Conflicts of interest
None.
Author contribution
Giovanni Damiani: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Visualization, Writing – original draft, Writing – review and editing. Alessia Pacifico: Investigation, Methodology, Project administration, Software, Validation. Writing – review and editing. Francesco Pelloni: Investigation, Project administration, Validation, Writing – review and editing. Matilde Iorizzo: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – review and editing.
Dear Editor,
During the COVID‐19 pandemics, the management of immunosuppressed patients, those affected by autoimmune bullous diseases (AIBDs), became a matter of particular concern for healthcare systems; dissemination of sound and updated information became mandatory to orient physicians and more generally healthcare policy.1, 2 Furthermore, AIBDs patients were encouraged to take advantage from teledermatological rather than in‐person consultations; also, they were encouraged to undergo COVID‐19 vaccination, preferably during remission periods or at least while under low immunosuppression.3, 4 To date, data regarding the effect of COVID vaccines on AIBDs are scanty. We present 5 cases (from 4 different centres) of bullous pemphigoid (BP) – respectively of pemphigus vulgaris (PV) – all confirmed by clinical, immunopathologic/serological findings, where the patients had undergone COVID vaccination during a period of remission of their bullous disease and have subsequently experienced a disease flare (Table 1).
Table 1.
Data of AIBD‐affected patients
| Sex/age | AIBD | Previous medications for AIBDs | Vaccine (2 doses) | New medications for flare up | Anti‐SARS‐CoV‐2 S1‐RBD IgG >50 UI/L | |
|---|---|---|---|---|---|---|
| 1 | F/75 | BP | Oral prednisone | Moderna | Oral prednisone | Yes |
| 2 | M/40 | PV | Rituximab | Moderna |
Oral prednisone Mycophenolate mofetil |
Yes |
| 3 | M/84 | BP |
Oral prednisone Azathioprine |
Moderna | Oral prednisone* | Yes |
| 4 | F/82 | BP |
Oral prednisone Mycophenolate mofetil |
Pfizer | Oral prednisone | Yes |
| 5 | M/80 | PV |
Oral prednisone Mycophenolate mofetil |
Pfizer | Oral prednisone | Yes |
Treatment started only after the 2nd dose.
Case 1. A 63‐year‐old female with bullous pemphigoid (BP) in clinical remission since 6 months after oral prednisone treatment was administered the first dose of Moderna mRNA‐1273 vaccine, and 3 days later, she experienced a flare of her disease: the latter started with a generalized erythema 1 day after the vaccination, and on the third day, several blisters appeared on the trunk. The diagnosis was made in a telemedical consultation and oral prednisone was prescribed. 28 days later, the patient was given the second dose of the vaccine, and no disease flares and no injection site reactions took place.
Case 2. A 40‐year‐old male, with pemphigus vulgaris (PV) in remission since one year after rituximab therapy, came to our attention 3 days after the first dose of the Moderna mRNA‐1273 vaccine because of a PV flare. He presented with several blisters on the back and on the upper limbs (Fig. 1) and started a therapy with mycophenolate mofetil and oral prednisone. After 28 days, he was given the second dose of the vaccine: he experienced only pain on the injection site for 2 days.
Fig. 1.
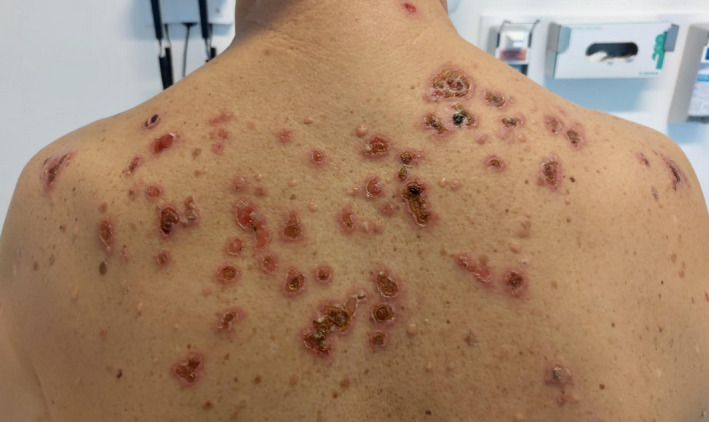
Patient number 2 showing a flare of PV after the 1st dose of Moderna mRNA‐1273 vaccine
Case 3. A 84‐year‐old male with BP in remission since 4 years after oral prednisone and azathioprine treatment was administered the first dose of the Moderna mRNA‐1273 vaccine. After 2 weeks, he started showing mild blistering lesions on the trunk, which were deemed not to require systemic immunosuppressive treatment. 28 days later, the patient was given the second dose; a worsening of the lesions which became more widespread, involving also the oral cavity, took place. A treatment with oral prednisone was started.
Case 4. A 82‐year‐old female with BP in remission since 3 years after oral prednisone and mycophenolate mofetil treatment was given the first dose of the Pfizer mRNABNT162b2 vaccine. 3 days later, the patient experienced a moderate BP flare in the form of small blisters on the arms and legs. The patient lived in a nursing home where only teledermatological consultations were possible. Oral prednisone was prescribed, and the second dose was administered 21 days later. No further BP flares and no injection site reactions occurred.
Case 5. A 80‐year‐old male with a history of PV remitted before 1 year after oral prednisone and mycophenolate mofetil treatment experienced a flare 3 days after the first dose of the mRNABNT162b2 vaccine. He was seen in a teledermatological consultation because of severe blisters on the back, and he was treated with oral prednisone. After 21 days, the patient was able to complete the second dose of Pfizer mRNABNT162b2 vaccination. No further BP flares and no injection site reactions occurred.
Despite the ongoing treatment with immunosuppressants, all patients developed IgG antibodies against the SARS‐CoV‐2 S1‐receptor binding domain (RBD) (>150 UI) 1 month after the second dose, which supports the assumption that the vaccination was effective in all cases presented.
These anecdotal data suggest that both mRNA vaccines may trigger relapses in AIBD patients. Vaccines are important in AIBDs patients, since the latter are characterized by a vulnerability against infections in the mucocutaneous barrier5: completion of vaccination is advisable, and patients should be treated for flares when needed. However, data on the effect on immunosuppressants on IgG antibodies titres in short and long term are needed. IgG S1‐RBD persistence in AIBD patients is also a matter of concern; this should encourage dermatologists to monitor anti‐COVID‐19 immunity.
Funding sources
None.
Acknowledgement
The patients presented in this paper have given written informed consent to the publication of their case details.
Data availability statement
Data available on request due to privacy/ethical restrictions.
References
- 1.Di Altobrando A, Patrizi A, Bardazzi F. Should SARS‐CoV‐2 influence immunosuppressive therapy for autoimmune blistering diseases? J Eur Acad Dermatol Venereol. 2020; 34(7): e295–e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. https://recovab.umcg.nl
- 3.Kasperkiewicz M, Schmidt E, Fairley JAet al. Expert recommendations for the management of autoimmune bullous diseases during the COVID‐19 pandemic. J Eur Acad Dermatol Venereol 2020; 34: e302‐e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasperkiewicz M, Schmidt E, Amagai Met al. Updated international expert recommendations for the management of autoimmune bullous diseases during the COVID‐19 pandemic. J Eur Acad Dermatol Venereol. 2021; 35(7): e412–e414. 10.1111/jdv.17207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laniosz V, Lehman JS, Poland GA, Wetter DA. Literature‐based immunization recommendations for patients requiring immunosuppressive medications for autoimmune bullous dermatoses. Int J Dermatol 2016; 55(6): 599–607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


