To the Editor:
Despite numerous studies on SARS‐CoV‐2‐induced inflammation, we still lack markers for rapid disease progression with admission to intensive care unit (ICU) or respiratory failure (RF). Few studies have evaluated the prognostic value of routine diagnostic repertoire available at most hospitals. The NOR‐Solidarity trial is an independent add‐on study to the WHO Solidarity trial, evaluating hydroxychloroquine (HCQ) and remdesivir compared to standard of care in hospitalized COVID‐19 patients [1]. We explored whether standard biomarkers in peripheral blood could give information on ICU admission and RF in hospitalized COVID‐19 patients.
Adult patients admitted to 23 Norwegian hospitals with PCR‐confirmed SARS‐2‐CoV‐2 infection were eligible for participation. In this substudy, the routine biochemistry was related to (i) the need for ICU admission or (ii) RF defined as pO2/FiO2 (P/F ratio) < 26.6 kPa during the first 10 days of hospitalization. Routine peripheral blood samples were collected at inclusion and daily until discharge from the hospital, and outpatients were followed up 3 months after discharge. Markers included were C‐reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), haemoglobin, fibrinogen, procalcitonin (PCT), D‐dimer, platelet count, total white blood cell count, monocyte, neutrophil and lymphocyte count. Exclusion criteria, intervention, ethical statement, details on viral load and SARS‐CoV‐2 antibodies and statistical analysis are given in the Supporting Information file.
The NOR‐Solidarity trial design and main results have recently been published. As reported, neither HCQ nor remdesivir had any significant impact on routine biochemistry, and laboratory data from all study arms were pooled prior to analysis [2].Of 184 randomized patients, 35 patients (19%) were admitted to ICU and 60 (33%) patients experienced RF.
We first assessed the discriminatory properties of admission levels of markers focusing on markers with AUC ≥ 0.70 (Table S2) and determined cutoffs with Youden's index followed by stepwise Cox regression to identify independent candidates. Thus, ferritin and neutrophil counts were risk factors for ICU admission, whilst PCT, LDH and neutrophil counts were independently associated with RF. Similar results were observed excluding patients with bacterial co‐infection (n = 4, Table S3). We calculated a lymphocyte–monocyte–neutrophil score [3], which gave good discrimination, but was not selected over neutrophil counts in multivariable analysis.
Precision–recall curves revealed no benefit in combining markers and poor discriminatory properties for ICU admission, whilst combinations of markers gave better discrimination than either marker alone for RF (Fig. 1a). Thus, having above threshold levels of two markers, around 80% of these patients could be identified with close to 70% true positives. Kaplan–Meier and Cox regression analyses confirmed a high risk of RF and ICU admission with increasing number of markers above threshold levels (Fig. 1b). However, as shown in Fig. 1c, no beneficial effects of treatment were observed when comparing patients with less than two versus two or more elevated markers. All markers except neutrophils remained markedly elevated in patients with outcome, with a decline towards the end of the 10‐day period (Fig. 1d).
Fig. 1.
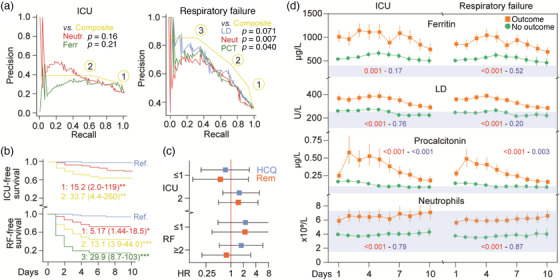
Standard biomarkers in relation to need for intensive care unit (ICU) admittance and respiratory failure (RF). (a) Precision–recall curves for admission levels of standard biomarkers in relation to ICU and RF (Neutr, neutrophils; Ferr, ferritin; LD, lactate dehydrogenase; PCT, procalcitonin). The yellow line reflects the combination of having elevated levels of one (1), two (2) or three (3) of these biomarkers (i.e., above the cutoff in Table S2). (b) Kaplan–Meier curve of having admission levels of one, two or three markers above cutoff (Ref., blue). The numbers shown are the hazard ratio and (95% confidence interval) from a Cox regression adjusting for age, gender and randomized treatment. *p < 0.05, **p < 0.01, ***p < 0.001. (c) Evaluation of treatment effects according to having high levels of two (i.e., above cutoff) or one or less markers. Hydroxychloroquine (HCQ) and remdesivir (Rem) as compared with their respective standard of care. (d) Temporal profile of the markers for which baseline levels were found to be associated with severe outcomes. Red squares/lines, unfavourable outcome (ICU admittance and RF); green circle/line, no unfavourable outcome. The red p‐values reflect the outcome effect from the repeated measures regression analysis, whilst the blue p‐values reflect the interaction between time and outcome. Grey areas reflect reference value range.
A total 121 patients completed 3‐month follow‐up. As shown in Table S4, a substantial number of patients had inflammatory markers above reference limits, in particular LDH (24%) and CRP (30%). We found no significant impact of treatment on clinical biomarkers at 3 months.
Admission levels of several routine biochemical parameters (i.e., neutrophil counts, LDH, PCT and ferritin) gave independent prognostic information on disease severity in hospitalized COVID‐19 patients. Flow cytometry of whole blood samples has shown that severe COVID‐19 infection is characterized by a dramatic increase in immature neutrophils, associated with augmented systemic inflammation [4]. Moreover, neutrophils have been linked with development of COVID‐19‐associated acute respiratory distress syndrome and induction of thrombus formation through neutrophil extracellular traps, representing a potential therapeutic target in COVID‐19 disease [5, 6]. Both ferritin, reflecting macrophage activation, and LDH, as a general marker of cell damage, correlate with severe disease manifestations and/or unfavourable outcome in COVID‐19 patients [7, 8]. PCT is suggested to be a specific marker of bacterial‐driven inflammation. However, we detected bacterial co‐infections only in a few patients (2.2%) with negligible influence on our findings. Although our study population was small, bacterial infections seem to be rare in hospitalized COVID‐19 patients, also reported in a large cohort study [9].
Approximately 25%–30% of the patients had persistent biochemical signs of systemic inflammation even 3 months after discharge, indicative of a long‐lasting, low‐grade systemic inflammation. Several reports suggest long‐term complications in hospitalized COVID‐19 patients several months after hospitalization [10].
Our findings suggest that routine biochemistry could give valuable prognostic information in these patients, both during the course of hospitalization and possibly during long‐term follow‐up.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
SUPPORTING INFORMATION
[Correction added on 19 April 2022 after first online publication: The copyright line was changed]
References
- 1. Pan H, Peto R, Henao‐Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, et al. Repurposed antiviral drugs for COVID‐19 ‐ Interim WHO Solidarity Trial results. N Engl J Med. 2021;384(6):497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barratt‐Due A, Olsen IC, Nezvalova‐Henriksen K, Kåsine T, Lund‐Johansen F, Hoel H, et al. Evaluation of the effects of remdesivir and hydroxychloroquine on viral clearance in COVID‐19. A randomized trial. Ann Intern Med. 2021; 174(9):1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qi Y, Jia JA, Li H, Wan N, Zhang S, Ma X. Lymphocyte–monocyte–neutrophil index: a predictor of severity of coronavirus disease 2019 patients produced by sparse principal component analysis. Virol J. 2021;18(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carissimo G, Xu W, Kwok I, Abdad MY, Chan YH, Fong SW, et al. Whole blood immunophenotyping uncovers immature neutrophil‐to‐VD2 T‐cell ratio as an early marker for severe COVID‐19. Nature Commun. 2020;11(1):5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiang CC, Korinek M, Cheng WJ, Hwang TL. Targeting neutrophils to treat acute respiratory distress syndrome in coronavirus disease. Front Pharmacol. 2020;11:572009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arcanjo A, Logullo J, Menezes CCB, de Souza Carvalho Giangiarulo TC, Dos Reis MC, de Castro GMM, et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID‐19). Sci Rep. 2020;10(1):19630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng L, Li H, Li L, Liu C, Yan S, Chen H, et al. Ferritin in the coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J Clin Lab Anal. 2020;34(10):e23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martha JW, Wibowo A, Pranata R. Prognostic value of elevated lactate dehydrogenase in patients with COVID‐19: a systematic review and meta‐analysis. Postgrad Med J. 2021. 10.1136/postgradmedj-2020-139542 [DOI] [PubMed] [Google Scholar]
- 9. Russell CD, Fairfield CJ, Drake TM, Turtle L, Seaton RA, Wootton DG, et al. Co‐infections, secondary infections, and antimicrobial use in patients hospitalised with COVID‐19 during the first pandemic wave from the ISARIC WHO CCP‐UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2(8):e354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lerum TV, Aaløkken TM, Brønstad E, Aarli B, Ikdahl E, Lund KMA, et al. Dyspnoea, lung function and CT findings three months after hospital admission for COVID‐19. Eur Respir J. 2021;57(4):2003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION


