Abstract
Background
Fibulin-1 is an extracellular matrix protein expressed at high levels in the placenta. Elevated circulating fibulin-1 have been observed in women with severe pre-eclampsia, whereas low levels have been found in the fetal membranes, prior to membrane rupture. The aim of the study was primarily to evaluate plasma fibulin-1 during expected normal pregnancy and delivery, and secondarily to explore fibulin-1 levels in women developing pre-eclampsia or preterm premature rupture of fetal membranes (PPROM).
Methods
From the historical longitudinal cohort originally consisting of 801 healthy Danish women with a singleton pregnancy, 128 women (632 samples) were selected. Of these, 107 women had normal pregnancies, nine experienced PPROM, and 12 pre-eclampsia. All samples were analyzed for fibulin-1, and levels were compared with blood donors. Differences in mean fibulin-1 between groups were estimated using a linear mixed model.
Results
The mean concentration of fibulin-1 in 120 blood donors was 15.7 µg/mL, (25th-75th-percentiles, 12.3–18.2), with no significant difference in groups stratified by gender or age. Compared to baseline levels in week 12–20, fibulin-1 levels increased significantly from week 29–34 (estimated difference, 5.6 µg/mL; standard error, 1.7; p < 0.001) and 35–42 (12.5 µg/mL; 1.6; p < 0.001) and normalized after birth. The decrease at delivery tended to be more pronounced after elective (-7.0 µg/mL; 2.3; p = 0.002) and emergency (-5.6 µg/mL; 2.9; p = 0.05) cesarean section than after vaginal delivery (reference group). Women who developed PPROM had lower fibulin-1 levels throughout their pregnancies (-11.6 µg/mL; 4.2; p = 0.006). We did not observe a correlate between late pre-eclampsia and fibulin-1 (-0.2 µg/mL; 3.0; p = 0.9).
Conclusions
Fibulin-1 was above non-pregnant levels at week 12 and increased significantly throughout pregnancy. We observed an association between low levels of fibulin-1 and PPROM. Further studies are needed to examine if fibulin-1 could serve as biomarker for the risk of PPROM. However, its role in late preeclampsia is doubtful.
Trial registration
The study was conducted in accordance with the Declaration of Helsinki. The participants provided written informed consent, including storage for future use. The study was approved on July 18, 2005 by The Danish National Committee on Bioethics (No. KA 05065 and S-20,090,061) and the Danish Data Protection Agency.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-021-04110-y.
Keywords: Fibulin, Humans, Plasma, Postpartum, Pre-eclampsia, Pregnancy, Preterm premature rupture of membranes
Background
Fibulin-1 is a member of a family comprised of eight extracellular matrix glycoproteins that are characterized by a molecular weight of ≈ 100 kDa and a modular structure. The gene encoding fibulin-1 (FBLN1) is located on the long arm of human chromosome 22 (22q13.3), and the protein is detected in the extracellular matrix in many organs, often in relation to membranes, elastic fibers, and other connective tissue structures, suggesting a role in matrix remodeling [1, 2]. Fibulin-1 is found in plasma and has been detected in fetuses during early embryonic development [3]. Fibulin-1 mRNA and protein is expressed at high level in the placenta, according to tissue-specific oligonucleotide arrays using human and mouse samples [4]. In addition to roles in the differentiation of heart, skeletal, and neuronal structures in the early human embryo, fibulin-1 is associated with a variety of conditions [5, 6], including cancer, cardiovascular events, impaired kidney function [7], diabetes [7] and polycystic ovary syndrome [8]. Higher plasma levels of fibulin-1 have been observed in women with severe pre-eclampsia (PE), compared to healthy, gestational age-matched controls [9]. In addition, fibulin-1 and two other members of the fibulin family, have been found to be decreased in the para-cervical weak zone of the fetal membranes, prior to rupture [10].
To our knowledge, no study published to date has performed a longitudinal analysis of plasma fibulin-1 levels in healthy pregnant women. We hypothesized that plasma fibulin-1 levels vary with the gestational age and explored if the levels differ in women with normal, PE, and preterm premature rupture of membranes (PPROM) pregnancies [9–12]. In the present study, we report gestational age-specific plasma fibulin-1 concentrations in Caucasian Danish women during normal pregnancy, at delivery, and in the early postpartum period.
Methods
Study design and participants
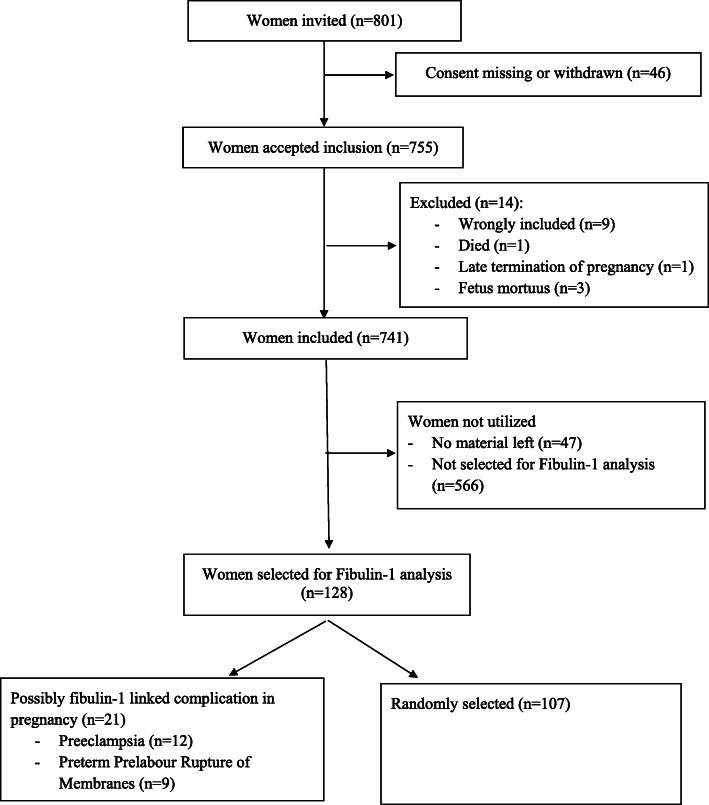
In the original cohort, 801 healthy Caucasian women aged greater than 18 years with an expected normal singleton pregnancy were recruited among 2147 women attending a first trimester screen (week 11 + 4 to 13 + 6) between June 2006 and October 2007 [13–18]. Clinical data were obtained from pregnancy charts and medical records. PE was defined as blood pressure above 140/90 mmHg debuting after week 20 + 0 and either proteinuria or signs of organ dysfunction. Early PE was defined as occurring before week 34 + 0, late PE after week 34 + 0. Severe PE was defined as blood pressure above 160/110, and/or subjective symptoms or laboratory findings consistent with severe organ dysfunction. PPROM was defined as rupture of membranes without uterine contractions before week 37 + 0. Preterm birth was defined as birth before week 37 + 0. Low birth weight was defined as a birth weight < 2500 g, regardless of gestational age. Postpartum hemorrhage was defined as blood loss of > 500 mL during a vaginal birth and > 1000 mL during cesarean section. Each woman was scheduled for seven blood collections at gestational weeks 13–20, 21–28, 29–34, and 35–42; at active labor or cesarean section; and on the first and second days after delivery. Blood samples were obtained and stored for future use. Due to financial restraint, only a subgroup of 128 women from the original cohort were selected for fibulin-1 quantification (Fig. 1). Of these, 107 women were randomly selected, as well as all available cases that developed PE (n = 12) or PPROM (n = 9).
Fig. 1.
Consort flow chart
Reference range of Fibulin-1 levels in blood donors
Serum samples were obtained from a cohort of healthy adult Danish blood donors in August and September 2011 at Odense University Hospital, Denmark. One hundred twenty donors were included, with a target of 30 donors in each subgroup stratified by gender and greater than and less than 40 years of age (numbers obtained 62 females, 58 males; age range: 19–66 years) [19]. Informed consent was obtained, and the study was performed according to the Declaration of Helsinki. Immediately after sampling, all samples were aliquoted and stored at -80 °C until analysis. The reference range of fibulin-1 levels was established in 2015 using an immunoassay kit (CY-8094, Circulex, Medical & Biological Laboratories, Nagoya, Japan) with a manual setup on a Victor X5 Multiplate Reader (PerkinElmer, Waltham, MA) and verified in 2020 on 20 newly collected normal samples (from 4 men and 16 women, age range: 23–60 years) on the current platform according to the CLSI EP28-A3c guideline. No difference in serum and plasma fibulin-1 concentrations have been observed according to the kit manufacturer.
Fibulin-1 analysis
Blood samples were collected and transported to the laboratory, where they were stored at -80 °C until use. Fibulin-1 levels were measured in ethylenediaminetetraacetic acid-treated plasma diluted 1:500 using the Circulex immunoassay kit on a Triturus 4-Plate platform (Grifols, Barcelona, Spain) according to the manufacturer’s instructions. The intra-assay and inter-assay coefficients of variation were < 8 % and < 12 % at a level of 10 µg/mL, respectively. The detection limit of the assay was 0.9 µg/mL, and serum samples were diluted in parallel to standard curve. The results were not available to the clinicians during the study.
Statistical analysis
Data were analyzed using SPSS version 25 (IBM Corp, Armonk, NY, USA) and R software [20]. Differences in mean fibulin-1 levels between groups and associations with predictors were estimated using a linear mixed model. The model was fitted using the restricted maximum likelihood estimation, and included all predictors simultaneously, with the only exception when a continuous predictor was replaced with its dichotomized version for age and body mass index (BMI), ensuring complete adjustment for measured confounders. The following predictors (fixed effects) were simultaneously investigated: gestational period, PPROM, PE, mode of delivery, bleeding at delivery, birth weight, parity, smoking, BMI or BMI-group, and age or age-group. Participant-specific random intercept effects were included in the linear mixed model to capture the biological between-participant variation. Estimated mean differences and slope coefficients were reported. Based on the linear mixed models, post hoc pairwise comparisons using approximate t-tests were performed to quantify relevant differences. Differences were considered significant at p < 0.05. P-values were not adjusted for multiple comparisons due to the exploratory and hypothesis-generating nature of the study. Univariate and multivariate linear regression analyses were performed to investigate any correlations between fibulin-1 levels measured around time of birth with birthweight, bleeding at delivery and mode of delivery.
Results
Fibulin-1 levels were measured in 632 samples from 128 of the 801 originally enrolled healthy pregnant Caucasian women. Five hundred forty-two of these samples were collected from 107 randomly selected women, 61 from 12 women with PE and 29 from 9 women with PPROM. Each woman contributed an average of 4.9 (range 1–8) samples. Five women attended their 1st trimester screening between week 12 + 2 to 12 + 6. The cohort had a mean (standard deviation) age of 32 (4.2) years, a BMI of 22 (2.7) kg/m2, a birth weight of 3442 (523) grams, and a gestational age at delivery of 39 + 5 (13) days.
Among the 107 randomly selected women, one woman was successfully administered tocolysis in week 29 + 5 due to premature contractions. None of the women experienced antepartum bleeding, glycosuria, isolated elevated blood pressure, isolated proteinuria, intrauterine growth restriction, or thrombosis in pregnancy. They all delivered at term: 24 by elective cesarean section (mainly due to maternal request and breech presentation), 12 by emergency cesarean section and the remaining 71 vaginally. Postpartum hemorrhage occurred in three women. Low birth weight occurred once (2450 g).
Among the 12 women with PE, one woman was treated with an anticoagulant in week 33 + 2 due to a suspicion of pulmonary embolism. None of the 12 women developed early PE. Two developed severe PE. All but one woman delivered at term, one delivered by elective cesarean section, two delivered by emergency cesarean section, and the remaining nine delivered vaginally. Postpartum hemorrhage occurred in two women, and a single child had low birth weight (1983 g).
Among the nine women with PPROM, all delivered vaginally and preterm (at gestational age 27 + 4 to 36 + 5). None experienced postpartum hemorrhaging. Low birth weight occurred in four infants (870, 1225, 1800, and 2042 g).
In blood donors, the mean concentration of fibulin-1 was 15.7 µg/mL, (SD 4.5, 25th -75th -percentiles, 12.3–18.2), with no significant difference in groups stratified by gender or age, and thus, a common reference interval was used.
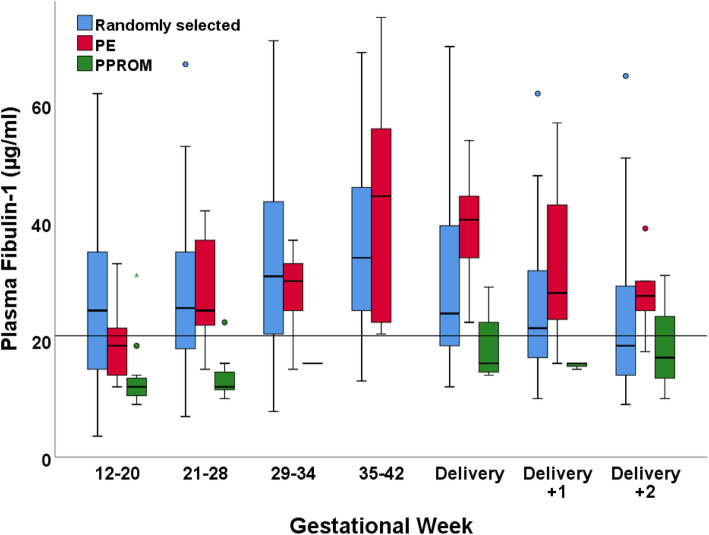
Fibulin-1 concentrations in different demographic subgroups is shown in Table 1. The mean concentration of fibulin-1 in the pregnancy cohort was 27.4 (17.0-35.5) µg/mL. The lowest and highest concentrations measured were 3.6 µg/mL and 75.0 µg/mL, respectively. Among the randomly selected women, women with PE and women with PPROM, the mean concentrations of fibulin-1 were 27.8 (17.0–36.0) µg/mL, 29.1 (19.0–37.0) µg/mL, and 15.4 (11.0-16.5) µg/mL, respectively. Gestational age-specific plasma concentrations are shown in Fig. 2. Women who developed PPROM generally presented with low plasma fibulin-1 levels throughout their pregnancies. Women who developed late PE did not differ in plasma fibulin-1 levels compared to the randomly selected women.
Table 1.
Characteristics of 128 healthy pregnant Caucasian women, 120 blood donors and their plasma Fibulin-1 concentrations
| Women | Samples | Fibulin-1, μg/mL | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | mean | SD | |
| Non-pregnant reference groupa | 62F/58M | 120 | 15.7 | 4.5 | ||
| Pregnant women overall | 128 | 632 | 27.4 | 13.2 | ||
| Age (years) | 128 | 632 | ||||
| 20-29 | 44 | 35 | 198 | 31 | 25.6 | 12.9 |
| 30-39 | 81 | 63 | 418 | 66 | 28.2 | 13.3 |
| ≥40 | 3 | 2 | 16 | 3 | 28.7 | 12.2 |
| Parity | 128 | 632 | ||||
| 0 | 51 | 40 | 255 | 40 | 25.1 | 12.8 |
| 1 | 59 | 46 | 289 | 46 | 28.2 | 13.4 |
| 2+ | 18 | 14 | 88 | 14 | 31.3 | 12.7 |
| Body mass index (kg/m2) | 127 | 628 | ||||
| Underweight <18.5 | 5 | 4 | 22 | 4 | 18.7 | 10.0 |
| Normal weight 18.5-24.9 | 98 | 77 | 491 | 78 | 27.8 | 13.1 |
| Overweight 25.0-29.9 | 22 | 17 | 107 | 17 | 27.9 | 13.9 |
| Obese 30.0-34.9 | 2 | 2 | 8 | 1 | 22.5 | 9.0 |
| Smoking habits | 128 | 632 | ||||
| Non-smoker | 107 | 84 | 531 | 84 | 28.2 | 13.7 |
| Smoker | 21 | 16 | 101 | 16 | 23.2 | 9.3 |
| Mode of conception | 128 | 632 | ||||
| Spontaneous | 126 | 98 | 625 | 98 | 27.5 | 13.2 |
| In-vitro fertilization | 1 | 1 | 3 | 1 | 16.3 | 1.1 |
| Insemination | 1 | 1 | 4 | 1 | 14.3 | 1.3 |
| Gestational weeks | - | 632 | ||||
| 12-20 | 66 | - | 93 | 15 | 23.4 | 12.2 |
| 21-28 | 90 | - | 121 | 19 | 26.4 | 11.4 |
| 29-34 | 59 | - | 60 | 10 | 32.8 | 14.7 |
| 35-42 | 50 | - | 63 | 10 | 36.0 | 14.3 |
| Delivery | 98 | - | 98 | 15 | 30.4 | 14.6 |
| 1st day after delivery | 101 | - | 101 | 16 | 25.2 | 10.9 |
| 2nd day after delivery | 96 | - | 96 | 15 | 22.7 | 10.8 |
| Pre-eclampsia | 128 | 632 | ||||
| Not present | 116 | 91 | 571 | 90 | 27.3 | 13.2 |
| Present | 12 | 9 | 61 | 10 | 29.1 | 13.1 |
| PPROM | 128 | 632 | ||||
| Not present | 119 | 93 | 603 | 95 | 28.0 | 13.1 |
| Present | 9 | 7 | 29 | 5 | 15.4 | 6.0 |
| Mode of delivery | 128 | 632 | ||||
| Vaginal birth | 88 | 69 | 396 | 63 | 28.5 | 13.1 |
| Elective cesarean section | 24 | 19 | 147 | 23 | 27.4 | 13.8 |
| Emergency cesarean section | 16 | 12 | 89 | 14 | 23.2 | 12.0 |
| Postpartum haemmorhageb | 128 | 632 | ||||
| Not present | 120 | 94 | 580 | 92 | 27.7 | 13.4 |
| Present | 8 | 6 | 52 | 8 | 23.4 | 9.4 |
| Birth weight (grams) | 126 | 621 | ||||
| <2500 | 6 | 5 | 20 | 3 | 23.6 | 14.3 |
| 2500-2999 | 18 | 14 | 84 | 13 | 24.6 | 11.3 |
| 3000-3499 | 42 | 33 | 215 | 35 | 27.3 | 13.4 |
| 3500-3999 | 43 | 34 | 224 | 36 | 29.7 | 14.1 |
| 3999> | 17 | 14 | 78 | 13 | 25.1 | 10.8 |
Fibulin-1 concentrations in different socio-demographic and clinical subgroups of the total study population. The data are presented as counts, percentages (%), or means and standard deviations (SD). aNon-pregnant reference group consist of 62 female (F) and 58 male (M) blood donors. b Defined as >500 mL during vaginal delivery and >1000 mL during cesarean section
Fig. 2.
Gestational age-specific variation in plasma fibulin-1 levels during pregnancy. Plasma fibulin-1 concentrations in 632 samples from 128 healthy pregnant Caucasian women. The box plots represent the range of data from the 25th to the 75th percentiles, while the bar in the middle of each box plot represents the median value. The whiskers extending from the box represent the range of values, excluding outliers. Circles indicate outliers (1.5 x the interquartile range). The line represents the 90th reference percentile in blood donors
Notably, two women with non-spontaneous conceptions for whom 1st or 2nd trimester samples were not available, presented with low fibulin-1 levels in the 3rd trimester and at delivery (maximal value, 17.0 µg/mL).
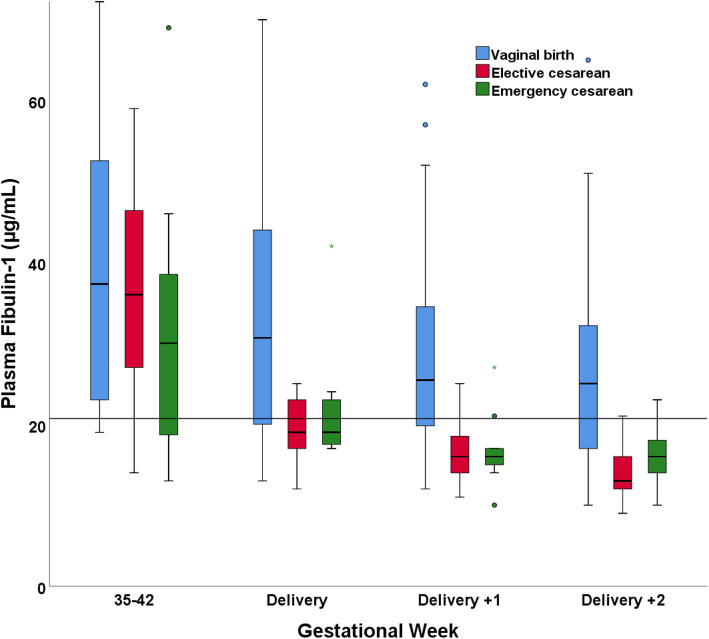
Compared to baseline levels measured in the early 2nd trimester and after adjusting for other variables, a significant increase in fibulin-1 concentrations was observed from week 29 throughout delivery (Table 2). An observed and predicted peak was observed in weeks 35–42. The level of fibulin-1 was significantly different between weeks 21–28 and 29–34 (p = 0.003), weeks 29–34 and 35–42 (p < 0.001), weeks 35–42 and delivery (p < 0.001), and delivery and the 1st day after delivery (p < 0.001). A significant estimated difference in fibulin-1 concentrations was identified between women with and without PPROM (-11.6 µg/mL; standard error (SE) 4.0; p = 0.006), where women with PPROM presented the lowest levels. A difference in fibulin-1 concentrations was not observed between women with and without PE (-0.2 µg/mL; SE 3.0; p = 0.9). Smokers had lower measured and predicted fibulin-1 concentrations than non-smokers (-5.2 µg/mL; SE 2.3; p = 0.02). In our main analysis, age, parity, BMI, mode of conception, bleeding at delivery, and birth weight did not correlate with fibulin-1 concentrations (Table 2). Additionally, in the late 3rd trimester and at delivery, we did not observe a correlation between fibulin-1 concentrations and bleeding at delivery or birth weight. On the other hand, women giving birth vaginally had higher estimated fibulin-1 concentrations than women who delivered by elective (-7.0 µg/mL; SE 2.3; p = 0.002) and emergency (-5.6 µg/mL; SE 2.9; p = 0.05) cesarean section (Table 2). In the late 3rd trimester, the mode of delivery was not correlated with fibulin-1 concentrations. After delivery, significantly lower fibulin-1 concentrations were detected in women who delivered by cesarean section, both elective (unadjusted, -13.2 µg/mL; SE 4.9; p = 0.008; adjusted, -14.0 µg/mL; SE 5.4; p = 0.01) and emergency (unadjusted, -11.2 µg/mL; SE 4.5; p = 0.01; adjusted, -9.1 µg/mL; SE 5.4; p = 0.09), than in women with vaginal delivery (reference group). See Fig. 3; Table 3 and supplementary material Figure S1 and Table S1.
Table 2.
Estimated differences in plasma fibulin-1 concentrations between various subgroups of participants
| Parameter | β-estimate (μg/mL)a |
SE b | p-value c | |
|---|---|---|---|---|
| Intercept | 31.9 | 11.2 | 0.004 ** | |
| Age | Per 1-year increase | 0.2 | 0.3 | 0.4 |
| Parity | 0 | Reference | ||
| 1 | 2.6 | 2.2 | 0.2 | |
| 2+ | 4.9 | 3.2 | 0.1 | |
| Body mass index | Per 1-unit increase (kg/m2) | -0.3 | 0.3 | 0.3 |
| Smoking habits | Non-smoker | Reference | ||
| Smoker | -5.2 | 2.3 | 0.02 * | |
| Mode of conception | Spontaneous | Reference | ||
| In-vitro fertilization | 0.4 | 10.3 | 1.0 | |
| Insemination | -18.1 | 9.5 | 0.06 | |
| Gestational weeks | 12-20 | Reference | ||
| 21-28 | 0.9 | 1.4 | 0.5 | |
| 29-34 | 5.6 | 1.7 | <0.001 *** | |
| 35-42 | 12.5 | 1.6 | <0.001 *** | |
| Delivery | 4.1 | 1.5 | 0.005 ** | |
| 1st day after delivery | -1.3 | 1.5 | 0.4 | |
| 2nd day after delivery | -3.4 | 1.5 | 0.002 * | |
| Pre-eclampsia | Not present | Reference | ||
| Present | -0.2 | 3.0 | 0.9 | |
| PPROM | Not present | Reference | ||
| Present | -11.6 | 4.2 | 0.006 ** | |
| Mode of delivery | Vaginal birth | Reference | ||
| Elective caesarean section | -7.0 | 2.3 | 0.002 ** | |
| Emergency caesarean section | -5.6 | 2.9 | 0.05 # | |
| Bleeding at delivery | Per 100mL increase | -0.03 | 0.4 | 0.9 |
| Birth weight | Per 100grams increase | -0.08 | 0.2 | 0.7 |
a Estimated slope coefficient using a linear mixed model and fitted with the restricted maximum likelihood approach. Tukey’s test was used for multiple comparisons of means. b Standard error. c Compared to the reference level of the variable, adjusted for all other variables in the table. Significance levels ‘***’0.001, ‘**’0.01, ‘*’0.05, ‘#’ 0.1
Fig. 3.
Fibulin-1 levels and mode of delivery. Plasma fibulin-1 concentrations in 3rd trimester and around delivery measured in participants grouped according to the mode of delivery. The box plots represent the range of data from the 25th to the 75th percentiles, while the bar in the middle of each box plot represents the median value. The whiskers extending from the box represent the range of values excluding outliers. Circles indicate outliers. The line represents the 90th reference percentile in blood donors
Table 3.
Fibulin-1 levels and mode of delivery
| Unadjusted | Adjusteda | |||||
|---|---|---|---|---|---|---|
| β-estimate (μg/mL)b | SEc | p-valued | β-estimate (μg/mL)b | SEc | p-value d | |
| Vaginal birth | Reference | Reference | ||||
| Elective caesarean section | -13.2 | 4.9 | 0.008 ** | -14.0 | 5.4 | 0.01 * |
| Emergency caesarean section | -11.2 | 4.5 | 0.01 * | -9.1 | 5.4 | 0.09 . |
| (Intercept) | 32.9 | 1.6 | <0.001 *** | 45.3 | 19.7 | 0.02 * |
a Adjusted for all other variables in the main regression analysis. b Estimated slope coefficient of fibulin-1 at birth, in groups stratified by mode of delivery. c Standard error. d Compared to vaginal delivery. Significance levels ‘***’0.001, ‘**’0.01, ‘*’0.05, ‘#’ 0.1
Discussion
The role of fibulin-1 in the developing pregnancy is by far fully understood. In this study, we examined fibulin-1 levels longitudinally in normal pregnancy. In addition, we explored potential factors influencing variability of fibulin-1 in maternal plasma.
Plasma fibulin-1 concentrations increased significantly during pregnancy and normalized quickly after birth in women with uncomplicated pregnancies. Fibulin-1 expression in the endometrium is regulated by progesterone in a cycle-dependent manner [21, 22], and its expression is upregulated 2.8-fold in the myometrium at term [23]. High expression rates of fibulin-1 mRNA is found in the placenta, and fibulin-1 is already detected in human embryos at the 4th gestational week. The increase in plasma fibulin-1 concentration with gestational age probably reflect the high expression rates in the growing uterus, placenta, and the fetus itself. A potential explanation for the rapid decrease in plasma fibulin-1 levels observed after delivery is the elimination of circulating fibulin-1 by hemostatic processes during delivery or because the placenta and fetus are expelled [24, 25].
Quite interestingly, women who underwent elective and emergency cesarean section presented a more rapid decrease in fibulin-1 levels after birth compared to women with vaginal delivery. A plausible explanation would be that more fibulin-1 is consumed in hemostatic processes after surgical trauma, by binding to fibrinogen and mediating platelet adhesion [24, 25]. We anticipated that low levels of fibulin-1 were associated with a higher degree of bleeding at delivery. Our regression model did not support this hypothesis, potentially due to collinearity with the mode of delivery.
In a study by Liu et al., higher levels of fibulin-1 were observed in pooled serum samples collected late in the 3rd trimester from five women with severe PE than in five healthy, gestational age-matched controls [9]. However, in the present study, no difference in plasma fibulin-1 levels could be observed between the 12 women who developed PE and women with uncomplicated pregnancies. The pathophysiological mechanisms involved in PE are unclear, but early and late PE appear to be different entities. Early PE is thought to result from deficient placentation, which induces extracellular matrix remodeling, among other processes. Late PE involves a complex interaction between stressed syncytiotrophoblasts and a susceptible maternal cardiovascular system [26, 27]. In our study, no women had early PE, and few developed severe PE. This difference might explain why we did not observe a correlation between the development of PE and fibulin-1 plasma concentrations.
In 40 % of preterm deliveries, rupture of membranes is the sentinel event [11]. The recurrence rate in a subsequent pregnancy is increased by 20-fold compared to women with no previous history of PPROM [28]. Rupture of the fetal membranes is precipitated by stretch forces acting upon biochemically mediated, pre-weakened areas overlying the cervix [11]. Fibulins are structural proteins that contribute to the elastic properties of connective tissue fibers [2]. Fibulin-1 is found to be down regulated in the para-cervical weak zone, prior to rupture, being consistent with decreased rupture strength [10]. Our nine women with PPROM presented significantly lower plasma fibulin-1 concentrations throughout their pregnancies. This lack of increase in plasma fibulin-1 and the known high recurrence rate of PPROM is suggestive of a genetic explanation. Larger scale prospective studies, including Mendelian randomization, are needed to evaluate fibulin-1 as a potential prognostic biomarker for the risk of PPROM.
The two cases of women with assisted pregnancies and low fibulin-1 levels indicate that the protein might have a role in fecundity. Fibulin-1 expression is regulated by progesterone. Progesterone is a key factor in the establishing and maintaining pregnancy, and inadequate levels of postovulatory progesterone are associated with infertility and recurrent miscarriage [29]. Fibulin-1 has fibronectin-and integrin-binding properties and a role in angiogenesis. It may therefore be important for both the architecture of the endometrium and the periodic neovascularization, and as such have a functional role in endometrial receptivity for embryo implantation [21]. It would be interesting to follow women referred to fertility treatment and measure fibulin-1 before, during and after treatment.
Our study has some strengths and limitations. To our knowledge, this study is the first to date to perform a longitudinal investigation of plasma fibulin-1 levels in pregnant women. The cohort consisted of healthy women, and data on pregnancy outcomes were available. However, the study is limited by its size and the lack of pre-conceptual and first trimester samples. The few women with PE (n = 12) and PPROM (n = 9), clearly hampers any definite conclusions regarding these subgroups. All participants were Caucasian, and predominantly normal- or slightly overweight, limiting the generalizability. A comparison group consisting of women, during subsequent stages of the menstrual cycle, would have been of value. Finally, our study design did not allow us to evaluate the fibulin-1 status and its importance in fetal development.
Conclusions
Plasma fibulin-1 levels increase significantly during pregnancy and normalize quickly after birth in women with uncomplicated pregnancies, more pronounced after elective and emergency cesarean section than after vaginal delivery. The women with late PE had fibulin-1 levels equivalent to women with uncomplicated pregnancies in our cohort. Although limited by sample size, this increase in fibulin-1 levels was not observed in pregnancies complicated with PPROM. Larger scale and validation studies are needed to determine whether fibulin-1 represents a potential prognostic biomarker for the risks of developing PPROM and PE.
Supplementary Information
Acknowledgements
We thank the entire staff at the Departments of Obstetrics and Clinical Biochemistry, Gentofte Hospital, for their expert assistance.
Abbreviations
- BMI
Body mass index
- SE
Standard error
- SD
Standard deviation
- PE
Preeclampsia
- PPROM
Preterm premature rupture of membranes
Authors’ contributions
PBS, SS, MRA, and ABO designed the study. MRA retrieved the samples. LP performed the laboratory analysis. ABO and CR performed the regression analysis. ABO and PBS conducted the study, interpreted the results, and drafted the manuscript. All of the authors approved the final version of the manuscript.
Funding
No funding was obtained.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. The participants provided written informed consent, including storage for future use. The study was approved on July 18, 2005 by the The Danish National Committee on Bioethics (No. KA 05065) and (S-20090061) and the Danish Data Protection Agency.
Consent for publication
NA.
Competing interests
None of the authors has any financial or other actual or potential conflicts of interest capable of influencing any part of this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kobayashi N, Kostka G, Garbe JH, Keene DR, Bachinger HP, Hanisch FG, et al. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J Biol Chem. 2007;282(16):11805–16. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- 2.Roark EF, Keene DR, Haudenschild CC, Godyna S, Little CD, Argraves WS. The association of human fibulin-1 with elastic fibers: an immunohistological, ultrastructural, and RNA study. J Histochem Cytochem. 1995;43(4):401–11. doi: 10.1177/43.4.7534784. [DOI] [PubMed] [Google Scholar]
- 3.Miosge N, Gotz W, Sasaki T, Chu ML, Timpl R, Herken R. The extracellular matrix proteins fibulin-1 and fibulin-2 in the early human embryo. Histochem J. 1996;28(2):109–16. doi: 10.1007/BF02331415. [DOI] [PubMed] [Google Scholar]
- 4.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 5.Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4(12):1127–31. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu ML, Tsuda T. Fibulins in development and heritable disease. Birth Defects Res C Embryo Today. 2004;72(1):25–36. doi: 10.1002/bdrc.20003. [DOI] [PubMed] [Google Scholar]
- 7.Scholze A, Bladbjerg EM, Sidelmann JJ, Diederichsen AC, Mickley H, Nybo M, et al. Plasma concentrations of extracellular matrix protein fibulin-1 are related to cardiovascular risk markers in chronic kidney disease and diabetes. Cardiovasc Diabetol. 2013;12:6. doi: 10.1186/1475-2840-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarinci E, Tropea A, Russo G, Notaristefano G, Messana C, Alesiani O, et al. Increased fibulin-1 plasma levels in polycystic ovary syndrome (PCOS) patients: possible contribution to the link between PCOS and cardiovascular risk. J Endocrinol Invest. 2019;42(1):91–6. doi: 10.1007/s40618-018-0891-3. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Zhang N, Yu H, Chen Y, Liang Y, Deng H, et al. Proteomic analysis of human serum for finding pathogenic factors and potential biomarkers in preeclampsia. Placenta. 2011;32(2):168–74. doi: 10.1016/j.placenta.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore RM, Redline RW, Kumar D, Mercer BM, Mansour JM, Yohannes E, et al. Differential expression of fibulin family proteins in the para-cervical weak zone and other areas of human fetal membranes. Placenta. 2009;30(4):335–41. doi: 10.1016/j.placenta.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar D, Moore RM, Mercer BM, Mansour JM, Redline RW, Moore JJ. The physiology of fetal membrane weakening and rupture: Insights gained from the determination of physical properties revisited. Placenta. 2016;42:59–73. doi: 10.1016/j.placenta.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Joyce EM, Moore JJ, Sacks MS. Biomechanics of the fetal membrane prior to mechanical failure: review and implications. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S121-7. doi: 10.1016/j.ejogrb.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szecsi PB, Jorgensen M, Klajnbard A, Andersen MR, Colov NP, Stender S. Haemostatic reference intervals in pregnancy. Thromb Haemost. 2010;103(4):718–27. doi: 10.1160/TH09-10-0704. [DOI] [PubMed] [Google Scholar]
- 14.Orvik AB, Andersen MR, Bratholm PS, Hedengran KK, Ritz C, Stender S, et al. Variation in plasma 25-hydroxyvitamin D2 and D3 in normal pregnancy with gestational age, sampling season, and complications: A longitudinal cohort study. PLoS One. 2020;15(4):e0231657. doi: 10.1371/journal.pone.0231657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klajnbard A, Szecsi PB, Colov NP, Andersen MR, Jorgensen M, Bjorngaard B, et al. Laboratory reference intervals during pregnancy, delivery and the early postpartum period. Clin Chem Lab Med. 2010;48(2):237–48. doi: 10.1515/CCLM.2010.033. [DOI] [PubMed] [Google Scholar]
- 16.Hedengran KK, Nelson D, Andersen MR, Stender S, Szecsi PB. Hepcidin levels are low during pregnancy and increase around delivery in women without iron deficiency - a prospective cohort study. J Matern Fetal Neonatal Med. 2016;29(9):1506–8. doi: 10.3109/14767058.2015.1052396. [DOI] [PubMed] [Google Scholar]
- 17.Hedengran KK, Andersen MR, Szecsi PB, Lindh C, Uldbjerg N, Stender S. Environmental tobacco smoke exposure during pregnancy has limited effect on infant birthweight and umbilical vein endothelial nitric oxide synthase. Acta Obstet Gynecol Scand. 2018;97(11):1309–16. doi: 10.1111/aogs.13419. [DOI] [PubMed] [Google Scholar]
- 18.Hedengran KK, Andersen MR, Stender S, Szecsi PB. Large D-Dimer Fluctuation in Normal Pregnancy: A Longitudinal Cohort Study of 4,117 Samples from 714 Healthy Danish Women. Obstet Gynecol Int. 2016;2016:3561675. doi: 10.1155/2016/3561675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen L, Pedersen SM, Brasen CL, Rasmussen LM. Soluble serum Klotho levels in healthy subjects. Comparison of two different immunoassays. Clin Biochem. 2013;46(12):1079–83. doi: 10.1016/j.clinbiochem.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 20.Team RC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 21.Haendler B, Yamanouchi H, Lessey BA, Chwalisz K, Hess-Stumpp H. Cycle-dependent endometrial expression and hormonal regulation of the fibulin-1 gene. Mol Reprod Dev. 2004;68(3):279–87. doi: 10.1002/mrd.20079. [DOI] [PubMed] [Google Scholar]
- 22.Nakamoto T, Okada H, Nakajima T, Ikuta A, Yasuda K, Kanzaki H. Progesterone induces the fibulin-1 expression in human endometrial stromal cells. Hum Reprod. 2005;20(6):1447–55. doi: 10.1093/humrep/deh841. [DOI] [PubMed] [Google Scholar]
- 23.Rehman KS, Yin S, Mayhew BA, Word RA, Rainey WE. Human myometrial adaptation to pregnancy: cDNA microarray gene expression profiling of myometrium from non-pregnant and pregnant women. Mol Hum Reprod. 2003;9(11):681–700. doi: 10.1093/molehr/gag078. [DOI] [PubMed] [Google Scholar]
- 24.Godyna S, Diaz-Ricart M, Argraves WS. Fibulin-1 mediates platelet adhesion via a bridge of fibrinogen. Blood. 1996;88(7):2569–77. doi: 10.1182/blood.V88.7.2569.bloodjournal8872569. [DOI] [PubMed] [Google Scholar]
- 25.Tran H, Tanaka A, Litvinovich SV, Medved LV, Haudenschild CC, Argraves WS. The interaction of fibulin-1 with fibrinogen. A potential role in hemostasis and thrombosis. J Biol Chem. 1995;270(33):19458–64. doi: 10.1074/jbc.270.33.19458. [DOI] [PubMed] [Google Scholar]
- 26.Shah DA, Khalil RA. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem Pharmacol. 2015;95(4):211–26. doi: 10.1016/j.bcp.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerdeira AS, Agrawal S, Staff AC, Redman CW, Vatish M. Angiogenic factors: potential to change clinical practice in pre-eclampsia? BJOG. 2018;125(11):1389–95. doi: 10.1111/1471-0528.15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee T, Carpenter MW, Heber WW, Silver HM. Preterm premature rupture of membranes: risks of recurrent complications in the next pregnancy among a population-based sample of gravid women. Am J Obstet Gynecol. 2003;188(1):209–13. doi: 10.1067/mob.2003.115. [DOI] [PubMed] [Google Scholar]
- 29.Okada H, Tsuzuki T, Shindoh H, Nishigaki A, Yasuda K, Kanzaki H. Regulation of decidualization and angiogenesis in the human endometrium: mini review. J Obstet Gynaecol Res. 2014;40(5):1180–7. doi: 10.1111/jog.12392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.