Abstract
We aimed to summarize the available evidence on cerebral blood flow (CBF) changes in normal aging and common cognitive disorders. We searched PubMed for studies on CBF changes in normal aging and cognitive disorders up to 1 January 2019. We summarized the milestones in the history of CBF assessment and reviewed the current evidence on the association between CBF and cognitive changes in normal aging, vascular cognitive impairment (VCI) and Alzheimer’s disease (AD). There is promising evidence regarding the utility of CBF studies in cognition research. Age-related CBF changes could be related to a progressive neuronal loss or diminished activity and synaptic density of neurons in the brain. While a similar cause or outcome theory applies to VCI and AD, it is possible that CBF reduction might precede cognitive decline. Despite the diversity of CBF research findings, its measurement could help early detection of cognitive disorders and also understanding their underlying etiology.
Keywords: Cerebrovascular circulation, neurovascular coupling, functional imaging, cognitive disorders, neuronal loss, aging
Introduction
Normal neuronal cell activity and brain function need a simultaneous increase in cerebral blood flow (CBF) in response to an increased energy demand. There is controversial evidence regarding the association between age-related CBF changes and the development and progression of cognitive impairment and dementia. Previous reviews of CBF studies and dementia focused on CBF regulation and neurovascular dysfunction,1 cardiovascular determinants of CBF in normal aging2 and regional changes in dementia subtypes, and discussed specific CBF assessment techniques.3–5 The main goal of this review is to provide a summary of age-related CBF changes compared with neurocognitive diseases.
Methods
For this narrative review, we searched PubMed and bibliographies of relevant articles, as well as book chapters up to 1 January 2019. We used Medical Subject Headings and entry terms related to cerebral circulation, aging and cognition and dementia to identify all relevant published studies. We summarized evidence on the history and utility of CBF in cognition research.
Results
Milestones in CBF study and measurement techniques
In 1870, Adolf Eugen Fick defined blood flow as the amount of oxygen used by an organ, such as the heart, over a certain time period.6 In the early 1880s, Angelo Mosso, an Italian physiologist, measured CBF by recording changes in brain pulsations in a 37-year-old farmer with a large skull fracture.7 He introduced the first medical device (plethysmograph) to measure CBF change. He was able to record and quantify brain volume changes in response to cognitive activities. Mosso’s method, in fact, is still the basis of current functional imaging studies, assessing CBF after cognitive tasks. In 1890, Roy and Sherrington8 realized that neuronal activities in the brain were associated with simultaneous changes in blood flow, the idea that led to the concept of ‘coupling’ in functional imaging studies. In 1928, Forbes9 used the cranial window to measure pial vessel flow, which later improved our understanding of the pial microcirculation and vascular physiology.10 In 1943, Dumke and Schmidt11 used an invasive quantitative measurement of CBF with the bubble-flow meter in a macaque monkey.
For the next 60 years, after the Mosso’s report, neuroscientists measured CBF in subjects with skull defects. In the 1940s, Kety and Schmidt12 used low concentrations of nitrous oxide to determine CBF in conscious human subjects. Despite its poor spatial and temporal resolution, this method remains as an important resource to study the physiology of cerebral circulation. With some modification in the Kety–Schmidt method, Niels Lassen13 managed to measure regional CBF (rCBF) with radioactive krypton. In 1955, focal brain circulation in cats was evaluated with trifluoroiodomethane as a tracer.14 Later, radionuclide techniques provided a more accurate evaluation of the regional functional activity via measurement of regional cerebral glucose consumption. Metabolic markers, including [14C] deoxyglucose and [18F] fluorodeoxyglucose (FDG), were employed in single photon emission computed tomography (SPECT) and positron emission tomography (PET) scan. In the 1980s, the transcranial doppler (TCD), as a non-invasive method, was developed15 and has been frequently used in CBF studies. In 1990, Seiji Ogawa showed the difference in magnetic properties of oxygenated and deoxygenated hemoglobin.16 This was a revolution in modern functional magnetic resonance imaging (MRI) and led to a technique known as blood oxygenation level-dependent or (BOLD) contrast. BOLD is a marker of neuronal activity and a good representative of CBF, cerebral blood volume (CBV) and cerebral metabolic rate of oxygen (CMRO2).17
In 1994, Kashimada et al.18 measured CBF with two-dimensional cine phase-contrast MRI in 24 healthy subjects. In 2002, Spilt et al.19 confirmed the accuracy of phase-contrast MRI in measuring total CBF in healthy individuals. The arterial spin labeling technique, including continuous and pseudo-continuous measurement, was also developed as a minimally invasive method, requiring no exogenous tracers, which makes it appropriate for dynamic CBF measurement in healthy individuals.20–22 Currently, PET using radiolabeled water (15O-water) is one of the best methods to measure CBF.23 CBF measurement with H215O‐PET needs an arterial input function (AIF). AIF requires continuous arterial blood sampling. However, a new technique using simultaneous PET and phase‐contrast MRI (PC‐MRI) is able to quantify CBF without blood sampling.19,24
In summary, since Mosso’s era, CBF measurement techniques have greatly improved from direct observations to indirect metabolic consumption measurement, and are still being used in daily practice and clinical research.
CBF changes in normal aging
Scientists have frequently swung their descriptions of dementia from ‘vascular hardening’ to ‘Alzhemirization’ and vice versa.25 In the late nineteenth and mid-twentieth centuries, there was a common belief that brain artery stiffness due to aging could cause chronic ischemia and brain failure.26 This theory resulted in an excessively global use of vasodilators in order to enhance CBF,26 which was later abandoned. In the mid-1970s, rCBF assessment showed a normal vasodilatation in response to changes in CO2 concentration in the brains of patients with primary degenerative dementia,27 indicating that there was no considerable vascular hardening and chronic ischemia. In 1981, Frackowiak et al. did not find an increase in global oxygen extraction ratio (as expected in ischemia) in PET scans of subjects with neurodegenerative dementia. Later, AD – which used to be regarded as a rare presenile disease – became synonymous with dementia, in the way that vascular hardening with low CBF had been.25 This paradigm shift with a bias toward Alzheimization or vascular hardening has remained a challenging issue in the pathophysiology of dementia. Nevertheless, blood flow studies may help in understanding pathophysiological processes of neurodegenerative and vascular cognitive disorders and elucidate their similarities and differences.
Learning patterns and rates of age-related blood flow change is the first step in CBF studies of cognitive disorders. Although CBF might remain unchanged or minimally diminished during normal aging,28,29 most studies found a gradual decline,30 ranging from 3.9 mL/min18 to 4.8 mL/min (0.52%) blood flow decline per year.31 An age-related CBF reduction occurs in the brain cortex (0.45% to 0.74% decline per year in gray matter) with only minimal changes in subcortical regions (0.3% decline per year in white matter).21,30,32–34 The normal blood supply of the brain tissues varies from 20 mL/100g/min in white matter to 70 mL/100g/min in gray matter.35
The reasons for age-related CBF reduction are still a matter of debate. There is a negative correlation between global CBF and subjective rates of cortical atrophy with aging.28,36 Some studies have shown that this reduction might be due to progressive neuronal loss, diminished neuronal activity and decreased synaptic density of brain neurons.33,37,38 Age-related CBF changes should be discussed according to the neurovascular system, from the smallest neurovascular unit to large cerebral arteries. There is a significant pressure gradient difference across cerebral arteries. The base of the brain (i.e., vascular centrencephalon) is supplied by relatively short and straight arteries. These are end arteries without substantial collateral supply, transmitting blood pressure directly to small vessels. In contrast, in the cerebral convexity, blood flow transfers from large and medium-sized cerebral vessels, passing through long arteries with many collateral to cerebral peripheral beds.39,40 In a normotensive person, a blood pressure of 117/75 mm Hg in brachial arteries is accompanied by a pressure of 59/38 mm Hg in small parietal arterioles.39 Such a low pressure would induce white matter lesions (WMLs), which explains the correlation between a low CBF and white matter changes.3 Further, high CBF is associated with less severe WMLs.41 According to the Rotterdam Study,42 those with a higher middle cerebral artery velocity have a lower chance of hippocampus atrophy and dementia. A study on 7700 brain images from the Alzheimer’s Disease Neuroimaging Initiative study showed a possible causative effect of CBF change on cognitive decline.43 It suggests that intra-brain vascular dysregulation is an early pathological event during the development of late onset AD. In addition to CBF changes related to hypotension, WMLs may happen due to dysfunction of the blood–brain barrier (BBB).44 Wong et al.44 showed a significant decrease in CBF with an increase in leakage volume in perilesional zones close to WMLs using dynamic susceptibility contrast and dynamic contrast-enhanced MRI in 27 cases with lacunar stroke or VCI. The presence of such leaky vessels may be explained by regional BBB permeability. As BBB and CBF are both regulated in the neurovascular unit (NVU), WMLs may be due to deterioration of this unit.1,44 In addition, it is hypothetically possible to have a reciprocal relationship between rCBF changes with autonomic control, modulating the cardiovascular system; i.e. insula45 and cingulate gyrus46 and further total and regional CBF changes.
Age-related cardiovascular dysfunction can change CBF.2 The rise in pulsatile hemodynamic stress to the brain may also play a role in age-related blood flow and cognitive changes. The aging process in large arteries, such as the aorta, may increase vascular stiffness, but reduce vascular compliance, which transfers more pulsatile energy to the brain.47 Theoretically, such a pulsatile energy might lead to cerebral microvascular disease, and consequently cognitive impairment.48 However, this association was not confirmed in a TCD study.49 With aging, a dramatic increase can also be seen in the prevalence of cardiovascular diseases. For example, CBF and its regulation could also be influenced by common comorbidities in the elderly, such as hypertension50 or antihypertensive medications.51,52
CBF changes in vascular and neurodegenerative dementia
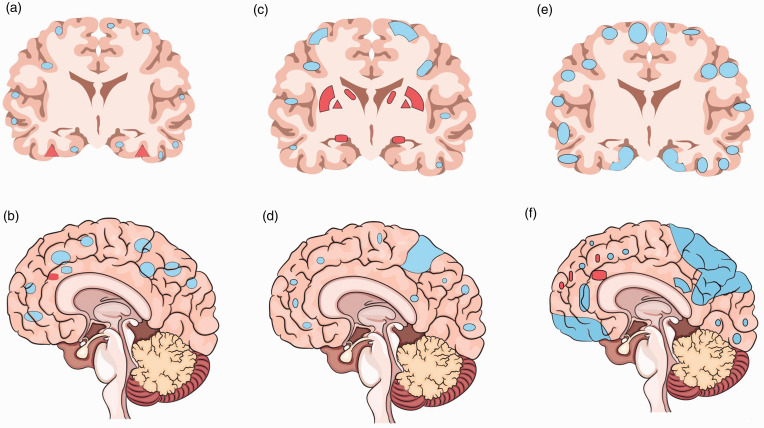
Compared to age-related CBF changes with trivial changes in subcortical areas, AD and neurodegenerative dementias have more specific regional changes in rCBF (Table 1) that could be related to underlying pathophysiology of the disease. While it can be debated that regional hypoperfusion in dementia reflects diminished demand due to brain atrophy and neuronal loss, several studies suggest that CBF changes may contribute to brain lesions, and thus precede cognitive impairments. Similar to clinical stages of AD, CBF may change in a stepwise pattern (Figure 1): asymptomatic stage (in APO ε4 carriers)53 and preclinical (prior to amyloid-β accumulation or brain atrophy).54–57 Compared to healthy volunteers, in cases with mild cognitive impairment (MCI)/early AD, a selective CBF reduction can be found in precuneus and posterior cingulate gyrus.58,59 While in MCI a compensatory high CBF may happen in several areas (i.e., hippocampus, amygdala, caudate, putamen and globus pallidus), such CBF changes may be negative or only found in limited (frontal lobe, anterior cingulate gyrus) brain regions of AD patients (Figure 2). During the transitional stage from MCI to AD, a decreased rCBF develops in other brain areas, including parietotemporal, parahippocampal gyrus, hippocampus, entorhinal cortex, frontal cortex and occipital cortex.54–57 Lower CBF in the posterior brain regions,60 including parietal61 and parieto-temporal lobes, is a predictor of a rapid conversion from MCI to AD.62
Table 1.
Blood flow changes in aging and dementias.
| Aging/cognitive disorders | Blood flow changes and brain regions | Other findings | Measurement techniques | |
|---|---|---|---|---|
| Aging | Reduced CBF in the cortex of lateral occipital, cingulate, precuneus,32 temporal,32,34 parietal,32,34 insular and frontal lobes32,33 | No CBF change in subcortical areas32 | ASL-MRI;32 PET;33 SPECT34 | |
| Vascular cognitive impairment | Multiple regional CBF reduction with a posterior–anterior gradient, sparing occipital lobe;63–66 extensive white matter involvement with a tendency toward subcortical circuit44 | NVU dysregulation due to a combination of hypoperfusion and BBB permeability44 | SPECT;63,64 ASL-MRI;65 PET;66 DCE/DSC-MRI44 | |
| Alzheimer’s disease | Asymptomatic phase | Regional blood flow changes in asymptomatic middle-aged adults with a maternal history of AD53 and APOε4 carriers67 | CBF difference between older and younger APOε4 carriers67 | ASL-MRI53,59,67 |
| MCI | Intra-brain vascular dysregulation as early pathological findings with disease development.43 Reduced CBF in the occipital,68 angular gyrus, temporal,62,68 posterior cingulate gyrus, cuneus,69 parietal62,70 and frontal lobes62 | Compensatory CBF increment in hippocampus, amygdala, caudate, putamen and globus pallidus;69 CMBs in 25% of cases71 | ASL-MRI;68–70 GE-MRI;62 2D phase-contrast MRI;54 SW-MRI71 | |
| Dementia | Regional CBF reduction beyond the MCI regions with a prominent decline in the medial temporal lobe,72 posterior cingulate gyrus,69,70 and inferior parietal cortex69 | Limited compensatory CBF increment in the anterior cingulate gyrus;69 lobar CMBs (78% of cases) | ASL-MRI;57,58,68–70,73 GE-MRI;62 SPECT;72,74 PET;58,66 7-tesla MRI75 | |
| Amyotrophic lateral sclerosis | Generalized CBF reduction (whole cortex and subcortical areas);76 regional CBF reduction in the frontal and parietal lobes77 | CMBs in motor cortex78 | ASL-MRI;77 CT;76 MRI78 | |
| Frontotemporal dementia | Reduced CBF in the frontal lobe73 | Increased CBF in medial parietal, posterior cingulate and precuneus73 | ASL-MRI | |
| Huntington's disease | Reduced CBF in the sensorimotor paracentral, temporal, occipital, postcentral gyrus and insula79 | ASL-MRI | ||
| Lewy body dementia | Reduced CBF in the parietal, temporal and occipital lobes;74 occipital hypoperfusion74,80 | SPECT;74 Radio pharmacological techniques74,80 | ||
| Multiple sclerosis | Reduced CBF in both white81,82 and gray matter82 | Increased BBB permeability;81 impaired cerebrovascular reactivity83 | DCE-MRI;81 DSC-MRI;82 ASL-MRI83 | |
| Parkinson’s disease | Reduced CBF in the parietal, occipital, frontal, cuneus,84 supramarginal gyrus,85 precuneus, temporal, cingulate84,85 and subcortical areas (thalamus and caudate)84 | CMBs in both white and gray matter;86,87 impaired whole brain cerebrovascular reactivity88 | T2-MRI and SWI-MRI;86,87 ASL-MRI84,85,88 | |
| Progressive supranuclear palsy | Reduced CBF in the frontal lobe89 | SPECT | ||
Abbreviations: 2D: two-dimensional; ASL-MRI: arterial spin labeling magnetic resonance imaging; BBB: blood–brain barrier; CBF: cerebral blood flow; CMBs: cerebral microbleeds; CT: computed tomography; DCE-MRI: dynamic contrast-enhanced magnetic resonance imaging; DSC-MRI: dynamic susceptibility contrast magnetic resonance imaging; GM: gray matter; MCI: mild cognitive impairment; MRI: magnetic resonance imaging; NVU: neurovascular unit; PET: positron emission tomography; rCBF: regional cerebral blood flow; SPECT: single photon emission computed tomography; SWI: susceptibility-weighted imaging; WM: white matter.
Figure 1.
Stepwise pattern of cerebral blood flow changes in Alzheimer's disease. (a) Sagittal view and (b) coronal view in asymptomatic APO ε4 carriers; (c) sagittal view and (d) coronal view in those with mild cognitive impairment; and (e) sagittal view and (f) coronal view in those with frank dementia.
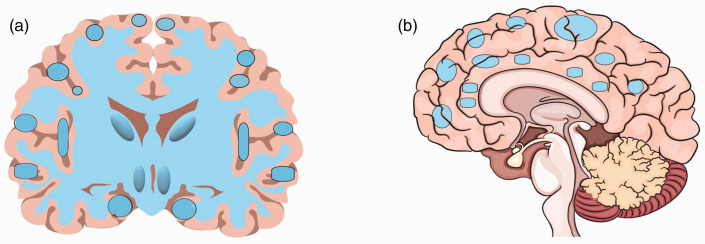
Figure 2.
Cerebral blood flow reduction in patients with vascular cognitive impairment. (a) Coronal view and (b) sagittal view.
Compared to AD, in VCI, various brain regions are involved with a gradient from posterior to anterior brain regions (Table 1, Figure 2).63 Blood flow may change in both gray matter63–66 and white matter,66 or merely white matter with a tendency toward extensive CBF changes in subcortical circuits44 (Figure 1). While regional CBF changes could be observed with aging (Table 1), such stepwise patterns of CBF changes do not happen in VCI. This important finding might provide opportunities for interventions prior to clinical symptoms.
Discussion
Knowledge about the patterns of CBF changes in cognitive decline and normal aging may provide a useful tool to assess individuals at risk and to identify the pathophysiology of cognitive changes. In the current review, we summarized different contributors of age-related CBF changes. They can be classified into non-modifiable age-related changes (i.e., cortical atrophy with aging28,36 with changes in synaptic density of brain neurons33,37,38) to modifiable cardiovascular determinants of CBF (i.e., blood pressure, changes in cerebral arteries and NVU). Such knowledge about the pattern of CBF in the elderly emphasizes the opportunity for prevention by controlling risk of cardiovascular diseases.2
The pattern of CBF changing could help differentiate between CBF changes in age-related and neurocognitive diseases. There is a stepwise pattern of CBF changes in AD. The pattern of regional CBF changes follows an almost similar model with progression of AD from asymptomatic phases to dementia. This finding has clinical implications including identifying cases with possible AD. This result may also guide clinicians and scientists in selecting cases for studies on changes of the baseline CBF.
Conclusion
In the current review, we summarized CBF changes that can be seen in different neurocognitive changes that could be matched with underlying diseases. However, it is not still clear that CBF changes in aging and neurocognitive diseases are the cause or outcome of neuronal loss. In AD, the stepwise changes in total and regional CBF are suggestive of a causal role for CBF changes in the pathophysiology of the disease. This theory needs to be tested for other neurocognitive diseases. Knowledge regarding CBF changes in different diseases and aging has clinical implications in understanding the pathophysiology of diseases, their diagnosis and prevention.
Highlights
Patterns of cerebral blood flow correspond with cognitive disorders.
Cerebral blood flow usually remains unchanged or minimally declines during aging.
Cerebral blood flow changes may precede cognitive decline.
Cerebral blood flow differs from asymptomatic AD to dementia.
In vascular cognitive impairment, most brain regions show declined blood flow.
Disclosures
The authors have no competing interests to declare.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Abolfazl Avan https://orcid.org/0000-0002-5537-6385
References
- 1.Kisler K, Nelson AR, Montagne A, et al. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci 2017; 18(7): 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarumi T, Zhang R.Cerebral blood flow in normal aging adults: cardiovascular determinants, clinical implications, and aerobic fitness. J Neurochem 2018; 144(5): 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Y, Thrippleton MJ, Makin SD, et al. Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J Cereb Blood Flow Metab 2016; 36(10): 1653–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang N, Gordon ML, Goldberg TE.Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neurosci Biobehav Rev 2017; 72: 168–175. [DOI] [PubMed] [Google Scholar]
- 5.Archer HA, Smailagic N, John C, et al. Regional cerebral blood flow single photon emission computed tomography for detection of Frontotemporal dementia in people with suspected dementia. Cochrane Database Syst Rev 2015;6: CD010896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fick A.Ueber die Messung des Blutquantums in der Herzenventrikeln. Sitzung der Phys Med Gesell zu Wurzburg 1870; Jul 9; 36. [Google Scholar]
- 7.Mosso A.Ueber den kreislauf des blutes im menschlichen gehirn: untersuchungen. Leipzig: Verlag von Veit & Comp., 1881. [Google Scholar]
- 8.Roy CS, Sherrington CS.On the regulation of the blood-supply of the brain. J Physiol (Lond) 1890; 11(1–2): 85–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forbes HS.The cerebral circulation: I. Observation and measurement of pial vessels. Arch Neurol Psychiatry 1928; 19(5): 751. [Google Scholar]
- 10.Fog M.Cerebral circulation: the reaction of the pial arteries to a fall in blood pressure. Arch Neurol Psychiatry 1937; 37(2): 351–364. [Google Scholar]
- 11.Dumke PR, Schmidt CF.Quantitative measurements of cerebral blood flow in the macaque monkey. Am J Physiol 1943; 138: 421–427. [Google Scholar]
- 12.Kety SS, Schmidt CF.The determination of cerebral blood flow in man by the use of nitrous oxide in low concentrations. Am J Physiol 1945; 143: 53–66. [Google Scholar]
- 13.Lassen NA, Fritts HW, Caldwell PR, et al. Intrapulmonary exchange of the stable isotope 18O2 injected intravenously in man. J Appl Physiol 1965; 20(5): 809–815. [DOI] [PubMed] [Google Scholar]
- 14.Landau WM, Freygang WH, Roland LP, et al. The local circulation of the living brain; values in the unanesthetized and anesthetized cat. Trans Am Neurol Assoc 1955; (80th Meeting): 125–129. [PubMed] [Google Scholar]
- 15.Aaslid R, Markwalder TM, Nornes H.Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 1982; 57(6): 769–774. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 1990; 87(24): 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buxton RB, Uludağ K, Dubowitz DJ, et al. Modeling the hemodynamic response to brain activation. Neuroimage 2004; 23 (Suppl 1): S220–233. [DOI] [PubMed] [Google Scholar]
- 18.Kashimada A, Machida K, Honda N, et al. [ Measurement of cerebral blood flow in normal subjects by phase contrast MR imaging]. Nippon Igaku Hoshasen Gakkai Zasshi 1994; 54(12): 1116–1125. [PubMed] [Google Scholar]
- 19.Spilt A, Box FMA, van der Geest RJ, et al. Reproducibility of total cerebral blood flow measurements using phase contrast magnetic resonance imaging. J Magn Reson Imaging 2002; 16(1): 1–5. [DOI] [PubMed] [Google Scholar]
- 20.Alsop DC, Detre JA, Grossman M.Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol 2000; 47(1): 93–100. [PubMed] [Google Scholar]
- 21.Parkes LM, Rashid W, Chard DT, et al. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 2004; 51(4): 736–743. [DOI] [PubMed] [Google Scholar]
- 22.Detre JA, Alsop DC.Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. Eur J Radiol 1999; 30(2): 115–124. [DOI] [PubMed] [Google Scholar]
- 23.Ssali T, Anazodo UC, Thiessen JD, et al. A noninvasive method for quantifying cerebral blood flow by hybrid PET/MRI. J Nucl Med 2018; 59(8): 1329–1334. [DOI] [PubMed] [Google Scholar]
- 24.Henriksen OM, Larsson HBW, Hansen AE, et al. Estimation of intersubject variability of cerebral blood flow measurements using MRI and positron emission tomography. J Magn Reson Imaging 2012; 35(6): 1290–1299. [DOI] [PubMed] [Google Scholar]
- 25.Azarpazhooh M, Hachinski V. Pathophysiology and epidemiology. In: Hachinski V (ed) Treatable and potentially preventable dementias. Cambridge: Cambridge University Press, 2018, pp. 1–22.
- 26.Lloyd-Evans S, Brocklehurst JC, Palmer MK.Assessment of drug therapy in chronic brain failure. Gerontology 1978; 24(4): 304–311. [DOI] [PubMed] [Google Scholar]
- 27.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975; 32(9): 632–637. [DOI] [PubMed] [Google Scholar]
- 28.Waldemar G, Hasselbalch SG, Andersen AR, et al. 99mTc-d,l-HMPAO and SPECT of the brain in normal aging. J Cereb Blood Flow Metab 1991; 11(3): 508–521. [DOI] [PubMed] [Google Scholar]
- 29.Marchal G, Rioux P, Petit-Taboué MC, et al. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol 1992; 49(10): 1013–1020. [DOI] [PubMed] [Google Scholar]
- 30.Shin W, Horowitz S, Ragin A, et al. Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: evaluation of reproducibility and age- and gender-dependence with fully automatic image postprocessing algorithm. Magn Reson Med 2007; 58(6): 1232–1241. [DOI] [PubMed] [Google Scholar]
- 31.Buijs PC, Krabbe-Hartkamp MJ, Bakker CJ, et al. Effect of age on cerebral blood flow: measurement with ungated two-dimensional phase-contrast MR angiography in 250 adults. Radiology 1998; 209(3): 667–674. [DOI] [PubMed] [Google Scholar]
- 32.Chen JJ, Rosas HD, Salat DH.Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 2011; 55(2): 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 1990; 113 (Pt 1): 27–47. [DOI] [PubMed] [Google Scholar]
- 34.Claus JJ, Breteler MM, Hasan D, et al. Regional cerebral blood flow and cerebrovascular risk factors in the elderly population. Neurobiol Aging 1998; 19(1): 57–64. [DOI] [PubMed] [Google Scholar]
- 35.Gilkes CE, Whitfield PC.Intracranial pressure and cerebral blood flow. A pathophysiological and clinical perspective. Surgery 2009; 27(3): 139–144. [Google Scholar]
- 36.Poels MMF, Ikram MA, Vernooij MW, et al. Total cerebral blood flow in relation to cognitive function: the Rotterdam Scan Study. J Cereb Blood Flow Metab 2008; 28(10): 1652–1655. [DOI] [PubMed] [Google Scholar]
- 37.Fierstra J, Poublanc J, Han JS, et al. Steal physiology is spatially associated with cortical thinning. J Neurol Neurosurg Psychiatr 2010; 81(3): 290–293. [DOI] [PubMed] [Google Scholar]
- 38.Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cereb Cortex 2004; 14(7): 721–730. [DOI] [PubMed] [Google Scholar]
- 39.Blanco PJ, Müller LO, Spence JD.Blood pressure gradients in cerebral arteries: a clue to pathogenesis of cerebral small vessel disease. Stroke Vasc Neurol 2017; 2(3): 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sörös P, Whitehead S, Spence JD, et al. Antihypertensive treatment can prevent stroke and cognitive decline. Nat Rev Neurol 2013; 9(3): 174–178. [DOI] [PubMed] [Google Scholar]
- 41.Bisschops RHC, van der Graaf Y, Mali WPTM, et al. SMART study group. High total cerebral blood flow is associated with a decrease of white matter lesions. J Neurol 2004; 251(12): 1481–1485. [DOI] [PubMed] [Google Scholar]
- 42.Ruitenberg A, den Heijer T, Bakker SLM, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 2005; 57(6): 789–794. [DOI] [PubMed] [Google Scholar]
- 43.Iturria-Medina Y, Sotero RC, Toussaint PJ, et al. Alzheimer’s Disease Neuroimaging Initiative. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 2016; 7: 11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong SM, Jansen JFA, Zhang CE, et al. Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology 2019; 92(15): e1669–e1677. [DOI] [PubMed] [Google Scholar]
- 45.Nagai M, Hoshide S, Kario K.The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J Am Soc Hypertens 2010; 4(4): 174–182. [DOI] [PubMed] [Google Scholar]
- 46.Cheung RT, Hachinski V.The insula and cerebrogenic sudden death. Arch Neurol 2000; 57(12): 1685–1688. [DOI] [PubMed] [Google Scholar]
- 47.Xu T-Y, Staessen JA, Wei F-F, et al. Blood flow pattern in the middle cerebral artery in relation to indices of arterial stiffness in the systemic circulation. Am J Hypertens 2012; 25(3): 319–324. [DOI] [PubMed] [Google Scholar]
- 48.O’Rourke MF, Safar ME.Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005; 46(1): 200–204. [DOI] [PubMed] [Google Scholar]
- 49.Pase MP, Grima NA, Stough C, et al. Association of pulsatile and mean cerebral blood flow velocity with age and neuropsychological performance. Physiol Behav 2014; 130: 23–27. [DOI] [PubMed] [Google Scholar]
- 50.Strandgaard S.Cerebral blood flow in the elderly: impact of hypertension and antihypertensive treatment. Cardiovasc Drugs Ther 1991; 4 (Suppl 6): 1217–1221. [DOI] [PubMed] [Google Scholar]
- 51.Traub YM, Shapiro AP, Dujovny M, et al. Cerebral blood flow changes with diuretic therapy in elderly subjects with systolic hypertension. Clin Exp Hypertens A 1982; 4(7): 1193–1201. [DOI] [PubMed] [Google Scholar]
- 52.Lipsitz LA, Gagnon M, Vyas M, et al. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension 2005; 45(2): 216–221. [DOI] [PubMed] [Google Scholar]
- 53.Okonkwo OC, Xu G, Oh JM, et al. Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer’s disease. Cereb Cortex 2014; 24(4): 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leijenaar JF, van Maurik IS, Kuijer JPA, et al. Lower cerebral blood flow in subjects with Alzheimer’s dementia, mild cognitive impairment, and subjective cognitive decline using two-dimensional phase-contrast magnetic resonance imaging. Alzheimers Dement (Amst )2017; 9: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suri S, Mackay CE, Kelly ME, et al. Reduced cerebrovascular reactivity in young adults carrying the APOE ε4 allele. Alzheimers Dement 2015; 11(6): 648–657.e1. [DOI] [PubMed] [Google Scholar]
- 56.Wirth M, Pichet Binette A, Brunecker P, et al. Divergent regional patterns of cerebral hypoperfusion and gray matter atrophy in mild cognitive impairment patients. J Cereb Blood Flow Metab 2017; 37(3): 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leeuwis AE, Benedictus MR, Kuijer JPA, et al. Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer’s disease. Alzheimers Dement 2017; 13(5): 531–540. [DOI] [PubMed] [Google Scholar]
- 58.Chen Y, Wolk DA, Reddin JS, et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology 2011; 77(22): 1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wierenga CE, Dev SI, Shin DD, et al. Effect of mild cognitive impairment and APOE genotype on resting cerebral blood flow and its association with cognition. J Cereb Blood Flow Metab 2012; 32(8): 1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benedictus MR, Leeuwis AE, Binnewijzend MAA, et al. Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer’s disease. Eur Radiol 2017; 27(3): 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirao K, Ohnishi T, Hirata Y, et al. The prediction of rapid conversion to Alzheimer’s disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage 2005; 28(4): 1014–1021. [DOI] [PubMed] [Google Scholar]
- 62.Lacalle-Aurioles M, Mateos-Pérez JM, Guzmán-De-Villoria JA, et al. Cerebral blood flow is an earlier indicator of perfusion abnormalities than cerebral blood volume in Alzheimer’s disease. J Cereb Blood Flow Metab 2014; 34(4): 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park SY, Yoon H, Lee N, et al. Analysis of cerebral blood flow with single photon emission computed tomography in mild subcortical ischemic vascular dementia. Nucl Med Mol Imaging 2014; 48(4): 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang D-W, Kim B-S, Park J-K, et al. Analysis of cerebral blood flow of subcortical vascular dementia with single photon emission computed tomography: adaptation of statistical parametric mapping. J Neurol Sci 2002; 203–204: 199–205. [DOI] [PubMed] [Google Scholar]
- 65.Sun Y, Cao W, Ding W, et al. Cerebral blood flow alterations as assessed by 3D ASL in cognitive impairment in patients with subcortical vascular cognitive impairment: a marker for disease severity. Front Aging Neurosci 2016; 8: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tohgi H, Yonezawa H, Takahashi S, et al. Cerebral blood flow and oxygen metabolism in senile dementia of Alzheimer’s type and vascular dementia with deep white matter changes. Neuroradiology 1998; 40(3): 131–137. [DOI] [PubMed] [Google Scholar]
- 67.Wierenga CE, Clark LR, Dev SI, et al. Interaction of age and APOE genotype on cerebral blood flow at rest. J Alzheimers Dis 2013; 34(4): 921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alexopoulos P, Sorg C, Förschler A, et al. Perfusion abnormalities in mild cognitive impairment and mild dementia in Alzheimer’s disease measured by pulsed arterial spin labeling MRI. Eur Arch Psychiatry Clin Neurosci 2012; 262(1): 69–77. [DOI] [PubMed] [Google Scholar]
- 69.Dai W, Lopez OL, Carmichael OT, et al. Mild cognitive impairment and Alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 2009; 250(3): 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson NA, Jahng G-H, Weiner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 2005; 234(3): 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yates PA, Desmond PM, Phal PM, et al. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology 2014; 82(14): 1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsuda H, Kanetaka H, Ohnishi T, et al. Brain SPET abnormalities in Alzheimer’s disease before and after atrophy correction. Eur J Nucl Med Mol Imaging 2002; 29(11): 1502–1505. [DOI] [PubMed] [Google Scholar]
- 73.Hu WT, Wang Z, Lee VMY, et al. Distinct cerebral perfusion patterns in FTLD and AD. Neurology 2010; 75(10): 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lobotesis K, Fenwick JD, Phipps A, et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology 2001; 56(5): 643–649. [DOI] [PubMed] [Google Scholar]
- 75.Brundel M, Heringa SM, de Bresser J, et al. High prevalence of cerebral microbleeds at 7Tesla MRI in patients with early Alzheimer’s disease. J Alzheimers Dis 2012; 31(2): 259–263. [DOI] [PubMed] [Google Scholar]
- 76.Murphy MJ, Grace GM, Tartaglia MC, et al. Widespread cerebral haemodynamics disturbances occur early in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2012; 13(2): 202–209. [DOI] [PubMed] [Google Scholar]
- 77.Rule RR, Schuff N, Miller RG, et al. Gray matter perfusion correlates with disease severity in ALS. Neurology 2010; 74(10): 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwan JY, Jeong SY, Van Gelderen P, et al. Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 2012. Apr 1; 7(4): e35241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen JJ, Salat DH, Rosas HD.Complex relationships between cerebral blood flow and brain atrophy in early Huntington’s disease. Neuroimage 2012; 59(2): 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanyu H, Shimizu S, Hirao K, et al. Differentiation of dementia with Lewy bodies from Alzheimer’s disease using Mini-Mental State Examination and brain perfusion SPECT. J Neurol Sci 2006; 250(1–2): 97–102. [DOI] [PubMed] [Google Scholar]
- 81.Ingrisch M, Sourbron S, Morhard D, et al. Quantification of perfusion and permeability in multiple sclerosis: dynamic contrast-enhanced MRI in 3D at 3T. Invest Radiol 2012; 47(4): 252–258. [DOI] [PubMed] [Google Scholar]
- 82.Hojjat SP, Kincal M, Vitorino R, et al. Cortical perfusion alteration in normal-appearing gray matter is most sensitive to disease progression in relapsing-remitting multiple sclerosis. AJNR Am J Neuroradiol 2016; 37(8): 1454–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marshall O, Chawla S, Lu H, et al. Cerebral blood flow modulation insufficiency in brain networks in multiple sclerosis: a hypercapnia MRI study. J Cereb Blood Flow Metab 2016; 36(12): 2087–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Melzer TR, Watts R, MacAskill MR, et al. Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson’s disease. Brain 2011; 134(Pt 3): 845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Syrimi ZJ, Vojtisek L, Eliasova I, et al. Arterial spin labelling detects posterior cortical hypoperfusion in non-demented patients with Parkinson’s disease. J Neural Transm 2017; 124(5): 551–557. [DOI] [PubMed] [Google Scholar]
- 86.Ham JH, Yi H, Sunwoo MK, et al. Cerebral microbleeds in patients with Parkinson’s disease. J Neurol 2014; 261(8): 1628–1635. [DOI] [PubMed] [Google Scholar]
- 87.Kim JH, Park J, Kim YH, et al. Characterization of cerebral microbleeds in idiopathic Parkinson’s disease. Eur J Neurol 2015; 22(2): 377–383. [DOI] [PubMed] [Google Scholar]
- 88.Al-Bachari S, Vidyasagar R, Emsley HC, et al. Structural and physiological neurovascular changes in idiopathic Parkinson’s disease and its clinical phenotypes. J Cereb Blood Flow Metab 2017; 37(10): 3409–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurata T, Hayashi T, Murakami T, et al. Differentiation of PA from early PSP with different patterns of symptoms and CBF reduction. Neurol Res 2008; 30(8): 860–867. [DOI] [PubMed] [Google Scholar]