Abstract
Objective
To assess the association between SARS‐CoV‐2 infection and decreased hand grip strength (HGS).
Design
Longitudinal population‐based study.
Setting
Community‐dwelling older adults (aged ≥60 years) living in a rural Ecuadorian village struck by the SARS‐CoV‐2 pandemic.
Participants
Of 282 enrolled individuals, 254 (90%) finished the study.
Measurements
HGS was measured 3 months before (January 2020) and 9 months after the introduction of the virus into the population (January 2021). SARS‐CoV‐2 antibody testing was performed in two rounds: in May–June (early) and September–November (late), 2020. An independent association between SARS‐CoV‐2 infection and HGS decline was assessed by fitting linear mixed models for longitudinal data. Changes in HGS scores in SARS‐CoV‐2 seropositive subjects, according to the time elapsed since seroconversion, were compared with those who remained seronegative.
Results
Overall, 149 (59%) individuals became seropositive for SARS‐CoV‐2. The mean HGS (in kg) was 25.3 ± 8.3 at baseline and 23.7 ± 8.1 at follow‐up (p = 0.028), with 140 individuals having >5% HGS decline between both measurements. The follow‐up HGS measurement decreased by 1.72 kg in seropositive individuals, and by 0.57 kg in their seronegative counterparts (p < 0.001). SARS‐CoV‐2 seropositive individuals were 2.27 times more likely (95% CI: 1.33–3.87) to have a lower HGS measurement at the time of follow‐up than those who remained seronegative. When compared with seronegative subjects, seropositive patients with early seroconversion were 3.41 times (95% CI: 1.73–6.74) more likely to have >5% HGS decline at the time of the follow‐up than those with later, i.e., more recent, infections.
Conclusions
This study shows an independent deleterious impact of SARS‐CoV‐2 on HGS that is more marked among individuals with infections that occurred more than 8 months before follow‐up HGS. Results suggest the possibility of chronic damage to skeletal muscles by SARS‐CoV‐2.
Keywords: COVID‐19, hand grip, older adults, rural communities, SARS‐CoV‐2
Key Points
This study shows an independent deleterious association between SARS‐CoV‐2 and decreased hand grip strength (HGS).
Decrease in HGS was only significant in those individuals whose SARS‐CoV‐2 infection occurred early in the study, allowing for a greater interval before follow‐up measurements.
Results suggest the possibility of chronic damage to skeletal muscles by SARS‐CoV‐2.
Why Does this Paper Matter?
Study results will help to better understand the decrease in muscle strength observed in subjects with history of SARS‐CoV‐2 infection, and to implement adequate programs of physical rehabilitation and nutrition therapy.
INTRODUCTION
The Coronavirus Disease 2019 (COVID‐19) pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) has affected almost 150 million people worldwide, a sizable proportion of whom are older adults. SARS‐CoV‐2‐infected individuals aged 60 years and older suffer the most devastating consequences of this pandemic, including higher mortality rates and more severe disease expression compared with younger affected individuals. 1 , 2 , 3 Age‐related comorbidities, e.g., arterial hypertension, diabetes, and cardiovascular disease, likely account for the grimmer prognosis of SARS‐CoV‐2 infections in older adults. 4 At the same time, it appears that this infection may exacerbate—or result in the development of—additional morbidities in people of this age group, many of whom are left with long‐lasting sequelae. 5 , 6 , 7 , 8 For example, a decrease in hand grip strength (HGS) has been associated with several chronic diseases, length of hospital stay, institutionalization and mortality, 9 and a decrease in muscle strength has been linked to SARS‐CoV‐2 infection. 10 Several studies have addressed the relationship between SARS‐CoV‐2 infection and decreased muscle strength, but the temporal relationship between them has not been well studied. Most available information derives from editorial comments or letters that provide no data or that report on hospitalized patients who did not have standardized assessments before and after the infection ocurred. 11 , 12 , 13 , 14
In January 2020, we commenced a door‐to‐door survey aimed at assessing clinical and neuroimaging correlates of HGS among community‐dwellers aged 60 years and older who were actively enrolled in the Atahualpa Project. The study had to be paused when the SARS‐CoV‐2 pandemic severely struck the village, resulting in a devastating mortality rate of 21.6 per 1000 population from March to April, 15 a seroprevalence of 45% among survivors, 16 and an incidence rate ratio of 7.4 per 100 person‐months of potential virus exposure. 17 Seizing on the unique opportunity presented by this ongoing prospective cohort, we evaluated differences in HGS before and after SARS‐CoV‐2 infection in older adults living in this rural setting.
METHODS
Study population
This study was conducted in community‐dwelling older adults residing in Atahualpa—a rural Ecuadorian village—where several neuroepidemiological studies have been carried out. 18 Residents are homogeneous regarding race/ethnicity (Amerindian ancestry), socioeconomic status (most men belong to the blue collar class and most women are homemakers), and dietary habits (rich in dietary oily fish intake, vegetables, and carbohydrates but poor in red meat and dairy products). In addition, the migration rate is low, which makes Atahualpa an optimal setting for the realization of population‐based cohort studies.
Study design
The study protocol and informed consent forms (signed by all participants) were approved by the I.R.B. of our Institution. This prospective longitudinal study is based on prior enrollment in the Atahualpa Project of 300 nondisabled, stroke‐free, community‐dwelling older adults who had clinical evaluations, a brain MRI, and HGS determinations 3 months before the onset of the SARS‐CoV‐2 pandemic in Atahualpa. For the purposes of studying the effect of SARS‐CoV‐2 infection on muscle strength in this cohort, we evaluated HGS 9 months after the start of the pandemic. We took into account the serological status of the individual as well as the presence of covariates that may have influenced changes between baseline and follow‐up HGS measurements. Due to a lack of normative data in the population of Amerindians and a lack of consensus for a cutoff indicating an abnormal loss of muscle strength, 9 we used HGS measurements as a continuous variable. Nevertheless, in order to define a significant reduction in HGS across baseline and follow‐up measurements, we selected a cutoff of greater than a 5% decrease, which is a percentage point more than the expected annual decline in HGS among older adults. 9
Cohort establishment
Out of 300 individuals receiving a baseline HGS measurement, 282 (94%) granted consent for the determination of SARS‐CoV‐2 IgM and IgG by the use of lateral flow‐based SARS‐CoV‐2 antibody testing (BIOHIT Health Care Ltd., Cheshire, UK). The antibody tests were performed from April to June, 2020.
This cohort was followed prospectively until January 2021 at which time HGS was again measured in those who were still active up to the censoring date. During the follow‐up period, participants received periodic home visits and clinical examinations as well as repeated serological tests in those who had previously tested negative. A subsequent round of tests was performed from September to November, 2020. Only individuals who remained seronegative in the first round received a subsequent serological test irrespective of whether they had asymptomatic infections or COVID‐19‐related symptomatology.
Hand grip strength protocol
HGS (recorded in kg) was measured by trained physicians using a Jamar© Smart Digital Hand Dynamometer (Performance Health, USA) at baseline and follow‐up. The device was calibrated according to procedures traceable to the National Institute of Standards & Technology (certification number: ASP010318‐0216). A variation of the Southampton protocol was adopted for study participants. 19 The main difference between our methodology and the Southampton protocol is that our subjects were tested while standing erect with legs straightened at 180° (Figure 1). Previous studies have shown no significant differences between the standing and the sitting position when measuring HGS so long as the elbows are flexed. 20 , 21 , 22 , 23 Consistent with the Southampton protocol, individuals had their shoulder adducted and neutrally rotated, with 90° flexion of the elbow, the forearm in neutral position, and the wrist with a mild extension of 15°–30°. Three trials were performed in the right and the left hand (alternating sides) with 10–15 s of rest per hand between each trial. Acquisition time for maximal strength was about 2–4 s, and the best of the six measurements was used for analysis. The device was always placed in the second position, 19 except for older individuals with small hand sizes that required the device to be placed in the first position. 23 Patients were actively encouraged to squeeze “hard enough to break the device” as this kind of verbal command increases HGS scores. 24 The same dynamometer was used for baseline and follow‐up HGS measurements, and the same trained physicians performed both assessments.
FIGURE 1.

Study participant at the time of hand grip strength measurement. Note the position of the body, arm, and hand
Covariates investigated
Demographics (age, gender), level of education (primary school or higher), physical activity, blood pressure, the body mass index, fasting glucose levels, oily fish intake, and neuroimaging evidence of white matter hyperintensities (WMH) of presumed vascular origin were selected as potentially confounding variables as they have been associated with frailty (a condition linked to decreased muscle strength) in previous studies conducted in the same population. 25 , 26 , 27 , 28 In addition, we took into account a covariate associated with the SARS‐CoV‐2 pandemic itself that may eventually modify HGS follow‐up measurements, such as home confinement for more than 2 months during the previous year.
According to the American Heart Association criteria, a level of poor physical activity was designated if the individual had no moderate or vigorous activity, a high blood pressure measuring ≥140/90 mmHg, a poor body mass index if ≥30 kg/m2, and a high fasting glucose if ≥126 mg/dl. 29 These cardiovascular risk factors were last updated in the study population during a door‐to‐door survey conducted in December 2019. Dietary oily fish intake (servings per week) was quantified based on a previously described questionnaire. 28
MRIs were performed in the year after enrollment in the Atahualpa Project cohort, with the use of a Philips Intera 1.5 T (Philips Medical Systems, Eindhoven, the Netherlands), following a previously detailed protocol. 27 MRI readings focused on WMH, which were defined as lesions appearing hyperintense on T2‐weighted images that remained bright on FLAIR (without cavitation) and graded according to the modified Fazekas scale in none‐to‐mild or moderate‐to‐severe. 30 MRIs were independently read by two study neurologists blinded to clinical information. The Kappa coefficient for interrater agreement was 0.93 for the presence of moderate‐to‐severe WMH; discrepancies were resolved by consensus, as previously described. 31
Statistical analysis
Data analyses were carried out using STATA version 16 (College Station, TX, USA). In univariate analyses, continuous variables were compared by linear models and categorical variables by x 2 or the Fisher exact test as appropriate. Changes in HGS between baseline and follow‐up measurements (as continuous variables), as well as the association between SARS‐CoV‐2 seropositivity and reductions in HGS, were assessed by means of linear mixed models for longitudinal data (random‐effects generalized least squares [GLS] regression), adjusted for relevant covariates. The independent relationship between SARS‐CoV‐2 infection and HGS decline greater than the 5% expected due to age—as the dependent variable—was assessed by fitting a logistic regression model. Estimated adjusted proportions were used to evaluate the differences in magnitude of the decline in HGS between seropositive and seronegative subjects, according to the time of seroconversion, after adjusting for all covariates.
RESULTS
The mean age of the 282 individuals enrolled in this cohort was 70.7 ± 7.8 years, and 171 (61%) were women. Ninety percent of the cohort (n = 254) remained in the study up to the censoring date and had follow‐up HGS. Eleven of the 28 individuals who lacked follow‐up HGS measurements died (five from COVID‐19), five were hospitalized due to severe COVID‐19 at the censoring date, three emigrated, one declined consent, and the remaining eight developed disabling conditions during the follow‐up period that precluded HGS measurements.
The 254 participants who completed both baseline and follow‐up HGS assessments had a mean age (at enrollment) of 70.2 ± 7.7 years; 153 (60%) were women; 190 (75%) had primary school education only; 12 (5%) reported no moderate or vigorous physical activity; 104 (41%) had blood pressures ≥140/90 mmHg; 56 (22%) had a body mass index ≥30 kg/m2; 72 (28%) had fasting glucose levels ≥126 mg/dl; the mean dietary oily fish intake was 9 ± 5.1 servings per week; and 42 (17%) individuals had moderate‐to‐severe WMH. A total of 122 (48%) subjects had been confined at home for at least 2 months.
Overall, 149 (58.7%; 95% CI: 52.5–64.5%) individuals became seropositive for SARS‐CoV‐2 antibodies. These included 120 of 254 (47.2%) individuals tested from May to June, and 29 of 134 (21%) tested from September to November. The mean interval between testing seropositive and the follow‐up HGS measurement in January 2021 was 8–9.2 months for those tested in May–June and 4–5.2 months for those tested in September–November.
The mean value of HGS measurements (in kg) was 25.3 ± 8.3 at baseline, and 23.7 ± 8.1 at the time of follow‐up. This difference represents an overall significant decline in HGS over time (p < 0.028). The longitudinal deficits in HGS persisted when men (32.9 ± 7 vs 31 ± 6.5; p = 0.047) and women (20.2 ± 4.3 vs 18.9 ± 4.6; p = 0.011) were analyzed separately, although the difference was more significant among women. A total of 140 (55%) individuals had more than a 5% decline in the follow‐up HGS measurement. This decline was evident in 87 of 153 (57%) women and in 53 of 101 (52%) men, but the difference between women and men was not significant (p = 0.491).
Table 1 depicts the characteristics of participants across categories of SARS‐CoV‐2 infection. Univariate analyses disclosed significant associations between HGS decline and SARS‐CoV‐2 seropositivity, but no association with any of the investigated covariates. Also in univariate analyses, the mean values of baseline HGS measurements did not differ between those who seroconverted during the follow‐up and those who remained seronegative (p = 0.189), whereas the mean value of follow‐up HGS measurements was significantly lower among seropositive individuals compared with their seronegative counterparts (p = 0.019). Similarly, the number of individuals who showed more than 5% HGS decline at follow‐up was significantly greater among seropositive individuals (p = 0.005).
TABLE 1.
Characteristics of Atahualpa residents aged 60 years and older across categories of SARS‐CoV‐2 serological status (univariate analyses)
| Variable | Total series (n = 254) | SARS‐CoV‐2 serological status | ||
|---|---|---|---|---|
| Seronegative (n = 105) | Seropositive (n = 149) | p Value | ||
| Age at enrollment, years (mean ± SD) | 70.2 ± 7.7 | 70.5 ± 8.4 | 69.9 ± 7.1 | 0.539 |
| Female, n (%) | 153 (60) | 60 (57) | 93 (62) | 0.398 |
| Primary school education, n (%) | 190 (75) | 77 (73) | 113 (76) | 0.651 |
| Poor physical activity, n (%) | 12 (5) | 7 (7) | 5 (3) | 0.221 |
| Blood pressure ≥ 140/90 mmHg, n (%) | 104 (41) | 46 (44) | 58 (39) | 0.059 |
| Body mass index ≥ 30 kg/m2, n (%) | 56 (22) | 27 (26) | 29 (19) | 0.237 |
| Fasting glucose ≥ 126 mg/dl, n (%) | 72 (28) | 28 (27) | 44 (30) | 0.618 |
| Oily fish intake, servings/week (mean ± SD) | 9 ± 5.1 | 8.9 ± 5 | 9.1 ± 5.2 | 0.759 |
| Moderate‐to‐severe WMH, n (%) | 42 (17) | 21 (20) | 21 (14) | 0.212 |
| Home confinement, n (%) | 122 (48) | 52 (50) | 70 (47) | 0.698 |
| Baseline hand grip, kg (mean ± SD) | 25.3 ± 8.3 | 26.1 ± 8.4 | 24.7 ± 8.3 | 0.189 |
| Follow‐up hand grip, kg (mean ± SD) | 23.7 ± 8.1 | 25.1 ± 8.1 | 22.7 ± 7.9 | 0.019* |
| Hand grip strength decline, n (%) | 140 (55) | 47 (45) | 93 (62) | 0.005* |
Statistically significant result.
A logistic regression model in which the dependent variable was whether a subject exhibited more than a 5% decline in HGS confirmed that SARS‐CoV‐2 seropositivity was the only predictor of a significant reduction in HGS between baseline and follow‐up determinations, after adjusting for all the investigated covariates. SARS‐CoV‐2 seropositive individuals were 2.27 times more likely (95% CI: 1.33–3.87) to have a lower HGS than those who remained seronegative (Table 2).
TABLE 2.
Logistic regression model showing the independent relationship between SARS‐CoV‐2 seropositivity and hand grip strength decline
| Decline in hand grip strength | Odds ratio | Standard error | 95% Confidence interval | p Value |
|---|---|---|---|---|
| SARS‐CoV‐2 seropositivity | 2.27 | 0.62 | 1.33–3.87 | 0.003* |
| Age at enrollment | 1.03 | 0.02 | 0.99–1.07 | 0.107 |
| Female gender | 0.90 | 0.26 | 0.51–1.60 | 0.722 |
| Primary school education | 1.02 | 0.32 | 0.55–1.89 | 0.953 |
| Poor physical activity | 0.60 | 0.38 | 0.17–2.11 | 0.421 |
| Blood pressure ≥ 140/90 mmHg | 1.32 | 0.37 | 0.76–2.29 | 0.320 |
| Fasting glucose ≥ 126 mg/dl | 0.72 | 0.22 | 0.40–1.30 | 0.275 |
| Body mass index ≥ 30 kg/m2 | 1.79 | 0.63 | 0.90–3.56 | 0.095 |
| Oily fish intake, servings/week | 1.04 | 0.03 | 0.99–1.09 | 0.158 |
| Home confinement | 1.47 | 0.44 | 0.82–2.64 | 0.195 |
| Moderate‐to‐severe WMH | 0.95 | 0.35 | 0.46–1.96 | 0.888 |
Statistically significant result.
Further analysis by means of mixed models (using continuous baseline and follow‐up values for each subject as his/her own control) demonstrated that the difference in baseline HGS among those who became seropositive compared with those who remained seronegative at follow‐up was not significant (β: −1.43; 95% CI: −3.51 to 0.66; p = 0.178). This finding confirms that the level of HGS at baseline was not associated with an increased risk of SARS‐CoV‐2 infection.
A random‐effects GLS regression that included the impact of time and seropositivity on the follow‐up HGS (as a continuous variable) showed that both variables contributed to a worsening in HGS, but the association with seropositivity was larger. Compared with baseline HGS measurements, the β coefficient for seropositive individuals was larger (β: −1.98; 95% CI: −2.47 to −1.48) than that attributable only to the follow‐up in their seronegative counterparts (β: −0.96; 95% CI: −1.54 to −0.37); however, both declines were significant. When all covariates were added to this model, the impact of time from seroconversion to follow‐up (early vs late) on HGS vanished, but the association with SARS‐CoV‐2 seropositivity remained highly significant. HGS measurements at follow‐up decreased by 1.72 kg in seropositive individuals compared with only 0.57 kg among those who remained seronegative. Increasing age, being female, having high fasting glucose levels, and the presence of moderate‐to‐severe WMH all contributed to a worsening in HGS measurements at follow‐up, whereas the amount of dietary intake of oily fish was a protective factor (Table 3).
TABLE 3.
Fully‐adjusted random‐effects GLS regression model that included the effect of time and SARS‐CoV‐2 seropositivity on hand grip strength at the follow‐up
| Hand grip strength | β coefficient | Standard error | 95% confidence interval | p Value |
|---|---|---|---|---|
| Hand grip strength at baseline | Referent category | |||
| Hand grip strength at follow‐up in seronegative subjects | −0.57 | 0.30 | −1.15 to 0.01 | 0.054 |
| Hand grip strength at follow‐up in seropositive subjects | −1.72 | 0.25 | −2.21 to −1.23 | <0.001* |
| Age at enrollment, years | −0.31 | 0.04 | −2.21 to −1.23 | <0.001* |
| Female gender | −12.1 | 0.59 | −13.2 to −10.9 | <0.001* |
| Primary school education | −0.09 | 0.68 | −1.42 to 1.23 | 0.886 |
| Poor physical activity | −0.39 | 1.35 | −3.04 to 2.25 | 0.769 |
| Blood pressure ≥ 140/90 mmHg | −0.27 | 0.59 | −1.42 to 0.89 | 0.652 |
| Body mass index ≥ 30 kg/m2 | 0.29 | 0.72 | −1.13 to 1.71 | 0.688 |
| Fasting glucose ≥ 126 mg/dl | −1.89 | 0.63 | −3.12 to −0.65 | 0.003* |
| Oily fish intake, servings/week | 0.14 | 0.05 | 0.03 to 0.24 | 0.013* |
| Moderate‐to‐severe WMH | −1.97 | 0.78 | −3.49 to −0.45 | 0.011* |
Statistically significant result.
When study participants were stratified according to whether they remained seronegative or became seropositive with a shorter or longer interval of time to follow‐up, a logistic regression model adjusted for relevant confounders showed that seropositive individuals with longer intervals yielded a significantly greater proportion of subjects with more than 5% HGS decline at the follow‐up by 3.41 times; 95% 1.73–6.70; p < 0.001 (Table S1). In this analysis, the difference between no infection (seronegative) and shorter duration of seropositivity was not significant (p = 0.127). Estimated proportions confirmed the significant association (p < 0.01; no overlapping 95% CI) between those who remained seronegative and those with long exposure (Figure 2).
FIGURE 2.
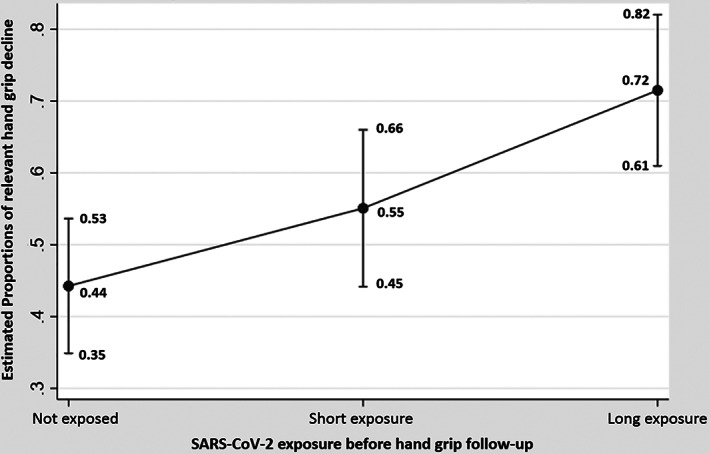
Graph plot showing differences in estimated proportions of hand grip strength decline >5% across individuals who remained seronegative versus those who had long exposure versus short exposure to SARS‐CoV‐2
An additional multivariate linear regression model using the within‐patient difference in continuous HGS measurement (instead of the categorized decline) also demonstrated a significant difference at follow‐up between individuals who remained seronegative and those who became seropositive with a longer duration (β: −1.02; 95% CI: −1.80 to −0.23; p = 0.011), but not with those who became seropositive with a shorter duration (β: −0.97; 95% CI: −1.94 to 0.02; p = 0.055). In none of these models did home confinement during the follow‐up prove to be independently significant.
DISCUSSION
This longitudinal prospective study demonstrates the impact of the SARS‐CoV‐2 pandemic on HGS. Although both seronegative and seropositive individuals had similar baseline HGS measurements before the pandemic, seropositive individuals showed a significantly greater decline in HGS at the end of the follow‐up period.
Our study found three important and distinct aspects of the association between SARS‐CoV‐2 infection and HGS. First, individuals with lower baseline measurements of HGS were not more prone to become infected with the virus or to develop COVID‐19 as evidenced by similar levels of HGS across subjects who subsequently became infected and those who did not. Second, once an individual became infected, the odds of developing a decline in HGS greater than that expected due to age alone were more than three times higher than if the subject was not infected. At the same time, the many covariates investigated had a minimal impact on this outcome. Third, the deleterious association between SARS‐CoV‐2 infection and HGS appears to worsen over time as evidence by a more significant decline found in those who seroconverted earlier versus later.
Study results are in line with the increasingly recognized delayed complications of SARS‐CoV‐2 infection. Indeed, the term “Long‐COVID” has been coined to define persistence of clinical manifestations or laboratory abnormalities beyond the acute phase of the disease. 32 , 33 A recent systematic review and meta‐analysis of studies identified 55 long‐term effects of COVID‐19, a sizable proportion of which involve the central or the peripheral nervous system. 34 According to this and other reviews, neurological sequelae include—but are not limited to—cognitive decline, sleep disorders, chronic headache, stroke sequelae (stroke often occurs during the acute phase of the disease), decreased muscle strength, chronic fatigue, ageusia and anosmia, hearing loos and tinnitus, and neuropathic sequelae of acute peripheral nerve abnormalities. 5 , 33 , 34 Some of these long‐term neurological sequelae may occur in patients who had experienced only mild disease yet they persist several months after the acute episode. 7 , 8 Similarly, other infections may contribute to a loss in muscle strength. 35 , 36
Whether decreased muscle strength predisposes to SARS‐CoV‐2 infection or more severe disease has been subject to debate. 37 , 38 It has been argued that decreased muscle strength is associated with decreased expression of the immune system, which may increase susceptibility to SARS‐CoV‐2 and other viral infections. 39 To our knowledge, however, no study has provided data on the relationship between decreased muscle strength and increased risk of SARS‐CoV‐2 infection. Results of the present study that show no increased risk of SARS‐CoV‐2 infection among individuals with lower baseline HGS measurements argue against this conjecture. These results are consistent with a previous survey conducted in the same population, which found that frailty (a condition closely related to decreased muscle strength) does not facilitate infection with SARS‐CoV‐2. 40
That the measured decline in HGS was similar between not infected individuals and those infected near the end of the study helps to differentiate decreased muscle strength from the frequently reported fatigue or pain‐related syndromes that occur during the first weeks after a symptomatic COVID‐19 episode. 41 It is possible that direct damage to muscle cells or the surrounding vasculature (with extension of the damage into skeletal muscle cells) by the virus may account for the delayed decrease in muscle strength found in our study. This hypothesis is supported by the demonstrated invasion of the myocardium by SARS‐CoV‐2. 42 The receptor used by this virus for cell entry—angiotensin‐converting enzyme‐2—is expressed in skeletal muscle cells as well as in endothelial cells, 43 and provides a possible mechanism for the loss of muscle strength in survivors of this infection that may not necessarily be related to the severity of acute COVID‐19. Viral particles in damaged muscle cells and surrounding endothelial cells have also been demonstrated in histopathological examinations of muscle tissue, thus providing further evidence for this hypothesis. 44
Interestingly, home confinement for more than 2 months was not associated with a decline in HGS measurements in any of the models. This can be explained by the characteristics of the study population. As previously noted, most men work as artisan carpenters in small carpentry shops located at their homes while most women are homemakers. For this reason, many of the subjects were able to continue with their usual level of activity despite confinement. This may not be the case for other populations where people suffer a decrease in muscle strength due to lockdown restrictions. 45 It must also be recognized that our study participants were taken from the community and not from a long‐term care facility where the residents do not engage in active physical activity, are often medicated with sedative drugs, and live in close quarters in a crowded environment.
This study provides a unique opportunity to prospectively evaluate HGS by means of an internationally accepted protocol, in a population of community‐dwelling older adults who underwent a formal evaluation both before and several months after the onset of the SARS‐CoV‐2 pandemic. In addition, we compared—in the same population—the association between older versus more recent infections and the decline in HGS at the time of follow‐up. This reduces the likelihood of unexpected confounders that may occur when two different populations are compared. Another strength of this study is the inclusion of long‐term participants in the Atahualpa Project cohort in whom several other risk factors have already been investigated. Nevertheless, the study has limitations. Significant findings in longitudinal studies may hide longer‐term effects and not be causal. The study population was limited to people aged 60 years and older. As such, we may have missed cases of HGS decline among middle‐aged adults with SARS‐CoV‐2 infection. In addition, although the lateral flow‐based antibody test we used is reported to have high diagnostic reliability, we cannot exclude the possibility of some degree of misclassification due to false positive or false negative results, 16 , 17 or the improbable scenario of cross‐reactions with other viruses that are endemic in the region. 46
In summary, this longitudinal study cohort demonstrated in a select cohort an independent detrimental association between SARS‐CoV‐2 and HGS worsening, which reached significance among individuals with older rather than more recent infections. Further long‐term prospective studies may be helpful to achieve a better understanding of the progression of HGS decline in subjects with history of SARS‐CoV‐2 infection. In addition, these findings may have implications for the design and implementation of programs of physical rehabilitation and improved nutrition in affected individuals.
CONFLICT OF INTEREST
The authors declare they do not have competing financial or nonfinancial conflict to disclose.
AUTHOR CONTRIBUTIONS
Oscar H. Del Brutto: conceived and designed the study, manuscript drafting; Robertino M. Mera: statistical analyses of data; Pedro Pérez, Bettsy Y. Recalde and Aldo F. Costa: data acquisition and analysis; Mark J. Sedler: significant intellectual contribution to study design and manuscript drafting.
SPONSOR'S ROLE
The sponsors had no role in the design of the study, in data collection or analyses, or in the decision to submit the study for publication.
Supporting information
Table S1: Logistic regression model showing that a longer exposure to SARS‐CoV‐2 increased the proportion of subjects with hand grip strength decline.
ACKNOWLEDGMENTS
This study was partly supported by Universidad Espiritu Santo—Ecuador, and by the Renaissance School of Medicine, Stony Brook University, New York, NY, USA.
Del Brutto OH, Mera RM, Pérez P, Recalde BY, Costa AF, Sedler MJ. Hand grip strength before and after SARS‐CoV‐2 infection in community‐dwelling older adults. J Am Geriatr Soc. 2021;69(10):2722–2731. 10.1111/jgs.17335
Funding information Renaissance School of Medicine, Stony Brook University, New York, NY, USA; Universidad Espiritu Santo ‐ Ecuador
REFERENCES
- 1. Bonanad C, García‐Blas S, Tarazona‐Santabalbina F, et al. The effect of age on mortality in patients with COVID‐19: a meta‐analysis with 611,583 subjects. J Am Med Dir Assoc. 2020;21:915‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang Z, Guo L, Huang L, et al. Distinct disease severity between children and older adults with COVID‐19: impacts of ACE2 expression, distribution, and lung progenitor cells. Clin Infect Dis. 2021. 10.1093/cid/ciaa1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68:926‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Honardoost M, Janani L, Aghili R, Emami Z, Khamseh ME. The association between presence of comorbidities and COVID‐19 severity: a systematic review and meta‐analysis. Cerebrovasc Dis. 2021;50:132‐140. 10.1159/000513288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang F, Kream RM, Stefano GB. Long‐term respiratory and neurological sequelae of COVID‐19. Med Sci Monitor. 2020;26:e928996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu J, Deswal A, Khalid U. COVID‐19 myocarditis and long‐term heart failure sequelae. Curr Opin Cardiol. 2021;36:234‐240. [DOI] [PubMed] [Google Scholar]
- 7. Del Brutto OH, Mera RM, Costa AF, Recalde BY, Castillo PR. Sleep quality deterioration in middle‐aged and older adults living in a rural Ecuadorian village severely struck by the SARS‐CoV‐2 pandemic. A population‐based longitudinal prospective study. Sleep. 2021. 10.1093/sleep/zsab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Del Brutto OH, Wu S, Mera RM, Costa AF, Recalde BY, Issa NP. Cognitive decline among individuals with history of mild symptomatic SARS‐CoV‐2 infection. A longitudinal prospective study nested to a population cohort. Eur J Neurol. 2021. 10.1111/ene.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cruz‐Jentoft AJ, Bahat G, Nauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morley JE, Kalantar‐Zadeh K, Anker SD. COVID‐19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle. 2020;11:863‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lim WS, Liang CK, Assantachai P, et al. COVID‐19 and older people in Asia: Asian working Group for Sarcopenia calls to actions. Geriatr Gerontol Int. 2020;20:547‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gadelha AB, Lima RM. COVID‐19 quarantine in older people: the need of thinking about sarcopenia‐related phenotypes. J Frailty Aging. 2020;9:244‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Welch C, Creig C, Masud T, Wilson D, Jackson TA. COVID‐19 and acute sarcopenia. Aging Dis. 2020;11:1345‐1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SH, Gong HS. Measurement and interpretation of handgrip strength for research on sarcopenia and osteoporosis. J Bone Metab. 2020;27:85‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Del Brutto OH, Costa AF, Mera RM, Recalde BY, Bustos JA, García HH. SARS‐CoV‐2‐related mortality in a rural Latin American population. Int J Infect Dis. 2020;99:226‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Del Brutto OH, Costa AF, Mera RM, Recalde BY, Bustos JA, García HH. SARS‐CoV‐2 in rural Latin America. A population‐based study in coastal Ecuador. Clin Infect Dis. 2020. 10.1093/cid/ciaa1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Del Brutto OH, Costa AF, Mera RM, Recalde BY, Bustos JA, García HH. Late incidence of SARS‐CoV‐2 infection in a highly endemic remote rural setting. A prospective population‐based cohort study. Pathog Glob Health. 2020;114:457‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Del Brutto OH, Mera RM, Castillo PR, Del Brutto VJ. Key findings from the Atahualpa project: what should be learn? Expert Rev Neurother. 2018;18:5‐8. [DOI] [PubMed] [Google Scholar]
- 19. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Aging. 2011;40:423‐429. [DOI] [PubMed] [Google Scholar]
- 20. El‐Sais WM, Mohammad WS. Influence of different testing postures on hand grip strength. Eur Sci J. 2014;10(36):290‐301. [Google Scholar]
- 21. Watanabe T, Owashi K, Kanauchi Y, Mura N, Takahara M, Ogino T. The short‐term reliability of grip strength measurement and the effects of posture and grip span. J Hand Surg Am. 2005;30(3):603‐609. [DOI] [PubMed] [Google Scholar]
- 22. De S, Sengupta P, Maity P, Pal A, Dhara P. Effect of body posture on hand grip strength in adult Bengalee population. JESP. 2011;7(2):79‐88. [Google Scholar]
- 23. Ruiz‐Ruiz J, Mesa JL, Gutiérrez A, Castillo MJ. Hand size influences optimal grip span in women but not in men. J Hand Surg Am. 2002;27:897‐901. [DOI] [PubMed] [Google Scholar]
- 24. Jung MC, Hallbeck MS. The effects of instruction, verbal encouragement, and visual feedback on static handgrip strength. Proc Hum Factors Ergon Soc Annu Meet. 1999;43:703‐707. [Google Scholar]
- 25. Del Brutto OH, Mera RM, Sedler JM, et al. The effect of age in the association between frailty and poor sleep quality: a population‐based study in community‐dwellers (the Atahualpa project). J Am Med Dir Assoc. 2016;17:269‐271. [DOI] [PubMed] [Google Scholar]
- 26. Del Brutto OH, Mera RM, Zambrano M, Sedler MJ. Influence of frailty on cognitive decline: a population‐based cohort study in rural Ecuador. J Am Med Dir Assoc. 2019;20:213‐216. [DOI] [PubMed] [Google Scholar]
- 27. Del Brutto OH, Mera RM, Cagino K, et al. Neuroimaging signatures of frailty: a population‐based study in community‐dwelling older adults (the Atahualpa project). Geriatr Gerontol Int. 2017;17:270‐276. [DOI] [PubMed] [Google Scholar]
- 28. Del Brutto OH, Mera RM, Ha JE, Gillman J, Zambrano M, Sedler MJ. Dietary oily fish intake and frailty. A population‐based study in frequent fish consumers living in rural coastal Ecuador (the Atahualpa project). J Nutr Gerontol Geriatr. 2020;39:88‐97. [DOI] [PubMed] [Google Scholar]
- 29. Lloyd‐Jones D, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion. The American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586‐613. [DOI] [PubMed] [Google Scholar]
- 30. Pantoni L, Basile AM, Pracucci G, et al. Impact of age‐related cerebral white matter changes on the transition to disability: the LADIS study: rationale, design and methodology. Neuroepidemiology. 2005;24:51‐62. [DOI] [PubMed] [Google Scholar]
- 31. Del Brutto OH, Mera RM, Recalde BY, Del Brutto VJ. Cerebral small vessel disease in community‐dwelling older adults living in remote rural settings. J Neurol Sci. 2020;416:117016. [DOI] [PubMed] [Google Scholar]
- 32. Mandal S, Barnett J, Brill SE, et al. “Long‐COVID”: a cross‐sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalization for COVID‐19. Thorax. 76:396‐398. 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626‐631. 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lopez‐Leon S, Wegman‐Ostrosky T, Perelman C, et al. More than 50 long‐term effects of COVID‐19: a systematic review and meta‐analysis. medRxiv. 10.1101/2021.01.27.21250617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang GC, Chiu YL. Walston JD (2019) CMV infection and frailty: immunologic consequences and disease pathogenesis. In: Fulop T, Franceschi C, Hirokawa K, Pawelec G, eds. Handbook of Immunosenescence. Cham: Springer; 2019. [Google Scholar]
- 36. Tang M, Quanstrom K, Jin C, Suskind AM. Recurrent urinary tract infections are associated with frailty in older adults. Urology. 2019;123:24‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang PY, Li Y, Wang Q. Sarcopenia: an underlying treatment target during the COVID‐19 pandemic. Nutrition. 2020;84:111104. 10.1016/j.nut.2020.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Silveiro R, Gonçalves DC, Andrade MF, Seelaender M. Coronavirus disease 2019 (COVID‐19) and nutritional status: the missing link? Adv Nutr. 2020;12:682‐692. 10.1093/advances/nmaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin‐6 and tumor necrosis factor‐alpha with muscle mass and muscle strength in elderly men and women: the health ABC study. J Gerontol A Biol Sci Med Sci. 2002;57:M326‐M332. [DOI] [PubMed] [Google Scholar]
- 40. Del Brutto OH, Costa AF, Recalde BY, Mera RM. Frailty and SARS‐CoV‐2 infection. A population study in a highly endemic village. J Neurol Sci. 2020;418:117136. 10.1016/j.jns.2020.117136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crokks MG. Post‐COVID‐19 symptom burden: what is long‐COVID and how should we manage it? Lung. 2021;199:113‐119. 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamamoto K, Takeshita H, Rakugi H. ACE2, angiotensin 1‐7 and skeletal muscle: review in the era of COVID‐19. Clin Sci (Lond). 2020;134:3047‐3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hooper JE, Uner M, Priemer DS, Rosenberg A, Chen L. Muscle biopsy findings in a case of SARS‐CoV‐2‐associated muscle injury. J Neuropathol Exp Neurol. 2020;80:377‐378. 10.1093/jnen/nlaa155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roshdy A, Zaher S, Fayed H, Coghlan JG. COVID‐19 and the heart: a systematic review of cardiac autopsies. Front Cardiovasc Med. 2021;7:626975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kirwan R, McCullough D, Butler T, Perez de Heredia F, Davies IG, Stewart C. Sarcopenia during COVID‐19 lockdown restrictions: long‐term health effects of short‐term muscle loss. Geroscience. 2020;42:1547‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spinici M, Bartoloni A, Mantella A, Zammarchi L, Rossolini GM, Antonelli A. Low risk of serological cross‐reactivity between dengue and COVID‐19. Mem Inst Oswaldo Cruz. 2020;115:e200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Logistic regression model showing that a longer exposure to SARS‐CoV‐2 increased the proportion of subjects with hand grip strength decline.


