Abstract
Hypersensitivity pneumonia is an immune-mediated inflammation of the lung parenchyma that occurs in previously susceptible individuals, after inhalation of antigens, usually organic. In recent years, various chemical agents have been described as inducers of hypersensitivity pneumonia, including exposure to high concentrations of pesticides. The objective of the present case report was to describe a possible association of hypersensitivity pneumonia with pesticide chronic inhalation and to draw attention to the importance of early diagnosis. The patient was 72-year-old man who worked for over 30 years as a health agent fumigating pesticides in rural and urban areas. He had progressive dyspnea and cough for the past 3 years. Chest tomography demonstrated parenchymal bands, honeycombing, and diffuse air trapping. Spirometry showed a severe restrictive pattern. Surgical lung biopsy was indicated, which confirmed the diagnosis of hypersensitivity pneumonia. Due to the wide use of pesticides in Brazil, the knowledge of their association with hypersensitivity pneumonia is of great importance in warning the teams involved in health care and surveillance of these workers, providing earlier diagnoses, with better prognosis. On the contrary, late diagnoses, such as that of the case reported, have important health impacts. As a priority, preventive measures must be taken to protect exposed individuals.
Keywords: pesticides, occupational exposure, hypersensitivity pneumonia
INTRODUCTION
Hypersensitivity pneumonia (HP) is an interstitial lung disease characterized pathologically by varying degrees of inflammation and/or fibrosis that result from the repeated inhalation of an antigen by susceptible individuals.1,2 Exposures can be both environmental and occupational. Initially, three large groups of organic agents were described as causative agents: fungi, bacteria, and avian protein. Over the last 15-20 years, continued interest in HP led to the development of experimental models of HP and to the identification of several new agents.3,4 Among the latter is exposure to pesticides5,6 such as pyrethroids,7,8 organochlorines,9,10 and carbamates.9
Data from registries of interstitial lung diseases in three European countries indicated that HP represents 4 to 15% of all interstitial diseases.2 In Brazil, it was the second most common disease (19%), after connective tissue diseases (21.7%), followed by idiopathic pulmonary fibrosis and sarcoidosis.11 The prevalence varies considerably from country to country, depending on disease definition, diagnostic methods, type and intensity of exposure, geographical conditions, agricultural and industrial practices, and host risk factors.12 In Brazil, the most common causes of HP are exposure to bird’s feathers and droppings (including feather pillows), molds at home, and exposure to isocyanates.11 In other countries, farmer’s lung and pigeon breeder’s lung are more common, whereas summer-type HP is registered in Japan.13
Conventionally, HP is classified as acute, subacute, and chronic. In general, both subacute and chronic HP develop after a low but long exposure to antigens. Recently, medical societies proposed to classify HP as non-fibrotic (pure inflammatory changes) and fibrotic (inflammatory changes associated with fibrosis or pure fibrosis), considering both exposure and specific radiological and histopathological changes. The main implication of this classification regards to the prognosis, significantly better in the non-fibrotic group. The radiological patterns were categorized as non-fibrotic and subdivided into typical, compatible and indeterminate for HP. The histopathology of HP was also redefined and classified into fibrotic and non-fibrotic patterns, which are subdivided into typical, probable and indeterminate. This document emphasizes the importance of multidisciplinary discussion, which is indispensable for better diagnostic clarification.4
Detecting relevant exposures and removing offending antigens have prognostic and therapeutic implications.14,15 Currently, courses of corticosteroids are frequently prescribed for HP patients, when there are symptoms and physiologic abnormalities.12
The workers health service at the hospital of the Universidade Federal de Minas Gerais (UFMG) is a reference center in occupational health, where great part of the medical appointments are about respiratory diseases. The objective of this report is to highlight the possible association of pesticides with HP and draw attention to the importance of early diagnosis.
CASE REPORT
This case report was approved by the ethic committee of the UFMG (process number 3.338.690). The patient is a 72-year old male who worked for over 30 years as a health care agent fumigating pesticides in both rural and urban areas. He was evaluated for progressive dyspnea on exertion and persistent cough for the last three years at the Occupational Lung Diseases Ambulatory. There was no history of allergies, bird or mold exposure, smoking or any other prior pulmonary diseases. Physical examination revealed bilateral velcro like crackles in lung fields and digital clubbing. Chemical exposure ceased in April 2017 and included pyrethroids (deltamethrin and cyphenothrin), organophosphates (malathion), and organochlorines (hexachlorobenzene, dichlorodiphenyltrichloroethane [DDT]). The patient declared a frequency of exposure around three-five times a week. According to him, he was instructed not to use personal protective equipment (PPE), because the population could be alarmed about the possible risks related to pesticides and respiratory health.
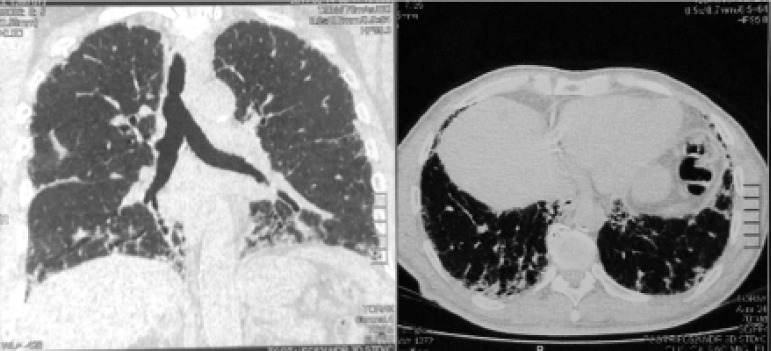
High resolution computed tomography (HRCT) of the chest demonstrated numerous parenchymal bands, bilateral honeycomb images, and diffuse air trapping, pattern compatible with fibrotic HP (Figure 1). Spirometry findings revealed a severe restrictive pattern. Forced vital capacity was 1.5 L (41% of expected), and forced expiratory volume in the first second was 1.41 L (50% of expected). Extended investigation included diffusing capacity of the lung for carbon monoxide of 43%. After a multidisciplinary discussion and the need for a definitive diagnosis, which could even have implications related to this patient’s work activity, surgical lung biopsy was indicated. It confirmed the presence of interstitial pneumonia with a bronchiolocentric distribution, old fibrosis areas and cholesterol crystals foci surrounded by giant cells, typical pattern for fibrotic HP (Figure 2). By associating exposure with radiological and histopathological changes, the final conclusion was chronic PH. Considering the ongoing inflammation process, an optimized inhaled dose of corticosteroid was prescribed. Endoscopy indicated no abnormalities. A 24-hour esophageal pH test was indicated as differential diagnosis for gastroesophageal reflux disease, despite the absence of pyrosis and dyspepsia. By the end of consultation, we explained the disease process to the patient, as well as his prognosis, the importance of preventive measures such as influenza and pneumonia vaccinations, and the risks of a new pesticide exposure.
Figure 1.
High resolution computed tomography of the chest demonstrating numerous parenchymal bands, bilateral honeycomb images and diffuse air trapping.
Figure 2.
Interstitial pneumonia with a bronchiolocentric distribution, old fibrosis areas (left) and cholesterol crystals foci surrounded by giant cells (arrow, right image).
DISCUSSION
This case illustrates ongoing occupational public-health impacts and brings attention to the importance of protective measures and early diagnosis. In any patient with HP, clinicians should aggressively search for antigen identification and exposure cessation. All efforts to avoid potential sources of antigen exposure should be made. Clinicians need reliable, validated, and more widely accessible methods for identification of chemicals that cause HP and for distinguishing disease from ordinary past exposure.4,15 A cross-sectional study of occupational risk factors for HP among 50,000 farmers and their wives found that self-declared high pesticide exposure events (odds ratio [OR] = 1.75, 95% confidence interval [95%CI] 1.39-2.21), and ever use of organochlorine (OR = 1.34, 95%CI 1.04-1.74) and carbamate pesticides (OR = 1.32, 95%CI 1.03-1.68) were associated with HP in mutually-adjusted models.9 DDT was positively associated with HP as well. Occupational exposure to pyrethroid was related to HP in a pet groomer.7 In addition, residential mosquito-coil (pyrethroid containing) smoke exposure was described as a cause of HP.
Damage from HP can be partially or completely reversed in cases of early diagnosis and exposure cessation. In a study of patients with chronic HP, the presence of lung fibrosis was strongly associated with increased rate of death. However, even after accounting for the presence of fibrosis, the inability to identify an inciting antigen was independently associated with shorter survival.15 Similarly, a Brazilian study in a cohort of 112 patients with chronic HP showed that withdrawal from exposure was a factor independently associated with better survival.14 Thus, the diagnosis of HP requires a high index of suspicion and should be considered in any patient presenting with clinical evidence of interstitial lung disease. Early diagnosis and avoidance of further exposure are keys in disease management.12 Although knowledge and use of PPE are widely spread, some workers and employers still inadequately adhere to basic safety and industrial hygiene protocols.
Footnotes
Funding: None
Conflicts of interest: None
REFERENCES
- 1.Chandra D, Cherian SV. StatPearls. Treasure Island: StatPearls Publishing; 2018. Hypersensitivity pneumonitis. [Google Scholar]
- 2.Lacasse Y, Girard M, Cormier Y. Recent advances in hypersensitivity pneumonitis. Chest. 2012;142(1):208–217. doi: 10.1378/chest.11-2479. [DOI] [PubMed] [Google Scholar]
- 3.Barber CM, Wiggans RE, Carder M, Agius R. Epidemiology of occupational hypersensitivity pneumonitis; reports from the SWORD scheme in the UK from 1996 to 2015. Occup Environ Med. 2017;74(7):528–530. doi: 10.1136/oemed-2016-103838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes H, Morisset J, Molyneaux P, Westall G, Glaspole I, Collard HR, et al. A systematically derived exposure assessment instrument for chronic hypersensitivity pneumonitis. Chest. 2020;157(6):1506–1512. doi: 10.1016/j.chest.2019.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wysong K, Phillips JA, Hammond S. Hypersensitivity pneumonitis. Workplace Health Saf. 2016;64(6):284–284. doi: 10.1177/2165079916640284. [DOI] [PubMed] [Google Scholar]
- 6.Nordgren TM, Bailey KL. Pulmonary health effects of agriculture. Curr Opin Pulm Med. 2016;22(2):144–149. doi: 10.1097/MCP.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pu CY, Rasheed MR, Sekosan M, Sharma V. Pet Groomer's Lung: a novel occupation related hypersensitivity pneumonitis related to pyrethrin exposure in a pet groomer. Am J Ind Med. 2017;60(1):141–145. doi: 10.1002/ajim.22664. [DOI] [PubMed] [Google Scholar]
- 8.Carlson JE, Villaveces JW. Hypersensitivity pneumonitis due to pyrethrum. Report of a case. JAMA. 1977;237(16):1718–1719. [PubMed] [Google Scholar]
- 9.Hoppin JA, Umbach DM, Kullman GJ, Henneberger PK, London SJ, Alavanja MC, et al. Pesticides and other agricultural factors associated with self-reported farmer's lung among farm residents in the Agricultural Health Study. Occup Environ Med. 2007;64(5):334–341. doi: 10.1136/oem.2006.028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen DH, Basten A, Williams GV, Woolcock AJ. Familial hypersensitivity pneumonitis. Am J Med. 1975;59(4):505–514. doi: 10.1016/0002-9343(75)90258-2. [DOI] [PubMed] [Google Scholar]
- 11.Pereira CA, Gimenez A, Kuranishi L, Storrer K. Chronic hypersensitivity pneumonitis. J Asthma Allergy. 2016;9:171–181. doi: 10.2147/JAA.S81540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selman M, Pardo A, King Jr TE. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186(4):314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 13.King Jr TE. Hypersensitivity pneumonitis (extrinsic allergic alveolitis): epidemiology, causes, and pathogenesis. UpToDate. 2019. [2019 Oct. 05]. Available from: https://www.uptodate.com/contents/hypersensitivity-pneumonitis-extrinsic-allergic-alveolitis-epidemiology-causes-and-pathogenesis.
- 14.Gimenez A, Storrer K, Kuranishi L, Soares MR, Ferreira RG, Pereira CAC. Change in FVC and survival in chronic fibrotic hypersensitivity pneumonitis. Thorax. 2018;73(4):391–392. doi: 10.1136/thoraxjnl-2017-210035. [DOI] [PubMed] [Google Scholar]
- 15.Pérez ERF, Swigris JJ, Forssén AV, Tourin O, Solomon JJ, Huie TJ, et al. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest. 2013;144(5):1644–1651. doi: 10.1378/chest.12-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]