Abstract
-
What is the topic of this review?
Human serum albumin (HSA) a common factor in COVID‐19 vulnerabilities.
-
What advances does it highlight?
Understanding of HSA capacity, and systemic vulnerabilities to COVID‐19. Raising HSA in COVID‐19 patients may alleviate systemic injury caused by diminished native HSA binding. A change in fluid therapy administration into the portal system of the liver is proposed to safely raise HSA levels.
Abstract
The specific nature of the vulnerabilities to COVID‐19 are an intrinsic part of COVID‐19 infection in many patients. This paper proposes that vulnerabilities to COVID‐19 may be intensified by a decrease in human serum albumin (HSA) as a ligand carrier for nutrients. A mechanism for COVID‐19 vulnerabilities is evident from consideration of ligand carriers such as HSA as intermediaries. We hypothesise that low levels of pool HSA binding, caused for whatever reason, affect the performance of albumin as a carrier protein reducing the availability of nutrients. Hypoalbuminaemia (low HSA) has been implicated as an indicator of COVID‐19 and long‐COVID‐19. The levels of HSA directly affect the immune system and vulnerabilities to age, diabetes and obesity in COVID‐19. Any slight reduction in available HSA has profound effects on ligand concentrations in the small capillaries where damage occurs in COVID‐19. The clinical implication is that attempts should be made to return HSA to clinical levels to compensate for the additional ligands caused by infection (SARS‐CoV‐2 virions, antibodies and cellular breakdown products). Therapeutic albumin is usually given peripherally, and usual preparations are unbound to ligands, but we suggest that a clinical trial of HSA therapy via the hepatic portal vein should be considered.
Keywords: albumin therapy, COVID‐19, human serum albumin, hypoalbuminemia, long‐COVID, nutrient distribution, portal system, SARS‐CoV‐2, virion vulnerabilities
1. INTRODUCTION
There is now considerable evidence that there are vulnerabilities to COVID‐19 exhibited by defined at‐risk groups. The vulnerabilities are most severe in the over 50s (Islam et al., 2021), and obese patients are at risk of severe disease (Ho et al., 2020; Huang et al., 2020b). Considerable attention has been concentrated on the infection and destruction of SARS‐CoV‐19 such that the question of why there are these vulnerabilities has been largely overlooked. The assumption that one cause (the virus) is the purveyor of all the systemic symptoms following COVID‐19 infection is inherent in almost all documentation. It is assumed that symptoms and mortality are always a direct result of an increase in virions over time. Recently we described a multipart model based upon peer reviewed material over the last 50 years which results in a far more complex and accurate model of the systems involved (Johnson et al., 2020). The main known factor affecting vulnerabilities to COVID‐19 is the availability of nutrient‐bound ligand carriers, but present methods of human serum albumin (HSA) therapy are insufficient to prevent morbidity (Boada et al., 2019; Caraceni et al., 2013). Furthermore, a recent paper by Xu et al. (2021, Kheir et al., 2021) demonstrates that low serum albumin levels are a predictor of COVID‐19 vulnerability. They studied a cohort of 79 COVID‐19 patients combined with a review of electronic laboratory records. This showed that hypoalbuminaemia was common in COVID‐19 patients, as has been demonstrated elsewhere (see Johnson et al., 2020 for review), and called for ‘dynamic monitoring of serum albumin’ to be ‘performed during COVID‐19 patient treatments’: we concur with their viewpoint.
COVID infection usually begins with the lungs but in younger people the infection is often asymptomatic or only localised. Complications that cause what is known as long‐COVID with symptoms lasting for many months after the infection, or death, occur at a later stage when other organs become infected until they reach a critical stage. The systemic nature of COVID‐19 (SARS‐CoV2) when presented as a serious condition indicates that the cause is mediated systemically with infection in multiple organs and subsequent multiple organ failure (De la Rica et al., 2020; Iwasaki et al., 2021).
Immediacy is always present in a clinical situation where a life‐threatening illness is involved. SARS‐CoV2 has been defined as an acute respiratory syndrome (Larsen et al., 2020) by the early symptoms of the disease with efforts to maintain oxygen concentrations critical to care. Low oxygen levels are only one aspect of respiration and in SARS‐CoV2 it is an indication of low lung performance due to damage. The ventilation system of the lungs is intricately tied to the functionality of the rest of the cardiovascular system and the flow of nutrients is a function of blood flow and concentration.
2. HYPOTHESIS
To summarise, HSA is the most abundant human plasma protein. It is the primary transporter of nutrients, fatty acids and hormones in the body and it maintains oncotic pressure. Given that HSA declines with ageing (Figure 1), we hypothesise that this makes older people more vulnerable to COVID‐19 infection. If this hypothesis is correct, treatment of COVID‐19 symptoms with HSA therapy should be considered, via an appropriate route of administration.
FIGURE 1.
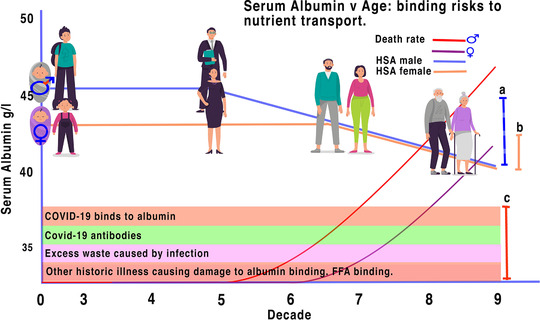
Illustrative profile match of albumin decrease with age and risk of COVID‐19. Levels of albumin binding changes during ageing making older males more vulnerable after 50 years and females after 65. Serum albumin levels of males (a) decrease with age earlier than those of females (b) (derived from Weaving et al., 2016). SARS‐CoV2 virions, antibodies, excess waste and factors from other illnesses reduce the tolerance of unbound albumin further (c). When the number of ligands caused by COVID‐19 (c) exceeds that of either the male HSA binding tolerance (a) or the female HSA binding tolerance (b), the ability of HSA to transport nutrients is exhausted. The implication is that as SARS‐CoV2 virions enter the system they, and the consequential antibodies and other created ligands, block the natural ability of HSA to bind the correct nutrients causing cellular stress and crisis in the systemic system affecting all organs, leading to excess death rates in both males and females as illustrated (curves derived from data of Islam et al., 2021). Human figures designed by Tartila/Freepik
3. LOW HSA MAY CAUSE THE CELLULAR STRESS SEEN DURING THE SYSTEMIC ACUTE STAGE OF COVID‐19
We recently argued that lack of pool HSA that circulates through the endothelial and interstitial structures, bound to ligands, may be the intermediary that leads to cell death and severe illness (Johnson & Winlow, 2020). We demonstrated that the clinical symptoms of SARS‐CoV‐2 infection occur at frequencies which depend upon available nutrients and infection rates of individual organs within the body. We divided the symptoms into localised and systemic and described the progress of the symptoms of the disease by known molecular events. Unlike the majority of research, which has been concentrated on reducing the virus replication or provision of antibodies (Planas et al., 2021) against variants (Salim et al., 2021), our focus has been on the systemic mechanisms that cause increased morbidity and mortality. This is a novel approach that assumes each infected site within an individual is capable of recovery if correct nutrients are present. The implication is that it is the body's lack of binding resources to contain the virus locally that allows the systemic spread of the virus particularly in vulnerable individuals who are over 50, obese and with underlying clinical conditions.
3.1. Localised infections
An initial infection occurs locally on an organ system, most usually the lungs. Initial spread may take place systemically with each subsequent organ infected locally. Localised infections are susceptible to both localised and systemic treatments – e.g., by topical sprays (Horby et al., 2021), or by systemic antiviral drugs (Lee et al., 2020a). Each localised infection is controlled according to nutrients and environment. Localised infections lead to localised symptoms.
3.2. Systemic infection
Nutrients and gases are dispersed throughout the body via all of the systemic fluids. In our model these are primarily the blood (both plasma and red blood cells), the lymph and importantly the interstitial fluids surrounding the cells, all of which form the free body fluids. Our model also includes the cerebrospinal fluid (CSF).
4. AGE AND VULNERABILITY
What is rarely considered is that in all epidemics including COVID‐19 a large proportion of the population survive with little or no long‐term effect. The normal physiology of the healthy human body is therefore well‐adapted to survive COVID‐19 and the norm is for patients to recover. This is easily demonstrated by the survival of young people. Vulnerabilities must therefore be the key to understanding the mechanisms behind long‐COVID, and mortality in COVID‐19. Bats are a well‐known host to coronaviruses, but they are able to defend themselves (Irving et al., 2021) as is most of the human population. Therefore, individual vulnerabilities are likely to indicate the mechanisms that cause long‐COVID‐19, and COVID‐19 mortality.
A feature of pandemics is their rates of vulnerability and mortality, both related to vulnerabilities in a population, which in turn are formed largely through individual physiologies where each individual has both environmental and genetic variations. Each vulnerability has its own level of harm as demonstrated in population statistics, and those of COVID‐19 are now well known (De Larochelambert et al., 2020). Unusually COVID‐19 affects the over 50s disproportionately, with children and young adults less susceptible (Crimmins, 2020). The obese are similarly affected (Tamara & Tahapary, 2020). These are common points of reference which we will return to. Thus, COVID‐19 does not follow the age profile of previous epidemics like 1918 flu that affected both young and old. A study of the system of nutrient transport led to our previous paper (Johnson et al., 2020) where we discussed HSA transport of nutrients that may affect the endothelial cellular structures – especially of the small capillaries (Figure 2b).
FIGURE 2.
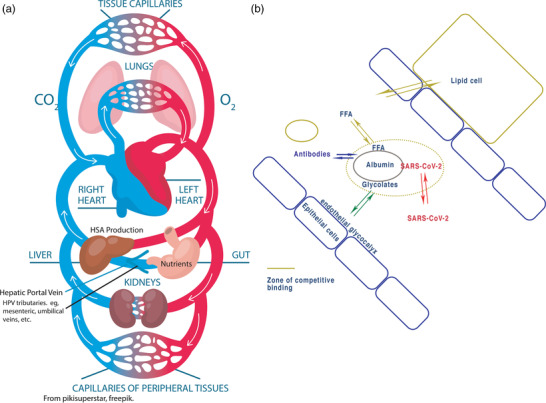
Illustration of direction of flow of nutrients from the gut and ligand exchange in the capillaries. (a) Circulatory system showing direction and flow of nutrients from the intestine through the heart and lungs. Fluid therapy to the periphery of unbound HSA will flow to the heart and then is concentrated in the lungs and may make many circulations before re‐entering the liver . HSA entering the liver is charged with ligands before circulating. Thus fresh, unbound HSA could be introduced into the hepatic portal vein using standard techniques as described in the discussion. (b) At the capillary level, concentrations, and therefore delivery of ligands (FFA – free fatty acids), are determined by relative concentrations across cells. Competition exists to maintain equilibrium and any outside element such as SARS‐CoV2 will interfere with this balance. (From Johnson et al., 2020 – reproduced under the Creative Commons Licence.) (a) modified from pikissuperstar freepik
5. LOCAL PLASMA CIRCULATION
Often the circulatory system is considered a single system, but it is better considered as not one circulatory system based on the heart, but an integrated mechanism of many: the pulmonary cardiac system – regulating gaseous supply; the intestinal–hepatic nutrient system – regulating other nutrients; the renal system – waste removal; etc. Thought of in those terms, each individual organ function has a different locus: lungs – gas exchange, liver – nutrients, kidneys – waste, etc. As plasma circulates, nutrients are removed changing relative pool levels of nutrients. Previously (Johnson et al., 2020) we discussed HSA in terms of its binding to SARS‐CoV‐2 at the sites of distribution. Recently evidence has shown that hypoalbuminaemia (which we interpret as whole‐body reduction in active HSA not just that in the bloodstream) can be used as a direct marker for prediction of vulnerabilities in long‐COVID and mortality confirming our hypothesis that hypoalbuminaemia is implicated (Huang et al., 2020a; Kheir et al., 2021; Viana‐Llamas et al., 2021; Xu et al., 2021). In these papers the authors suggest using hypoalbuminemia as a clinical marker for serious COVID‐19 infection.
6. THE NUTRIENT CIRCULATORY SYSTEM AND TRANSPORT‐PROTEIN MAINTENANCE
Nutrients are provided to the body almost exclusively through the intestines where they pass through the hepatic portal vein into the liver. The hepatic portal vein (Okudaira, 1991) passes quite close to the hepatic artery as it enters the capillary structures ensuring a mixing of nutrient‐rich blood and nutrient‐poor blood, diluting nutrients, moderating concentrations and binding HSA in the liver to the necessary nutrients to supply the systemic system. It is in the liver that a large proportion of nutrients can be stored in real time to be used for controlled release by various mechanisms, for example insulin modulation of glucose and glycogen. The liver also produces and regulates the concentrations of many other nutrients in the plasma including HSA (Lee et al., 2020b). Liver function tests have recently revealed that 33% of COVID‐19 patients suffer from hypoalbuminaemia (Weber et al., 2021) and patients with acute liver injury are known to suffer from acute hypoalbuminaemia (Signorini et al., 2021). Thus, acute damage to the liver may have lethal consequences following COVID‐19 infection. From the liver, plasma rich nutrients, modulated by the liver (Levitt, & Levitt, 2016), maintain relatively stable concentrations of ligands, passing into the hepatic vein, vena cava and heart where nutrients are further diluted. Importantly for our research, nutrients then pass into the capillaries of the lungs before continuing back to the heart and then on to the tissue capillaries.
In the tissue capillaries, nutrient concentrations change in the plasma as it passes from arterial to venous as nutrients are absorbed. Boden (2008) showed that levels of fatty acids in the plasma corresponded to levels of fat in cells. The levels of nutrients in the plasma and in the capillaries decrease according to relative usage in adjacent cells. The HSA levels are set between the plasma and intracellular levels. The level of passively transferred nutrients in tissue corresponds to the relative level in the plasma in adjacent cells (Levitt & Levitt, 2016). Nutrients that do not flow directly into cells pass into the interstitial spaces between cells. The flow of plasma in interstitial spaces is greatly reduced, for example causing the half‐life of large molecular structures like HSA to be 20 days (Moman et al., 2020). Large molecules like HSA, the ‘sediment’ (Moman et al., 2020), travel more slowly than the plasma, hence a half‐life of 20 hours. HSA circulation is thus separate from the fluid plasma. Interstitial fluid eventually passes into the lymph, finally re‐joining the venous flow to the heart via the lymphatic ducts. The implication of the lungs being fed first is that any large nutrient like HSA will remain in the lungs, where up to 80% may remain in the interstitial fluid (Weaving et al., 2016).
7. PLASMA TRANSPORT AND MODULATION OF LIGANDS
A large proportion of ligands carried in the blood are not in the fluid plasma but become bound reversibly to each other and to large ligands. HSA, prealbumin and gamma globulins are some of the ligand carriers (Collins, 2001; Levitt, & Levitt, 2016; Moman et al., 2020). However, HSA has by far the highest concentration and must be the first candidate for examination.
The reversible nature of this binding is such that a large ligand carrier molecule can carry many ligands. Ligands are carried therefore in two separate mechanisms in the plasma: in the liquid portion and bound to large ligand carriers such as HSA. Because many ligands like glycolates are almost entirely bound to carrier proteins such as HSA, ligands carried in the fluid are many times less concentrated than are the protein‐bound ligands. This has the following advantages.
Per volume, plasma can carry a far greater concentration of ligands than carried in just the fluid.
The relative oncotic pressure change produced from the ligand binding maintains pressure.
There is a modulatory effect when ligand concentration diminishes in the fluid portion in the capillaries; replacement of ligands automatically follows from the ligand carriers, thus maintaining a relatively stable concentration. For example if 10 times as many ligands exist bound to a carrier as in the fluid, if all of the fluid ligand is removed and the equilibrium restored, the concentration will only have dropped by 1/10 of its value. Conversely a drop of 1/10 of the carrier protein will result in a drop in total of 1/10 of total ligand concentration. This provides a buffer where the total content of the ligand in plasma is related to the concentration of ligand carriers. High ligand carrier concentrations have little effect, but any loss results in an immediate fall in ligand availability: this is most relevant to individuals whose HSA levels are close to the physiological minimum.
Any change in the status of the ligand carrier will result in changes in the plasma concentration of the ligand, affecting its mobility and in consequence its actions at its destination. Many of these ligands have homeostatic regulation of their own and form their own circulatory systems, HSA, for example, binds with glucose. This is not a simple system as multiple ligand carriers will have different affinities and binding capacities for their respective ligands. For example, although prealbumin only represents a tiny fraction of binding compared to HSA, it may bind preferentially to some ligands, which may be critical. In the case of COVID‐19 we present evidence of how this transport system functions in relation to HSA.
8. HUMAN SERUM ALBUMIN
Age and sex variation of HSA concentration confirms that ‘The mean population serum HSA concentration increases to peak at around age 20 years and then decreases with increasing age. Initially higher, values in females decrease more rapidly but become close to male values at 60 years’ (Weaving et al., 2016). HSA constitutes almost 50% of all protein found in the blood. It is synthesised in the liver and has a half‐life of 20 days in the plasma (Moman et al., 2020). Up to 80% of HSA is not contained in the plasma but in a serum pool of free body fluids: the lymph, interstitial fluids, CSF (Suárez‐Gonzalez et al., 2020), there is a pool for the placenta, the testis and ovaries, everywhere there is a barrier, where it feely circulates and mixes with the plasma with a half‐life of about 8 h (Levitt & Levitt, 2016). Hypoalbuminaemia can also lead to hypertension via the immune system, which is dysregulated in hypertension and SARS‐CoV2 infection (Drummond et al., 2019).
COVID‐19 complications occur at the systemic stage of the infection and then lead to the integral collapse of the endothelial cellular structure leading to the more serious systemic symptoms and death. We propose that this is caused by a similar system to that of sepsis (Johnson et al., 2020; Roger, 2021; Stasi et al., 2021), but which we have redefined more specifically for operational purposes as being ‘the symptoms caused by an inoperative systemic nutrient transport system’. We provide evidence that the main cause of this is insufficient unbound HSA. Although other proteins may be involved, 80% of all ligands are carried in the blood on HSA (Moman et al., 2020). We have identified a reduction in active HSA as being the common point in sepsis and in SARS‐CoV‐2 major systemic failure. In brief:
HSA is reduced by up to 33% in patients over 50 (De la Rica et al., 2020; Ghahramani et al., 2020; Weaving et al., 2016; Weber et al., 2021).
Low Serum HSA predicts severe COVID‐19 (Huang et al., 2020a; Kheir et al., 2021; Viana‐Llamas et al., 2021; Xu et al., 2021) and can be used as triage for serious illness (Viana‐Llamas et al., 2021; Xu et al., 2021).
Hypoalbuminaemia is also a known factor in sepsis, acute respiratory distress syndrome (ARDS) and COVID (Roger, 2021; Stasi et al., 2021).
Eighty per cent of HSA resides in the pool of interstitial spaces and lymph. It is the main provider of proteins forming the endothelial glycocalyx layer – the inherent stabiliser of endothelial cell connectivity maintaining the structure and placement of endothelial cells (Johnson et al., 2020).
Any externally applied ligand may bind competitively to HSA and displace the transport‐nutrients necessary for the cell. These include viruses like SARS‐CoV‐2 and antibodies, both of which reduce binding capacity for other ligands as COVID‐19 reaches a critical point (Johnson et al., 2020). Levels of available binding decrease upon increase in levels of free fatty acids (Boden, 2008) – and the SARS‐CoV‐2 virions. Reduction in binding leads to sepsis.
Plasma HSA may also affect gaseous nutrients; for example, haem is also transported on HSA along with units of oxygen (Ascenzi et al., 2015).
Low serum HSA level predicts mortality in dialysis patients (Mehrotra et al., 2011).
Without knowledge of how drugs operate as ligands when transported, topically localised drugs may increase recovery time. However, in HSA‐compromised individuals, systemically applied drugs will inevitably have greater effects on symptoms and mortality (Guzik et al., 2020).
Levels of HSA in the free body fluids, including interstitial fluid and the CSF, affect the concentrations of proteins within a localised area. We suggest that the presence of SARS‐COV‐2 binding to HSA displaces and reduces the ligands available for transport. This depletes nutrients at their sites of requirement. This indicates that, since HSA is the main transport mediator of the body, its relative concentration is a part of the regulatory mechanism that defines cellular integrity (Apte, 2020). Transport of ligands by other sources such as gamma globulins also acts in competition with HSA. However, HSA administered in high doses has been examined for its therapeutic value in models of some CNS diseases. In models of ischaemia (e.g., global, transient focal, or permanent focal) or traumatic brain injury, administration of HSA resulted in protection (LeVine, 2016).
8.1. Other properties of HSA add evidence to the hypothesis
It has been demonstrated that when more ligands are present after injury, HSA concentration is increased (Ishida et al., 2014; Pérez‐Guisado et al., 2013). Indications in COVID‐19 implicate degraded liver function maintaining or reducing HSA levels (Crea et al., 2020; Guzik et al., 2020; Paliogiannis et al., 2020). There is evidence that declining HSA is associated with nutritional risk, physiology and system inflammation (Almasaudi et al., 2020; Iba et al., 2020; Iwasaki et al., 2021) as well as rheumatic heart disease (Wei et al., 2017). Long‐term administration of human HSA improves survival in patients with cirrhosis and refractory ascites (Di Pascoli et al., 2019) and hospital mortality (Akirov et al., 2017).
It is important to note that HSA maintains the fluid balance in the body and any condition that results in a decrease in plasma volume will cause falsely elevated HSA levels (Ishida et al., 2014). The serum HSA test only looks at the levels of HSA in a person's blood, not in the rest of the body fluids. HSA concentrations rise slowly during nutritional therapy (refeeding) and in patients recovering from stress (Caraceni et al., 2013). Changes in HSA can be acquired by at least 2–3 weeks of nutritional intervention. Diseases such as COVID‐19 cause the liver cells to lose the ability to synthesize HSA (Guzik et al., 2020; Paliogiannis et al., 2020).
In our hypothesis (Johnson et al., 2020), we proposed that the serious symptoms of COVID‐19 are caused by hypoalbuminaemia (Gounden et al., 2020; Larsen et al., 2020). Maintenance of systemic homeostasis of nutrients requires a certain level of HSA, which may become saturated on addition of SARS‐CoV‐19 virions as well as subsequent antibodies that bind to its structure. Thus, the concentration in the blood plasma is only one representation of effective HSA, because any other previous infection or illness that affects blood concentrations of ligands bound to HSA will have moderating effects on cell physiology, e.g., insulin, fatty acids, which increase in diabetes (Boden, 2008), many antibiotics and other drugs. Furthermore, recent infections will generate antibodies that will bind to HSA.
9. DISCUSSION
Here, we have explained how HSA production and subsequent binding to ligands is managed by the liver and explain the potential positive effects of HSA therapy applied to the portal system. The evidence we have presented suggests that COVID‐19 presents initially as a symptomatic respiratory disease, which then distributes systemically causing organ failure. We have also demonstrated that organ failure in COVID‐19 is due to localised hypoalbuminaemia caused by competition on HSA‐binding sites by SARS‐CoV‐19 and the body's immune response. We have also shown how HSA binding capacity can be influenced by the body's own decrease in the over 50s: obesity by the action of FFA competition for albumin. Other comorbidities, such as organ damage, will change the equilibrium of bound ligands to carrier proteins affecting the same systemic transport system. The nutrient transport system of the human body is constructed so that only a very minor quantity of nutrient will be transported in the fluid portion of the plasma; almost all ligands are transported bound to ligand carriers creating large buffers of competitive nutrient ligands in equilibrium between the fluid and the ‘sediment’ containing ligand carriers. HSA is by far the largest ligand carrier.
The circulation of HSA begins in the liver where nutrient supply into the plasma is moderated. At this point HSA is pre‐bound, and in healthy subjects all ligands are in an equilibrium, which will transport ligands to their sites of absorption, in the correct concentrations for heathy cellular activity. HSA that leaves the liver is ‘loaded’ to its optimal extent with a variety of ligands representative of the state of the liver and its supply (Figure 2a). As HSA passes through the circulation and especially the small capillary and interstitial spaces, the relative concentrations of ligands bound to HSA change as the cellular structures acquire ligands. This will change both current binding and oncotic pressure according to what nutrients are released and waste binds to HSA and other carriers. Any slight decrease in HSA concentration will produce a corresponding large decrease in available ligands especially in the distal capillaries and if HSA is near minimum. This will cause many of the blood markers (Kim et al., 1999) for inflammation and autoimmunity (Lee et al., 2020b) to change value as has been seen in COVID‐19 (Thwaites et al., 2021). These changing markers may be a signal of HSA binding deficiency as SARS‐CoV2 virions displace other ligands from binding sites. Any foreign ligand or destabilisation of the HSA binding will reduce the active level of HSA further as it is charged in the liver or interacts throughout its circulation. When a SARS‐CoV2 virion binds to HSA, this reduces the binding activity for other ligands, which then have a corresponding drop in concentrations in the distal small capillaries.
10. HSA THERAPY AND THE APPROPRIATE SITE OF HSA ADMINISTRATION
Infection in COVID‐19 is usually via the lungs, which contain a large proportion of HSA; low HSA in the system will therefore preferentially bind SARS‐CoV2 virions. HSA remains in the body with a half‐life of 20 days, much of it resident in the interstitial spaces from which it moves slowly. The symptoms of long‐COVID follow a similar pattern expected from hypoalbuminaemia caused by SARS‐CoV2 binding to HSA, which remains in the interstitial spaces. One method of reducing the risk of vulnerabilities to COVID‐19 might be HSA therapy (Mani Mishra et al., 2020; Mendez et al., 2005), but we need to know the most appropriate site of administration as this is critical.
Currently HSA therapy is given into a peripheral vein that then flows to the vena cava, heart and then lungs. HSA given in this manner is effectively in an unnatural state as any binding of HSA is unrepresentative of the ligand–HSA equilibrium formed on loading in the liver. Any addition of HSA into a peripheral vein is preferentially absorbed into the interstitial spaces of the lungs for the half‐life of HSA in the pool, about 20 h, before passing to the rest of the body, concentrating the unbound albumin in one place. Of course, binding will also occur between the administered HSA and ligands in competition with other HSA already bound, but this will further distort the nutrients delivered with potentially many days before re‐equilibrium throughout the systemic system; this problem is exacerbated with higher HSA concentrations and a longer time period. On administration, the HSA passes into the capillaries and is slowed in movement as it passes into the interstitial spaces causing any new HSA to congregate in the lungs. The equilibrium of the ligands bound to existing HSA in the body changes to reflect the state of the new unbound HSA, reducing the overall concentration of ligands in the plasma as described above. For ARDS diseases like COVID‐19 this is especially problematic when the lungs are already inflamed and nutrients low. There is also a risk that unbound HSA will preferentially bind to SARS‐CoV2 virions in the lungs and become systemic. A more appropriate site of administration is therefore required.
Safely raising the levels of healthy bound HSA in plasma will depend upon the site of administration and the form in which it is given: whether it enters the liver to be charged by nutrient‐ligands or the lungs where it may cause damage. HSA to be given correctly would require pre‐ligand‐binding at least representative of the ligand equilibrium formed in the liver where binding takes place. The liver is a highly evolved organ and its capacity for HSA synthesis and moderation of correct nutrient ligands must not be underestimated; in a functioning liver any amount of HSA should be preferentially bound to the correct ligands. Ideally, then, HSA should be given into the portal system via the hepatic portal vein or its tributaries (Okudaira, 1991) or the umbilical vein, which is accessible in most adults and ‘can be cannulated in a superficial position in the upper abdomen’ (Braastad et al., 1967). Other entry sites to the hepatic portal vein exist via catheterisation through jugular, femoral or humeral veins (Butzow & Novak, 1977; Lebrec, 1991). Giving HSA to the portal system should therefore provide greater effectiveness and control over administration of HSA for both oncotic pressure and ligand binding, thus providing a more stable environment in which to raise HSA. The evidence presented demonstrates that clinical trials should be carried out to test this hypothesis as soon as possible with the aim of reducing vulnerabilities to COVID‐19.
11. CONCLUSION
There is evidence to support the hypothesis that low binding of nutrients in the HSA pool may be a contributor to severe COVID‐19 at the systemic stage. Raising the HSA binding in the pool level causes complications by disturbing both the oncotic balance and also the equilibrium of ligands bound to HSA carrier proteins. Thus, HSA should be given bound to ligands or through the hepatic portal vein and tributaries such as the umbilical vein at more than minimal levels. Addition of HSA directly to the liver has the advantage that the resultant albumin delivered to the systemic system will have been bound to appropriate ligands. This process may help alleviate severe COVID‐19 symptoms. We call for detailed clinical studies of this hypothesis.
COMPETING INTERESTS
No competing interests.
AUTHOR CONTRIBUTIONS
Both authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Johnson, A. S. , & Winlow, W. (2022). COVID‐19 vulnerabilities are intensified by declining human serum albumin levels. Experimental Physiology, 107, 674–682. 10.1113/EP089703
Edited by: Jeremy Ward
REFERENCES
- Akirov, A. , Masri‐Iraqi, H. , Atamna, A. , & Shimon, I. (2017). Low albumin levels are associated with mortality risk in hospitalized patients. American Journal of Medicine, 130, 1465.e11–1465.e19. 10.1016/j.amjmed.2017.07.020 [DOI] [PubMed] [Google Scholar]
- Almasaudi, A. S. , Dolan, R. D. , Edwards, C. A. , & McMillan, D. C. (2020). Hypoalbuminemia reflects nutritional risk, body composition and systemic inflammation and is independently associated with survival in patients with colorectal cancer. Cancers, 12, 1986. 10.3390/cancers12071986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte, S. P. (2020). Examining the nuances of the Albumin enigma in SARS CoV‐2 infections. Journal of Excipients and Food Chemicals, 11, 58–61 [Google Scholar]
- Ascenzi, P. , di Masi, A. , Fanali, G. , & Fasano, M. (2015). Heme‐based catalytic properties of human serum albumin. Cell Death Discovery, 1, 15025. 10.1038/cddiscovery.2015.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada, M. , López, O. , Núñez, L. , Szczepiorkowski, Z. M. , Torres, M. , Grifols, C. , & Páez, A. (2019). Plasma exchange for Alzheimer's disease Management by Albumin Replacement (AMBAR) trial: Study design and progress. Alzheimer's & Dementia, 5, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden, G. (2008). Obesity and free fatty acids. Endocrinology and Metabolism Clinics of North America, 37, 635–646. 10.1016/j.ecl.2008.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braastad, F. W. , Condon, R. E. , & Gyorkey, F. (1967). The umbilical vein. Archives of Surgery, 95, 948. 10.1001/archsurg.1967.01330180096018 [DOI] [PubMed] [Google Scholar]
- Bützow, G. H. , & Novak, D. (1977). Clinical value of hepatic vein catheterization. Gastrointestinal Radiology, 2, 153–161. 10.1007/BF02256490 [DOI] [PubMed] [Google Scholar]
- Caraceni, P. , Tufoni, M. , & Bonavita, M. E. (2013). Clinical use of albumin. Blood Transfusion, 11(Suppl 4), s18–s25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, N. (2001). The difference between albumin and prealbumin. Advances in Skin & Wound Care, 14, 235–236. [DOI] [PubMed] [Google Scholar]
- Crea, F. , Thomson, E. C. , & McInnes, I. B. (2020). COVID‐19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovascular Research, 116, 1666–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins, E. M. (2020). Age‐related vulnerability to coronavirus disease 2019 (COVID‐19): Biological, contextual, and policy‐related factors. Public Policy & Aging Report, 30, 142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Larochelambert, Q. , Marc, A. , Antero, J. , Le Bourg, E. , & Toussaint, J.‐.F. (2020). Covid‐19 mortality: A matter of vulnerability among nations facing limited margins of adaptation. Frontiers in Public Health, 8, 604339. 10.3389/fpubh.2020.604339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Rica, R. , Borges, M. , Aranda, M. , Del Castillo, A. , Socias, A. , Payeras, G. R. , Socias, L. , Masmiquel, L. , & Gonzalez‐Freire, M. (2020). Low albumin levels are associated with poorer outcomes in a case series of COVID‐19 patients in Spain: A retrospective cohort study. Microorganisms, 8, 1106. 10.3390/microorganisms8081106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pascoli, M. , Fasolato, S. , Piano, S. , Bolognesi, M. , & Angeli, P. (2019). Long‐term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver International, 39, 98–105. 10.1111/liv.13968 [DOI] [PubMed] [Google Scholar]
- Drummond, G. R. , Vinh, A. , Guzik, T. J. , & Sobey, C. G. (2019). Immune mechanisms of hypertension. Nature Reviews. Immunology, 19, 517–532. 10.1038/s41577-019-0160-5 [DOI] [PubMed] [Google Scholar]
- Ghahramani, S. , Tabrizi, R. , Lankarani, K. B. , Kashani, S. M. A. , Rezaei, S. , Zeidi, N. , Akbari, M. , Heydari, S. T. , Akbari, H. , Nowrouzi‐Sohrabi, P. , & Ahmadizar, F. (2020). Laboratory features of severe vs. non‐severe COVID‐19 patients in Asian populations: A systematic review and meta‐analysis. European Journal of Medical Research, 25, 30. 10.1186/s40001-020-00432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounden, V. , Vashisht, R. , & Jialal, I. (2020) Hypoalbuminemia. In: StatPearls [Internet]. Treasure Island, FL, USA: StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK526080/ [PubMed] [Google Scholar]
- Guzik, T. G. , Mohiddin, S. A. , Dimarco, A. , Patel, V. , Savvatis, K. , Marelli‐Berg, F. M. , Madhur, M. S. , Tomaszewski, M. , Maffia, P. , D'Acquisto, F. , Nicklin, S. A. , Marian, A. J. , Nosalski, R. , Murray, E. C. , Guzik, B. , Berry, C. , Touyz, R. M. , Kreutz, R. , Wang, D. W. , … McInnes, I. B. (2020). COVID‐19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovascular Research, 116, 1666–1687. 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, J. S. Y. , Fernando, D. I. , Chan, M. Y. , & Sia, C. H. (2020). Obesity in COVID‐19: A systematic review and meta‐analysis. Annals of the Academy of Medicine, Singapore, 49, 996–1008. 10.47102/annals-acadmedsg.2020299 [DOI] [PubMed] [Google Scholar]
- Horby, P. , Lim, W. S. , Emberson, J. R. , Mafham, M. , Bell, J. L. , Linsell, L. , Staplin, N. , … Landray, M. J. (2021). Dexamethasone in hospitalized patients with Covid‐19. New England Journal of Medicine, 384, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J. , Cheng, A. , Kumar, R. , Fang, Y. , Chen, G. , Zhu, Y. , & Lin, S. (2020a). Hypoalbuminemia predicts the outcome of COVID‐19 independent of age and co‐morbidity. Journal of Medical Virology, 92, 2152–2158. 10.1002/jmv.26003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, T. , Lu, Y. , Huang, Y.‐M. , Wang, M. , Ling, W. , Siu, Y. , & Zhao, H.‐L. (2020b). Obesity in patients with COVID‐19: A systematic review and meta‐analysis. Metabolism, 113, 154378. 10.1016/j.metabol.2020.154378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba, T. , Connors, J. M. , & Levy, J. H. (2020). The coagulopathy, endotheliopathy, and vasculitis of COVID‐19. Inflammation Research, 69, 1181–1189. 10.1007/s00011-020-01401-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving, A. T. , Ahn, M. , Goh, G. , Anderson, D. E. , & Wang, L. F. (2021). Lessons from the host defences of bats, a unique viral reservoir. Nature, 589, 363–370. 10.1038/s41586-020-03128-0 [DOI] [PubMed] [Google Scholar]
- Islam, N. , Shkolnikov, V. M. , Acosta, R. J. , Klimkin, I. , Kawachi, I. , Irizarry, R. A. , Alicandro, G. , Khunti, K. , Yates, T. , Jdanov, D. A. , White, M. , Lewington, S. , & Lacey, B. (2021). Excess deaths associated with covid‐19 pandemic in 2020: Age and sex disaggregated time series analysis in 29 high income countries. BMJ, 373, n1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida, S. , Hashimoto, I. , Seike, T. , Abe, Y. , Nakaya, Y. , & Nakanishi, H. (2014). Serum albumin levels correlate with inflammation rather than nutrition supply in burns patients: A retrospective study. Journal of Medical Investigation, 61, 361–368 . 10.2152/jmi.61.361 [DOI] [PubMed] [Google Scholar]
- Iwasaki, M. , Saito, J. , Zhao, H. , Sakamoto, A. , Hirota, K. , & Ma, D. (2021). Inflammation triggered by SARS‐CoV‐2 and ACE2 augment drives multiple organ failure of severe COVID‐19: molecular mechanisms and implications. Inflammation, 44, 13–34. 10.1007/s10753-020-01337-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, A. S. , Fatemi, R. , & Winlow, W. (2020) SARS‐CoV‐2 bound human serum albumin and systemic septic shock. Frontiers in Cardiovascular Medicine, 7, 153. 10.3389/fcvm.2020.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheir, M. , Saleem, F. , Wang, C. , Mann, A. , & Chua, J. (2021). Higher albumin levels on admission predict better prognosis in patients with confirmed COVID‐19. PLoS One, 16, e0248358. 10.1371/journal.pone.0248358] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. B. , Chi, H. S. , Park, J. S. , Hong, C. D. , & Yang, W. S. (1999). Effect of increasing serum albumin on plasma D‐dimer, von Willebrand factor, and platelet aggregation in CAPD patients. American Journal of Kidney Diseases, 33, 312–317. 10.1016/S0272-6386(99)70306-9 [DOI] [PubMed] [Google Scholar]
- Larsen, J. R. , Martin, M. R. , Martin, J. D. , Kuhn, P. , & Hicks, J. B. (2020). Modeling the onset of symptoms of COVID‐19. Frontiers in Public Health, 8, 473. 10.3389/fpubh.2020.00473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrec, D. (1991). Hepatic vein catheterization. In Okuda K. (ed.), Portal hypertension. Tokyo: Springer Japan. [Google Scholar]
- Lee, N. , Ison, M. , & Dunning, J. (2020a). Early triple antiviral therapy for COVID‐19. Lancet, 396, 1487–1488. 10.1016/S0140-6736(20)32274-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. , Achuthan, A. A. , & Hamilton, J. A. (2020b). GM‐CSF: A promising target in inflammation and autoimmunity. ImmunoTargets and Therapy, 9, 225–240. 10.2147/ITT.S262566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine, S. M. (2016). Albumin and multiple sclerosis. BMC Neurology, 16, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt, D. G. , & Levitt, M. D. (2016). Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. International Journal of General Medicine, 9, 229–255. 10.2147/IJGM.S102819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani Mishra, P. , Uversky, V. N. , & Nandi, C. K. (2020). Serum albumin‐mediated strategy for the effective targeting of SARS‐CoV‐2. Medical Hypotheses, 140, 109790. 10.1016/j.mehy.2020.109790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra, R. , Duong, U. , Jiwakanon, S. , Kovesdy, C. P. , Moran, J. , Kopple, J. D. , & Kalantar‐Zadeh, K. (2011). Serum albumin as a predictor of mortality in peritoneal dialysis: Comparisons with hemodialysis. American Journal of Kidney Diseases, 58, 418–428. 10.1053/j.ajkd.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez, C. M. , McClain, C. J. , & Marsano, L. S. (2005). Albumin therapy in clinical practice. Nutrition in Clinical Practice, 20, 314–320. 10.1177/0115426505020003314 [DOI] [PubMed] [Google Scholar]
- Moman, R. N. , Gupta, N. , Sheikh, N. S. , Rajat, N. , & Varacallo, M. (2020). Physiology, albumin. In: StatPearls [Internet]. Treasure Island, FL, USA: StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK459198/ [PubMed] [Google Scholar]
- Okudaira, M. (1991). Anatomy of the portal vein system and hepatic vasculature. In Okuda K. & Benhamou J. P. (eds.). Portal hypertension. Tokyo: Springer Japan. 10.1007/978-4-431-68361-2_1 [DOI] [Google Scholar]
- Paliogiannis P., Zinellu, A. , Scano, V. , Mulas, G. , De Riu, G. , Pascale, R. M. , Arru L. B., Carru C., Pirina P., Mangoni A. A., & Fois A. G. (2020). Laboratory test alterations in patients with COVID‐19 and non COVID‐19 interstitial pneumonia: a preliminary report. Journal of Infection in Developing Countries, 685–690. 10.3855/jidc.12879 [DOI] [PubMed] [Google Scholar]
- Pérez‐Guisado, J. , de Haro‐Padilla, J. M. , Rioja, L. F. , Derosier, L. C. , & de la Torre, J. I. (2013). Serum albumin levels in burn people are associated to the total body surface burned and the length of hospital stay but not to the initiation of the oral/enteral nutrition. International Journal of Burns and Trauma, 3, 159–163. [PMC free article] [PubMed] [Google Scholar]
- Planas, D. , Brue, T.l. , Grzelak, L. , Guivel‐Benhassine, F. , Staropoli, I. , Porrot, F. , Planchais, C. , Buchrieser, J. , Rajah, M. M. , Bishop, E. , Albert, M. , Donati, F. , Prot, M. , Behillil, S. , Enouf, V. , Maquart, M. , Smati‐Lafarge, M. , Varon, E. , Schortgen, F. , …, & Schwartz, O. (2021). Sensitivity of infectious SARS‐CoV‐2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nature Medicine,27, 917–924 . 10.1038/s41591-021-01318-5 [DOI] [PubMed] [Google Scholar]
- Roger, C. (2021) COVID‐19: Should we consider it as a septic shock? (The treatment of COVID‐19 patients in the ICU). Current Opinion in Anesthesiology, 34, 119–124. 10.1097/ACO.0000000000000956 [DOI] [PubMed] [Google Scholar]
- Salim, S. , Karim, A. , de Oliviera, T. , & Loots, G. (2021). Appropriate names for COVID‐19 variants. Science, 371, 1215. [DOI] [PubMed] [Google Scholar]
- Signorini, C. , Pignatti, P. , & Coccini, T. (2021). How do inflammatory mediators, immune response and air pollution contribute to COVID‐19 disease severity? A lesson to learn. Life, 11, 182. 10.3390/life11030182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasi, A. , Franzin, R. , Fiorentino, M. , Squiccimarro, E. , Castellano, G. , & Gesualdo, L. (2021). Multifaced roles of HDL in sepsis and SARS‐CoV‐2 infection: Renal implications. International Journal of Molecular Sciences, 22, 5980. 10.3390/ijms22115980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez‐González, A. , Livingston, G. , Low, L. F. , Cahill, S. , Hennelly, N. , Dawson, W. D. , Weidner, W. , Bocchetta, M. , Ferri, C. P. , Matias‐Guiu, J. A. , Alladi, S. , Musyimi, C. W. , & Comas‐Herrera, A. (2020) Impact and mortality of COVID‐19 on people living with dementia: Cross‐country report. https://ltccovid.org/wp-content/uploads/2020/08/International-report-on-the-impact-of-COVID-19-on-people-living-with-dementia-19-August-2020.pdf
- Tamara, A. , & Tahapary, D. L. (2020). Obesity as a predictor for a poor prognosis of COVID‐19: A systematic review. Diabetes & Metabolic Syndrome, 14, 655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites, R. S. , Sanchez Sevilla Uruchurtu, A. , Siggins, M. K. , Liew, F. , Russell, C. D. , Moore, S. C. , Fairfield, C. , Carter, E. , Abrams, S. , Short, C.‐.E. , Thaventhiran, T. , Bergstrom, E. , Gardener, Z. , Ascough, S. , Chiu, C. , Docherty, A. B. , Hunt, D. , Crow, Y. J. , Solomon, T. , … Openshaw, P. J. (2021). ISARIC4C investigators. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM‐CSF in severe COVID‐19. Science Immunology, 6, eabg9873. 10.1126/sciimmunol.abg9873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana‐Llamas, M. C. , Arroyo‐Espliguero, R. , Silva‐Obregón, J. A. , Uribe‐Heredia, G. , Núñez‐Gil, I. , García‐Magallón, B. , Torán‐Martínez, C. G. , Castillo‐Sandoval, A. , Díaz‐Caraballo, E. , Rodríguez‐Guinea, I. , & Domínguez‐López, J. (2021). Hypoalbuminemia on admission in COVID‐19 infection: An early predictor of mortality and adverse events. A retrospective observational study. Medicina Clinica, 156, 428–436. 10.1016/j.medcli.2020.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaving, G. , Batstone, G. F. , & Jones, R. G. (2016). Age and sex variation in serum albumin concentration: An observational study. Annals of Clinical Biochemistry, 53, 106–111. 10.1177/0004563215593561 [DOI] [PubMed] [Google Scholar]
- Weber, S. , Hellmuth, J. C. , Scherer, C. , Muenchhoff, M. , Mayerle, J. , & Gerbes, A. L. (2021). Liver function test abnormalities at hospital admission are associated with severe course of SARS‐COV‐2 infection: A prospective cohort study. Gut, 10.1136/gutjnl-2020-323800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, X. B. , Jiang, L. , Liu, Y. H. , Feng, D. , He, P. C. , Chen, J. Y. , Yu, D.‐Q. , & Tan, N. (2017). Prognostic value of hypoalbuminemia for adverse outcomes in patients with rheumatic heart disease undergoing valve replacement surgery. Scientific Reports, 7, 1958. 10.1038/s41598-017-02185-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y. , Yang, H. , Wang, J. , Li, X. , Xue, C. , Niu, C. , & Liao, P. (2021). Serum albumin levels are a predictor of COVID‐19 patient prognosis: Evidence from a single cohort in Chongqing, China. International Journal of General Medicin, 14, 2785–2797. 10.2147/IJGM.S312521 [DOI] [PMC free article] [PubMed] [Google Scholar]


