Abstract
Foreign body embolization is a rare and potentially under-recognized complication of neuroendovascular procedures. This complication should be considered in the differential diagnosis for clinical or radiological deterioration following neurovascular interventions. We report a case of foreign body hydrophilic coating embolization that occurred following an attempted flow diversion of an intracranial aneurysm with dramatic flare-up after repeat exposure. We also provide a literature review of all reported cases of hydrophilic polymer embolization following flow diversion procedures.
Keywords: Aneurysms, flow diversion, embolization, hydrophilic
Introduction
Hydrophilic polymer embolism has been reported as a potential complication of endovascular flow diversion in the treatment of intracranial aneurysms.1–3 Particulates of the hydrophilic device coatings may strip off secondary to mechanical friction/abrasion and embolize to downstream vascular territories causing a granulomatous inflammatory reaction with a wide spectrum of clinical and radiological manifestations.4,5 Commonly reported complications of flow diversion include hemorrhagic and ischemic complications.6–10 Recently, hydrophilic polymer embolism has been reported as an additional potential complication of flow diversion of cerebral aneurysms.1–3 This rare and potentially under-recognized complication should be considered in the differential diagnosis of new neurological findings following neurovascular interventions, particularly if there is a delay in the development of clinical symptoms after the procedure.
Case description
We report a woman with HIV and recurrence of a ruptured posterior communicating artery aneurysm 10 years after coiling with Micrus coils (Codman Neuro, Raynham, MA). The recurrent aneurysm was detected on imaging performed for headache. Strategies to retreat recurrent aneurysms include repeat coiling, stent assisted coiling, or endovascular flow diversion, or surgical clipping. Challenges associated with repeat coiling are the potential for coil migration in wide-neck aneurysms with shallow luminal recurrences.11,12 Given the unfavorable anatomy for repeat coiling and the difficulty of surgical treatment, a flow diverter stent was recommended. A Flexor® Shuttle® Sheath (Cook Medical, Bloomington, IN) was placed in the right internal carotid artery. An intermediate Navien™ 058 catheter was used to cannulate the petrous segment over a Marksman™ microcatheter (Medtronic, Irvine, CA) and Synchro™ 0.014-in microguidewire (Stryker, Kalamazoo, MI). Multiple attempts were made to deploy a Pipeline Flex device™ (Medtronic, Irvine, CA) (Figure 1). However, due to unfavorable positioning of the device and repetitive prolapse in the aneurysm cavity, deployment was abandoned and the device was removed. She was discharged without complications. One-month post discharge, the patient presented with partial seizures involving her left arm and leg. MRI showed multifocal micro-nodular areas of enhancement surrounded by vasogenic edema involving the right frontal and parietal regions (Figure 2). Results of an extensive work-up to identify an underlying cause were negative. The working diagnosis was delayed inflammatory reaction secondary to hydrophilic polymer embolization. Steroid treatment was considered but ultimately not prescribed due to compound risks of immunosuppression in setting of HIV. The seizures were adequately controlled with antiepileptic medications. Eighteen months later, the patient elected to have a repeated attempt of flow diversion. Using different microcatheter and guide catheters, the patient underwent successful placement of two overlapping Pipeline Flex devices™ that were delivered via Neuron Max 6 French, 088 guide catheter (Penumbra, Inc., Alameda, CA), Sofia (Microvention-Terumo, Inc.) intermediate catheter, Phenom microcatheter (Medtronic) and Synchro 0.014-in microguidewire (Stryker) (Figure 3). The procedure was uneventful without immediate complications. Two days later, the patient had focal seizures and left-sided weakness. Brain MRI exam showed worsened significant FLAIR hyperintensities and nodular enhancement in the right frontal and parietal lobes suggestive of recurrent granulomatous reaction (Figure 4). Given significant clinical worsening, treatment with corticosteroids was initiated. The patient was treated with dexamethasone (4 mg, PO, every 6 hours) for 5 days with excellent response; dexamethasone was transitioned to oral prednisone 60 mg daily with weekly decrease by 10 mg. Follow up brain MRI performed 2.5 months later showed near-complete resolution of the hyperintensities and abnormal enhancement. Repeat angiogram 6 months after successful flow diversion showed complete resolution of the recurrent aneurysm (Figure 5).
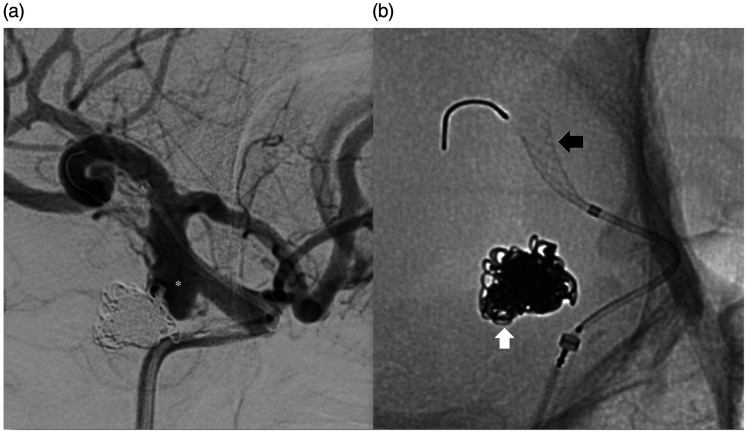
Figure 1.
Angiographic images at the time of attempted flow diverter placement. Digital subtraction angiogram (right anterior oblique projection) of the right internal carotid artery (a) shows the recurrence of the previously coiled right posterior communicating aneurysm (asterisk). Unsubtracted image (b) shows the previous coil mass (white arrow) and the partially deployed flow diverter in the supraclinoid internal carotid artery (black arrow).
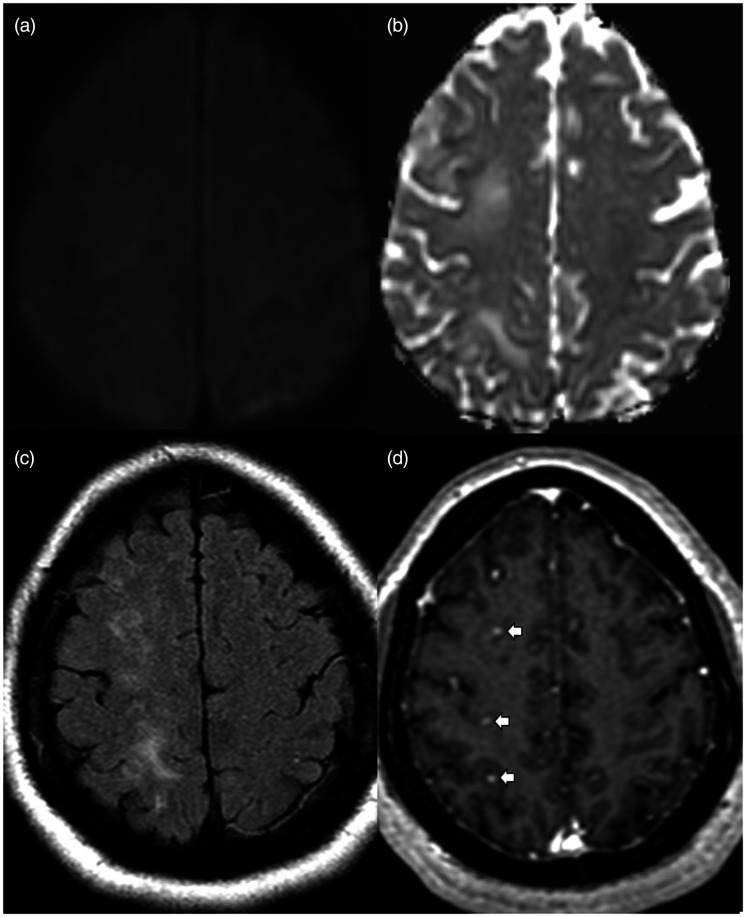
Figure 2.
MRI brain exam performed 1 month after aborted flow diverter placement. Axial diffusion weighted images (a) with apparent diffusion coefficient (b) showing no restricted diffusion. Axial FLAIR image (c) shows multifocal hyperintensities in the subcortical white matter of the right frontal and parietal lobes. Axial gadolinium-enhanced T1 image (d) shows multiple small enhancing lesions (white arrows).
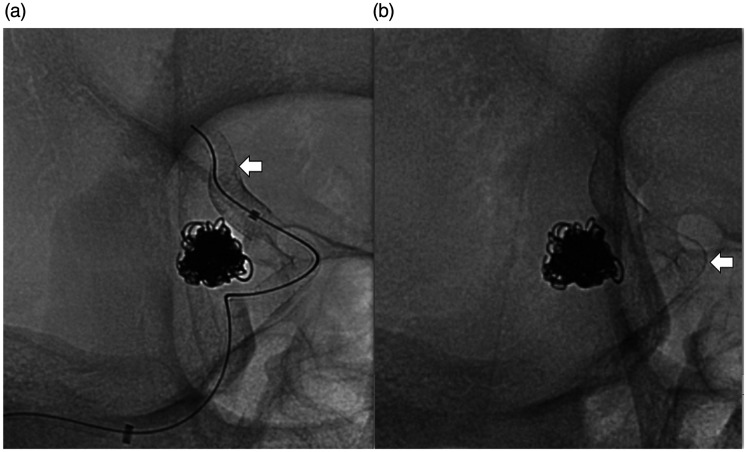
Figure 3.
Angiographic images at the time of subsequent flow diverter placement. Unsubstracted angiograms (right anterior oblique projection) of the right internal carotid artery (a) and (b) showing successful deployment of two telescoping flow diverters (white arrows) across the neck of the aneurysm with the distal end of the distal flow diverter noted in the proximal M1 segment to ensure stability.
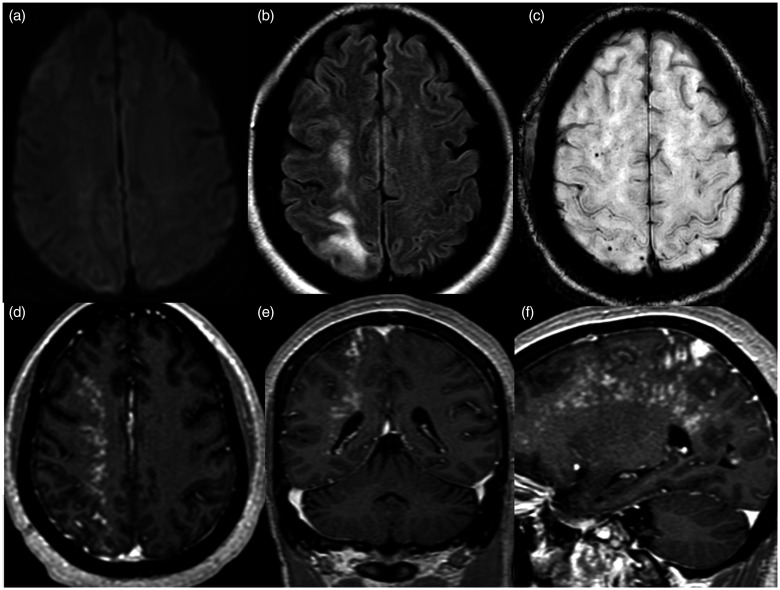
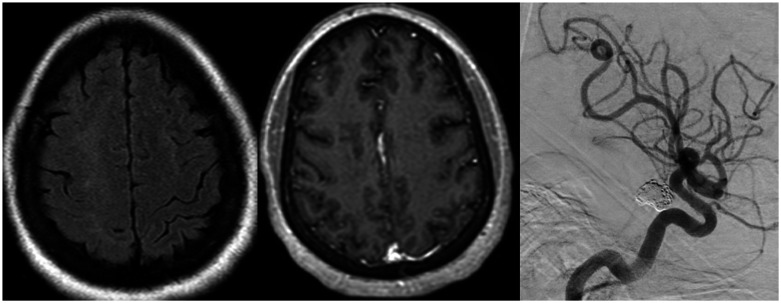
Figure 4.
MRI brain exam performed two days after successful placement of the flow diverters. Axial diffusion weighted images (a) showing no restricted diffusion. Axial FLAIR image (b) shows worsened hyperintensities in the right frontal and parietal subcortical white matter. Axial susceptibility weighted image (c) shows a few associated punctate signal voids and axial (d), coronal (e) and sagittal (f) gadolinium-enhanced T1 images show a dramatic increase in extent of multinodular enhancement.
Figure 5.
Follow up MRI brain exam and angiogram performed after flow diverter placement. Axial FLAIR (a) and gadolinium-enhanced T1 (b) images 2.5 months later show near-complete resolution of the subcortical hyperintensities and enhancement in the right frontal and parietal lobes. Digital subtraction angiogram (lateral projection) of the right internal carotid artery (c) 6 months later shows occlusion of the residual aneurysm with reconstruction of the supraclinoid carotid artery.
Discussion
Recurrence after endovascular coiling of intracranial aneurysms is a known phenomenon associated with treatment of brain aneurysms and is more commonly encountered in patients who present in a ruptured state, with wide-neck aneurysms, larger sized aneurysms, or incompletely occluded aneurysms at the initial treatment.12–14 Endovascular flow diversion has been shown to have high technical success in the definitive closure of intracranial aneurysms.15–18 As our patient demonstrated major recurrence of her aneurysm several years after coiling of her initially ruptured aneurysm, endovascular flow diversion was selected as the preferred modality to achieve aneurysm closure.
Hydrophilic polymers (especially polyvinylpyrrolidone-PVP) are widely used as surface coatings on neurovascular medical devices including guidewires, catheters, coils, stents, stent retrievers and flow diverters to enhance biocompatibility and maneuverability, and reduce friction during vessel navigation and between the co-axial devices.19 Despite the advantages of surface coatings on neurovascular tools, particulates of these coatings may strip off the devices and embolize to downstream vascular territories causing reactive granulomatous inflammation to the foreign bodies.4,5 This complication is known as hydrophilic polymer embolization (HPE). Data about this phenomenon in neurointervention is limited to small cases series and case reports.1,3,4,7,20–22
The true incidence of HPE is unknown because MRI is not systematically obtained after endovascular procedures and there may be asymptomatic cases that go unnoticed. Clinical, histopathologic and radiological manifestations of HPE are secondary to small-sized distal cerebral vessel occlusion evolving into subacute granulomatous angiitis.4,5 Symptoms vary depending on the vascular territory involved and the extent of the inflammatory reaction. HPE may be asymptomatic or may present with non-specific neurological symptoms (such as headache), fatigue, seizure or focal neurological symptoms with stroke-like symptoms corresponding to the territory involved. The time frame for clinical presentation is usually between a few weeks to a few months with most occurring within 2 weeks after the procedures. Radiologically, foreign body emboli manifest as nodular enhancing lesions in the vascular territory of the catheterized arteries at the cortico-subcortical regions where small emboli usually lodge. These lesions, also known as non-ischemic cerebral enhancing lesions (NICE), are usually surrounded by white matter edematous changes characterized by T2/FLAIR hyperintensities.21 Restricted diffusion is not typically described with these lesions, likely because of the subacute or late presentation. There may also be associated microhemorrhages characterized by blooming artifacts on gradient echo or susceptibility images as seen in our patient.4 HPE was also reported to be associated with delayed intracranial hemorrhage after flow diversion and post-procedural ischemia.7 HPE should be a consideration in patients with radiological findings in a vascular territory following endovascular procedures. It may mimic multifocal ischemic or hemorrhagic complications, meningitis or other inflammatory or malignant conditions.
In our patient, although not confirmed by biopsy, a presumptive diagnosis of a foreign body reaction due to HPE was made based on the timeline of symptoms and of the imaging findings seen exclusively within the right cerebral hemisphere. Since all neuroendovascular tools used in both procedures were coated with hydrophilic materials, it is not possible to know the exact source of the embolic material. We hypothesize that the source may have been the flow diverter, since the first attempt to treat the aneurysm was technically challenging and required multiple attempts of partial unsheathing/re-sheathing, and back and forth movement of the densely coated flow diverter in the dysplastic supraclinoid internal carotid artery. This may have caused the hydrophilic coating of the flow diverter to dislodge and shower distally. The patient had uneventful coiling with hydrophilic tools 10 years previously. In addition, the only common tool between the initial attempted flow diversion and the repeated procedure 18 months later was the pipeline flow diverter. We intentionally changed the guide catheter (from Shuttle to Neuron max), the intermediate catheter (from Navien to Sofia) and the microcatheter (Marksman to Phenom) on the second deployment.
HPE has been reported after placement of flow diverter devices1–4,20,21,23–25 (Table 1). To our knowledge, this is the first case of HPE after an aborted flow diversion. Eighteen months after the first attempt and soon after the successful deployment of the flow diverter, brain MRI showed significant worsening of the inflammatory reaction in the same territory. The rapid flare-up after the second exposure is typical of a type IV granulomatous hypersensitivity reaction when re-exposure to the foreign body reactivates specific memory T-cells triggering an accelerated reaction.26 Treatment of reactions to HPE is mainly conservative symptomatic treatment and includes corticosteroids to decrease the inflammatory reaction and the associated vasogenic edema. Our patient did not receive corticosteroids initially because of her HIV status. However, the flare-up after the successful deployment was severe enough requiring initiating steroids treatment to decrease the edema and was associated with marked clinical improvement.
Table 1.
Table showing all the reported cases of hydrophilic polymer embolization following flow diversion of cerebral aneurysms.
| Author, year | Study type | Number of cases | Aneurysm location | Symptoms | Symptoms onset | Diagnosis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Cruz,7 2014 | Case series | Patient # 1 | Supraclinoid aneurysm | Not available | 4 months | MRI: NICE | N/A | N/A |
| Patient # 2 | Cavernous ICA aneurysm | Asymptomatic | 3 months | MRI: NICE | Antibiotics | N/A | ||
| Hu,23 2014 | Case series | Patient # 1 | Paraophthalmic aneurysm | Parenchymal hemorrhage | 3 days | Autopsy: foreign body reaction | None | Death |
| Patient # 2 | Paraophthalmic aneurysm | Parenchymal hemorrhage | 6 days | Autopsy: foreign body embolic | None | Death | ||
| Patient # 3 | Paraophthalmic aneurysm | Parenchymal hemorrhage | 2 weeks | Foreign body embolic occlusion | None | Death | ||
| Shapiro,4 2015 | Case series | Patient # 1 | Trigeminal aneurysm | Fatigue, left-sided weakness and quadrantanopsia | 8 weeks | NICE with SWI artifacts | Antibiotics and steroids | Recovery |
| Patient # 2 | Right ICA aneurysm | Fatigue, headache | 8 weeks | MRI: right parieto-occipital hematoma, with NICE | Dexamethasone | Recovered | ||
| Patient # 3 | Paraophthalmic aneurysms | Headache | 2 weeks | MRI: NICE Biopsy: giant cell granulomas | Six-week course of corticosteroids | Improvement | ||
| Patient # 4 | Paraophthalmic aneurysm | Seizure | 3 months | MRI: NICE and SWI. Biopsy: granulomatous angiitis | Antiepileptics and steroids | Not available | ||
| Shotar,21 2016 | Case report | Patient # 1 | Ophthalmic aneurysm | Seizure at 17 months | 17 months | Biopsy: leukocytoclastic vasculitis | Antiepileptics and steroids | Recovery |
| Lorentzen,3 2016 | Case report | Patient # 1 | Paraophthalmic aneurysm | Headache followed by aphasia and hemiparesis | 3 months | MRI: NICE Biopsy: non-polarizable foreign material surrounded by giant cell granulomas | Steroids | Improvement with residual headache |
| Sablani,1 2018 | Case report | Patient # 1 | Supraclinoid carotid | Headache, weakness and numbness | 3 days | MRI: NICE | Steroids | Recovery |
| Brinjikji,13 2018 | Case report | Patient # 1 | Paraophthalmic aneurysm | Headache and word-finding difficulty | 2 months | MRI: NICE | Steroids | Recovery |
| Daneshmand,25 2018 | Case report | Patient # 1 | Supraclinoid carotid artery | Seizure | 1 year | MRI: NICE | Steroids | Recovery |
| Geisbush,2 2019 | Case report | Patient # 1 | Supraclinoid aneurysm | Headache, numbness and weakness | 1–2 months | MRI: NICE | Steroids | Improvement with residual headache |
Abbreviations: MRI: magnetic resonance imaging; NICE: non-ischemic contrast enhancement with surrounding edema; ICA: internal carotid artery; SWI: susceptibility weighted images; N/A: not available.
Conclusion
HPE remains a clinically and radiologically challenging and under-recognized complication of neuroendovascular procedures. We report the first case in which an aborted flow diverter deployment was associated with this complication followed by recurrence of symptoms and imaging findings following subsequent successful deployment. It is important to raise awareness of this potential complication after neuroendovascular procedures to allow for appropriate clinical management and to prevent unnecessary investigations. Also, controlling polymer coating emboli by mitigating or eliminating particulates from intravascular devices should become a focus of interest for engineers and manufacturers.
Footnotes
Conflict(s) of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Mohamad Abdalkader https://orcid.org/0000-0002-9528-301X
Anvitha Sathya https://orcid.org/0000-0001-8858-7851
References
- 1.Sablani N, Hasan MM, Shrestha A, et al. Delayed neurological deficits after endovascular placement of a pipeline embolisation device: clinical manifestation and treatment. BMJ Case Rep 2018; 2018. doi:10.1136/bcr-2016-216580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geisbush TR, Marks MP, Heit JJ.Cerebral foreign body reaction due to hydrophilic polymer embolization following aneurysm treatment by pipeline flow diversion device. Interv Neuroradiol 2019; 25(4): 447–453. doi:10.1177/1591019919830767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorentzen AO, Nome T, Bakke SJ, et al. Cerebral foreign body reaction after carotid aneurysm stenting. Interv Neuroradiol 2016; 22(1): 53–57. doi:10.1177/1591019915609171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro M, Ollenschleger MD, Baccin C, et al. Foreign body emboli following cerebrovascular interventions: clinical, radiographic, and histopathologic features. Am J Neuroradiol 2015; 36(11): 2121–2126. doi:10.3174/ajnr.A4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta RI, Mehta RI.Polymer-induced central nervous system complications following vascular procedures: spectrum of iatrogenic injuries and review of outcomes. Hum Pathol 2016; 53: 178–190. doi:10.1016/j.humpath.2016.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou G, Su M, Yin Y-L, et al. Complications associated with the use of flow-diverting devices for cerebral aneurysms: a systematic review and meta-analysis. Neurosurg Focus 2017; 42(6): E17. doi:10.3171/2017.3.FOCUS16450 [DOI] [PubMed] [Google Scholar]
- 7.Cruz JP, Chow M, O’Kelly C, et al. Delayed ipsilateral parenchymal hemorrhage following flow diversion for the treatment of anterior circulation aneurysms. AJNR Am J Neuroradiol 2012; 33(4): 603–608. doi:10.3174/ajnr.A3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv X, Yang H, Liu P, et al. Flow-diverter devices in the treatment of intracranial aneurysms: a meta-analysis and systematic review. Neuroradiol J 2016; 29(1): 66–71. doi:10.1177/1971400915621321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahed R, Darsaut TE, Kotowski M, et al. Re-treatment of residual aneurysms after flow diversion: an experimental study. Neuroradiol J 2018; 31(3): 270–279. doi:10.1177/1971400918763198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmoud M, Farag A, Farid M, et al. Application of flow diverters in the treatment of aneurysms in the internal carotid artery bifurcation region. Neuroradiol J 2020; 33(4): 297–305. doi:10.1177/1971400920924840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdalkader M, Piotin M, Chen M, et al. Coil migration during or after endovascular coiling of cerebral aneurysms. J NeuroInterv Surg 2020; 12(5): 505–511. doi:10.1136/neurintsurg-2019-015278 [DOI] [PubMed] [Google Scholar]
- 12.Abdalkader M, Raymond J, Mian A, et al. Early major recurrence of cerebral aneurysms after satisfactory initial coiling. Interv Neuroradiol. Epub ahead of print 20 October 2020. doi:10.1177/1591019920968370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen TN, Hoh BL, Amin-Hanjani S, et al. Comparison of ruptured vs unruptured aneurysms in recanalization after coil embolization. Surg Neurol 2007; 68(1): 19–23. doi:10.1016/j.surneu.2006.10.021 [DOI] [PubMed] [Google Scholar]
- 14.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003; 34(6): 1398–1403. doi:10.1161/01.STR.0000073841.88563.E9 [DOI] [PubMed] [Google Scholar]
- 15.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013; 267(3): 858–868. doi:10.1148/radiol.13120099 [DOI] [PubMed] [Google Scholar]
- 16.Eskey CJ, Meyers PM, Nguyen TN, et al. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association. Circulation 2018; 137(21): e661–e689. doi:10.1161/CIR.0000000000000567 [DOI] [PubMed] [Google Scholar]
- 17.Ravindran K, Casabella AM, Cebral J, et al. Mechanism of action and biology of flow diverters in the treatment of intracranial aneurysms. Neurosurgery 2020; 86(Suppl 1): S13–S19. doi:10.1093/neuros/nyz324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 2013; 44(2): 442–447. doi:10.1161/STROKEAHA.112.678151 [DOI] [PubMed] [Google Scholar]
- 19.Chopra AM, Mehta M, Bismuth J, et al. Polymer coating embolism from intravascular medical devices – a clinical literature review. Cardiovasc Pathol 2017; 30: 45–54. doi:10.1016/j.carpath.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 20.Cruz JP, Marotta T, O’Kelly C, et al. Enhancing brain lesions after endovascular treatment of aneurysms. AJNR Am J Neuroradiol 2014; 35(10): 1954–1958. doi:10.3174/ajnr.A3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shotar E, Law-Ye B, Baronnet-Chauvet F, et al. Non-ischemic cerebral enhancing lesions secondary to endovascular aneurysm therapy: nickel allergy or foreign body reaction? Case series and review of the literature. Neuroradiology 2016; 58(9): 877–885. doi:10.1007/s00234-016-1699-5 [DOI] [PubMed] [Google Scholar]
- 22.Shannon P, Billbao JM, Marotta T, et al. Inadvertent foreign body embolization in diagnostic and therapeutic cerebral angiography. AJNR Am J Neuroradiol 2006; 27(2): 278–282. [PMC free article] [PubMed] [Google Scholar]
- 23.Hu YC, Deshmukh VR, Albuquerque FC, et al. Histopathological assessment of fatal ipsilateral intraparenchymal hemorrhages after the treatment of supraclinoid aneurysms with the Pipeline Embolization Device: report of 3 cases. J Neurosurg 2014; 120(2): 365–374. doi:10.3171/2013.11.JNS131599 [DOI] [PubMed] [Google Scholar]
- 24.Giordan E, Brinjikji W, Lanzino G.Teaching NeuroImages: intracranial foreign body reaction after endovascular procedures. Neurology 2018; 90(6): 296–297. doi:10.1212/WNL.0000000000004938 [DOI] [PubMed] [Google Scholar]
- 25.Daneshmand A, Krecke KN, Wijdicks EFM.Plastic in the brain: delayed recognition of progressive unilateral hemispheric lesions. Neurocrit Care 2019; 31(1): 222–224. doi:10.1007/s12028-018-0576-y [DOI] [PubMed] [Google Scholar]
- 26.Kitagaki H, Fujisawa S, Watanabe K, et al. Immediate-type hypersensitivity response followed by a late reaction is induced by repeated epicutaneous application of contact sensitizing agents in mice. J Invest Dermatol 1995; 105(6): 749–755. doi:10.1111/1523-1747.ep12325538 [DOI] [PubMed] [Google Scholar]