Abstract
Background
The outbreak of a SARS‐CoV‐2 resulted in a massive afflux of patients in hospital and intensive care units with many challenges. Blood transfusion was one of them regarding both blood banks (safety, collection, and stocks) and consumption (usual care and unknown specific demand of COVID‐19 patients). The risk of mismatch was sufficient to plan blood transfusion restrictions if stocks became limited.
Study design and methods
Analyses of blood transfusion in a tertiary hospital and blood collection in the referring blood bank between February 24 and May 31, 2020.
Results
Withdrawal of elective surgery and non‐urgent care and admission of 2291 COVID‐19 patients reduced global activity by 33% but transfusion by 17% only. Only 237 (10.3) % of COVID‐19 patients required blood transfusion, including 45 (2.0%) with acute bleeding. Lockdown and cancellation of mobile collection resulted in an 11% reduction in blood donation compared to 2019. The ratio of reduction in blood transfusion to blood donation remained positive and stocks were slightly enhanced.
Discussion
Reduction of admissions due to SARS‐CoV‐2 pandemic results only in a moderate decrease of blood transfusion. Incompressible blood transfusions concern urgent surgery, acute bleeding (including some patients with COVID‐19, especially under high anticoagulation), or are supportive for chemotherapy‐induced aplasia or chronic anemia. Lockdown results in a decrease of blood donation by cancellation of mobile donation but with little impact on a short period by mobilization of usual donors. No mismatch between demand and donation was evidenced and no planned restriction to blood transfusion was necessary.
Keywords: blood donation, COVID‐19, lockdown, transfusion
Abbreviations
- A&E
accident and emergencies department
- EFS
Établissement Français du Sang
- FFP
fresh frozen plasma
- HUS
Hôpitaux Universitaires de Strasbourg
- ICANS
Institut de Cancérologie Strasbourg Europe
- LFB
Laboratoire Français du Fractionnement et des Biotechnologies
- MICUs
medical intensive care units
- OT
operating theater
- PC
platelet concentrates
- RBC
red blood cell packs
- SICUs
surgical intensive care units
1. INTRODUCTION
A new coronavirus called SARS‐CoV‐2, has emerged in China during the second semester of 2019, and is responsible for a new pandemic disease referred to as COVID‐19.1, 2, 3, 4, 5
Among the many problems associated with this pandemic resulting in a significant increase in hospitalizations, and especially in critical care units due to acute respiratory failure, availability and/or demand of blood products for transfusion could become critical and was unknown at this time.6 Blood banks faced a risk of a dramatic reduction in blood stocks due to exclusion of potentially infected donors, destruction of unsecured blood products, and reduction in blood collection (especially blood donation services) with lockdown, which were decided in many countries to stop the virus spread. Reduction of hospital activities and especially elective surgery on one hand and strict blood patient management are thought to reduce demand in blood transfusion.7 Some hospitals have planned to reserve blood products for only staff‐approved transfusion under 20% of the usual stock.8
In France, massive COVID‐19 admissions in hospitals began in late February and national lockdown was effective from March 17 to May 10, 2020. In this study, the authors analyze the consequences of lockdown and reduction of hospital activities on blood consumption with a focus on COVID‐19 patients in a tertiary teaching hospital and on collection, stocks, and delivery of blood products by the blood bank during spring COVID‐19 outbreak and compared them to the same period in 2019.
2. METHODS
2.1. Study design
In this study, the authors retrospectively analyzed blood transfusion in a tertiary teaching hospital (Hôpitaux Universitaires de Strasbourg—HUS), which had more than 2600 beds. The labile blood products were delivered by the French public blood bank (EFS Grand‐Est—Établissement Français du Sang), which is responsible for the collection, preparation and distribution of labile blood products. This study was approved by The Ethics Committee of Strasbourg University School of medicine (decision CE‐2020‐139 on August 26, 2020).
The study period (February 24 to May 31) was divided into three subperiods regarding closure and resumption of activities:
Feb 24 to Mar 15 (21 days): from the admission of the first COVID‐19 patients to the cessation of all non‐urgent activities,
Mar 16 to Apr 13 (29 days): withdrawal of all non‐urgent activities,
Apr 14 to May 31 (48 days): few new COVID‐19 patients and the gradual resumption of non‐urgent activities.
These three periods in 2020 were compared with the same periods in 2019. Mean activity of 2019 was used as reference (100%) for the 2020 periods.
2.2. Data collection
The Department for Medical Information provided non‐nominative data on both activity (number of admissions, surgical procedures, hemodialysis, radiotherapy, and out clinic patients) and blood transfusion (including the type of blood product and known side effects). For COVID‐19 patients, large‐volume was defined as the transfusion of five or more blood products over 24 h.
2.3. Blood bank data
Data concerning the nature of blood donation (whole blood, apheresis, or plasmapheresis), the delivery of blood products, and evaluation of regional stock were provided by EFS Grand Est, an administrative region of northern France (including Alsace, Champagne‐Ardenne, and Lorraine with 5,518,000 inhabitants). Only regional data were available regarding blood collection, but both regional and local (Strasbourg‐restricted) were available for delivery.
2.4. Statistical analysis
Quantitative data were expressed as mean ± SD or median [IQR], depending on the normality of the distribution. Comparisons of quantitative variables between groups were fulfilled using Student's t‐test or Wilcoxon test depending on the normality of the distribution. The normality of the distribution was assessed graphically and using Shapiro–Wilk test. Categorical variables were described as frequency, and comparisons were performed using χ2 test or Fisher's exact test according to the effectiveness.
A p < .05 was considered statistically significant. All statistics and graphs were performed with GraphPad Prism version 9.0.2 for Mac OS, GraphPad Software, San Diego, California USA.
3. RESULTS
3.1. Global activity
SARS‐CoV‐2 pandemic resulted in a 33.4% reduction in global hospital activity compared to 2019 despite many COVID‐19 patients admitted in general ward and ICUs (Figure 1).
FIGURE 1.
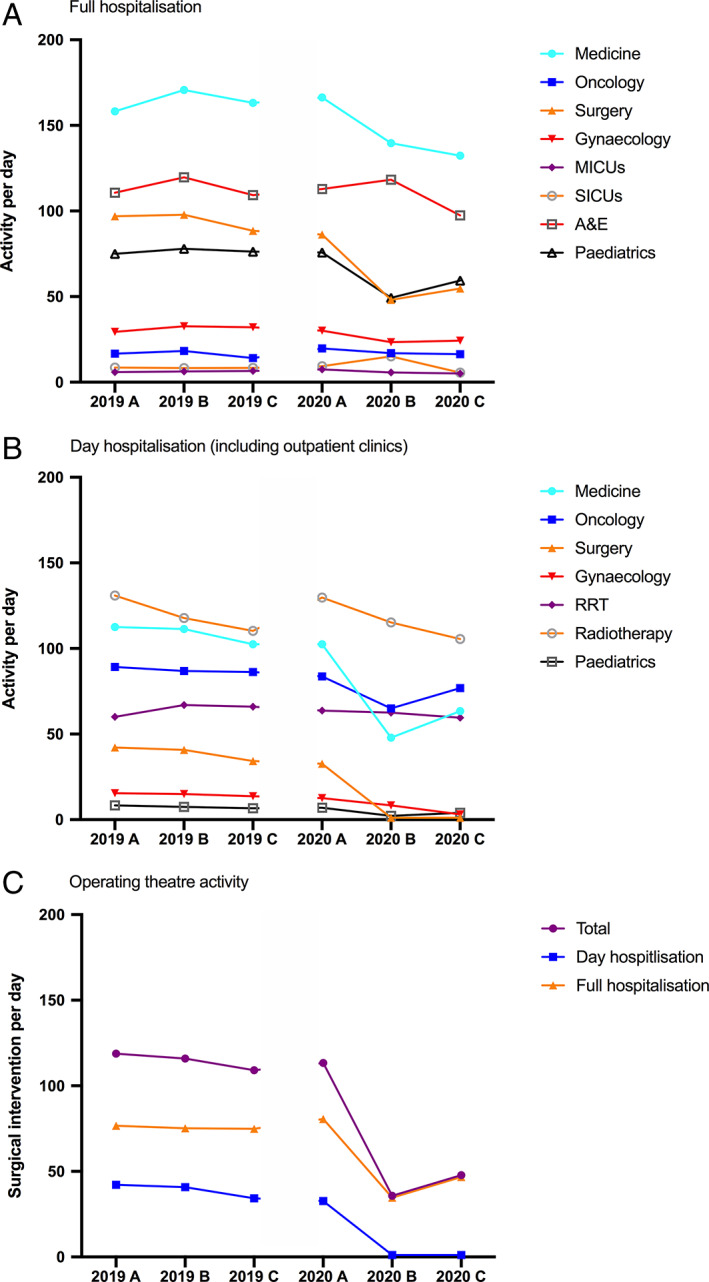
Mean activity per day for 2019 and 2020. Hospital activity is expressed as mean per day for (A) full hospitalization and (B) for day hospitalization for each subperiod A (24 Feb to 15 Mar), B (16 Mar to 13 Apr), and C (14 Apr to 31 May). (C) Mean number of surgical intervention per day. A&E, accident and emergency department; MICUs, medical ICUs, RRT: renal replacement therapy unit; SICUs, surgical ICUs [Color figure can be viewed at wileyonlinelibrary.com]
The most affected activities were day hospitalization (including outpatient clinics) and surgery, with a 62% and 57% reduction in activity, respectively, compared to 2019. Ambulatory surgery almost ceased during period B with less than 3% of 2019 activity. During this period, only urgent surgery (including transplantation) was performed. Activities that cannot be postponed such as hemodialysis, obstetrics, and oncology/hematology (including radiotherapy and out‐clinic chemotherapy or transfusion activities) remained constant during the COVID‐19 epidemic. As expected, ICU activity increased significantly during Period B, as many patients were hospitalized with severe COVID‐19 and with the creation of new ICU beds (from 97 to 207 beds, +113%). The third period (2020C) refers to decreased COVID‐19 patients' admissions after lockdown and resumption of surgical (up to 45% of 2019 activity for the same period), out clinic (50%), and medical (60%) activities.
3.2. Transfusion in COVID‐19 era
In 2019, about 180 blood products were transfused daily in our hospital: 120 packed RBC, 30 units of PC and 30 units of FFP.
In 2020, during SARS‐CoV‐2 spring outbreak, the reduction of blood transfusion was 14.2% for RBC, 23.5% for PC, and 11.2% for FFP. There was a great heterogeneity regarding medical and surgical specialities (Figures 2 and 3). Transfusion demand followed global activity of the different departments. Oncology and hematology, gynecology and obstetrics, and pediatrics were slightly affected by lockdown and SARS‐CoV‐2 pandemic and transfusion rates remained in the usual range. Altogether, these departments represent one‐third of daily RBC and half PC transfused in 2020. On the other hand, surgical activities were dramatically reduced. Only a mean of 30 operations were performed daily versus 120 in 2019 (−75%) and a mean of 6 RBC packs were transfused instead of 16 in 2019 (−63%). Only urgent surgeries were done, especially heart, vascular, and liver surgery—including transplantations and ventricular assist devices, with high hemorrhagic risk and requiring (massive) transfusion (Figure 3).
FIGURE 2.
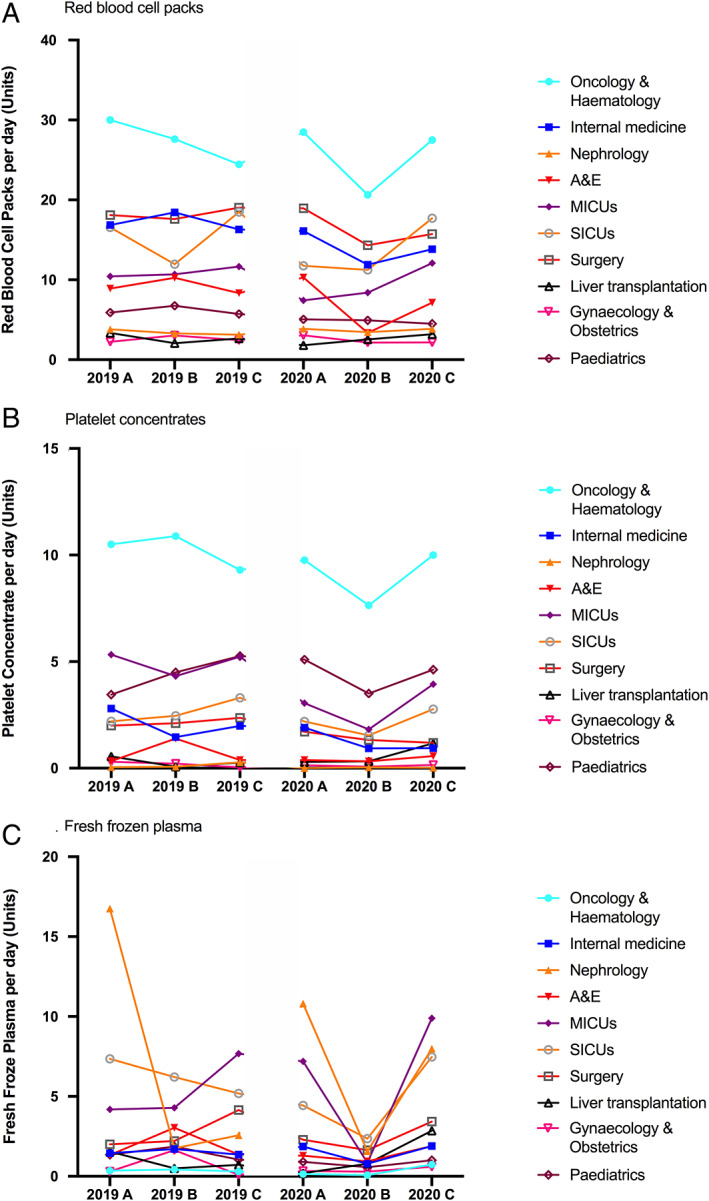
Mean transfusion activity per day for 2019 and 2020. Transfusion of (A) Red blood cell packs, (B) platelet concentrates and (C) fresh frozen plasma. A&E, accident and emergency department; MICUs, medical ICUs; SICUs, surgical ICUs [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
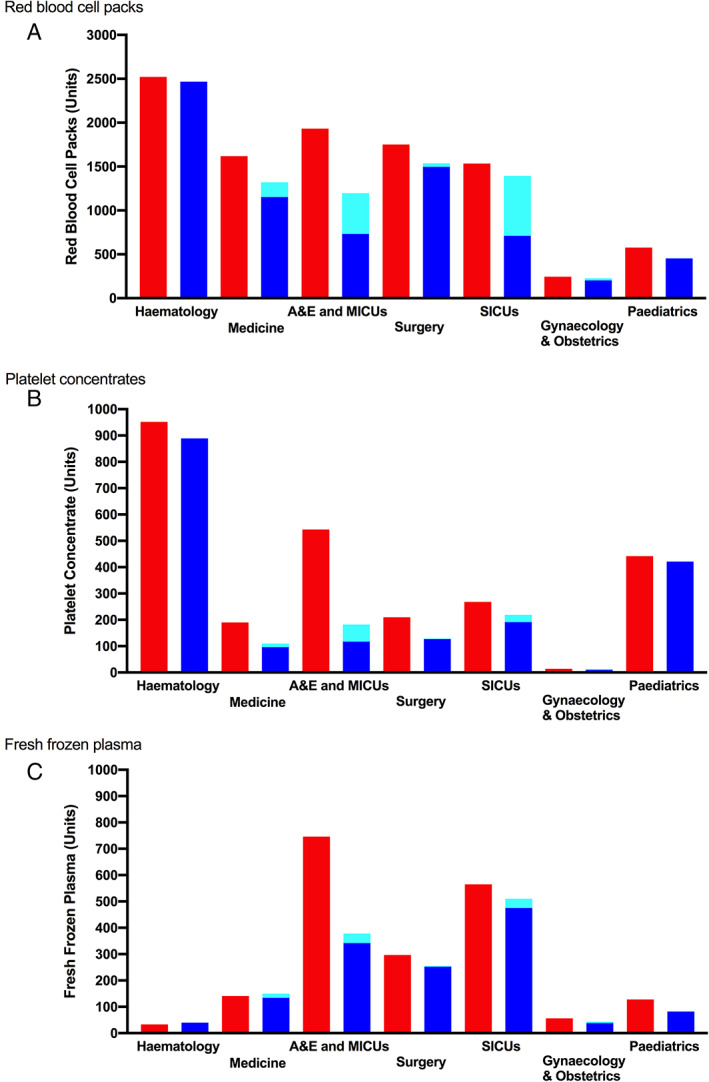
Transfusion in non‐COVID‐19 and COVID‐19 patients (16 March to 31 May, 2019 and 2020). Transfusion of (A) red blood cell packs, (B) platelet concentrates and (C) fresh frozen plasma in 2019 (red) and in 2020 (non‐COVID‐19 patients in dark blue and COVID‐19 patients in light blue). A&E, accident and emergency department; MICUs, medical ICUs; SICUs, surgical ICUs [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Transfusion and COVID‐19
Between February 24 and May 31, 2291 patients were admitted for COVID‐19 including 489 in ICU (21.3%). Among all COVID‐19 patients, 237 (10.3%) have been transfused (Table 1 and Figure 4). Forty‐five patients with massive bleeding (19.0% of transfused patients) received about half of all blood products transfused to COVID‐19 patients. Patients without acute bleeding (192) received a median of 3 and a mean of 4 transfusions with less than two blood products each (Figure 4).
TABLE 1.
Blood transfusion in transfused COVID‐19 patients
| Red blood cell packs | Fresh frozen plasma | Platelet concentrates | Transfusion | ||||
|---|---|---|---|---|---|---|---|
| Median [IQ] | Products | Median [IQ] | Products | Median [IQ] | Products | ||
| All patients (n = 237) | 4.0 [2.0–7.0] (n = 234) | 1384 | 2.5 [2.0–4.8] (n = 24) | 97 | 2.0 [1.0–4.3] (n = 30) | 110 | 732 |
| Large volume (n = 45) | 12.0 [7.0–21.8] (n = 44) | 610 (44.1%) | 3.0 [2.0–7.8] (n = 20) | 91 (93.8%) | 2.0 [1.0–5.5] (n = 21) | 81 (73.6%) | 291 (39.8%) |
| Non‐large volume (n = 216) | 3.0 [2.0–5.3] (n = 190) | 774 (55.9%) | 1.5 [1.0–2.0] (n = 4) | 6 (6.2%) | 2.0 [1.0–3.0] (n = 9) | 29 (26.4%) | 441 (60.2%) |
FIGURE 4.
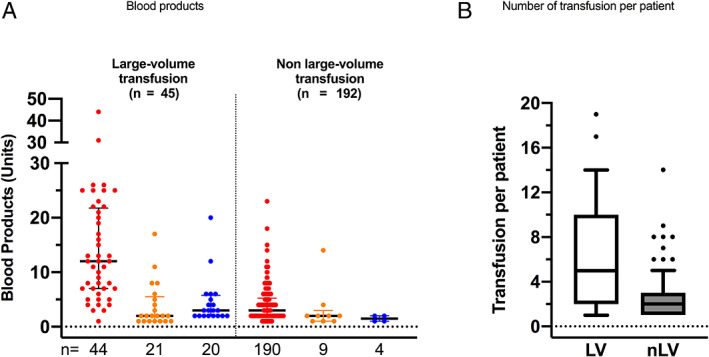
Transfusion in transfused COVID‐19 patients. (A) Scatter plot with median and interquartile of large‐volume (left) and non‐large‐volume (right) transfused products: red blood cell packs in red, platelet concentrates in orange and fresh frozen plasma in blue and (B) whisker boxes (horizontal line inside box median, upper and lower box limits 25–75 percentiles and T‐bars 10–90 percentiles respectively) of number of transfusion per patients during all the stay (LV, large‐volume; nLV, non‐volume transfused patients) [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Blood banking
To face lockdown, the blood bank had to reorganize blood collection to ensure availability of blood products. The priority was collection of whole blood, maintained above 83–97% compared to 2019, to produce RBC packs, PC, and plasma. Apheresis (for one‐donor platelet concentrate) was marginal and plasmapheresis was reduced by one‐third during period B but maintained in the two others periods (Table 2). Of note, mobile blood collection was canceled in universities, schools, and factories (closed) and reduced in cities but EFS sites remained open for blood donation. Overall, the authors evidenced an 11% reduction in blood donations compared to 2019. As described above, blood transfusion was reduced in Strasbourg University Hospitals (Figure 5A). Figure 5B shows the evolution of weekly available stocks always higher than in 2019 except for RBC at the very beginning of lockdown period. Interestingly, decrease in transfusion activity was more important than decrease in blood collection and the high level of reserves allowed providing enough blood products when “routine” surgical and medical activities recovered.
TABLE 2.
Mean donation per day to EfS grand Est regarding type of donation
| Whole blood | Apheresis | Plasmapheresis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | Variation (%) | 2019 | 2020 | Variation (%) | 2019 | 2020 | Variation (%) | |
| A | 861.71 | 731.52 | −15.1 | 15.33 | 14.38 | −6.2 | 205.4 | 193.62 | −5.7 |
| B | 908.64 | 752.96 | −17.1 | 15.04 | 15.14 | +0.7 | 202.68 | 134.18 | −33.8 |
| C | 713.98 | 691.62 | −3.1 | 17.72 | 15.43 | −12.9 | 187.94 | 179.6 | −4.4 |
FIGURE 5.
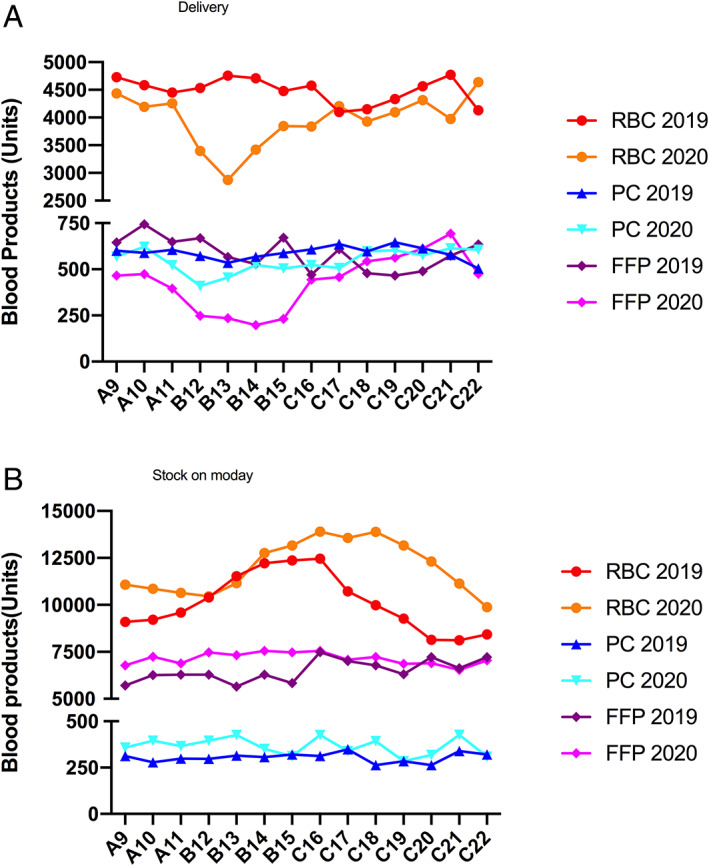
Blood bank (EFS grand‐Est) activity. Results are given by weeks for 2019 and in 2020 by sub‐period A (weeks 9–11), B (weeks 12–15), and C (weeks 16–22). (A) delivery and (B) regional stock of Monday of each week [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Among the many fears facing a new infectious agent (SARS‐CoV‐2) and disease (COVID‐19), one concerns transfusion. Blood transfusion is mandatory to support patients with hematological diseases, during surgery with high blood loss, or when acute bleeding occurs. However, blood transfusion is limited by blood products availability depending on blood donation. Reduction in blood donation (due to lockdown and cancellation of mobile collection) and the deferral of donors suspected of being infected to prevent disease transmission could create a mismatch between demand and supply, especially considering that the specific demand for blood transfusion in COVID‐19 patients was unknown. In this context, a reflection on blood transfusion was initiated in some countries or hospitals. The literature reports guidelines but no paper to date depicts blood transfusion and blood banking in the COVID‐19 era.6 In this study, the authors were able to evidence that:
Despite a significant reduction in hospital activities, including elective surgery, blood transfusion remains high due to nonadjustable transfusion in hematology, pediatrics, or during urgent surgery,
About 10% of patients admitted with COVID‐19 required transfusion,
Reorganization of blood banks allows a high level of donation despite closure of mobile collection with extended stocks regarding the greater reduction of blood transfusion,
Resumption of medical activities after lockdown results in increased blood transfusion prior to resumption of mobile blood collection but stocks were sufficient to support activity.
Reduction, then cancellation, of elective surgery was the first reflex to face COVID‐19 pandemic and lockdown to release anesthesiologists and nurses to make them available for patients' care in regular and newly‐opened ICUs and to reduce surgical admission in ICUs. Despite a dramatic reduction in number of surgeries, blood transfusions remained high, mandated by urgent or uncancellable bleeding surgical procedures, especially heart and liver transplantations, aortic repair for ruptured aneurysms, etc. The impact of elective surgery cancellation on blood transfusion was limited to 14%, as transfusions during these surgeries are greatly reduced by the blood patient management (BPM) program.9 Elective admissions were also canceled in medical specialities (except in hematology and oncology) to release physicians and nurses in COVID‐19 general wards, with few if any impact on blood transfusion.
Blood transfusion requirement during SARS‐CoV‐2 infection was unknown at the beginning of the pandemic outbreak. In our study, only 10% of our COVID‐19 patients required blood transfusion. The authors evoke four reasons for blood transfusion:
Clinically relevant bleeding,
Hemolysis during extracorporeal membrane oxygenation(ECMO) and renal replacement therapy(RRT),
Inflammatory anemia,
Blood spoliation for routine biology.
Interestingly, blood transfusion does not reflect bleeding. In the literature, hemorrhagic complications were about 8–14%10, 11, 12, 13 but only massive bleeding requires transfusion. In one study including 187 patients, 15 patients presented a hemorrhage (six gastro‐intestinal, five intracranial, and four other sites); only 7 (3.8%) patients were transfused—but the authors do not know if patients without hemorrhagic complications were transfused.11 The study from DeSimone et al.,14 including 11,041 patients in New York, reported that only 3.3% of COVID‐19 patients required transfusion (vs. 10% in the present study), and essentially RBC. The number of blood products per patient was lower than in our study (4.8 vs. 6.7) but the distribution among RBC, PC, and FFP was the same. One explanation could be that patients were less severe in their study (7.3% of patients in ICU vs. 21.3%). Interestingly, the authors transferred some of the less severe patients requiring critical care to others ICU in France, Switzerland or Germany.15
Rapidly, COVID‐19 was identified as a prothrombotic inflammatory disease associated with Lupus anticoagulant13 and is now considered as one therapeutic target.16 Incidence of thrombotic events (including pulmonary embolism) is not known precisely but seems to be about 15–20%.17, 18, 19, 20 Soon after, several scientific societies proposed to increase the level of prophylactic anticoagulation in COVID‐19 patients, sometimes to therapeutic anticoagulation.21 Hemorrhagic complications of full‐dose anticoagulation are not frequently reported in the literature and seemed to be about 4–8%.11, 22, 23 They are about 7% and supervene after the eighth day of anticoagulation in COVICLOT, a French national survey of COVID‐19's patients admitted in some surgical ICUs.24 ECMO can be associated with acute bleeding (mostly at the time of insertion) or with progressive anemia due to hemolysis.25, 26
Specific recommendations were made to lower blood transfusion threshold during the pandemic in accordance with clinical assessment rather than hemoglobin level to preserve stocks.27 Moreover, global recommendations are now available for patient blood management in the COVID‐19 era.28
Another challenging issue was blood products safety regarding SASR‐CoV‐2 transmission. Selection of SARS‐CoV‐2 free donors was challenging, as only PCR test in nasal swab was available to detect acute disease. Implementation of selection criteria on fever or respiratory symptoms was proposed and donors were invited to contact blood bank if those symptoms appear in the days following donation. Inactivation of pathogens in blood products is effective to reduce transmission. It can be achieved by treatment with Amotosalen and UV‐A light with proven efficacy over many pathogens including Chikungunya virus, SARS‐CoV, and MERS‐CoV.4, 5, 29, 30, 31 All platelet concentrates (either single donor or pooled) are treated by Amotosalen and UV‐A light in France and there is great evidence that this treatment will secure platelet concentrates during SARS‐CoV‐2 outbreak. This result has been confirmed with SARS‐CoV‐2 (Azhar EI, accepted abstract 2020; Cerus Inc. personal communication). Plasma can also be secured by Amotosalen and UV‐A illumination for blood transfusion and by nanofiltration since 1995 for protein extraction by LFB.32, 33, 34, 35 Red blood cell packs are not treated but treatment by Amotosalen and UV‐A light is under evaluation by drug agencies and seems to be effective for blood‐borne infection prevention and transfusion efficacy. Finally, viremia was not reported during symptomatic COVID‐19 but early phase was not explored. Late in May 2020, a first report confirms that SARS‐CoV‐2 RNA was considered absent or of very low level in donors without symptoms of COVID‐19 among blood donors.36 Only few data are available about transfusion from donors with acute (asymptomatic) illness but the risk of transmission seems to be very low if any, even in immunocompromised host.37, 38 This subject is not closed and inactivation of pathogens is mandatory when feasible.
Despite cancellation of several mobile blood donations, blood donation remained high. Regular donors were called by EFS and most of them agreed to go to the EFS fixed blood donation centers (whole blood, platelet apheresis, or plasmapheresis) and communications were attended to promote donation as recommended by WHO (WHO/2019‐nCoV/BloodSupply/2020.1). This activity may have been facilitated by the lockdown, as most of the donors were at home rest. Another way to increase blood donors is to revise exclusion criteria. Men having sex with men (MSM), excluded from blood donation since 1985, were allowed to give blood in France since 2016 if they had no sexual intercourse during 1 year. This period has been reduced to 3 or 4 months in some countries. A recent study confirms that such a reduction is safe39 and could be promoted during SARS‐CoV‐2 outbreak to face the loss of mobile collection40 and to promote the use of “therapeutic” plasma from patients who have recovered from COVID‐19.41
This study depicts the absence of mismatch in blood demand and blood supply during the first outbreak of COVID‐19 in a French tertiary hospital. Synergy of decrease in blood collection (−10%) with a more important decrease in blood products use in hospitals during COVID‐19 outbreak in spring 2020 (−17%) helped to maintain self‐sufficiency and supply of blood products in other regions in France. As a comparison, a report from 15 Eastern Mediterranean countries evidenced a net mismatch of more than 10% in 11 countries with a reduction of less than 10% in blood collection only in 2 countries, between 10 and 25% in 4, between 26 and 50% in 7 and above 50% in 2. Interestingly, three countries increased more than 10% of blood transfusion from baseline.42 In this study, most of the countries allowed family replacement (reduced by the lockdown), and some have hospital‐based blood bank limiting the possibility of supply from another blood bank (medical insurance). Five centers experienced reagent shortages and loss of staff due to illness. Moreover, one indication for RBC transfusion was hemoglobinopathies (sickle cell disease and thalassemia), frequent in these countries but rare in Grand‐Est. To improve blood donation, Saudi Arabia reduced deferral after tattoo and piercing (12–3 months), and five countries reduced inter‐donation deferral at 8 weeks (instead of 12) for men with hemoglobin over 12.5 g/dL. No information was available regarding MSM deferral. Nevertheless, repetition of lockdown periods could have a greater impact on blood donation and stocks over time especially if patients' care cannot be delayed and resumption of care supervenes before resumption of blood donation.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Delabranche X, Kientz D, Tacquard C, et al. Impact of COVID‐19 and lockdown regarding blood transfusion. Transfusion. 2021;61:2327–2335. 10.1111/trf.16422
REFERENCES
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinna D, Sampson‐Johannes A, Clementi M, Poli G, Rossini S, Lin L, et al. Amotosalen photochemical inactivation of severe acute respiratory syndrome coronavirus in human platelet concentrates. Transfus Med. 2005;15(4):269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasongles P, Angelini‐Tibert MF, Simon P, Currie C, Isola H, Kientz D, et al. Transfusion of platelet components prepared with photochemical pathogen inactivation treatment during a Chikungunya virus epidemic in Ile de La Reunion. Transfusion. 2009;49(6):1083–91. [DOI] [PubMed] [Google Scholar]
- 6.Stanworth SJ, New HV, Apelseth TO, Brunskill S, Cardigan R, Doree C, et al. Effects of the COVID‐19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020;7(10):e756–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gehrie EA, Frank SM, Goobie SM. Balancing supply and demand for blood during the COVID‐19 pandemic. Anesthesiology. 2020;133(1):16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vossoughi S, Fischkoff K, DeSimone RA, Schwartz J. Blood stewardship: conservation and supply of blood components during the SARS‐CoV‐2 pandemic. Transfusion. 2020;60(9):2156–2158. 10.1111/trf.15995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchini M, Marano G, Veropalumbo E, Masiello F, Pati I, Candura F, et al. Patient blood management: a revolutionary approach to transfusion medicine. Blood Transfus. 2019;17(3):191–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barriteau CM, Bochey P, Lindholm PF, Hartman K, Sumugod R, Ramsey G. Blood transfusion utilization in hospitalized COVID‐19 patients. Transfusion. 2020;60(9):1919–1923. 10.1111/trf.15947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah A, Donovan K, McHugh A, Pandey M, Aaron L, Bradbury CA, et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID‐19: a multicentre observational study. Crit Care. 2020;24(1):561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helms J, Tacquard C, Severac F, Leonard‐Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeSimone RA, Costa VA, Kane K, Sepulveda JL, Ellsworth GB, Gulick RM, et al. Blood component utilization in COVID‐19 patients in New York City: transfusions do not follow the curve. Transfusion. 2021;61(3):692–698. 10.1111/trf.16202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collange O, Sammour Y, Soulie R, Castelain V, Mertes PM. ICU re‐organisation to face the first COVID‐19 epidemic wave in a tertiary hospital. Anaesth Crit Care Pain Med. 2020;39(6):731–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48(9):1358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard‐Lorant I, Delabranche X, Severac F, Helms J, Pauzet C, Collange O, et al. Acute pulmonary embolism in patients with COVID‐19 at CT angiography and relationship to d‐dimer levels. Radiology. 2020;296(3):E189–E91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID‐19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296(3):E186–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res. 2020;191:148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Susen S, Tacquard CA, Godon A, Mansour A, Garrigue D, Nguyen P, et al. Prevention of thrombotic risk in hospitalized patients with COVID‐19 and hemostasis monitoring. Crit Care. 2020;24(1):364. 10.1186/s13054-020-03000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musoke N, Lo KB, Albano J, Peterson E, Bhargav R, Gul F, et al. Anticoagulation and bleeding risk in patients with COVID‐19. Thromb Res. 2020;196:227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al‐Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood. 2020;136(4):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tacquard C, Mansour A, Godon A, Godet J, Poissy J, Garrigue D, et al. Impact of high dose prophylactic anticoagulation in critically ill patients with COVID‐19 pneumonia. Chest. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munshi L, Walkey A, Goligher E, Pham T, Uleryk EM, Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta‐analysis. Lancet Respir Med. 2019;7(2):163–72. [DOI] [PubMed] [Google Scholar]
- 26.Falcoz PE, Monnier A, Puyraveau M, Perrier S, Ludes PO, Olland A, et al. Extracorporeal membrane oxygenation for critically ill patients with COVID‐19‐related acute respiratory distress syndrome: worth the effort? Am J Respir Criti Care Med. 2020;202(3):460–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh G, Nahirniak S, Arora R, Legare JF, Kanji HD, Nagpal D, et al. Transfusion thresholds for adult respiratory extracorporeal life support: an expert consensus document. Can J Cardiol. 2020;36(9):1550–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron DM, Franchini M, Goobie SM, Javidroozi M, Klein AA, Lasocki S, et al. Patient blood management during the COVID‐19 pandemic: a narrative review. Anaesthesia. 2020;75(8):1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashem AM, Hassan AM, Tolah AM, Alsaadi MA, Abunada Q, Damanhouri GA, et al. Amotosalen and ultraviolet a light efficiently inactivate MERS‐coronavirus in human platelet concentrates. Transfus Med. 2019;29(6):434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hindawi SI, Hashem AM, Damanhouri GA, El‐Kafrawy SA, Tolah AM, Hassan AM, et al. Inactivation of Middle East respiratory syndrome‐coronavirus in human plasma using amotosalen and ultraviolet a light. Transfusion. 2018;58(1):52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gehrie EA, Rutter SJ, Snyder EL. Pathogen reduction: the state of the science in 2019. Hematol Oncol Clin North Am. 2019;33(5):749–66. [DOI] [PubMed] [Google Scholar]
- 32.Burnouf‐Radosevich M, Appourchaux P, Huart JJ, Burnouf T. Nanofiltration, a new specific virus elimination method applied to high‐purity factor IX and factor XI concentrates. Vox Sang. 1994;67(2):132–8. [DOI] [PubMed] [Google Scholar]
- 33.Burnouf T, Radosevich M. Reducing the risk of infection from plasma products: specific preventative strategies. Blood Rev. 2000;14(2):94–110. [DOI] [PubMed] [Google Scholar]
- 34.Schrader T, Koch J, Ross R, Schafer W, Keiner B, Roth NJ, et al. Effective coronavirus reduction by various production steps during the manufacture of plasma‐derived medicinal products. Transfusion. 2020;60(6):1334–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth NJ, Dichtelmuller HO, Fabbrizzi F, Flechsig E, Groner A, Gustafson M, et al. Nanofiltration as a robust method contributing to viral safety of plasma‐derived therapeutics: 20years' experience of the plasma protein manufacturers. Transfusion. 2020;60(11):2661–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang L, Yan Y, Zhao L, Hu G, Deng L, Su D, et al. No evidence of SARS‐CoV‐2 RNA among blood donors: a multicenter study in Hubei, China. Transfusion. 2020;60(9):2038–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liapis K, Papoutselis M, Vrachiolias G, Misidou C, Spanoudakis E, Bezirgiannidou Z, et al. Blood and platelet transfusion from a donor with presymptomatic Covid‐19. Ann Hematol. 2020(Nov 13):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho HJ, Koo JW, Roh SK, Kim YK, Suh JS, Moon JH, et al. COVID‐19 transmission and blood transfusion: a case report. J Infect Public Health. 2020;13(11):1678–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pillonel J, Pelat C, Tiberghien P, Sauvage C, Danic B, Martinaud C, et al. The evolving blood donor deferral policy for men who have sex with men: impact on the risk of HIV transmission by transfusion in France. Transfusion. 2020;60(3):525–34. [DOI] [PubMed] [Google Scholar]
- 40.Skelly AN, Kolla L, Tamburro MK, Bar KJ. Science over stigma: the need for evidence‐based blood donation policies for men who have sex with men in the USA. Lancet Haematol. 2020;7(11):e779–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasavda C, Ho BK, Snyder SH. Ease restrictions on U.S. blood donations. Science. 2020;368(6494):957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riyami AZA, Abdella YE, Badawi MA, Panchatcharam SM, Ghaleb Y, Maghsudlu M, et al. The impact of COVID‐19 pandemic on blood supplies and transfusion services in eastern Mediterranean region. Transfus Clin Biol. 2020;28(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


