Author contributions
N.D., S.G. and B.O. designed the study. E.A. N.D., B.G., N.F., S.A., S.G. and B.O. consulted with the different patients. E.A., S.G. and B.O collected the data. P.S. performed the pathological analysis. E.A., N.D., S.G. and B.O. wrote the manuscript with the input from all the other authors.
Conflict of interest
None.
Funding sources
None.
Dear Editor,
Among the vaccines against coronavirus disease 2019 (COVID‐19), BNT162b2 from BioNtech‐Pfizer and mRNA‐1273 from Moderna are mRNA vaccines targeting the spike protein of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The ChAdOx1 nCoV‐19 (AZD1222) from Oxford‐AstraZeneca is based on adenovirus expressing the full‐length spike protein. Clinical trials reported different cutaneous adverse events, mainly local injection site reactions, either immediate or delayed on/after 8 days. 1 , 2 , 3
We conducted a retrospective observational study among patients referred to the Dermatology Department of Cochin Hospital from January 2021 to April 2021 who presented with skin manifestations induced by COVID‐19 vaccines. We excluded patients with immediate and/or delayed local site injection reactions.
We included 8 consecutive cases, 3 men and 5 women, aged from 44 to 80 years, with no history of prior SARS‐CoV‐2 infection (6 patients had negative SARS‐CoV‐2 serology) or prior vaccine/drug‐induced manifestations (Table 1). Five, 1 and 2 patients received Pfizer, Moderna or Oxford‐AstraZeneca vaccine, respectively. We observed various skin reactions on average 6 days after the first dose: 2 morbilliform exanthemas, diffuse cutaneous erythema, acute generalized exanthematous pustulosis (AGEP), localized oedematous infiltrated plaque, erythematous indurated nodules or livedo racemosa (Fig. 1, Table 1). Patient 6 developed fixed drug eruption (FDE) 2 days after the second dose without previous history of FDE. No associated systemic manifestation was observed except in Patients 7 and 8 who presented with eosinophilia and fever, respectively. Patient 5 showed a livedo racemosa mimicking erythema ab igne without history of chronic heat exposure. A skin biopsy was performed in 7 patients (Table 1). Pathological examination showed several different non‐specific patterns including association of features of spongiotic and interface dermatitis. Several cases resembled cutaneous drug reactions and 2 had inflammatory infiltrate with numerous eosinophils. In one case, skin biopsy displayed a superficial and deep perivascular and perieccrine lymphocytic infiltrate similar to those of chilblains or chilblain‐like lesions. Symptoms subsided within 8–30 days in 6 patients; mild symptoms persisted in 2 cases. Relapse or new skin manifestations occurred in 2 patients following the second dose without worsening of symptoms. Patient 3 relapsed 4 days after the second dose with diffuse cutaneous erythema, also treated with topical corticosteroids and UVB phototherapy. Patient 2 presented with 2 erythematous nodules 4 days after the first dose and chilblains 5 days after the second dose without nodules relapse. A serology performed 2 days after chilblains onset revealed high levels of spike‐specific IgG antibodies. Patient 7 presenting with AGEP did not relapse upon second dose of Pfizer vaccine (Table 1).
Table 1.
Clinical and pathological characteristics of cutaneous adverse events induced by COVID‐19 vaccines
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Sex, Age (y) | F,72 | M, 55 | M,76 | F, 67 | F, 55 | F, 80 | F, 43 | M, 44 |
| Comorbidities | Hypothyroidism, depression | None | None | Idiopathic CD4 immunodeficiency, thyroiditis, cutaneous vasculitis | Familial myoclonic dystonia | Mycosis fungoides, hypothyroidism, depression | HIV, Kaposi’s disease | None |
| Prior COVID‐19 infection | No | No | No | No | No | No | No | No |
| Vaccine | Pfizer | Pfizer | Pfizer | Pfizer | Pfizer | Moderna | Astra Zeneca | Astra Zeneca |
| Time from 1st (or 2nd where indicated) dose to skin reaction onset (d) | 7 | 4 / 5 (after 2nd injection) | 5 | 8 | 12 | 2 (after 2nd injection) | 3 | 3 |
| Cutaneous manifestations | Morbilliform rash (50% of BSA) | Erythematous indurated nodules/chilblains | Diffuse erythematous rash (80% of BSA) | Morbilliform rash, pathergy reaction (50% of BSA) | Livedo racemosa of thighs | Fixed drug eruption | Diffuse maculopapular pustular exanthema (>80% of BSA) | Oedematous infiltrated plaque of buttock and thigh |
| Histopathological features | Spongiotic dermatitis | NA / papillary dermal oedema, superficial and deep perivascular and perieccrine lymphocytic infiltrate | Vacuolar interface dermatitis, spongiosis, perivascular superficial lymphocytic infiltrate | NA | Epidermal dysmaturation, vacuolization of basal keratinocytes, apoptotic cells | Vacuolar interface dermatitis, perivascular superficial lymphocytic infiltrate with numerous eosinophils | Lichenoid interface dermatitis, intracorneal pustules, lymphocytic infiltrate with numerous eosinophils | Papillary dermal oedema, superficial and deep perivascular lymphoplasmocytic infiltrate |
| Systemic manifestation | None | None for both manifestations | None | None | None | None | Eosinophilia, leucocytosis | Fever |
| Specific treatment | None | None for bothmanifestations | Topical CS, phototherapy | Topical CS | None | Topical CS | topical CS | none |
| Evolution | Resolution within 8d | Resolution within 7d for both manifestations | Improvement | Resolution within 15d | Persistence of post‐inflammatory pigmented lesions at 2 months | Resolution within 5d | Resolution within 30d | Resolution within 16d |
| Relapse following the 2nd dose | No | Yes (but different manifestation) | Yes | No | No | Appeared after the second dose | No (received Pfizer vaccine) | No |
F, female; M, male; y, year; d, day; BSA, body surface area; NA, non‐available; CS, corticosteroids.
Figure 1.
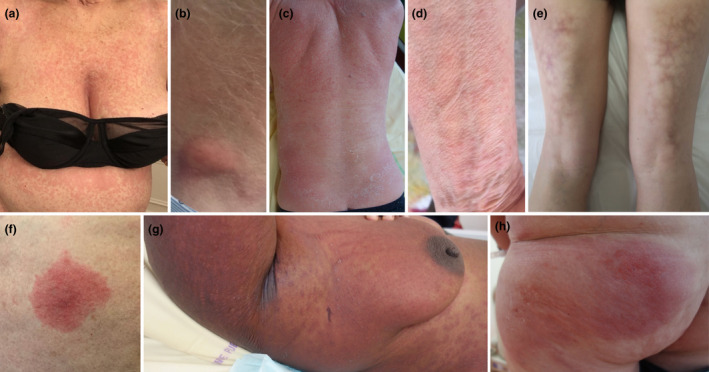
Cutaneous manifestations induced by COVID‐19 vaccines. Clinical pictures of morbilliform rashes (a, Patient 1; d, Patient 4), cervical erythematous indurated nodule (b, Patient 2), a diffuse erythematous rash (c, Patient 3), livedo racemosa (e, Patient 5), FDE (f, Patient 6), AGEP (g, Patient 7) and oedematous infiltrated plaque (h, Patient 8).
Various skin reactions (local site and delayed large local reaction, urticaria, morbilliform purpuric and/or oedematous rash, erythromelalgia, pernio/chilblains, vasculitis) were recently described following Pfizer or Moderna COVID‐19 vaccine. 3 , 4 , 5 We report for the first time vaccine‐induced livedo, FDE, AGEP or distant localized oedematous infiltrated plaques or nodules. Relapse occurred in 2 of our patients following the second dose without worsening of symptoms. One relapsing morbilliform rash and 2 cases of recurrent chilblains were described in patients following Moderna and Pfizer vaccine. 6 , 7 , 8 In McMahon study, 43% of patients with first‐dose reactions experienced second‐dose recurrence with similar, milder or more severe reactions in 28%, 28% and 45% of cases, respectively. 4 Practitioners should be aware of these side‐effects of COVID‐19 vaccines which do not require vaccination discontinuation.
Similar COVID‐19‐associated manifestations have been described in a registry of 716 cases: morbilliform rash (22%), pernio‐like lesions (18%), urticaria (16%), retiform purpura (6.4%), and macular erythematous (13%), or vesicular (11%), or papulo‐squamous lesions (9.9%). 9 , 10 One could hypothesize a common immune response directed against the spike RNA or protein inducing vaccine and virus‐associated skin lesions.
Compliance with ethical standards
The patients in this manuscript have given written informed consent to publication of their case details in accordance with French Bioethics Law for retrospective non‐interventional research studies.
Acknowledgement
The authors would like to thank all members of the Dermatology medical team of Cochin Hospital. Of note, Patient 2 case report has previously been published (PMID 34396420).
E. Annabi and N. Dupin contributed equally to this report as first authors.
S. Guégan and B. Oulès contributed equally to this report as co‐senior authors.
References
- 1. Baden LR, El Sahly HM, Essink B et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 Vaccine. N Engl J Med 2021; 384: 403–416. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N Engl J Med 2020; 383: 2603–2615. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Menni C, Klaser K, May A et al. Vaccine side‐effects and SARS‐CoV‐2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 2021; 21: 939–949. 10.1016/S1473-3099(21)00224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McMahon DE, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 cases. J Am Acad Dermatol 2021; 85: 46–55. 10.1016/j.jaad.2021.03.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corbeddu M, Diociaiuti A, Vinci MR et al. Transient cutaneous manifestations after administration of Pfizer‐BioNTech COVID‐19 Vaccine: an Italian single‐centre case series. J Eur Acad Dermatology Venereol 2021; 35: e483–e485. 10.1111/jdv.17268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jedlowski PM, Jedlowski MF. Morbilliform rash after administration of Pfizer‐BioNTech COVID‐19 mRNA vaccine. Dermatol Online J 2021; 27: 13030. 10.5070/D3271052044 [DOI] [PubMed] [Google Scholar]
- 7. Kha C, Itkin A. New‐onset chilblains in close temporal association to mRNA‐1273 vaccination. JAAD Case Reports 2021; 12: 12–14. 10.1016/j.jdcr.2021.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piccolo V, Bassi A, Argenziano G et al. BNT162b2 mRNA Covid‐19 Vaccine‐induced chilblain‐like lesions reinforces the hypothesis of their relationship with SARS‐CoV‐2. J Eur Acad Dermatol Venereol 2021; 35: e493–e494. 10.1111/jdv.17320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freeman EE, McMahon DE, Lipoff JB et al. The spectrum of COVID‐19–associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol 2020; 83: 1118–1129. 10.1016/j.jaad.2020.06.1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matar S, Oulès B, Sohier P et al. Cutaneous manifestations in SARS‐CoV‐2 infection (COVID‐19): a French experience and a systematic review of the literature. J Eur Acad Dermatol Venereol 2020; 34: e686–e689. 10.1111/jdv.16775 [DOI] [PMC free article] [PubMed] [Google Scholar]


