Dear Editor,
We report data on the antibody response against the SARS‐CoV‐2 receptor‐binding domain (RBD) in healthcare professionals from two hospitals in Copenhagen at baseline and 3 weeks post the first injection with the BNT162b2 mRNA COVID‐19 vaccine. The response was assessed using assays measuring antibody neutralizing capacity (NAbs) [1] and direct binding of IgG, IgM and IgA [2]. Moreover, we tested antibody responses against the nucleocapsid (N) protein, which is not a part of the vaccine and used as a proxy for natural infection. For more details, see the Supporting Information.
Out of the 1460 participants, 1227 were females (Table S1). The included subjects were categorized as SARS‐CoV‐2 primed with a natural infection if they were positive at day 0 (baseline). At baseline, 108 participants (7.4%) and approximately 3 weeks after the first vaccination, 122 participants (8.3%) were N protein seropositive, respectively (Fisher's exact test, p < 0.0001). A total of 65 of the 108 (60.2%) seropositive patients had received reverse transcription polymerase chain reaction (RT‐PCR) laboratory confirmation of SARS‐CoV‐2 infection before inclusion in the study. Divided according to age, seropositivity at baseline showed that the prevalence was highest among the participants below 30 years of age (17.3%) and decreased (3.9%) in those 60 years of age and above (Table S1; X 2, p < 0.0001).
Thirty participants (2.1%) remained IgG RBD seronegative after 3 weeks. In individuals with a previous SARS‐CoV‐2 infection compared with infection naïve, the vaccine responses were significantly increased for both NAbs, IgG, IgA and IgM (Mann–Whitney U test, p < 0.0001, p < 0.0001, p < 0.0001 and p = 0.0001, respectively; Fig. 1a–d). Among previously SARS‐CoV‐2 infected individuals, 98.4% were positive for NAbs compared with 75.8% in the infection naïve group 3 weeks post‐vaccination (Mann–Whitney U test, p < 0.0001) (Fig. 1a).
Fig. 1.
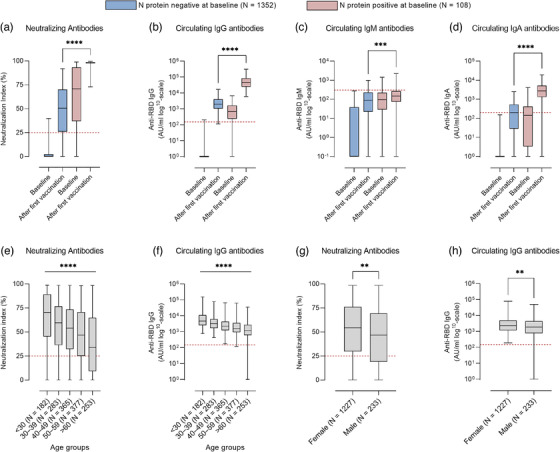
Immune response in participants according to age, sex and with or without previous SARS‐CoV‐2 infection at baseline and after the first vaccination. Box plots display the median values with the interquartile range (lower and upper hinge) and the 2.5%–97.5% (lower and upper whiskers): neutralizing antibodies (a), anti‐receptor‐binding domain (anti‐RBD) IgG antibodies (b), anti‐RBD IgM antibodies (c) and anti‐RBD IgA antibodies (d). The participants are divided into two groups based on N protein seropositivity at baseline, the seropositive individuals (red, N = 108) and seronegative (blue, N = 1352). Shown are neutralizing antibodies according to age groups (e), anti‐RBD IgG antibodies according to age groups (f), neutralizing antibodies according to sex (g) and anti‐RBD IgG antibodies according to sex (h). Age groups: <30 (N = 182), 30–39 (N = 283), 40–49 (N = 365), 50–59 (N = 377) and >60 (N = 253). Sex distribution: females (N = 1227) and males (N = 233). Significance levels are as follows: *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001; p < 0.05 was considered statistically significant. Horizontal dotted lines indicate estimated threshold between antibody positive and negative samples.
The vaccine antibody responses decreased gradually by increasing age (Kruskal–Wallis test, p < 0.0001) (Fig. 1e,f). Females had a higher neutralizing and IgG antibody vaccine response than males (Mann–Whitney U test, p = 0.0017 and 0.0085, respectively; Fig. 1g,h). No significant difference in vaccine responses was reflected in body mass index (BMI) nor in the time since the positive PCR test (Figs S1 and S2).
Multiple regression analysis (Table S2) revealed that with increasing age, the IgG antibody response after the first vaccination declined significantly compared to the youngest age group (<30 years). Previous SARS‐CoV‐2 infection was associated with the most pronounced IgG antibody response (p < 0.0001). Male sex was associated with a lower IgG antibody response in all regression models (p = 0.0002, p < 0.0001 and p = 0.001). Furthermore, when dividing the participants into BMI groups, we found no significant association to IgG antibody responses (p > 0.05).
This study shows that a single dose of the BNT162b2 vaccine induces a robust antibody response in individuals that have experienced previous COVID‐19 infection. This is compatible with a recent observation that prior infection equals a double vaccine dose of the BNT162b2 [3].
In general, low responsiveness to vaccines increases with age, and our observation that the antibody responses to the BNT162b2 vaccine decrease by each centile are compatible with this knowledge [4]. Furthermore, sex appeared to play a significant but less pronounced role since females mounted a significantly higher antibody response, which has been reported previously regarding other viral vaccines [5].
Fourteen individuals (1.1%) became N protein‐positive during the 3‐week observation period, indicating that they had contracted COVID‐19 after the first injection. This agrees with initial findings in the BNT162b2 phase three trial that there is a lag phase of around 10–14 days before a robust protective response is generated [6]. Thirty individuals (2.1%) of the vaccinated participants did not generate any vaccine antibody response against RBD. These individuals were also negative for N protein responses. Our results are in line with previous observations that about 2%–10% of healthy individuals fail to mount antibody responses to routine vaccines [7]. However, it might be possible that some individuals may need a second booster to mount a detectable immunological response.
Obese individuals may have increased susceptibility to infections [7], and for some vaccines elevated BMI is associated with poor vaccine responsiveness [8, 9]. We did not find an association of vaccine responses with BMI in this cohort. However, this should be interpreted with some caution. The median BMI in our study was 23.9, and 90% of the included subjects had a BMI below 30 and were thus not obese. Therefore, our study might have been underpowered to detect an effect of obesity.
We conclude that age and sex appear to be significant factors in how well a vaccine response is mounted. Previous natural SARS‐CoV‐2 infection is associated with a more rapid and robust response to a COVID‐19 vaccine than individuals who were not infected.
Conflict of interest
The authors declare to have no conflict of interest.
Supporting information
Supporting Information
Contributor Information
Cecilie Bo Hansen, Email: cecilie.bo.hansen@regionh.dk.
Peter Garred, Email: peter.garred@regionh.dk.
References
- 1. Bayarri‐Olmos R, Idorn M, Rosbjerg A, Pérez‐Alós L, Hansen CB, Johnsen LB, et al. SARS‐CoV‐2 neutralizing antibody responses towards full‐length spike protein and the receptor‐binding domain. J Immunol. 2021;207:878–87. [DOI] [PubMed] [Google Scholar]
- 2. Hansen CB, Jarlhelt I, Perez‐Alos L, Hummelshoj LL, Loftager M, Rosbjerg A, et al. SARS‐CoV‐2 antibody responses are correlated to disease severity in COVID‐19 convalescent individuals. J Immunol. 2021;206(1):109–17. [DOI] [PubMed] [Google Scholar]
- 3. Ebinger JE, Fert‐Bober J, Printsev I, Wu M, Sun N, Prostko JC, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS‐CoV‐2. Nat Med. 2021;27(6):981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grubeck‐Loebenstein B, Della BS, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009;21(3):201–9. [DOI] [PubMed] [Google Scholar]
- 5. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wiedermann U, Garner‐Spitzer E, Wagner A. Primary vaccine failure to routine vaccines: why and what to do? Hum Vaccin Immunother. 2016;12(1):239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weber DJ, Rutala WA, Samsa GP, Bradshaw SE, Lemon SM. Impaired immunogenicity of hepatitis B vaccine in obese persons. N Engl J Med. 1986;314(21):1393. [DOI] [PubMed] [Google Scholar]
- 9. Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes. 2012;36(8):1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


