Dear Editor,
Obesity has been identified as an important risk factor for COVID‐19 severity [1]. Besides, several reports also suggested that obesity (or higher body mass index [BMI]) may also be associated with a higher susceptibility to SARS‐CoV‐2 infection [1, 2], although this remains unclear. Going beyond BMI, this research letter provides insights into the associations between several anthropometric characteristics and the risk of SARS‐CoV‐2 infection using exhaustive seroprevalence data acquired in a large population‐based sample.
NutriNet‐Santé is a French web‐based cohort launched in 2009 [3] (#NCT03335644; ethical authorizations available at https://info.etude‐nutrinet‐sante.fr/node/12). At baseline and every 6–12 months, participants receive questionnaires based on socioeconomic status, lifestyle, physical activity and health. A subset of participants also underwent body composition measurements (BC‐418MA, TANITA; 2011–2014). In April 2020, a questionnaire investigated a wide range of aspects related to the COVID‐19 crisis (SAPRIS‐SERO project [4]), including bodyweight just before the March 2020 lockdown. In June 2020, self‐reported measures of current waist and hip circumference were provided following detailed instructions. Participants involved in the SAPRIS‐SERO project returned self‐sampled dried‐blood spots in May–October 2020 that were analysed to detect anti‐SARS‐CoV‐2 antibodies directed against the spike protein (ELISA‐S) and the nucleocapsid protein (ELISA‐NP), as well as neutralizing antibodies (SN) [5]. More details regarding the tests are provided in the Supporting Information. The main outcome was a positive ELISA‐S test, while being positive to all three ELISA‐S, ELISA‐NP and SN tests (likely to reflect more symptomatic forms of SARS‐CoV‐2 infection [5]) constituted the secondary outcome. Associations between the seroprevalence of SARS‐CoV‐2 and anthropometric characteristics were assessed using multi‐adjusted logistic regression models (two‐sided tests, p < 0.05 considered as statistically significant; SAS 9.4).
The main analyses included 1027 ELISA‐S‐positive (among which 2.6% declared a COVID‐19‐related hospitalization) and 20,349 ELISA‐S‐negative participants (flowchart in the Supporting Information and characteristics of the participants in Table S1). A positive yet unclear association was observed between BMI before the March 2020 lockdown (self‐reported in April 2020) and SARS‐CoV‐2 seroprevalence in women: A positive linear trend for ELISA‐S (p 1‐SD‐increment = 0.07) significant when using restricted cubic splines (p = 0.04, with no evidence of non‐linearity, p non‐linearity = 0.1, Fig. S1) and for ELISA‐S‐NP/SN (p 1‐SD‐increment = 0.02), but no association with standard categories of BMI. No association was observed in men (Fig. 1). Waist circumference (p = 0.04) and waist‐to‐hip ratio (p = 0.01; self‐reported in June 2020) were associated with a higher seroprevalence in women, whereas opposite trends were observed in men (p = 0.08 and 0.03, respectively; restricted cubic splines in Fig. S2). Body, trunk and visceral fats (all p < 0.05) but not lean or muscle mass (measured in 2011–2014) were associated with higher seroprevalence, with similar trends in women only. Overall, results were similar considering the positivity to all three ELISA‐S‐NP/SN tests, with yet some loss of significance following a reduced number of cases (Table S2).
Fig. 1.
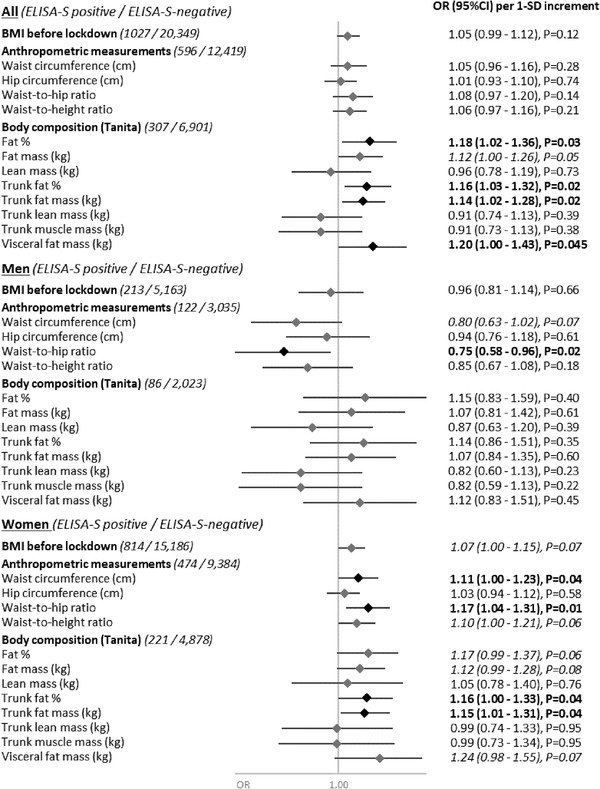
Anthropometric characteristics and SARS‐CoV‐2 infection (ELISA‐S), NutriNet‐Santé cohort–SAPRIS‐SERO, 2009–2020. Odds ratios and 95% confidence intervals obtained from multi‐adjusted logistic regression models assessing the association between anthropometric characteristics (BMI just before lockdown: body weight before the start of the lockdown in France in 17 March 2020, self‐reported in April 2020; anthropometric measurements: self‐measured and reported in June 2020; body composition: measured during a clinical examination between 2011 and 2014) and the seroprevalence of IgG antibodies directed against the S1 domain of the SARS‐CoV‐2 spike protein (positive vs. negative ELISA test; Euroimmun, Germany). Models included the following covariates: sex (men/women), age, educational level (< high‐school degree/high‐school degree/undergraduate degree/graduate degree), professional activity during the lockdown (no professional activity prior to lockdown: unemployed, retired, homemaker/partially unemployed/working outside home/working from home/student, trainee and other), physical activity level (high, moderate, low), smoking status (non‐smoker, former smoker, smoker), children and/or grandchildren aged under 18 years at home during the lockdown (yes/no), residential area during the lockdown (rural area/city < 20,000 inhabitants/city ≥ 20,000 to 100,000 inhabitants/city > 100,000 inhabitants), frequency of going out over the past week during the lockdown (never/once/two to five times/6–10 times/>10 times), prevalent chronic disease (cancer, cardiovascular disease, high blood pressure, diabetes, dyslipidemia: yes/no), regional residential area during the lockdown (Paris Basin/Center‐East/East/Mediterranean/North/West/Paris region/ Southwest) and month of blood draw (May–June/July/August–September–October). SD values were as follows: BMI before lockdown, SD: 4.5 kg/m²; waist circumference, SD: 13.0 cm; hip circumference, SD: 10.0 cm; waist‐to‐hip ratio, SD: 0.09; waist‐to‐height ratio, SD: 0.08; fat percentage, SD: 8.5; fat mass, SD: 8.5 kg; lean mass, SD: 9.7 kg; trunk fat percentage, SD: 8.7; trunk fat mass, SD: 4.7 kg; trunk lean mass, SD: 5.1 kg; trunk muscle mass, SD: 5.0 kg; and visceral fat mass, SD: 3.9 kg. Corresponding OR (95% CI) using standard categories of BMI and BMI [18.5,25.0] as reference were ORBMI<18.5 = 0.83 (0.60–1.15), ORBMI [25.0,30.0] = 1.05 (0.89–1.23), ORBMI≥30 = 0.98 (0.78–1.23) and p‐trend = 0.61.
Our results suggest a higher susceptibility to SARS‐CoV‐2 infection in women with higher body fat, displaying a moderate association with BMI, consistent with some prior reports [1, 2], but clearer associations with more specific markers of central adiposity.
The higher risk of SARS‐CoV‐2 infection associated with central adiposity in women may relate to impaired immune response, chronic low‐grade inflammation or a higher prevalence of angiotensin‐converting enzyme 2 receptors (SARS‐CoV‐2 entry point) in the adipose tissue [1]. In men, most associations were non‐significant (more limited sample size and power), except some inverse associations warranting further investigations, yet with a potential underlying mechanistic hypothesis involving reduced testosterone levels in obese men while testosterone would facilitate SARS‐CoV‐2 interaction with the angiotensin‐converting enzyme 2 receptor [6]. The study strengths are the exhaustive seroprevalence assessment with highly sensitive assays (able to detect antibodies even in asymptomatic/mild cases) [5, 7], independent of whether or not the participant sought testing in a large population‐based sample with detailed phenotyping (including anthropometric data). Yet, there are some limitations: The imperfect sensitivity of the ELISA‐S test [5, 7] and a possible decrease in antibodies over time potentially resulting in some misclassification. Waist and hip circumference data were collected in June 2020 and may be either post‐, contemporary to or pre‐SARS‐CoV‐2 infection (whereas most anthropometric data were collected before potential SARS‐CoV‐2 infection, allowing a prospective design: self‐reported but validated [8] body weight measures, and measured body composition data but acquired long before the COVID‐19 crisis), potentially resulting in some reverse causality bias if SARS‐CoV‐2 infection led to weight loss.
This study highlights central adiposity as an important factor to consider when looking at factors associated with a higher susceptibility to SARS‐CoV‐2 infection (e.g., for risk stratification in the population). This is all the more relevant since the excess weight has been shown to increase the risk for developing severe forms of COVID‐19, once infected.
Conflict of interest
Prof. Fabrice Carrat reports personal fees from Imaxio and Sanofi, outside the submitted work. All other authors declare no conflict of interest.
Supporting information
Supporting Information
References
- 1. Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID‐19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ho FK, Celis‐Morales CA, Gray SR, Katikireddi SV, Niedzwiedz CL, Hastie C, et al. Modifiable and non‐modifiable risk factors for COVID‐19, and comparison to risk factors for influenza and pneumonia: results from a UK Biobank prospective cohort study. BMJ Open. 2020;10:e040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hercberg S, Castetbon K, Czernichow S, Malon A, Mejean C, Kesse E, et al. The NutriNet‐Santé study: a web‐based prospective study on the relationship between nutrition and health and determinants of dietary patterns and nutritional status. BMC Public Health. 2010;10:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carrat F, Touvier M, Severi G, Meyer L, Jusot F, Lapidus N, et al. Incidence and risk factors of COVID‐19‐like symptoms in the French general population during the lockdown period: a multi‐cohort study. BMC Infect Dis. 2021;21:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carrat F, de Lamballerie X, Rahib D, Blanché H, Lapidus N, Artaud F, et al. Antibody status and cumulative incidence of SARS‐CoV‐2 infection among adults in three regions of France following the first lockdown and associated risk factors: a multicohort study. Int J Epidemiol. 2021. 10.1093/ije/dyab110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalra S, Bhattacharya S, Kalhan A. Testosterone in COVID‐19—foe, friend or fatal victim? Eur Endocrinol. 2020;16:88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cramer A, Goodman N, Cross T, Gant V, Dziadzio M. Analytical evaluation and critical appraisal of early commercial SARS‐CoV‐2 immunoassays for routine use in a diagnostic laboratory. Br J Biomed Sci. 2021;78:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lassale C, Péneau S, Touvier M, Julia C, Galan P, Hercberg S, et al. Validity of web‐based self‐reported weight and height: results of the NutriNet‐Sante study. J Med Internet Res. 2013;15:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


