Abstract
The burden of senescent cells (SnCs), which do not divide but are metabolically active and resistant to death by apoptosis, is increased in older adults and those with chronic diseases. These individuals are also at the greatest risk for morbidity and mortality from SARS‐CoV‐2 infection. SARS‐CoV‐2 complications include cytokine storm and multiorgan failure mediated by the same factors as often produced by SnCs through their senescence‐associated secretory phenotype (SASP). The SASP can be amplified by infection‐related pathogen‐associated molecular profile factors. Senolytic agents, such as Fisetin, selectively eliminate SnCs and delay, prevent, or alleviate multiple disorders in aged experimental animals and animal models of human chronic diseases, including obesity, diabetes, and respiratory diseases. Senolytics are now in clinical trials for multiple conditions linked to SnCs, including frailty; obesity/diabetes; osteoporosis; and cardiovascular, kidney, and lung diseases, which are also risk factors for SARS‐CoV‐2 morbidity and mortality. A clinical trial is underway to test if senolytics decrease SARS‐CoV‐2 progression and morbidity in hospitalized older adults. We describe here a National Institutes of Health‐funded, multicenter, placebo‐controlled clinical trial of Fisetin for older adult skilled nursing facility (SNF) residents who have been, or become, SARS‐CoV‐2 rtPCR‐positive, including the rationale for targeting fundamental aging mechanisms in such patients. We consider logistic challenges of conducting trials in long‐term care settings in the SARS‐CoV‐2 era, including restricted access, consent procedures, methods for obtaining biospecimens and clinical data, staffing, investigational product administration issues, and potential solutions for these challenges. We propose developing a national network of SNFs engaged in interventional clinical trials.
Keywords: cellular senescence, facility for geroscience analysis, SARS‐CoV‐2, senolytics, Translational Geroscience Network
Key Points
Cellular senescence may accentuate morbidity and mortality in older adults with SARS‐CoV‐2 infection.
Design and initiation of a trial of Fisetin to target senescent cells in SARS‐CoV‐2‐infected SNF residents is presented.
Why Does this Paper Matter?
This trial is a step toward a Translational Geroscience SNF Network.
INTRODUCTION
By July 2021, over 185 million confirmed cases and 4 million deaths had occurred worldwide in the β‐coronavirus‐19 (SARS‐CoV‐2) pandemic, mostly in older adults. In the United States, 81% of SARS‐CoV‐2 deaths have occurred in those older than 65 years. 1 , 2 Residence in a skilled nursing facility (SNF) increases mortality risk related in part to increased transmission inherent in tightly confined congregate living environments with a high percentage of cognitively impaired residents, in addition to system factors, including underfunding, inadequate staffing, suboptimal infection control, and lack of personal protective equipment. 3 , 4 Approximately 20% of SNF residents who test positive for SARS‐CoV‐2 by quantitative real‐time polymerase chain reaction (rtPCR) die. 5 Of ~600,000 U.S. SARS‐CoV‐2 deaths by July 2021, residents of long‐term care facilities accounted for 32%, despite comprising only 5% of cases. 6
Although vaccination 7 and evolving SARS‐CoV‐2 treatment, including monoclonal antibodies (Mab), have improved the situation in SNFs, SARS‐CoV‐2 will remain a major concern for the foreseeable future. Some SARS‐CoV‐2 variants appear to evade Mab efficacy. 8 Vaccines are not 100% effective 9 and some residents may refuse vaccination or have contraindications to vaccines, so a minority of SNF residents will remain vulnerable to SARS‐CoV‐2 infection and related morbidity/mortality. There remain uncertainties about duration of vaccine protection, and emerging variants may pose risk to vaccine efficacy. Hesitancy about vaccination is a barrier, including in SNF staff members. 10 , 11 , 12 Moreover, SNF residents who have been infected by SARS‐CoV‐2 before vaccination might still develop long‐hauler syndrome. Thus, the search for better SARS‐CoV‐2 treatments for SNF residents remains critical.
National Institutes of Health (NIH)‐funded, U.S. Food and Drug Administration (FDA)‐regulated, placebo‐controlled, randomized, double‐blind, multisite trial of the senolytic agent, Fisetin, has commenced for older adults in SNFs who are SARS‐CoV‐2 rtPCR test‐positive. Herein, we discuss the geroscience rationale for conducting such a trial, design and implementation strategies, logistical challenges to conducting trials in long‐term care settings in the SARS‐CoV‐2 era, and the need for a national network of SNFs engaged in interventional clinical trials, a Translational Geroscience SNF Network.
GEROSCIENCE AND COVID
General geroscience
The Geroscience Hypothesis posits that biological drivers of aging, including cellular senescence, are root cause contributors to multiple chronic disorders and diseases. 13 Senescent cell (SnC) burden is low in healthy young individuals but increases with aging in many tissues. SnCs also appear at pathogenic sites of many diseases, including lung in COPD, asthma, smoking, and idiopathic pulmonary fibrosis; adipose and other tissues in obesity/diabetes; heart and vessels in cardiovascular diseases; brain in Alzheimer's disease; around cancers; bone in osteoporosis; bone and synovium in osteoarthritis; kidney in renal disease; and liver in cirrhosis and steatosis.
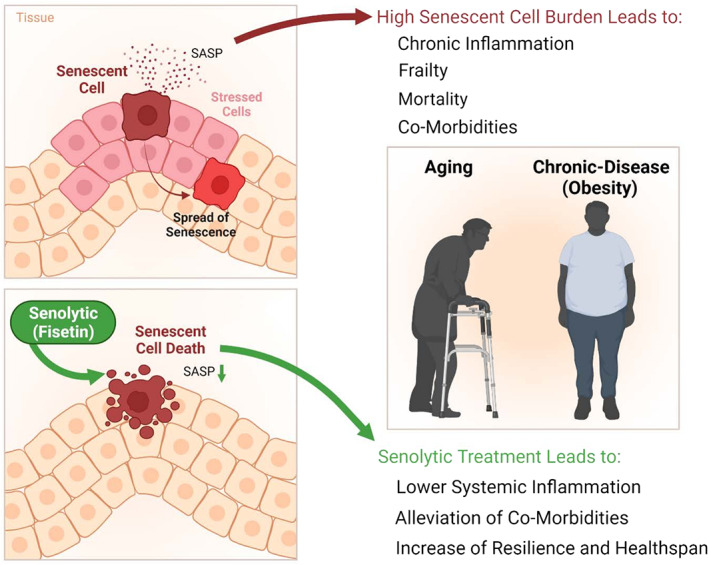
Cellular senescence is a cell fate, such as differentiation, proliferation, apoptosis, or necrosis. SnCs are in essentially irreversible replicative arrest, yet remain viable and metabolically active. External and internal signals, including genotoxic, metabolic, and mechanical/shear stresses can drive senescence through transcription factor cascades (e.g., p16INK4A/retinoblastoma protein and/or p53/p21CIP1). This causes extensive changes in gene expression and organelle function (e.g., mitochondria, lysosomes), profound morphological and metabolic shifts, and resistance to apoptosis. SnCs often develop a senescence‐associated secretory phenotype (SASP). This can include proinflammatory; proapoptotic cytokines (TNF‐α, IL‐1α, IL‐6, etc.); chemokines that attract, activate, and anchor immune cells (IP‐10, MCP's, etc.); tissue‐destroying proteases (e.g., MMP‐3, 9, or 12); prothrombotic factors (PAI‐1, etc.); factors that impair stem cell/progenitor function and cause fibrosis (e.g., Activin‐A, TGF‐β‐related proteins); and ferritin. In addition to proteins and peptides, the SASP can include bioactive lipids (e.g., bradykinins, ceramides, prostaglandins), tissue damaging, proinflammatory, noncoding nucleotides (including micro‐RNAs, mitochondrial DNA), exosomes, and other factors that contribute to tissue necrosis, fibrosis, systemic inflammation, immune dysfunction, and spread of senescence (Figure 1). 14 , 15
FIGURE 1.
Fisetin mechanism of action
The impact of SnCs on health has driven the search for and discovery of senolytics, drugs that selectively eliminate SnCs. Short‐acting senolytics are effective even if administered intermittently, once every few days or weeks in a “hit‐and‐run” approach, potentially reducing side effects. Brief disruption of prosurvival pathways is sufficient to kill SnCs in human cell cultures, in vivo in mice, and in SnC‐containing human tissue explants. After drug‐induced depletion, SnCs take >1 week to re‐accumulate if inducers remain present, at least in vitro. Monthly senolytic administration is as effective as daily doses for alleviating some age‐related diseases, for example, osteoporosis in mice. These points, together with satisfying a modified set of Koch's postulates, indicate senolytics alleviate dysfunction by removing SnCs, not through other off‐target mechanisms that require continuous occupancy of a receptor or engagement of an enzyme. Senolytics alleviate multiple conditions in mice, including lung fibrosis, heart failure, vascular dysfunction, dementias, diabetes and its complications; frailty, kidney, liver, and intestinal damage/dysfunction; osteoporosis; osteoarthritis; delay cancer; and extend healthspan and median lifespan.
Rationale for using geroscience interventions to treat COVID‐19 infection and its sequelae
SARS‐CoV‐2 can cause hyperinflammation, cytokine storm, acute respiratory distress syndrome (ARDS), myocarditis, widespread thrombosis, persistent symptoms and morbidity (long‐hauler syndrome), and multiorgan failure, particularly in older or chronically ill individuals. SARS‐CoV‐2 morbidity and mortality are strongly associated with age and are also increased in younger individuals with chronic conditions, including obesity, diabetes, chronic lung disease, smoking, asthma, atherosclerosis, hypertension, and immunological, hepatic, and kidney diseases. 16 Consistent with the Geroscience Hypothesis, older adults and patients with senescence‐associated diseases are more susceptible to adverse outcomes in response to infections (e.g., SARS‐CoV‐2) and frequently develop a more exaggerated inflammatory state than young patients without pre‐existing senescence‐associated chronic diseases. This has generally been attributed to immune system dysfunction or chronic inflammation. Our “Amplifier/Rheostat” Hypothesis is that pathogen‐associated molecular pattern factors (PAMPs), such as viral antigens, cause a shift in the SASP of pre‐existing SnCs into a more highly inflammatory, proapoptotic, profibrotic SASP, 17 potentially exacerbating systemic inflammatory responses and also amplifying spread of senescence to other cells. The resulting additional SnCs may exacerbate and prolong inflammation, attenuate or delay recovery, lead to persistent frailty, cause long‐term tissue fibrosis, and contribute to long‐hauler syndrome, multiorgan failure, and death.
Agents that target fundamental aging mechanisms, such as senolytics, hold potential to enhance resilience and prevent, delay, or alleviate morbidity and mortality due to infections such as SARS‐CoV‐2. 14 , 15 , 18 , 19 The senolytic Fisetin (3,3′,4′,7‐tetrahydroxyflavone, a flavonoid in many fruits and vegetables that is available as a dietary supplement) attenuates multiple senescence‐associated disorders in mice, physical dysfunction in old mice, and age‐related tissue dysfunction and pathology. 20 Studies in a mouse model indicate that Fisetin enhances antibody response and improves clinical outcomes (including mortality) in old mice that are exposed to fomites containing a murine β‐coronavirus, as does another senolytic regimen, Dasatinib plus Quercetin (D + Q). 17 Although ongoing clinical trials of these agents (Table S1) have revealed few if any serious side effects directly related to the senolytic drugs, it remains unknown if senolytics will be safe and effective for older adults with SARS‐CoV‐2 and they should not be used outside a carefully supervised clinical trial. Given the high morbidity and mortality resulting from SARS‐CoV‐2 in older adults and chronically ill patients, however, a randomized, placebo‐controlled, double‐blind trial of Fisetin for SNF residents who have a positive rtPCR test for SARS‐CoV‐2 appears important.
THE COVID‐FIS CLINICAL TRIAL
Study design
For this trial, “COVID‐FIS: A Phase 2 Placebo‐Controlled Pilot Study in COVID‐19 of Fisetin to Alleviate Dysfunction and Excessive Inflammatory Response in Older Adults in Nursing Homes” (NIH R01AG72301; FDA IND149813; ClinicalTrials.gov Identifier: NCT04537299), we focused on candidate senolytics that have a history of safe human use, can be administered orally, and have a short elimination half‐life. Fisetin met these criteria. The main hypothesis of the trial is that Fisetin can delay, prevent, or treat complications of SARS‐CoV‐2 infection in SNF residents. The main trial objectives are to reduce progression of SARS‐CoV‐2 severity; prevent SARS‐CoV‐2‐related complications; evaluate safety and tolerability of Fisetin; and to reduce SnCs, inflammation, and physical dysfunction (frailty).
COVID‐FIS (Table 1) will enroll SNF residents (either short term or long term) residing at facilities associated with participating academic medical centers. Inclusion criteria are straightforward: age >65 years, positive rtPCR for SARS‐CoV‐2, and oxygen saturation >85% on 2 L/min or less of supplemental oxygen at enrollment. Several exclusion criteria exist (Table 2), including unstable major medical conditions, severe laboratory abnormalities, and certain medications (Tables S2 and S3), but most SNF residents, even those with several chronic diseases and polypharmacy, should not be excluded. Use of other agents to treat SARS‐CoV‐2, either previously to or concurrently with Fisetin, is not an exclusion criterion. Subjects will be asked to participate in the study for 6 months (Table S4), and after enrollment will be randomized in a 1:1 manner to receive either Fisetin 20 mg/kg or placebo, both of which will be administered orally or by feeding tube on study days 0, 1, 8, and 9. Both Fisetin and the placebo have no odor or taste, and can easily be mixed with food or beverages.
TABLE 1.
Study summary
| Title | COVID‐FIS: A Phase 2 Placebo‐Controlled Pilot Study in COVID‐19 of Fisetin to Alleviate Dysfunction and Excessive Inflammatory Response in Older Adults in Nursing Homes. |
| Running title | COVID‐FIS: COVID‐19 Pilot Study of Fisetin to Alleviate Dysfunction and Inflammation. |
| Phase | Phase II. |
| Methodology | Randomized, placebo‐controlled, double‐blind secondary prevention trial. |
| Overall study duration | 32 months. |
| Subject participation duration | Subject participation duration of 6 months (screening, 4 days treatment, follow‐up visits, unblinded at the end of the study). |
| Single or multi‐site | Multi‐Site: Mayo Clinic Rochester and the other Translational Geroscience Network sites. |
| Objectives |
|
| Primary outcome | 7‐point ordinal severity scale adapted from the World Health Organization Ordinal Scale for Clinical improvement of SARS‐CoV‐2. |
| Secondary outcomes | Measures of senescent cell abundance/inflammation, physical dysfunction/frailty, safety/tolerability, progression to severe/critical SARS‐CoV‐2, oxygenation and oxygen requirement, cell lysis syndrome, various laboratory parameters, chest imaging, need for acute hospital transfer, palliative care, intubation or intensive care unit admission, and mortality. |
| Number of subjects | 250 enrolled and screened, 150 accrued and randomized. |
| Diagnosis and main inclusion criteria | Age ≥65 years in a skilled nursing facility with test‐proven SARS‐CoV‐2 infection. SpO2 ≥85% (on room air or ≤2 L supplemental O2) at enrollment. |
| Study product, dose, route, regimen | This study will involve a 2‐day oral or feeding tube course of Fisetin twice (~20 mg/kg/day for 2 consecutive days; Days 0, 1, 8, and 9). |
| Duration of administration | 4 out of 10 days. |
| Reference therapy | Placebo controlled. |
| Statistical methodology | Assuming 75 subjects/group with a significance level of 0.05, the study will have 80% power to detect an odds ratio of 2.24 comparing the placebo‐ to Fisetin‐treated group, using the 7‐point severity score at Day 14 as the endpoint in an ordinal logistic regression model. |
TABLE 2.
Exclusion criteria
| General |
|
| Laboratory |
|
| Clinical history |
|
| Medication |
|
The primary outcome of COVID‐FIS is incidence of progression on a 7‐point ordinal severity scale adapted from the WHO Ordinal Scale for Clinical improvement of SARS‐CoV‐2 (Table S5). Secondary outcomes include measures of SnC abundance/inflammation, physical dysfunction/frailty, safety/tolerability, various laboratory parameters, chest imaging, need for acute hospital transfer, ICU care, intubation, and mortality. We aim to screen 250 participants and ultimately enroll and randomize 150. Assuming 75 subjects per group with a significance level of 0.05, the study will have 80% power to detect an odds ratio of 2.24 comparing the placebo‐ to Fisetin‐treated group, using the 7‐point severity score at Day 14 as the endpoint in an ordinal logistic regression model.
Implementation strategies and early lessons
Clinical trials often exclude older adults, particularly those with frailty and multiple comorbidities (almost universally the case for SNF residents). 21 , 22 , 23 Consensus recommendations regarding recruitment and retention strategies to overcome this bias, all of which were incorporated into the design and implementation of COVID‐FIS, include using clear, simple inclusion/exclusion criteria aimed at limiting ineligibility due to comorbidity, developing procedures that minimize drop‐out risk (e.g., avoiding requirement to travel to study visits), using informed consent processes that can be administered remotely and account for cognitive impairment, and incorporating endpoints relevant to older adults (including function and quality of life). 24
Even with close attention paid to the abovementioned factors, the prospect of conducting a clinical trial of a new drug in SNFs during the SARS‐CoV‐2 pandemic is daunting. 23 Multiple potential barriers and solutions are outlined in Table 3. Barriers include SNF understaffing (which has worsened during the pandemic 25 , 26 ), concern of SNF leaders regarding liability, ability of the research team to enter the facility, storing and delivering Fisetin, and lack of familiarity with clinical trials on the part of SNF leaders and staff. We used multiple strategies to address these potential barriers in the design of COVID‐FIS. All study visits are conducted at the SNF by a research nurse, which should minimize dropouts due to logistical inconvenience. The research nurse gathers most data, including vital signs, clinical information, and biospecimens. SNF staff have no active involvement and will be spared work as they will not need to obtain vital signs on days when the research nurse visits. The only “ask” from SNF staff is to share results of Minimum Data Set (MDS) item GG (Functional Abilities, including Self‐Care and Mobility) assessments with the research nurse. Since access is typically restricted to only facility staff, essential healthcare workers, and close family (“essential caregivers,” “compassionate care visits”) when cases of SARS‐CoV‐2 are active in an SNF, 27 COVID‐FIS was initiated at a point in the pandemic when vaccination had become more widespread and case numbers had diminished greatly. Research personnel will be screened at facility entry as stipulated by the Centers for Medicare and Medicaid Services and state health departments and have been trained in infection control and personal protective equipment use. To overcome any concerns about drug storage, Fisetin will be stored at an off‐site research pharmacy and picked up by the research team to deliver to the patient for administration.
TABLE 3.
Barriers to implementing a senolytic trial in skilled nursing facilities and potential solutions
| Skilled nursing facilities are often understaffed | Trial design that involves research team member entering the facility to administer drug/placebo and perform assessments, then monitoring for any adverse events remotely via health system electronic medical record. Other than sharing a portion of new minimum data set assessments with the research team when they occur, there is no work required by facility staff for study participants. |
| Potential skilled nursing facility leadership concerns about liability | Implementation of a memorandum of understanding, signed by the sponsoring institution for the study as well as facility leadership, outlining expectations of each party and making clear that the facility is not the research participant (rather the patient/resident is) and bears no legal responsibility for any trial outcomes. |
| Challenges in obtaining consent for institutionalized patients, potentially with cognitive impairment | Protocol allows consent to be obtained via phone and/or electronically, utilizing the participant's legally authorized representative when appropriate. |
| Concerns about ability of research team to enter facility during pandemic restrictions | Trial conducted at a point during the pandemic when visitation/entry restrictions have relaxed due to fairly widespread vaccination and improving case/mortality numbers. Ensuring research team members are trained in infection control and personal protective equipment use. |
| Challenges in delivering and storing Fisetin | Trial medication will be picked up from the research pharmacy by the study research nurse and delivered to the subject for administration. Fisetin is stored refrigerated for stability, once dispensed, Fisetin is stable in ambient conditions until administration, typically within 2–4 h. |
| Potential for participants, particularly if in the facility only for a short‐term stay, to relocate during the trial | Protocol design to minimize in‐person assessments and sample collection to only those instances that are strictly necessary. After initial 14 days of the study, follow‐up is very infrequent, and can largely be accomplished remotely. |
| Lack of familiarity with clinical trials among facility staff/leadership | Inclusion of skilled nursing facility medical directors on the research team and early engagement (months before enrollment begins) by said medical directors with facility leadership, including multiple methods of communication (written and verbal) as well as question/answer sessions to ensure facility leadership are comfortable with the study. |
Some early lessons have emerged. First, involvement of multiple SNF medical directors on the study team has provided practical perspectives about the protocol (i.e., will this be feasible in SNFs?). The simplicity of this trial, where a once daily oral drug is administered on only 4 days, facilitates involvement of the many SNFs unfamiliar with clinical trials. Although trials involving SNF residents do not require specific consent from SNFs, a collaborative approach is critical to optimize the process for residents and the facility. Early collaboration with leaders at candidate SNFs (months before trial commencement) allowed rapport building and alleviated concerns regarding burden for staff and potential liability. Ultimately, a memorandum of understanding making clear that SNFs do not take on risk when residents in their facility choose to participate (Table S6) is critical. We developed several other tools including a basic, one‐page frequently asked questions (FAQ) document for patients/families and SNF staff (Table 4) and a summary of the trial and lengthier FAQ document for SNF medical directors that includes scripting for discussing the trial with SNF leaders (Table S7). With these tools and strategies in place, 9 of 10 facilities approached about the trial thus far have expressed strong interest.
TABLE 4.
Frequently asked questions (FAQ) document for patients, families, and SNF staff
| Who is being asked to participate in this trial and why? | The study aims to enroll nursing home residents, not nursing homes themselves. COVID‐19 has had a disproportionate negative impact on older adults residing in nursing homes, who have historically been excluded from clinical trials and thus unable to fully benefit from medical advances. The research team is studying a promising therapy (Fisetin) that may help improve outcomes after COVID‐19 infection in nursing home residents. |
| What is Fisetin? | Fisetin is a substance with anti‐aging and anti‐inflammatory properties found in some fruits, vegetables, nuts, and wine. The dose being studied is much higher than that occurring in typical portions of these foods. Fisetin has shown benefit against the effects of viral infection in human cells and in an animal model of infection. It is administered orally as a pill or powder that can be mixed with food. |
| If I choose to participate, what will I need to do? | You will be randomly assigned to receive Fisetin or placebo, which you will take daily on days 0, 1, 8, and 9 of the study. You will not know which you are receiving. Mayo Clinic researchers will review your medical record frequently to check on your health status. Blood/urine collections will occur twice during the initial 14 days of the study, and if possible, at 3 months and 6 months after enrollment. You will not need to leave the nursing home—the research team will come to you. |
| How will I benefit from participating? | While it is not clear if receiving Fisetin after COVID‐19 infection will reduce your chance of dying or having worsening health, there is enough promising data from human cells and animals to suggest this is possible. If the trial is successful, and you are in the group assigned to receive Fisetin, your chance of dying or having worsening health after COVID‐19 infection may decrease. Even if this does not happen, you will be playing a role in advancing medical care for older adults living in nursing homes by helping accelerate new discoveries and treatments. |
| Who is conducting and paying for the trial? | The trial is conducted by Mayo Clinic researchers, funded by the National Institute on Aging (NIA)/National Institutes of Health (NIH), and approved by the NIA and Mayo Institutional Review Board (IRB). |
| Are there risks? | Fisetin is a naturally occurring substance found in many foods (though in much lower levels that used in this study). In other recent and ongoing clinical trials at Mayo Clinic, several dozen older adults have received Fisetin at the dose that will be used in this study. No substantial adverse effects have been seen. While there is no guarantee that side effects will not occur, the study team will be monitoring closely for these in real‐time. |
| What extra work will be required of SNF staff? How will the study drug be delivered and ordered? | We recognize that your staff are very busy and intend to avoid introducing extra work when residents in your facility are participating in the trial. The drug will be provided and delivered by the Mayo research pharmacy. It will be secured on site at your facility using storage provided by the research team. Your medical director will provide orders. A research team nurse will come to your facility to administer the drug, perform any needed assessments, and collect any samples when needed. |
Note: This sample is for SNFs near Mayo Clinic. Similar forms will be used for SNFs near other Translational Geroscience Network institutions (Harvard [Hebrew SeniorLife], Johns Hopkins, and Wake Forest Universities, Universities of Connecticut, Michigan, and Minnesota, and University of Texas Health Sciences Center at San Antonio).
BEYOND COVID‐FIS: WHERE DO WE GO FROM HERE
Future studies of geroscience interventions in COVID‐19 infection
Multiple geroscience approaches for treating SARS‐CoV‐2 infection are currently under investigation, including agents that increase innate viral defenses (e.g., mTOR inhibitors 28 ), metformin, 29 NAD+ precursors, and senolytics. Combinations of these and other interventions may ultimately alleviate morbidity, prevent progression, treat complications, and attenuate mortality, as in other potentially severe viral infections, such as influenza. It is not currently known what dose of Fisetin is effective at targeting SnCs in humans, unlike for D + Q. If the Fisetin dose used in COVID‐FIS does not reduce markers of senescence, additional studies examining different Fisetin doses and D + Q may be necessary.
If successful, COVID‐FIS would provide rationale for larger‐scale clinical trials to determine the effects of Fisetin and other senolytics on the following outcomes: (1) delay or prevention of complications from SARS‐CoV‐2 (or other potentially severe infections such as influenza), including cytokine storm, ARDS, multi‐organ failure, lung fibrosis, and Long‐hauler Syndrome, (2) improved response of older adults to SARS‐CoV‐2 vaccination, and (3) delay, prevention, or treatment of multiple senescence‐associated conditions in older adults and those with chronic diseases. The role of interventions that target fundamental aging mechanisms needs to be ascertained in SNF populations as a defense against potential future pandemics, including new SARS‐CoV‐2 mutants and influenza.
Studying geroscience interventions in SNF populations
The SARS‐CoV‐2 pandemic presents a unique opportunity to establish a Translational Geroscience SNF Network. Given the disproportionate impact of SARS‐CoV‐2, motivation for improvement and change in the industry is high. SNFs are prominently in the public eye and find themselves at the forefront for adoption of new therapies, as evidenced by the roll‐out of the first SARS‐CoV‐2 vaccines when SNF residents and staff were first‐in‐line. 7 Clinical trials of new drugs carry the potential of improved health outcomes and quality of life for a group whose vulnerability has been laid bare by the pandemic. Moreover, therapies that reduce functional decline and prevent hospitalization offer financial benefit to SNFs as alternative payment models are advanced. While challenges remain, the benefit‐to‐barrier ratio for creating a Translational Geroscience SNF Network is higher now than ever before.
We propose that a national Translational Geroscience SNF Network be established, perhaps by building on the NIH‐funded Translational Geroscience Network (TGN; currently includes Mayo Clinic, Harvard, Johns Hopkins, and Wake Forest Universities, and the Universities of Minnesota, Michigan, Connecticut, and Texas [San Antonio]; NIH R33AG61456). The network would include willing SNFs with medical or nursing directors cross‐appointed to current or future TGN institutions. As an initial step toward creating such a network, a blog is being developed through the NIH, as is a TGN website housing publicly available consent forms, generic examples of contracts, standard operating protocols (SOPs) for geroscience‐based laboratory specimen collection and analysis, and other tools (https://www.gerosciencenetwork.org). These web‐based resources may become a forum for sharing comments and advice about geroscience‐based interventions and designing and conducting clinical trials in SNFs and other long‐term care settings.
Developing an effective Translational Geroscience SNF Network will likely be incremental and challenging. Initiating clinical trials of geroscience‐based interventions in SNFs, like COVID‐FIS, should serve to reveal unforeseen obstacles that could inform future trial protocols and conduct. Engaging members of the geriatrics community, including representatives of SNF staff and administrators, may also accelerate developing such a network. The NIH blog and TGN website described earlier are examples of such engagement efforts. The role that regulatory bodies such as CMS and state health departments might play in the development of a network is not currently clear, though certainly any research efforts in SNFs will need to be compliant with federal SNF regulations and state operations manuals. We could envision Translational Geroscience SNF Network participation becoming a publicly reported data point (a quality measure of sorts) in the future, perhaps acting as a marker for potential residents and their family members that a facility is actively engaged in efforts to innovatively improve function and quality of life for their residents.
Ultimately, we view COVID‐FIS as a catalyst for creating a Translational Geroscience SNF Network and a template for future clinical trials in SNFs. Therefore, even if COVID‐FIS does not show that Fisetin improves SARS‐CoV‐2 outcomes in SNF residents, it could help facilitate clinical trials of other interventions that ultimately provide reliable evidence‐based information on which to base clinical practice.
CONCLUSIONS
Despite the introduction of effective vaccines, interventions beyond vaccination are very likely to be needed as new SARS‐CoV‐2 variants appear and since some SNF residents and their caregivers may not be vaccinated. Geroscience‐related interventions, especially senolytics, may prove to be beneficial for preventing, treating, or alleviating long‐term complications of SARS‐CoV‐2 and other viruses in SNF populations. However, these interventions should not be introduced into general clinical practice unless and until carefully monitored clinical trials have demonstrated safety and effectiveness. This is especially the case for SNF residents, who frequently have complex co‐morbidities and are already taking multiple, potentially interacting medications. These patients are generally understudied. Inferences from findings of studies in other, healthier populations tend to be extended without good evidence to these patients, perhaps compounding their risk of morbidity. A Translational Geroscience SNF Network would be invaluable for ascertaining the safety and effectiveness of new interventions targeting fundamental aging processes that may delay, prevent, or treat multiple age‐related diseases and disorders. Such a network could conduct trials of drugs and lifestyle interventions designed to meet the complex needs of SNF patients.
CONFLICT OF INTEREST
Patents on senolytic drugs and their uses are held by Mayo Clinic and the University of Minnesota. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic conflict of interest policies.
AUTHOR CONTRIBUTIONS
All authors were involved in designing the COVID‐FIS trial and developing the Translational Geroscience Network. Brandon P. Verdoorn, Tamara K. Evans, and James L. Kirkland prepared the initial draft and all authors revised and edited the article. The authors are grateful for advice and ideas generated in developing this project from Irina Sazonova, PhD, Sergei V. Romashkan, MD, PhD, and Evan Hadley, MD in the Division of Geriatrics and Clinical Gerontology, National Institute on Aging, NIH, Bethesda, MD.
SPONSOR'S ROLE
NIH staff have been active participants in advising about the COVID‐FIS trial. The other sponsors provided funding but have not been involved in developing this trial or preparing this article.
Supporting information
Table S1. Selected translational geroscience network clinical trials of senolytics.
Table S2. Excluded medications and dosing modifications.
Table S3. Tyrosine kinase inhibitor list (CYP2C8).
Table S4. Schedule of events.
Table S5. Primary outcome scale.
Table S6. Points to consider for inclusion in memoranda of understanding with SNFs.
Table S7. Frequently asked questions (FAQ) and scripting for SNF Medical Director.
Table S8. Additional references.
ACKNOWLEDGMENTS
The authors would like to acknowledge the support received from NIH grants R01 AG72301 (PI: James L. Kirkland); R33 AG61456 (Translational Geroscience Network; PI: James L. Kirkland, Stephen B. Kritchevsky, George A. Kuchel, Tamara Tchkonia); R37 AG13925 (PI: James L. Kirkland); P01 AG62413 (Co‐PIs: Sundeep Khosla and James L. Kirkland), U19 AG056278 (PI: Paul D. Robbins), R01 AG063543‐S1 (PI: Laura J. Niedernhofer), the Connor Fund (James L. Kirkland, Tamara Tchkonia), Robert J. and Theresa W. Ryan (James L. Kirkland, Tamara Tchkonia), and the Noaber Foundation (James L. Kirkland, Tamara Tchkonia).
Verdoorn BP, Evans TK, Hanson GJ, et al. Fisetin for COVID‐19 in skilled nursing facilities: Senolytic trials in the COVID era. J Am Geriatr Soc. 2021;69(11):3023-3033. doi: 10.1111/jgs.17416
Funding information National Institutes of Health, Grant/Award Numbers: P01 AG62413, R01 AG063543‐S1, R01 AG72301, R33 AG61456, R37 AG13925, U19 AG056278; Noaber Foundation; Robert J. and Theresa W. Ryan; The Connor Fund
REFERENCES
- 1. Provisional Death Counts for Coronavirus Disease 2019 (COVID‐19). Centers for Disease Control and Prevention (CDC) National Center for Health Statistics, 2020. https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm#AgeAndSex. Accessed January 29, 2021.
- 2. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fisman DN, Bogoch I, Lapointe‐Shaw L, McCready J, Tuite AR. Risk factors associated with mortality among residents with coronavirus disease 2019 (COVID‐19) in long‐term care facilities in Ontario. Can JAMA Netw Open. 2020;3:e2015957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ouslander JG, Grabowski DC. COVID‐19 in nursing homes: calming the perfect storm. J Am Geriatr Soc. 2020;68:2153‐2162. [DOI] [PubMed] [Google Scholar]
- 5. COVID‐19 Nursing Home Data . Data.CMS.Gov. https://data.cms.gov/stories/s/bkwz-xpvg. Accessed April 11, 2021.
- 6. State COVID‐19 Data and Policy Actions . Kaiser Family Foundation. https://www.kff.org/coronavirus-covid-19/issue-brief/state-covid-19-data-and-policy-actions/#longtermcare. Accessed April 11, 2021.
- 7. The Advisory Committee on Immunization Practices' Updated Interim Recommendation for Allocation of COVID‐19 Vaccine—United States, December 2020. Centers for Disease Control and Prevention. https://www.cdc.gov/mmwr/volumes/69/wr/mm695152e2.htm?s_cid=mm695152e2_w. Accessed January 1, 2021. [DOI] [PMC free article] [PubMed]
- 8. Wibmer CK, Ayres F, Hermanus T, et al. SARS‐CoV‐2 501Y.V2 escapes neutralization by South African COVID‐19 donor plasma. Nat Med. 2021;27(4):622‐625. [DOI] [PubMed] [Google Scholar]
- 9. Cavanaugh AM, Fortier S, Lewis P, et al. COVID‐19 outbreak associated with a SARS‐CoV‐2 R.1 lineage variant in a skilled nursing facility after vaccination program—Kentucky, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:639‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes toward a potential SARS‐CoV‐2 vaccine: a survey of U.S. adults. Ann Intern Med. 2020;173:964‐973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin C, Tu P, Beitsch LM. Confidence and receptivity for COVID‐19 vaccines: a rapid systematic review. Vaccines (Basel). 2020;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gharpure RGA, Bishnoi CK, et al. Early COVID‐19 first‐dose vaccination coverage among residents and staff members of skilled nursing facilities participating in the pharmacy Partnership for Long‐Term Care Program—United States, December 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:178‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iske J, Seyda M, Heinbokel T, et al. Senolytics prevent mt‐DNA‐induced inflammation and promote the survival of aged organs following transplantation. Nat Commun. 2020;11:4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Song S, Lam EW, Tchkonia T, Kirkland JL, Sun Y. Senescent cells: emerging targets for human aging and age‐related diseases. Trends Biochem Sci. 2020;45:578‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou Y, Yang Q, Chi J, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta‐analysis. Int J Infect Dis. 2020;99:47‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Camell MJY CD, Zhu Y, Langhi Prata LGP, et al. Senolytics reduce coronavirus‐related mortality in old mice. Science. 2021;eabe4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirkland JL, Stout MB, Sierra F. Resilience in aging mice. J Gerontol A Biol Sci Med Sci. 2016;71:1407‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol. 2013;48:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu Y, Doornebal EJ, Pirtskhalava T, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL‐XL inhibitors, A1331852 and A1155463. Aging. 2017;9:955‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kennedy‐Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high‐impact general medical journals: a systematic sampling review. JAMA. 2007;297:1233‐1240. [DOI] [PubMed] [Google Scholar]
- 23. Quinn CC, Adams AS, Magaziner JS, Gurwitz JH. Coronavirus disease 2019 and clinical research in U.S. nursing homes. J Am Geriatr Soc. 2021;69:1748‐1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cherubini A, Del Signore S, Ouslander J, Semla T, Michel JP. Fighting against age discrimination in clinical trials. J Am Geriatr Soc. 2010;58:1791‐1796. [DOI] [PubMed] [Google Scholar]
- 25. Bostick JE, Rantz MJ, Flesner MK, Riggs CJ. Systematic review of studies of staffing and quality in nursing homes. J Am Med Dir Assoc. 2006;7:366‐376. [DOI] [PubMed] [Google Scholar]
- 26. COVID‐19 Intensifies Nursing Home Workforce Challenges . Office of the Assistant Secretary for Planning and Evaluation. https://aspe.hhs.gov/basic-report/covid-19-intensifies-nursing-home-workforce-challenges. Accessed February 5, 21.
- 27. Nursing Home Visitation‐COVID‐19 . Centers for Medicare & Medicaid Services. https://www.cms.gov/files/document/qso-20-39-nh.pdf. February 5, 21.
- 28. Mannick JB, Del Giudice G, Lattanzi M, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6:268ra179. [DOI] [PubMed] [Google Scholar]
- 29. Bramante CT, Buse J, Tamaritz L, et al. Outpatient metformin use is associated with reduced severity of COVID‐19 disease in adults with overweight or obesity. J Med Virol. 2021;93:4273‐4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Selected translational geroscience network clinical trials of senolytics.
Table S2. Excluded medications and dosing modifications.
Table S3. Tyrosine kinase inhibitor list (CYP2C8).
Table S4. Schedule of events.
Table S5. Primary outcome scale.
Table S6. Points to consider for inclusion in memoranda of understanding with SNFs.
Table S7. Frequently asked questions (FAQ) and scripting for SNF Medical Director.
Table S8. Additional references.


