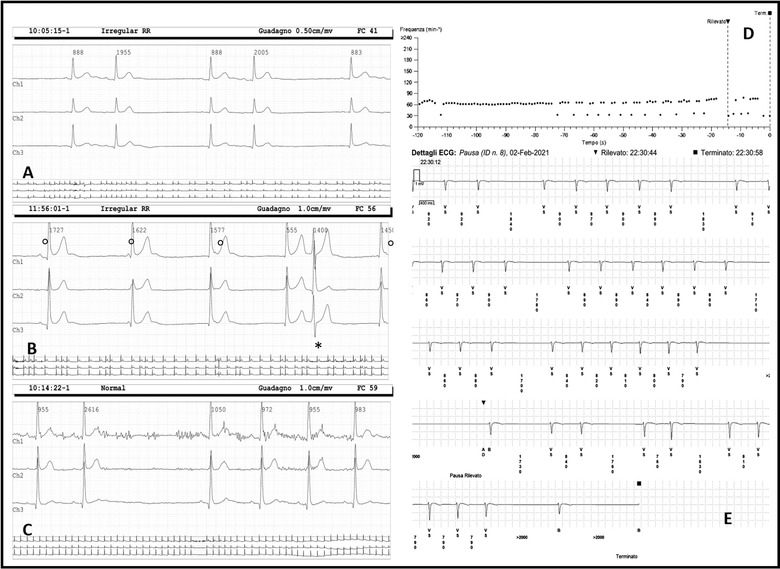
Holter EKG records: (A) sinus arrhythmia; (B) severe daytime bradycardia with atrioventricular isorhythmic dissociation and juntional escape rhythm, ° indicates dissociated P wave and * indicates an ectopic supraventricular early beat; (C) sinus pause of 2616 ms. Implanted Cardiac Monitor recording: (D) tachogram with revealed events; (E) sinus arrest, max R‐R interval 4200 ms
The patient underwent implantation of a loop recorder with remote monitoring. At 1‐month follow‐up, although the patient did not complain of symptoms, numerous electrical pauses, especially during night‐time, the longest of 4.2 s, were recorded (Figure 1 d,e). The patient is daily controlled by remote monitoring and follow‐up is still ongoing in order to evaluate the indication to permanent pacemaker implantation.
3. REVIEW OF THE LITERATURE
3.1. Pathophysiology
The bradyarrhythmias observed in COVID‐19 include SSS, sinus node disfunction, second and third degree AVB. Although not completely elucidated, the pathogenesis of bradyarrhythmias has been explained with several conditions occurring during SARS‐CoV‐2 infection, such as increased level of ACE‐2,15, 16, 31 direct injury of virus to cardiac cells,6, 20, 29, 30, 31, 32, 33 hyperinflammatory status,6, 16, 17, 21, 22, 27, 31 hypoxemia and electrolytic disorders,19, 21, 29 imbalance of autonomic nervous system likely due to involvement of nervous system by virus infection6, 19 and side effect of medications.24, 25, 26 A pivotal role is represented by the pro‐inflammatory cytokine IL‐6, which is the main responsible for cytokine storm during COVID and also is able to imbalance autonomic nervous system increasing vagal tone36, 37 (Figure 2). Among the included studies, an important insights on pathophysiology of bradyarrhythmias could be found in the article by Goette et al. were autopsy showed microangiopathy, myocardial necrosis, nerval ganglion cells with lymphocyte infiltration and viral alterations of right atrium, all conditions possibly implicated in bradyarrhythmias development.6
FIGURE 2.
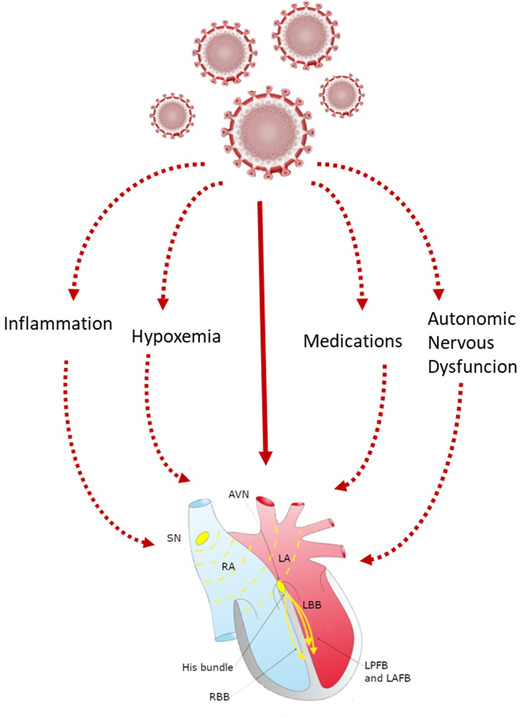
Proposed mechanisms of cardiac conduction system damage in SARS‐COV‐2 infection. ANS, autonomic nervous system; AVN, Atrio‐ventricular node; SN, sinus node; RA, right atrium; LA, left atrium; RBB: right bundle branch; LBB, Left bundle branch; LPFB, left posterior fascicular bundle; LAFB, left anterior fascicular bundle [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Assessment and diagnosis
A total of 38 cases, derived from 23 articles, of hypokinetic arrhythmias in patients with SARS‐CoV‐2 infection have been described so far (Table 1). Among the available data, mean age (±SD) of the patients was 59 ± 19 years old, with the youngest patient being 10 years old. Out of 31 patients, 21 (68%) were male, 10/31 (32%) female, in 7/38 (19%) this data were not available. In 31/38 (91%) medical history has been reported and cardiovascular risk factors such as diabetes, hypertension, obstructive sleep apnea or previous cardiovascular events were described in 11/31 (35%) of subjects. All the patients were hospitalized, with 23/38 (60%) developing severe respiratory failure. Worsening of clinical conditions occurred about 6 ± 2 days after first symptoms and 11/31 (35%) of patients required endotracheal intubation. CRP values at hospitalization was reported in 23/38 (61%) of cases and the mean value (±SD) was 18 ± 14 mg/dL. The most frequently reported bradyarrhythmias were second and third degree AVB in 26/38 (68%) patients and in 11/26 (42%) there was associated a BBB (46% left BBB, 54% right BBB). A marked bradycardia was described in all cases, in 12/38 (32%) of them sinus arrest or SSS were described, rarely associated with BBB (8%). The onset of cardiac conduction disorders was very variable, ranging from 0 to 30 days from hospitalization, with a median of 3.5 days. Although bradyarrhythmias are rarely described, relative bradycardia is a common characteristic in patients with SARS‐CoV‐2 infection.38, 39, 40, 41, 42 The described bradyarrhythmias related to COVID‐19 are summarized in Figure 3a and 3b.
FIGURE 3.
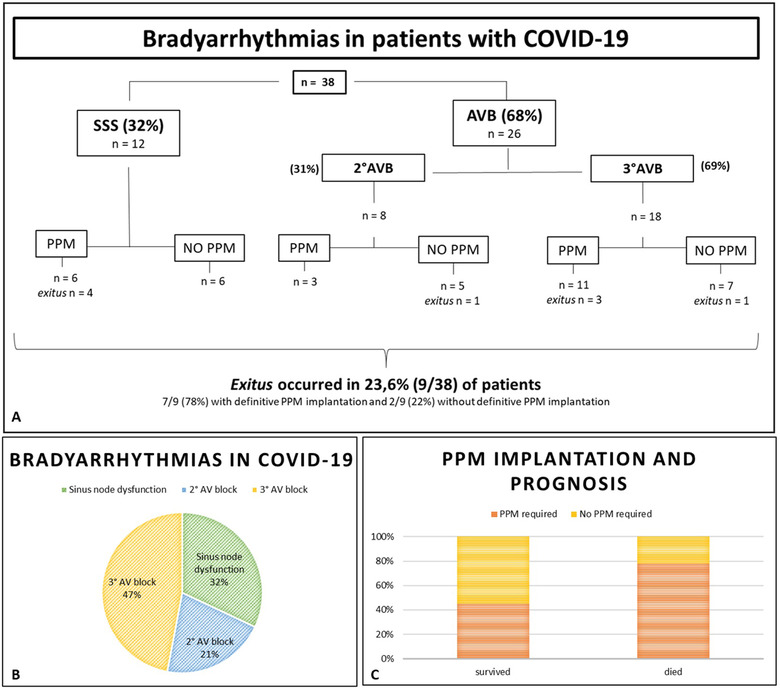
Bradyarrhythmias in patients with COVID‐19 (A); percentage of heart conduction disorders (B); PPM implantation and prognosis (C); PPM, permanent pacemaker implantation; SSS, sick sinus syndrome; AVB, atrio‐ventricular block [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Treatment and prognosis
In 17/38 (44.7%) cases, the bradyarrhythmias were described as reversible, while in 20/38 (52.6%) patients pacemaker implantation was required (70% of patients with AVB and 30% of patients with SSS). When necessary, temporary pacemaker implantation was considered a reasonable option prior to implanting a permanent device due to the possible transient nature of bradyarrhythmias observed during COVID‐19 and the risk of device infection.13, 23, 37, 38, 39 The mean (±SD) age of patients who required definitive pacemaker implantation was 60 ± 18 years old, with the youngest patient being 34 years old. Nine out of 38 (23.6%) died during hospitalization due to refractory respiratory failure and 7/9 (77%) have had a definitive pacemaker implantation. Clinical studies addressing bradyarrhythmias and COVID‐19 any are not available and major scientific evidences are based on case series. In the report by Gupta et al. two patients with SSS and five patients with atrioventricular block are described; in view of symptomatic bradyarrhythmias and the uncertainty of clinical course of COVID‐19, the patients received emergent temporary transvenous pacing; all patients were strictly monitored for 10‐14 days; 5/7 (71%) (mean age 62.6 ± 10.9 years old) with pacing dependent and symptomatic complete heart block underwent dual‐chamber permanent pacemaker implantation, while 3/7 (29%) (mean age 57 ± 16.9 years old) with SSS were kept under medical follow‐up.
In the report of Chinitz et al. 5/7 (71%) of patients undergone definitive pacemaker implantation and leadless pacemaker was chosen as best option due to lower probability of device infection; in 2/7 (28%) patients a temporary or semipermanent pacemaker was implanted.23 In this population 4/7 (57%) of patients died and the patients with definitive pacemaker implantation were 4/5 (80%); exitus occurred between 1 and 32 days after the procedure (median 9.5 days). A recent study reported a low percentage (9/700, 1.2%) of bradyarrhythmias in hospitalized patient with COVID‐19, and these seem not be related to acute mortality.11 In the present report we described 38 patients with bradyarrhythmias and we found that exitus occurred in 23.6% (9/38) of patients, among these, 7/9 (78%) with definitive pacemaker.
The prognosis in patients with persistent bradyarrhythmias is not clearly defined, while some studies showed that clinical outcome (intensive care unit admission, intubation, death) was similar in patients with fever and relative bradycardia and in patients with fever and appropriate heart rate response.41, 42
The percentage of pacemaker implantation in patients with heart conduction disorders and COVID‐19, divided into those who survived and those who died, are reported in Figure 3c. Although not statistically significant, patients who did not survive to SARS‐CoV‐2 infection apparently more frequently had pacemaker implantation during hospitalization.
3.4. Discussion and conclusions
Several cardiovascular diseases related to COVID‐19 have been described so far, including, although rarely, bradyarrhythmias and heart conduction disorders such as bundle branch blocks, with the latter correlating with worse prognosis.11, 12, 13 Moreover, the lack of the physiological heart rate increase during fever, named as “relative bradycardia,” has been shown to represent an early sign of SARS‐CoV‐2 infection.11, 41 This condition had yet an acknowledged diagnostic significance in infectious diseases and has been associated also with typhoid fever, Legionnaire's disease, leptospirosis and certain viral infections.43, 44 Some years ago, relative bradycardia was described also during Middle Eastern respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS), more often related to medications as ribavirin and sometimes was related to poor prognosis.45, 46
It now appears that patients with relative bradycardia should be monitored during the course of infection and follow‐up. Nevertheless, all the bradyarrhythmias described so far occurred during hospitalization in the acute phase of the infection. To the best of our knowledge, we describe the first case of SSS diagnosed in an outpatient setting likely developed as a consequence of mild SARS‐CoV‐2 infection, since previous EKGs were normal and no causative conditions other than the recent SARS‐CoV‐2 infection were present.
Although severe SARS‐CoV‐2 disease affected many people worldwide and critically ill patients are usually ECG‐monitored 24 h/d, only few cases of bradyarrhythmias have been described so far. It is likely that the onset of cardiac conduction disorders may have been underestimated and not correlated with COVID‐19 only, since critical patients were mostly elder and with several comorbidities, conditions that may explain per se the occurrence of heart conduction disorders, while cases of transient bradyarrhythmias may have not been judged clinically relevant and therefore may have not been reported. In addition, beyond relative bradycardia, which has been described to occur either at hospital admission and during hospitalization in patients with COVID‐19,41, 42 apparently no other comprehensive study regarding cardiac conduction disorders during COVID‐19 has been carried out so far and, currently, evidence is mostly provided by case reports.
From the available data in the literature, it is still unknown whether it is necessary to implant a permanent pacemaker and which would be the optimal timing of the procedure. Since we could not define with certainty the stability of the arrhythmic condition of our patient, and considering that he had never experienced syncope, we decided to implant a loop recorder with home monitoring in order to closely unveil the occurrence of severe bradyarrhythmias events which might subsequently require a definitive pacemaker implantation. The occurrence of heart conduction alterations following asymptomatic or mild COVID‐19, as it happened in our case, should focus the attention towards possible late sequelae of SARS‐COV‐2 infection, suggesting that the virus itself should be considered an additional risk factor for cardiovascular disease.
The case herein presented highlights that cardiological evaluation during follow‐up of outpatients with previous SARS‐CoV‐2 infection may be desirable, especially in the presence of symptoms suggesting a possible heart involvement. Although the proposal may seem unsustainable during a pandemic, it is also known that a not negligible rate of patients still experience symptoms (i.e., fatigue, pre‐syncope, shortness of breath) even after SARS‐CoV‐2 infection resolution, and this is true not only for severe but also for mild infections, as it is the present case. Therefore, in order to screen for heart rhythm disorders a cardiological follow up may be reasonable in these patients.
In conclusion, heart conduction disorders may occur during the course of COVID‐19 and, although less commonly, even after resolution of SARS‐CoV‐2 infection. The decision to pacemaker implantation should be based on accurate analysis aimed at evaluating the reversibility of this disorder, especially in young patients.
Gatto MC, Persi A, Tung M, Masi R, Canitano S, Kol A. Bradyarrhythmias in patients with SARS‐CoV‐2 infection: A narrative review and a clinical report.. Pacing Clin Electrophysiol. 2021;44:1607–1615. 10.1111/pace.14308
DATA AVAILABILITY STATEMENT
Data are available upon request from corresponding author.
REFERENCES
- 1.Dhakal BP, Sweitzer NK, Indik JH, Acharya D, William P. SARS‐CoV‐2 infection and cardiovascular disease: COVID‐19 heart. Heart Lung Circ. 2020;29(7):973‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID‐19. Am J Emerg Med. 2020;38:1504‐1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Violi F, Oliva A, Cangemi R, et al. Nox2 activation in Covid‐19. Redox Biol. 2020;36:101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbasi J. Researchers investigate what COVID‐19 does to the heart. JAMA. 2021;325(9):808‐811. [DOI] [PubMed] [Google Scholar]
- 5.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goette A, Patscheke M, Henschke F, Hammwöhner M. COVID‐19‐Induced cytokine release syndrome associated with pulmonary vein thromboses, atrial cardiomyopathy, and arterial intima inflammation. TH Open. 2020;4:e271‐e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Violi F, Ceccarelli G, Cangemi R, et al. Hypoalbuminemia, coagulopathy, and vascular disease in COVID‐19. Circ Res. 2020;127:400‐401. [DOI] [PubMed] [Google Scholar]
- 8.Inciardi RM, Adamo M, Lupi L, Metra M. Atrial fibrillation in the COVID‐19 era: simple bystander or marker of increased risk?. Eur Heart J. 2020:3094‐3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchis‐Gomar F, Perez‐Quilis C, Lavie CJ. Should atrial fibrillation be considered a cardiovascular risk factor for a worse prognosis in COVID‐19 patients?. Eur Heart J. 2020:3092‐3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vetta F, Marinaccio L, Vetta G, Marchese D. Electrical storm in a patient with COVID ‐19 treated with hydroxychloroquine: a case report. SAGE Open Med Case Rep. 2020;8:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Wang Z, Tse G, et al. Cardiac arrhythmias in patients with COVID‐19. J Arrhythm. 2020:827‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatla A, Mayer MM, Adusumalli S, et al. COVID‐19 and cardiac arrhythmias. Heart Rhythm. 2020:1439‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dherange P, Lang J, Qian P, et al. Arrhythmias and COVID‐19. JACC Clin Electrophysiol. 2020:1193‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonsoz MR, Oncul A, Cevik E, et al. Wide QRS complex and lateral ST‐T segment abnormality are associated with worse clinical outcomes in COVID‐19 patients. Am J Med Sci. 2021;361(5):591‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kir D, Mohan C, Sancassani RHeart Brake. JACC Case Rep. 2020:1252‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al‐assaf O, Mirza M, Musa A. Atypical presentation of COVID‐19 as subclinical myocarditis with persistent high‐degree atrioventricular block treated with pacemaker implant. HeartRhythm Case Rep. 2020:884‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El‐Assaad I, Hood‐Pishchany MI, Kheir J, et al. Complete heart block, severe ventricular dysfunction, and myocardial inflammation in a child with COVID‐19 infection. JACC Case Rep. 2020:1351‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azarkish M, Laleh Far V, Eslami M, Mollazadeh R. Transient complete heart block in a patient with critical COVID‐19. Eur Heart J. 2020:2131‐2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eneizat Mahdawi T, Wang H, Haddadin FI, Al‐Qaysi D, Wylie JV. Heart block in patients with coronavirus disease 2019: a case series of 3 patients infected with SARS‐CoV‐2. HeartRhythm Case Rep. 2020:652‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gubitosa JC, Xu P, Ahmed A, Pergament K. Incomplete trifascicular block and mobitz type II atrioventricular block in COVID‐19. Cureus. 2020;12:e10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malekrah A, Fatahian A. A case report of a rare cardiac complication in novel coronavirus disease. Eur Heart J Case Rep. 2020:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domico M, McCanta AC, Hunt JL, Ashouri N, Nugent D, Kelly RB. High‐grade heart block requiring transvenous pacing associated with multisystem inflammatory syndrome in children during the COVID‐19 pandemic. HeartRhythm Case Rep. 2020:811‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chinitz JS, Goyal R, et al. Bradyarrhythmias in patients with COVID‐19: marker of poor prognosis?. Pacing Clin Electrophysiol. 2020:1199‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cimino G, Pascariello G, Bernardi N, et al. Sinus node dysfunction in a young patient with COVID‐19. JACC Case Rep. 2020:1240‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eid MM. COVID‐19 patient with symptomatic bradycardia. Vis J Emerg Med. 2021:100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang LY‐T, Ng GYP. COVID‐19 treatment with lopinavir–Ritonavir resulting in sick sinus syndrome: a case report. Eur Heart J Case Rep. 2020:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pecora D. COVID‐19 e coinvolgimento cardiaco: una presentazione inusuale. Giornale Italiano di Cardiologia. 2020;21:594‐597. [DOI] [PubMed] [Google Scholar]
- 28.Peigh G, Leya MV, Baman JR, Cantey EP, Knight BP, Flaherty JD. Novel coronavirus 19 (COVID‐19) associated sinus node dysfunction: a case series. Eur Heart J Case Rep. 2020:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochav SM, Coromilas E, Nalbandian A, et al. Cardiac arrhythmias in COVID‐19 infection. Circ:Arrhythm Electrophysiol. 2020;13:e008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babapoor‐Farrokhran S, Batnyam U, Wiener PC, et al. Atrioventricular and sinus node dysfunction in stable COVID‐19 Patients. SN Compr. Clin Med. 2020:1955‐1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta MD, Qamar A, Mp G, Al SafalSet. Bradyarrhythmias in patients with COVID‐19: a case series. Indian Pacing Electrophysiol J. 2020:211‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashok V, Loke WI. Case report: high‐grade atrioventricular block in suspected COVID‐19 myocarditis. Eur Heart J Case Rep. 2020:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivetti L, Mantovan R, Sitta N, et al. Management of pacemaker implantation during COVID‐19 infection. Case Rep Cardiol. 2020;2020:8833660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haddadin FI, Mahdawi TE, Hattar L, Beydoun H, Fram F, Homoud M. A case of complete heart block in a COVID‐19 infected patient. J Cardiol Cases. 2021:27‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `cytokine storm' in COVID‐19. J Infect. 2020;80:607‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hajiasgharzadeh K, Mirnajafi‐Zadeh J, Mani AR. Interleukin‐6 impairs chronotropic responsiveness to cholinergic stimulation and decreases heart rate variability in mice. Eur J Pharmacol. 2011;673:70‐77. [DOI] [PubMed] [Google Scholar]
- 37.Ikeuchi K, Saito M, Yamamoto S, Nagai H, Adachi E. Relative bradycardia in patients with mild‐to‐moderate coronavirus disease, Japan. Emerg Infect Dis. 2020;26:2504‐2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu L, Gong L, Jiang Z, Wang Q, Zou Y, Zhu L. Clinical analysis of sinus bradycardia in patients with severe COVID‐19 pneumonia. Crit Care. 2020;24:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ESC . Guidance for the Diagnosis and Management of CV Disease during the COVID‐19 Pandemic. France: ESC. [Google Scholar]
- 40.Desai AD, Boursiquot BC, Melki L, Wan EY. Management of arrhythmias associated with COVID‐19. Curr Cardiol Rep. 2021;23:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capoferri G, Osthoff M, Egli A, Stoeckle M, Bassetti S. Relative bradycardia in patients with COVID‐19. Clin Microbiol Infect. 2021;27(2):295–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliva A, Franchi C, Gatto MC, Galardo G, Pugliese F, Mastroianni C. Prevalence and clinical significance of relative bradycardia at hospital admission in patients with coronavirus disease 2019 (COVID‐19). Clin Microbiol Infect. 2021. 10.1016/j.cmi.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunha BA. The diagnostic significance of relative bradycardia in infectious disease. Clin Microbiol Infect. 2000;6:633‐634. [DOI] [PubMed] [Google Scholar]
- 44.Ye F, Hatahet M, Mohamed A, Youniss HZT, Mazza JJ, Yale S. The clinical significance of relative bradycardia. WMJ. 2018;117:73‐78. [PubMed] [Google Scholar]
- 45.Zhong H, Wang Y, Zhang ZL, et al. Efficacy and safety of current therapeutic options for COVID‐19—Lessons to be learnt from SARS and MERS epidemic: a systematic review and meta‐analysis. Pharmacol Res. 2020;157:104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller MP, Dresser L, Raboud J, McGeer A, Rea E, Richardson SE. Adverse events associated with high‐dose ribavirin: evidence from the Toronto outbreak of severe acute respiratory syndrome. Pharmacotherapy. 2007;27:494‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from corresponding author.


