Patient consent
The patients in this manuscript have given written informed consent to the publication of their case details.
Conflict of interest
None to declare.
Dear Editor,
Immediate and delayed local injection site reactions to mRNA vaccines against SARS‐CoV‐2 have been previously reported. 1 , 2 , 3 However, eczematous cutaneous reactions after COVID‐19 mRNA vaccines have only been described once in the literature. 4 The previously described patient had moderate‐to‐severe atopic dermatitis on dupilumab and flared after vaccination. His atopic dermatitis flare resolved with oral glucocorticoids. Here, we present two patients who developed generalized eczematous eruptions after both doses of the (Pfizer‐BioNTech, Brooklyn, NY, USA) COVID‐19 vaccine.
This first patient was a 43‐year‐old man with a history of seasonal allergies who developed a pruritic eruption on his extremities 3 days after his first dose of the Pfizer‐BioNTech COVID‐19 vaccine. He presented to the dermatology clinic on day 5 postvaccine due to progression of the eruption. He had a family history of atopic dermatitis but no personal history of atopic dermatitis. On examination, he had pink patches and papules with overlying excoriation on his back, arms and legs (Fig. 1). A skin biopsy showed spongiosis, acanthosis, parakeratosis and an inflammatory infiltrate consistent with eczematous dermatitis. He was treated with topical glucocorticoids and oral antihistamines and improved over several weeks. He received the second vaccine dose 7 weeks after his first dose, and two weeks after the second dose, he had a recurrence of the eruption that again responded to topical glucocorticoids.
Figure 1.
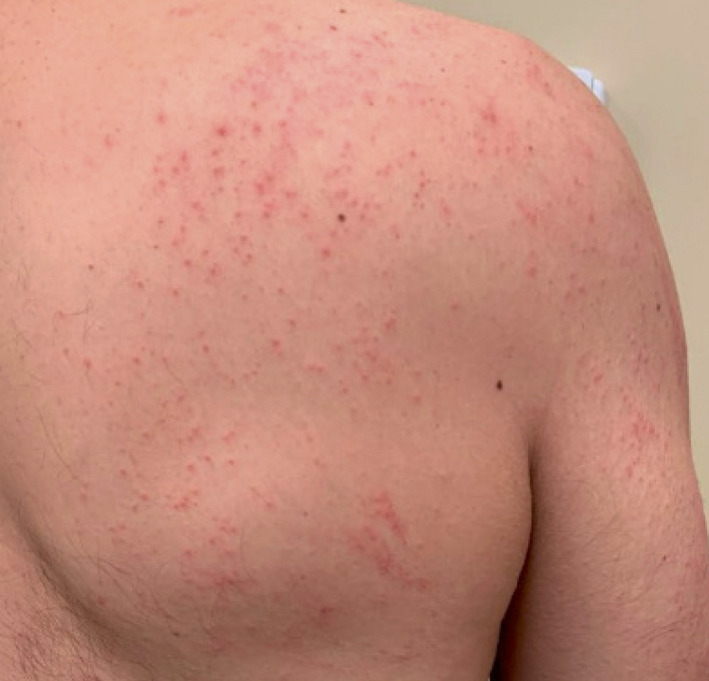
Generalized eczematous reaction on the back and shoulder.
The second patient was a 51‐year‐old woman with a history of dyshidrotic eczema who presented with a pruritic eruption 4 days after her first Pfizer‐BioNTech COVID‐19 vaccine dose. She developed pruritus on her lower back hours after receiving the vaccine. The rash progressed over several days. On examination, pink papules coalescing into plaques were noted on the left lower back (Fig. 2). Skin biopsy revealed spongiosis with a mainly superficial perivascular lymphocytic infiltrate consistent with eczematous dermatitis. She was treated with topical glucocorticoids and improved. She received the second Pfizer‐BioNTech COVID‐19 vaccine dose 4 weeks after the first dose and had a recurrence of the rash 4 days later; the eruption was more generalized and involved the posterior neck, chest, flanks, back and lower extremities. She was treated with an oral prednisone taper and her symptoms resolved.
Figure 2.
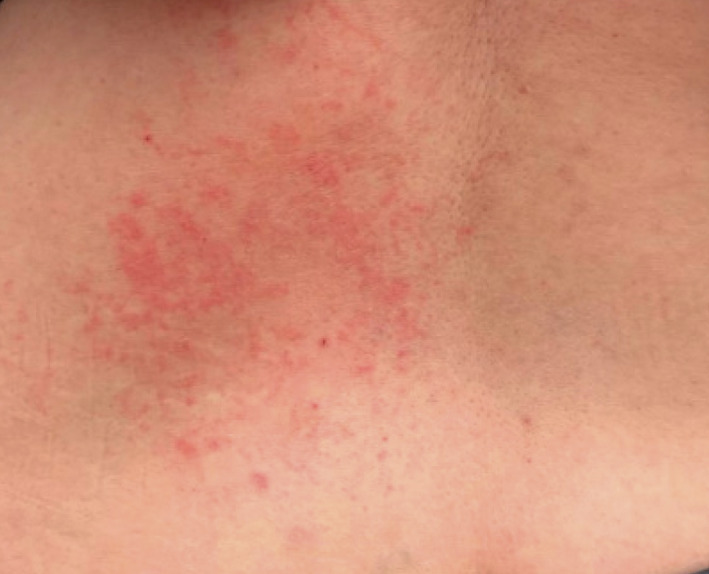
Generalized eczematous reaction on the lower back.
Despite experiencing generalized reactions, neither patient developed serious adverse events after the first or second vaccine dose. Both of these cases suggest that patients who develop generalized eczematous reactions to the Pfizer‐BioNTech COVID‐19 vaccine can safely receive the second dose. We did observe a recurrence in the eczematous dermatitis with the second vaccine dose, but these recurrences were responsive to topical and oral glucocorticoids. This contrasts with what has been observed with delayed large local reactions, in which most patients with a first‐dose reaction did not develop a second‐dose reaction. 2 Interestingly, both patients in this series had no involvement of the site of injection.
While the cause of these generalized eczematous reactions is unknown, the Pfizer‐BioNTech vaccine may act as an environmental trigger in a genetically susceptible individual. In both cases, the patients had a personal or family history of atopy, perhaps making them more susceptible to an eczematous reaction. It is unknown if this reaction may also occur with the Moderna mRNA COVID‐19 vaccine. As more of the population is vaccinated against COVID‐19, rare reactions are likely to surface and it is important to study these reactions to facilitate optimal management of patients who develop them. In this series of two patients who developed generalized eczematous reactions to the Pfizer‐BioNTech COVID‐19 vaccine, the reaction occurred with both doses, was managed by oral or systemic glucocorticoids and did not prevent safe administration of both doses of the vaccine.
References
- 1. Blumenthal KG, Freeman EE, Saff RR et al. Delayed large local reactions to mRNA‐1273 vaccine against SARS‐CoV‐2. N Engl J Med 2021; 384: 1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMahon D, Amerson E, Rosenbach M et al. Cutaneous reactions reported after Moderna and Pfizer COVID‐19 vaccination: a registry‐based study of 414 case. J Am Acad Dermatol 2021; 85: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localized hypersensitivity reactions to the Moderna COVID‐19 vaccine: a case series. JAMA Dermatol 2021; 157: 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corbeddu M, Diociaiuti A, Vinci MR et al. Transient cutaneous manifestations after administration of Pfizer‐BioNTech COVID‐19 vaccine: an Italian single centre case series. J Eur Acad Dermatol Venereol 2021; 35: e483–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]


