Abstract
The Fas receptor (FasR) is an important physiological mediator of apoptosis in various tissues and cells. However, there are also many FasR-expressing cell types that are normally resistant to apoptotic signaling through this receptor. The mitogen-activated protein kinase (MAPK) signaling cascade has, apart from being a growth-stimulating factor, lately received attention as an inhibitory factor in apoptosis. In this study, we examined whether MAPK signaling could be involved in protecting FasR-insensitive cells. To this end, we used different approaches to inhibit MAPK signaling in HeLa cells, including treatment with the MAPK kinase inhibitor PD 98059, serum withdrawal, and expression of dominant-interfering MAPK kinase mutant protein. All of these treatments were effective in sensitizing the cells to FasR-induced apoptosis, demonstrating that MAPK indeed is involved in the control of FasR responses. The MAPK-mediated control seemed to occur at or upstream of caspase 8, the initiator caspase in apoptotic FasR responses. Transfection with the constitutively active MAPK kinase abrogated FasR-induced apoptosis also in the presence of cycloheximide, indicating that the MAPK-generated suppression of FasR-mediated apoptotic signaling is protein synthesis independent. In cells insensitive to FasR-induced apoptosis, stimulation of the FasR with an agonistic antibody resulted in significant MAPK activation, which was inhibited by PD 98059. When different cell types were compared, the FasR-mediated MAPK activation seemed proportional to the degree of FasR insensitivity. These results suggest that the FasR insensitivity is likely to be a consequence of FasR-induced MAPK activation, which in turn interferes with caspase activation.
Apoptosis, or programmed cell death, is an essential mechanism for maintaining homeostasis in multicellular organisms (76). There are a number of cell surface receptors that act as physiological mediators of apoptosis, and activation of these receptors rapidly triggers the apoptotic signaling and effector machinery. One of these receptors is the Fas receptor (FasR) (26), which belongs to the increasing number of receptors in the tumor necrosis factor (TNF) receptor family (60). The FasR is a 48-kDa transmembrane protein (79), the cytoplasmic region of which contains a death domain essential for transducing the apoptotic signal (27). Upon oligomerization, several proteins with distinct functions (33), among them a cytosolic adaptor protein, FADD (8), are recruited to the cytoplasmic domain of the FasR. FADD in turn contains a death effector domain, which binds to the cystein protease caspase 8 (6). This binding results in cleavage and activation of caspase 8 (44). The activated caspase-8 triggers a strictly regulated process, which involves activation of effector caspases, eventually leading to the characteristic signs of apoptosis, such as disruption of normal cell and nuclear morphology, followed by DNA fragmentation (9, 55). However, oligomerization of the FasR also leads to recruitment of proteins other than those directly associated with the caspase effector machinery (55). Consequently, FasR activation has effects other than activation of the caspase cascade. Several recent studies have shown that stimulation of the FasR and TNF receptor I (TNF R1) involves activation of multiple kinases (38, 65, 66), among them the c-Jun N-terminal kinase (78) and mitogen-activated protein kinase (MAPK) (59). The activated FasR also recruits an inhibitory phosphatase (55), and it has been suggested that the phosphatidylinositol 3-kinase (PI-3K) pathway may have a role in regulating the functions of the FasR (25). Taken together, these studies indicate that phosphorylation-based signaling is likely to be involved in some aspects of FasR activation and regulation.
For continued growth and/or differentiation, vertebrate cells depend on survival factors that activate signal transduction pathways suppressing apoptosis. However, the identities and targets of these inhibitory signals have not been ascertained. Defects in apoptotic and antiapoptotic signaling pathways have been implicated in many pathological conditions, including cancer (12). Transformed cells benefit from oncogenes as well as extracellular signals that activate cell proliferation and/or inhibit apoptosis (11). One way for cytotoxic T cells to terminate cancer cells is by Fas ligand (FasL) expression, as the majority of tumor cells do express the FasR. A possible strategy for cancer cells to escape this type of immune system-mediated apoptosis is inhibited FasR expression along with increased expression of the FasL (22, 58, 62). Alternatively, cells may modulate their FasR responses. There are indeed studies indicating that FasR-expressing tumor cells may be completely insensitive to FasR-induced apoptosis (52, 68). Interestingly, some cell lines have even been shown to respond to FasR stimuli by accelerated cell growth (1, 14, 29, 53). A possible signaling candidate which could generate this kind of response is the MAPK pathway. Activation of the MAPK pathway is often associated with increased cell division rates (36) and could thereby also protect tumor cells from apoptosis. Further evidence for this assumption is provided by studies showing elevated MAPK activities in tumor cells (35, 37, 50). The paradigm of MAPK as an inhibitory pathway in regulation of apoptosis is supported by a number of studies showing that activation of MAPK can protect against various apoptotic stimuli in different cell types (9, 16, 77). Furthermore, we have also shown that MAPK specifically protects Jurkat T cells from FasR-induced apoptosis (23). There are several studies indicating that MAPK is activated in a variety of cell types upon TNF R1 stimulation (21, 59, 74). This kind of activation could also apply for FasR signaling and lead to inhibited FasR responsiveness.
In the present study, we examined the role of MAPK signaling in the sensitivity of various tumor cell lines against FasR-induced apoptosis. We were especially interested in possible FasR-mediated MAPK activation and the extent to which inhibition of MAPK, by either pharmacological or molecular biology-derived means, could sensitize the cells to FasR-induced apoptosis. Our results show that MAPK inhibition results in a dramatic increase in FasR sensitivity in the FasR-insensitive cell lines. Furthermore, we show that the MAPK-mediated suppression of FasR-induced apoptosis is protein synthesis independent and occurs at or upstream of the initiating caspase, caspase 8.
MATERIALS AND METHODS
Cell culture.
The mouse cell line NIH 3T3 and the human cell lines HeLa, HL60, Jurkat, and U937 were obtained from the American Type Cell Collection (Rockville, Md.). The HeLa and NIH 3T3 cell lines were cultured in Dulbecco modified Eagle medium (DMEM), whereas the HL60, Jurkat, and U937 cell lines were cultured in RPMI 1640 in a humidified incubator with 5% CO2 in air at 37°C. The cell culture medium was supplemented with 10% inactivated fetal calf serum (FCS), 2 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). HeLa, HL60, and Jurkat cells were incubated with an agonistic anti-human FasR immunoglobulin M antibody (100 ng/ml; Kamiya Biomedical Company, Thousand Oaks, Calif.), whereas NIH 3T3 cells were incubated with an agonistic anti-mouse hamster FasR immunoglobulin G antibody (clone Jo2; Pharmingen, San Diego, Calif.), for the indicated time periods in the absence or presence of 30 μM PD 98059 (Calbiochem, La Jolla, Calif.). HeLa cells were also incubated with 10 nM cycloheximide (CHX; Sigma, St. Louis, Mo.) 10 nM LY294002 (Calbiochem), and 100 nM wortmannin (Calbiochem).
Analysis of nuclear morphology.
For confocal microscopy, cells were cultured on coverslips and subjected to different treatments as previously described (24). After incubation, cells were fixed in 3% formaldehyde in phosphate-buffered saline (PBS), washed once, preincubated with 50 μM RNase A (Sigma), and stained for 30 min with propidium iodide (PI; 10 μg/ml; Molecular Probes, Eugene, Oreg.) in PBS. The cells were washed once with PBS before mounting with Mowiol (Sigma) on coverslips and viewed under a Leica TCS40 confocal laser microscope (Leica, Wetzlar, Germany).
Analysis of DNA fragmentation.
Detection of DNA fragmentation into oligonucleosomal DNA fragments by agarose gel electrophoresis was performed as described elsewhere (61). To detect DNA fragmentation by flow cytometry, purified nuclei were stained with PI and analyzed on a FACScan flow cytometer (Becton Dickinson, Lincoln Park, N.J.) as previously described (15, 49); the results were plotted as means ± standard errors of the means (SEM).
Immunoblotting techniques for analysis of caspase activation and presence of the FasR.
HeLa cells (106/ml) were subjected to various treatments, scraped off the culture plate with a rubber policeman, and washed once with PBS. The cell pellets were lysed in 30 μl of resuspension buffer (150 mM NaCl, 1 mM EDTA, 10 mM Tris HCl [pH 7.6]) containing 10 mM phenylmethylsulfonyl fluoride. The protein concentration of the total cell lysates was determined by the Bradford assay with bovine serum albumin as a standard. The total cell lysates (25 μg) were boiled with Laemmli sample buffer and electrophoresed on a sodium dodecyl sulfate (SDS)–13% polyacrylamide gel. The separated proteins were transferred to a nitrocellulose membrane, probed with antibodies to caspase-3 (gift from Donald Nicholson, Merck Frosst, Quebec, Quebec, Canada) and caspase-8 (Santa Cruz Biotechnology, Santa Cruz, Calif.), followed by the appropriate horseradish peroxidase-conjugated secondary antibodies. Detection was carried out by chemiluminescence (Supersignal; Pierce, Rockford, Ill.) according to manufacturer’s specifications.
MAPK activity assays.
Cells (2 × 106/sample) were lysed with 400 μl of lysis buffer (PBS [pH 7.4], 1% Nonidet P-40, 0.5% sodium deoxycholate, 1 mM Na3VO4, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 20 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 μg each of aprotinin, leupeptin, and pepstatin per ml). For MAPK immunoprecipitation, cell lysates were centrifuged (3,000 × g for 15 min), and the supernatant was incubated with a mouse antibody generated against human p42 MAPK or ERK2 (Transduction Laboratories, Lexington, Ky.) coupled to protein A- or protein G-Sepharose (Sigma). Immunoprecipitates were then washed three times in lysis buffer and three times in kinase assay buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 10 mM MgCl2, 0.5 mM dithiothreitol). The kinase reaction was carried out by adding 20 μl of kinase assay buffer to the immunoprecipitate. The kinase assay buffer included 25 μM ATP, 2.5 μCi of [γ-32P]ATP (Amersham, Buckinghamshire, United Kingdom), and myelin basic protein (MBP; 1 mg/ml; Sigma) as the substrate. The reaction was carried out for 15 min at 37°C and stopped by addition of 3× Laemmli sample buffer. The samples were resolved on an SDS–12.5% polyacrylamide gel, and MBP phosphorylation was quantified with a phosphorimager (Bio Rad Laboratories, Hercules, Calif.). In parallel with the immunocomplex kinase assays, we determined the amount of ERK2 in the immunoprecipitates by using a rabbit antibody specific for the human ERK1- and -2 isoforms (New England Biolabs, Boston, Mass.). After coupling to a secondary antibody (Zymed, San Francisco, Calif.), the proteins were visualized with the Amersham ECL (enhanced chemiluminescence) system.
Expression of mutated forms of MKK1.
Cells were transiently transfected by electroporation (200 V, 960 μF), washed twice with DMEM, and allowed to rest for 20 h before treatment. The DNA constructs used were pMCL-HA-MKK1-S218E/S222D and pMCL-HA-MKK1-K97M, expressing hemagglutinin (HA)-tagged constitutively active (S218E/S222D) and dominant negative (K97M) forms of the MAPK kinase (MKK1) (39, 40), respectively. In transient cotransfection experiments, 20 μg of an HA-tagged wild-type ERK2 construct (pMCL4-wtERK; a kind gift from Melanie Cobb, Texas Southwestern Medical Center, Dallas) was used together with 20 μg of S218E/S222D or K97M MKK1 before immunoprecipitation with a monoclonal HA-specific antibody (12CA5; Boehringer, Mannheim, Germany), subsequently followed by the MAPK activity assay.
For some experiments, we used a cell line with inducible expression of constitutively active MKK1. This cell line was established as follows. The constitutively active HA-tagged MKK1-S218E/S222D cDNA was cloned into the NotI/SalI sites of plasmid pBI-4 (3) under the control of the tetracycline transactivator (tTA)-regulated cytomegalovirus promoter. HeLa cells were first transfected with both pUHD15-1, coding for tTA (19), and pTK-Hyg (Clontech, Palo Alto, Calif.). Clones were selected for 2 weeks on hygromycin (250 μg/ml; Calbiochem) and submitted to luciferase assay after transient transfection of the reporter construct pBI-1 (3). Positive HeLa-tTA clones were simultaneously transfected with the pBI-4/MKK1-S218E/S222D construct and the pSV2NEO marker. Colonies were grown in tetracycline (1 μg/ml; Sigma), selected 2 weeks on G418 (500 μg/ml; Calbiochem), and cloned by limiting dilution. Positive double stable clones were assayed 48 h after removal of tetracycline by HA immunostaining and Western blotting with antibodies against HA or phospho-MAPK (data not shown). In this cell line, we have, after depletion of tetracycline, routinely observed 20 to 30% HA-MKK1-positive cells. We do not know why we do not get 100% penetrance of HA-MKK1 expression.
For detection of transfected cells, the cells were fixed for 30 min with 3% formaldehyde in PBS. The cells were then washed once with PBS and permeabilized with 0.1% Nonidet P-40 (Sigma) for 10 min at room temperature. After washing with PBS, cells were incubated for 2 h at room temperature with 10 μg of a monoclonal HA-specific antibody (12CA5; Boehringer Mannheim) per ml in PBS with 1% bovine serum albumin (Sigma). Cells were then washed three times with PBS and incubated further for 1 h at room temperature with tetramethyl rhodamine isothiocyanate-conjugated anti-mouse secondary antibody (Zymed) and 10 mg of Hoechst 33342 (Molecular Probes) or 10 μg of PI per ml in PBS with 1% bovine serum albumin. Cells were mounted in 50% glycerol (Sigma) and viewed under a Leica RMB epifluorescence microscope. For confocal images, cells were stained for HA as described above, and the DNA was visualized by PI staining and viewed under a Leica TCS40 confocal laser microscope.
RESULTS
Inhibition of MAPK signaling sensitizes HeLa cells to FasR-induced apoptosis.
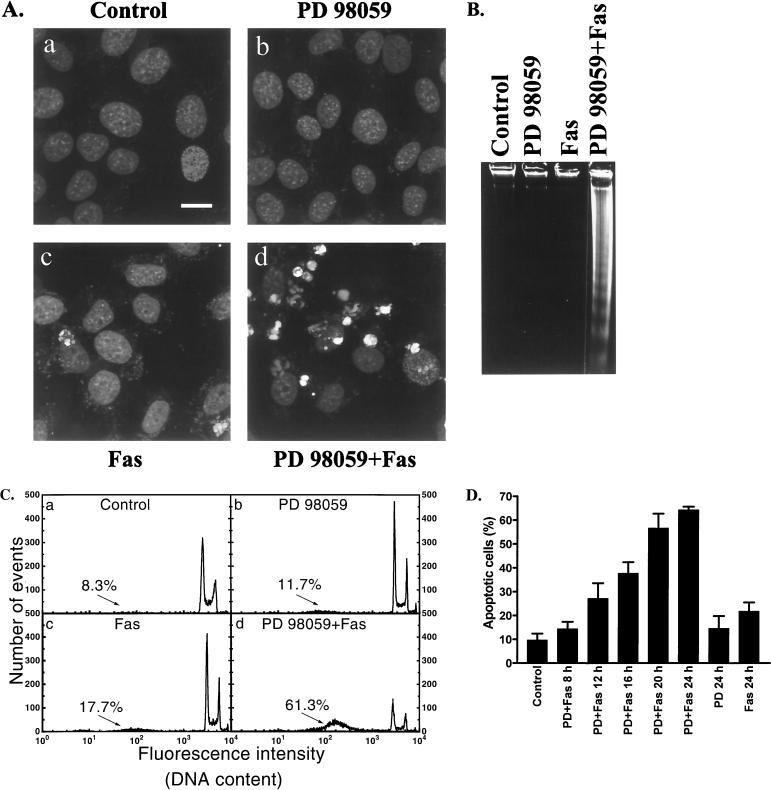
As mitogenic signaling through the MAPK cascade has been shown to have an inhibitory effect on the induction of apoptosis, we wanted to determine whether MAPK-mediated signaling could be involved in protection of FasR-expressing cells that are normally insensitive to FasR activation. To examine if inhibition of MAPK signaling would affect FasR-induced apoptosis, we pretreated HeLa cells with the specific MKK1 inhibitor PD 98059 (2) for 30 min before addition of the agonistic anti-Fas antibody for the indicated time periods. Normally, these cells were not sensitive to stimulation of the FasR, as both control cells and cells incubated with the agonistic FasR antibody displayed a normal chromatin pattern after staining with PI (Fig. 1A). In contrast, cells pretreated with PD 98059 before addition of the agonistic FasR antibody displayed apoptosis-specific alterations of the chromatin structure, including condensation and fragmentation of nuclei (Fig. 1). Treatment with PD 98059 alone did not induce apoptosis in these cells (Fig. 1). The sensitizing effect of PD 98059 was further confirmed by conventional agarose gel electrophoresis, showing the distinct apoptotic DNA laddering in HeLa cells pretreated with PD 98059 before addition of anti-FasR antibody, whereas no effects were observed with PD 98059 or FasR antibody alone (Fig. 1B).
FIG. 1.
Specific inhibition of MAPK signaling sensitizes HeLa cells to FasR-induced apoptosis. HeLa cells, which normally are not responsive to FasR stimulation, were preincubated with the specific MKK1 inhibitor PD 98059. This caused an almost complete sensitization to FasR stimulation. (A) Representative confocal micrographs of cells incubated for 24 h with medium alone (a) or in the presence of 30 μM PD 98059 (b) 100 ng of anti-FasR antibody per ml (c), and 30 μM PD 98059 plus 100 ng of anti-FasR antibody per ml (d). Nuclear alterations were visualized by PI staining. The micrographs show that nuclei of apoptotic cells are fragmented into apoptotic bodies with intense staining, whereas the nuclei of normal interphase cells show a uniform and dimer staining. Bar = 10 μm. (B) The formation of oligonucleosome-sized DNA fragments in cells subjected to the same treatments as above was studied by agarose gel electrophoresis. (C) Apoptotic DNA fragmentation was determined in PI-stained nuclei by flow cytometric analysis. (D) Analysis of the time course of FasR-induced apoptosis in HeLa cells in the presence of PD 98059. After treatments, aliquots of cells were stained with a hypotonic PI solution, and the proportion of apoptotic nuclei was determined with a FACScan flow cytometer. The data represent means ± SEM from a minimum of three separate experiments.
To quantify the amount of apoptotic cells at different time points and to compare the induction of DNA fragmentation by different treatments, we used flow cytometric (fluorescence-activated cell sorter [FACS]) analysis of isolated nuclei as detected with the DNA stain PI (49). The measurement is based on the fact that apoptotic nuclei show a lower DNA content due to leakage of DNA fragments from the nuclei. The amount of apoptotic cells is indicated by a large subdiploid peak on the FACS histogram, the events of which represent apoptotic nuclei and nuclear fragments (Fig. 1C). Cells treated with PD 98059 for 24 h did not show any significant induction of apoptosis compared to the control (Fig. 1C and D). In samples pretreated with PD 98059 before addition of anti-FasR antibody, the number of apoptotic cells started to rise after incubation for 8 to 12 h and was approximately 65 to 70% after 24 h. In contrast, cells incubated with the anti-FasR antibody alone displayed only a minor increase in the number of apoptotic cells (Fig. 1D).
Inhibition of mitogenic signals by serum starvation potentiates the effects of PD 98059.
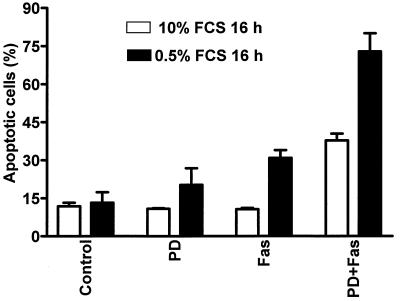
The major source of growth signals in cultured cells is supplied by the addition of serum to the cell culture medium. To investigate if a general inhibition of growth signals could affect FasR-induced apoptosis, we preincubated HeLa cells for 1 h in a medium containing 0.5% FCS before addition of agonistic anti-FasR antibody and analyzed the samples for apoptotic cells by flow cytometry. These analyses were carried out at 16 h, as the potentiating effects were most obvious at the initial stages of the triggering process (data not shown). Serum-starved cells were clearly more sensitive to FasR stimulation than cells grown with 10% FCS (Fig. 2). Serum starvation also induced a potentiation of the PD 98059-induced FasR sensitization at 16 h, as the number of apoptotic cells was approximately doubled in FasR-stimulated cells preincubated both with low serum and in the presence of PD 98059 (Fig. 2).
FIG. 2.
Inhibition of mitogenic stimuli by serum starvation increases the FasR responsiveness in HeLa cells. HeLa cells were incubated for 16 h in medium containing 0.5 or 10% FCS and in the presence of PD 98059 (30 μM), anti-FasR antibody (100 ng/ml), or PD 98059 (30 μM) plus anti-FasR antibody (100 ng/ml), and the number of apoptotic cells was determined by flow cytometric analysis as for Fig. 1C. The data represent means ± SEM from a minimum of three separate experiments.
Transient transfection with dominant negative MKK1 confirms the involvement of MAPK signaling in FasR insensitivity.
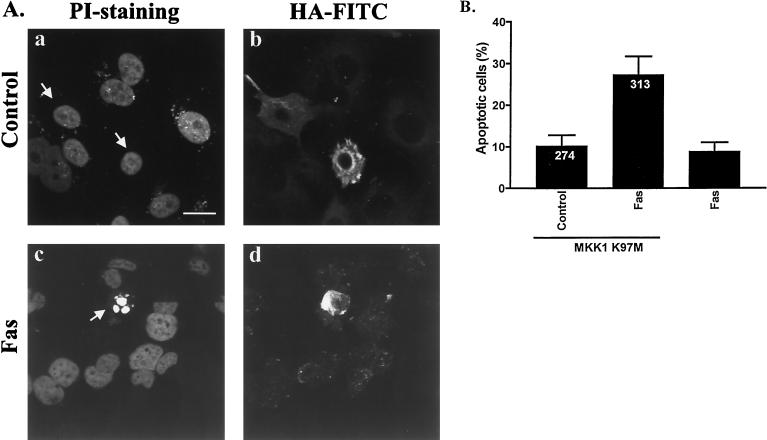
To further demonstrate that the observed PD 98059-mediated effect relates to signaling by the MAPK cascade, we transfected HeLa cells transiently with a dominant negative mutant of MKK1. While the dominant negative (K97M [39, 40]) form of MKK1 by itself did not elevate the number of apoptotic cells among the transfected cell population, it clearly made the transfected cells more sensitive to FasR-induced apoptosis (Fig. 3). Note that the incubation time in these experiments was 16 h, as the longer 24-h incubations resulted in loss of apoptotic cells from the coverslips. These results further corroborate the assumption that inhibition of MAPK is a key feature of the sensitization to FasR-induced apoptosis.
FIG. 3.
Transfection with dominant negative MKK1 sensitizes HeLa cells to FasR-induced apoptosis. (A) Representative immunofluorescence micrographs of cells transfected with a dominant negative (MKK1 K97M) MKK1 construct and incubated for 16 h in the absence or presence of anti-FasR antibody. PI staining (a and c) was used to detect alterations in the nuclei, and a monoclonal anti-HA antibody linked to an FITC-conjugated secondary antibody (b and d) was used to detect the presence of the HA-tagged MKK1 in transfected cells. The arrows indicate transfected cells. Bar = 10 μm. (B) Percentage of apoptosis in transfected cells after treatment with an anti-FasR antibody. Number on bars indicate how many transfected cells were counted. The data represent means ± SEM from a minimum of three separate experiments. Note that the incubation time with the agonistic FasR antibody is 16 h.
MAPK-mediated resistance to FasR-induced apoptosis is not dependent on protein synthesis.
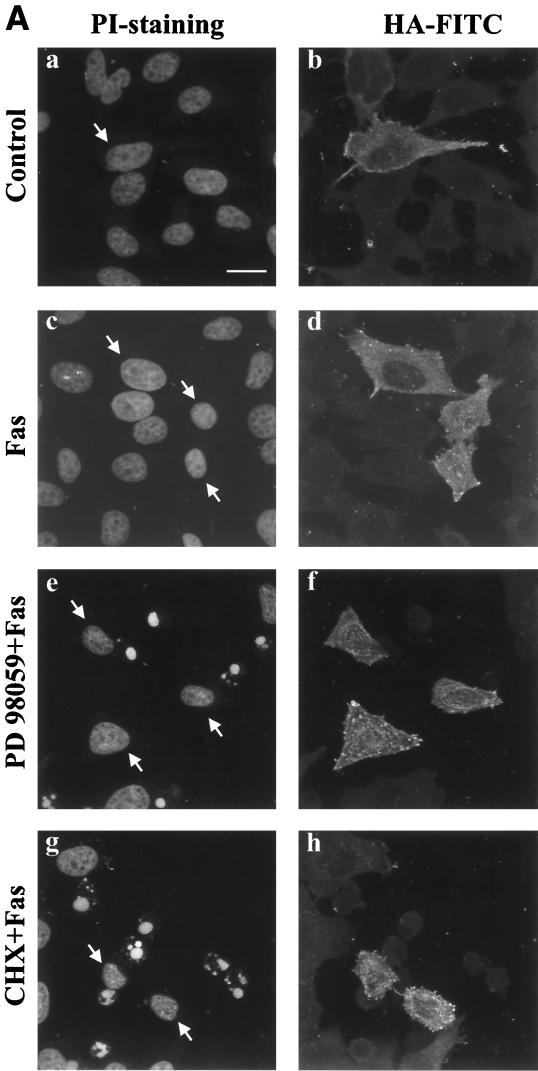
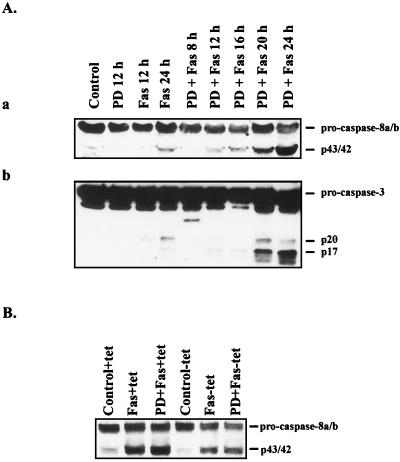
Many cells that are normally resistant to FasR or TNF R1-induced apoptosis can be sensitized by inhibition of protein synthesis (50, 69). Since we observed that inhibition of MAPK signaling sensitizes HeLa cells to FasR-induced apoptosis, we wanted to determine whether the MAPK-generated protection requires protein synthesis. It is plausible that inhibition of MAPK activation could inhibit the synthesis of some crucial inhibitor proteins. For these experiments, we used a HeLa cell line with inducible expression of a constitutively active mutant form of MKK1 (S218E/S222D [39, 40]) and pretreated the cells with CHX before stimulation of the FasR. By using this cell line, we also wanted convincingly confirm that the sensitization obtained with PD 98059 was indeed MAPK dependent by determining whether the sensitization could be reversed by the constitutively active MKK1, which should not be affected by this inhibitor. When cells had been incubated for 48 h in the absence of tetracycline, 20 to 30% of the cells were positive for the mutant MKK1, as indicated by HA immunoreactivity (Fig. 4). Immunoblotting with phospho-MAPK antibodies confirmed that the cell line showed increased MAPK activity along with increasing HA-MKK1 expression (data not shown). The PD 98059-induced effect was completely reversed in cells positive for constitutively active HA-MKK1 (Fig. 4). Furthermore, HA-MKK1-positive cells were efficiently protected against CHX-mediated sensitization in FasR-stimulated cells (Fig. 4). Identical results were obtained with transient transfections with the constitutively active MKK1 (Fig. 4B). Taken together, these results indicate that the MAPK-generated protection is not likely to be protein synthesis dependent.
FIG. 4.
Constitutively active MKK1 inhibits FasR-induced apoptosis in the presence of the protein synthesis inhibitor CHX. (A) An HeLa cell line with the constitutively active MKK1 (MKK1 S218E/S222D) in a tTA-regulated expression vector was incubated for 48 h in the absence of tetracycline to yield maximal MKK1 expression. To assess the effect of MKK1-induced MAPK activation on FasR-mediated apoptosis, cells were incubated for 16 h in the absence (a and b) or presence (c to h) of anti-FasR, and with preincubation with PD 98059 (e and f) or CHX (g and h). Arrows indicate HA-MKK1-positive cells. Bar = 10 μm. (B) The effect of expression of constitutively active MKK1 was quantified by counting apoptotic and nonapoptotic transfected cells viewed under an epifluorescence microscope. The data represent means ± SEM from a minimum of three separate experiments. Numbers on bars indicate total of counted cells. Note that the incubation time with the different compounds was 16 h. Similar results were obtained both by transient transfections and with the inducible MKK1 construct.
Inhibition of PI-3K does not sensitize HeLa cells to FasR-induced apoptosis.
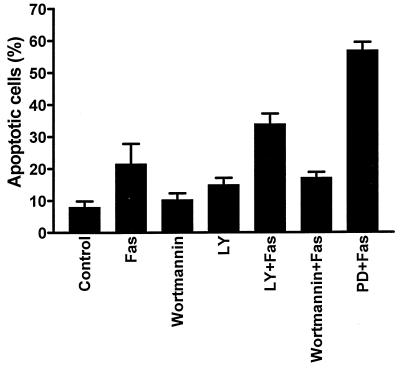
Since activation of PI-3K has been shown to inhibit different forms of apoptosis (10, 30, 33, 34), we investigated if PI-3K could be involved in the protection of FasR-insensitive cells. HeLa cells were preincubated for 30 min with the specific PI-3K inhibitors LY294002 and wortmannin before addition of anti-FasR antibody. Determination of the number of apoptotic cells after 24 h by FACS analysis indicated that neither LY294002 nor wortmannin sensitized HeLa cells to FasR-induced apoptosis (Fig. 5). Thus, it appears that PI-3K signaling is not involved in the observed protection of HeLa cells against FasR-induced apoptosis.
FIG. 5.
Inhibition of PI-3K by LY294002 and wortmannin does not sensitize HeLa cells to FasR-induced apoptosis. HeLa cells were treated for 24 h with LY294002 (LY; 10 μM), wortmannin (100 nM), LY294002 plus anti-FasR antibody (100 ng/ml), wortmannin plus anti-FasR antibody, anti-FasR antibody, or PD 98059 (PD; 30 μM) plus anti-FasR antibody. The degree of apoptosis was quantified by flow cytometric analysis of apoptotic nuclei. The data represent means ± SEM from a minimum of three separate experiments.
The MAPK-dependent FasR insensitivity is maintained upstream from the caspase effector machinery.
A key feature of FasR-mediated apoptosis is activation of different caspases. All caspases are activated by cleavage of inactive proforms (42). To assess if the MAPK-mediated inhibition occurs at the initial phases of FasR signaling, we analyzed the cleavage and activation of caspase 8 and caspase 3 by immunoblotting with specific antibodies. Caspase 8 is the initiator of the FasR-induced apoptotic effector and signaling machinery. This protein exists as 55- and 54-kDa inactive proforms that are cleaved and activated when recruited by adaptor proteins to the oligomerized FasR complex (43, 47). The antibody that we used for caspase 8 recognizes both of the 55- and 54-kDa proforms of caspase 8 as well as the intermediate cleavage form corresponding to ∼43/42 kDa. By using the fragmentation pattern as an indicator of activation, we could observe a clear caspase 8 cleavage after 12 h in FasR-stimulated HeLa cells incubated in the presence of PD 98059 (Fig. 6Aa). With this treatment, the caspase 8 cleavage products had dramatically accumulated after 24 h of incubation. Caspase 3 has been shown to be activated during the earliest phases of apoptotic induction (59). The 32-kDa precursor protein of caspase 3 is rapidly cleaved via an intermediate step into two subunits of 12 and 17 kDa (57). The antibody that we used for detection of caspase 3 recognizes the 32-kDa proform, the 20-kDa intermediate, and the 17-kDa subunit of activated caspase 3. When cell extracts were immunoblotted with this antibody, the first signs of accumulation of the activated 17-kDa subunit could be observed 12 h following FasR stimulation in PD 98059-treated cells (Fig. 6Ab). In contrast to the PD 98059-sensitized cells, treatment with anti-FasR antibody alone induced a minor cleavage and activation of caspase 8 and caspase 3 after 24 h (Fig. 6A), corresponding to the small number of apoptotic cells seen after this treatment. The activation kinetics of both the initiator, caspase 8, and the effector, caspase 3, corresponds very well to the time course of apoptosis induction in these cells. As FasR-stimulated HeLa cells incubated in the absence of PD 98059 showed only an insignificant activation of caspases, the observed MAPK-mediated protection seemed to occur at the level of or upstream of caspase 8. To confirm this assumption, we used the above-mentioned HeLa cell line with inducible expression of the constitutively active form of MKK1 (S218E/S222D) and tested the MKK1-positive cells for activation of caspase 8. The cells were incubated in the presence of FasR-stimulating antibody and PD 98059 for 24 h. As the penetrance in this cell line is relatively low (20 to 30%), most of the cells detached during this treatment. However, the MKK1-positive cells remained attached and viable, as shown in Fig. 4. The samples from the attached MKK1-positive cells showed a clearly suppressed activity of the triggering factor, caspase 8 (Fig. 6B). Since the attached cells also contained a contaminating fraction of apoptotic cells that could not be shaken off, the attached cells showed some degree of caspase-8 activation (Fig. 6B). The inducible cell line seemed to be somewhat more sensitive to FasR stimulation (in the absence of PD 98059) than normal HeLa cells, as there was a more pronounced caspase 8 cleavage in these cells in the presence of tetracycline (Fig. 6B) than in the parent cell line. We have obtained identical results with adenovirus-based gene delivery of the constitutively active MKK1 (S218E/S222D), which very efficiently suppressed FasR-induced caspase 8 activation in HeLa cells sensitized with PD 98059 (67). Taken together, these experiments indicate that the MAPK-mediated protection occurs upstream of caspase 8 activation.
FIG. 6.
Caspase 8 and caspase 3 activities in FasR-stimulated cells are affected by modulation of MAPK activity. (A) To analyze the kinetics of FasR-induced caspase activation in the presence and absence PD 98059, HeLa cells were treated as outlined in the legend to Fig. 1. Caspase activation can be observed as the appearance of active fragments of the caspase proforms of caspase 8 (a) and caspase 3 (b) (see text for details). No significant activation occurred in the absence of PD 98059. (B) To test the effect of MAPK activation on the activity of the apoptotic initiator, caspase 8, the cell line with the constitutively active MKK1 (MKK1 S218E/S222D) was incubated for 3 days in the presence or absence of tetracycline and then treated for 24 h as described for Fig. 1. The MKK1-negative cells detached during this treatment and were washed off. The remaining cells were scraped off the culture flasks, centrifuged, washed, and lysed prior to immunoblotting with antibodies to caspase 8 as described in Materials and Methods. Each well was loaded with 25 μg of protein.
Inhibition of the MAPK cascade does not affect the abundance or distribution of the FasR.
One possible mechanism by which MAPK inhibition could affect the Fas response is by altering the levels of accumulated FasR protein or by changing the cellular compartmentalization of the receptor. To exclude the possibility that inhibition of mitogenic stimuli could affect the levels of receptor protein, we analyzed the amounts of protein by Western blotting of whole-cell extracts and the presence of the FasR on the cell surface by FACS analysis in PD 98059-treated samples. Neither the amounts of accumulated FasR nor the FasR levels on the cell surface were altered by PD 98059 treatment (data not shown). These results exclude alterations in FasR levels or organization as a mechanism behind the sensitization of HeLa cells to FasR-induced apoptosis.
Stimulation of the FasR activates MAPK.
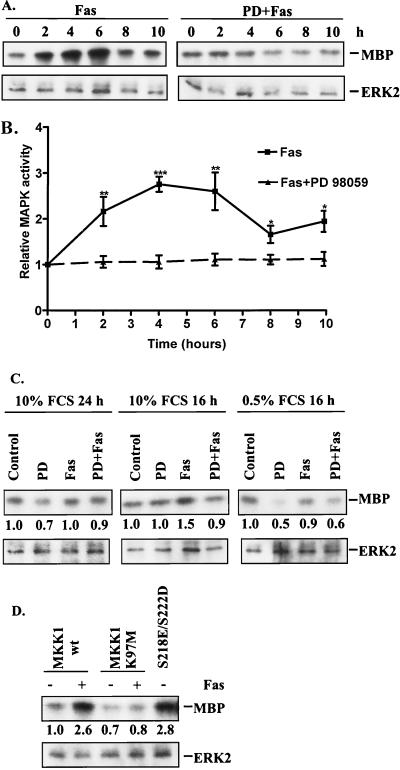
The synthetic MKK1 inhibitor PD 98059 has been shown to inhibit MKK1 activation induced by Raf, probably by competitive inhibition of Raf binding to the activation site on MKK1 (2). Since PD 98059 does not inhibit MKK1 activity per se, we wanted to investigate if stimulation of the FasR could activate MAPK. This kind of activation could provide an explanation why a compound such as PD 98059 is so efficient in rendering cells sensitive to FasR-induced apoptosis. MAPK activity was analyzed by an immunocomplex kinase assay. The results show that stimulation of HeLa cells with anti-FasR antibody induced a clear increase in MAPK activity starting 2 h after stimulation of the FasR and lasting up to 8 to 10 h after stimulation (Fig. 7A). The increase in MAPK activity peaked at 4 to 6 h, being approximately 2.5-fold higher than the control values (Fig. 7B). This activation was not due to increased synthesis of MAPK, as the MAPK protein levels remained constant following FasR stimulation (Fig. 7A). The FasR-induced MAPK activation was completely inhibited by pretreatment of HeLa cells with 30 μM PD 98059 before addition of anti-FasR antibody (Fig. 7A and B).
FIG. 7.
Stimulation of the FasR activates MAPK in HeLa cells. The effect of FasR stimulation on MAPK activity was followed 10 h after addition of the agonistic FasR antibody in cells incubated in the presence or absence of PD 98059. (A) The activation was measured by an immunocomplex kinase assay with MBP as a substrate. To confirm equal loading of MAPK and to check whether the observed effect could be due to changes in the amounts of MAPK, the immunoprecipitates were immunoblotted for the presence of ERK2 protein. A representative autoradiograph and an ERK2 immunoblot of the immunocomplex kinase assay are shown. The immunocomplex kinase assay with MBP as the substrate showed that MAPK is significantly activated by FasR stimulation, without any detectable changes in MAPK protein levels. (B) Quantification from multiple parallel samples of the relative MAPK activities (control = 1) at different time points was performed by phosphorimager analysis of the MBP-associated 32P labeling. The data represent means ± SEM from a minimum of five separate experiments. The statistical significance between Fas- and PD 98059-plus-Fas-treated samples was tested by Student’s t test (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001). (C) The effect of FasR stimulation and treatment with PD 98059 was also analyzed in cells cultured in medium containing 0.5 or 10% FCS and treated in the same way as for Fig. 2. Numbers below the lanes indicate relative MAPK activity (control = 1). ERK2 immunoblotting was used to confirm equal loading. Serum starvation seemed to amplify the PD 98059-induced MAPK inhibition. (D) The efficacy of transfection with dominant negative (K97M) and constitutively active (S218E/S222D) MKK1 on the FasR-induced MAPK activation and on overall MAPK activity was tested by MAPK activity measurements of cells cotransfected with HA-tagged ERK2. The HA-ERK2 was immunoprecipitated with an HA-specific antibody, and the immunocomplex assays were carried out as indicated above. Western blotting of the immunoprecipitates with an ERK2-specific antibody was used to confirm equal loading. wt, wild type.
To further relate the degree of MAPK activity to the observed FasR resistance, we wanted to determine the efficacy of our attempts to modulate MAPK activity by the other methods used, apart from employing PD 98059. In Fig. 2, we observed that serum starvation induced some degree of sensitization and enhanced the effect of PD 98059. MAPK activity measurements of cells treated as in Fig. 2 showed that the serum starvation to some extent abrogated the FasR-induced MAPK activation on its own (Fig. 7C). The combination of serum starvation and treatment with PD 98059 abrogated the FasR-induced MAPK activation even more efficiently (Fig. 7C). Hence, although these results were obtained at the later stages of exposure and by the time at which the differences in MAPK activities have leveled, it seems that the effect of serum starvation is likely based on its inhibition of MAPK activity. In Fig. 3 and 4, transfection and inducible expression of dominant negative (K97M) and constitutively active (S218E/S222D) forms of MKK1 were used to modulate MAPK activities. We could verify the efficacy of these constructs in modulating MAPK activity, as cotransfection with HA-tagged ERK2 showed that the activities of the immunoprecipitated HA-ERK2 were significantly affected by both constructs (Fig. 7D). Furthermore, the FasR-induced MAPK activation could be abolished with the dominant negative form of MKK1 (Fig. 7D).
Resistance to FasR-mediated apoptosis correlates with induction of MAPK activity in different cell lines.
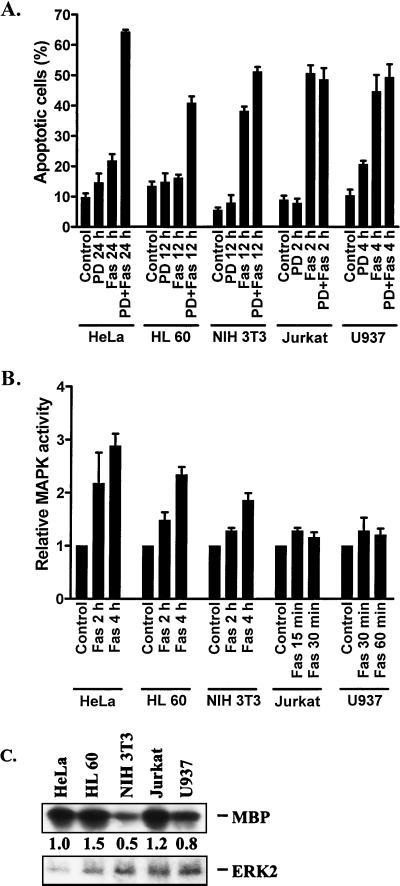
To correlate the PD 98059-induced sensitization to FasR-mediated apoptosis with induction of MAPK activity and to further confirm that the observed sensitization in HeLa cells is not a cell-type-specific phenomenon, we treated HL60, NIH 3T3, Jurkat, and U937 cells (Fig. 8A and B) with anti-FasR antibody with or without PD 98059, in the same manner as with HeLa cells. All of these cell lines showed different degrees of FasR sensitivity. In accordance with HeLa cells, HL60 cells were resistant to FasR stimulation. Anti-FasR antibody treatment for 12 h did not induce apoptosis in these cells. However, approximately 45% of HL60 cells pretreated with PD 98059 were apoptotic (Fig. 8A). NIH 3T3 cells were moderately sensitive to FasR stimulation. When these cells were incubated for 24 h with mouse anti-FasR, approximately 40% of the cells were apoptotic (Fig. 8A). However, pretreatment of NIH 3T3 cells with PD 98059 before addition of anti-FasR antibody increased the amount of apoptotic cells to approximately 55% (Fig. 8A). Both Jurkat and U937 cells are known to be sensitive to FasR-mediated apoptosis; correspondingly, incubation of Jurkat cells for 2 h and U937 cells for 4 h with anti-Fas antibody induced apoptosis in 50% of the cells (Fig. 8A). In Jurkat and U937 cells, pretreatment with PD 98059 did not affect the sensitivity to FasR-mediated apoptosis (Fig. 8A). To examine how the degree of apoptosis relates to the degree of MAPK activation following FasR stimulation, we measured the MAPK activity after addition of anti-Fas antibody (Fig. 8B) in the different cell types. HeLa cells showed the strongest response, with up to a threefold activation after 4 h. Treatment of HL60 cells with anti-Fas antibody increased MAPK activity almost 2.5-fold compared to untreated cells after 4 h, whereas the increase in MAPK activity was approximately twofold in NIH 3T3 cells (Fig. 8B). In Jurkat and U937 cells, treatment with anti-Fas antibody induced only a minimal MAPK activity, being approximately 1.2-fold after 15 to 30 min (Fig. 8B). The MAPK activation was not elevated after these time points (data not shown). The earlier time points, presented for Jurkat and U937 cells, were chosen to reflect the faster kinetics of apoptotic induction in these cells than in the other cell types.
FIG. 8.
Correlation between FasR-induced MAPK activity and resistance to FasR-mediated apoptosis in different cell lines. HeLa, HL60, NIH 3T3, Jurkat, and U937 cells were incubated for the indicated time periods with medium alone and in the presence of anti-FasR antibody, PD 98059 (PD; 30 μM), and PD 98059 plus anti-FasR antibody (100 ng of anti-human FasR per ml was used for all cell lines except NIH 3T3, which was incubated with 10 μg of Jo2 anti-mouse FasR per ml). The degree of apoptosis was quantified by flow cytometric analysis of apoptotic nuclei (A), and MAPK activity from multiple parallel samples was quantified by phosphorimager analysis of the MBP-associated 32P-labeling (B). (C) The basal MAPK activity in each cell line was measured by immunocomplex assays as indicated above. Western blotting of the immunoprecipitates with an ERK2-specific antibody was used to confirm equal loading. The results show that the degree of MAPK activation upon FasR stimulation correlates with the degree of FasR resistance, whereas the basal activity of each cell line does not correlate with the sensitivity to FasR-mediated apoptosis. The data represent means ± SEM from a minimum of three separate experiments.
The induction of MAPK activity correlated well with the degree of FasR sensitization observed with PD 98059 pretreatment. Both of the cell lines (Jurkat and U937) showing only minor MAPK activation upon FasR stimulation were highly sensitive to FasR stimulation even without PD 98059 treatment (Fig. 8A and B). The HeLa, HL60, and NIH 3T3 cells were all clearly sensitized to FasR-mediated apoptosis in the presence of PD 98059. Furthermore, in the latter three cell lines, the degree of FasR-mediated apoptosis following treatment with PD 98059 showed a strong negative correlation to the degree of FasR-induced MAPK activation in the absence of PD 98059 (Fig. 8A and B).
We examined whether the differences between the tested cell lines could be due to differences in the basal activities of MAPK. When equal amounts of MAPK from the different cell lines were used for immunocomplex kinase assays, HeLa, Jurkat, and U937 cells showed similar basal kinase activities (Fig. 8C). The HL60 and NIH 3T3 cells showed somewhat higher and lower activities, respectively, than the other cell lines. However, observed small differences in the basal MAPK activities did not correlate with the degree of FasR insensitivity. Hence, the crucial parameter in regulation of FasR responses seems to be the FasR-induced activation of MAPK, not the basal MAPK activity.
DISCUSSION
Inhibition of mitogenic signals sensitizes cells to FasR-induced apoptosis.
Mitogenic signals are usually associated with induction of cell growth or differentiation (36). However, recent studies have indicated that such signals may also be involved in inhibition of apoptosis. In this respect, it has been shown that activation of two signaling cascades involved in mitogenic stimuli, namely, the MAPK (9, 16, 23, 77) and PI-3K (10, 30, 33, 34) signaling pathways, are able to suppress apoptosis in different cell lines. Our data show that inhibition of MAPK signaling by a specific MKK1 inhibitor, by serum withdrawal, and by transfection with dominant negative MKK1 renders HeLa cells susceptible to FasR-induced apoptosis. Furthermore, we show that in HeLa, HL60, and NIH 3T3 cells, the FasR is able to induce a significant MAPK activation. This kind of MAPK activation is likely to be involved in protecting cells from FasR-induced apoptosis. These results could also provide an explanation for why some tumor cells expressing the FasR are resistant to FasR-induced apoptosis (52, 68). Furthermore, our observations may explain why stimulation of the FasR with agonistic FasR antibodies has been associated with stimulated cell growth in some cell lines (1, 14, 29, 53). Recent reports have indicated the PI-3K signaling cascade as a major antiapoptotic pathway (13, 41). Our results indicate that the PI-3K pathway is not involved in the MAPK-dependent regulation of FasR responses that we observed.
The FasR is able to generate a MAPK-mediated signal which suppresses activation of caspases.
Our data quite conclusively show that the FasR is in some cells able to generate a dominant survival signal. All of the included studies indicate that the activation of MAPK induced by the FasR is the key factor in the MAPK-mediated protection, not the levels of basal MAPK activity. This result, showing that the FasR would be able to generate its own survival signal, is rather surprising. There have been indications that the FasR could activate the Ras pathway and that this activation would have an apoptosis-promoting function (19). However, a later study suggested that the Ras effect could perhaps be accounted for by Rac (20). This could explain the seeming contradiction between these studies and our study. The suggested mandatory role of Ras would relate not to a requirement of the MAPK cascade for apoptotic FasR responses but to some other Rac-induced signaling pathway. Furthermore, our results do not favor the hypothesis of a requirement of MAPK signaling in apoptotic FasR responses, as transfections with the dominant negative MKK1 had only a sensitizing effect without any inhibitory effect.
In the present study, the increased sensitivity to FasR-induced apoptosis by MAPK inhibition could be detected as caspase 8 and caspase 3 activation. Conversely, untreated cells displayed only a minor caspase activation upon FasR stimulation, which was proportional to the small number of apoptotic cells that were detected under these conditions. Furthermore, cells expressing constitutively active MKK1 also showed abrogated caspase 8 activity when they were stimulated with PD 98059 and a FasR-specific antibody. These results are in accordance with our previous results (23), indicating that MAPK-mediated inhibition of FasR-induced apoptosis in Jurkat cells occurs upstream or at the level of caspase activation. Activation of caspase 8 has been considered to be the first caspase activated following FasR stimulation with the subsequent assembly of the death-inducing signaling complex (44). Our results imply that in cells insensitive to FasR-induced apoptosis, stimulation of the FasR averts the FasR-mediated signal from the caspase effector machinery. In cells insensitive to FasR-induced apoptosis, FasR stimulation results in a significant MAPK activation, instead of activation of the caspase cascade. The resulting MAPK activation seems to interfere with some critical function required for caspase activation. The inhibition of apoptosis at early steps in the apoptotic pathway, rather than at some intermediate stages in the caspase cascade, is likely desirable, as only in this way cells could escape from any detrimental effects of a partially activated apoptotic effector machinery.
Possible downstream targets of MAPK.
The MAPKs are known to phosphorylate many different proteins involved in various signaling and regulatory pathways as well as in transcriptional regulation (72). While the role of MAPKs in regulation of transcription factors has received special attention, there is much less information on MAPK-induced phosphorylation as a bona fide regulatory factor in determining the functions of cytosolic targets. Phosphorylation of the epidermal growth factor receptor by the MAPK pathway has been suggested to down-regulate the receptor tyrosine kinase activation complex (51, 74). According to this scheme, MAPK activation would function as an autoregulatory loop for signaling through the epidermal growth factor receptor (18, 47). Interestingly, our results point to a similar autoregulatory loop, whereby the FasR would generate an intervention of its own apoptotic signaling by activation of MAPK. However, our experimental setup is not able to distinguish whether MAPK signaling affects the receptor complex directly or indirectly. An attractive scenario is that the target for the MAPK-mediated regulation of FasR responses will be found among the protein(s) in the death-inducing signaling complex. We are currently testing whether the MAPK-mediated signal can affect the phosphorylation state of some of the proteins required for caspase activation. For example, both FasR (31) and FADD have been shown to be phosphorylated (31, 32, 80) and hence possible targets for this kind of regulation. Another possibility is that some known or unknown inhibitory protein, with constitutive expression in the cells, is activated by a phosphorylation event generated by the FasR-induced MAPK signal.
MAPK-mediated protection of FasR-induced apoptosis is not dependent on protein synthesis.
It has been well established that many different cell types that are resistant to FasR- or TNF R1-induced apoptosis can be made sensitive when the cells are preincubated with protein synthesis inhibitors such as CHX. In view of these observations, it has been suggested that FasR and TNF R1 can elicit a protein synthesis-dependent signal (69), which would be distinct from the apoptotic signal. A number of studies also suggest an inhibitory effect of viral as well as cellular proteins such as p35 (5), CrmA, (63), NF-κB (4, 70, 71), Bcl-2 (28, 56), inhibitor of apoptosis proteins (43, 54) c-FLIP (25), and v-FLIP (64) in FasR-induced apoptosis. On the other hand, there is evidence for protective signals occurring postranslationally through phosphorylation-based signaling pathways such as MAPK (9, 16, 23, 77) and PI-3K (10, 30, 33, 34). In the present study, CHX-treated HeLa cells transfected with constitutively active MKK1 were not sensitized to FasR-induced apoptosis. This result precludes the requirement for protein synthesis in protection against FasR-induced apoptosis. This hypothesis of a posttranslationally induced modulation of FasR signaling is corroborated by our previous study, showing that MAPK activation in the presence of CHX protects Jurkat cells efficiently against FasR-induced apoptosis (23). Thus, a high constitutive MAPK activity seems to be sufficient for inhibition of the FasR-mediated apoptotic signal. Taken together, our results strongly support the hypothesis of direct phosphorylation-based modulation of FasR-mediated signaling.
The possible role of MAPK in resistance to FasR-induced apoptosis.
During development, cells most likely need to modulate their responsiveness to apoptosis-inducing cytokines, such as FasL and TNF-α. Direct modulation by phosphorylation-based signaling could have evolved to rapidly modulate FasR responsiveness in situations where a cell is in an environment or developmental stage with rapidly fluctuating conditions. Posttranslational regulation of receptor functions is likely to be significantly faster and more dynamic than regulation through, for example, protein inhibitors or activators that are synthesized after transcriptional activation.
Cytotoxic T lymphocytes (CTLs) and natural killer cells are mediators of immune responses against tumor cells and other potential target cells (7, 73, 75). The mechanism by which CTLs mediate their cytotoxicity can be divided into two pathways, FasR and perforin dependent, whereas natural killer cells use only the FasR pathway (48). Upon recognizing tumor cells, CTLs are activated and start to express FasL, and binding of FasL to FasR induces apoptosis in the target cells (48). Resistance to FasR-induced apoptosis is beneficial for tumor cells, as this enables them to escape immune responses. According to this view, decreased susceptibility of tumor cells to FasR-induced apoptosis has been shown to play a role in tumor growth (45, 46, 75). These studies have indicated high expression of Bcl-2 or FAP-1 or down-regulation of the FasR as a possible cause for the observed insensitivity. Apart from these inhibitory proteins, various FasR-resistant tumor cells likely need signaling mechanisms that participate both in induction of cell growth and in suppression of apoptosis (11). Little is known about the signaling mechanisms that protect cells from apoptosis and how they act, although especially the PI-3K and to some extent also the MAPK pathways have been recognized as potential inhibitory factors. The inhibition of FasR-induced apoptosis by activation of MAPK could both during specific stages of normal development and during tumorigenesis be an important factor in making cells resistant to immune surveillance. This inhibition seems to be independent of protein synthesis, which indicates that in any given cell there are direct regulatory signaling mechanisms at work, constantly integrating the sum of incoming stimulatory and inhibitory signals and thereby determining whether a cell will continue proliferation or differentiation or whether it will undergo apoptosis.
ACKNOWLEDGMENTS
We thank Donald Nicholson, Merck-Frosst (Quebec, Quebec, Canada) for caspase antibodies, Melanie Cobb (Southwestern Medical Center, Dallas, Tex.) for the pMCL-wtERK2 construct, Päivi Koskinen (Turku Centre for Biotechnology, Turku, Finland) for the pSV2NEO construct, and Poul Jørgensen (University of Århus, Århus, Denmark) for the pBI-1, pBI-4, and pUHD15-1 constructs. We also thank Lea Sistonen and other members of our laboratories for critical comments on the manuscript.
Financial support from the Academy of Finland (grant 35718), Sigrid Jusélius Foundation, Erna and Victor Hasselblad Foundation, Finnish Cancer Foundation, Nordic Academy for Advanced Study (NorFA), and Cell Signaling Program of Åbo Akademi University is gratefully acknowledged. T.H.H. is supported by the Turku Graduate School of Biomedical Sciences. V.L.J. is a holder of an MRC studentship (United Kingdom).
REFERENCES
- 1.Aggarwal B B, Singh S, LaPushin R, Totpal K. Fas antigen signals proliferation of normal human diploid fibroblast and its mechanism is different from tumor necrosis factor receptor. FEBS Lett. 1995;364:5–12. doi: 10.1016/0014-5793(95)00339-b. [DOI] [PubMed] [Google Scholar]
- 2.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;46:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 3.Baron U, Freundlieb S, Bujard H. Co-regulation of two gene activities by tetracycline via a bidirectional promoter. Nucleic Acids Res. 1995;23:3605–3606. doi: 10.1093/nar/23.17.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1995;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 5.Beidler D R, Tewari M, Friesen P D, Poirier G, Dixit V M. The baculovirus p35 protein inhibits Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem. 1995;270:16526–16528. doi: 10.1074/jbc.270.28.16526. [DOI] [PubMed] [Google Scholar]
- 6.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH a novel MORT/Fadd-interacting protease in Fas/APO-1 and TNF receptor induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 7.Bursch W, Oberhammer F, Schulte-Hermann R. Cell death by apoptosis and its protective role against disease. Trends Pharmacol Sci. 1992;13:243–251. doi: 10.1016/0165-6147(92)90077-j. [DOI] [PubMed] [Google Scholar]
- 8.Chinnaiyan A M, O’Rourke K, Tewari M, Dixit V M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 9.Cuvillier O, Pirianov G, Kleuser B, Vanek P G, Coso O A, Gutkind J S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 10.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R M, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 11.Evan G I, Brown L, Whyte M, Harrington E. Apoptosis and the cell cycle. Curr Opin Cell Biol. 1995;7:825–834. doi: 10.1016/0955-0674(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 12.Fisher D E. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 13.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 14.Freiberg R A, Spencer D M, Choate K A, Duh H J, Schreiber S L, Crabtree G R, Khavari P A. Fas signal transduction triggers either proliferation or apoptosis in human fibroblasts. J Investig Dermatol. 1997;108:215–219. doi: 10.1111/1523-1747.ep12334273. [DOI] [PubMed] [Google Scholar]
- 15.Fried J, Perez A G, Clarkson B-D. Rapid hypotonic method for flow cytofluorometry of monolayer cell cultures, some pitfalls in staining and data analysis. J Histochem Cytochem. 1978;26:921–933. doi: 10.1177/26.11.82573. [DOI] [PubMed] [Google Scholar]
- 16.Gardner A M, Johnson G L. Fibroblast growth factor-2 suppression of tumor necrosis factor α-mediated apoptosis requires Ras and the activation of mitogen-activated protein kinase. J Biol Chem. 1996;271:14560–14566. doi: 10.1074/jbc.271.24.14560. [DOI] [PubMed] [Google Scholar]
- 17.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griswold-Prenner I, Carlin C R, Rosner M R. Mitogen-activated protein kinase regulates the epidermal growth factor receptor through activation of a tyrosine kinase phosphatase. J Biol Chem. 1993;268:13050–13054. [PubMed] [Google Scholar]
- 19.Gulbins E, Bissonnette R, Mahboubi A, Martin S, Nishioka W, Brunner T, Baier G, Baier-Bitterlich G, Byrd C, Lang F, Kolesnick R, Altman A, Green D. FAS-induced apoptosis is mediated via a ceramide-initiated RAS signaling pathway. Immunity. 1995;2:341–351. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 20.Gulbins E, Coggeshall K M, Brenner B, Schlottmann K, Linderkamp O, Lang F. Fas-induced Apoptosis is Mediated by activation of a Ras and Rac protein-regulated signaling pathway. J Biol Chem. 1996;271:26389–26394. doi: 10.1074/jbc.271.42.26389. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y-L, Kang B, Williamson J R. Inhibition of the expression of mitogen activated protein phosphatase-1 potentiates apoptosis induced by tumor necrosis factor-α in rat mesengial cells. J Biol Chem. 1998;273:10362–10366. doi: 10.1074/jbc.273.17.10362. [DOI] [PubMed] [Google Scholar]
- 22.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French L E, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J, Tschopp J. Melanoma cell expression of Fas(Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 23.Holmström T H, Chow S C, Elo I, Coffey E, Orrenius S, Sistonen L, Eriksson J E. Suppression of Fas/APO-1-mediated apoptosis by mitogen-activated kinase signaling. J Immunol. 1998;160:2626–2636. [PubMed] [Google Scholar]
- 24.Holt K H, Kasson B G, Pessin J E. Insulin stimulation of a MEK-dependent but ERK-independent SOS protein kinase. Mol Cell Biol. 1996;16:577–586. doi: 10.1128/mcb.16.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J-L, Schröter M, Burns K, Mattmann C, Rimoldi D, French L E, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–194. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 26.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S-I, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 27.Itoh N, Nagata S. A novel protein domain required for apoptosis. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- 28.Itoh N, Tsujimoto Y, Nagata S. Effect of bcl-2 on Fas antigen-mediated cell death. J Immunol. 1993;151:621–627. [PubMed] [Google Scholar]
- 29.Jelaska A, Korn J H. Anti-Fas induces apoptosis and proliferation in human dermal fibroblasts: differences between foreskin and adult fibroblasts. J Cell Physiol. 1998;175:19–29. doi: 10.1002/(SICI)1097-4652(199804)175:1<19::AID-JCP3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Kaufmann-Zeh A, Rodriquez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy N J, Budd R C. Phosphorylation of FADD/MORT1 and Fas by kinases that associate with the membrane-proximal cytoplasmic domain of Fas. J Immunol. 1998;160:4881–4888. [PubMed] [Google Scholar]
- 32.Kischkel F C, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer P H, Peter M E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khwaja A, Rodriquez-Viciana P, Wennström S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulik G, Klippel A, Weber M J. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lengyel E, Wang H, Gum R, Simon C, Wang Y, Boyd D. Elevated urokinase-type plasminogen activator receptor expression in a colon cancer cell line is due to a constitutively actived extracellular signal regulated kinase-1-dependent signaling cascade. Oncogene. 1997;29:2563–2573. doi: 10.1038/sj.onc.1201098. [DOI] [PubMed] [Google Scholar]
- 36.Lewis T S, Shapiro P S, Ahn N G. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 37.Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Horvat G, Farahani R, McLean M, Ikeda J E, McKenzie A, Korneluk R G. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 38.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 39.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande Woude G F, Ahn N G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Nature. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 40.Mansour S J, Candia J M, Matsuura J E, Manning M C, Ahn N G. Interdependent domains controlling the enzymatic activity of mitogen-activated protein kinase kinase 1. Biochem. 1996;35:15529–15536. doi: 10.1021/bi961854s. [DOI] [PubMed] [Google Scholar]
- 41.Marte B M, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 42.Martins L M, Earnshaw W C. Apoptosis: live and kicking in 1997. Trends Cell Biol. 1997;7:111–114. doi: 10.1016/S0962-8924(96)10053-2. [DOI] [PubMed] [Google Scholar]
- 43.McKillop I H, Schmidt C M, Cahill P A, Sitzmann J V. Altered expression of mitogen-activated protein kinases in a rat model of experimental hepatocellular carcinoma. Hepatology. 1997;6:1484–1491. doi: 10.1002/hep.510260615. [DOI] [PubMed] [Google Scholar]
- 44.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mori S, Murakami-Mori K, Jewett A, Nakamura S, Bonavida B. Resistance of AIDS-associated Kaposi’s sarcoma cells to Fas-mediated apoptosis. Cancer Res. 1996;56:1874–1879. [PubMed] [Google Scholar]
- 46.Morimoto H, Yonehara S, Bonavida B. Overcoming tumor necrosis factor and drug resistance of human tumor cell lines by combination treatment with anti-Fas antibody and drugs or toxins. Cancer Res. 1993;53:2591–2596. [PubMed] [Google Scholar]
- 47.Morrison P, Saltiel A R, Rosner M R. Role of mitogen-activated protein kinase kinase in regulation of the epidermal growth factor receptor by protein kinase C. J Biol Chem. 1996;271:12891–12896. doi: 10.1074/jbc.271.22.12891. [DOI] [PubMed] [Google Scholar]
- 48.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 49.Nicoletti I, Migliorati G, Pagliacci C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;13:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 50.Nophar Y, Holtmann H, Ber R, Wallach D. Dominance of resistance to the cytocidal effect of tumor necrosis factor in heterokaryons formed by fusion of resistant and sensitive cells. J Immunol. 1988;140:3456–3460. [PubMed] [Google Scholar]
- 51.Northwood I C, Gonzalez F A, Wartmann F A, Raden D L, Davis R J. Isolation and characterization of two growth factor-stimulated protein kinases that phosphorylate the epidermal growth factor receptor at threonine 669. J Biol Chem. 1991;266:15266–15276. [PubMed] [Google Scholar]
- 52.O’Connell J, O’Sullivan G C, Collins J K, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peter M E, Kischkel F C, Hellbardt S, Chinnaiyan A M, Krammer P H, Dixit V M. CD95 (APO-1/Fas)-associating signalling proteins. Cell Death Differ. 1996;3:161–165. [PubMed] [Google Scholar]
- 54.Rothe M, Pan M G, Henzel W J, Ayres T M, Goeddel D V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 55.Sato T, Irie S, Kitada S, Reed J C. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995;268:411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- 56.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K-M, Krammer P H, Peter M E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlegel J, Peters I, Orrenius S, Miller D K, Thornberry N A, Yamin T-T, Nicholson D W. CPP32/Apopain is a key interleukin 1β converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem. 1996;4:1841–1844. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- 58.Seino K-I, Kayagaki N, Okumura K, Yagita H. Antitumor effect of locally produced CD95 ligand. Nat Med. 1997;3:165–170. doi: 10.1038/nm0297-165. [DOI] [PubMed] [Google Scholar]
- 59.Shievella A R, Chen J H, Graham J R, Lin L-L. MADD, a novel death domain protein that interacts with the type 1 tumor necrosis factor receptor and activates mitogen-activated protein kinase. J Biol Chem. 1997;272:12069–12075. doi: 10.1074/jbc.272.18.12069. [DOI] [PubMed] [Google Scholar]
- 60.Smith C A, Farrah T, Goodwin R G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 61.Sorenson C M, Barry M A, Eastman A. Analysis of events associated with cell cycle arrest at G2 phase and cell death induced by cisplatin. J Natl Cancer Inst. 1990;8:749–755. doi: 10.1093/jnci/82.9.749. [DOI] [PubMed] [Google Scholar]
- 62.Strand S, Hofmann W J, Hug H, Muller M, Otto G, Strand D, Mariani S M, Stremmel W, Krammer P H. Lymphocyte apoptosis induced by CD95 (Apo-1/Fas) ligand expressing tumor cells—a mechanism of immune evasion? Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 63.Tewari M, Dixit V M. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 64.Thome M, Schneider P, Hofmann K, Fickenscher H, Meini E, Neipel F, Mattmann C, Burns K, Bodmer J-L, Schröter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 65.Tian Q, Taupin J-L, Elledge S, Robertson M, Anderson P. Fas-activated serine/threonine kinase phosphorylates TIA-1 during Fas-mediated apoptosis. J Exp Med. 1995;182:865–874. doi: 10.1084/jem.182.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toyoshima F, Moriguchi T, Nishida E. Fas induces cytoplasmic apoptotic responses and activation of the MKK7-JNK/SAPK and MKK6-p38 pathways independent of CPP32-like proteases. J Cell Biol. 1997;139:1005–1015. doi: 10.1083/jcb.139.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tran, S. E. F., T. H. Holmström, V. L. Johnson, M. Ahonen, S. C. Chow, V.-M. Kähäri, and J. E. Eriksson. Unpublished observations.
- 68.Ungefroren H, Voss M, Jansen M, Roeder C, Henne-Bruns D, Kremer B, Kalthoff H. Human pancreatic adenocarcinomas express Fas and Fas ligand yet are resistant to Fas-mediated apoptosis. Cancer Res. 1998;15:1741–1749. [PubMed] [Google Scholar]
- 69.Wallach D. Cell death induction by TNF: a matter of self control. Trends Biochem Sci. 1997;22:107–109. doi: 10.1016/s0968-0004(97)01015-3. [DOI] [PubMed] [Google Scholar]
- 70.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 71.Wang C-Y, Mayo M W, Baldwin A S., Jr TNF- and cancer-therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 72.Waskiewicz A J, Cooper J. Mitogen and stress response pathway: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:7981–7989. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 73.Williams G T. Programmed cell death: apoptosis or oncogenesis. Cell. 1991;65:1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- 74.Williams R, Sanghera J, Wu F, Carbonaro-Hall D, Campbell D L, Warburton D, Pelech S, Hall F. Identification of a human epidermal growth factor receptor-associated protein kinase as a new member of the mitogen-activated protein kinase/extracellular signal-regulated protein kinase family. J Biol Chem. 1993;268:18213–18217. [PubMed] [Google Scholar]
- 75.Wright S C, Zhong J, Larrick J W. Inhibition of apoptosis as a mechanism of tumor promotion. FASEB J. 1994;8:654–660. doi: 10.1096/fasebj.8.9.8005393. [DOI] [PubMed] [Google Scholar]
- 76.Wyllie A H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 77.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 78.Yang X, Khosravi-Far R, Chang H Y, Baltimore D. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell. 1997;89:1067–1076. doi: 10.1016/s0092-8674(00)80294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (Anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J, Winoto A. A mouse Fas-associated protein with homology to the Mort1/FADD protein is essential for Fas-induced apoptosis. Mol Cell Biol. 1996;16:2756–2763. doi: 10.1128/mcb.16.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]