Conflict of interest
None.
Funding sources
None.
Dear Editor,
Since December 2020, COVID‐19 vaccination started around the world. Messenger RNA (mRNA)‐based and adenovirus vector vaccines have received authorization for their use. A wide variety of cutaneous reactions after all the COVID‐19 vaccines have been reported. 1 We describe an unusual case of disseminated annular lesions triggered by m‐RNA COVID‐19 vaccine BioNTech/Pfizer (Mainz, Germany; New York, NY, USA).
A 75‐year‐old woman presented to the emergencies department with a 7‐day history of widespread mildly pruritic lesions. She had personal history of arterial hypertension, diabetes mellitus, auricular fibrillation and chronic cardiac insufficiency. Physical examination revealed erythematous annular patches on the trunk and lower limbs, with purpuric peripheral areas and central clearing, predominantly located on the abdominal area (Fig. 1). No other mucocutaneous involvement was observed. The patient denied any previous infectious disease or recent changes in her medications. However, she had received mRNA Pfizer COVID‐19 vaccine 4 days before the onset of the skin lesions.
Figure 1.
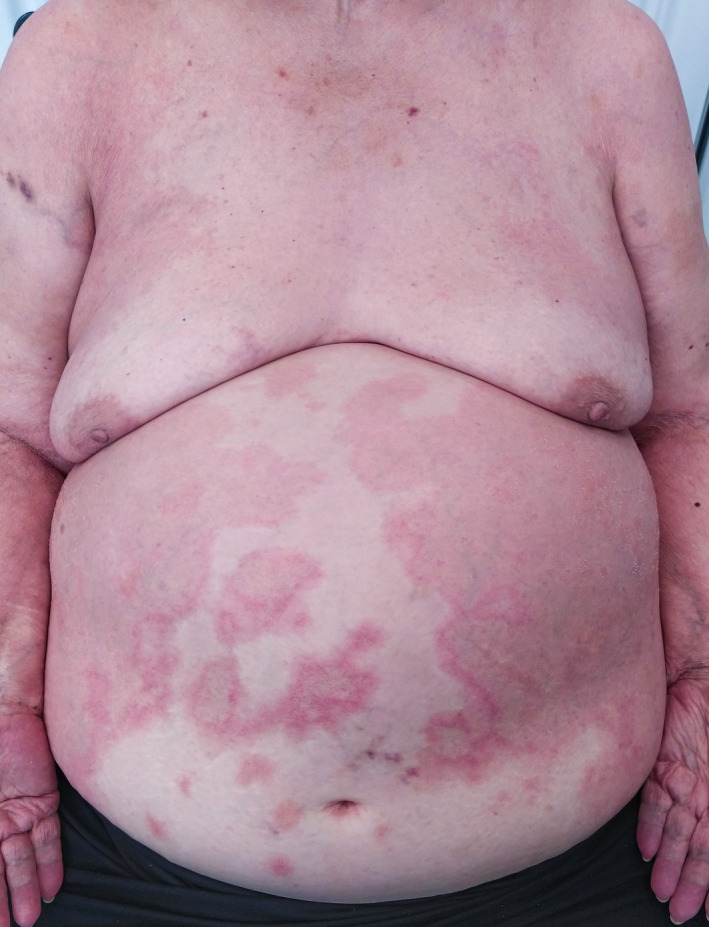
Erythematous patches located on the trunk, mainly on the abdominal region. The lesions present annular form, with a purpuric/petechial peripheral area and central clearing. Some lesions are confluent, forming big erythematous brownish patches.
Blood analysis revealed C‐reactive protein elevation (1.1 mg/dL). Blood cell count and the rest of the biochemical profile were normal. A skin biopsy was performed (Fig. 2), which showed dermal perivascular lymphocytic infiltrate and extravasated erythrocytes. These findings were consistent with purpuric pigmented dermatosis. Consequently, following the clinical and histopathological findings, a diagnosis of purpura annularis telangiectodes of Majocchi was made.
Figure 2.
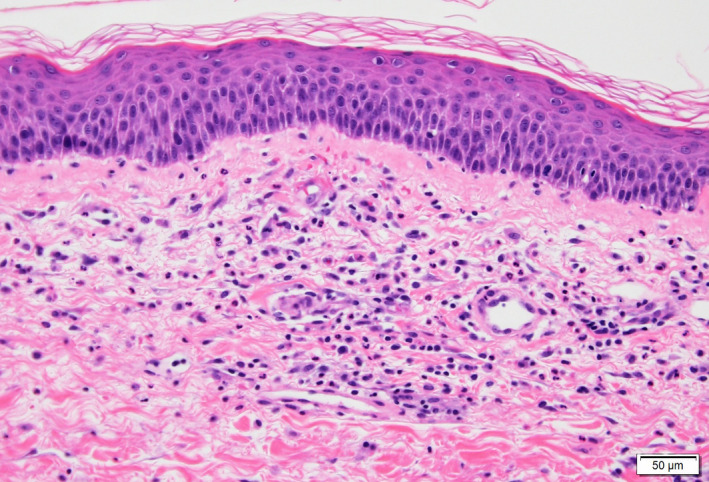
Skin biopsy of annular lesion (Hematoxylin‐Eosin stain ×20): No epidermal changes are observed. A perivascular lymphocytic infiltrate can be appreciated in the dermis, with focal neutrophils and extravasated red blood cells. Absence of fibrinoid necrosis or fibrin deposition. These findings are consistent with purpuric pigmented dermatosis.
Treatment with oral prednisone and topical methylprednisolone was initiated. Three weeks later, the patient presented resolution of the skin eruption. No new outbreaks of lesions were observed after 2 months follow‐up period.
Purpura annularis telangiectodes of Majocchi is a rare variant of pigmented purpuric dermatosis. 2 It is characterized by punctate telangiectatic macules progressing to annular patches with central regression, usually distributed on the legs. The lesions are occasionally pruritic. Although its aetiology remains unknown, a possible role of the immune system is widely assumed, 2 and some proposed trigger factors in its physiopathology are medications, infections and immune dysregulations (which are usually present after vaccinations).
A wide variety of cutaneous adverse effects after COVID‐19 vaccination has been described in the literature. McMahon et al. 1 reported a study of 414 cases of dermatological reactions to mRNA COVID‐19 vaccines: the most common cutaneous findings were local reactions, followed by urticarial and morbilliform rash. Farinazzo et al. 3 described similar results in their study about dermatological adverse effects to Pfizer vaccine, being local reactions and urticarial eruptions the most frequent dermatological related entities. Sometimes, COVID‐19 vaccination secondary reactions mimic SARS‐CoV‐2 infection itself, such as pernio/chilblains or vesicular eruptions. 1 , 4 Other less common associated inflammatory conditions to mRNA COVID‐19 vaccines have been reported, like leukocytoclastic vasculitis 5 and erythema multiforme. 6
To the best of our knowledge, there are no reports in the current literature of pigmented purpuric dermatosis outbreaks following SARS‐CoV‐2 vaccination. Recently, a case of a widespread annular eruption 48 h after Ad26.COV2.S COVID‐19 (adenovirus vector) vaccine has been described. 7 The skin biopsy was not conclusive, and the dermatosis resolved in 2 weeks with topical corticosteroids.
We describe a prominent case of purpura annularis telangiectodes of Majocchi after mRNA COVID‐19 Pfizer vaccine, presenting with widespread annular lesions. This is, to the best of our knowledge, an unusual non‐previously reported secondary effect of COVID‐19 vaccination. This dermatosis was probably caused by an immune dysregulation following the vaccination, similarly to leukocytoclastic vasculitis flares. 5 Since COVID‐19 global immunization started, new postvaccination adverse events and their treatment continue to be described by clinicians. We report this case in order to contribute to a better characterization and diagnosis of the wide spectrum of COVID‐19 vaccination dermatological side effects, a key point for the management of these secondary reactions.
Acknowledgements
The patients in this manuscript have given written informed consent to the publication of their case details. The authors confirm that the manuscript has been submitted solely to this journal and is not submitted, in press, or published in any language elsewhere. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content.
References
- 1. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoesly FJ, Huerter CJ, Shehan JM. Purpura annularis telangiectodes of Majocchi: case report and review of the literature. Int J Dermatol 2009; 48: 1129–1133. [DOI] [PubMed] [Google Scholar]
- 3. Farinazzo E, Ponis G, Zelin E et al. Cutaneous adverse reactions after m‐RNA COVID‐19 vaccine: early reports from Northeast Italy. J Eur Acad Dermatol Venereol 2021; 35: e548–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Piccolo V, Bassi A, Argenziano G et al. BNT162b2 mRNA COVID‐19 vaccine‐induced chilblain‐like lesions reinforces the hypothesis of their relationship with SARS‐CoV‐2. J Eur Acad Dermatol Venereol. 2021; 35: e493–e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen SR, Prussick L, Kahn JS, Gao DX, Radfar A, Rosmarin D. Leukocytoclastic vasculitis flare following the COVID‐19 vaccine. Int J Dermatol 2021; 60: 1032–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lavery MJ, Nawimana S, Parslew R, Stewart L. A flare of pre‐existing erythema multiforme following BNT162b2 (Pfizer‐BioNTech) COVID‐19 vaccine. Clin Exp Dermatol 2021; 46: 1325–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song EJ, Wong AJS. Widespread annular eruption after Ad26.COV2.S COVID‐19 vaccine. JAAD Case Rep 2021; 13: 30–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


