To the editor
Early detection of melanoma is an important intervention to reduce morbidity and mortality. 1 , 2 The COVID‐19 pandemic has affected timely access to health care, potentially affecting patient outcomes. Marson et al. 3 showed that the incidence of melanoma decreased during the pandemic using the United States data. Lallas et al. 4 demonstrated an overall 30.1% decrease in cancers diagnosed during the pandemic in Greece. We sought to evaluate whether melanomas diagnosed during the pandemic at our medical centre differed in stage compared to the prepandemic time period.
This was an IRB‐approved, retrospective study. We reviewed consecutive melanoma biopsy reports performed from January 2019 to March 2021 from Pontificia Universidad Catolica de Chile. We included adult (≥18 years) patients with histopathology‐confirmed diagnosis of melanoma. We excluded patients that were not evaluated at our institution (e.g. tissue slides sent for consultation) and non‐cutaneous melanomas. Patients’ demographics and pathological characteristics were recorded. For study purposes and based on our local epidemiology, ‘pre‐COVID period (preCP)’ ranged between January 2019 and March 2020. ‘COVID period (CP)’ ranged between April 2020 and March 2021. Means, medians and proportions were calculated. The chi‐squared test was used for categorical variables. For continuous variables, student’s t‐test was used. All tests were two‐sided and statistical significance was set at P < 0.05.
A total of 296 cases of melanoma were included in the study period (Table 1 and Fig. 1). The cases per month decreased from 12.7 in the preCP to 8.8 in the CP (P = 0.013); this reduction was primarily due to a decrease in early stage melanoma (i.e., in situ, stages I‐II, and ≤2 mm melanomas). The number of in situ melanomas per month decreased from 5.1 to 2.3 (P = 0.0009). The number of ≤2 mm melanomas per month decreased from 4.8 to 3 (P = 0.02) and the number of stage I–II cases per month decreased from 6.2 to 3.8 (P = 0.025). There was a trend towards more advanced melanomas during the CP period. During the preCP, 26.3% of melanomas were >2 mm vs. 41.3% during CP (P = 0.046). During the preCP, 14.1% were diagnosed at an advanced stage (III & IV) vs. 27.8% during CP (P = 0.008; Table 1).
Table 1.
Demographics and tumour characteristics
| Variable | Pre‐COVID‐19 | COVID | P‐value |
|---|---|---|---|
| Cases (n) | 191 | 105 | |
| Cases per month [mean (SD)] | 12.73 (3.45) | 8.75 (4.33) | 0.013 |
| Mean age (SD) | 52.75 (17.21) | 53.28 (16.36) | 0.79 |
| Gender, male [n(%)] | 80 (41.9) | 54 (51.4) | 0.11 |
| Gender, female [n(%)] | 111 (58.1) | 51 (48.6) | |
| Melanoma characteristics: | |||
| Breslow, mm (median, p25‐75) | 1 (0.5‐2.2) | 1.5 (0.5‐4.0) | 0.153 |
| Breslow ≤ 2 mm [n(%)] | 73 (73.7) | 37 (58.7) | 0.046 |
| Breslow > 2 mm [n(%)] | 26 (26.3) | 26 (41.3) | |
| Melanoma, in situ [n(%)] | 76 (39.8) | 28 (26.7) | 0.023 |
| Melanoma, Invasive [n(%)] | 115 (60.2) | 77 (73.3) | |
| Melanoma in situ per month [mean (SD)] | 5.06 (2.15) | 2.33 (1.43) | 0.0009 |
| Localized stage 0, I, II [n(%)] | 152 (85.9) | 57 (72.2) | 0.008 |
| Advanced stage III, IV [n(%)] | 25 (14.1) | 22 (27.8) | |
| Stage I & II [n(%)] | 76 (75.2) | 30 (57.7) | 0.025 |
| Stage III & IV [n(%)] | 25 (24.8) | 22 (42.3) | |
| Stage I & II per month [mean (SD)] | 6.2 (1.85) | 3.83 (3.24) | 0.025 |
| Stage III & IV per month [mean (SD)] | 1.73 (1.62) | 1.91 (1.5) | 0.765 |
Complete staging (0, I, II, III & IV) was available for 256 cases.
Figure 1.
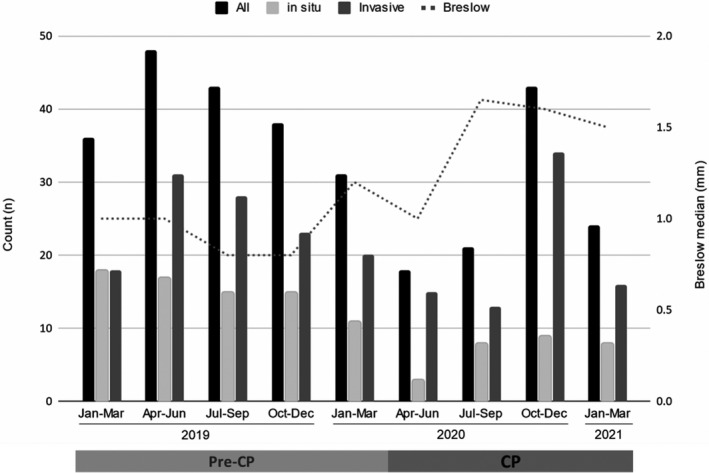
Diagnosis of melanoma and Breslow per quarter. Total cases of melanoma (all), in situ, invasive and Breslow median per quarter between January 2019 and March 2021. Pre‐COVID period (preCP) ranges from January 2019 to March 2020, while COVID period (CP) is from April 2020 to March 2021.
In this study, there was a 31.2% reduction in the melanoma cases diagnosed per month during CP with a decrease in the proportion and counts of localized and thin melanomas. The most probable explanation for this was lack of access to healthcare during the pandemic’s lockdowns in association with patient reluctance to present for examination of both symptomatic lesions and screening examinations. Marson et al. showed a 43% decrease in melanoma diagnosis in the COVID period and estimated that 19 600 melanomas would be delayed in initial diagnosis/treatment in the United States. Lallas et al. 4 demonstrated a 36.4% reduction in melanoma diagnosis in Greece. This might be critical since melanoma is a highly curable disease in early stages and this window might be lost. Tejera‐Vaquerizo et al. 5 estimated a 45% risk of upstaging after a 3‐month delay in diagnosis using melanoma models; highlighting the potential future implications of our results. Limitations of our study include single institution and relatively low number of patients with short follow‐up.
Despite the population‐based skin cancer screening being not currently recommended, 6 hampering access to health care when needed might affect melanoma stage at diagnosis and mortality rates. COVID‐19 pandemic has served as a model that highlights the importance of universal and streamlined healthcare access.
Conflict of interest
Dr Koch received grants from Novartis and honoraria as speaker and travel fees from Novartis, Bristol Myers Squibb, Roche and Merck & Co. Dr Mondaca has received consulting fees from Roche and Foundation Medicine and honoraria as speaker from Bristol Myers Squibb and Merck & Co.
Funding sources
Dr Marchetti’s research is funded in part through the Memorial Sloan Kettering Cancer Center Institutional National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Erica Koch and Francisco Villanueva Shared first authorship.
References
- 1. Breitbart EW, Waldmann A, Nolte S et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol 2012; 66: 201–211. [DOI] [PubMed] [Google Scholar]
- 2. Balch CM, Gershenwald JE, Soong SJ et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27: 6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marson JW, Maner BS, Harding TP et al. The magnitude of COVID‐19's effect on the timely management of melanoma and nonmelanoma skin cancers. J Am Acad Dermatol 2021; 84: 1100–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lallas A, Kyrgidis A, Manoli SM et al. Delayed skin cancer diagnosis in 2020 because of the COVID‐19‐related restrictions: data from an institutional registry. J Am Acad Dermatol 2021. In press. 10.1016/j.jaad.2021.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tejera‐Vaquerizo A, Nagore E. Estimated effect of COVID‐19 lockdown on melanoma thickness and prognosis: a rate of growth model. J Eur Acad Dermatol Venereol 2020; 34: e351–e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Force USPST, Bibbins‐Domingo K, Grossman DC et al. Screening for skin cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016; 316: 429–435. [DOI] [PubMed] [Google Scholar]


