Abstract
The disease caused by the new coronavirus, initially described in China in December 2019, became known as coronavirus disease 2019 and quickly spread to countries on all continents, becoming a pandemic with an important global impact. Despite being a virus that typically affects the respiratory tract, some studies have already described neurological manifestations associated with this infection, including acute ischaemic vascular insult. We report a case series including 30 patients, who presented with neurological symptoms during admission to our service, being diagnosed with ischaemic stroke and, concomitantly, coronavirus disease 2019. In the subgroup of patients analysed, a state of hypercoagulability and pro thrombosis was observed through laboratory tests, probably related to the cytokine storm syndrome associated with infection by this virus. With that, we discussed the possibility of this finding being an aggravating factor in the occurrence of stroke in these patients.
Keywords: COVID-19, neuroradiology, stroke
Introduction
A new virus, called severe acute respiratory syndrome coronavirus (SARS-CoV-2), was identified in the city of Wuhan, China, in December 2019, causing coronavirus 2019 disease (COVID-19) that soon spread to other countries, becoming a pandemic that continues to have a far-reaching impact on almost all aspects of society.1,2
COVID-19 is transmitted mainly by respiratory droplets and has the potential to cause severe respiratory disorders, especially in patients at risk, resulting in pneumonia, multiple organ dysfunction and even death.3 The neuroinvasive potential of the new coronavirus has also been described, demonstrating its ability to spread from the airways to the central nervous system (CNS).4–6 Two main mechanisms by which the coronavirus can invade the CNS have been suggested: haematogenic or neuronal. However, post-mortem studies in humans have generated evidence against the viability of the haematogenous pathway in coronavirus neuroinvasion.7
Coagulation disorders and vascular endothelial dysfunction have also been proposed as complications of COVID-19.8 Studies described in the literature suggest that subgroups of critically ill patients may have a cytokine storm syndrome, which can trigger ischaemic strokes, probably related to the prothrombotic effect of the inflammatory response.9,10 There is growing evidence that thrombi are a major cause of multisystem organ dysfunction.11,12
The infection by SARS-CoV-2 was associated with neurological symptoms in about 36.4% of patients hospitalised with COVID-19, according to Mao et al.13 In that study, it was observed that most neurological manifestations occurred at the beginning of the disease. Some patients without typical symptoms (fever, cough, anorexia and diarrhoea) of COVID-19, presented only with neurological manifestations at admission. It was also observed that patients with severe infection were more likely to develop neurological disorders, including acute cerebrovascular disease.13
As COVID-19 continues to spread around the world, when treating patients with neurological manifestations, doctors should consider SARS-CoV-2 infection as a concomitant diagnosis in order to avoid delay in diagnosis or diagnostic errors, as well as the prevention of transmission. In addition, it is especially significant to understand that in patients with severe COVID-19, rapid deterioration or clinical worsening may be associated with a neurological event such as stroke, which could contribute to an unfavourable outcome.13
Case series
We report a series of cases with 30 patients admitted to a hospital in Fortaleza, Brazil, from 25 April to 30 June 2020, who presented with acute neurological symptoms during admission, being diagnosed with ischaemic stroke and, concomitantly, COVID-19. In all 30 patients, COVID-19 was confirmed by either reverse transcription–polymerase chain reaction (RT–PCR) assay from nasopharyngeal swab samples or rapid testing for SARS-CoV-2. There is no history of recent hospital stay (in the past 2 months) in this hospital and no report of hospitalisation elsewhere by patients during admission. All patients underwent chest computed tomography (CT) without contrast and CT without contrast or magnetic resonance imaging of the brain. Twenty-six patients presented with hypodensities compatible with acute/subacute ischaemic insult on cranial CT. Twelve patients underwent angiotomography of the intracranial vessels, and thrombus was observed in six patients (Figure 1). Brain magnetic resonance imaging was performed in four patients, showing an area of diffusion restriction compatible with acute/subacute ischaemic insult (Figure 2). Changes suggestive of COVID-19 were evidenced in the chest tomography of 25 patients. One patient had no known medical history, three denied previous comorbidities and 26 had at least one of the following chronic disorders: cerebrovascular disease, four (13.3%); hypertension, 24 (80%); diabetes mellitus, 11 (36.7%); and hyperlipidemia, four (13.3%). The main feature of neuroimaging was acute ischaemic infarctions. Of these infarctions, three (10%) were in the territory of the anterior cerebral artery (Figure 3), 24 (80%) in the territory of the middle cerebral artery (Figure 4) and five (16.7%) in the territory of the posterior cerebral artery (Figure 5) (Table 1). Seven of the patients included in the study died during hospitalisation. Twenty-six patients reported flu-like symptoms; of these, eight had severe acute respiratory syndrome (SARS) during hospitalisation. Nineteen patients had laboratory tests in their charts that included D-dimer, lactate dehydrogenase (LDH), C-reactive protein (CRP) and ferritin. Of these, 18 had high levels of the afore-mentioned parameters, suggesting a cytokine storm syndrome. Ten patients presented with glycemia and controlled blood pressure at admission, which may be related to an adequate control of the underlying diseases, and eight had these parameters altered, with values reaching 379 mg/dl. Controlled blood pressure was considered for levels below 140 × 90 and controlled blood glucose for capillary blood glucose values below 180 mg/dL. Eleven patients did not present reports of laboratory values for the tests mentioned above in their medical records.
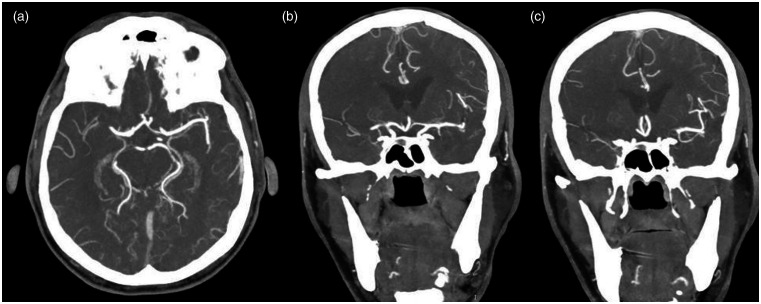
Figure 1.
Computed angiotomographic cranial images in axial (a) and coronal (b) and (c) sections show occlusion of the M1 segment of the right middle cerebral artery.
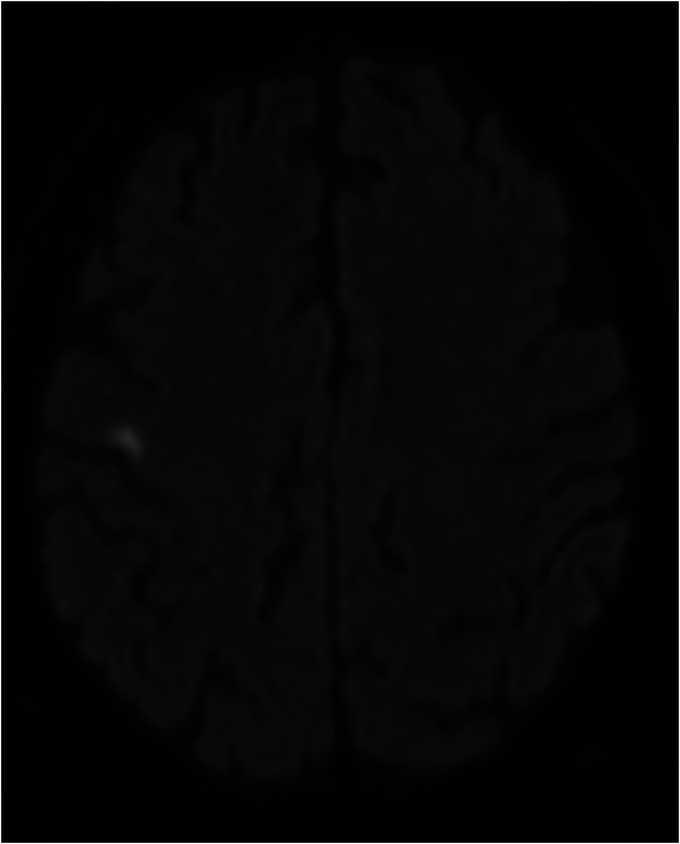
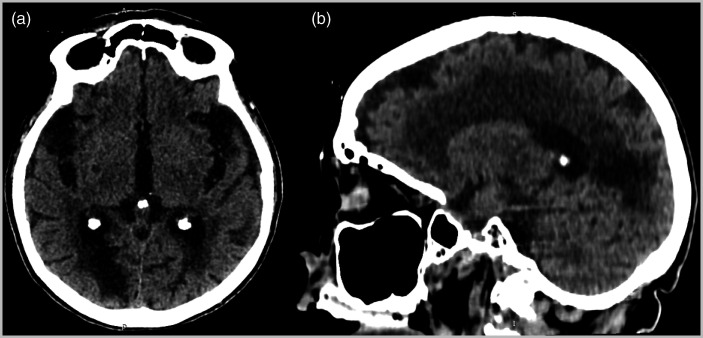
Figure 2.
Brain magnetic resonance imaging shows an area of diffusion restriction in the right frontal lobe compatible with acute/subacute ischaemic insult.
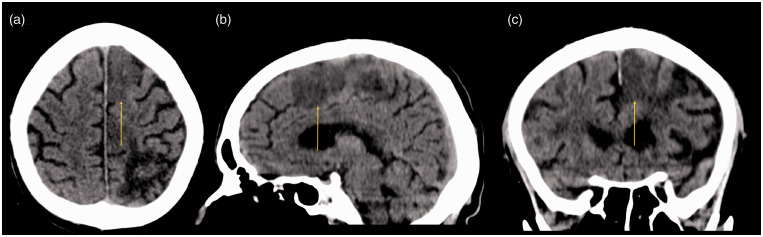
Figure 3.
Computed tomographic cranial images in axial (a), sagittal (b) and coronal (c) sections show an area of hypodensity in the territory of the left anterior cerebral artery, suggestive of infarction.
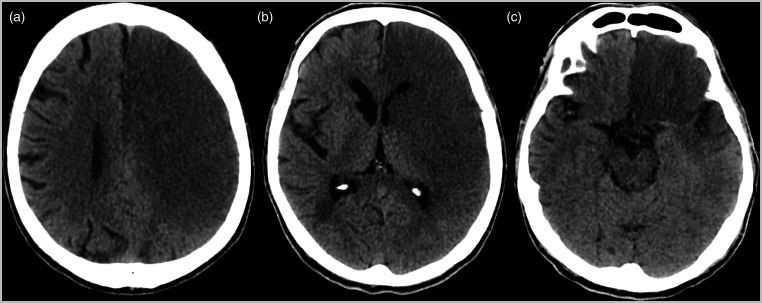
Figure 4.
Computed tomographic cranial images in axial sections show an extensive area of infarction in the territory of the left middle cerebral artery (a), causing compression of the adjacent lateral ventricle (b). (c) Hyperdensity of the left middle cerebral artery is also observed.
Figure 5.
Computed tomographic cranial images in axial (a) and sagittal (b) sections show an area of hypodensity in the right occipital region, suggestive of subacute ischaemic insult.
Table 1.
Cerebral vascular territories in acute ischaemic stroke.
| All patients (N = 30) | |
|---|---|
| Anterior cerebral artery | 3 (10) |
| Middle cerebral artery | 24 (80) |
| >1/3 | 13 (43.3) |
| <1/3 | 11 (36.7) |
| Posterior cerebral artery | 5 (16.7) |
Numbers in parentheses are percentages.
Discussion
The potential for involvement of the CNS in COVID-19 is of great concern and has already been described in several studies published in the literature. Occasionally, CNS symptoms precede other manifestations and may be the only indicators of the disease.13
Currently, the exact pathophysiology involved in strokes in patients infected with COVID-19 has yet to be determined, but an affinity of the virus for receptors for angiotensin-converting enzyme 2 (ACE 2) and a cytokine storm syndrome, especially in critically ill patients has been reported.14,15 However, we must be aware of the potential for injury to the CNS, either directly by viral replication in the cells or indirectly, due to the host’s immune response. Additional studies are still needed for a comprehensive understanding of the neurological pathology of COVID-19 and its effects on the nervous system.
Some studies have already described neurological manifestations related to the coronavirus, with emphasis on the ischaemic vascular insult, in some cases reported as the initial manifestations of the disease. In addition, a greater occurrence of neurological symptoms in patients with severe disease caused by SARS-CoV-2 is also described in the literature.
In our study, the patients selected presented with acute neurological symptoms during admission. All patients included in the study tested positive for SARS-CoV-2, either by RT–PCR of nasopharyngeal samples or rapid test. We noticed that these events occurred mainly in patients over 50 years of age and with previous comorbidities such as hypertension and diabetes.
Nineteen patients had reports of D-dimer, CRP, LDH and ferritin in their medical records, among which 18 had high values and one had normal results. In this subgroup composed of 18 patients with changes in the values of D-dimer, CRP, LDH and ferritin in their medical records, 10 patients had blood pressure and blood glucose levels considered controlled for their underlying diseases during admission, which are well reported in the literature as risk factors for the occurrence of usual stroke. Thus, the authors questioned the fact that the cytokine storm syndrome and its inherent prothrombotic state, associated with COVID-19 infection, is a likely aggravating factor in the occurrence of stroke in these patients. However, studies with a larger sample size and number of events are needed to confirm our findings.
As COVID-19 continues to spread around the world, when treating patients with neurological manifestations, doctors should consider SARS-CoV-2 infection as a concomitant diagnosis in order to avoid delay in diagnosis or diagnostic errors, as well as the prevention of transmission. In addition, it is especially significant to understand that in patients with severe COVID-19, rapid deterioration or clinical worsening may be associated with a neurological event such as stroke, which could contribute to an unfavourable outcome.13
As limitations of our study, we can highlight the fact that patients who tested positive for COVID-19 could be false positive, even with the high incidence of the disease in the city of Fortaleza during the study period. In addition, we should also mention the small sample size and case analysis only in a hospital. Laboratory tests that could reflect a state of systemic hypercoagulability were not performed in all patients.
Conclusion
In this pandemic, when treating patients with acute stroke, doctors should consider SARS-CoV-2 infection as a diagnostic possibility, especially in those with other risk factors associated with cerebrovascular disease.
Adequate control of underlying diseases in the population with previous comorbidities, especially hypertension and diabetes, is of paramount importance in the context of infection with the new coronavirus, as the state of systemic hypercoagulabity, probably related to this virus, may represent an aggravating factor in the occurrence stroke in these patients.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Isabella B Oliveira https://orcid.org/0000-0002-5154-9373
References
- 1.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. DOI: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris M, Zohrabian VM.Neuroradiologists, Be mindful of the neuroinvasive potential of COVID-19. AJNR Am J Neuroradiol 2020; 41: E37–E39. 10.3174/ajnr.A6551 (accessed 29 December 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W, Ni Z, Hu Y, et al. of 2019 in China. N Engl J Med 2020; 382: 1708–1720. 10.1056/NEJMoa2002032 (accessed 29 December 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desforges M, Le Coupanec A, Brison E, et al. Neuroinvasive and in humans. Adv Exp Med Biol 2014; 807: 75–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2019; 12: 14. DOI: 10.3390/v12010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zochodne DW.SARS, SIRS, and neurological disease. Arch Neurol 2004; 61: 1647–1648. DOI: 10.1001/archneur.61.11.1647 [DOI] [PubMed] [Google Scholar]
- 7.Li YC, Bai WZ, Hashikawa T.The of SARS-CoV2 may play a role in the of. J Med Virol 2020; 92(6): 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oxley T, Mocco J, Majidi S, et al. Large-vessel stroke as a of Covid-19 in the young. N Engl J Med 2020; 382(20): e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Wang M, Zhou Y, et al. Acute cerebrovascular disease following COVID-19: a single center retrospective, observational study. SSRN Electronic J 2020; 5(3): 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: P1033–1034. 10.1016/S0140-6736(20)30628-0 (accessed 29 December 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadman M.How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. Science 2020; Available at: https://www.sciencemag.org/news/2020/04/how-does-coronavirus-kill-clinicians-trace-ferocious-rampage-through-body-brain-toes (accessed 6 January 2021). [Google Scholar]
- 12.Poor HD, Ventetuolo CE, Tolbert T, et al. COVID-19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis. Clin Translat Med 2020; 10: e44. 10.1002/ctm2.44 (accessed 29 December 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77(6): 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus: a first step in understanding SARS pathogenesis. J Pathol 2004; 203: 631–637. DOI: 10.1002/path.1570 pmid:15141377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta P, McAuley DF, Brown M, et al.; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–1034. DOI: 10.1016/S0140- 6736(20)30628-0 pmid:32192578 [DOI] [PMC free article] [PubMed] [Google Scholar]