Abstract
Background
The occurrence of colorectal cancer has doubled over the last 50 years and many people are living with the disease in the palliative phase. Therefore, it is important that healthcare personnel have knowledge about the patient’s health-related quality of life (HRQoL). The aim of this review is to investigate how HRQoL is reported by means of different measures for patients in the palliative phase of colorectal cancer and examine which sociodemographic and clinical factors are associated with the mean scores reported for HRQoL.
Method
A systematic review and meta-analysis using forest plots in STATA were conducted. The databases MEDLINE, CINAHL, Embase, Amed, and SveMed+ were used for the systematic searches with combinations of terms for colorectal cancer, the palliative phase and HRQoL. The Cochrane handbook and the PRISMA checklist from 2009 were utilised.
Results
In total, 710 articles were identified. Eleven quantitative studies met the inclusion criteria and six were included in the meta-analysis. Five of the 11 studies had a longitudinal design, while the other six had a cross-sectional design. The meta-analyzes shows that the average HRQoL in palliative phase was 62.9 (56.8–69.0) 15D was 0.76 (0.73–0.79), EQ-5D was 0.67 (0.62–0.73), and VAS was 64.1 (53.7–74.4). Multiple sociodemographic and clinical variables were associated with HRQoL and a higher prevalence of common cancer symptoms were reported than gastrointestinal symptoms.
Conclusion
This systematic review revealed that patients with colorectal cancer report low HRQoL. Furthermore, it shows that what affects HRQoL is complicated, including multiple clinical and sociodemographic variables. This underlines the need for further research. To ensure the best possible care, it is important that all healthcare professionals have easy access to knowledge about HRQoL in patients with colorectal cancer, and what impacts it in the last phase of life.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12904-021-00837-9.
Keywords: Colorectal neoplasms, Palliative care, Nursing, Health-related quality of life, Systematic review, Meta-analysis
Background
Cancer is one of the most common diseases in the world, and 17 million new cases of cancer were registered worldwide in 2018 [1]. It is estimated that one in three Norwegians will develop cancer before the age of 75. For those who develop cancer in old age, the colorectal site is one of the top three cancer types in terms of incidence, and Norway is one of the countries with the highest rates of such cancer in the world [2]. In 2019, 4295 new cases of colorectal cancer (CRC) were registered in Norway [3]. The term CRC refers to colon cancer, rectum cancer or both [4]. Despite the fact that the five-year survival rate has increased consistently since 1970, more people are living longer with the disease in the palliative phase, a phase in which they are no longer responding to curative treatment, and life-prolonging approaches, symptom management and optimising quality of life (QoL) take over as goals for treatment and care [3, 5]. According to the World Health Organization, the goal of palliative care is symptom management and promoting QoL for patients and their families [6]. QoL includes the individual’s perception of their personal situation in their own life such as physical, social, mental and spiritual dimensions [7]. HRQoL is one component of QoL [8]; QoL with a specific health component [9]. The term HRQoL refers to a person’s subjective rating of their satisfaction with general health, and their level of well-being [9]. Since HRQoL is a subjective measure, one of the best ways to measure people’s subjective HRQoL is through questionnaires or patient-reported outcomes (PROMS) – the gold standard in assessment. Several different psychometric tested questionnaires exist [9].
The Norwegian strategy for cancer treatment and care also illustrates the importance of maintaining the best possible QoL for cancer patients [10]. To achieve this goal, it is important to relieve the patient’s physical, psychosocial and spiritual symptoms, and to support the families and next-of-kin [6]. According to the International Council of Nurses (ICN), all nurses are responsible for providing care and alleviating suffering [11], which includes contributing to a natural and dignified death. In order to provide care and to maintain HRQoL even in the palliative phase, nurses must have knowledge and understand what is important for the patient in the palliative phase, what creates security and how to approach people in their final phase of life. The most common symptoms experienced by patients in the palliative phase include: pain, fatigue, depression, anxiety, confusion, breathlessness, insomnia, nausea, constipation, diarrhea and loss of appetite [12]. These symptoms affect the patient’s HRQoL, and may be due to their current condition, other chronic diseases and previous treatment such as chemotherapy [13]. Before starting this review, we searched for systematic reviews in the database of the Center for Reviews and Disseminations (CRD) and in the Prospero database for ongoing reviews [14]. We also searched in the McMasterPLUS database where we identified one UpToDate article regarding the HRQoL of patients with CRC. However, the article focused only on patients who no longer had any signs of active disease [15]. Several reviews with a similar focus also exist i.e., HRQoL in patients with CRC. However, these reviews focus on survivors, not on patients in the palliative phase [16–19], or the studies aim to test different interventions [20]. As described, patients in the palliative phase have a lot of unique and distressing symptoms compared to survivors or patients in active treatment. Therefore, the symptoms and HRQoL of patients in the palliative phase of CRC are not necessarily comparable to that of the survivors of CRC. Since cure is not an option for patients in the palliative phase, HRQoL is often considered the outcome for treatment and follow-up, and thereby especially important to review for this patient group. To our knowledge, no systematic reviews have been published in the last 10 years about HRQoL in patients with CRC in the palliative phase. To close this gap, this study will therefore focus on HRQoL in patients in the palliative phase of CRC. The results will give clinicians easier access to relevant and quality-controlled research, thereby giving them a better opportunity to optimise treatment and care for these patients.
The aim of this systematic review is to investigate how HRQoL is reported by means of different measures for patients in the palliative phase of CRC, and examine which sociodemographic and clinical factors are associated with the mean scores reported for HRQoL.
For this review, the following research questions were formulated: (a) What questionnaires are used to measure HRQoL in the different studies? (b) What was the average score for HRQoL for each of the included questionnaires? (c) Which symptoms are reported to impact the patients? (d) Which sociodemographic and clinical factors are found to be statistically significantly correlated with the average HRQoL score in each study?
Method
Our research team conducted a systematic review and meta-analysis inspired by the Cochrane handbook [21]. The guidelines for systematic reviews and meta-analyses (PRISMA), including the checklist (see Additional file 2) and flowchart were also followed throughout the process [22].
The systematic search
A systematic search was performed in MEDLINE (Ovid), CINAHL (EBSCOhost), Embase (Ovid), Amed (Ovid), and SveMed+. MeSH terms and keywords for CRC (e.g., colorectal cancer, colon carcinoma, colorectal neoplasms), the palliative phase (e.g., palliative therapy, terminal care, palliative, incurable) and HRQoL (e.g., quality of life, wellbeing, vitality) were included in the search strategy. All the systematic search details are presented in additional file 3. The searches were filtered by publishing year only, 2009–2019. We only included studies from the last 10 years because the field of palliative medicine and care is rapidly changing, and new medicines and methods will have influenced the patients’ HRQoL and the most recent research will therefore be the most relevant for this review. Updated searches were executed for MEDLINE, CINAHL and Embase in March 2020. These three databases were selected for updated searches in communication with the specialist librarian because, together, they cover most of the medical and nursing research and it is therefore likely that any new articles would be identified by this strategy.
Selection of eligible studies
Two researchers (IRF and SM) separately reviewed all the references by abstract and full text. This is in accordance with the PRISMA standard of systematic reviews [22]. The studies were included if they fulfilled the following criteria: [1] sample over 18 years of age; [2] focused on CRC, the palliative phase and HRQoL; [3] reported results of different patient groups, i.e., different cancer types and disease stages, separately; [4] written in Norwegian, Swedish, Danish or English; [5] original study; and [6] quantitative study design except RCTs. The RCT design was excluded because it has been criticised for being less transferable to real-world settings and may cause artificially positive results by including fewer severely sick individuals [23, 24]. Disagreements on eligibility were resolved by discussion and consulting the full text if necessary. The researchers only disagreed about a few articles, and all of these disagreements were resolved by initial discussion.
Assessing the quality of the studies
In total, 12 quantitative studies were assessed according to the Critical Appraisal Skills Programme (CASP) for cohort studies [25]. Additional file 4 shows the CASP checklist in more detail. Each study was assessed by three researchers (IRF/SM/EKG and IRF/SM/PJ) to minimise the risk of bias and to increase the validity of the review [23]. The plan was to discuss disagreements and resolve them by consulting with a fourth researcher and reaching consensus through discussion; however, this was not necessary because we agreed on the decisions. The CASP criteria were selected from the appraisal tool and included the factors: [1] a clearly focused issue; [2] an acceptable recruitment strategy; [3] accurate measurement of exposure to minimise bias; [4] accurate measurement of outcome to minimise bias; [5] identifying all important confounding factors and accounting for them in design and analysis; [6] complete follow up of the subjects; [9] is the result believable; [10] relevance for the local population/situation [25]. A score of 8 for the longitudinal studies was considered high, 6–7 moderate and a score of < 6 was considered low. For the cross-sectional studies, a high score was 7, moderate 5–6 and low < 5.
Data extraction and analysis
In this analysis, we included studies that reported the HRQoL of patients in the palliative phase of CRC. Of the 11 studies included in this review, six studies had appropriate data for the meta-analysis [26–31]. From these six studies, a meta-analysis was conducted, following both the process described in the Cochrane handbook [21] and the reference manual for STATA 16 [32]. For the studies which include multiple measuring times, we have utilised the second line data. For Adamowicz and Baczkowska-Waliszewska [29] and Stein et al. [30], we used the post-treatment and post-progression data.
The study characteristics of each included study and data on which variables were included in the analysis of correlation with the HRQoL score, as well as the statistical significance in each article (Table 4), were extracted and controlled by two researchers (IRF and SM). Data on mean HRQoL, SD, number of participants and type of questionnaire were extracted from each study by two researchers separately (IRF & SM) and controlled by a third researcher (PJ). From this, SE was calculated. The summarised data were rechecked for transcription errors. Data were then managed using Excel version 1902, and all data analysis was carried out in STATA version 16.
Table 4.
Sociodemographic and clinical variables
| Adamowicz (2018) [29] | Asplund (2017) [36] | Färkkilä (2013) [28] | Färkkilä (2014) [27] | Jasinska (2010) [37] | Kim (2013) [38] | Koskinen (2019) [39] |
Mayrbäurl (2016) [31] | Selby (2010) [40] | Stein (2014) [30] |
Teker (2015) [26] |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Education | No β | – | – | Yes μ (b) | – | No α | Yes μ (b) a | – | – | – | – |
| Age | No β | – | Yesd, μ,α (c) | – | – | No α | Yes μ (c)a | – | No Ω | – | Noa β |
| Sex | No β | – | – | Yes μ,α (b) | – | – | – | – | Yes Ω (c) | – | – |
| Marital status | – | – | Yesd, α, (b) | Yes α (b) | – | – | – | – | – | – | – |
| Place of residence | No β | – | – | – | – | – | – | – | – | – | – |
| Financial difficulties | – | – | Yesd, μ,α (c) | Yes α (c) | – | – | Yes μ (c)a | – | – | – | Noa β |
| Stage | – | Yes α (c) | Yesd, μ,α (c) | – | – | – | Yes μ (c)a | – | – | Nod, α | Noa β |
| Type of Chemotherapy /treatment | – | – | – | – | – | – | – | – | No Ω | – | Noa β |
| Chemotherapy Line/ Duration of treatment | – | – | – | – | – | – | – | Yes β (c) | YesΩ (c) No∑ | – | No β |
| ECOG-score | Yes β (c) | – | – | – | – | – | – | – | No Ω | – | – |
| Targeted treatment | No β | – | Yes α, (c) | – | No π | – | – | – | No Ω | – | Noa β |
| Smoking status | No β | – | – | – | – | – | – | – | – | – | – |
| Response to treatment | Yes β (b) | – | – | – | – | – | – | Yes β (b) | – | – | – |
| Oncology knowledge | Yes β (b) | – | – | – | – | – | – | – | – | – | – |
| Unconventional treatment | Yes β (c) | – | – | – | – | – | – | – | – | – | – |
| Out-of-pocket cost | – | – | – | – | – | – | Yes μ (c) a | – | – | – | – |
| Total economic costs | – | – | – | – | – | – | Yes μ (c) a | – | – | – | – |
| Comorbidities | – | – | – | – | – | – | – | – | – | – | Noa β |
| Depression | – | – | – | Yes α (c) | – | Yes α (c) | – | – | – | – | – |
| Feeling of Coherence | – | Yes α (b) | – | – | – | – | – | – | – | – | – |
| Intrusive thoughts | – | Yes α (c) | – | – | – | – | – | – | – | – | – |
| Time from diagnosis | – | – | – | Yes μ (c) | – | – | – | – | – | – | – |
| Hospital stay | – | – | – | – | No π | – | – | – | – | – | – |
| Awareness of terminal status | – | – | – | – | – | Yes α (c) | – | – | – | – | – |
| Metastasis to lungs | – | – | – | – | – | No α | – | – | – | – | – |
| Treatment at enrolment | – | – | – | – | – | – | – | – | – | Nod | – |
| Treatment at enrolment + stage | – | – | – | – | – | – | – | – | – | Yesd (b) | – |
Yes = p < .05. No = p > .05. --- = Not analyzed
a = Not only patients with CRC in the palliative phase in the analysis
b Indicates a positive correlation between the variable and the HRQoL -score
c Indicates a negative correlation between the variable and the HRQoL -score
d = EQ-5D, μ = 15D, α = VAS, β = QLQ-C30 π = QLQ-C15 Ω = RSCL ∑ = ESAS
The heterogeneity between studies was primarily evaluated by I2 values, reference 0–100%, where 25% indicates low heterogeneity, 50% moderate and 75% indicates high heterogeneity [33]. In addition, the value for Cochran’s Q is referred to when appropriate. To explore the heterogeneity, subgroup analyses were performed based on comparison of outcomes for the individual questionnaires. Forest plots were created, showing the effect estimate, level of variability around the estimate for each study and the weight given to each study in the meta-analysis along with the overall result of all studies together [34]. Funnel plots were also created, so that potential publication bias and small-study effects could be assessed visually [32].
Five studies also included the symptom scale from the QLQ-C30 questionnaire but two of these studies only included graphs, not the numerical data [27, 28]. The authors of these articles were contacted but did not respond and some relevant data were therefore not available. Data from the three studies with numerical data were collected (by IRF and SM) and a forest plot was created (Fig. 5). P-values < .05 were considered statistically significant [23].
Fig. 5.
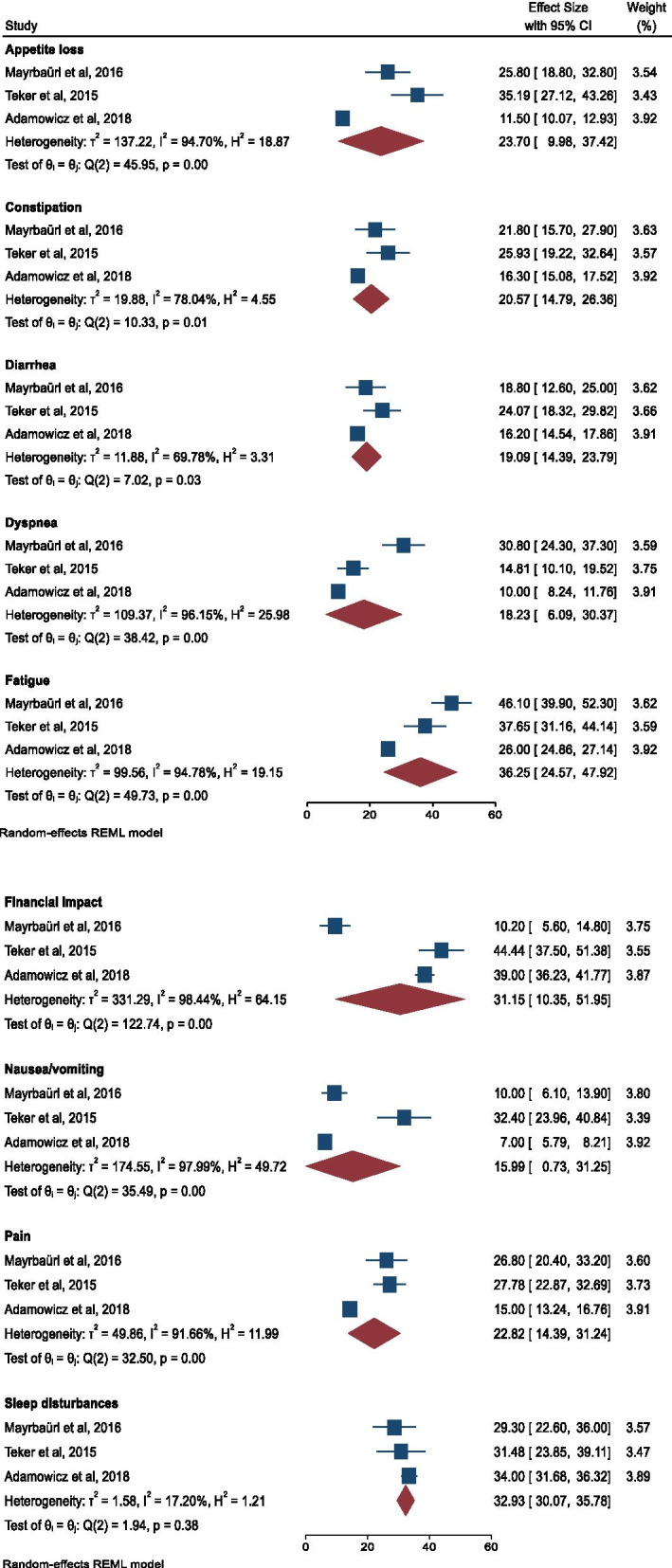
Forest plot for the QLQ-C30 symptom scale
Results
In total, we identified 710 potentially relevant articles, after excluding duplicates. Out of these, 11 were deemed eligible. No relevant articles were identified in the updated searches executed in March 2020. The selection process is shown in Fig. 1. Through the analysis for risk of bias, no publication bias was identified. Two researchers (IRF and SM) collected the characteristics of each included article, shown in Table 2. The cumulative sample of patients with CRC in all disease stages was 4629. This review focuses on patients in the palliative phase and therefore the relevant sample is 839. Five of the studies had a longitudinal design, while the other six had a cross-sectional design. The studies also differ in their samples with respect to the level of functioning and the number of deaths or other dropouts during data collection. Moreover, an overview was made of the mean HRQoL scores in different disease stages for all studies included in the meta-analysis, which is presented in Table 5, see additional file 1 for details.
Fig. 1.
Flowchart of the selection process
Table 2.
Presentation of included studies
| Reference | Aim | Design | Population/time | ECOG/time before death/stage | Questionnaire |
|---|---|---|---|---|---|
|
Adamowicz (2018) [29] Poland Journal of Cancer Education |
To investigate which environmental factors, incl. Unconventional methods of treatment and dietary supplementation, influenced patients’ QoL. | Prospective |
N = 330 palliative colon cancer patients Data collection Jan. 2010 to Dec. 2016 |
50% had an ECOG performance status of 1–2. | QLQ-C30 |
|
Asplund (2017) [36] Denmark &Sweden International Journal of Colorectal Disease |
To investigate the association of intrusive thoughts and the patients’ sense of coherence with pretreatment QoL in patients with newly diagnosed rectal cancer. | Cross-sectional |
N = 1085 patients with rectal cancer, all stages. N = 73 palliative patients Data collection Feb. 2012 to Sep. 2015 |
VAS | |
|
Färkkilä (2013) [28] Helsinki, Finland. Colorectal Disease |
To assess the HRQoL among various disease states of CRC in real-world settings using three standard instruments and to compare it with the HRQoL of the general population. Furthermore, to explore clinical and demographic factors determening HRQoL in CRC. | Cross -sectional |
N = 508 CRC patients N = 41 CRC palliative patients Data collection Oct. 2009 to Feb. 2011. |
EQ-5D VAS QLQ-C30 15D |
|
|
Färkkilä (2014) [27] Helsinki, Finland Quality of Life Research |
To explore HRQoL and assess utility in BC, PCa and CRC patients during the final stages of their disease, to compare the results obtained by different HRQoL instruments, and to explore factors related to impaired HRQoL. | Cross-sectional |
N = 114 N = 57 CRC Data collection Sep. 2009 to Apr. 2011. |
Months until death for the CRC group: < 3 = 17 (30%) 3–6 = 15 (26%) > 6 = 25 (44%) |
EQ-5D VAS QLQ-C30 15D |
|
Selby (2010) [40] Canada Palliative Medicine |
To track changes in symptoms and RSCL subscale scores from presentation to 3 months of follow-up. | Prospective, Longitudinal |
N = 35 patients with non-curable metastatic cancer N = 19 mCRC Data collection Mar. 2006 to Mar. 2008 |
ECOG of all included patients: 0 = 14.3% 1 = 28.6% 2 = 25.7% 3 = 28.6% 4 = 2.9% During the study, 25 patients died. |
Edmonton Symptom Assessment System (ESAS) Rotterdam Symptom Checklist (RSCL) |
|
Stein (2014) [30] The Netherlands and the United Kingdom International Journal of Colorectal Disease |
To elicit EQ-5D utility values from real-world mCRC patients receiving second and subsequent lines of therapy both pre- and post-progression in a real-world setting. | Cross-sectional |
N = 75 mCRC N = 33 post- progression/ palliative Data collection Apr. 2012- Dec. 2012 |
ECOG of all included patients (for palliative): 0 = 24% (21.2%) 1 = 66.7% (66.7%) 2 = 9.3% (12.1%) |
EQ-5D VAS |
|
Teker (2015) [26] Turkey JBUON- Open Acess Journal amied at the diffusion of scientific knowledge in oncology. |
To evaluate the QoL in CRC patients undergoing chemotherapy and to explore the relationship between QoL and patient characteristics and to evaluate the relationship between QoL and different chemotherapy regimens. | Cross-sectional |
N = 101 CRC N = 73 CRC palliative Timeframe unknown |
ECOG: All patients between 0 and 2 |
QLQ-C30 |
|
Mayrbäurl (2016) [31] Austria Support Care Cancer |
To compare HRQoL as reported by patients measured by computer-assisted completion of validated questionnaires in patients with nonresectable advanced CRC while they were undergoing treatment with several palliative chemotherapy lines. | Prospective, longitudinal |
N = 100 Patients with nonresectable advanced CRC. Data collection Feb. 2007- Sep. 2011 |
25% of the patients died during the first year of the study. 29% died during the second year and 26% died during the third year. | QLQ-C30 |
|
Jasinska (2010) [37] Poland Contemporary Oncology |
To assess the effectiveness of palliative care during the hospitalization period in palliative oncological, end-of-life patients with lung, prostate, breast and colon cancer. Considering the linkage between the outcomes and type of tumor. | Prospective, longitudinal |
N = 41 N = 16 CRC palliative Data collection Feb. 2007 to Apr. 2009 |
ECOG of all included patients: 1 = 0% 2 = 4.88% 3 = 53.66% 4 = 41.46% 20 patients died during the study. 37.5% of the 16 CRC patients died. |
EORTC QLQ-C15-PAL |
|
Kim (2013) [38] South Korea Psycho-Oncology |
To investigate the impact of patients’ awareness of their terminal status on their survival and QoL. | Prospective cohort |
N = 262 N = 56 = metastatic, terminal CRC Data collection Mar. 2009 to Aug. 2011 |
ECOG of all included patients: 0 = 5.7% 1 = 7.3% 2 = 29.8% 3 = 40.8% 4 = 16.4% |
VAS |
|
Koskinen (2019) [39] Finland ACTA ONCOLOGICA |
To examine the relationship between financial difficulties and HRQoL among breast, prostate and colon cancer patients in different stages of the disease as well as to calculate the total burden to patients caused by cancer. Also, to identify patient characteristics that are associated with financial difficulties and HRQoL and identify factors affecting the cost. | Cross-sectional |
N = 1.978 N = 508 CRC N = 41 CRC palliative Data collection 2009–2011 |
EQ-5D VAS QLQ-C30 15D |
Results of the quality assessment
Our review only include studies with moderate or high quality (Table 1). Only one study: The estimation of quality of life of the hospitalized terminally ill palliative patients with lung, breast, colon or prostate cancer, by Jasinska et al. [35] was excluded because of methodological weaknesses. This study had an average CASP score (6/8); however, had only included two individuals in the colorectal group. Eleven studies were included [26–31, 36–40], and these are presented in Table 2.
Table 1.
Quality assessment of included studies
| Reference | Criteria | Tot | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 9 | 10 | |||
| Adamowicz [29] | + | + | + | + | + | + | + | + | 8/8 | High |
| Asplund [36] | + | + | + | + | + | / | + | + | 7/7 | High |
| Färkkilä [28] | + | + | + | + | + | / | + | + | 7/7 | High |
| Färkkilä [27] | + | + | + | + | + | / | + | + | 7/7 | High |
| Jasinska [37] | + | + | + | + | – | + | + | + | 7/8 | Moderate |
| Jasinska [35] | + | + | + | + | – | – | + | + | 6/8 | Moderate |
| Kim [38] | + | + | + | + | + | + | + | + | 8/8 | High |
| Koskinen [39] | + | + | + | + | + | / | + | + | 7/7 | High |
| Mayrbäurl [31] | + | + | + | + | – | + | + | + | 7/8 | Moderate |
| Selby [40] | + | + | + | + | – | + | + | + | 7/8 | Moderate |
| Stein [30] | + | + | + | + | + | / | + | + | 7/7 | High |
| Teker [26] | + | + | + | + | – | / | + | + | 6/7 | Moderate |
Question 6 is not relevant for the cross-sectional designs, and this is symbolised by (/). Questions 11 and 12 in the CASP tool are not relevant for the quality score and are therefore omitted from the table
High quality for the cross-sectional studies = 7, moderate = 5–6, low < 5. High quality for the longitudinal studies = 8, moderate = 6–7, low < 6. Jasinska et al. [35] is included in the table, however, excluded for further analyses because of methodological weakness (see ‘Results of the quality assessment’ for details)
Methods for measuring HRQoL
There are different levels of HRQoL, and the levels presented in this article refer to the conceptual model by Spilker from 1996 [9]. Furthermore, a variety of questionnaires are relevant for measuring HRQoL. The European Organization for Research and Treatment of Cancers’ (EORTC) quality of life questionnaire, QLQ-C30, is a disease-specific questionnaire with focus on cancer, with a possible score range of 0–100, with higher scores indicating better HRQoL. The questionnaire includes questions about five function domains and eight questions about the person’s symptoms [41]. Although the questionnaire is disease-specific, the scores we have used in our meta-analysis refer to the person’s overall perception of their health status and QoL. Therefore, we acknowledge that such scores belong to the category of HRQoL. The study by Aronson et al. [41] considered the psychometric properties (reliability and validity) of the QLQ-C30 among the three language-cultural groups: patients from English-speaking countries, Northern Europe and Southern Europe. The results of the study support the questionnaire as a reliable and valid measure of the QoL of cancer patients in different clinical research settings. The EuroQoL EQ-5D and the 15D questionnaire are two validated questionnaires that measure the individual’s HRQoL. The EQ-5D questionnaire includes questions on the person’s mobility, self-care, pain, usual activities and psychological status, with three possible answers for each item [42]. Feng et al. [43] show in their article that the EQ-5D is a valid and reliable generic HRQoL instrument across a broad range of populations, settings and conditions. The 15D questionnaire consists of 15 dimensions: breathing, mental function, speech (communication), vision, mobility, regular activities, hearing, nutrition, elimination, sleep, distress, discomfort and symptoms, as well as sexual activity. Each dimension is divided into five levels, each with an answer option [44]. Sintonen [44] examines in his study the psychometric properties of acceptability, validity, reliability, responsiveness and discriminatory ability of the 15D instrument, and asserts that its properties are superior in several respects to existing generally used profile and single index score instruments. Both of the questionnaires give a single index score, with a value range between 0 and 1 [42, 44].
The Visual Analog Scale (VAS) is a visual scale where a score of 100 indicates the best imaginable health state and 0 the worst [42]. VAS is also considered a strong, clinically useful, valid and reliable instrument, for its measure of symptom intensity [45].
Meta-analysis
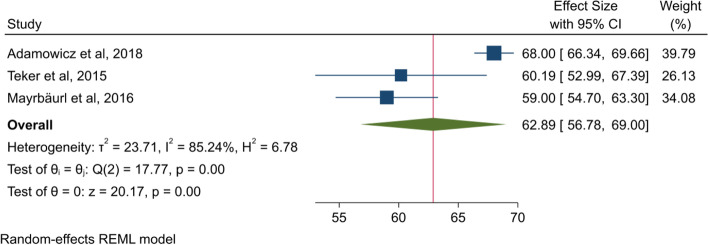
We analysed the QLQ-C30 results as one subgroup (Fig. 2). The mean HRQoL score found in this meta-analysis indicates that the patients in the palliative phase of CRC included in the present studies on average scored 2.19 points higher than the reported reference value for patients with recurrent or metastatic CRC using the QLQ-C30 by Scott et al. [46]. The heterogeneity between the studies included in this analysis is high (I2 = 85.24%).
Fig. 2.
Forest plot for QLQ-C30
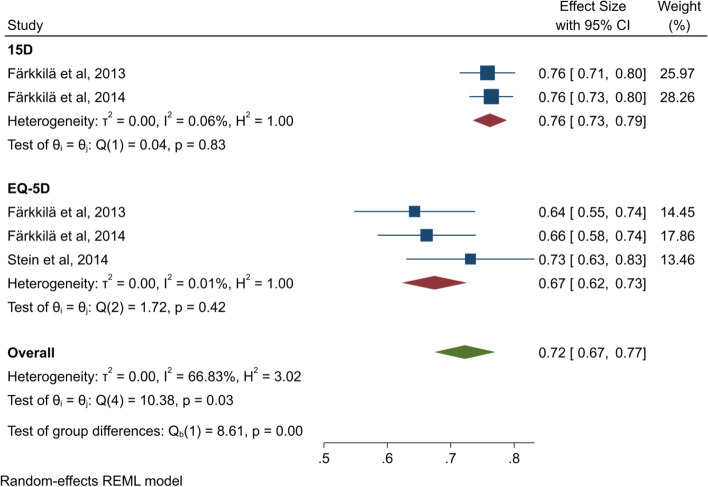
As shown in Fig. 3, the heterogeneity is in the moderate to high range for the overall estimate of EQ-5D and 15D (I2 = 66.83%). When the questionnaires are analysed separately, the heterogeneity decreases to a low level (I2: 15D = 0.06 and EQ-5D = 0.01). This decrease in heterogeneity when the analysis is split into subgroups strengthens the validity of the result from each subgroup. The analysis for the EQ-5D questionnaire shows that the score is 0.083 points lower than the reference score for the general population (+ 75 years) in Denmark [42].
Fig. 3.
Forest plot for 15D and EQ-5D
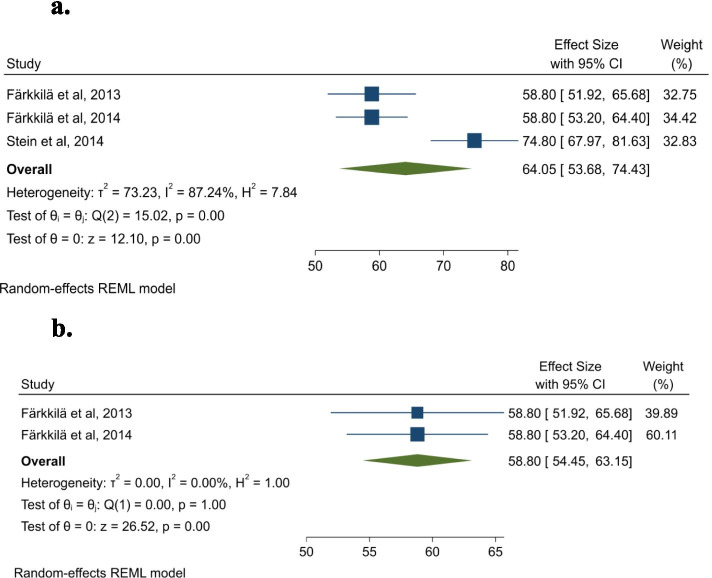
Figure 4a shows that our results for VAS were 12.15 points lower than the reference value from Denmark for the general population, 75+ years group [42]. This indicates that this patient group has lower HRQoL compared to the general population. The heterogeneity for this analysis is in the high range (I2 = 87.24%). The p-value for the Cochran’s Q (p < .005) also indicates heterogeneity [33].
Fig. 4.
a Forest plot for Visual analog scale with Stein et al., [30] included. b Forest plot for Visual analog scale (Stein et al., [30] excluded)
Meta-analysis of the QLQ-C30 symptoms scale
Three of the studies included in this review included data on the symptom scale from the QLQ-C30 questionnaire. The results of a meta-analysis of these data are shown in Fig. 5. Fatigue, financial impact and sleep disturbance are the symptoms that score the highest, with an overall score of over 30 points. Nausea and vomiting are the symptoms with the lowest score, with diarrhea and constipation as third and fourth lowest. Appetite loss and pain are reported with a higher score than the other gastrointestinal (GI) symptoms. Financial impact, nausea and vomiting, pain, appetite loss, dyspnea, constipation and fatigue all have high heterogeneity, over 75%. Diarrhea has a I2 value indicating a moderate to high amount of heterogeneity (I2 = 69.78%), while sleep disturbance is the only symptom showing low heterogeneity (I2 = 17.20%).
Sensitivity analysis
Our meta-analysis found problems with clinical and methodological heterogeneity related to the article by Adamowicz and Baczkowska-Waliszewska [29]. Methodological because it is not clear which data should be used from the study. While Adamowicz and Baczkowska-Waliszewska [29] report pre- and post-treatment, the other study included in the analysis report on overall HRQoL and symptoms in 1st, 2nd and 3rd line (we use the data from 2nd line). In the sensitivity analysis, we therefore report both for pre- and post-data from Adamowicz and Baczkowska-Waliszewska [29].
There might also be clinical heterogeneity because the patients in the Adamowicz and Baczkowska-Waliszewska [29] article have a longer survival time than normal compared to stage IV patients, and therefore appear to tolerate CRC and/or treatment better. In the sensitivity analysis, we therefore also estimate the result without data from Adamowicz and Baczkowska-Waliszewska [29]. The results of these sensitivity analyses based on QLQ-C30 are shown in Table 3. It is clear that the heterogeneity of the mean HRQoL and many of the symptoms change depending on how data from Adamowicz and Baczkowska-Waliszewska [29] are handled, while the estimates of the mean of e.g., HRQoL vary more modestly, from 62 to 59. If we exclude the data from Adamowicz and Baczkowska-Waliszewska [29], the analysis generally results in a worse mean HRQoL and more negative symptoms. For more details, see additional file 5.
Table 3.
Sensitivity for meta-analysis based on QLQ-C30
| Including Adamowicz et.al.:data pre-treatment | Including Adamowicz et.al.: data post-treatment | Excluding data from Adamowicz et.al. | |
|---|---|---|---|
| Overall HRQoL | |||
| Mean (CI) | 62.01 (57.72–66.29) | 62.89 (56.78–69.00) | 59.31 (55.62–63.00) |
| Heterogeneity: Q | 7.47, p = 0.02 | 17.77, p = 0.00 | 0.08, p = 0.78 |
| Heterogeneity: I2 | 69.60% | 85.24% | 0.00% |
| Symptoms | |||
| Appetite loss | |||
| Mean (CI) | 23.61 (9.72–37.51) | 23.70 (9.98–37.42) | 30.27 (21.08–39.46) |
| Heterogeneity: Q | 45.47, p = 0.00 | 45.95, p = 0.00 | 2.97, p = 0.08 |
| Heterogeneity: I2 | 94.67% | 94.70% | 66.29% |
| Constipation | |||
| Mean (CI) | 20.77 (15.49–26.05) | 20.57 (14.79–26.36) | 23.67 (19.15–28.18) |
| Heterogeneity: Q | 8.43, p = 0.01 | 10.33, p = 0.01 | 0.80, p = 0.37 |
| Heterogeneity: I2 | 73.89% | 78.04% | 0.00% |
| Diarrhea | |||
| Mean (CI) | 16.20 (5.86–26.54) | 19.09 (14.39–23-79) | 21.57 (16.41–26.73) |
| Heterogeneity: Q | 47.50, p 0 0.00 | 7.02, p = 0.03 | 1.49, p = 0.22 |
| Heterogeneity: I2 | 94.23% | 69.78% | 33.00% |
| Dyspnea | |||
| Mean (CI) | 17.97 (5.45–30.49) | 18.23 (6.09–30.37) | 22.64 (6.97–38.31) |
| Heterogeneity: Q | 41.63, p = 0.00 | 38.42, p = 0.00 | 15.25, p = 0.00 |
| Heterogeneity: I2 | 96.33% | 96.15% | 93.44% |
| Fatigue | |||
| Mean (CI) | 34.64 (19.96–49.33) | 36.25 (24.57–47.92) | 41.93 (33.65–50.21) |
| Heterogeneity: Q | 80.88, p = 0.00 | 49.73, p = 0.00 | 3.40, p = 0.07 |
| Heterogeneity: I2 | 96.65% | 94.78% | 70.62% |
| Financial impact | |||
| Mean (CI) | 30.81 (10.25–51.37) | 31.15 (10.35–51.95) | 27.22 (−6.34–60.77) |
| Heterogeneity: Q | 115.79, p = 0.00 | 122.74, p = 0.00 | 64.98, p = 0.00 |
| Heterogeneity: I2 | 98.39% | 98.44% | 98.46% |
| Nausea/vomiting | |||
| Mean (CI) | 15.51 (−0.39–31.41) | 15.99 (0.73–31.25) | 20.87 (−1.07–42.82) |
| Heterogeneity: Q | 41.26, p = 0.00 | 35.49, p = 0.00 | 22.28, p = 0.00 |
| Heterogeneity: I2 | 98.10% | 97.99% | 95.51% |
| Pain | |||
| Mean (CI) | 24.59 (19.89–29.29) | 22.82 (14.39–31.24) | 27.42 (23.52–31.31) |
| Heterogeneity: Q | 8.15, p = 0.02 | 32.50, p = 0.00 | 0.06, p = 0.81 |
| Heterogeneity: I2 | 71.76% | 91.66% | 0.00% |
| Sleep disturbance | |||
| Mean (CI) | 33.27 (28.77–37.76) | 32.93 (30.07–35.78) | 30.25 (25.21–35.28) |
| Heterogeneity: Q | 4.28, p = 0.12 | 1.94, p = 0.38 | 0.18, p = 0.67 |
| Heterogeneity: I2 | 53.09% | 17.20% | 0.00% |
Q: Cochran’s Q. I2 = 100% x (Q-dt)/Q, where df is the degrees of freedom and Q the Cochran’s heterogeneity statistics
The forest plot of VAS showed high heterogeneity. One possible explanation for this can be difference in patients’ performance status. Stein et al. [30] enrolled patients from second or subsequent lines of palliative therapy which had an ECOG performance status score of 0, 1 or 2 when initiation of second-line therapy. While Färkkilä et al. [27] only included patients that had died from cancer within 6 months after finishing the questionnaire. From Färkkilä et al. [28], we found no information about the patients’ performance status. Figure 4b shows the VAS score for Stein et al. [30] excluded. The overall VAS score changed from 64.05 to 58.80 and the heterogeneity decreased.
Sociodemographic and clinical variables influencing HRQoL
Table 4 shows the variables in all the included studies which are statistically significantly correlated with HRQoL. All of the studies that include different cancer types or stages in their sample do not clarify whether the analysis was conducted for the whole group or for the subgroups. We therefore decided to include all the variables but have noted where there are differences.
Education was analysed for in four studies, but only two showed that higher education was significantly correlated with higher HRQoL using the 15D questionnaire [27, 39]. Age and sex were analysed for in six and three studies respectively. For age, one study using 15D and one study using 15D, EQ-5D and VAS indicated that higher age was significantly correlated with lower HRQoL [28, 39], while sex was significant in two of the studies. One of these studies [27] using VAS and 15D indicates that the female gender was significantly correlated with higher HRQoL. On the other hand, Selby et al. [40] report the female gender to be statistically significantly correlated with a lower score on the psychological subscale of the Rotterdam Symptom Checklist (RSCL).
Five studies analysed for stage and three of them indicate that more severe disease state statistically correlates with HRQoL [28, 36, 39]. Five studies analysed for the effect of targeted treatment, but only one [28] showed a significant negative correlation between HRQoL and radiotherapy. Both studies that analysed for depression found that higher levels of depression were statistically significantly correlated with lower HRQoL [27, 38]. Financial difficulties were associated with lower HRQoL in three [27, 28, 39] of the four studies that analysed for this. Marital status, response to treatment, feeling of coherence, higher levels of oncology knowledge and treatment in correlation with stage were positively correlated with HRQoL.
Adamowicz and Baczkowska-Waliszewska [29] found that a better performance status (ECOG score = 0) indicated a better HRQoL. Selby et al. [40], on the other hand, did not find any correlation between performance status and HRQoL. Mayrbäurl et al. [31] indicate that HRQoL increased from first-line chemotherapy to post first-line, but then decreased from post first-line to post third-line, from 65.3 to 44.2 (P < .001). Selby et al. [40] indicate that the ESAS score significantly decreased from the baseline value to the one-month assessment point. The results of the RSCL questionnaire showed no significant change in HRQoL over time. Teker, Demirag, Erdem, Kemal and Yucel [26] found no correlation between the chemotherapy line and HRQoL. The presence of intrusive thoughts, time from diagnosis, awareness of terminal disease and use of unconventional treatment, out-of-pocket cost and total cost were statistically negatively correlated with HRQoL. Comorbidities, staying at a hospital, metastasis to lungs, smoking status, place of residence, type of chemotherapy or treatment and treatment at enrolment did not significantly correlate with HRQoL.
Discussion
The results of this review show that the average HRQoL score of patients in the palliative phase of CRC are comparable to that of long-term survivors of CRC from previous studies, but lower than for the general population. Multiple sociodemographic and clinical variables were statistically significantly associated with HRQoL. The highest scored symptoms were symptoms generally associated with cancer, such as pain, fatigue and sleep disturbances, while GI symptoms such as diarrhea and constipation were scored somewhat lower. Overall, this review shows that there are both similarities and differences between the HRQoL in CRC survivors and patients in the palliative phase, which is an important finding which healthcare professionals should be aware of when caring for this patient group. Including, but not limited to identification of the patients’ symptoms and tailoring a treatment plan in collaboration with the individual, is essential. The findings from this study are valuable in terms of creating a plan with focus on the most relevant aspects of HRQoL for colorectal cancer patients. The differences found between the two patient groups in this review underpin its importance, given that no review has previously been published on this specific patient group. Furthermore, policy makers need to be aware of these differences to optimize the care strategy offered this patient group through specified guidelines.
Which HRQoL questionnaires are commonly used and what is the average HRQoL score in the palliative phase of CRC?
With respect to the first research question; which questionnaires are used to measure HRQoL in the included studies, we found that the most common questionnaires used to measure HRQoL in our sample of studies were the QLQ-C30, the 15D, the EQ-5D and the VAS scale. In addition, the ESAS questionnaire, the RSCL and the QLQ-C15-PAL were used in the included studies.
With respect to the question; what is the average score for HRQoL for each of the included questionnaires, we found that compared to the results of Nolte et al. [47], the sample in this review had a lower HRQoL than that of the general population in 13 European countries. On the other hand, the average score for the QLQ-C30 data in this review was only a few points higher than the reported reference value for patients with recurrent or metastatic CRC using the same questionnaire as reported by Scott et al. [46].
Furthermore, heterogeneity is high in the meta-analysis of QLQ-C30. The patients in Adamowicz and Baczkowska-Waliszewska [29] have a longer survival time than normal compared to stage IV patients, and both Adamowicz and Baczkowska-Waliszewska [29] and Teker et al. [26] appear to have healthier samples with an ECOG score between 0 and 2, with over half of the sample in Adamowicz and Baczkowska-Waliszewska [29] scoring 0 (no problems). On the other hand, Mayrbäurl et al. [31] do not refer to an ECOG score, but the median survival time after study inclusion was 21.8 months (51 dropouts due to death). This seems to indicate that this sample was more affected by their illness, which can explain the between-study heterogeneity. In addition to this clinical heterogeneity, we also argue that there is methodological heterogeneity. This is due to certain ambiguities regarding at what stage of the palliative care measurements of QLQ-C30 were performed. The sensitivity analyses we have performed show estimates of what variations in outcome the aforementioned types of heterogeneity can cause.
The large sample size and small confidence interval (CI) identified in the study by Adamowicz and Baczkowska-Waliszewska [29] mean this study is given more weight in the meta-analysis (Fig. 2). Furthermore, this sample has a higher mean HRQoL and thereby raises the average score for the analysis [32]. Both Adamowicz and Baczkowska-Waliszewska [29] and Stein et al. [30] include more female participants compared to the other two studies included in each analysis; Teker et al. [26], Mayrbäurl et al. [31], Färkkilä et al. [27] and Färkkilä et al. [28], respectively. Thus, compared with Nolte et al. [47], our review showed that women report higher HRQoL than men. On the other hand, the review by Bours et al. [16] on survivors of CRC found inconclusive evidence for the association between gender and HRQoL, with several studies associating each gender with lower HRQoL. The performed sensitivity analyses indicate variations in HRQoL score that may have been caused by clinical and methodological heterogeneity. With respect to both QLQ-C30 and HRQoL measurements, we recognise the importance of the information in the original studies about the health state of the patients and when the measurements were performed during the palliative treatment.
We also found that when the 15D and the EQ-5D were analysed together, the heterogeneity was moderate to high, but when analysed separately the heterogeneity decreased to a low level. This indicates that this methodological heterogeneity was caused by the use of different questionnaires [21]. Results for the EQ-5D indicate that our sample had a lower HRQoL (0.083) than the general population (+ 75 years) in Denmark [42]. Furthermore, our results are similar to (0.03 lower than) those of Rodriguez, Hawkins, Berkowitz and Li [48], who investigated the HRQoL in survivors of CRC. This indicates that the HRQoL in palliative CRC patients is similar to that of people no longer struggling with the disease. The comparable HRQoL of these different patient groups can be explained by CRC survivors struggling with late effects as reported by Haggstrom and Cheung [15], as well as depression and anxiety about experiencing recurrence as expressed by the patients in the systematic review by Jansen, Koch, Brenner and Arndt [19].
Similar to the results of EQ-5D, the results of the meta-analysis including Färkkilä et al. [27], Färkkilä et al. [28] and Stein et al. [30] show that the sample measured using VAS had a 12.15-point lower HRQoL than the Danish general population [42]. On the other hand, more than three quarters of the sample in Stein et al. [30] had an ECOG score of 0–1, indicating almost no impact on their performance status. In Färkkilä et al. [27], over half of the sample died within 6 months, indicating that the sample was in a late stage of their disease. This difference in health state can explain why the participants in the Stein et al. [30] study reported a higher average HRQoL.
Which symptoms are linked to HRQoL in the palliative phase of CRC?
With respect to the analysis linked to the question; which symptoms are reported to impact the patients with CRC in the palliative phase, it is interesting to note that the included patients reported lower GI symptoms, which are often especially associated with CRC, than the more general cancer-related symptoms such as fatigue, pain and sleep disturbance [12]. This can be explained by the fact that the GI symptoms are among the most common symptoms for CRC patients [49], and are therefore well managed. This is somewhat in accordance with the result of the review by Bours et al. [16], which found that GI symptoms was weakly associated with HRQoL in survivors of CRC, while fatigue, anxiety and depression were strongly associated with HRQoL. On the other hand, compared to our results, Bours et al. [16] found that pain was only weakly associated with HRQoL.
Furthermore, the analysis of nausea and vomiting, appetite loss, constipation, dyspnea, fatigue, pain and financial impact were all highly heterogeneous. In all of the heterogeneous analyses, apart from the financial impact variable, Adamowicz and Baczkowska-Waliszewska [29] scored the best out of the three studies. This can be explained by the fact that the sample participants in Adamowicz and Baczkowska-Waliszewska [29] seem to be less affected by their disease. The sample also has a higher percentage of highly educated individuals. Interestingly, similar to the overall HRQoL score discussed above, Adamowicz and Baczkowska-Waliszewska [29] have the highest percentage of women of the three studies included in the symptom analysis but still have the best score despite the female gender often being linked to more symptoms and lower HRQoL as in the study by Nolte et al. [47].
Teker et al. [26] have the highest score on most of the variables as shown in Fig. 5 (excl. Dyspnea, sleep disturbance and fatigue). Overall, this sample has lower education and scores the highest on the financial impact variable, which can explain why these individuals seem to have poorer symptom management. These associations are in accordance with the results of Jansen et al. [19], while Lathan et al. [50] found the association with financial difficulties.
As mentioned, the sample in Mayrbäurl et al. [31] seems to be most affected by their disease, which explains why this sample has the highest dyspnea and fatigue scores. These symptoms are typical of the last part of life as explained in the literature summary by Chang [12]. Mayrbäurl et al. [31] also have the lowest score on financial impact, which can be explained by the fact that the study is from Austria, where there is a lower share of households with impoverishing health spending (health spending that causes the household to drop below the poverty line) as described by the World Health Organization Regional Office for Europe [51], compared to the study by Teker et al. [26] from Turkey and the study by Adamowicz and Baczkowska-Waliszewska [29] from Poland. In accordance with our results, Bours et al. [16] found that lower household income was associated with HRQoL in CRC survivors.
Which sociodemographic and clinical factors are correlated with HRQoL?
With respect to the question; which sociodemographic and clinical factors are found to be statistically significantly correlated with the average HRQoL score in each study, we found that similar to the results for the average HRQoL, Selby et al. [40] report that the female gender was associated with a lower score on the psychosocial scale of the RSCL instrument. This is in accordance with the results of a study by Paika et al. [52] investigating HRQoL in patients with CRC using the World Health Organization Quality of Life Instrument, Short-Form. Färkkilä et al. [27] on the other hand, found the female gender to be associated with higher HRQoL using VAS and the 15D instrument. The different measuring instruments used and the fact that the psychosocial scale is only a subscale of the full RSCL measurement can explain the difference.
The association between HRQoL and age, more severe disease stage, time since diagnosis, chemotherapy line and response to treatment are substantiated by physiological mechanisms such as age-related homeostenosis [53], more invasive tumours and spreading to other organs or lymph nodes [49] and the side-effects of chemotherapy [15]. The use of targeted treatment (radiation) was associated with lower HRQoL, which is explained by the side effects of radiation [54]. On the other hand, radiation is often considered a palliative treatment to reduce pain and other symptoms [55]. In this study, we did not find any significant association between comorbidities or smoking with HRQoL, which deviates from the results of the review by Bours et al. [16], which found several studies that identified an association between comorbidities and smoking status with lower HRQoL in CRC survivors. Only two studies had analysed for the correlation between the ECOG score and HRQoL in our review. In accordance with the results of Bours et al. [16], who found that poor performance status was associated with lower HRQoL in survivors of CRC, one study in our review found that ECOG score was statistically significant, linking better performance status to higher HRQoL. ECOG is a score that indicates the patient’s performance status, which Chang [12] calls a key indicator of prognosis in patients with terminal disease. This variable should be included more often in studies investigating HRQoL.
The use of unconventional treatment was found to be associated with lower HRQoL. This might of course be explained by the fact that these treatments are not necessarily thoroughly tested and can give unknown side effects. Another possible explanation is that the individual is very ill, and therefore open to any treatment that could help. The impact of the disease might cause the association with lower HRQoL. On the other hand, we could hypothesise that these patients would be hopeful of the unconventional treatment working, and therefore report higher HRQoL.
The association between HRQoL and higher education, being married, higher levels of oncology knowledge and feeling of coherence can be explained by the fact that all of these variables, which Lazarus [56] calls personal resources, affect the individual’s ability to cope with stress in a productive manner. In accordance with our results, Jansen et al. [19] also found that education and higher income were associated with higher QoL. On the other hand, financial difficulties, higher out-of-pocket payments, higher total costs, intrusive thoughts, awareness of terminal disease and more depression can negatively affect the individual’s ability to cope with stress [56], and therefore be associated with lower HRQoL. In accordance with the results in this review, Bours et al. [16] found that depression and feelings of coherence were associated with HRQoL. While Haverfield et al. [57] found financial cost to be a worry for oncology patients.
Strengths, limitations and future direction
We decided to analyse the symptoms scale for the QLQ-C30 only. The other questionnaires also include information of interest in this area and this is a relevant topic to include in future meta-analyses. We excluded studies with an RCT design, which might have excluded some relevant studies. On the other hand, RCTs tend to include healthier individuals with fewer comorbidities. RCTs are also usually executed in controlled environments with personnel who have specialised knowledge and a heightened focus on observation and care [18]. This can lead to HRQoL being skewed to a higher score than is the case for average CRC patients in palliative care.
The studies included are somewhat heterogeneous with respect to the measurements used, and the sociodemographic and clinical variables of the samples. This affects comparison of the studies. The number of studies included in the review is also limited. However, this is due to a rigorous search with a focus on including the most relevant and recent studies.
The most severely ill patients often decline participation in studies. Non-compliance can be due to many factors, such as ethical considerations or that the patient is too frail to complete the questionnaires. This may have affected the average HRQoL score by excluding patients with the lowest scores. The quality of the studies included was also thoroughly assessed by three independent researchers. The fact that we performed a meta-analysis strengthens the results of this review.
Considering the limitations, and the small number of relevant studies, further research is needed. A meta-analysis should be performed highlighting data from all the instruments including symptoms. ECOG is one variable that should be included more often in studies investigating HRQoL. Since the results for both CRC patients in the palliative phase and CRC survivors show an association between psychological distress and HRQoL, it would also be interesting to understand and elucidate the cause of both groups’ depression or anxiety, linked to their different disease stages. This would give healthcare personnel more knowledge to properly tailor their psychological support to each patient group.
Conclusion
In this systematic review, we found that QLQ-C30, the 15D, the EQ-5D and VAS were the most commonly used questionnaires for measuring HRQoL in the included studies. Furthermore, we found that the average HRQoL score of patients with CRC in the palliative phase is comparable to that of long-term survivors of CRC from previous studies, but lower than for the general population. The highest scored symptoms were symptoms generally associated with cancer, while GI symptoms such as diarrhea and constipation were scored somewhat lower. Education, financial difficulties and performance status were correlated with the patients’ HRQoL. Unlike previous research, no statistically significant association between sex and HRQoL was found in the studies we analysed.
Supplementary Information
Additional file 1: Table 5. Mean HRQoL-scores in different disease stages. Mean (SD/CI).
Acknowledgements
We would like to thank senior librarian, Kari Kalland at the Oslo Metropolitan University Library for help and guidance with our search strategies.
Abbreviations
- HRQoL
Health-Related Quality of Life
- QOL
Quality of Life
- CRC
Colorectal Cancer
- PROMS
Patient Reported Outcomes
- ICN
International Council of Nurses
- CRD
Center for Reviews and Disseminations
- CASP
Critical Appraisal Skills Programme
- EORTC
The European Organization for Research and Treatment of Cancers
- VAS
The Visual Analog Scale
- GI
Gastrointestinal
- RSCL
Rotterdam Symptom Checklist
- CI
Confidence interval.
Authors’ contributions
IRF and SM collected and analysed all the relevant data from all the included articles. Both authors contributed to the systematic search and all parts of the written article. EKG and PJ contributed to the conceptualisation of the study design and provided continuous feedback on both content and language clarifications. All authors read and approved the final manuscript for publication.
Funding
The authors declare that there is no funding to declare for this review.
Availability of data and materials
The data used in this review are collected from the included research articles. A list is shown below:
Asplund D, Bisgaard T, Bock D, Burcharth J, Gonzalez E, Haglind E, et al. Pretreatment quality of life in patients with rectal cancer is associated with intrusive thoughts and sense of coherence. International Journal of Colorectal Disease. 2017;32:1639–47 [36].
Adamowicz K, Baczkowska-Waliszewska Z. Prognostic Value of Knowledge of Cancer and Used Unconventional Therapy Methods on Quality of Life in Advanced, Metastatic Colorectal Cancer in Clinical Practice. J Cancer Educ. 2018;06:06 [29].
Färkkilä N, Sintonen H, Saarto T, Jarvinen H, Hanninen J, Taari K, et al. Health-related quality of life in colorectal cancer. Colorectal Disease. 2013;15(5):215–22 [28].
Färkkilä N, Torvinen S, Roine RP, Sintonen H, Hanninen J, Taari K, et al. Health-related quality of life among breast, prostate, and colorectal cancer patients with end-stage disease. Qual Life Res. 2014;23:1387–94 [27].
Jasinska M, Tracz M, Kurczewska U, Orszulak-Michalak D. Assessment of change of quality of life in hospitalized terminally ill cancer patients. Wspolczesna Onkologia. 2010;14(5):333–9 [37].
Kim SY, Kim JM, Kim SW, Shin IS, Bae KY, Shim HJ, et al. Does awareness of terminal status influence survival and quality of life in terminally ill cancer patients? Psycho-Oncology. 2013;22(10):2206–13 [38].
Koskinen J-P, Färkkilä N, Sintonen H, Saarto T, Taari K, Roine RP. The association of financial difficulties and out-ofpocket payments with health-related quality of life among breast, prostate and colorectal cancer patients. Acta Oncol. 2019;58(7):1062–8 [39].
Mayrbäurl B, Giesinger J, Burgstaller S, Piringer G, Holzner B, Thaler J, et al. Quality of life across chemotherapy lines in patients with advanced colorectal cancer: a prospective single-center observational study. Supportive Care in Cancer. 2016;24(2):667–74 [31].
Selby D, Wright F, Stilos K, Daines P, Moravan V, Gill A, et al. Room for improvement? A quality-of-life assessment in patients with malignant bowel obstruction. Palliative Medicine. 2010;24(1):38–45 [40].
Stein D, Joulain F, Naoshy S, Iqbal U, Muszbek N, Payne KA, et al. Assessing health-state utility values in patients with metastatic colorectal cancer: a utility study in the United Kingdom and the Netherlands. International Journal of Colorectal Disease. 2014;29:1203–10 [30].
Teker F, Demirag G, Erdem D, Kemal Y, Yucel I. Quality of life in colorectal cancer patients during chemotherapy in the era of monoclonal antibody therapies. Journal of BUON. 2015;20(2):443–51 [26].
Declarations
Ethics approval and consent to participate:
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ida Røed Flyum and Seila Mahic contributed equally to this work.
Contributor Information
Ida Røed Flyum, Email: Ida.r.flyum@ldh.no.
Seila Mahic, Email: Seila.Mahic@vid.no.
References
- 1.Cancer-Research-UK. Worldwide cancer statistics n.d [Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer?fbclid=IwAR2jJIBUnwXd7szLqIK_9PEwV-xpBNrjkgfrTZj6bpsVqkx94fvx3A5furM#heading-One.
- 2.Cancer Registry of Norway. Cancer in Norway 2018: Cancer incidence, mortality, survival and prevalence in Norway2019. Available from: https://www.kreftregisteret.no/globalassets/cancer-in-norway/2018/cin2018.pdf.
- 3.Cancer Registery of Norway. Cancer in Norway 2019- Cancer incidence, mortality, survival and prevalence in Norway.2020. Available from: https://www.kreftregisteret.no/globalassets/cancer-in-norway/2019/cin_report.pdf.
- 4.BMJ: Best-practice. Patient information from BMJ- Bowel cancer: what is it? 2020 [Available from: https://bestpractice.bmj.com/patient-leaflets/en-gb/pdf/1183382360493.pdf.
- 5.Grov EK. The Cancer trajectory — a model of phases. Vård i Norden. 2014;34(1):46–47. [Google Scholar]
- 6.World Health Organization. Palliative Care 2018 [Available from: https://www.who.int/news-room/fact-sheets/detail/palliative-care.
- 7.World Health Organization. WHOQOL: Measuring Quality of Life n.d. [Available from: https://www.who.int/healthinfo/survey/whoqol-qualityoflife/en/.
- 8.Gurková E. Issues in the definitions of HRQoL. Journal of Nursing, Social Studies, Public Health and Rehabilitation [Internet]. 2011; 3(4):[190–97 pp.]. Available from: http://casopis-zsfju.zsf.jcu.cz/journal-of-nursing-social-studies-public-health-and-rehabilitation/administrace/clankyfile/20120430140614748509.pdf.
- 9.Spilker B. Quality of life and Pharmacoeconomics in clinical trials. 2. Philadelphia: Lippincott Williams and Wilkins; 1996. [Google Scholar]
- 10.Helse og omsorgsdepartementet . Lev med kreft: Nasjonal kreftstrategi (2018–2022) 2018. [Google Scholar]
- 11.International Council of Nurses . The ICN code of ethics for nurses. Geneva: International Council of Nurses; 2012. [Google Scholar]
- 12.Chang VT. Approach to symptom assessment in palliative care. In: Smith TJ, Givens J, editors. UpToDate2020.
- 13.Helsedirektoratet. Nasjonalt handlingsprogram for palliasjon i kreftomsorgen: Nasjonal faglig retningslinje. In: Avdeling for spesialisthelsetjenester, editor. Oslo2019.
- 14.National institute for health research. PROSPERO: International prospective register of systematic reviews n.d. [Available from: https://www.crd.york.ac.uk/PROSPERO/.
- 15.Haggstrom DA, Cheung WY. Approach to the long-term survivor of colorectal cancer. In: Nekhlyudov L, Savarese DMF, editors. UpToDate2020.
- 16.Bours MJL, van der Linden BWA, Winkels RM, van Duijnhoven FJ, Mols F, van Roekel EH, et al. Candidate predictors of health-related quality of life of colorectal Cancer survivors: a systematic review. Oncologist. 2016;21(4):433–452. doi: 10.1634/theoncologist.2015-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lapinsky E, Man LC, MacKenzie AR. Health-related quality of life in older adults with colorectal Cancer. Curr Oncol Rep. 2019;21(9):81. doi: 10.1007/s11912-019-0830-2. [DOI] [PubMed] [Google Scholar]
- 18.Eyl RE, Xie K, Koch-Gallenkamp L, Brenner H, Arndt V. Quality of life and physical activity in long-term (≥5 years post-diagnosis) colorectal cancer survivors - systematic review. Health Qual Life Outcomes. 2018;16(1):112. doi: 10.1186/s12955-018-0934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen L, Koch L, Brenner H, Arndt V. Quality of life among long-term (⩾5years) colorectal cancer survivors – systematic review. Eur J Cancer. 2010;46(16):2879–2888. doi: 10.1016/j.ejca.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Balhareth A, Aldossary MY, McNamara D. Impact of physical activity and diet on colorectal cancer survivors’ quality of life: a systematic review. World J Surg Oncol. 2019;17(1):153. doi: 10.1186/s12957-019-1697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deeks JJ, Higgins JPT, Altman DG, JPT H, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 60: Cochrane. 2019. Analysing data and undertaking meta-analyses. [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, ThePRISMAGroup Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polit DF, Beck CT. Nursing research : generating and assessing evidence for nursing practice. 10. Philadelphia: Wolters Kluwer; 2017. [Google Scholar]
- 24.Efficace F, Bottomley A, Vanvoorden V, Blazeby JM. Methodological issues in assessing health-related quality of life of colorectal cancer patients in randomised controlled trials. Eur J Cancer. 2004;40(2):187–197. doi: 10.1016/j.ejca.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Critical appraisal skills programme. CASP Checklists n.d [Available from: https://casp-uk.net/casp-tools-checklists/.
- 26.Teker F, Demirag G, Erdem D, Kemal Y, Yucel I. Quality of life in colorectal cancer patients during chemotherapy in the era of monoclonal antibody therapies. J BUON. 2015;20(2):443–451. [PubMed] [Google Scholar]
- 27.Färkkilä N, Torvinen S, Roine RP, Sintonen H, Hänninen J, Taari K, et al. Health-related quality of life among breast, prostate, and colorectal cancer patients with end-stage disease. Qual Life Res. 2014;23(4):1387–1394. doi: 10.1007/s11136-013-0562-y. [DOI] [PubMed] [Google Scholar]
- 28.Färkkilä N, Sintonen H, Saarto T, Jarvinen H, Hänninen J, Taari K, et al. Health-related quality of life in colorectal cancer. Color Dis. 2013;15(5):215–222. doi: 10.1111/codi.12143. [DOI] [PubMed] [Google Scholar]
- 29.Adamowicz K, Baczkowska-Waliszewska Z. Prognostic value of knowledge of Cancer and used unconventional therapy methods on quality of life in advanced, metastatic colorectal cancer in clinical practice. J Cancer Educ. 2018;06:06. doi: 10.1007/s13187-018-1454-1. [DOI] [PubMed] [Google Scholar]
- 30.Stein D, Joulain F, Naoshy S, Iqbal U, Muszbek N, Payne KA, et al. Assessing health-state utility values in patients with metastatic colorectal cancer: a utility study in the United Kingdom and the Netherlands. Int J Color Dis. 2014;29:1203–1210. doi: 10.1007/s00384-014-1980-1. [DOI] [PubMed] [Google Scholar]
- 31.Mayrbäurl B, Giesinger JM, Burgstaller S, Piringer G, Holzner B, Thaler J. Quality of life across chemotherapy lines in patients with advanced colorectal cancer: a prospective single-center observational study. Support Care Cancer. 2016;24(2):667–674. doi: 10.1007/s00520-015-2828-0. [DOI] [PubMed] [Google Scholar]
- 32.StataCorp.LLC. Stata meta-analysis reference manual: Release 16. Texas: Stata Press; 2019. Available from: https://www.stata.com/manuals/meta.pdf.
- 33.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. Br Med J. 2001;322:1479–1480. doi: 10.1136/bmj.322.7300.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jasinska M, Kurczewska U, Owczarek J, Tracz M, Piaskowska A, Orszulak-Michalak D. The estimation of quality of life of the hospitalized terminally ill palliative patients with lung, breast, colon or prostate cancer. Clin Exp Med Letters. 2010;51(1):29–33. [Google Scholar]
- 36.Asplund D, Bisgaard T, Bock D, Burcharth J, Gonzalez E, Haglind E, et al. Pretreatment quality of life in patients with rectal cancer is associated with intrusive thoughts and sense of coherence. Int J Color Dis. 2017;32:1639–1647. doi: 10.1007/s00384-017-2900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jasinska M, Tracz M, Kurczewska U, Orszulak-Michalak D. Assessment of change of quality of life in hospitalized terminally ill cancer patients. Wspolczesna Onkol. 2010;14(5):333–339. doi: 10.5114/wo.2010.17298. [DOI] [Google Scholar]
- 38.Kim S-Y, Kim J-M, Kim S-W, Shin I-S, Bae K-Y, Shim H-J, et al. Does awareness of terminal status influence survival and quality of life in terminally ill cancer patients? Psycho Oncol. 2013;22(10):2206–2213. doi: 10.1002/pon.3275. [DOI] [PubMed] [Google Scholar]
- 39.Koskinen J-P, Färkkilä N, Sintonen H, Saarto T, Taari K, Roine RP. The association of financial difficulties and out-of-pocket payments with health-related quality of life among breast, prostate and colorectal cancer patients. Acta Oncol. 2019;58(7):1062–1068. doi: 10.1080/0284186X.2019.1592218. [DOI] [PubMed] [Google Scholar]
- 40.Selby D, Wright F, Stilos K, Daines P, Moravan V, Gill A, et al. Room for improvement? A quality-of-life assessment in patients with malignant bowel obstruction. Palliat Med. 2010;24(1):38–45. doi: 10.1177/0269216309346544. [DOI] [PubMed] [Google Scholar]
- 41.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 42.Szende A, Janssen B, Cabases J. Self-reported population health: an international perspective based on EQ-5D. 2014. [PubMed] [Google Scholar]
- 43.Feng YS, Kohlmann T, Janssen MF, Buchholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021;30(3):647–673. doi: 10.1007/s11136-020-02688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33(5):328–336. doi: 10.3109/07853890109002086. [DOI] [PubMed] [Google Scholar]
- 45.Kahl C, Cleland JA. Visual analogue scale, numeric pain rating scale and the McGill pain questionnaire: an overview of psychometric properties. Phys Ther Rev. 2005;10(2):123–128. doi: 10.1179/108331905X55776. [DOI] [Google Scholar]
- 46.Scott NW, Fayers PM, Aaronson NK, Bottomley A, de Graeff A, Groenvold M, et al. EORTC QLQ-C30 reference values Brussels: European Organisation for Research and Treatment of Cancer (EORTC): quality of life group. 2008. [Google Scholar]
- 47.Nolte S, Liegl G, Petersen MA, Aaronson NK, Costantini A, Fayers PM, et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the unites states. Eur J Cancer. 2019;107:153–163. doi: 10.1016/j.ejca.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez JL, Hawkins NA, Berkowitz Z, Li C. Factors associated with health-related quality of life among colorectal Cancer survivors. Am J Prev Med. 2015;49(6):518–527. doi: 10.1016/j.amepre.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macrae FA, Bendell J. In: Clinical presentation, diagnosis, and staging of colorectal cancer. Tanabe KK, DMF S, Grover S, editors. 2020. [Google Scholar]
- 50.Lathan CS, Cronin A, Tucker-Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of Financial Strain with Symptom Burden and Quality of life for patients with lung or colorectal Cancer. J Clin Oncol. 2016;34(15):1732–1740. doi: 10.1200/JCO.2015.63.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization Regional Office for Europe. Can people aford to pay for health care? New evidence on financial protection in Europe. Regional report2019. Available from: https://apps.who.int/iris/bitstream/handle/10665/311654/9789289054058-eng.pdf?sequence=1&isAllowed=y.
- 52.Paika V, Almyroudi A, Tomenson B, Creed F, Kampletsas EO, Siafaka V, et al. Personality variables are associated with colorectal cancer patients' quality of life independent of psychological distress and disease severity. Psycho Oncol. 2010;19(3):273–282. doi: 10.1002/pon.1563. [DOI] [PubMed] [Google Scholar]
- 53.Taffet GE. In: Normal aging. Schmader KE, Givens J, editors. 2020. [Google Scholar]
- 54.O'neill BDP, Tait DM. Radiotherapy in colorectal cancer. In: Brown G, editor. Colorectal Cancer. Cambridge: Cambridge University Press; 2007. pp. 110–135. [Google Scholar]
- 55.Van Cutsem E, Nordlinger B, Cervantes A. Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol. 2010;21:93–97. doi: 10.1093/annonc/mdq222. [DOI] [PubMed] [Google Scholar]
- 56.Lazarus RS. Stress and emotion : a new synthesis. New York: Springer; 1999. [Google Scholar]
- 57.Haverfield MC, Singer AE, Gray C, Shelley A, Nash A, Lorenz KA. Implementing routine communication about costs of cancer treatment: perspectives of providers, patients, and caregivers. Support Care Cancer. 2020. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table 5. Mean HRQoL-scores in different disease stages. Mean (SD/CI).
Data Availability Statement
The data used in this review are collected from the included research articles. A list is shown below:
Asplund D, Bisgaard T, Bock D, Burcharth J, Gonzalez E, Haglind E, et al. Pretreatment quality of life in patients with rectal cancer is associated with intrusive thoughts and sense of coherence. International Journal of Colorectal Disease. 2017;32:1639–47 [36].
Adamowicz K, Baczkowska-Waliszewska Z. Prognostic Value of Knowledge of Cancer and Used Unconventional Therapy Methods on Quality of Life in Advanced, Metastatic Colorectal Cancer in Clinical Practice. J Cancer Educ. 2018;06:06 [29].
Färkkilä N, Sintonen H, Saarto T, Jarvinen H, Hanninen J, Taari K, et al. Health-related quality of life in colorectal cancer. Colorectal Disease. 2013;15(5):215–22 [28].
Färkkilä N, Torvinen S, Roine RP, Sintonen H, Hanninen J, Taari K, et al. Health-related quality of life among breast, prostate, and colorectal cancer patients with end-stage disease. Qual Life Res. 2014;23:1387–94 [27].
Jasinska M, Tracz M, Kurczewska U, Orszulak-Michalak D. Assessment of change of quality of life in hospitalized terminally ill cancer patients. Wspolczesna Onkologia. 2010;14(5):333–9 [37].
Kim SY, Kim JM, Kim SW, Shin IS, Bae KY, Shim HJ, et al. Does awareness of terminal status influence survival and quality of life in terminally ill cancer patients? Psycho-Oncology. 2013;22(10):2206–13 [38].
Koskinen J-P, Färkkilä N, Sintonen H, Saarto T, Taari K, Roine RP. The association of financial difficulties and out-ofpocket payments with health-related quality of life among breast, prostate and colorectal cancer patients. Acta Oncol. 2019;58(7):1062–8 [39].
Mayrbäurl B, Giesinger J, Burgstaller S, Piringer G, Holzner B, Thaler J, et al. Quality of life across chemotherapy lines in patients with advanced colorectal cancer: a prospective single-center observational study. Supportive Care in Cancer. 2016;24(2):667–74 [31].
Selby D, Wright F, Stilos K, Daines P, Moravan V, Gill A, et al. Room for improvement? A quality-of-life assessment in patients with malignant bowel obstruction. Palliative Medicine. 2010;24(1):38–45 [40].
Stein D, Joulain F, Naoshy S, Iqbal U, Muszbek N, Payne KA, et al. Assessing health-state utility values in patients with metastatic colorectal cancer: a utility study in the United Kingdom and the Netherlands. International Journal of Colorectal Disease. 2014;29:1203–10 [30].
Teker F, Demirag G, Erdem D, Kemal Y, Yucel I. Quality of life in colorectal cancer patients during chemotherapy in the era of monoclonal antibody therapies. Journal of BUON. 2015;20(2):443–51 [26].