Abstract
In response to extracellular and intracellular stressors, the nucleus and nuclear compartments undergo distinct molecular changes to maintain cell homeostasis. In the context of Alzheimer’s disease, misfolded proteins and various cellular stressors lead to profound structural and molecular changes at the nucleus. This review summarizes recent research on nuclear alterations in AD development, from the nuclear envelope changes to chromatin and epigenetic regulation and then to common nuclear stress responses. Finally, we provide our thoughts on the importance of understanding cell-type-specific changes and identifying upstream causal events in AD pathogenesis and highlight novel sequencing and gene perturbation technologies to address those challenges.
Keywords: Nucleus, Alzheimer’s disease, Chromatin, Gene regulations, Cell cycle deregulation
Background
The membrane-bound nucleus is one of the complex features that arose from prokaryotic to eukaryotic cells through evolution. It contains genetic materials and acts as the control center of the cell to control the synthesis of ribosomes and proteins. Under normal circumstances, the nucleus regulates gene expression to maintain cell homeostasis. In response to environmental and intracellular insults, cells relay the “stress signals” through various signaling pathways to the nucleus to defend cells against stress and restore homeostasis.
An emerging concept that unifies Alzheimer’s disease (AD) and other neurodegenerative diseases is that chronic response to oxidative stress and misfolded proteins disrupts neuronal function, leading eventually to neurodegeneration. In AD, cellular stress is often initiated by oxidative stress and further enhanced by neurotoxic amyloid-beta (Aβ) oligomers and phosphorylated tau (p-tau), as well as the release of inflammatory mediators [1]. With the nucleus being a point of convergence for stress response, a better understanding of its structural, molecular and functional changes would highlight intracellular underpinnings of AD pathogenic processes.
Accumulated studies have shown that cellular insults induce profound changes to the nuclear structure, as well as the epigenome and transcriptome in AD brains [2]. Here we summarize the recent literature on these nuclear changes in animal models of AD and AD postmortem brain tissue. We first outline the nuclear structure changes from the nuclear envelope and nuclear pore complexes (NPCs) to the nucleolus, then elaborate on multiple layers of epigenetic regulation of gene expression. Furthermore, we discuss DNA damage response (DDR) and cell cycle deregulation in AD pathogenesis. Lastly, we provide our thoughts on refining the molecular signature of AD and identifying the causal genes for therapeutic intervention.
Main text
Nuclear envelope and nucleolus in AD
Nuclear envelope in AD
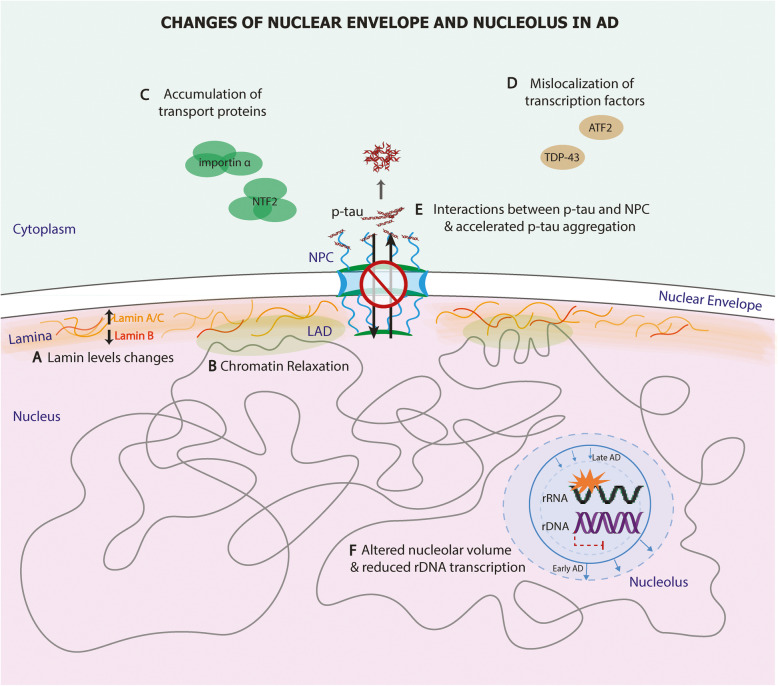
The nuclear envelope is a highly dynamic structure, consisting of the nuclear lamina and a double membrane connected at specific points where the NPCs form [3]. The nuclear lamina forms a dense fibrillar network regulating important cellular events such as DNA replication, gene regulation, and signal transduction. Lamins, the major architectural proteins of the lamina, also serve as a scaffold to tether chromatin-protein complexes to the nuclear lamina, thereby sustaining genomic stability. Lamina-associated domains (LADs), the chromatin positioned close to the nuclear lamina, display typical heterochromatin features and are usually flanked by insulator protein CTCF-binding sites [4]. Increased lamin A and lamin C levels have been detected with the aggravation of AD pathology in postmortem hippocampus [5], whereas lamin B levels are reduced in AD frontal cortices (Fig. 1A) [6]. In the same study, lamin dysfunction in a tau-transgenic Drosophila melanogaster AD model led to heterochromatin relaxation (Fig. 1B), DNA damage, and neuronal degeneration [6]. Interestingly, pharmacologic and genetic inhibition of thioredoxin1, an antioxidant, enhanced caspase-6 activity in serum-deprived SH-SY5Y neurons, which resulted in the degradation of lamin B1 and nuclear envelop invagination [7]. This study indicates that thioredoxin1 a key regulator for nuclear lamina integrity. Consistently, reduced thioredoxin1 was detected in AD mouse brain, a finding also reported in AD postmortem brains [8].
Fig. 1.
Nuclear envelope and nucleolus changes in AD. A In the nuclear lamina, lamin A/C expression is increased, whereas lamin B is reduced in the AD cortex. B Dysfunctional lamina causes pathological chromatin relaxation at lamina-associated domains (LAD). C Abnormal accumulation of nuclear pore complex (NPC)-associated proteins and other nuclear transport factors, i.e. NTF2 and importin α, compromises nucleocytoplasmic transport. D Various transcription factors are found mislocalized to the cytoplasm. E NPC components are found mislocalized to the cytoplasm, interacting with neurofibrillary tangles (NFTs), leading to accelerated phosphorylated tau aggregation and eventually impaired nucleoplasmic transport. F The volume of the nucleolus increases at the early stage of AD but decreases as AD progresses. In the nucleolus, ribosomal DNA (rDNA) transcription reduces, and ribosomal RNA (rRNA) is damaged by oxidative stress
The NPC is embedded in the nuclear envelope, containing more than 500 copies of 30 distinct nucleoporin proteins (Nups). NPCs mediate selective nucleocytoplasmic transport by forming a permeability barrier with the intrinsically disordered phenylalanine-glycine-rich Nups (FG-Nups) in the center and scaffold Nups in the periphery [9]. Nups also play an essential role in transcriptional regulation to determine cellular fate and identity of various cell types in the brain [10], likely through coordinating super-enhancers [11].
Multiple studies have shown that the NPC structure and nucleocytoplasmic transport are altered in AD. Initial evidence came from the immunolabeling of nucleoporins and NPC-associated proteins on postmortem hippocampal sections. This study demonstrated increased nuclear irregularity accompanying intracellular neurofibrillary tangles (NFTs) in AD hippocampal neurons [12]. They also observed abnormal perinuclear accumulation of nuclear transport factor 2 (NTF2), a critical NPC-associated protein, in scattered CA1 neurons in AD (Fig. 1C). Furthermore, importin α, an essential protein of cytoplasmic-nuclear transport, was also found accumulated in human AD hippocampal CA1 neurons [13] (Fig. 1C). Lastly, various transcription factors, such as TDP-43 [14] and ATF2 [14], were found mislocalized to the cytoplasm of AD neurons (Fig. 1D). These studies indicate the dysfunction of nucleocytoplasmic transport in AD. A recent study provided further evidence that pathological tau directly interacts with components of the NPC, including Nup98, leading to accelerated tau aggregation in the cytoplasm and impaired nucleocytoplasmic transport [15] (Fig. 1E). As expected, reducing soluble p-tau and Nup98 can restore nucleocytoplasmic transport in rTg4510 mice [15]. Concordantly, Paonessa et al. showed that tau mutations resulted in its hyperphosphorylation and mislocalization from axons to cell bodies and dendrites in stem cell-derived neurons, leading to nuclear membrane deformation and nucleocytoplasmic transport defect [14].
Nucleolus in AD
The nucleolus, consisting of ribosomal DNA (rDNA), ribosomal RNA (rRNA), and proteins, is the site for ribosome biogenesis [16]. The nucleolus is compartmentalized into the fibrillar center, the dense fibrillar center, and the granular component for pre-rRNA transcription, processing, and ribosomal ribonucleoprotein (RNP) assembly respectively [17, 18]. Ribosomal biogenesis requires 80% of the cellular energy; therefore, cellular metabolism can directly affect nucleolar activities.
Abnormal nucleoli morphology and function have been implicated in AD [19]. Using design-based stereology, Iacono et al. measured the volumes of neuronal cell bodies, nuclei, and nucleoli in postmortem cortex and hippocampus [20, 21]. Interestingly, asymptomatic AD demonstrated significant neuronal hypertrophy, especially profound nucleoli hypertrophy in CA1 neurons of the hippocampus, compared with mild cognitive impairment (MCI) cases with a similar load of AD pathology (Braak III-V) [20, 21], indicating a compensatory mechanism that prevents the disease progression into dementia. In contrast, definitive AD cases (Braak IV-VI) demonstrated significant atrophy of the neuronal cell bodies and nucleoli in the CA1 region [20, 21]. In line with this, Tagliavini et al. found significantly reduced nucleolar volume in the basal nucleus of Meynert, and the percentage volume reduction correlated with the percentage of cell loss in this region [20–22] (Fig. 1F).
Accumulated studies have indicated that rDNA transcription is regulated by different tau species. Immunogold labeling of human brain sections has shown that tau is expressed within the nucleolus and colocalizes with TIP5, a key player in heterochromatin stability, indicating a potential role for tau in rDNA transcriptional repression. Indeed, depleting tau in SH-SY5Y neuroblastoma cells decreases heterochromatin and DNA methylation, increasing rDNA transcription [23]. Federico et al. studied the cellular localization of the phosphorylated AT8 (Ser202/Thr205) and unphosphorylated Tau1 (Pro189/Gly207) epitopes of tau protein in the SK-N-BE cell line. They detected punctated staining for Tau1 in nucleoli of both proliferative and differentiated cells, whereas diffused AT8 staining in the entire nucleolus of only differentiated cells [24]. Since the transcriptional activity is reduced in differentiated cells, this study also supports a possible role of rDNA silencing for p-tau during neuronal differentiation. It has been reported that AD patients have hypermethylated rDNA promoters and reduced rDNA transcription [25] (Fig. 1F). Whether this reduced rDNA transcription results from tau or p-tau is yet to be studied. Nevertheless, nuclear tau species may function differently under cellular stress. For example, glutamate-induced cellular stress triggered the redistribution of nucleolar tau, but not p-tau [23]. Recently, Gil et al. conducted immunohistochemistry on postmortem brains at different ages and revealed that p-tau, AT100 (Thr212/Ser214), progressively increased in nuclei during aging and co-localized with the DAPI-positive heterochromatin [26]. Interestingly, AT100 was also detected in the nucleolus of pyramidal neurons in the CA1 region, with its highest expression in senescent cells in early AD stages and disappearing in more advanced stages [26] (Fig. 1F). In the same study pronounced AT100 expression in nucleoli at Braak stage I was in concordance with nucleolar hypertrophy while the absence of AT100 matched the drastic reduction in nucleolar volume observed in stages IV-V [26] (Fig. 1F).
In vitro culture and animal studies have also demonstrated the nucleolar responses to Aβ-related pathologies. Incubation of SH-SY5Y neuroblastoma cells with Αβ oligomers for 24 h altered distribution of nucleolar tau, induced nucleolar stress and a reduction of rRNA synthesis and protein production [27]. Garcia-Esparcia et al. conducted a comparative study on nucleolar and ribosomal molecules in the cortex of postmortem AD individuals (Braak stage V-VI) versus APP/PS1 mice, and they detected significant but divergent protein and gene alternations related to the protein synthesis machinery from the nucleolus to the ribosome [28]. Furthermore, a recent study identified a long nucleolus-specific lncRNA (LoNA) that can serve as a sensor of neuronal activities, and its activity-dependent decrease leads to elevated rRNA levels, ribosome biosynthesis, and protein translation [29]. Notably, LoNA expression was elevated in the hippocampus of APP/PS1 mice, accompanied by reduced levels of rRNAs, and knockdown of LoNA restored rRNA expression and rescued cognitive and memory impairments in the same AD mouse model [29].
Recent studies have attributed nucleolar stress response as a novel signaling pathway in early AD development (reviewed in [30]). For example, SHSY5Y cells treated with Aβ42 oligomers for 2 h showed oxidative stress and a significant reduction in UBF, a nucleolar transcription factor that drives the transcription of rDNA [30]. Furthermore, oxidative stress can directly affect rRNA, contributing to ribosome dysfunction by increasing the iron-binding capacity of rRNA. Consistent with this, ribosomes purified from the AD hippocampus contained significantly higher levels of RNase-sensitive iron and redox activity [31]. In addition, AD and MCI cortices demonstrated elevated rRNA oxidation and reduced rRNA level [32, 33] (Fig. 1D). Lastly, the application of DNA damage reagents or blocking rRNA synthesis reduced nucleolar rRNA transcription, leading to p53-dependent protracted neuronal degeneration in vitro [31, 34]. Therefore, the nucleolus may serve as a critical stress-sensor and gatekeeper to maintain the cell homeostatic state, initiating neurodegenerative molecular changes upon cellular stress.
Nuclear chromatin in AD
Histone modifications in AD
Histone post-translational modifications (PTMs) are a significant contributor to the epigenetic regulation of gene expression. Histone methylation and histone acetylation are the two common but distinct forms of histone PTMs. Histone methylation, catalyzed by histone methyltransferases, occurs on specific N-terminus lysines of histones H3 and H4 to either increase or repress transcription of the nearby genes [35]. Histone acetylation, executed by histone acetyltransferases (HATs), generally results in transcriptional activation; conversely, histone deacetylases (HDACs) reverse histone acetylation and suppress transcription.
Histone methylation changes linked to heterochromatin state have been implicated in AD but remain inconclusive. Frost et al. examined the H3K9me2, a heterochromatin mark for constitutive telomeric and pericentromeric heterochromatin along with the heterochromatin protein 1α (HP1α) in tau-induced neurodegeneration [36]. They found widespread loss of these heterochromatin marks and aberrant gene expression in tau transgenic Drosophila and mice, and in the human AD hippocampus (Braak stages V/VI). Leveraging public chromatin immunoprecipitation followed by sequencing (ChIP-seq) datasets from human AD brains, they also revealed a widespread transcriptional increase in genes silenced in controls due to heterochromatin state [36]. On the contrary, Zheng and others used similar experimental approaches but detected significant elevation of H3K9me2 in 5XFAD mouse model and the prefrontal cortex of postmortem human AD brains. Concomitantly, H3K9me2 at glutamate receptors was increased in the prefrontal cortex of aged 5XFAD mice; treating FAD mice with specific histone methyltransferase inhibitors, reversed histone hypermethylation, restored glutamate receptor expression and cognitive impairment [37]. Meanwhile, Lee and others discovered that H3K9me3-mediated heterochromatin condensation was also elevated in sporadic AD postmortem cortices (Braak stages V/VI). By combining H3K9me3 ChIP-seq and mRNA-seq, they discovered that epigenomes highly occupied by H3K9me3 were inversely correlated with their mRNA expression levels in AD, and the downregulated genes were mainly involved in synaptic function and neuronal differentiation [38].
Histone acetylation changes have also been implicated in AD pathogenesis (Fig. 2A). Early work from the Johnson group reported elevated expression of HDAC6 in human AD cortices and hippocampi [39]. Interestingly, they showed that HDAC6 interacted with tau, and inhibition of HDAC6 in HEK cells did not disrupt HDAC6-tau interaction but attenuated tau phosphorylation [39]. Tsai group conducted ChIP-PCR on the hippocampal CA1 tissue of the CK-p25 AD mouse model and revealed loss of H2BK5ac, H3K14ac, H4K5ac, and H4K12ac on neuroplasticity genes. They further experimentally validated that this epigenetic blockade was mediated by elevated HDAC2, which was also detected in the CA1 area of 5XFAD mice and in AD patients (Braak I–VI) [40]. The initial effort of using targeted proteomics to measure histone acetylation was made to measure H3K18/K23ac in a limited set of human samples and found a significant reduction of H3K18/K23ac levels in the AD temporal cortex [41]. A recent study demonstrated that astrocytic ApoE particles promote acetylation of H3K9, H3K27, H4K5, and H4K12 in cultured neurons, which subsequently enhanced transcription of neuronal immediate early genes (IEGs) that favor memory consolidation [42]. Indeed, ApoE knockout mice showed drastically reduced H3K27ac marks on the promoter regions of IEGs in response to a learning and memory training paradigm, and human ApoE4 targeted replacement (TR) mice demonstrated less enriched H3K27ac than ApoE3 TR mice, indicating that ApoE4 is less capable of promoting histone acetylation [42]. In line with those studies, HDAC inhibitors have shown promise as a therapeutic approach to combat the cognitive impairment associated with aging and neurodegenerative disease [43–45].
Fig. 2.
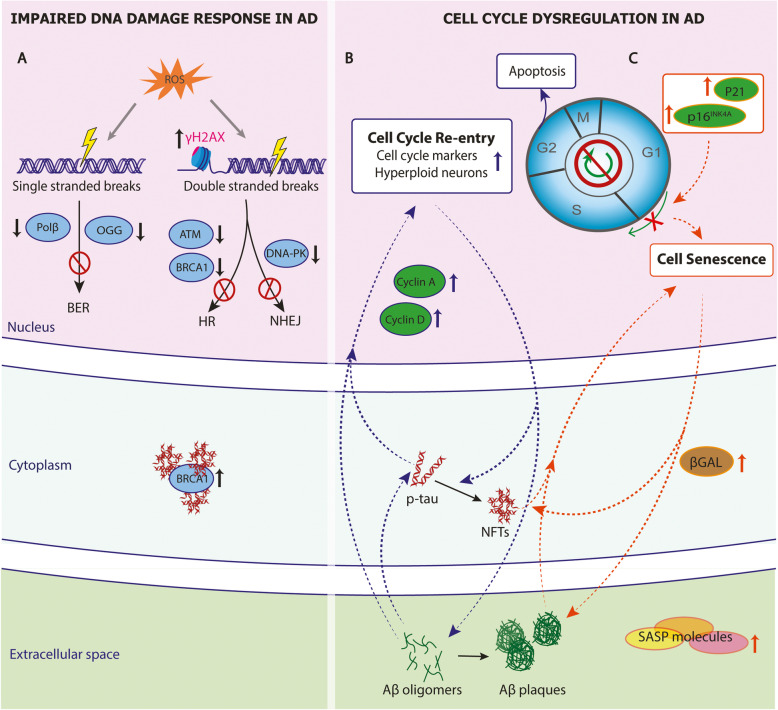
DNA damage and cell cycle dysregulation in AD. A Reactive oxygen species (ROS) cause DNA single- or double-stranded DNA breaks in AD. The histone variant H2AX (γH2AX), a marker of DNA double breaks, is increased. The enzymes and pivotal molecules for base-excision pair (BER), homologous recombination (HR), and non-homologous end-joining (NHEJ) repairing pathways are reduced, leading to reduced DNA damage response in AD. BRCA1, a pivotal molecule for HR, is downregulated in the nuclei but increased in the cytosol, interacting with neurofibrillary tangles (NFTs). B Dysregulation of cell cycle regulators result in cell cycle reentry (blue labeling) or C cell senescence (orange labeling) in AD. Soluble forms of Aβ and tau increase cyclin A and cyclin D, leading to cell cycle reentry and cell apoptosis. The upregulation of P16, P21 likely induces cell senescence. Senescent cells also express SA-βGal and release pro-inflammatory, senescence-associated secretory phenotype (SASP) molecules. The CCR and cell senescence are likely to form feedback loops with AD pathology. Notably, Aβ oligomers and phosphorylated tau (p-tau) in their soluble forms lead to cell cycle reentry
The application of ChIP-seq has enabled genome-wide analysis of acetylation patterns in postmortem AD brain tissue. In this regard, Nativio et al. conducted the first ChIP-seq for H4K16ac, a key modification related to aging and cellular senescence [46], on the lateral temporal lobe of 31 younger and elderly cognitively normal controls as well as AD patients. They found that H4K16ac peaks were predominantly increased with normal aging but lost in AD. Notably, altered H4K16ac peaks in the AD cortex were enriched for AD-associated single nucleotide polymorphisms (SNPs) and expression quantitative trait loci (eQTL) [46]. Recently, the Mill group conducted ChIP-seq for H3K27ac, a robust mark of active enhancers and promoters, on the entorhinal cortex of 47 elderly individuals comprising of both AD cases (Braak VI) and controls. They identified thousands of differential peaks in AD brains associated with transcriptional alterations at nearby genes [47]. Consistent with the H4K16ac study, those H3K27ac differential peaks also represented a significant enrichment of AD risk variants, including genetic regions involved in AD neuropathology such as APP, PSEN1, PSEN2, and MAPT. With a sample size of 669 cases from the ROSMAP cohort, Klein et al. conducted ChIP-seq for H3K9ac, another histone mark for transcriptionally active open chromatin, in the dorsolateral prefrontal cortex of control and AD individuals. They found that tau protein burden, but not Aβ, coincided with widespread H3K9ac chromatin remodeling, and the majority of H3K9ac domains resided in the open chromatin region and were positively correlated with transcriptional changes in AD brains [48].
DNA methylation in AD
The most abundant and broadly studied DNA modification, 5-methylcytosine (5mC), is the addition of a methyl group at the cytosine in a CpG dinucleotide. Another stable epigenetic mark that is abundant in the brain is 5-hydroxymethylcytosine (5hmC), an oxidized form of the canonical 5mC catalyzed by ten-eleven translocation (TET) enzymes [49]. Although less prevalent, non-CpG (CpH) methylation also plays a critical role in many biological processes. It is widely accepted that increased methylation in promoter regions results in transcriptional repression, whereas hydroxymethylation of the same loci is associated with transcriptional activation [50].
Global methylation was initially assessed by immunohistochemistry but with inconclusive results. Mastroeni et al. first reported significantly reduced immunoreactivity of 5mC in tangle-bearing neurons of the temporal cortex of AD individuals [51, 52]. However, results from other studies using similar antibody-based methods showed either increased [53–55] or unaltered DNA methylation in the AD cortex [54]. With the advent of bead-based methylation arrays, extensive genome-wide profiling of DNA methylation was conducted in multiple brain regions of individuals with AD. Much like the immunohistochemistry results from global DNA methylation studies, the results have been inconclusive. Nevertheless, these methylation-wide association studies (MWAS) have revealed common methylation changes at a number of AD risk loci such as ANK1, BIN1, RHBDF2, HOXA3, CDH23, and RPL13 [56–61], providing relatively strong evidence that methylation of these genetic loci may be altered in AD. Recently, Zhang et al. conducted a meta-analysis of more than 1000 prefrontal cortex brain samples and identified 119 differentially methylated loci significantly associated with Braak stage progression; the most significant locus is the MAMSTR gene, a cofactor that regulates PU.1, a central gene hub in the AD [57]. Furthermore, Smith et al. combined the data of three cortical regions from six independent AD MWAS and identified 220 differentially methylated CpGs associated with the Braak stage, provided additional significant new differentially methylated loci, including PPT2/PRRT1, AGAP2, SLC44A2, and ADAM10 [62].
The majority of published AD MWAS studies are performed in bulk brain tissue that contains multiple cell types, including neurons, astrocytes, and microglia, all with potential distinct methylation patterns. Although corrections for cell-type composition through reference-based algorithms are applied, establishing cell-type-specific methylation changes is still challenging due to the different ratios between neurons and glia across brain regions. An exciting improvement in recent studies is to conduct methylation assays on enriched cell types isolated by fluorescence-activated cell sorting or laser-assisted microdissection [63–66]. Based on these studies, neurons and astrocytes each demonstrate thousands of differentially methylated CpGs associated with Braak stages but with only ~ 5% overlapping. Glia, especially microglia, primarily exhibit prominent CpG methylation in the ANK1 gene, whereas CpG methylation in neurons occurs in the BIN1, SEC14L1, BRCA, and MCF2L genes [63–66]. Differentially methylated sites in AD neurons are primarily hypomethylated at CpH sites in the enhancer regions, associated with upregulated ∝-secretase 1 and increased plaque and tangle formation [67].
Association studies of 5hmC with AD neuropathology have been sprouting in the past couple of years. Coppieters et al. reported a global increase of both 5mC and 5hmC in neurons (but not glia) of AD frontal and temporal cortex using immunohistochemistry, correlated with AD pathology load [53]. While still lacking power and sample size for meta-analyses, some interesting findings have emerged. In postmortem AD brains, locus-specific changes in 5hmC have been associated with AD pathology [68]. By simultaneously profiling 5mC and 5hmC levels, Smith et al. discovered hypermethylation and hypohydroxymethylation at the ANK1 promoter in AD brains [69]. Recently, Lardenoije et al. revealed a novel differentially hydroxymethylated region in the CHRNB1 gene that encodes acetylcholine receptor beta subunit, crucial for cholinergic neurotransmission [60]. Moreover, Zhao et al. performed 5hmC-capture sequencing and identified various differentially hydroxymethylated regions associated with plaques or neurofibrillary tangles. They also developed differential co-methylation network analysis and identified various modules with unique hub genes that drive AD pathology [68].
Enhancers in AD
Enhancers are gene regulatory elements where transcription factors bind to influence spatiotemporal gene expression programs [70]. Enhancers can undergo three-dimensional interactions with promoters either locally or over large distances to regulate gene transcription [71–73]. In addition, enhancers often show tissue- and cell-type-specific activities [74–76], and neuronal enhancers are also regulated by cell activities [77].
SNPs in enhancer regions can influence the expression of genes and predispose individuals to AD [78]. Gjoneska et al. profiled seven chromatin states and transcriptional changes during the pathological progression of the hippocampus in the CK-p25 AD mouse model. They mapped orthologous genes in noncoding regions between mouse and human and found strong conservation of gene expression and epigenomic signatures. Notably, AD-associated SNPs were specifically enriched in increased-level enhancer orthologues with immune function, implicating immune processes in AD predisposition [79]. By integrating AD SNPs with publicly available data for enhancers that were annotated from 127 human tissues or cell types, a recent study revealed that about 96% of AD SNPs localize in non-coding regions, and 27% in enhancers [80]. Among those enhancer SNPs, 95% reside in the same topological associated domains with their eQTL genes and genes associated with synaptic transmission, immune responses, and Aβ metabolism [78, 80, 81].
Although the field just started to understand how enhancer variants affect gene expression in AD, some exciting studies have emerged. For instance, some AD enhancer variants regulate multiple eQTL genes by affecting the binding of CTCF or other cohesin complex subunits and chromatin looping [80]. The rs7364180 AD variant alters the expression of the transcription factor SREFB2 and then indirectly regulates 20 AD risk genes through a cascade of transcriptional events [80]. The CLU intron variant rs2279590 affects CLU expression and two other AD risk genes EPHX2 and PTK2B, by eliminating a transcription factor binding site for heat shock factor 1 (HSF1) [82]. Since most enhancers are unique to specific cell types, AD enhancer SNPs likely confer their functions in a cell-type-specific manner [83]. A powerful method to map active promotor-enhancer interactome in specific cell types is to utilize proximity ligation-assisted ChIP-seq (PLAC-seq) in which proximity ligation preceded an enrichment for active promoters by H3K4me3 ChIP-seq. Using this approach, Nott et al. identified AD candidate causal variants in microglia-specific enhancers that were looped to corresponding active promoters. Indeed, deletion of a BIN1 microglia-specific enhancer harboring AD-risk variants ablated BIN1 expression in iPSC-derived microglia but not in neurons or astrocytes [81].
Nuclear stress responses in AD
DNA damage response
DNA lesions are sites of damage in the base-pairing or structure of DNA, classified as single-strand breaks (SSBs) and double-strand breaks (DSBs). They occur as either physiological or pathological cellular processes. Nevertheless, cells often initiate various mechanisms, termed DNA-damage response (DDR), to recognize and repair these incidents. Specifically, SSBs are usually recognized and corrected by the base excision repair (BER), and DSBs by either the error-prone non-homologous end-joining (NHEJ) or the homologous recombination (HR). If the damage remains unrepaired, genome instability, cellular senescence, and cell death can subsequently occur [84, 85] .
In AD, multiple brain cell types have been reported to harbor DNA damage due to oxidative stress and the inefficient DDR [85]. Evidence for DNA aberrations dates back to 1999, when DSBs and SSBs were detected in hippocampi of AD brains [86]. More recently, studies also showed increased levels of γH2AX, a well-established marker of DSBs, in neurons and astrocytes of AD hippocampi and cortices [87, 88] (Fig. 2A). Interestingly, the elevation of γH2AX expression was detected in brains with MCI and preclinical AD, suggesting an early contribution of DNA damage to AD pathophysiology [88]. As endogenous reactive oxygen species are the major source of DNA damage, cerebrospinal fluid (CSF) levels of DNA oxidation marker, the 8-OHdG has been proposed as a biomarker for AD early diagnosis in multiple studies [89].
DNA BER pathway is the primary pathway to repair oxidized bases and subsequent DNA SSBs. In general, DNA glycosylase recognizes and removes the oxidized base, and then APE1 endonuclease, PNK kinase, DNA polymerase β (Polβ), and ligases III/I complete the repair. The major enzymes involved in BER have been found downregulated in AD [85] (Fig. 2A), and their changes correlate with the clinical manifestations and AD CSF biomarkers [90]. For instance, MCI and AD brains show decreased levels of Polβ, a DNA polymerase primarily responsible for replacing single nucleotides during BER [91]. Interestingly, a recent study has shown that loss of Polβ is enough to drive cells into senescence [92], another potential mechanism contributing to AD pathophysiology (see cell senescence section). In the brains of both AD and MCI patients, there is a significant reduction of 8-oxoguanine DNA glycosylase (OGG), which excises oxidized DNA thereby preventing its accumulation [91]. Therefore, the profound changes of OGG, and Polβ at the MCI stage suggest that the impaired BER responses could occur before overt AD pathology.
Poly (ADP-ribose) polymerase 1 (PARP1) also contributes to BER by detecting an SSB and then signaling other DNA-repairing enzymes. In AD brains, elevated levels of poly (ADP-ribosylated) proteins, products synthesized by PARP1, have been detected [93]. Furthermore, DNA damage caused by Aβ can activate PARP-1 in astrocytes, dopaminergic neurons, and hippocampal slices, which can further induce the p53 and reduce the Bcl-2 protein expression, leading to cell apoptosis [94, 95].
DSB repair pathways mediated by HR or NHEJ are also involved in AD (Fig. 2A). For instance, ATM and BRCA1, two pivotal molecules for HR, have been found downregulated in AD brains and iPSC-derived neurons [96, 97]. A postmortem neuron-specific DNA methylome study revealed that the BRCA1 promoter was hypomethylated in AD, accompanied by a reduced BRCA1 expression in the nuclei but an increased expression in the cytosol, especially in tau-bearing insoluble aggregates [65]. Likewise, literature also suggests a compromised NHEJ-mediated repair pathway in AD. First, end-joining activity and protein levels of DNA-dependent protein kinase (DNA-PK), a kinase involved in repairing DSBs through NHEJ, were found reduced in AD cortices [98]. Moreover, the MRE11, a protein complex essential for NHEJ responses, is also decreased in AD cortical neurons [99].
Cell cycle deregulation
Cell cycle re-entry
In eukaryotes, the cell cycle consists of four discrete phases: G1, S, G2, and M. Progression through these phases is regulated by cyclin-dependent kinases (CDKs) [100]. Neurons in the adult brain are terminally differentiated and generally thought to be incapable of re-entering the cell cycle. However, multiple studies suggest that neurons can re-enter the cell cycle from their quiescence G0 to G1 phase upon cellular stress and then continue into S, G2, or M phase [101–106]. However, only a small number of those neurons eventually divide [106], and most of them undergo apoptosis [107]. Cell cycle re-entry (CCR) is likely mediated by multiple signaling pathways [104, 108–112].
Accumulated studies have detected cell cycle markers and regulatory proteins in postmortem brain tissue, supporting CCR present across multiple brain regions in all AD stages [113, 114] (Fig. 2B). As a result of CCR, hyperploid neurons are drastically increased at preclinical stages of AD, indicating CCR is a potentially causal event in AD pathogenesis [115]. Indeed, SV40 large T antigen-induced CCR was reported to cause cortical deposition of Aβ plaques and NFT pathology [116], in addition to neuronal degeneration [117]. Similarly, CCR induced by c-myc and ras oncogenes also increases p-tau levels in cultured primary cortical neurons [118]. Furthermore, overexpressing denticle-less (DTL), a potent cell cycle regulator, induces CCR and subsequent tau hyperphosphorylation, Aβ production, and cognitive impairment in mice [119]. Consistently, many other studies also demonstrate that CDKs can drive the Aβ plaque formation [120–123] and tau phosphorylation [124, 125] (Fig. 2B).
On the other end, research evidence also shows that AD pathology triggers neuronal CCR. First, knock-in mice harboring human APP and PSEN1 show increased cyclin A and cyclin D1 in hippocampal and cortical neurons, leading to CCR and cell apoptosis [103, 126]. Moreover, oligomeric Aβ induces dose-dependent neuronal CCR, driving neurons into different cell cycle phases or apoptosis [111, 127]. This Aβ-induced CCR depends on tau phosphorylation by multiple protein kinases activated by Aβ, indicating soluble forms of Aβ and tau are the essential elements for CCR [128]. Genetically perturbing cell-cycle progression in tau-expressing Drosophila models can reduce tau-induced neuronal apoptosis [129]. As such, Aβ oligomers, phosphorylated tau, and CCR are likely to form feedback loops at the early stage of AD and ultimately lead to neuronal apoptosis (Fig. 2B).
Cell senescence
Senescence is an irreversible cell cycle arrest due to the blockade to the S phase of the cycle. Cell senescence related to aging and neurodegeneration is often chronic. It includes replicative senescence [130], stress-induced premature senescence [131], and mitochondrial dysfunction-associated senescence [132]. Despite different categories, chronic cell senescence is generally characterized by a proinflammatory senescence-associated secretory phenotype (SASP), altered mitochondrial function, cellular metabolism, and DNA damage [133, 134].
Cell types in the central nervous system, including neurons, astrocytes, microglia, oligodendrocytes, have been reported to undergo cell senescence during aging. In AD, Aβ plaques and NFTs, along with other cellular stressors, have been shown to induce DNA damage and alter chromatin structure, and subsequently leading to cell senescence [36, 135–143] (Fig. 2C). In postmortem AD brains, Aβ plaques are commonly associated with oligodendrocyte precursor cells expressing senescent markers SA-βGal, p21 (CDKN1A), and p16INK4(CDKN2A) [144] (Fig. 2C). Moreover, laser-dissected neurons from AD brains also bear a transcriptomic profile characteristic of cell senescence, including proinflammatory cytokines and senescence-related upstream regulators [141] (Fig. 2C). Furthermore, senescent astrocytes marked by p16INK4A and MMP-1 are increased with age and were more prominent in age-matched AD cortices [145]. Finally, microglia with dystrophic morphology and shorter telomeres also increases with age, but with a significantly higher number in AD brains [146–148]. Interestingly, a recent study indicates that increased myelin breakdown with age overwhelms microglial phagocytosis function, contributing to microglial senescence [149].
Mouse studies also provided evidence of senescent glia and neurons near Aβ plaques or NFTs, but the cell types undergoing senescence varied among animal models [141, 144, 145, 150, 151]. However, regardless of cell types affected, ablation of senescent cells using either chemical or genetic approaches was protective against AD progression, indicating that cell senescence causally contributes to AD pathogenesis (Fig. 2C). For instance, selectively ablating senescent cells by senolytics in AD mouse models reduces SASP, neuroinflammation, plaque size, NFT burden, and alleviates cognitive declines [141, 144, 151]. Because senescent cells undergo profound chromatin and gene alterations [152–154], perturbing related factors have been shown to alter cell senescence and AD phenotype. For example, mice lacking one allele of Bmi1, a core component of the polycomb repressive complex, shows relaxed heterochromatin, cellular senescence, amyloid plaque, and p-tau formation; meanwhile, introducing mutant APP to Bmi1-deficient mice exacerbates amyloid and tau pathology [155].
The fate choice for stressed cells toward CCR or cell senescence is yet to be investigated in AD. Stressors such as oxidative stress, neuroinflammation, hypoxia, and DNA damage can affect nuclear integrity and regulation, inducing CCR or cell senescence [156–160]. Recent studies suggest that senescent cells result from insoluble NFT formation [141, 151], whereas CCR occurs before NFT and plaque formation [128], indicating the solubility of AD-related proteins as a potential determinant toward cell senescence or CCR. Thus, understanding the molecular underpinning of cell senescence and CCR will help us develop therapeutic strategies to mitigate cell cycle dysregulations in AD development.
Conclusions
This review discussed nuclear dynamics and nuclear stress response in AD, focusing on nuclear architecture, chromatin modifications, and nuclear stress responses. These nuclear characteristics are dynamically regulated to collectively maintain cellular homeostasis. Therefore, abnormal changes reviewed here present the major nuclear perspectives of the AD pathological process. While this review focuses on nuclear mechanisms, multiple gene regulations outside the scope of this review have also shown emerging evidence of their implications in AD, including transcriptional factors [161–163], RNA splicing [164–166], RNA editing [167], RNA binding proteins [168], microRNAs [169], nuclear non-coding RNAs (ncRNAs) [170], and enhancer RNAs (eRNAs) [170, 171]. These gene regulators have been shown to shape gene expression and modulate chromatin architecture [170], but their precise mechanisms in AD remain to be explored [172, 173].
It is worth noting that molecular changes in AD often intertwine and occur concurrently to mediate AD progression. For instance, lamin dysfunction in a tau-transgenic fly model of AD leads to heterochromatin relaxation and DDR [6]. DDR is also reported to trigger CCR and cell senescence [174–177] and reduces nucleolar rRNA transcription [31, 34]. Multi-omics studies from AD mouse models and postmortem brain tissue showed concordant changes among chromatin states, DNA accessibility, transcriptomics [48, 79], and widespread loss of CpH methylation at enhancers of AD neurons significantly converge on transcriptomic changes related to abnormal CCR, apoptotic and inflammatory pathways [67]. Furthermore, SNPs influencing epigenomic marks (xQTLs) overlap significantly with splicing QTLs in AD, and there is significant sharing of xQTL SNPs across the AD molecular phenotypes [165, 178]. Repressor element 1-silencing transcription factor (REST), mediates active epigenetic repression of many genes that promote cell death and AD pathology, and at the same time, induces the expression of stress response genes [179].
How the different nuclear regulations occur coherently within the nucleus is still not clear. An emerging concept is that most nuclear regulatory processes occur through dynamic nuclear condensates that compartmentalize regulatory proteins and RNA molecules to proper genomic loci for coordinated nuclear regulations [180, 181]. The way the condensates are involved in disease progress is yet to be investigated. Notably, a recent study has revealed that the causal mutation of the methyl CpG binding protein 2 (MeCP2) disrupts its ability to form heterochromatin condensates, suggesting a novel mechanism for Rett syndrome [182].
One major challenge in AD research is understanding cell-type-specific molecular changes and their responses to intra- and extracellular pathology. We have seen exciting advances in applying single-cell biology and spatial transcriptomics in AD postmortem tissue and animal models in the past couple of years. These studies have provided invaluable information on cell-type-specific transcriptomic changes and revealed cell types implicated in early AD [183–186]. Furthermore, single-soma transcriptomics of tangle-bearing neurons directly maps tangle pathology to gene changes, proving an exciting approach to understanding pathology heterogeneity of single neurons in AD [186]. Lastly, a recent spatial transcriptomics study provides the first spatial map of transcriptional changes in the vicinity of AD pathogenic hallmarks and identifies plaques-induced gene networks in the early and late AD phases, respectively [183]. With these technologies rapidly evolving, we expect to see more impressive research systematically mapping multidimensional molecular changes in AD with unprecedented cellular, spatial and temporal resolution.
Another challenge is that epigenetic and transcriptomic changes in AD could result from genetic variants or/and pathological insults. Therefore, identifying the causal genetic variants and variant-driven transcriptional changes will allow us to construct the genetic circuitry of AD pathogenesis, thereby providing a better strategy for early AD intervention. By combining CRISPR gene editing with iPSC-based cell models, numerous studies have provided significant insights into the role of genetic variants in AD development [187–189]. Genetic perturbations can also be implemented in massively parallel genetic screens to interrogate gene functions in iPSC-derived neural cell types [190]. Elegant genetic screening studies have been conducted in iPSC-derived neurons to identify causal genes for cell survival, oxidative stress, and lysosome dynamics [191, 192]. The advent of base editors [192, 193] and prime editing [194] technologies enable all possible single-base transition and transversion, providing a powerful platform to interrogate the function of genetic variants in AD development and establish the causal links from genetics to various intermediate molecular phenotypes.
Lastly, the dynamic nuclear structure alterations that contribute to AD can be investigated using experimental and computational approaches developed by the 4D nucleome project [195]. Visualizing chromatin contact sites with super-resolution microscopy [196] or sequencing [197, 198] has started to reveal exciting insight into chromatin structure changes in AD [199]. Implementation of these state-of-the-art technologies will help explain how the nuclear genome is maintained and regulated in AD progression, providing novel mechanistic insights into the molecular events and their dynamic progression.
Acknowledgements
Not applicable.
Abbreviations
- 5hmC
5-hydroxymethylcytosine
- 5mC
5-methylcytosine
- Aβ
Amyloid-beta
- AD
Alzheimer’s disease
- BER
Base excision repair
- CCR
Cell cycle re-entry
- CDKs
Cyclin-dependent kinases
- ChIP-seq
Chromatin immunoprecipitation followed by sequencing
- CSF
Cerebrospinal fluid
- DDR
DNA-damage response
- DNA-PK
DNA-dependent protein kinase
- DSBs
Double-strand breaks
- DTL
Denticle-less
- eRNA
Enhancer RNA
- eQTL
Expression quantitative trait loci
- HATs
Histone acetyltransferases
- HDACs
Histone deacetylases
- HP1α
Heterochromatin protein 1α
- HR
Homologous recombination
- HSF1
Heat shock factor 1
- LADs
Lamina-associated domains
- lncRNA
Long noncoding RNA
- LoNA
Long nucleolus-specific lncRNA
- MAPT
Methyl CpG binding protein 2
- MCI
Mild cognitive impairment
- MWAS
Methylation-wide association studies
- ncRNAs
Non-coding RNAs
- NFTs
Neurofibrillary tangles
- NHEJ
Non-homologous end-joining
- NPC
Nuclear pore complex
- NTF2
Nuclear transport factor 2
- Nups
Nucleoporin proteins
- OGG
8-oxoguanine DNA glycosylase
- PLAC-seq
Proximity ligation-assisted ChIP-seq
- Polβ
DNA polymerase β
- PPARγ
Peroxisome proliferator-activated receptor γ
- p-tau
Phosphorylated tau
- PTMs
Post-translational modifications
- rDNA
Ribosomal DNA
- REST
Repressor element 1-silencing transcription factor
- RNP
Ribosomal ribonucleoprotein
- rRNA
Ribosomal RNA
- SASP
Senescence-associated secretory phenotype
- SNPs
Single-nucleotide polymorphisms
- SSBs
Single-strand breaks
- TET
Ten-eleven translocation
Authors’ contributions
YW contributed to the conception, design and the structure of the manuscript. AI and EMC did the literature search and contributed equally to the writing of the manuscript. YW substantially revised and edited the manuscript. All authors read and approved the final manuscript.
Authors’ information
AI and EMC are Postdoctoral Scientists, and YW is an Associate Professor at Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, USA.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Artemis Iatrou and Eric M. Clark contributed equally to this work.
Contributor Information
Artemis Iatrou, Email: Artemis_Iatrou@rush.edu.
Eric M. Clark, Email: Eric_M_Clark@rush.edu
Yanling Wang, Email: Yanling_Wang@rush.edu.
References
- 1.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagyinszky E, Giau VV, An SA. Transcriptomics in Alzheimer’s disease: aspects and challenges. Int J Mol Sci. 2020;21(10):3517. doi: 10.3390/ijms21103517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero-Bueno R, Ruiz PC, Artal-Sanz M, Askjaer P, Dobrzynska A. Nuclear organization in stress and aging. Cells. 2019;8(7):664. doi: 10.3390/cells8070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Steensel B, Belmont AS. Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell. 2017;169(5):780–791. doi: 10.1016/j.cell.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Méndez-López I, Blanco-Luquin I, Sánchez-Ruiz de Gordoa J, Urdánoz-Casado A, Roldán M, Acha B, et al. Hippocampal LMNA gene expression is increased in late-stage Alzheimer’s disease. Int J Mol Sci. 2019;20(4):878. doi: 10.3390/ijms20040878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frost B, Bardai FH, Feany MB. Lamin dysfunction mediates neurodegeneration in tauopathies. Curr Biol. 2016;26(1):129–136. doi: 10.1016/j.cub.2015.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islam MI, Nagakannan P, Ogungbola O, Djordjevic J, Albensi BC, Eftekharpour E. Thioredoxin system as a gatekeeper in caspase-6 activation and nuclear lamina integrity: implications for Alzheimer’s disease. Free Radic Biol Med. 2019;134:567–580. doi: 10.1016/j.freeradbiomed.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Akterin S, Cowburn RF, Miranda-Vizuete A, Jiménez A, Bogdanovic N, Winblad B, et al. Involvement of glutaredoxin-1 and thioredoxin-1 in β-amyloid toxicity and Alzheimer’s disease. Cell Death Differ. 2006;13(9):1454–1465. doi: 10.1038/sj.cdd.4401818. [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Fernandez-Martinez J, Nudelman I, Shi Y, Zhang W, Raveh B, et al. Integrative structure and functional anatomy of a nuclear pore complex. Nature. 2018;555(7697):475–482. doi: 10.1038/nature26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Angelo MA, Gomez-Cavazos JS, Mei A, Lackner DH, Hetzer MW. A change in nuclear pore complex composition regulates cell differentiation. Dev Cell. 2012;22(2):446–458. doi: 10.1016/j.devcel.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibarra A, Benner C, Tyagi S, Cool J, Hetzer MW. Nucleoporin-mediated regulation of cell identity genes. Genes Dev. 2016;30(20):2253–2258. doi: 10.1101/gad.287417.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheffield LG, Miskiewicz HB, Tannenbaum LB, Mirra SS. Nuclear pore complex proteins in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65(1):45–54. doi: 10.1097/01.jnen.0000195939.40410.08. [DOI] [PubMed] [Google Scholar]
- 13.Lee HG, Ueda M, Miyamoto Y, Yoneda Y, Perry G, Smith MA, et al. Aberrant localization of importin alpha1 in hippocampal neurons in Alzheimer disease. Brain Res. 2006;1124(1):1–4. doi: 10.1016/j.brainres.2006.09.084. [DOI] [PubMed] [Google Scholar]
- 14.Davidson Y, Amin H, Kelley T, Shi J, Tian J, Kumaran R, et al. TDP-43 in ubiquitinated inclusions in the inferior olives in frontotemporal lobar degeneration and in other neurodegenerative diseases: a degenerative process distinct from normal ageing. Acta Neuropathol. 2009;118(3):359–369. doi: 10.1007/s00401-009-0526-z. [DOI] [PubMed] [Google Scholar]
- 15.Eftekharzadeh B, Daigle JG, Kapinos LE, Coyne A, Schiantarelli J, Carlomagno Y, et al. Tau protein disrupts nucleocytoplasmic transport in Alzheimer’s disease. Neuron. 2018;99(5):925–40. e7. doi: 10.1016/j.neuron.2018.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lempiäinen H, Shore D. Growth control and ribosome biogenesis. Curr Opin Cell Biol. 2009;21(6):855–863. doi: 10.1016/j.ceb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, et al. Coexisting liquid phases underlie nucleolar subcompartments. Cell. 2016;165(7):1686–1697. doi: 10.1016/j.cell.2016.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kressler D, Hurt E, Baβler J. Driving ribosome assembly. Biochim Biophys Acta, Mol Cell Res. 2010;1803(6):673–683. doi: 10.1016/j.bbamcr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Hetman M, Pietrzak M. Emerging roles of the neuronal nucleolus. Trends Neurosci. 2012;35(5):305–314. doi: 10.1016/j.tins.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iacono D, Markesbery W, Gross M, Pletnikova O, Rudow G, Zandi P, et al. The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology. 2009;73(9):665–673. doi: 10.1212/WNL.0b013e3181b01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iacono D, O'Brien R, Resnick SM, Zonderman AB, Pletnikova O, Rudow G, et al. Neuronal hypertrophy in asymptomatic Alzheimer disease. J Neuropathol Exp Neurol. 2008;67(6):578–589. doi: 10.1097/NEN.0b013e3181772794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tagliavini F, Pilleri G. Basal nucleus of Meynert: a neuropathological study in Alzheimer’s disease, simple senile dementia, Pick's disease and Huntington's chorea. J Neurol Sci. 1983;62(1–3):243–260. doi: 10.1016/0022-510X(83)90203-4. [DOI] [PubMed] [Google Scholar]
- 23.Maina MB, Bailey LJ, Wagih S, Biasetti L, Pollack SJ, Quinn JP, et al. The involvement of tau in nucleolar transcription and the stress response. Acta Neuropathol Commun. 2018;6(1):70. doi: 10.1186/s40478-018-0565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Federico C, Gil L, Bruno F, D'Amico AG, D'Agata V, Saccone S. Phosphorylated nucleolar Tau protein is related to the neuronal in vitro differentiation. Gene. 2018;664:1–11. doi: 10.1016/j.gene.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 25.Pietrzak M, Rempala G, Nelson PT, Zheng J-J, Hetman M. Epigenetic silencing of nucleolar rRNA genes in Alzheimer’s disease. PLoS One. 2011;6(7):e22585. doi: 10.1371/journal.pone.0022585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gil L, Federico C, Pinedo F, Bruno F, Rebolledo AB, Montoya JJ, et al. Aging dependent effect of nuclear tau. Brain Res. 1677;2017:129–137. doi: 10.1016/j.brainres.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Maina MB, Bailey LJ, Doherty AJ, Serpell LC. The involvement of Aβ42 and Tau in nucleolar and protein synthesis machinery dysfunction. Front Cell Neurosci. 2018;12:220. doi: 10.3389/fncel.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Esparcia P, Sideris-Lampretsas G, Hernandez-Ortega K, Grau-Rivera O, Sklaviadis T, Gelpi E, et al. Altered mechanisms of protein synthesis in frontal cortex in Alzheimer disease and a mouse model. Am J Neurodegener Dis. 2017;6(2):15–25. [PMC free article] [PubMed] [Google Scholar]
- 29.Li D, Zhang J, Wang M, Li X, Gong H, Tang H, et al. Activity dependent LoNA regulates translation by coordinating rRNA transcription and methylation. Nat Commun. 2018;9(1):1726. doi: 10.1038/s41467-018-04072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyhus C, Pihl M, Hyttel P, Hall VJ. Evidence for nucleolar dysfunction in Alzheimer’s disease. Nat Rev Neurosci. 2019;30(7):685–700. doi: 10.1515/revneuro-2018-0104. [DOI] [PubMed] [Google Scholar]
- 31.Parlato R, Kreiner G, Erdmann G, Rieker C, Stotz S, Savenkova E, et al. Activation of an endogenous suicide response after perturbation of rRNA synthesis leads to neurodegeneration in mice. J Neurosci. 2008;28(48):12759–12764. doi: 10.1523/JNEUROSCI.2439-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honda K, Smith MA, Zhu X, Baus D, Merrick WC, Tartakoff AM, et al. Ribosomal RNA in Alzheimer disease is oxidized by bound redox-active iron. J Biol Chem. 2005;280(22):20978–20986. doi: 10.1074/jbc.M500526200. [DOI] [PubMed] [Google Scholar]
- 33.Ding Q, Markesbery WR, Cecarini V, Keller JN. Decreased RNA, and increased RNA oxidation, in ribosomes from early Alzheimer’s disease. Neurochem Res. 2006;31(5):705–710. doi: 10.1007/s11064-006-9071-5. [DOI] [PubMed] [Google Scholar]
- 34.Kalita K, Makonchuk D, Gomes C, Zheng JJ, Hetman M. Inhibition of nucleolar transcription as a trigger for neuronal apoptosis. J Neurochem. 2008;105(6):2286–2299. doi: 10.1111/j.1471-4159.2008.05316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos AL, Lindner AB. Protein posttranslational modifications: roles in aging and age-related disease. Oxid Med Cell Longev. 2017;2017:5716409. doi: 10.1155/2017/5716409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frost B, Hemberg M, Lewis J, Feany MB. Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci. 2014;17(3):357–366. doi: 10.1038/nn.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Y, Liu A, Wang ZJ, Cao Q, Wang W, Lin L, et al. Inhibition of EHMT1/2 rescues synaptic and cognitive functions for Alzheimer’s disease. Brain. 2019;142(3):787–807. doi: 10.1093/brain/awy354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee MY, Lee J, Hyeon SJ, Cho H, Hwang YJ, Shin JY, et al. Epigenome signatures landscaped by histone H3K9me3 are associated with the synaptic dysfunction in Alzheimer’s disease. Aging Cell. 2020;19:e13153. doi: 10.1111/acel.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding H, Dolan PJ, Johnson GV. Histone deacetylase 6 interacts with the microtubule-associated protein tau. J Neurochem. 2008;106(5):2119–2130. doi: 10.1111/j.1471-4159.2008.05564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gräff J, Rei D, Guan J-S, Wang W-Y, Seo J, Hennig KM, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483(7388):222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang K, Schrag M, Crofton A, Trivedi R, Vinters H, Kirsch W. Targeted proteomics for quantification of histone acetylation in a lzheimer's disease. Proteomics. 2012;12(8):1261–1268. doi: 10.1002/pmic.201200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Zhang J, Li D, He C, He K, Xue T, et al. Astrocytic ApoE reprograms neuronal cholesterol metabolism and histone-acetylation-mediated memory. Neuron. 2021;109(6):957–70.e8. doi: 10.1016/j.neuron.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Yang S-s, Zhang R, Wang G, Y-f Z. The development prospection of HDAC inhibitors as a potential therapeutic direction in Alzheimer’s disease. Transl Neurodegener. 2017;6(1):1–6. doi: 10.1186/s40035-017-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sung YM, Lee T, Yoon H, DiBattista AM, Song JM, Sohn Y, et al. Mercaptoacetamide-based class II HDAC inhibitor lowers Aβ levels and improves learning and memory in a mouse model of Alzheimer’s disease. Exp Neurol. 2013;239:192–201. doi: 10.1016/j.expneurol.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner F, Zhang Y-L, Fass D, Joseph N, Gale J, Weïwer M, et al. Kinetically selective inhibitors of histone deacetylase 2 (HDAC2) as cognition enhancers. Chem Sci. 2015;6(1):804–815. doi: 10.1039/C4SC02130D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, et al. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459(7248):802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marzi SJ, Leung SK, Ribarska T, Hannon E, Smith AR, Pishva E, et al. A histone acetylome-wide association study of Alzheimer’s disease identifies disease-associated H3K27ac differences in the entorhinal cortex. Nat Neurosci. 2018;21(11):1618–1627. doi: 10.1038/s41593-018-0253-7. [DOI] [PubMed] [Google Scholar]
- 48.Klein H-U, McCabe C, Gjoneska E, Sullivan SE, Kaskow BJ, Tang A, et al. Epigenome-wide study uncovers large-scale changes in histone acetylation driven by tau pathology in aging and Alzheimer’s human brains. Nat Neurosci. 2019;22(1):37–46. doi: 10.1038/s41593-018-0291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324(5929):929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, Mastroeni D, et al. The epigenetics of aging and neurodegeneration. Prog Neurobiol. 2015;131:21–64. doi: 10.1016/j.pneurobio.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J. Epigenetic changes in Alzheimer’s disease: decrements in DNA methylation. Neurobiol Aging. 2010;31(12):2025–2037. doi: 10.1016/j.neurobiolaging.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mastroeni D, McKee A, Grover A, Rogers J, Coleman PD. Epigenetic differences in cortical neurons from a pair of monozygotic twins discordant for Alzheimer’s disease. PLoS One. 2009;4(8):e6617. doi: 10.1371/journal.pone.0006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coppieters N, Dieriks BV, Lill C, Faull RL, Curtis MA, Dragunow M. Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain. Neurobiol Aging. 2014;35(6):1334–1344. doi: 10.1016/j.neurobiolaging.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 54.Lashley T, Gami P, Valizadeh N, Li A, Revesz T, Balazs R. Alterations in global DNA methylation and hydroxymethylation are not detected in Alzheimer’s disease. Neuropathol Appl Neurobiol. 2015;41(4):497–506. doi: 10.1111/nan.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao J, Keleshian V, Klein S, Rapoport S. Epigenetic modifications in frontal cortex from Alzheimer’s disease and bipolar disorder patients. Transl Psychiatry. 2012;2(7):e132-e. doi: 10.1038/tp.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Jager PL, Srivastava G, Lunnon K, Burgess J, Schalkwyk LC, Yu L, et al. Alzheimer’s disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Neurosci. 2014;17(9):1156–1163. doi: 10.1038/nn.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu L, Chibnik LB, Srivastava GP, Pochet N, Yang J, Xu J, et al. Association of Brain DNA methylation in SORL1, ABCA7, HLA-DRB5, SLC24A4, and BIN1 with pathological diagnosis of Alzheimer disease. JAMA Neurol. 2015;72(1):15–24. doi: 10.1001/jamaneurol.2014.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lunnon K, Smith R, Hannon E, De Jager PL, Srivastava G, Volta M, et al. Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer’s disease. Nat Neurosci. 2014;17(9):1164–1170. doi: 10.1038/nn.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith RG, Hannon E, De Jager PL, Chibnik L, Lott SJ, Condliffe D, et al. Elevated DNA methylation across a 48-kb region spanning the HOXA gene cluster is associated with Alzheimer’s disease neuropathology. Alzheimers Dement. 2018;14(12):1580–1588. doi: 10.1016/j.jalz.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lardenoije R, Roubroeks JA, Pishva E, Leber M, Wagner H, Iatrou A, et al. Alzheimer’s disease-associated (hydroxy) methylomic changes in the brain and blood. Clin Epigenetics. 2019;11(1):164. doi: 10.1186/s13148-019-0755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watson CT, Roussos P, Garg P, Ho DJ, Azam N, Katsel PL, et al. Genome-wide DNA methylation profiling in the superior temporal gyrus reveals epigenetic signatures associated with Alzheimer’s disease. Genome Med. 2016;8(1):1–14. doi: 10.1186/s13073-015-0258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith RG, Pishva E, Shireby G, Smith AR, Roubroeks JA, Hannon E, et al. A meta-analysis of epigenome-wide association studies in Alzheimer’s disease highlights novel differentially methylated loci across cortex. Nat Commun. 2021;12(1):1–13. doi: 10.1038/s41467-020-20314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gasparoni G, Bultmann S, Lutsik P, Kraus TF, Sordon S, Vlcek J, et al. DNA methylation analysis on purified neurons and glia dissects age and Alzheimer’s disease-specific changes in the human cortex. Epigenetics Chromatin. 2018;11(1):41. doi: 10.1186/s13072-018-0211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hernández HG, Sandoval-Hernández AG, Garrido-Gil P, Labandeira-Garcia JL, Zelaya MV, Bayon GF, et al. Alzheimer’s disease DNA methylome of pyramidal layers in frontal cortex: laser-assisted microdissection study. Epigenomics. 2018;10(11):1365–1382. doi: 10.2217/epi-2017-0160. [DOI] [PubMed] [Google Scholar]
- 65.Mano T, Nagata K, Nonaka T, Tarutani A, Imamura T, Hashimoto T, et al. Neuron-specific methylome analysis reveals epigenetic regulation and tau-related dysfunction of BRCA1 in Alzheimer’s disease. Proc Natl Acad Sci. 2017;114(45):E9645–E9E54. doi: 10.1073/pnas.1707151114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mastroeni D, Sekar S, Nolz J, Delvaux E, Lunnon K, Mill J, et al. ANK1 is up-regulated in laser captured microglia in Alzheimer’s brain; the importance of addressing cellular heterogeneity. PLoS One. 2017;12(7):e0177814. doi: 10.1371/journal.pone.0177814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li P, Marshall L, Oh G, Jakubowski JL, Groot D, He Y, et al. Epigenetic dysregulation of enhancers in neurons is associated with Alzheimer’s disease pathology and cognitive symptoms. Nat Commun. 2019;10(1):1–14. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao J, Zhu Y, Yang J, Li L, Wu H, De Jager PL, et al. A genome-wide profiling of brain DNA hydroxymethylation in Alzheimer’s disease. Alzheimers Dement. 2017;13(6):674–688. doi: 10.1016/j.jalz.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith AR, Smith RG, Pishva E, Hannon E, Roubroeks JA, Burrage J, et al. Parallel profiling of DNA methylation and hydroxymethylation highlights neuropathology-associated epigenetic variation in Alzheimer’s disease. Clin Epigenetics. 2019;11(1):1–13. doi: 10.1186/s13148-019-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ong C-T, Corces VG. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat Rev Genet. 2011;12(4):283–293. doi: 10.1038/nrg2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dowen JM, Fan ZP, Hnisz D, Ren G, Abraham BJ, Zhang LN, et al. Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell. 2014;159(2):374–387. doi: 10.1016/j.cell.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467(7314):430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schoenfelder S, Fraser P. Long-range enhancer–promoter contacts in gene expression control. Nat Rev Genet. 2019;20(8):437–55. [DOI] [PubMed]
- 74.Blankvoort S, Witter MP, Noonan J, Cotney J, Kentros C. Marked diversity of unique cortical enhancers enables neuron-specific tools by enhancer-driven gene expression. Curr Biol. 2018;28(13):2103–14. e5. [DOI] [PMC free article] [PubMed]
- 75.Dong X, Liao Z, Gritsch D, Hadzhiev Y, Bai Y, Locascio JJ, et al. Enhancers active in dopamine neurons are a primary link between genetic variation and neuropsychiatric disease. Nat Neurosci. 2018;21(10):1482–92. [DOI] [PMC free article] [PubMed]
- 76.Fullard JF, Hauberg ME, Bendl J, Egervari G, Cirnaru M-D, Reach SM, et al. An atlas of chromatin accessibility in the adult human brain. Genome Res. 2018;28(8):1243–1252. doi: 10.1101/gr.232488.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gray JM, Kim T-K, West AE, Nord AS, Markenscoff-Papadimitriou E, Lomvardas S. Genomic views of transcriptional enhancers: essential determinants of cellular identity and activity-dependent responses in the CNS. J Neurosci. 2015;35(41):13819–13826. doi: 10.1523/JNEUROSCI.2622-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carullo NV, Day JJ. Genomic enhancers in brain health and disease. Genes. 2019;10(1):43. doi: 10.3390/genes10010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gjoneska E, Pfenning AR, Mathys H, Quon G, Kundaje A, Tsai L-H, et al. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature. 2015;518(7539):365–369. doi: 10.1038/nature14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kikuchi M, Hara N, Hasegawa M, Miyashita A, Kuwano R, Ikeuchi T, et al. Enhancer variants associated with Alzheimer’s disease affect gene expression via chromatin looping. BMC Med Genet. 2019;12(1):128. doi: 10.1186/s12920-019-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nott A, Holtman IR, Coufal NG, Schlachetzki JC, Yu M, Hu R, et al. Brain cell type–specific enhancer–promoter interactome maps and disease-risk association. Science. 2019;366(6469):1134–1139. doi: 10.1126/science.aay0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Padhy B, Hayat B, Nanda GG, Mohanty PP, Alone DP. Pseudoexfoliation and Alzheimer’s associated CLU risk variant, rs2279590, lies within an enhancer element and regulates CLU, EPHX2 and PTK2B gene expression. Hum Mol Genet. 2017;26(22):4519–4529. doi: 10.1093/hmg/ddx329. [DOI] [PubMed] [Google Scholar]
- 83.Heinz S, Romanoski CE, Benner C, Glass CK. The selection and function of cell type-specific enhancers. Nat Rev Mol Cell Biol. 2015;16(3):144–154. doi: 10.1038/nrm3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Surova O, Zhivotovsky B. Various modes of cell death induced by DNA damage. Oncogene. 2013;32(33):3789–3797. doi: 10.1038/onc.2012.556. [DOI] [PubMed] [Google Scholar]
- 85.Lin X, Kapoor A, Gu Y, Chow MJ, Peng J, Zhao K, et al. Contributions of DNA damage to Alzheimer’s disease. Int J Mol Sci. 2020;21(5):1666. doi: 10.3390/ijms21051666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adamec E, Vonsattel JP, Nixon RA. DNA strand breaks in Alzheimer’s disease. Brain Res. 1999;849(1–2):67–77. doi: 10.1016/S0006-8993(99)02004-1. [DOI] [PubMed] [Google Scholar]
- 87.Myung N-H, Zhu X, Kruman II, Castellani RJ, Petersen RB, Siedlak SL, et al. Evidence of DNA damage in Alzheimer disease: phosphorylation of histone H2AX in astrocytes. Age. 2008;30(4):209–215. doi: 10.1007/s11357-008-9050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shanbhag NM, Evans MD, Mao W, Nana AL, Seeley WW, Adame A, et al. Early neuronal accumulation of DNA double strand breaks in Alzheimer’s disease. Acta Neuropathol Commun. 2019;7(1):77. doi: 10.1186/s40478-019-0723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peña-Bautista C, Tirle T, López-Nogueroles M, Vento M, Baquero M, Cháfer-Pericás C. Oxidative damage of DNA as early marker of Alzheimer’s disease. Int J Mol Sci. 2019;20(24):6136. doi: 10.3390/ijms20246136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lillenes MS, Rabano A, Støen M, Riaz T, Misaghian D, Møllersen L, et al. Altered DNA base excision repair profile in brain tissue and blood in Alzheimer’s disease. Mol Brain. 2016;9(1):61. doi: 10.1186/s13041-016-0237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weissman L, Jo D-G, Sørensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, et al. Defective DNA base excision repair in brain from individuals with Alzheimer’s disease and amnestic mild cognitive impairment. Nucleic Acids Res. 2007;35(16):5545–5555. doi: 10.1093/nar/gkm605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahmed AA, Smoczer C, Pace B, Patterson D, Cress CD. Loss of DNA polymerase β induces cellular senescence. Environ Mol Mutagen. 2018;59(7):603–612. doi: 10.1002/em.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Love S, Barber R, Wilcock GK. Increased poly (ADP-ribosyl) ation of nuclear proteins in Alzheimer’s disease. Brain. 1999;122(2):247–253. doi: 10.1093/brain/122.2.247. [DOI] [PubMed] [Google Scholar]
- 94.Strosznajder JB, Czapski GA, Adamczyk A, Strosznajder RP. Poly (ADP-ribose) polymerase-1 in amyloid beta toxicity and Alzheimer’s disease. Mol Neurobiol. 2012;46(1):78–84. doi: 10.1007/s12035-012-8258-9. [DOI] [PubMed] [Google Scholar]
- 95.Martire S, Fuso A, Rotili D, Tempera I, Giordano C, De Zottis I, et al. PARP-1 modulates amyloid beta peptide-induced neuronal damage. PLoS One. 2013;8(9):e72169-e. doi: 10.1371/journal.pone.0072169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen X, Chen J, Li J, Kofler J, Herrup K. Neurons in vulnerable regions of the Alzheimer’s disease brain display reduced ATM signaling. eNeuro. 2016;3(1):ENEURO.0124-15.2016. doi: 10.1523/ENEURO.0124-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wezyk M, Szybinska A, Wojsiat J, Szczerba M, Day K, Ronnholm H, et al. Overactive BRCA1 affects presenilin 1 in induced pluripotent stem cell-derived neurons in Alzheimer’s disease. J Alzheimers Dis. 2018;62(1):175–202. doi: 10.3233/JAD-170830. [DOI] [PubMed] [Google Scholar]
- 98.Shackelford DA. DNA end joining activity is reduced in Alzheimer’s disease. Neurobiol Aging. 2006;27(4):596–605. doi: 10.1016/j.neurobiolaging.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 99.Jacobsen E, Beach T, Shen Y, Li R, Chang Y. Deficiency of the Mre11 DNA repair complex in Alzheimer’s disease brains. Mol Brain Res. 2004;128(1):1–7. doi: 10.1016/j.molbrainres.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 100.Barnum KJ, O'Connell MJ. Cell cycle regulation by checkpoints. Methods Mol Biol. 2014;1170:29–40. doi: 10.1007/978-1-4939-0888-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu W, Giangrande PH, Nevins JR. E2Fs link the control of G1/S and G2/M transcription. EMBO J. 2004;23(23):4615–4626. doi: 10.1038/sj.emboj.7600459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer’s disease. J Neurosci. 2001;21(8):2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Malik B, Currais A, Andres A, Towlson C, Pitsi D, Nunes A, et al. Loss of neuronal cell cycle control as a mechanism of neurodegeneration in the presenilin-1 Alzheimer’s disease brain. Cell Cycle. 2008;7(5):637–646. doi: 10.4161/cc.7.5.5427. [DOI] [PubMed] [Google Scholar]
- 104.Chao A-C, Chen C-H, Chang S-H, Huang C-T, Hwang W-C, Yang D-I. Id1 and sonic hedgehog mediate cell cycle reentry and apoptosis induced by amyloid beta-peptide in post-mitotic cortical neurons. Mol Neurobiol. 2019;56(1):465–489. doi: 10.1007/s12035-018-1098-5. [DOI] [PubMed] [Google Scholar]
- 105.Vincent I, Jicha G, Rosado M, Dickson DW. Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer’s disease brain. J Neurosci. 1997;17(10):3588–3598. doi: 10.1523/JNEUROSCI.17-10-03588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Walton CC, Zhang W, Patiño-Parrado I, Barrio-Alonso E, Garrido J-J, Frade JM. Primary neurons can enter M-phase. Sci Rep. 2019;9(1):1–15. doi: 10.1038/s41598-019-40462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Copani A, Sortino MA, Nicoletti F, Bruno V, Ubertia D, Memo M. Activation of cell-cycle-associated proteins in neuronal death: a mandatory or dispensable path? Trends Neurosci. 2001;24(1):25–31. doi: 10.1016/S0166-2236(00)01663-5. [DOI] [PubMed] [Google Scholar]
- 108.Chao A-C, Chen C-H, Wu M-H, Hou B-Y, Yang D-I. Roles of Id1/HIF-1 and CDK5/HIF-1 in cell cycle reentry induced by amyloid-beta peptide in post-mitotic cortical neuron. Biochim Biophys Acta, Mol Cell Res. 1867;2020(4):118628. doi: 10.1016/j.bbamcr.2019.118628. [DOI] [PubMed] [Google Scholar]
- 109.Hung Y-H, Chang S-H, Huang C-T, Yin J-H, Hwang C-S, Yang L-Y, et al. Inhibitor of differentiation-1 and hypoxia-inducible factor-1 mediate sonic hedgehog induction by amyloid beta-peptide in rat cortical neurons. Mol Neurobiol. 2016;53(2):793–809. doi: 10.1007/s12035-014-9046-5. [DOI] [PubMed] [Google Scholar]
- 110.Giovanni A, Wirtz-Brugger F, Keramaris E, Slack R, Park DS. Involvement of cell cycle elements, cyclin-dependent kinases, pRB, and E2F· DP, in B-amyloid-induced neuronal death. J Biol Chem. 1999;274(27):19011–19016. doi: 10.1074/jbc.274.27.19011. [DOI] [PubMed] [Google Scholar]
- 111.Majd S, Zarifkar A, Rastegar K, Takhshid MA. Different fibrillar Aβ 1–42 concentrations induce adult hippocampal neurons to reenter various phases of the cell cycle. Brain Res. 2008;1218:224–229. doi: 10.1016/j.brainres.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 112.Lopes JP, Oliveira CR, Agostinho P. Cdk5 acts as a mediator of neuronal cell cycle re-entry triggered by amyloid-β and prion peptides. Cell Cycle. 2009;8(1):97–104. doi: 10.4161/cc.8.1.7506. [DOI] [PubMed] [Google Scholar]
- 113.Keeney JT, Swomley AM, Harris JL, Fiorini A, Mitov MI, Perluigi M, et al. Cell cycle proteins in brain in mild cognitive impairment: insights into progression to Alzheimer disease. Neurotox Res. 2012;22(3):220–230. doi: 10.1007/s12640-011-9287-2. [DOI] [PubMed] [Google Scholar]
- 114.Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J Neurosci. 2003;23(7):2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Arendt T, Brückner MK, Mosch B, Lösche A. Selective cell death of hyperploid neurons in Alzheimer’s disease. Am J Pathol. 2010;177(1):15–20. doi: 10.2353/ajpath.2010.090955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park KH, Hallows JL, Chakrabarty P, Davies P, Vincent I. Conditional neuronal simian virus 40 T antigen expression induces Alzheimer-like tau and amyloid pathology in mice. J Neurosci. 2007;27(11):2969–2978. doi: 10.1523/JNEUROSCI.0186-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barrio-Alonso E, Hernández-Vivanco A, Walton CC, Perea G, Frade J. Cell cycle reentry triggers hyperploidization and synaptic dysfunction followed by delayed cell death in differentiated cortical neurons. Sci Rep. 2018;8(1):1–14. doi: 10.1038/s41598-018-32708-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McShea A, Lee HG, Petersen RB, Casadesus G, Vincent I, Linford NJ, et al. Neuronal cell cycle re-entry mediates Alzheimer disease-type changes. Biochim Biophys Acta, Mol Cell Res. 2007;1772(4):467–472. doi: 10.1016/j.bbadis.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 119.Huang F, Wang M, Liu R, Wang J-Z, Schadt E, Haroutunian V, et al. CDT2-controlled cell cycle reentry regulates the pathogenesis of Alzheimer’s disease. Alzheimers Dement. 2019;15(2):217–231. doi: 10.1016/j.jalz.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ki I, Ando K, Takeda S, Satoh Y, Seki T, Itohara S, et al. Neuron-specific phosphorylation of Alzheimer’s β-amyloid precursor protein by cyclin-dependent kinase 5. J Neurochem. 2000;75(3):1085–1091. doi: 10.1046/j.1471-4159.2000.0751085.x. [DOI] [PubMed] [Google Scholar]
- 121.Lee M-S, Kao S-C, Lemere CA, Xia W, Tseng H-C, Zhou Y, et al. APP processing is regulated by cytoplasmic phosphorylation. J Cell Biol. 2003;163(1):83–95. doi: 10.1083/jcb.200301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu F, Su Y, Li B, Zhou Y, Ryder J, Gonzalez-DeWhitt P, et al. Regulation of amyloid precursor protein (APP) phosphorylation and processing by p35/Cdk5 and p25/Cdk5. FEBS Lett. 2003;547(1–3):193–196. doi: 10.1016/S0014-5793(03)00714-2. [DOI] [PubMed] [Google Scholar]
- 123.Suzuki T, Oishi M, Marshak D, Czernik A, Nairn A, Greengard P. Cell cycle-dependent regulation of the phosphorylation and metabolism of the Alzheimer amyloid precursor protein. EMBO J. 1994;13(5):1114–1122. doi: 10.1002/j.1460-2075.1994.tb06360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kimura T, Ishiguro K, Hisanaga S-i. Physiological and pathological phosphorylation of tau by Cdk5. Front Mol Neurosci. 2014;7:65. doi: 10.3389/fnmol.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patrick GN, Zukerberg L, Nikolic M, de La Monte S, Dikkes P, Tsai L-H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402(6762):615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 126.Yang K, Hitomi M, Stacey DW. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006;1:32. doi: 10.1186/1747-1028-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Varvel NH, Bhaskar K, Patil AR, Pimplikar SW, Herrup K, Lamb BT. Aβ oligomers induce neuronal cell cycle events in Alzheimer’s disease. J Neurosci. 2008;28(43):10786–10793. doi: 10.1523/JNEUROSCI.2441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seward ME, Swanson E, Norambuena A, Reimann A, Cochran JN, Li R, et al. Amyloid-β signals through tau to drive ectopic neuronal cell cycle re-entry in Alzheimer’s disease. J Cell Sci. 2013;126(5):1278–1286. doi: 10.1242/jcs.1125880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Khurana V, Lu Y, Steinhilb ML, Oldham S, Shulman JM, Feany MB. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr Biol. 2006;16(3):230–241. doi: 10.1016/j.cub.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 130.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 131.Toussaint O, Royer V, Salmon M, Remacle J. Stress-induced premature senescence and tissue ageing. Biochem Pharmacol. 2002;64(5–6):1007–1009. doi: 10.1016/S0006-2952(02)01170-X. [DOI] [PubMed] [Google Scholar]
- 132.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24(22):2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Coppé J-P, Desprez P-Y, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509(7501):439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol. 2004;6(2):168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 136.Nakamura AJ, Chiang YJ, Hathcock KS, Horikawa I, Sedelnikova OA, Hodes RJ, et al. Both telomeric and non-telomeric DNA damage are determinants of mammalian cellular senescence. Epigenetics Chromatin. 2008;1(1):6. doi: 10.1186/1756-8935-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rodier F, Coppé J-P, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kang HT, Lee KB, Kim SY, Choi HR, Park SC. Autophagy impairment induces premature senescence in primary human fibroblasts. PLoS One. 2011;6(8):e23367. doi: 10.1371/journal.pone.0023367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Matos L, Gouveia A, Almeida H. Copper ability to induce premature senescence in human fibroblasts. Age. 2012;34(4):783–794. doi: 10.1007/s11357-011-9276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Crowe EP, Tuzer F, Gregory BD, Donahue G, Gosai SJ, Cohen J, et al. Changes in the transcriptome of human astrocytes accompanying oxidative stress-induced senescence. Front Aging Neurosci. 2016;8:208. doi: 10.3389/fnagi.2016.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018;17(6):e12840. doi: 10.1111/acel.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ishikawa S, Ishikawa F. Proteostasis failure and cellular senescence in long-term cultured postmitotic rat neurons. Aging Cell. 2020;19(1):e13071. doi: 10.1111/acel.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–344. doi: 10.1016/S0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 144.Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci. 2019;22(5):719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, et al. Astrocyte senescence as a component of Alzheimer’s disease. PLoS One. 2012;7(9):e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Streit WJ, Braak H, Xue Q-S, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118(4):475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 2007;10(1):61–74. doi: 10.1089/rej.2006.9096. [DOI] [PubMed] [Google Scholar]
- 148.Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1(1):14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci. 2016;19(8):995–998. doi: 10.1038/nn.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Amram S, Iram T, Lazdon E, Vassar R, Porath IB, Frenkel D. Astrocyte senescence in an Alzheimer’s disease mouse model is mediated by TGF-β1 and results in neurotoxicity. bioRxiv. 2019:700013.
- 151.Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562(7728):578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sun L, Yu R, Dang W. Chromatin architectural changes during cellular senescence and aging. Genes. 2018;9(4):211. doi: 10.3390/genes9040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang N, Sen P. The senescent cell epigenome. Aging (Albany NY) 2018;10(11):3590. doi: 10.18632/aging.101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Casella G, Munk R, Kim KM, Piao Y, De S, Abdelmohsen K, et al. Transcriptome signature of cellular senescence. Nucleic Acids Res. 2019;47(14):7294–7305. doi: 10.1093/nar/gkz555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.El Hajjar J, Chatoo W, Hanna R, Nkanza P, Tétreault N, Tse YC, et al. Heterochromatic genome instability and neurodegeneration sharing similarities with Alzheimer’s disease in old Bmi1+/− mice. Sci Rep. 2019;9(1):1–16. doi: 10.1038/s41598-018-37444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nguyen MD, Boudreau M, Kriz J, Couillard-Després S, Kaplan DR, Julien J-P. Cell cycle regulators in the neuronal death pathway of amyotrophic lateral sclerosis caused by mutant superoxide dismutase 1. J Neurosci. 2003;23(6):2131–2140. doi: 10.1523/JNEUROSCI.23-06-02131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhu X, Rottkamp CA, Boux H, Takeda A, Perry G, Smith MA. Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J Neuropathol Exp Neurol. 2000;59(10):880–888. doi: 10.1093/jnen/59.10.880. [DOI] [PubMed] [Google Scholar]
- 158.Kirouac L, Rajic AJ, Cribbs DH, Padmanabhan J. Activation of Ras-ERK signaling and GSK-3 by amyloid precursor protein and amyloid beta facilitates neurodegeneration in Alzheimer’s disease. eNeuro. 2017;4(2):ENEURO.0149-16.2017. doi: 10.1523/ENEURO.0149-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ, et al. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41(4):549–561. doi: 10.1016/S0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- 160.Gil L, Niño SA, Chi-Ahumada E, Rodríguez-Leyva I, Guerrero C, Rebolledo AB, et al. Perinuclear lamin A and nucleoplasmic lamin B2 characterize two types of hippocampal neurons through Alzheimer’s disease progression. Int J Mol Sci. 2020;21(5):1841. doi: 10.3390/ijms21051841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Canchi S, Raao B, Masliah D, Rosenthal SB, Sasik R, Fisch KM, et al. Integrating gene and protein expression reveals perturbed functional networks in Alzheimer’s disease. Cell Rep. 2019;28(4):1103-16. e4. doi: 10.1016/j.celrep.2019.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Potashkin JA, Bottero V, Santiago JA, Quinn JP. Computational identification of key genes that may regulate gene expression reprogramming in Alzheimer’s patients. PLoS One. 2019;14(9):e0222921. doi: 10.1371/journal.pone.0222921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vargas DM, De Bastiani MA, Zimmer ER, Klamt F. Alzheimer’s disease master regulators analysis: search for potential molecular targets and drug repositioning candidates. Alzheimers Res Ther. 2018;10(1):59. doi: 10.1186/s13195-018-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bai B, Hales CM, Chen P-C, Gozal Y, Dammer EB, Fritz JJ, et al. U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer’s disease. Proc Natl Acad Sci. 2013;110(41):16562–16567. doi: 10.1073/pnas.1310249110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Raj T, Li YI, Wong G, Humphrey J, Wang M, Ramdhani S, et al. Integrative transcriptome analyses of the aging brain implicate altered splicing in Alzheimer’s disease susceptibility. Nat Genet. 2018;50(11):1584–1592. doi: 10.1038/s41588-018-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hsieh Y-C, Guo C, Yalamanchili HK, Abreha M, Al-Ouran R, Li Y, et al. Tau-mediated disruption of the spliceosome triggers cryptic RNA splicing and neurodegeneration in Alzheimer’s disease. Cell Rep. 2019;29(2):301-16. e10. doi: 10.1016/j.celrep.2019.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Khermesh K, D'Erchia AM, Barak M, Annese A, Wachtel C, Levanon EY, et al. Reduced levels of protein recoding by A-to-I RNA editing in Alzheimer’s disease. Rna. 2016;22(2):290–302. doi: 10.1261/rna.054627.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Montalbano M, McAllen S, Puangmalai N, Sengupta U, Bhatt N, Johnson OD, et al. RNA-binding proteins Musashi and tau soluble aggregates initiate nuclear dysfunction. Nat Commun. 2020;11(1):1–16. doi: 10.1038/s41467-020-18022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang M, Qin L, Tang B. MicroRNAs in Alzheimer’s disease. Front Genet. 2019;10:153. doi: 10.3389/fgene.2019.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sun Q, Hao Q, Prasanth KV. Nuclear long noncoding RNAs: key regulators of gene expression. Trends Genet. 2018;34(2):142–157. doi: 10.1016/j.tig.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carullo NVN, Phillips Iii RA, Simon RC, Soto SAR, Hinds JE, Salisbury AJ, et al. Enhancer RNAs predict enhancer–gene regulatory links and are critical for enhancer function in neuronal systems. Nucleic Acids Res. 2020;48:9550. doi: 10.1093/nar/gkaa671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of β-secretase. Nat Med. 2008;14(7):723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Faghihi MA, Zhang M, Huang J, Modarresi F, Van der Brug MP, Nalls MA, et al. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol. 2010;11(5):R56. doi: 10.1186/gb-2010-11-5-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sola M, Magrin C, Pedrioli G, Pinton S, Salvadè A, Papin S, et al. Tau affects P53 function and cell fate during the DNA damage response. Commun Biol. 2020;3(1):1–15. doi: 10.1038/s42003-020-0975-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Martínez-Cué C, Rueda N. Cellular senescence in neurodegenerative diseases. Front Cell Neurosci. 2020;14:16. doi: 10.3389/fncel.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tokarz P, Kaarniranta K, Blasiak J. Role of the cell cycle re-initiation in DNA damage response of post-mitotic cells and its implication in the pathogenesis of neurodegenerative diseases. Rejuvenation Res. 2016;19(2):131–139. doi: 10.1089/rej.2015.1717. [DOI] [PubMed] [Google Scholar]
- 177.Fielder E, Von Zglinicki T, Jurk D. The DNA damage response in neurons: die by apoptosis or survive in a senescence-like state? J Alzheimers Dis. 2017;60(s1):S107–SS31. doi: 10.3233/JAD-161221. [DOI] [PubMed] [Google Scholar]
- 178.Ng B, White CC, Klein HU, Sieberts SK, McCabe C, Patrick E, et al. An xQTL map integrates the genetic architecture of the human brain's transcriptome and epigenome. Nat Neurosci. 2017;20(10):1418–1426. doi: 10.1038/nn.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, et al. REST and stress resistance in ageing and Alzheimer’s disease. Nature. 2014;507(7493):448–454. doi: 10.1038/nature13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Meyer-Nava S, Nieto-Caballero VE, Zurita M, Valadez-Graham V. Insights into HP1a-chromatin interactions. Cells. 2020;9(8):1866. doi: 10.3390/cells9081866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sabari BR, Dall’Agnese A, Young RA. Biomolecular condensates in the nucleus. Trends Biochem Sci. 2020;45:961. doi: 10.1016/j.tibs.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li CH, Coffey EL, Dall'Agnese A, Hannett NM, Tang X, Henninger JE, et al. MeCP2 links heterochromatin condensates and neurodevelopmental disease. Nature. 2020;586(7829):440–444. doi: 10.1038/s41586-020-2574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen W-T, Lu A, Craessaerts K, Pavie B, Frigerio CS, Corthout N, et al. Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell. 2020;182(4):976-91. e19. doi: 10.1016/j.cell.2020.06.038. [DOI] [PubMed] [Google Scholar]
- 184.Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019;570(7761):332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Johnson TS, Xiang S, Helm BR, Abrams ZB, Neidecker P, Machiraju R, et al. Spatial cell type composition in normal and Alzheimers human brains is revealed using integrated mouse and human single cell RNA sequencing. Sci Rep. 2020;10(1):1–14. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Otero-Garcia M, Xue Y-Q, Shakouri T, Deng Y, Morabito S, Allison T, et al. Single-soma transcriptomics of tangle-bearing neurons in Alzheimer’s disease reveals the signatures of tau-associated synaptic dysfunction. BioRxiv. 2020:2020.05.11.088591.
- 187.Lin Y-T, Seo J, Gao F, Feldman HM, Wen H-L, Penney J, et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron. 2018;98(6):1141-54. e7. doi: 10.1016/j.neuron.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhao J, Fu Y, Yamazaki Y, Ren Y, Davis MD, Liu C-C, et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat Commun. 2020;11(1):1–14. doi: 10.1038/s41467-019-13993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Martins S, Müller-Schiffmann A, Erichsen L, Bohndorf M, Wruck W, Sleegers K, et al. IPSC-derived neuronal cultures carrying the Alzheimer’s disease associated TREM2 R47H variant enables the construction of an Aβ-induced gene regulatory network. Int J Mol Sci. 2020;21(12):4516. doi: 10.3390/ijms21124516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kampmann M. CRISPR-based functional genomics for neurological disease. Nat Rev Neurol. 2020;16(9):465–480. doi: 10.1038/s41582-020-0373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tian R, Gachechiladze MA, Ludwig CH, Laurie MT, Hong JY, Nathaniel D, et al. CRISPR interference-based platform for multimodal genetic screens in human iPSC-derived neurons. Neuron. 2019;104(2):239-55. e12. doi: 10.1016/j.neuron.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tian R, Abarientos A, Hong J, Hashemi SH, Yan R, Dräger N, et al. Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat Neurosci. 2021;24(7):1020–34. [DOI] [PMC free article] [PubMed]
- 193.Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, et al. Programmable base editing of a• T to G• C in genomic DNA without DNA cleavage. Nature. 2017;551(7681):464–471. doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576(7785):149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dekker J, Belmont AS, Guttman M, Leshyk VO, Lis JT, Lomvardas S, et al. The 4D nucleome project. Nature. 2017;549(7671):219–226. doi: 10.1038/nature23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Boettiger A, Murphy S. Advances in chromatin imaging at kilobase-scale resolution. Trends Genet. 2020;36(4):273–287. doi: 10.1016/j.tig.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tang Z, Luo OJ, Li X, Zheng M, Zhu JJ, Szalaj P, et al. CTCF-mediated human 3D genome architecture reveals chromatin topology for transcription. Cell. 2015;163(7):1611–1627. doi: 10.1016/j.cell.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326(5950):289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Winick-Ng W, Rylett RJ. Into the fourth dimension: dysregulation of genome architecture in aging and Alzheimer’s disease. Front Mol Neurosci. 2018;11:60. doi: 10.3389/fnmol.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.