Abstract
Multilocus enzyme electrophoresis (MLEE) of 397 Vibrio cholerae isolates, including 143 serogroup reference strains and 244 strains from Mexico and Guatemala, identified 279 electrophoretic types (ETs) distributed in two major divisions (I and II). Linkage disequilibrium was demonstrated in both divisions and in subdivision Ic of division I but not in subdivision Ia, which includes 76% of the ETs. Despite this evidence of relatively frequent recombination, clonal lineages may persist for periods of time measured in at least decades. In addition to the pandemic clones of serogroups O1 and O139, which form a tight cluster of four ETs in subdivision Ia, MLEE analysis identified numerous apparent clonal lineages of non-O1 strains with intercontinental distributions. A clone of serogroup O37 that demonstrated epidemic potential in the 1960s is closely related to the pandemic O1/O139 clones, but the nontoxigenic O1 Inaba El Tor reference strain is not. A strain of serogroup O22, which has been identified as the most likely donor of exogenous rfb region DNA to the O1 progenitor of the O139 clone, is distantly related to the O1/O139 clones. The close evolutionary relationships of the O1, O139, and O37 epidemic clones indicates that new cholera clones are likely to arise by the modification of a lineage that is already epidemic or is closely related to such a clone.
For Vibrio cholerae, the causative agent of cholera and a natural inhabitant of aquatic environments, the conventional method of identifying and classifying strains is a serotyping scheme in which nearly 200 serogroups (or serovars) have been distinguished on the basis of epitopic variation in the cell surface lipopolysaccharide (LPS) (52). From an epidemiological standpoint, the species has been divided into serogroup O1 and serogroup non-O1 strains, which were long believed to differ in ability to cause epidemic cholera (4, 10). Historically, O1 strains have been responsible for all major epidemics, including seven pandemics (19), but in 1992 an epidemic clone of serogroup O139 (Bengal) appeared in southern India (30). It rapidly spread throughout much of Southeast Asia (1, 9) and reached western Africa in 1994 (37).
The emergence of the O139 clone with pandemic potential stimulated increased interest in the molecular basis of pathogenesis in V. cholerae and the degree to which genes determining serotype and virulence properties are subject to horizontal transfer and recombination among strains (17). Molecular genetic studies have shown that the origin of the O139 clone involved a complex rearrangement of the rfb region in a strain of O1 El Tor, which included deletion of genes responsible for the biosynthesis and assembly of the side chains of the O1 cell surface LPS and insertion of exogenous DNA mediating synthesis of the O139 LPS core (2, 3, 40–42) and a capsule (12, 45). The observations that strains with identical nucleotide sequences of the aspartate semialdehyde dehydrogenase gene (asd) may express different O antigens and that O1 isolates are heterogeneous in sequence provided further evidence of the horizontal transfer of genes mediating O-antigen synthesis (19). Most surprisingly, it was discovered that the CTX element, which includes the structural genes (ctxA and ctxB) for the subunits of cholera toxin, is the integrated genome of a filamentous bacteriophage, CTXø, and is transmissible (31, 46). Moreover, the bacterial receptor for CTXø, the toxin-coregulated pilus, is encoded by an operon (tcp) that is part of a transmissible pathogenicity island (20, 21). These findings raise the possibility that all strains of V. cholerae have the potential to become agents of epidemic cholera.
Previous research on the evolutionary genetics of V. cholerae has been primarily concerned with the identification and epidemiology of O1 and O139 strains that are responsible for cholera epidemics and pandemics. This work includes the application of multilocus enzyme electrophoresis (MLEE) to assess genotypic diversity in a collection of 181 O1 and 79 non-O1 strains (33) and the extensive use of this technique, in conjunction with ribotyping and restriction fragment length polymorphism analysis of the ctxA gene, to study various aspects of the molecular epidemiology of cholera in Latin America and elsewhere (8, 15, 43, 44). Additionally, ribotyping and comparative sequence analysis of the asd gene have been employed to reconstruct the evolutionary history of O1 clones involved in the sixth and seventh pandemics (18, 19).
We report here the results of an analysis of 397 isolates of V. cholerae by MLEE undertaken to determine the extent of genetic diversity in the species as a whole, the relationships of the epidemic O1 and O139 clones to strains of other serogroups, and the genetic population structure of the non-O1 segment of the species.
MATERIALS AND METHODS
Bacterial strains.
This study was based on 397 strains received as V. cholerae. The sample included 143 strains in the serogroup reference collection maintained at the National Institute of Infectious Diseases in Japan (52). These strains were isolated from worldwide sources in the period from 1932 to 1993; 117 of them were recovered from humans, 13 from animals, 6 from river water, 3 from seawater, and 3 from unknown sources. The reference strains for serogroups O1 through O83 were provided by T. Cheasty, and those for serogroup O155 and serogroups O84 through O140, together with strain CA-385 (rough), which is used in serotyping, were obtained from T. Shimada.
A collection of 191 strains from Sonora, Tabasco, and 14 other states in Mexico was provided by the Instituto Nacional de Diagnóstico y Referencia Epidemiológicos, the Laboratorio Estatal de Salud Pública (Sonora), and the Laboratorio Regional de Salud Pública (Tabasco). A sample of 53 strains recovered from humans in Guatemala was obtained from the Instituto de Nutrición de Centroamérica y Panamá. Of the total of 244 strains from Mexico and Guatemala, 172 were recovered from humans, 41 from well and sewage water, 15 from fish, 7 from other environmental sources, 2 from food, and 7 from unspecified sources.
Five serogroup O139 strains from Thailand were furnished by P. Echeverria. From the Centers for Disease Control and Prevention (CDC), we received single isolates of O1 El Tor from Australia, Romania, Peru, and Louisiana and an O139 isolate from an imported human case of cholera in the United States in 1993.
The strains from Mexico and Guatemala, almost all of which were isolated in the period from 1991 to 1995, were serotyped in our laboratory by the standard method of Sakazaki and Donovan (32). Eight of these strains were of a serotype that was not then represented in the reference collection; for purposes of this paper, we have designated this serotype OA.
All of the strains used in this study have been deposited in the collection of the Facultad de Medicina, Universidad Nacional Autónoma de México (FMU).
MLEE.
MLEE was performed by the methods described by Selander et al. (34). Seventeen enzyme loci were assayed for allelic variation: 6PG (6-phosphogluconate dehydrogenase), G6P (glucose 6-phosphate dehydrogenase), IDH (isocitrate dehydrogenase), NSP (nucleoside phosphorylase), ALD (alanine dehydrogenase), SHK (shikimate dehydrogenase), CAT (catalase), LAP (leucine aminopeptidase), GOT (glutamic-oxalacetic transaminase), ME (malic enzyme), MDH (malate dehydrogenase), PLP (phenylalanyl-leucine peptidase), PGI (phosphoglucose isomerase), HEX (hexokinase), PGM (phosphoglucomutase), IPO (indophenol oxidase), and THD (threonine dehydrogenase).
Electromorphs (mobility variants) were equated with alleles, an absence of enzyme activity was scored as a null allele, and distinctive allele profiles for the 17 loci were designated electrophoretic types (ETs).
Statistical analysis.
From the allele profiles of the ETs, mean genetic diversity per locus (H) and pairwise genetic distance were calculated as described by Selander et al. (34), and dendrograms were constructed by the unweighted pair-group method with arithmetic mean (UPGMA). As a measure of multilocus population structure (linkage disequilibrium), we calculated an index of association of alleles (IA) by using the equation 1 − VO/VE, where VO is the variance of the observed distribution of number of mismatched alleles between ET pairs and VE is the mismatch variance expected when allele associations are random (linkage equilibrium) (24, 49). Calculations were made with computer programs written by T. S. Whittam (Pennsylvania State University, University Park).
Detection of the ctxA gene.
Tests for the presence of the ctxA gene, which encodes a subunit protein of cholera toxin, were performed by colony blot assay with a digoxigenin-labeled oligonucleotide probe, according to the procedure of Maniatis et al. (23), and by PCR amplification. The nucleotide sequences of the probe and the primers were those specified by Shirai et al. (39).
RESULTS
Serogroups.
Of the 244 strains from Mexico and Guatemala, 230 were of 59 serogroups and 14 were serologically nontypeable (NT). The reference collection currently includes nearly 200 serogroups (52), and thus about 30% of the described serotypic diversity of the species is exhibited by the Mexico-Guatemala strains.
In this paper, individual reference strains are referred to by their serogroup designations, marked with an asterisk (e.g., O155*).
Multilocus enzyme genotypes.
All 17 loci assayed were polymorphic, with an average of 9.5 alleles per locus and a range of 4 (IPO) to 15 (CAT and LAP). Among the total sample of 397 isolates, 279 ETs, each representing a distinctive allele profile, were distinguished. The 142 serogroup reference strains were of 133 ETs, and the 244 Mexico-Guatemala strains were assigned to 154 ETs.
For each of the 17 enzymes assayed, an absence of activity was rare. Among the 279 ETs, only 26 null alleles, distributed over 12 of the loci, were recorded. Thus, only 0.5% of a total of 4,743 (17 × 279) alleles scored were nulls, and 10 of them were represented in the profiles of the two strains (ETs 276 and 277) that form evolutionary subdivision x (see below).
The mean genetic diversities per locus were 0.436 for all ETs and 0.430 for the ETs of the reference strains (Table 1).
TABLE 1.
Measures of mean genetic diversity per locus and multilocus linkage equilibrium, based on ETs
| Sample | No. of:
|
Mean no. of alleles | Ha | IAb | |
|---|---|---|---|---|---|
| Isolates | ETs | ||||
| All strains | |||||
| Total | 397 | 279 | 9.5 | 0.436 | 1.248 ± 0.082* |
| Divisions I and II | 392 | 275 | 7.5 | 0.421 | 0.728 ± 0.083* |
| Division I | 380 | 263 | 6.7 | 0.397 | 0.237 ± 0.085* |
| Subdivision Ia | |||||
| Group A | 103 | 37 | 3.4 | 0.297 | 0.248 ± 0.228 |
| Group B | 148 | 123 | 4.8 | 0.309 | −0.048 ± 0.125 |
| Group C | 55 | 44 | 3.3 | 0.274 | 0.053 ± 0.210 |
| Subdivision Ic | 47 | 40 | 3.2 | 0.384 | 0.931 ± 0.217* |
| Division II | 12 | 12 | 2.8 | 0.375 | 1.204 ± 0.399* |
| Reference strainsc | |||||
| Total | 142 | 133 | 7.5 | 0.441 | 1.759 ± 0.120* |
H, mean genetic diversity per locus.
IA, index of association of alleles. *, P ≤0.05.
Strain CA-385 (rough) not included.
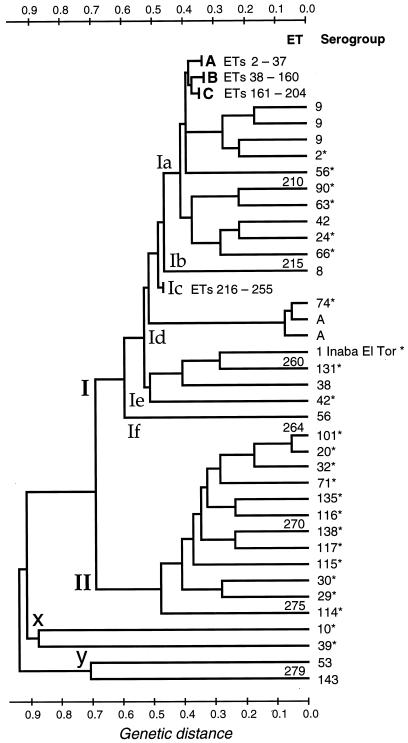
Estimates of genetic distance among the 279 ETs are summarized in the dendrograms presented in Fig. 1 and 2. All but four of the ETs are members of two major divisions (designated I and II) that diverge from one another at a distance of about 0.7, which roughly corresponds to an 11-locus difference (Fig. 1). In addition, there are two lineages (labeled x and y) that branch from the major divisions and from one other at distances greater than 0.9. Each of these lineages includes two distantly related ETs.
FIG. 1.
Dendrogram showing genetic relationships of the ETs of V. cholerae, based on MLEE analysis (17 loci). The dendrogram was constructed from a matrix of pairwise genetic distances by the UPGMA method. The lineages of subdivision Ic and of groups A, B, and C of subdivision Ia are truncated. The relationships of the 37 ETs in group A are shown in the dendrogram in Fig. 2.
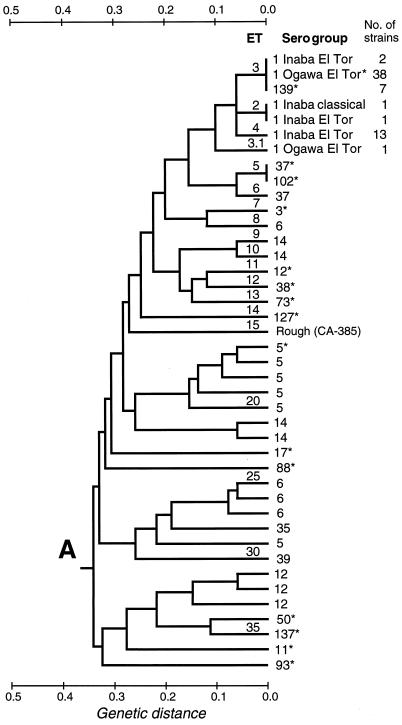
FIG. 2.
Dendrogram showing genetic relationships of the 37 ETs of V. cholerae in group A of subdivision Ia of division I, based on MLEE analysis (17 loci). The dendrogram was constructed from a matrix of pairwise genetic distances by the UPGMA method.
Division II consists of 12 ETs, each of which is represented by a single serogroup reference strain. Seven of these strains are from India and the Far East, as follows: India, two strains, both from humans; China, one human isolate; the Philippines, one human strain; and Japan, two strains, from a crab and from river water. Three strains (O114*, O115*, and O116*) were cultured from river water (1979), a human (1980), and seawater (1978) in the United States; one isolate (O71*) was recovered from a bird in Denmark (1978); and one strain (O29*) was isolated from a human at an unspecified locality (1968).
The vast majority of strains are of ETs in division I, in which for purposes of reference we have designated six subdivisions (Ia to If), each consisting of a single ET or a group of related ETs.
Most of the 51 strains of the 40 ETs that form subdivision Ic are from Mexico and Guatemala, but 8 strains (representing 8 ETs) are from the serogroup reference collection, as follows: O50*, O75*, O78*, O82*, and O126* from India; O155* from Thailand; and O107* and O92* from Japan.
Subdivision Ia includes 82% of the reference collection strains and 78% of the Mexico-Guatemala strains. All but 10 of the 214 ETs in this subdivision form three branches (designated A, B, and C in Fig. 1) that diverge from one another at a genetic distance of about 0.4. With the singular exception of the O1 Inaba El Tor reference strain (ET 259, in subdivision Ie), all O1 strains in our sample, together with all O139 isolates, are of 4 ETs that form a tight cluster in group A of subdivision Ia, which consists of a total of 37 ETs (Fig. 2).
Multilocus population structure.
Estimates of multilocus association of alleles for all 279 ETs and for the ETs in several segments of the dendrograms (Fig. 1 and 2) are shown in Table 1. For the total sample of 279 ETs, the 133 ETs of the reference strains, and the ETs of each of the divisions I and II and subdivision Ic, there is evidence of significant nonrandom associations of alleles (linkage disequilibrium), but allele associations are not demonstrably nonrandom for the ETs of groups A, B, and C in subdivision Ia.
Relationships of O1 and O139 epidemic strains.
With allowance for a difference in the panels of enzymes employed in MLEE analysis, our findings for the epidemic O1 and O139 strains are fully consistent with those reported by Evins et al. (15) and in earlier studies by Wachsmuth’s group at the CDC (43, 44). These workers assayed variation in 16 enzymes, only 9 of which were included in our panel of 17 enzymes. In the interest of consistency, we have numbered the ETs of our O1 and O139 isolates to correspond to the ET designations of the CDC group (Table 2; Fig. 2).
TABLE 2.
Allele profiles of 16 ETs in division I
| ET | Serogroup or reference strain | n | Allele at indicated enzyme locusa
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6PG | G6P | IDH | NSP | ALD | SHK | CAT | LAP | GOT | ME | MDH | PLP | PGI | HEX | PGM | IPO | THD | |||
| Subdivision Ia, group A | |||||||||||||||||||
| 3 | O1b/O139 | 47 | 3 | 4 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 2 | 4 | 3 | 3.5 | 3 | 3 | 1 |
| 2 | O1c | 2 | 3 | 4 | 3 | 3 | 3 | 1 | 3 | 4 | 3 | 3 | 2 | 4 | 3 | 3.5 | 3 | 3 | 1 |
| 4 | O1d | 13 | 3 | 4 | 3 | 3 | 3 | 1 | 3 | 4.5 | 3 | 3 | 2 | 4 | 3 | 3.5 | 3 | 3 | 1 |
| 3.1 | O1e | 1 | 3 | 3.8 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 2 | 4 | 3 | 3.5 | 3 | 3 | 1 |
| 5 | O37*/O102* | 2 | 3 | 4 | 3 | 3 | 3 | 1 | 3 | 4 | 3 | 3 | 2 | 4 | 4 | 3.5 | 3 | 3 | 1 |
| 6 | O37 | 1 | 3 | 4 | 3 | 3 | 3 | 1 | 3 | 4 | 4 | 3 | 2 | 4 | 4 | 3.5 | 3 | 3 | 1 |
| 7 | O3* | 1 | 3 | 4 | 3 | 3 | 3 | 1 | 3 | 3.5 | 3 | 3 | 2 | 4 | 4 | 3.5 | 5 | 3 | 1 |
| 8 | O6 | 1 | 2 | 4 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 2 | 4 | 4 | 3.5 | 5 | 3 | 1 |
| 9 | O14 | 1 | 3 | 4 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | 4 | 3.5 | 3 | 1 |
| 10 | O14 | 1 | 3 | 4 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | 4 | 3 | 3 | 1 |
| 11 | O12* | 1 | 3 | 4 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 2 | 5.5 | 4 | 3.5 | 3 | 3 | 1 |
| 12 | O38* | 1 | 3 | 4 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 2 | 4 | 4 | 3 | 3 | 3 | 1 |
| 13 | O73* | 1 | 3 | 4 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 6 | 4 | 3 | 3 | 3 | 1 |
| 14 | O127* | 1 | 4 | 4 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 2 | 2 | 4 | 4 | 4 | 3 | 3 | 1 |
| 15 | Roughf | 1 | 2 | 4 | 3 | 3 | 3 | 1 | 3 | 4 | 4 | 3 | 2 | 4 | 4 | 3 | 3 | 3 | 1 |
| Subdivision Ie | |||||||||||||||||||
| 259 | O1*g | 1 | 3.5 | 2 | 3 | 3 | 0 | 1 | 1 | 4.5 | 3 | 3 | 2 | 5 | 3 | 1 | 3 | 3 | 1 |
Minority alleles are indicated in boldface type.
Thirty-nine isolates of O1 Ogawa El Tor and 2 isolates of O1 Inaba El Tor.
One isolate of O1 Inaba classical and 1 isolate of O1 Inaba El Tor.
Thirteen isolates of O1 Inaba El Tor from Mexico, Guatemala, and Peru.
One isolate of O1 Ogawa El Tor from Tabasco.
Strain CA-385.
O1 Inaba El Tor reference strain.
ET 4 is represented by 13 isolates of O1 Inaba El Tor. Eleven of these strains are from Mexico (Quintana Roo, Campeche, Tabasco, Veracruz, Puebla, and Hidalgo), one is from Guatemala, and one is from Peru. ET 4 marks the original, or first-wave, Latin American epidemic clone (15).
ET 3 includes 2 strains of O1 Inaba El Tor, 38 strains of O1 Ogawa El Tor, and 7 strains of O139. The two Inaba El Tor strains (FMU strains 90501 and 90500) were recovered from humans in Tabasco in 1991 and 1993. The sample of O1 Ogawa El Tor isolates includes the reference strain, 35 isolates from Mexico (Tabasco, Morelos, and the state of México), a strain from Australia, and an isolate from Romania. ET 3 is the seventh-pandemic type, a clone that in Latin America was first identified in Mexico in 1991 and is now widely distributed in Mexico and Central America (15). The Australian isolate (CDC 2463-88) was distinguished, as ET 1, from isolates of ET 3 by Evins et al. (15) on basis of its carrying a different allele for diaphosrase 1. ET 1 marks a distinctive Australian toxigenic clone (13, 43).
Included in ET 2 are an O1 Inaba El Tor isolate (CDC 2164-78) collected in Louisiana in 1978 and an O1 Inaba classical strain (FMU 87295/0) recovered from a tourist returning to the United States from Cancún, Quintana Roo, in 1983. ET 2 marks a toxigenic clone that is endemic to the Gulf Coast of Mexico and the United States (15).
With our panel of 17 enzymes, the sole basis for distinguishing ETs 2, 3, and 4 is allelic variation at the LAP locus (Table 2). One O1 Ogawa El Tor isolate (from Tabasco) is ET 3.1, which differs from ET 3 in having a distinctive G6P allele. This variant genotype was not detected in previous studies.
Relationship of serogroup O37 strains.
The reference strains O37* (India, 1969) and O102* (China, 1988), both of which are of ET 5 (Fig. 2), differ from strains of the O1/O139 cluster (ETs 2 to 4) at a single locus, that for PGI, and share the LAP 4 allele with strains of ET 2 (Table 2). A second O37 strain (from Guatemala) is of ET 6, which differs from ET 5 in having a 4 allele rather than a 3 allele at the GOT locus. Two other O37 isolates in the collection, both of which were cultured from well water in Campeche, represent ETs 75 and 149, which are in group B of subdivision Ia.
Serotypic diversity among non-O1 strains of the same ET.
In addition to ETs 2 to 4 of the epidemic O1 and O139 clones, MLEE identified nine ETs that are each represented by non-O1 isolates from different continents or other major land masses (Table 3). In all cases, the strains from different continents are of different serogroups. For example, ET 196 (a member of group C of subdivision Ia) is represented by a total of seven isolates, including four (O15*, O47*, O51*, and O53*) recovered from humans in India between 1968 and 1974, a strain (O68*) obtained from seawater in Japan in 1978, and two isolates (both O7) cultured from well water in Campeche and a fish in Sonora in 1992 and 1993. The inference is that ET 196 marks a widely distributed clone that had persisted for at least 24 years, with several modifications in serotype through mutation or recombination of genes of the rfb region.
TABLE 3.
ETs represented by non-O1 isolates from different continents
| ET | No. of isolates | Serogroup | Locality (date) | Source | FMU strain no. |
|---|---|---|---|---|---|
| 196 | 7 | O15* | India (1968) | Human | 88554 |
| O47* | India (1973) | Human | 88586 | ||
| O51* | India (1973) | Human | 88590 | ||
| O53* | India (1974) | Human | 88592 | ||
| O68* | Japan (1978) | Seawater | 88607 | ||
| O7 | Campeche (1992) | Well water | 87242 | ||
| O7 | Sonora (1993) | Fish | 88354 | ||
| 256 | 6 | O74* | India (1979) | Human | 88613 |
| OA | Hidalgo (1991) | Human | 87250 | ||
| OA | Hidalgo (1991) | Human | 87256 | ||
| OA | Veracruz (1991) | Human | 87264 | ||
| OA | Puebla (1991) | Human | 87246 | ||
| OA | Guatemala (1993a) | Human | 88778 | ||
| 128 | 2 | O22* | India (1968) | Human | 88561 |
| O6 | Campeche (1991) | Human | 87268 | ||
| 131b | 2 | O34* | India (1968) | Human | 88573 |
| O14 | Guatemala (1993a) | Human | 88729 | ||
| 247 | 2 | O92* | Japan (1987) | River water | 88631 |
| O68 | Tabasco (1992) | Human | 87311 | ||
| 73 | 2 | O23* | India (1971) | Human | 88562 |
| O26* | Philippines (1972) | Human | 88565 | ||
| 5 | 2 | O37* | India (1969) | Human | 88576 |
| O102* | China (1988) | Human | 88641 | ||
| 129 | 2 | O130* | India (1981) | Human | 88669 |
| O104* | China (1988) | Human | 88643 | ||
| 65 | 2 | O125* | India (1981) | Human | 88664 |
| O132* | Thailand (1981) | Human | 88671 |
Date of receipt at the Departamento de Salud Pública de la Facultad de Medicina.
ET 131 differs from ET 132 of strain O14* (India, 1964) at a single locus (that for LAP) (Table 5).
ETs 128 and 131 are each represented by a strain collected in India in 1968 and an isolate recovered in Campeche or Guatemala in the early 1990s. It is noteworthy that ET 131 differs at only a single locus (that for LAP) from ET 132 of the O14 reference strain, which was recovered in India in 1964 (see Table 5). Thus, ETs 131 and 132 are members of a clonal lineage that was extant for at least 28 years.
TABLE 5.
Serogroups and geographic sources of non-O1 strains representing pairs of ETs that differ at a single locus
| Strains from different continents | Strains from same continent | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ET | Serogroup | Locality (date) | FMU strain no. | Locus | ET | Serogroup | Locality (date) | FMU strain no. | Locus | |
| 17 | O5* | India (1964) | 88544 | PLP | 194 | O4* | India (1932) | 88543 | LAP | |
| 16 | O5 | Hidalgo (1991)a | 87243 | PLP | 193 | O96* | India (1976) | 88635 | LAP | |
| 132 | O14* | India (1964) | 88553 | LAP | 166 | O6* | India (1962) | 88545 | PGM | |
| 131 | O14 | Guatemala (1993b) | 88729 | LAP | 165 | O79* | India (1976) | 88618 | PGM | |
| 5 | O37* | India (1969)c | 88576 | GOT | 132 | O14* | India (1964) | 88553 | LAP | |
| 6 | O37 | Guatemala (1993b) | 88777 | GOT | 131 | O34* | India (1968) | 88573 | LAP | |
| 109 | O44* | India (1973) | 88583 | PLP | 196 | O15* | India (1968) | 88554 | MDH | |
| 108 | O44 | Chiapas (1991) | 87245 | PLP | 195 | O35* | India (1969) | 88574 | MDH | |
| 102 | O18* | India (1964) | 88557 | PGI | 115 | O54* | India (1974) | 88593 | LAP | |
| 101 | O14 | Campeche (1992) | 87440 | PGI | 114 | O94* | India (1976) | 88633 | LAP | |
| 152 | O16* | India (1971) | 88555 | PGI | 168 | O95* | India (1976) | 88634 | CAT | |
| 151 | O48 | Tabasco (1991) | 87675 | PGI | 167 | O129* | India (1981) | 88668 | CAT | |
| 169 | O134* | India (1991) | 88673 | CAT | ||||||
| 122 | O49* | India (1974) | 88588 | 6PG | ||||||
| 121 | NT | Michoacan (1992) | 87293 | 6PG | 155 | O112* | Japan (1989)h | 88651 | IDH | |
| 123 | NT | Sonora (1993) | 88352 | 6PG | 154 | O113* | Japan (1989)h | 88652 | IDH | |
| 192 | O60* | India (1975) | 88599 | IDH | 31 | O12 | Tabasco (1992) | 87432 | G6P | |
| 191 | O68 | Sonora (1993) | 88392 | IDH | 32 | O12 | Guatemala (1993b) | 88750 | G6P | |
| 134 | O55* | India (1975) | 88594 | LAP | 107 | O64 | Veracruz (1991) | 87257 | LAP | |
| 133 | NT | Hidalgo (1991) | 87263 | LAP | 106 | O64 | Chiapas (1991) | 87272 | LAP | |
| 186 | O81* | India (1978) | 88620 | 6PG | 229 | O149 | Guanajuato (1991) | 87290 | CAT | |
| 185 | O80 | Campeche (1992) | 87437 | 6PG | 228 | O149 | Guanajuato (1991) | 87297 | CAT | |
| 230 | O155 | Campeche (1992) | 90594 | CAT | ||||||
| 73 | O23* | India (1971)d | 88562 | G6P | ||||||
| 72 | O93 | Guatemala (1993b) | 88773 | G6P | 22 | O14 | Campeche (1992) | 87661 | PGM | |
| 21 | O14 | Campeche (1992) | 87778 | PGM | ||||||
| 256 | O74*c | India (1979) | 88613 | IPO | ||||||
| 257 | OA | Guatemala (1993b) | 88733 | IPO | 59 | O44 | Sonora (1993) | 88380 | PGM | |
| 58 | O5 | Sonora (1993) | 88382 | PGM | ||||||
| 247 | O92* | Japan (1987)f | 88631 | CAT | ||||||
| 246 | O31 | Tabasco (1992) | 87303 | CAT | 157 | O141 | Hidalgo (1991) | 87274 | LAP | |
| 156 | O82 | Sonora (1993) | 88389 | LAP | ||||||
| 136 | O120* | Japan (1991) | 88659 | PGM | ||||||
| 135 | O52 | Sonora (1993) | 88356 | PGM | 233 | NT | Hidalgo (1991) | 87298 | CAT | |
| 234 | O5 | Campeche (1992) | 87240 | CAT | ||||||
| 53 | O108* | Japan (1989) | 88637 | PGM | ||||||
| 52 | O79 | Zacatecas (1991) | 87295 | PGM | 225 | O41 | Tabasco (1992) | 87309 | LAP | |
| 54 | O105 | Sonora (1993) | 88387 | PGM | 226 | NT | Tabasco (1992) | 87305 | LAP | |
| 98 | O122* | Romania (1980) | 88661 | CAT | 221 | O35 | Veracruz (1991) | 87279 | THD | |
| 97 | O97 | Veracruz (1991) | 87283 | CAT | 220 | O151 | Sonora (1993) | 88367 | THD | |
| 265 | O20* | India (1962) | 88559 | IDH | 51 | O43 | Guatemala (1993b) | 88727 | CAT | |
| 264 | O101* | China (1988) | 88640 | IDH | 50 | O40 | Guatemala (1993b) | 88744 | CAT | |
| 128 | O22* | India (1968)g | 88561 | PGM | ||||||
| 127 | O83* | India (1978) | 88622 | PGM | ||||||
| 129 | O104* | China (1988) | 88643 | PGM | ||||||
| 200 | O86* | Philippines (1981) | 88625 | GOT | ||||||
| 199 | O133* | India (1991) | 88672 | GOT | ||||||
There are six additional O5 isolates of ET 16 from Hidalgo, Yucatán, Tabasco, and “Mexico” (Table 4).
Date of receipt at the Departamento de Salud Pública de la Facultad de Medicina, UNAM.
Strain O37* (India, 1969) is of the same ET as O102* from China (1988) (Table 3).
Strain O23* (India, 1971) is of the same ET as strain O16* from the Philippines (1972) (Table 3).
Five additional OA isolates from Mexico and Guatemala (1991 to 1993) are of the same ET as strain O74* from India (1979) (Table 3).
Strain O92* (Japan, 1987) is of the same ET as a strain of serotype O68 from Tabasco (1992) (Table 3).
Strain O22* (India, 1968) is of the same ET as an O6 strain from Campeche (1991) (Table 3).
Source: rat.
Nine ETs are represented by pairs or multiple strains from different states in Mexico (Table 4). Five of these ETs are represented by strains of the same serogroup, and four of them are represented by strains of different serogroups. For example, ET 16 was represented by seven O5 isolates from Hidalgo, Tabasco, Yucatán, and an unspecified locality in Mexico. Significantly, ET 16 differs by only one locus (that for PLP) from ET 17 of the O5 reference strain, which was collected in India in 1964 (see Table 5).
TABLE 4.
ETs represented by isolates from two or more states in Mexico
| ET | No. of isolates | Serogroup | Locality (date) | Source | FMU strain no. |
|---|---|---|---|---|---|
| Of same serogroup | |||||
| 16a | 7 | O5 | Hidalgo (1991) | Human | 87243 |
| O5 | Hidalgo (1991) | Human | 87259 | ||
| O5 | Tabasco (1991) | Human | 87139 | ||
| O5 | Tabasco (1991) | Human | 87672 | ||
| O5 | Mexico (1991) | Human | 87288 | ||
| O5 | Tabasco (1992) | Human | 87306 | ||
| O5 | Yucatán (1992) | Human | 87291 | ||
| 228 | 2 | O149 | Guanajuato (1991) | Human | 87297 |
| O149 | Tabasco (1991) | Human | 87673 | ||
| 219 | 2 | O149 | Yucatán (1991) | Human | 87262 |
| O149 | Zacatecas (1991) | 87299 | |||
| 107 | 2 | O64 | Veracruz (1991) | Human | 87257 |
| O64 | Hidalgo (1991) | Human | 87289 | ||
| 124 | 2 | O24 | Veracruz (1991) | Human | 87282 |
| O24 | Tabasco (1992) | Human | 87434 | ||
| Of different serogroups | |||||
| 181 | 4 | O35 | Sonora (1993) | Sewage water | 88374 |
| O35 | Sonora (1993) | Sewage water | 88375 | ||
| O42 | Sonora (1993) | Fish | 88351 | ||
| NT | Guanajuato (1991) | Human | 87287 | ||
| 52 | 3 | O79 | Zacatecas (1991) | Human | 87295 |
| O43 | Tabasco (1992) | Human | 87307 | ||
| O29 | Sonora (1993) | Sewage water | 88371 | ||
| 234 | 2 | O5 | Campeche (1992) | Well water | 87240 |
| O62 | Tabasco (1992) | Human | 87304 | ||
| 171 | 2 | O41 | Sonora (1993) | Septic tank | 88366 |
| NT | Guerrero (1991) | 87271 |
ET 16 differs from ET 17 of strain O5* (India, 1964) at a single locus (that for PLP) (Table 5).
Serotypic diversity among strains of closely related ETs.
We identified 19 cases in which pairs of ETs that differ at a single enzyme locus are represented by strains collected on different continents or major land masses (Table 5). In four of these cases, the strains are of the same serotype; these are O5* from India (1964) and seven O5 isolates from Hidalgo and other states in Mexico (1991 and 1992), O14* from India (1964) and O14 from Guatemala (1993), O37* from India (1969) and O37 from Guatemala (1993), and O44* from India (1973) and O44 from Chiapas (1991). In all other cases, the strains are of different serotypes or one of them was NT.
Genetic diversity within serogroups.
MLEE analysis demonstrated that strains of the same serogroup may belong to two or more widely divergent ET lineages. Thus, for example, strains of serogroup O29 were assigned to four ETs that occur in division I (ET 50 and ET 52 in group B and ET 173 in group C) and division II (ET 274), and O53 strains are found in group C of division I (ET 196) and also in lineage y (ET 278). Some estimated levels of genetic diversity between or among the ETs of strains of the same serogroup are shown in Table 6. In several cases, the estimated diversity is at least equivalent to that obtained for the 279 ETs identified among all 397 isolates.
TABLE 6.
Mean genetic diversity per locus among multiple ETs of the same serogroup
| Serogroup | No. of:
|
Mean genetic diversity per locus | |
|---|---|---|---|
| Isolates | ETs | ||
| O12 | 4 | 4 | 0.206 |
| O37 | 4 | 4 | 0.314 |
| O6 | 12 | 10 | 0.316 |
| O8 | 14 | 4 | 0.333 |
| O5 | 14 | 8 | 0.345 |
| O38 | 4 | 2 | 0.412 |
| O155 | 9 | 7 | 0.423 |
| O42 | 3 | 3 | 0.490 |
| O29 | 4 | 4 | 0.559 |
| O39 | 5 | 4 | 0.618 |
| O30 | 2 | 2 | 0.824 |
| O53 | 3 | 2 | 1.000 |
Distribution of the ctxA gene.
When tested with the ctxA probe, 13 of the 143 reference strains were positive, as shown in Table 7. With PCR amplification of the gene, the same strains were positive, with the exception of O1 Inaba El Tor*. Among 104 of 254 nonreference strains that were randomly selected for testing, 2 isolates of O1 Ogawa El Tor, 3 isolates of O1 Inaba El Tor, an isolate of O1 Inaba classical, and an isolate of O6 were positive for ctxA by both colony blot assay and PCR amplification.
TABLE 7.
Strains testing positive for ctxAa
| Serogroup | FMU strain no. | ET | Division or subdivision (group) | Source |
|---|---|---|---|---|
| Reference | ||||
| O1 Ogawa El Tor* | 3 | Ia (A) | India (1941), human | |
| O1 Inaba El Tor* | 259 | Ie | India (1942), human | |
| O19* | 41 | Ia (B) | ||
| O33* | 204 | Ia (C) | India (1968), human | |
| O37* | 5 | Ia (A) | India (1969), human | |
| O43* | 43 | Ia (B) | India (1973), human | |
| O44* | 109 | Ia (B) | India (1972), human | |
| O54* | 115 | Ia (B) | India (1974), human | |
| O105* | 116 | Ia (B) | India (1988), human | |
| O106* | 64 | Ia (B) | China (1988), human | |
| O135* | 268 | II | India (1992), human | |
| O138* | 270 | II | Japan (1992), crab | |
| O139* | 3 | Ia (A) | India (1993), human | |
| Nonreference | ||||
| O1 Ogawa El Tor | 87668 | 3 | Ia (A) | Morelos, human |
| O1 Ogawa El Tor | 90334 | 3 | Ia (A) | Mexico, human |
| O1 Inaba El Tor | 87269 | 4 | Ia (A) | Campeche, human |
| O1 Inaba El Tor | 88696 | 2 | Ia (A) | Louisiana, human |
| O1 Inaba El Tor | 90500 | 3 | Ia (A) | Tabasco, human |
| O1 Inaba classical | 87395/0 | 2 | Ia (A) | Quintana Roo, human |
| O6 | 88751 | 27 | Ia (A) | Guatemala, human |
| O139 | 88230 | 3 | Ia (A) | United States, imported case |
All strains were positive when tested with the ctxA probe and by PCR, with the exception of O1 Inaba El Tor*, which was probe positive but PCR negative.
All but 3 of the total of 21 ctxA-positive strains are members of subdivision Ia of division I (Fig. 1 and 2); the exceptions are the reference strains O1 Inaba El Tor*, in subdivision Ie, and O135* and O138*, in division II.
DISCUSSION
Species limits.
Strains of ETs 276 to 279 in the deep lineages x and y (Fig. 1) are sufficiently differentiated from all other strains as to raise the question of whether they should be included in the species V. cholerae. It is likely that assessment of genomic relatedness by DNA-DNA hybridization would show relative degrees of annealing with other strains somewhat below the 70% standard adopted for species inclusion by the CDC (6).
Genetic diversity.
The estimate of 0.436 for the mean genetic diversity per locus among the 279 ETs of V. cholerae represented in the present study is larger than the comparable value of 0.343 reported for the Escherichia coli reference collection (35) but smaller than the corresponding value of 0.627 obtained for Salmonella enterica (36). In a previous MLEE study of allelic variation at 13 enzyme loci among 260 isolates of V. cholerae (most of which were serogroup O1), Salles and Momen (33) detected an average of 4.3 alleles per locus, identified 73 ETs, and estimated the mean genetic diversity per locus as 0.326.
Genetic structure of populations.
Comparisons of the observed and expected variances of the mismatch distributions for ETs at several hierarchical levels of dendrogram structure yielded only limited evidence of linkage disequilibrium (Table 1). The cases in which the observed variance exceeded the upper 95% confidence limit of the variance expected under random association of alleles were those involving all 279 ETs, the 275 ETs of divisions I and II combined, the 263 ETs of division I, the 12 ETs of division II, and the 40 ETs of subdivision Ic of division I. Within each of the groups A, B, and C of subdivision Ia, which include 204 ETs (Fig. 1), significant levels of nonrandom association were not demonstrable. The inference is that, at least among the strains of ETs in subdivision Ia, the rate of horizontal transfer and recombination of housekeeping enzyme genes is sufficiently high to prevent the development and long-term maintenance of distinctive allele complexes. On the whole, it seems likely that the frequency of recombination, both intragenic and assortative (50), of housekeeping genes in V. cholerae is somewhat higher than in either E. coli (48) or S. enterica (36), a conclusion also reached by Karaolis et al. (19) from a comparative sequence analysis of the asd gene. But even within subdivision Ia, clonal lineages may persist for periods of time measured in at least decades. The most obvious examples are the epidemic and pandemic strains of serogroups O1 and O139 (ETs 2 to 4), but our analysis identified numerous clones and clonal lineages of non-O1 strains with widespread, if not global, distributions (Tables 3 to 5).
A factor that has not been evaluated in studies of the genetic structure of bacterial populations on the basis of MLEE data is the convergent evolution of electromorphs, which cannot be equated with isoalleles. Similarity in electrophoretic mobility resulting from convergence in the net electrostatic charge of an enzyme will lessen the likelihood that linkage disequilibrium is detected from the analysis of MLEE data. Studies of sequence variation in several housekeeping enzymes among multiple strains of E. coli and S. enterica (5, 26–28, 47) have shown that individual electromorphs may exhibit substantial heterogeneity in amino acid sequence, much of which clearly stems from convergence rather than mutational divergence from a common ancestral sequence.
There was already evidence for recombination of genes of the rfb region of V. cholerae, based on studies of relatively small numbers of strains and serogroups. Our observation that strains of the same serogroup frequently are found in divergent, even distantly related, lineages supports earlier evidence (2, 19) that the rfb genes are subject to horizontal transfer and further suggests that this process occurs with relatively high frequency. Convergence in serotype is, of course, an alternative explanation, but reasoning by analogy from the lack of evidence for convergence in epitope structure in the serologically diverse flagellins of S. enterica (22), we favor the first hypothesis. The issue can be settled by comparative sequencing of the epitope-encoding segments of the rfb region.
The fact that strains of the same ET may express different O antigens can be explained by recombination or by spontaneous mutation of the genes encoding O somatic properties.
Epidemic non-O1 clones.
There are two examples of epidemic V. cholerae expressing a non-O1 antigen. The first is the serogroup O139 clone, which emerged in India and Bangladesh through modification of the El Tor O1 pandemic strain by acquisition of genes mediating the synthesis of the O139 LPS and a polysaccharide capsule. The second is the O37 strain that reportedly was responsible for a large outbreak of cholera in the Sudan in 1968 (54). By IS1004 fingerprinting, Bik et al. (3) determined that an O37 isolate from the Sudan is closely related to classical O1 strains. The O37 reference strain (ET 5), which was recovered from a patient in India in 1969, presumably represents the same clone as the O37 Sudan strain. As determined by MLEE, it is closely related to O1 El Tor and other epidemic O1 strains (Fig. 2), thus confirming the result obtained by IS1004 fingerprinting. In fact, ET 5 is distinguished from ETs 2 to 4 of the O1/O139 cluster solely by possession of a 4 allele (versus a 3 allele) at the PGI locus. It carries the ctxA gene and expresses cholera toxin. Yamamoto et al. (53) reported that the amino acid sequence of the cholera toxin produced by O37 strain S7 differs from that of most O1 strains in having single substitutions in both the CtxA and CtxB segments (29), which are presumed to cause the formation of an unusually large subunit B oligomer. Recently, Karaolis et al. (20) reported that, almost uniquely among non-O1 strains, a Sudan 1968 outbreak strain carries a chromosomal pathogenicity island that is characteristic of epidemic and pandemic strains.
Honma et al. (16) studied an O37 isolate that produces a hemolysin (O37-Hly) that is antigenically similar to O1 El Tor hemolysin (El Tor-Hly) but different in molecular size, hemolytic activity, and glucose-binding capacity. The gene encoding O37-Hly differs from that encoding O1 El Tor-Hly by the presence of a 4-bp insertion that generates a premature stop codon in the downstream region. Thus, the O37-Hly is a truncated derivative of O1 El Tor-Hly, sharing 90% of the N-terminal region.
In the Mexico-Guatemala collection, there are three O37 isolates, one of which (ET 6) is almost identical in MLEE genotype (it carries a GOT 4 rather than a GOT 3 allele) to the O37 reference strain (ET 5) from India but apparently lacks the ctxA gene. It was isolated from a patient in Guatemala. The two other O37 isolates, both of which were cultured from well water in Campeche, are distantly related (six- and seven-locus differences) to both the reference O37 and Guatemala O37 strains, as well as to one another (four-locus difference), and neither one carries the ctxA gene.
It is noteworthy that strain O102*, which was recovered from a patient with diarrhea in China in 1988, is identical in MLEE genotype (ET 5) to strain O37* but apparently does not carry the ctxA gene.
In sum, there is a clone of serogroup O37 that has epidemic potential and was present in Africa and India in 1968 and 1969. Because it is closely related to O1 El Tor and the other O1 pandemic clones, it apparently represents a case similar to that of the O139 clone, in which an already-established pathogenic lineage of serogroup O1 acquired a new serotype by horizontal DNA transfer and rearrangement of the rfb region genes. The O37 strain from Guatemala may be an offshoot of this clone in which the CTX genetic element has been deleted. The two O37 strains from Campeche presumably have independent acquisitions of the O37 polysaccharide gene region.
O1 Inaba El Tor reference strain.
The O1 Inaba El Tor reference strain (ET 259), which does not produce cholera toxin although it carries at least part of the ctxA gene, is not closely related to the O1/O139 cluster of pandemic strains or to the toxigenic O37 clone (ET 5). According to T. Shimada (38a), the reference strain is the NIH 35-a-3 isolate listed by Burrows et al. (7) as one of the strains used for vaccine preparation by the U.S. Army in the early 1940s. It was received from the Central Research Institute in Kasauli, India, in 1942, without indication of collection date or source of isolation. Perhaps it is related to the O1 strain that caused a cholera-like disease in Hong Kong in the 1950s (43).
Relationships of serogroup O1 strains.
Colwell et al. (11) hypothesized that non-O1 cells may convert to the O1 serotype and vice versa under suitable conditions, a possible strategy for survival in the environment. As noted earlier, O37* and O102* (both of ET 5) may represent cases in which O1 clones have acquired new serotypes. In our collection, the only apparent case of conversion of a non-O1 strain to the O1 serotype (apart from O139) is the O1 Inaba El Tor reference strain (ET 259), which occurs in subdivision Ie (Fig. 1) and is distantly related to the O1 epidemic strains (ETs 2 to 4) in group A of subdivision Ia (Fig. 2).
Source of rfb region DNA in the emergence of the epidemic O139 clone.
The putative source of the exogenous rfb region DNA that was involved in the transformation of an O1 El Tor strain to the epidemic O139 clone has been identified as a strain of serogroup O22, O141, or O155 on the basis of serotypic cross-reactions with O139 (2, 38, 42). Molecular analysis showed that, in common with O139, they have two open reading frames in the rfaD region that are lacking in O1 strains.
Through study of the gene content and organization of the rfb region adjacent to IS1358 in strains of O139 and 13 other serogroups, including O22 and O155, Dumontier and Berche (14) recently identified the clone represented by strain O22* (Shimada strain 169-68, from India) as the most likely donor, although the possibility of a multistep rearrangement in the recipient O1 strain cannot be excluded. As determined by MLEE analysis, O22* (ET 128) falls in group B of subdivision Ia and differs in ET from the epidemic O1 and O139 strains at four or five loci.
In our sample of strains, there were nine serogroup O155 isolates belonging to seven ETs. ET 224 of the O155 reference strain (Thailand, 1993) and five other ETs (represented by isolates from Tabasco and Campeche) are in subdivision Ic, where, however, they do not form a tight cluster. The remaining ET, which is represented by two isolates from Sonora, is in group B of subdivision Ia. Thus, strains of serogroup O155 belong to a moderately diverse group of ETs, none of which is closely related to the ETs of the epidemic O1 and O139 clones. This suggests that genes mediating expression of the O155 LPS antigen are transferred with relatively high frequency.
Genesis of epidemic clones.
Because genes for the major virulence factors can be transferred horizontally and antigenic conversion can be achieved by the acquisition and loss of rfb genes, there is the formal possibility that any V. cholerae cell could be transformed into a virulent strain, even an epidemic one (19, 25). However, the close evolutionary relationships of the O1, O139, and O37 epidemic clones indicate that new epidemic or other strongly virulent clones are likely to arise by the modification of a lineage that is already epidemic or is closely related to such a clone. Thus, O139 evolved from an El Tor O1 clone by acquisition of a transposon carrying genes for the O139 LPS and a polysaccharide capsule and deletion of most of the genes mediating synthesis of the serotype O1 LPS (see the review by Rubin et al. [31]). Also, the toxigenic O37 clone that caused outbreaks in the Sudan and India in the late 1960s is closely related to the cluster of O1/O139 epidemic clones. Analogously, E. coli O157:H7, which emerged as an agent of hemorrhagic colitis by acquisition of the O157 antigen, a Shiga-like toxin, and the enterohemorrhagic E. coli plasmid, is an evolutionary derivative of an O55:H7 clone that is associated with infantile diarrhea (51).
ACKNOWLEDGMENTS
We thank Thomas Cheasty, Peter Echeverria, Alma Rosa González, Sergio León, Claudio Lezana, José Luis Navarro-Heinze, and Toshio Shimada for supplying strains and Delia Licona and José Luis Méndez for technical assistance in the laboratory.
This research was supported by grants from the Consejo Nacional de Ciencia y Tecnología (project 2397PB); the Dirección General de Apoyo al Personal Académico, UNAM (project IN211496); and the National Institutes of Health (AI-22144).
REFERENCES
- 1.Albert M J, Siddique A K, Islam M S, Faruque A S, Ansaruzzaman M, Faruque S M, Sack R B. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet. 1993;341:704. doi: 10.1016/0140-6736(93)90481-u. [DOI] [PubMed] [Google Scholar]
- 2.Bik E M, Bunschoten A E, Gouw R D, Mooi F R. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 1995;14:209–216. doi: 10.1002/j.1460-2075.1995.tb06993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bik E M, Gouw R D, Mooi F R. DNA fingerprinting of Vibrio cholerae strains with a novel insertion sequence element: a tool to identify epidemic strains. J Clin Microbiol. 1996;34:1453–1461. doi: 10.1128/jcm.34.6.1453-1461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake P A, Weaver R E, Hollis D G. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- 5.Boyd E F, Nelson K, Wang F-S, Whittam T S, Selander R K. Molecular genetic basis of allelic polymorphism in malate dehydrogenase (mdh) in natural populations of Escherichia coli and Salmonella enterica. Proc Natl Acad Sci USA. 1994;91:1280–1284. doi: 10.1073/pnas.91.4.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner D J, Steigerwalt A G, Epple P, Bibb W F, McKinney R M, Starnes R W, Colville J M, Selander R K, Edelstein P H, Moss C W. Legionella pneumophila serogroup Lansing 3 isolated from a patient with fatal pneumonia, and descriptions of L. pneumophila subsp. pneumophila subsp. nov., L. pneumophila subsp. fraseri subsp. nov., and L. pneumophila subsp. pascullei subsp. nov. J Clin Microbiol. 1988;26:1695–1703. doi: 10.1128/jcm.26.9.1695-1703.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burrows W, Mather A N, Elliott M E, Wagner S M. Studies on immunity to Asiatic cholera. J Infect Dis. 1946;79:159–167. doi: 10.1093/infdis/79.2.159. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Evins G M, Cook W L, Almeida R, Hargrett-Bean N, Wachsmuth K. Genetic diversity among toxigenic and nontoxigenic Vibrio cholerae O1 isolated from the Western Hemisphere. Epidemiol Infect. 1991;107:225–233. doi: 10.1017/s0950268800048846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chongsa-nguan M, Chaicumpa W, Moolasart P, Kandhasingha P, Shimada T, Kurazono H, Takeda Y. Vibrio cholerae O139 Bengal in Bangkok. Lancet. 1993;342:430–431. doi: 10.1016/0140-6736(93)92841-g. [DOI] [PubMed] [Google Scholar]
- 10.Colwell R R. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 11.Colwell R R, Huq A, Chowdhury M A R, Brayton P R, Xu B. Serogroup conversion of Vibrio cholerae. Can J Microbiol. 1995;41:946–950. doi: 10.1139/m95-131. [DOI] [PubMed] [Google Scholar]
- 12.Comstock L E, Maneval D, Jr, Panigrahi P, Joseph A, Levine M M, Kaper J B, Morris J G, Jr, Johnson J A. The capsule and O antigen in Vibrio cholerae O139 Bengal are associated with a genetic region not present in Vibrio cholerae O1. Infect Immun. 1995;63:317–323. doi: 10.1128/iai.63.1.317-323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desmarchelier P M, Momen H, Salles C A. A zymovar analysis of Vibrio cholerae isolated in Australia. Trans R Soc Trop Med Hyg. 1988;82:914–917. doi: 10.1016/0035-9203(88)90041-7. [DOI] [PubMed] [Google Scholar]
- 14.Dumontier S, Berche P. Vibrio cholerae O22 might be a putative source of exogenous DNA resulting in the emergence of the new strain of Vibrio cholerae O139. FEMS Microbiol Lett. 1998;164:91–98. doi: 10.1111/j.1574-6968.1998.tb13072.x. [DOI] [PubMed] [Google Scholar]
- 15.Evins G M, Cameron D N, Wells J G, Greene K D, Popovic T, Giono-Cerezo S, Wachsmuth I K, Tauxe R V. The emerging diversity of the electrophoretic types of Vibrio cholerae in the Western Hemisphere. J Infect Dis. 1995;172:173–179. doi: 10.1093/infdis/172.1.173. [DOI] [PubMed] [Google Scholar]
- 16.Honma Y, Yamamoto K, Iwanaga M. Aberrant gene for El Tor hemolysin from Vibrio cholerae non-O1, N037. FEMS Microbiol Lett. 1995;133:151–154. doi: 10.1111/j.1574-6968.1995.tb07876.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaper J B, Morris J G, Jr, Levine M M. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaolis D K R, Lan R, Reeves P R. Molecular evolution of the seventh-pandemic clone of Vibrio cholerae and its relationship to other pandemic and epidemic V. cholerae isolates. J Bacteriol. 1994;176:6199–6206. doi: 10.1128/jb.176.20.6199-6206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karaolis D K R, Lan R, Reeves P R. The sixth and seventh cholera pandemics are due to independent clones separately derived from environmental, nontoxigenic, non-O1 Vibrio cholerae. J Bacteriol. 1995;177:3191–3198. doi: 10.1128/jb.177.11.3191-3198.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karaolis D K R, Johnson J A, Bailey C C, Boedeker E C, Kaper J B, Reeves P R. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc Natl Acad Sci USA. 1998;95:3134–3139. doi: 10.1073/pnas.95.6.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovach M E, Shaffer M D, Peterson K M. A putative integrase gene defines the distal end of a large cluster of ToxR-regulated colonization genes in Vibrio cholerae. Microbiology. 1996;142:2165–2174. doi: 10.1099/13500872-142-8-2165. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Nelson K, McWhorter-Murlin A C, Whittam T S, Selander R K. Recombinational basis of serovar diversity in Salmonella enterica. Proc Natl Acad Sci USA. 1994;91:2552–2556. doi: 10.1073/pnas.91.7.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 24.Maynard Smith J, Smith N H, O’Rourke M, Spratt B G. How clonal are bacteria? Proc Natl Acad Sci USA. 1993;90:4384–4388. doi: 10.1073/pnas.90.10.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mekalanos J J, Rubin E J, Waldor M K. Cholera: molecular basis for emergence and pathogenesis. FEMS Immunol Med Microbiol. 1997;18:241–248. doi: 10.1111/j.1574-695X.1997.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 26.Nelson K, Whittam T S, Selander R K. Nucleotide polymorphism and evolution in the glyceraldehyde-3-phosphate dehydrogenase gene (gapA) in natural populations of Salmonella and Escherichia coli. Proc Natl Acad Sci USA. 1991;88:6667–6671. doi: 10.1073/pnas.88.15.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson K, Selander R K. Evolutionary genetics of the proline permease gene (putP) and the control region of the proline utilization operon in populations of Salmonella and Escherichia coli. J Bacteriol. 1992;174:6886–6895. doi: 10.1128/jb.174.21.6886-6895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson K, Selander R K. Intergeneric transfer and recombination of the 6-phosphogluconate dehydrogenase gene (gnd) in enteric bacteria. Proc Natl Acad Sci USA. 1994;91:10227–10231. doi: 10.1073/pnas.91.21.10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsvik Ø, Wahlberg J, Petterson B, Uhlen M, Popovic T, Wachsmuth I K, Fields P I. Use of automated sequencing of polymerase chain reaction-generated amplicons to identify three types of cholera toxin subunit B in Vibrio cholerae O1 strains. J Clin Microbiol. 1993;31:22–25. doi: 10.1128/jcm.31.1.22-25.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramamurthy T, Garb S, Sharma R, Bhattacharya S K, Nair G B, Shimada T, Takeda T, Karasawa T, Kurazano H, Pal A, Takeda Y. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet. 1993;341:703–704. doi: 10.1016/0140-6736(93)90480-5. [DOI] [PubMed] [Google Scholar]
- 31.Rubin E J, Waldor M K, Mekalanos J J. Mobile genetic elements and the evolution of new epidemic strains of Vibrio cholerae. In: Krause R M, editor. Emerging infections. San Diego, Calif: Academic Press; 1998. pp. 147–161. [Google Scholar]
- 32.Sakazaki R, Donovan T J. Serology and epidemiology of Vibrio cholerae and Vibrio mimicus. Methods Microbiol. 1984;16:271–289. [Google Scholar]
- 33.Salles C A, Momen H. Identification of Vibrio cholerae by enzyme electrophoresis. Trans R Soc Trop Med Hyg. 1991;85:544–547. doi: 10.1016/0035-9203(91)90251-s. [DOI] [PubMed] [Google Scholar]
- 34.Selander R K, Caugant D A, Ochman H, Musser J M, Gilmour M N, Whittam T S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986;51:873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selander R K, Caugant D A, Whittam T S. Genetic structure and variation in natural populations of Escherichia coli. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 2. Washington, D.C: American Society for Microbiology; 1987. pp. 1625–1648. [Google Scholar]
- 36.Selander R K, Li J, Nelson K. Evolutionary genetics of Salmonella enterica. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C: ASM Press; 1996. pp. 2691–2707. [Google Scholar]
- 37.Sharma C, Ghosh A, Dalsgaard A, Forslund A, Ghosh R K, Bhattacharya S K, Nair G B. Molecular evidence that a distinct Vibrio cholerae O1 biotype El Tor strain in Calcutta may have spread to the African continent. J Clin Microbiol. 1998;36:843–844. doi: 10.1128/jcm.36.3.843-844.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimada T, Arakawa E, Itoh K, Nakazato T, Okitsu T, Yamai S, Kusum M, Nair G B, Takeda Y. Two strains of Vibrio cholerae non-O1 possessing somatic (O) antigen factors in common with V. cholerae serogroup O139 synonym “Bengal.”. Curr Microbiol. 1994;29:331–333. [Google Scholar]
- 38a.Shimada, T. Personal communication.
- 39.Shirai H, Nishibuchi M, Ramamurthy T, Bhattacharya S K, Pal S C, Takeda Y. Polymerase chain reaction for detection of the cholera enterotoxin operon of Vibrio cholerae. J Clin Microbiol. 1991;29:2517–2521. doi: 10.1128/jcm.29.11.2517-2521.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stroeher U H, Jedani K E, Dredge B K, Morona R, Brown M H, Karageorgos L E, Albert M J, Manning P A. Genetic rearrangements in the rfb regions of Vibrio cholerae O1 and O139. Proc Natl Acad Sci USA. 1995;92:10374–10378. doi: 10.1073/pnas.92.22.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stroeher U H, Parasivam G, Dredge B K, Manning P A. Novel Vibrio cholerae O139 genes involved in lipopolysaccharide biosynthesis. J Bacteriol. 1997;179:2740–2747. doi: 10.1128/jb.179.8.2740-2747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vimont S, Dumontier S, Escuyer V, Berche P. The rfaD locus: a region of rearrangement in Vibrio cholerae O139. Gene. 1997;185:43–47. doi: 10.1016/s0378-1119(96)00625-7. [DOI] [PubMed] [Google Scholar]
- 43.Wachsmuth I K, Evins G M, Fields P I, Olsvik Ø, Popovic T, Bopp C A, Wells J G, Carrillo C, Blake P A. The molecular epidemiology of cholera in Latin America. J Infect Dis. 1993;167:621–626. doi: 10.1093/infdis/167.3.621. [DOI] [PubMed] [Google Scholar]
- 44.Wachsmuth K, Olsvik Ø, Evins G M, Popovic T. Molecular epidemiology of cholera. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: ASM Press; 1994. pp. 357–370. [Google Scholar]
- 45.Waldor M K, Colwell R, Mekalanos J J. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc Natl Acad Sci USA. 1994;91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 47.Wang F-S, Whittam T S, Selander R K. Evolutionary genetics of the isocitrate dehydrogenase gene (icd) in Escherichia coli and Salmonella enterica. J Bacteriol. 1997;179:6551–6559. doi: 10.1128/jb.179.21.6551-6559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whittam T S. Genetic variation and evolutionary processes in natural populations of Escherichia coli. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C: ASM Press; 1996. pp. 2708–2720. [Google Scholar]
- 49.Whittam T S, Ochman H, Selander R K. Multilocus genetic structure in natural populations of Escherichia coli. Proc Natl Acad Sci USA. 1983;80:1751–1755. doi: 10.1073/pnas.80.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whittam T S, Ake S E. Genetic polymorphisms and recombination in natural populations of Escherichia coli. In: Takahata N, Clark A G, editors. Mechanisms of molecular evolution. Sunderland, Mass: Sinauer Associates, Inc.; 1993. pp. 223–245. [Google Scholar]
- 51.Whittam T S, McGraw E A, Reid S D. Pathogenic Escherichia coli O157:H7: a model for emerging infectious diseases. In: Krause R M, editor. Emerging infections. San Diego, Calif: Academic Press; 1998. pp. 163–183. [Google Scholar]
- 52.Yamai S, Okitsu T, Shimada T, Katsube Y. Distribution of serogroups of Vibrio cholerae non-O1 non-O139 with specific reference to their ability to produce cholera toxin, and addition of novel serogroups. Kansenshogaku Zasshi. 1997;71:1037–1045. doi: 10.11150/kansenshogakuzasshi1970.71.1037. . (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto K, Do Valle G R F, Xu M, Miwatani T, Honda T. Amino acids of the cholera toxin from Vibrio cholerae O37 strain S7 which differ from those of strain O1. Gene. 1995;163:155–156. doi: 10.1016/0378-1119(95)00415-3. [DOI] [PubMed] [Google Scholar]
- 54.Zinnaka Y, Carpenter C C., Jr An enterotoxin produced by noncholera vibrios. Johns Hopkins Med J. 1972;131:403–411. [PubMed] [Google Scholar]