Abstract
Background
Catheter ablation is an established therapy for atrial fibrillation but is limited by recurrence; efforts have been made to identify biomarkers that predict recurrence. We investigated the effect of baseline NT-proBNP on AF recurrence following catheter ablation in patients randomized to aggressive (< 120/80 mmHg) or standard blood pressure management (< 140/90 mmHg) in the Substrate Modification with Aggressive Blood Pressure Control trial (SMAC-AF).
Methods
The SMAC-AF study included 173 patients resistant or intolerant to at least one class I or III antiarrhythmic drug. We studied the effect of baseline NT-proBNP on the primary outcome of AF recurrence > 3 months post-ablation.
Results
Of the 173 patients, 88 were randomized to the aggressive cohort, and 85 into the standard group. The primary outcome occurred in 61.4% of those in the aggressive arm, versus 61.2% in the standard arm. In the aggressive group, logNT-proBNP predicted recurrence (HR 1.28, p = 0.04, adjusted HR 1.43, p = 0.03), while in the standard cohort, it did not (HR 0.94, p = 0.62, adjusted HR 0.83, p = 0.22). NT-proBNP ≥ 280 pg/mL also predicted occurrence in the aggressive (HR 1.98, p = 0.02) but not the standard cohort (HR 1.00, p = 1.00).
Conclusion
We conclude that pre-ablation NT-proBNP may be useful in predicting recurrence in hypertensive patients and identifying patients who benefit from aggressive blood control and upstream therapies.
Trial registration: NCT00438113, registered February 21, 2007.
Keywords: Atrial fibrillation, Catheter ablation, Recurrence, Biomarker
Introduction
Radiofrequency catheter ablation has been established as a valuable therapeutic option for atrial fibrillation (AF), although high rates of recurrence after ablation are a limitation. Factors predicting recurrence include type of AF (paroxysmal versus persistent), ejection fraction, structural heart disease and hypertension [1, 2]. The recent Substrate Modification with Aggressive Blood Pressure Control (SMAC-AF) randomized control trial did not find that aggressive blood pressure control prior to ablation had an effect on recurrence rates of AF post ablation [3]. Atrial fibrosis and remodeling is thought to play a role in predisposition to AF [4], and there has been developing interest in identifying biomarkers associated with fibrosis that may predict response to therapy for AF [5–7]. Brain natriuretic peptide (NT-proBNP), is commonly used to guide clinical decision in heart failure management, and has been studied as a biomarker [8, 9]. NT-proBNP is released by myocytes in response to adverse hemodynamic conditions [10], and is also associated with cardiac fibrosis [11]. Thus, it has been theorized that NT-proBNP levels may be reflective of underlying predisposition to AF, as well as being a marker of atrial fibrosis remodeling. Pre-ablation NT-proBNP has been shown in some populations to be a predictor of recurrence post-ablation [12–14].
The purpose of this study was to explore whether NT-proBNP is useful as a marker of recurrence post-ablation in hypertensive patients. NT-proBNP has not been shown to be elevated in those with hypertension without cardiac dysfunction [15]. Furthermore, the randomized nature of SMAC-AF allows us to investigate how the association of NT-proBNP and outcomes differed with the effect of the additional blood pressure management.
Methods
This is a secondary analysis of the SMAC-AF study, the details of which have been published previously (NCT00438113). [3] In brief, 173 hypertensive patients (defined as Bp ≥ 130/80 mmHg), resistant or intolerant to at least one class I or III antiarrhythmic drug, and scheduled to undergo catheter ablation, were randomized to undergo aggressive blood pressure management (target of 120/80 mmHg) or standard management (target of 140/90 mmHg), in the time leading up to ablation (0–6 months), and throughout study follow-up. The primary outcome was time to symptomatic AF, atrial tachycardia, or atrial flutter lasting more than 30 s, 3 months after ablation. The study took place from July 2011 to October 2015; the median follow-up was 14.0 months. The protocol of the original study was approved by the local ethics review board at all participating institutions (QEII Health Sciences Centre, Halifax, NS; Centre Hospitalier Universitaire de Montreal, Montreal Heart Institute, Montreal, QC; Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, QC; London Health Sciences Center, London, ON; Quebec Heart Institute, Quebec City, QC; Hamilton Health Sciences, Hamilton, ON; Royal Jubilee Hospital, Victoria, BC; University of Ottawa Heart Institute, Ottawa, ON; Toronto General Hospital, St Michael’s Hospital, Toronto, ON; Libin Cardiovascular Institute of Alberta, Mazankowski Alberta Heart Institute, Edmonton, AB.) All methods were carried out in accordance with relevant guidelines and regulations.
Ablation protocol
All patients underwent pulmonary vein isolation with either cryoballoon therapy or radiofrequency ablation. All ablations were performed within 0–3 months of randomization. Antiarrhythmic medications were discontinued 5 half-lives prior to the ablation with the exception of amiodarone, which was discontinued for four weeks prior to ablation. Peri-procedural anticoagulation was instituted in all patients for one month prior to the procedure, and for three months post procedure. Long-term anticoagulation was left to the discretion of the investigator. Three-dimensional maps of the left atrium were constructed with the use of a nonfluoroscopic navigation system (CARTO, Biosense Webster, Diamond Bar, CA, USA, or ESI, St. Jude Medical, St. Paul, Minnesota, USA). Continuous wide-area circumferential lesions were created encircling right and left pulmonary venous ostia guided by electroanatomic mapping with an irrigated, cooled-tip radiofrequency ablation catheter with pulmonary vein isolation being the endpoint. A maximum temperature of 40 °C with maximum power of 40 W was to be delivered, except for the posterior wall, where the power was not to exceed 25 W. Lesions were complete when electrogram amplitude was reduced by ≥ 80% of baseline. Additional non-pulmonary vein ablation was left to the discretion of the operator, including complex fractional atrial electrogram ablation and additional linear ablation.
Serum markers
NT-proBNP and C-reactive protein (CRP) levels were pre-specified in the trial protocol as a secondary outcome. The biomarker levels were measured at time of randomization, as well as 12 months after randomization, using a standard CRP and NT-proBNP assay (Cobas, Roche Diagnostics GmbH). These assays reliably measure NT-proBNP concentrations from 0.6–4130 g/mL, and CRP concentrations between 0.3 and 350 mg/L.
Data collection and follow-up
Baseline clinical characteristics were measured at time of randomization. Patients were seen in clinic with a 12-lead electrocardiogram at 3 months, 6 months and every 6 months thereafter for a maximum of 24 months. Recurrence of AF was documented using transtelephonic monitoring, performed routinely twice weekly for 2 weeks, then every 3 months for the duration of the study until a primary outcome was reached. The primary outcome was atrial fibrillation, atrial tachycardia or atrial flutter (atrial cycle length ≥ 220 ms) lasting longer than 30 s and was adjudicated by a blinded committee.
Analysis
Baseline characteristics are described as mean (standard deviation) or median (first quartile, third quartile) for continuous variables, and count (percent) for categorical variables, where appropriate. Comparisons between groups were made using t-tests for continuous variables, and chi-square tests for categorical variables. We examined for correlation between baseline logNT-proBNP and baseline characteristics, using Pearson coefficient method for continuous variables, and chi-square for categorical variables.
To investigate the effect of baseline NT-proBNP on the primary outcome, a Cox proportional hazard model was used.. A receiver operator curve was used to determine a stratification level for NT-proBNP; a cutoff of 280 pg/mL was found to have maximum area-under-curve. Kaplan–Meier product-limit estimates were used to compare those with elevated NT-proBNP (≥ 280 pg/mL) and without (< 280 pg/mL); hazard ratios were calculated using a Cox proportional model. This was repeated for those in the aggressive and standard pressure control cohorts. Adjusted analysis was performed, using baseline characteristics that were clinically relevant, including sex and age, as well as those characteristics that differed between the two groups determined by p-value of < 0.10 in univariable testing, in a multivariable Cox proportional hazard model. Change in NT-proBNP from baseline to 12 months was also tested using Cox proportional hazards modeling on the primary outcome.
Results
There were 173 patients included for analysis, 88 of whom were randomized to the aggressive cohort, and 85 to the standard group. The baseline characteristics for these patients is shown in Table 1. The majority of the patients had a pre-existing diagnosis of hypertension; the remainder had a documented blood pressure ≥ 130/80 mmHg to gain entry into the study.
Table 1.
Baseline characteristics are shown in the cohort, and in patients with NT-proBNP measured, separated by treatment group
| Variable: | Overall (n = 173) |
Standard group (n = 75) | Aggressive group (n = 78) | ||||
|---|---|---|---|---|---|---|---|
| NT-proBNP ≥ 280* (n = 24) |
NT-proBNP < 280 (n = 51) |
p value | NT-proBNP ≥ 280 (n = 25) |
NT-proBNP < 280 (n = 53) |
p value | ||
| Age (years, mean ± SD) | 59.7 ± 8.7 | 62.7 ± 6.8 | 57.5 ± 9.9 | 0.02 | 62.2 ± 7.4 | 58.7 ± 8.8 | 0.09 |
| Women n (%) | 45 (26.0) | 9 (37.5) | 12 (23.5) | 0.21 | 10 (40) | 9 (17) | 0.03 |
| Type of AF n (%): | 0.09 | 0.06 | |||||
| Persistent | 74 (42.8) | 10 (41.7) | 32 (63) | 11 (44) | 35 (66) | ||
| Paroxysmal | 99 (57.2) | 14 (58.3) | 19 (37.3) | 14 (56) | 18 (34) | ||
| AF duration (months, mean ± SD) | 57.2 ± 71.5 | 42.2 ± 35.6 | 65.5 ± 79.8 | 0.12 | 67.3 ± 99.8 | 51.1 ± 58.7 | 0.52 |
| Systolic Blood Pressure (mmHg, mean ± SD) | 142.6 ± 12.0 | 144.0 ± 16.6 | 141.5 ± 11.0 | 0.50 | 145.5 ± 11.8 | 141.3 ± 10.7 | 0.13 |
| Hypertension n (%) | 130 (75.0) | 20 (83.3) | 36 (70.6) | 0.24 | 18 (72.0) | 42 (79.3) | 0.50 |
| Diabetes n (%) | 22 (12.7) | 2 (8.3) | 7 (13.7) | 0.49 | 3 (12) | 8 (15.1) | 0.71 |
| CHADS2 n (%): | 0.61 | 0.73 | |||||
| 0 | 39 (22.5) | 3 (12.5) | 13 (25.5) | 7 (28) | 11 (20.8) | ||
| 1 | 108 (62.4) | 18 (75.0) | 31 (60.8) | 13 (52) | 33 (62.3) | ||
| 2 | 18 (10.4) | 2 (8.3) | 5 (9.8) | 3 (12) | 7 (13.2) | ||
| > 2 | 8 (4.6) | 1 (4.2) | 2 (3.9) | 2 (8) | 2 (3.8) | ||
| LVEF (%, mean ± SD) | 58.6 ± 8.2 | 58.3 ± 9.4 | 60.1 ± 6.1 | 0.43 | 55.9 ± 12.1 | 59.7 ± 7.6 | 0.19 |
Characteristics are compared between those with NT-proBNP ≥ 280 pg/mL and < 280 pg/mL, within each treatment group. The aggressive cohort had a target blood pressure of < 120/80 mmHg, and the standard cohort had a target of < 140/90 mmHg
AF, atrial fibrillation; SBP, systolic blood pressure; LVEF, left ventricular ejection fraction
*pg/mL
The primary outcome occurred in 61.4% of those in the aggressive arm, versus 61.2% in the standard arm (p = 0.76). NT-proBNP data was available for 153 patients (88.4%). There was no difference in baseline NT-proBNP (p = 0.73), LVEF (p = 0.72), age (p = 0.70), sex (p = 0.51), AF duration (p = 0.14) or baseline systolic blood pressure (p = 0.67) between aggressive and standard groups. AF type, mean blood pressure and number of antihypertensives agents were similar at baseline. Patients in the aggressive blood pressure arm were treated for a median of 3.5 months (interquartile range, 2.5–4.2 months) before ablation; the standard blood pressure arm received usual care for a similar duration (median of 3.1 months, interquartile range 2.6–4.2 months, p = 0.578). At time of ablation, patients in the aggressive arm were on more antihypertensives (4.61 vs 3.00, p < 0.0001). At 6 months, compared to the standard cohort, the aggressive cohort had lower blood pressure (systolic 123.2 ± 13.2 mmHg vs 135.4 ± 15.7 mmHg, diastolic 76.7 ± 11.4 mmHg vs 80.8 ± 10.2 mmHg, p < 0.001).
NT-proBNP and baseline characteristics
There was a correlation between logNT-proBNP and age (r = 0.43, p < 0.0001), LVEF (r = 0.26, p = 0.003). LogNT-proBNP was higher in those with persistent AF (p = 0.0018). There was no relationship found between logNT-proBNP and pre-randomization AF duration (p = 0.43), LA size (p = 0.54), systolic blood pressure (SBP) (p = 0.17). Women had higher NT-proBNP levels: 38.8% of those with NT-proBNP ≥ 280 pg/mL were female, compared with 20.2% of those with NT-proBNP < 280 pg/mL (p = 0.01).
NT-proBNP and outcomes
The correlation between baseline logNT-proBNP and outcomes is shown in Table 2. In the aggressive blood pressure cohort, baseline logNT-proBNP predicted recurrence (HR 1.28, p = 0.04), while in the standard cohort, it did not (HR 0.94, p = 0.62). After controlling for age, sex, LVEF, baseline SBP, and AF type, logNT-proBNP was still a predictor in the aggressive group (HR 1.43, p = 0.03), but not in the standard group (HR 0.83, p = 0.22) (Table 3), or in the entire study cohort (HR 1.04, p = 0.74). The test for interaction between treatment arm and baseline logNT-proBNP on the primary outcome demonstrated a trend to statistical significance (p = 0.07).
Table 2.
Univariable predictors of post-ablation recurrence of atrial fibrillation are shown
| Aggressive | Standard | Whole cohort | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) | 0.99 (0.96–1.03) | 0.69 | 1.03 (1.00–1.07) | 0.04 | 1.01 (0.99–1.03) | 0.35 |
| Sex (Female) | 1.17 (0.63–2.18) | 0.62 | 1.4 (0.78–2.5) | 0.26 | 1.29 (0.84–1.96) | 0.25 |
| Persistent AF | 1.06 (0.62–1.81) | 0.83 | 0.75 (0.43–1.3) | 0.3 | 1.13 (0.77–1.66) | 0.54 |
| Duration of AF | 1.00 (0.99–1.01) | 0.81 | 1.00 (1.00–1.01) | 0.07 | 1.00 (1.00–1.01) | 0.29 |
| Baseline SBP (mmHg) | 1.03 (1.00–1.05) | 0.02 | 1.01 (0.99–1.03) | 0.38 | 1.02 (1.00–1.03) | 0.02 |
| Baseline SBP ≥ 140 mmHg | 2.14 (1.22–3.73) | 0.01 | 0.83 (0.48–1.43) | 0.49 | 0.76 (0.51–1.11) | 0.15 |
| Diabetes | 0.76 (0.34–1.67) | 0.49 | 1.58 (0.71–3.51) | 0.26 | 1.04 (0.59–1.82) | 0.89 |
| LA Size | 1 (0.95–1.05) | 0.91 | 0.99 (0.94–1.04) | 0.74 | 0.99 (0.96–1.03) | 0.74 |
| Baseline NT-proBNP ≥ 280 (pg/ml) | 1.98 (1.13–3.51) | 0.02 | 1.00 (0.55–1.83) | 0.99 | 1.40 (0.92–2.12) | 0.10 |
| Log-NT-proBNP (pg/ml) | 1.28 (1.01–1.62) | 0.04 | 0.94 (0.74–1.20) | 0.62 | 1.09 (0.92–1.29) | 0.31 |
| CRP | 1.00 (0.95–1.05) | 0.89 | 0.99 (0.92–1.07) | 0.82 | 1.00 (0.96–1.04) | 0.81 |
Significant results (p < 0.05) are bolded
All characteristics were measured at time of randomization
AF, atrial fibrillation; SBP, systolic blood pressure; LVEF, left ventricular ejection fraction; LA, left atrium; CRP, C-reactive protein
Table 3.
Multivariable predictor predictors of post-ablation recurrence of atrial fibrillation are shown, by blood pressure treatment group, with corresponding adjusted hazard ratios
| Predictor | Aggressive cohort (n = 88) | Standard cohort (n = 85) | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| logNT-proBNP | 1.43 (1.03–1.99) | 0.03 | 0.83 (0.61–1.12) | 0.22 |
| Age | 0.96 (0.92–1.01) | 0.12 | 1.05 (1.01–1.10) | 0.01 |
| Sex | 0.90 (0.40–2.00 | 0.79 | 1.08 (0.47–2.45) | 0.86 |
| SBP | 1.03 (1.00–1.05) | 0.045 | 1.01 (0.98–1.04) | 0.48 |
| LVEF | 1.02 (0.98–1.06) | 0.35 | 1.00 (0.95–1.06) | 0.94 |
| AF type | 0.95 (0.48–1.88) | 0.87 | 1.04 (0.53–2.05) | 0.89 |
Significant results (p < 0.05) are bolded
All characteristics were measured at time of randomization. The aggressive cohort had a target blood pressure of < 120/80 mmHg, and the standard cohort had a target of < 140/90 mmHg
AF, atrial fibrillation; SBP, systolic blood pressure; LVEF, left ventricular ejection fraction
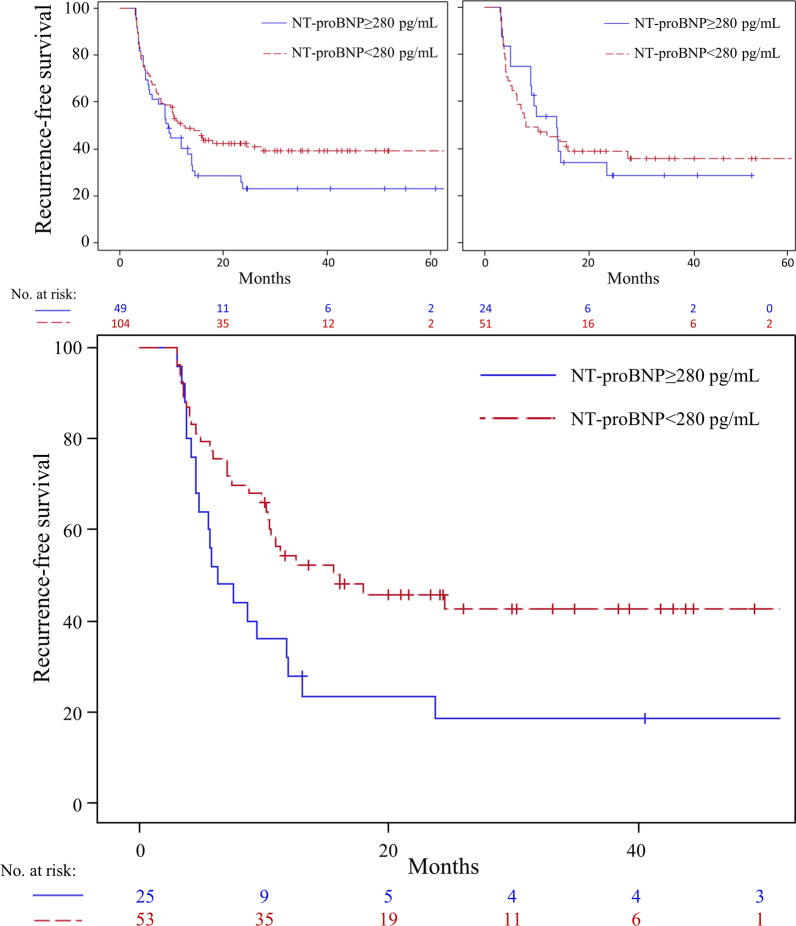
Stratifying by baseline NT-proBNP using a cutoff of 280 pg/mL, there was no difference in AF recurrence in the entire cohort (HR 1.40, p = 0.11) (Fig. 1). In the aggressive group, those with NT-proBNP ≥ 280 pg/mL had increased recurrence (HR 1.98, p = 0.02); in the standard group, there was no difference (HR 1.00, p = 0.998) (Fig. 1). After adjusting for age, sex, baseline SBP, LVEF, and AF type, baseline NT-proBNP ≥ 280 pg/mL was still a predictor in the aggressive cohort (HR 2.04, p = 0.0498) but not in the standard group (HR 0.82, p = 0.59).
Fig. 1.
NT-proBNP as predictor of recurrence after atrial fibrillation ablation. Atrial fibrillation recurrence-free survival after ablation, stratified by baseline NT-proBNP ≥ 280 pg/mL and < 280 pg/mL. Top left: among the overall cohort (n = 173), there was no difference in recurrence between the groups (for NT-proBNP ≥ 280 pg/mL, HR 1.40, 95%CI 0.92–2.12, p = 0.10). Top right: among patients (n = 85) undergoing standard blood pressure management, < 140/90 mmHg, there was no difference (for NT-proBNP ≥ 280 pg/mL, HR 1.00, 95%CI 0.55–1.83, p = 0.998). Bottom: among patients (n = 88) undergoing aggressive blood pressure management, < 120/80 mmHg, those with baseline NT-proBNP ≥ 280 pg/mL had increased recurrence (HR 1.98, 95%CI 1.13–3.51, p = 0.02)
Decrease in logNT-proBNP at 12 months compared to baseline was associated with decreased AF recurrence in the standard group (HR 0.69, p = 0.001), but not in the aggressive group (HR 0.89, p = 0.41).
Patients with baseline NT-proBNP ≥ 280 pg/mL had more additional (non-pulmonary vein) ablation (40.8% vs 19.2%, p = 0.005).
Univariable predictors
Univariable predictors of recurrence in each group are shown in Table 2. Only baseline SBP was a predictor in the overall cohort. Predictors of recurrence in the aggressive group included baseline SBP (HR 1.03, p = 0.02), but not age (HR 0.99, p = 0.69). In the standard group, age (HR 1.03 p = 0.04) predicted outcome, but not SBP (HR = 1.01, p = 0.38).
Multivariable analysis
In the whole study cohort, in multivariable analysis, logNT-proBNP (HR 1.03, 95%CI 0.84–1.27, p = 0.77), age (HR 1.02, 95%CI 0.99–1.05, p = 0.29), LVEF (HR 1.02, 95%CI 0.99–1.05, p = 0.19), AF type (HR 0.98, 95%CI 0.63–1.55, p = 0.94), sex (HR 1.04, 95%CI 0.6–1.80, p = 0.89) and baseline systolic pressure (HR 1.02, 95%CI 1.00–1.03, p = 0.08) were not predictors. Table 3 shows the multivariable predictors in each cohort. In the aggressive group, logNT-proBNP and baseline SBP were predictors. In the standard group, age was a predictor. NT-proBNP ≥ 280 pg/mL was also a predictor (adjusted HR 2.04, 95%CI 1.00–4.18, p = 0.0498) in the aggressive group, but not the standard group (adjusted HR 0.82, 95%CI 0.40–1.69, p = 0.59).
Discussion
This study was a post-hoc analysis of the SMAC-AF, which randomized patients undergoing AF ablation to aggressive versus standard blood pressure management. We examined the association between NT-proBNP and AF outcomes. We found that in the overall study population, baseline logNT-proBNP was not associated with recurrence post-ablation. However, in patients who underwent aggressive BP control, after adjusting for baseline LVEF, SBP, age, and AF type, logNT-proBNP was associated with recurrence. We determined that a NT-proBNP concentration of above 280 pg/mL was associated with increased AF recurrence. This association was not found in the standard arm.
Our results indicate that in a population receiving AF ablation and aggressive BP control, NT-proBNP is a marker for recurrence; this association was not seen in those undergoing standard blood pressure control. NT-proBNP can be elevated by various mechanisms, including AF, LA and LV stretch [16]; it is known to increase with age, but not by isolated hypertension [8]. We did find a correlation between baseline NT-proBNP and type of AF, Age, and LVEF, but not with duration of AF, LA size, SBP. We also found that women had a higher baseline level of NT-proBNP, concordant with a recent study of biomarker differences between sexes [17].
Prior studies have shown association between pre-ablation NT-proBNP and recurrence [18], as well as in lone AF [14, 19]. However, we only found this in patients undergoing aggressive blood pressure control. This suggests that when hypertension is aggressively controlled to 120/80 mmHg, pre-ablation NT-proBNP becomes a more important marker of recurrence, indicating that other factors that play a role in determining AF recurrence; the mechanism of this is still unclear. Prior meta-analyses of NT-proBNP and AF recurrence have shown marked heterogeneity, suggesting that NT-proBNP may reflect several underlying factors [12, 13]. Left atrial stretch and remodeling, increased left ventricular filling pressures, and subclinical heart failure may all play a part in explaining this observation: higher NT-proBNP levels could reflect a more diseased substrate, dysfunctional hemodynamics, or diastolic dysfunction, which may have been less responsive to aggressive blood pressure lowering [20]. It has previously been shown that uncontrolled BP after ablation is associated with recurrence post-ablation [21]. Benefits of blood pressure control as upstream therapy for AF is thought to occur through substrate modification in the LA, and improved hemodynamics [22]. In our study, aggressive blood pressure control may have treated factors associated with hypertension, reducing their effect on recurrence, thus factors associated with high NT-proBNP became more important in predicting recurrence in this group. Meanwhile, in the standard treatment group, hypertension may still have had a stronger effect on recurrence.
A “J-curve” phenomenon has been described with BP lowering, where targeting levels too low results in harm [23, 24]. For patients being treated for hypertension, blood pressure under 120 systolic has been shown to be associated with a higher risk of AF [25]. Thus, there may be some patients with hypertension who benefit less from aggressive blood control. Our finding that higher NT-proBNP levels were only associated with recurrence in those undergoing aggressive BP control, suggests that higher NT-proBNP levels could help identify these patients so they could be targeted for additional screening and upstream therapy aimed at altering the atrial substrate to reduce recurrence of AF. Our finding that baseline SBP was associated with recurrence in the aggressive group (adjusted HR 1.03, p = 0.045) but not in the standard group (adjusted HR 1.01, p = 0.48) suggests that aggressive blood pressure control may be more beneficial in reducing AF recurrence in those with milder forms of hypertension.
The extent to which structural remodeling in AF is related to inflammation has been of significant interest. Elevation of CRP, interleukin-6 (IL-6), atrial and brain natriuretic peptide (ANP/NT-proBNP), and apelin in patients with AF suggests the presence of systemic inflammation in these patients; whether this is due to structural remodeling or not is unclear [7, 26–30]. Damage of atrial myocardium by repetitive rapid atrial activation may result in low grade inflammation which may further damage atrial myocardium resulting in further structural and electrophysiologic changes needed to maintain AF. Baseline CRP was not associated with recurrence in our study. Another example is a recently identified measure of collagen type I cross-linking and deposition that can reflect excessive atrial myocardial interstitial fibrosis [31]. Altered levels were shown to predict recurrence of AF post-ablation [32]. However, this novel biomarker is not widely available by common lab assays, and its clinical utility is unknown. These factors require further exploration to understand the effects that such processes may have on propagation and recurrence of AF post ablation. This may lead to personalization of therapies in patients that may have different underlying factors resulting in AF.
There are some limitations to our observations. The outcome of NT-proBNP was a prespecified secondary outcome; however, since this was a secondary analysis, and we did not correct for multiple testing, the usual limitations of a secondary analysis apply. The results should be considered hypothesis-generating, however raises the issue of whether or not patients with AF and hypertension have early or subclinical heart failure, and if this contributes to their symptoms and clinical outcomes; this will require further study. Not all patients (11.6%) in the cohort had a baseline NT-proBNP checked. The relatively few number of patients who had a low NT-proBNP level prior to ablation prevented us from investigating if the randomized intervention of aggressive blood control decreased recurrence in this population.
Conclusion
In a hypertensive population undergoing AF ablation, baseline NT-proBNP levels predict recurrence in patients receiving aggressive blood pressure control, an association not seen in those receiving standard blood pressure control. This suggests that NT-proBNP levels may be useful as a biomarker to help select a subset of hypertensive patients who would benefit from aggressive blood pressure management, or other upstream therapies to target atrial substrate, to reduce AF recurrence post-ablation.
Acknowledgements
None.
Abbreviations
- AF
Atrial fibrillation
- BP
Blood pressure
- SBP
Systolic blood pressure
- CRP
C-reactive protein
- LVEF
Left ventricular ejection fraction
Authors' contributions
WW: Data interpretation, manuscript draft and revision. RC, JS, AT, JH, IN, LR, IG: Data interpretation, manuscript revision. JB: Statistical analysis, manuscript revision. RP: study conception and design, data interpretation, manuscript revision. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data will be made available upon reasonable request to the corresponding author.
Declarations
Ethics approval and consent to participate
The protocol of the original study was approved by the local ethics review board at all participating institutions. Informed consent was obtained for all patients.
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Balk EM, Garlitski AC, Alsheikh-Ali AA, Terasawa T, Chung M, Ip S. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J Cardiovasc Electrophysiol. 2010;21:1208–1216. doi: 10.1111/j.1540-8167.2010.01798.x. [DOI] [PubMed] [Google Scholar]
- 2.Epicoco G, Sorgente A. Predictors of Atrial Fibrillation Recurrence after Catheter Ablation. J Atr Fibrillation. 2014;6:1016. doi: 10.4022/jafib.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkash R, Wells GA, Sapp JL, Healey JS, Tardif JC, Greiss I, et al. Effect of aggressive blood pressure control on the recurrence of atrial fibrillation after catheter ablation. Circulation. 2017;135:1788–1798. doi: 10.1161/CIRCULATIONAHA.116.026230. [DOI] [PubMed] [Google Scholar]
- 4.Dzeshka MS, Lip GYH, Snezhitskiy V, Shantsila E. Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. J Am Coll Cardiol. 2015;66:943–959. doi: 10.1016/j.jacc.2015.06.1313. [DOI] [PubMed] [Google Scholar]
- 5.Howlett PJ, Hatch FS, Alexeenko V, Jabr RI, Leatham EW, Fry CH. Diagnosing paroxysmal atrial fibrillation: are biomarkers the solution to this elusive arrhythmia? BioMed Res Int. 2015;2015. [DOI] [PMC free article] [PubMed]
- 6.Staerk L, Preis SR, Lin H, Lubitz SA, Ellinor PT, Levy D, et al. Protein biomarkers and risk of atrial fibrillation: the framingham heart study. Circ Arrhythmia Electrophysiol. 2020;13(2):e007607. doi: 10.1161/CIRCEP.119.007607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Büttner P, Schumacher K, Dinov B, Zeynalova S, Sommer P, Bollmann A, et al. Role of NT-proANP and NT-proBNP in patients with atrial fibrillation: Association with atrial fibrillation progression phenotypes. Hear Rhythm. 2018;15:1132–1137. doi: 10.1016/j.hrthm.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Jiang H, Wang W, Wang C, Xie X, Hou Y. Associationofpre-ablation levelofpotential blood markers with atrial fibrillation recurrence after catheter ablation: a meta-analysis. Europace. 2017;19:392–400. doi: 10.1093/europace/euw335. [DOI] [PubMed] [Google Scholar]
- 9.Inohara T, Kim S, Pieper K, Blanco RG, Allen LA, Fonarow GC, et al. B-type natriuretic peptide, disease progression and clinical outcomes in atrial fibrillation. Heart. 2019;105:370–377. doi: 10.1136/heartjnl-2018-313642. [DOI] [PubMed] [Google Scholar]
- 10.Murakami Y, Shimada T, Inoue SI, Shimizu H, Ohta Y, Katoh H, et al. New insights into the mechanism of the elevation of plasma brain natriuretic polypeptide levels in patients with left ventricular hypertrophy. Can J Cardiol. 2002;18:1294–1300. [PubMed] [Google Scholar]
- 11.Liu C-Y, Heckbert SR, Lai S, Ambale-Venkatesh B, Ostovaneh MR, McClelland RL, et al. Association of elevated NT-proBNP with myocardial fibrosis in the multi-ethnic study of atherosclerosis (MESA) J Am Coll Cardiol. 2017;70:3102–3109. doi: 10.1016/j.jacc.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Chen A, Song L, Li M, Chen Y, He B. Association between baseline Natriuretic peptides and Atrial fibrillation recurrence after catheter ablation a meta-analysis. Int Heart J. 2016;57:183–189. doi: 10.1536/ihj.15-355. [DOI] [PubMed] [Google Scholar]
- 13.Hwang HJ, Son JW, Nam BH, Joung B, Lee B, Kim JB, et al. Incremental predictive value of pre-procedural N-terminal pro-B-type natriuretic peptide for short-term recurrence in atrial fibrillation ablation. Clin Res Cardiol. 2009;98:213–218. doi: 10.1007/s00392-009-0744-3. [DOI] [PubMed] [Google Scholar]
- 14.Hussein AA, Saliba WI, Barakat A, Bassiouny M, Chamsi-Pasha M, Al-Bawardy R, et al. Radiofrequency ablation of persistent atrial fibrillation: diagnosis-to-ablation time, markers of pathways of atrial remodeling, and outcomes. Circ Arrhythm Electrophysiol. 2016;9:e003669. doi: 10.1161/CIRCEP.115.003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaff MR, Bates M, Sullivan T, Popma J, Gao X, Zaugg M, et al. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: Results from the HERCULES trial. Catheter Cardiovasc Interv. 2012;80:343–350. doi: 10.1002/ccd.24449. [DOI] [PubMed] [Google Scholar]
- 16.Inoue SI, Murakami Y, Sano K, Katoh H, Shimada T. Atruim as a source of brain natriuetic polypeptide patients with atrial fibrillation. J Card Fail. 2000;6:92–96. doi: 10.1054/jcaf.2000.8185. [DOI] [PubMed] [Google Scholar]
- 17.Lau ES, Paniagua SM, Guseh JS, Bhambhani V, Zanni MV, Courchesne P, et al. Sex differences in circulating biomarkers of cardiovascular disease. J Am Coll Cardiol. 2019;74:1543–1553. doi: 10.1016/j.jacc.2019.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miake J, Kato M, Ogura K, Iitsuka K, Okamura A, Tomomori T, et al. Pre-ablation levels of brain natriuretic peptide are independently associated with the recurrence of atrial fibrillation after radiofrequency catheter ablation in patients with nonvalvular atrial fibrillation. Heart Vessels. 2019;34:517–526. doi: 10.1007/s00380-018-1267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussein AA, Saliba WI, Martin DO, Shadman M, Kanj M, Bhargava M, et al. Plasma B-type natriuretic peptide levels and recurrent arrhythmia after successful ablation of lone atrial fibrillation. Circulation. 2011;123:2077–2082. doi: 10.1161/CIRCULATIONAHA.110.007252. [DOI] [PubMed] [Google Scholar]
- 20.Casaclang-Verzosa G, Gersh BJ, Tsang TSM. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008;51:1–11. doi: 10.1016/j.jacc.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Kamioka M, Hijioka N, Matsumoto Y, Nodera M, Kaneshiro T, Suzuki H, et al. Uncontrolled blood pressure affects atrial remodeling and adverse clinical outcome in paroxysmal atrial fibrillation. PACE Pacing Clin Electrophysiol. 2018;41:402–410. doi: 10.1111/pace.13311. [DOI] [PubMed] [Google Scholar]
- 22.Dorian P, Singh BN. Upstream therapies to prevent atrial fibrillation. Eur Heart J Supplement. 2008;10:H11–H31. doi: 10.1093/eurheartj/sun033. [DOI] [Google Scholar]
- 23.Rahman F, McEvoy JW. Dangers of overly aggressive blood pressure control. Curr Cardiol Rep. 2018;20(11):108. doi: 10.1007/s11886-018-1063-y. [DOI] [PubMed] [Google Scholar]
- 24.Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243–251. doi: 10.1056/NEJMoa1803180. [DOI] [PubMed] [Google Scholar]
- 25.Thomas MC, Dublin S, Kaplan RC, Glazer NL, Lumley T, Longstreth WT, et al. Blood pressure control and risk of incident atrial fibrillation. Am J Hypertens. 2008;21:1111–1116. doi: 10.1038/ajh.2008.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelmann MDM, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005;26:2083–2092. doi: 10.1093/eurheartj/ehi350. [DOI] [PubMed] [Google Scholar]
- 27.Malouf JF, Kanagala R, Al Atawi FO, Rosales AG, Davison DE, Murali NS, et al. High sensitivity C-reactive protein: a novel predictor for recurrence of atrial fibrillation after successful cardioversion. J Am Coll Cardiol. 2005;46:1284–1287. doi: 10.1016/j.jacc.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 28.Conway DSG, Buggins P, Hughes E, Lip GYH. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol. 2004;43:2075–2082. doi: 10.1016/j.jacc.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 29.Psychari SN, Apostolou TS, Sinos L, Hamodraka E, Liakos G, Kremastinos DT. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am J Cardiol. 2005;95:764–767. doi: 10.1016/j.amjcard.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Ellinor PT, Low AF, Patton KK, Shea MA, MacRae CA. Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. J Am Coll Cardiol. 2005;45:82–86. doi: 10.1016/j.jacc.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 31.López B, González A, Díez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation. 2010;121:1645–1654. doi: 10.1161/CIRCULATIONAHA.109.912774. [DOI] [PubMed] [Google Scholar]
- 32.Ravassa S, Ballesteros G, López B, Ramos P, Bragard J, González A, et al. Combination of circulating type I collagen-related biomarkers is associated with atrial fibrillation. J Am Coll Cardiol. 2019;73:1398–1410. doi: 10.1016/j.jacc.2018.12.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request to the corresponding author.