Abstract
Background
Inhaled beclomethasone dipropionate (BDP) has been, together with inhaled budesonide, the mainstay of anti‐inflammatory therapy for asthma for many years. A range of new prophylactic therapies for asthma is becoming available and BDP has been reformulated using a hydrofluoroalkane‐134a (HFA) propellant which is free from chlorofluorocarbon (CFC).
Objectives
The objectives of this review were to: (1) Compare the efficacy of BDP with placebo with both CFC and HFA propellants in the treatment of chronic asthma. (2) Explore the possibility that a dose response relationship exists for BDP in the treatment of chronic asthma. (3) To provide the best estimate of the efficacy of BDP as a benchmark for evaluation of newer asthma therapies.
Search methods
Electronic searches were current as of January 2003.
Selection criteria
Randomised parallel group design trials for a minimum period of four weeks, in children and adults comparing CFC‐BDP or HFA‐BDP with placebo in the treatment of chronic asthma. Two reviewers independently assessed articles for inclusion and methodological quality.
Data collection and analysis
One reviewer extracted data; authors were contacted to clarify missing information. We analysed data with RevMan Analyses 1.0.2.
Main results
60 studies recruiting 6542 participants met the inclusion criteria. CFC‐BDP (57 studies): In non‐oral steroid treated patients, at doses of 400mcg/day or less CFC‐BDP produced significant improvements from baseline in a number of efficacy measures compared with placebo, including forced expiratory volume in one second (FEV1) 360 ml (95% CI 260 to 460); FEV1 (% predicted) WMD 12.41% (95% CI 8.18 to 16.64) and morning peak expiratory flow rate (am PEF) WMD 35.95 L/min (95% CI 27.85 to 44.04). BDP also led to reductions in rescue beta‐2 agonist use compared with placebo of ‐2.32 puffs/d (95% CI ‐2.55 to ‐2.09) and reduced the relative risk (RR) of trial withdrawal due to an asthma exacerbation 0.25 (95% CI 0.12 to 0.51). Subgroup analyses based on treatment duration provide support to the proposal that a treatment period of greater than four weeks is required to realise a fuller treatment effect. In oral steroid treated patients BDP led to significantly greater reductions in oral prednisolone use WMD ‐4.91 mg/d (95% CI ‐5.88 to ‐3.94 mg/d) and greater likelihood of withdrawing oral steroid treatment RR 8.02 (95% CI 3.23 to 19.92). HFA‐BDP (3 studies): In non‐oral steroid‐treated patients, HFA‐BDP was significantly more effective than placebo in improving FEV1, morning and evening PEF, FEF25 to 75%, reduced asthma symptoms and beta2‐agonists daily consumption. Significant effects for such outcomes were apparent after six weeks of treatment. In oral steroid treated patients, HFA‐BDP improved significantly FEV1 and am PEF. The summary estimates for these outcomes suggested a high level of heterogeneity, and divergent aims of the studies may contribute to the variation we observed. Limited data on adverse events were reported.
Authors' conclusions
This review has quantified the efficacy of CFC‐BDP and HFA‐BDP in the treatment of chronic asthma and strongly supports its use. Current asthma guidelines recommend titration of dose to individual patient response, but the published data provide little support for dose titration above 400 mcg/d in patients with mild to moderate asthma. There are insufficient data to draw any conclusions concerning dose‐response in people with severe asthma.
Plain language summary
Inhaled beclomethasone compared with placebo for chronic asthma
During an asthma attack, the airways narrow, causing breathing problems, wheezing and coughing. Inhaled corticosteroids (ICS) are used to reduce swelling of the airways. Inhaled beclomethasone dipropionate (BDP) is commonly used. There is now a new formulation of the drug which uses a new propellant (HFA‐BDP). This review of trials found that BDP delivered with the old and new propellant is effective in helping people with chronic asthma. BDP at all doses improves airflow, reduces symptoms and the need for rescue bronchodilators. The review only included studies conducted for more than 4 weeks. The drug was well tolerated and the safety profile was comparable with placebo. The findings apply to both children and adults. The effects of the new propellant suggest that it could be more effective than the older version, although a different review will address that particular question.
Background
Inhaled corticosteroids (ICS) have been available for the treatment of asthma for over 40 years, and are recommended at an early stage in national and international management guidelines (BTS 2003). Greatest experience has been gained with beclomethasone dipropionate (BDP) which has been available since the early 1970's. Many controlled and uncontrolled studies assessing the clinical effectiveness and safety of the drug have been conducted and there is a consensus opinion that BDP has a favourable clinical therapeutic index (i.e. effective with low risk of side effects) when treating chronic asthma. A number of issues remain to be clarified however. These include the extent to which the size of clinical response is influenced by treatment dose, patient age, asthma severity and treatment duration.
There has been a recent drive to produce aerosol consumables, including pressurised metered dose inhalers (pMDIs) that are free of chlorofluorocarbon (CFC) propellants. CFCs are being phased out in all manufactured products because of environmental concerns. Therefore BDP delivered via pMDI has been reformulated using a hydrofluoroalkane‐134a (HFA) propellant. In this formulation BDP is delivered in a solution, rather than in suspension as is the case with conventional CFC‐BDP. HFA‐BDP with its small particle size allows a higher proportion of the drug to deposit in the lung small airways. The changeover policy from CFC‐BDP into HFA‐BDP has been established in many countries.
This review aims to synthesise the findings from studies that have compared BDP, with it's two different propellants, HFA and CFC, to placebo in the treatment of chronic asthma.
Objectives
The objectives of this review were to:
(1) Compare the efficacy of inhaled beclomethasone dipropionate (CFC‐BDP) with placebo in the treatment of chronic asthma.
(2) Compare the efficacy of inhaled beclomethasone dipropionate (HFA‐BDP) with placebo in the treatment of chronic asthma. (3) Explore whether a dose response relationship exists for BDP in the treatment of chronic asthma.
(4) Provide the best estimate of efficacy of BDP as a benchmark for evaluation of newer asthma therapies.
Methods
Criteria for considering studies for this review
Types of studies
Studies were selected for this review if they were randomised double, single or unblinded controlled studies. We made an amendment to the entry criteria for this review. One reviewer felt that study design was an important factor in affecting the validity of pooled effect estimates from individual studies. In view of the uncertainty surrounding the influence of carryover effects in studies of crossover design, whereby effect estimates may be affected by an inadequate washout phase, we have retained crossover studies previously included, but switched off pooled totals. We restricted the inclusion of new studies to those with a parallel group design.
Types of participants
We included studies recruiting children and adults. To be eligible participants had to have a clinical diagnosis of chronic asthma. We included trials in which the diagnosis was based upon physician opinion only, as well as those in which further clinical, physiological and/or immunological features in keeping with asthma were stated as inclusion criteria. Only patients over the age of two years were included. We included studies involving patients with both asthma and chronic obstructive pulmonary disease (COPD) provided that the data for asthmatic patients were available separately. We considered studies conducted in a primary care, hospital outpatient and institutional care setting.
Types of interventions
BDP delivered by mouth inhalation versus placebo. Any dose of beclomethasone was considered, but nominal daily dose had to be stated. Nominal daily dose is calculated as valve dose (UK, Europe) or the actuator dose (USA) multiplied by the number of actuations per day. In the initial version of the review treatment needed to be for at least one week. Subsequent to the initial version of this review, one reviewer felt that studies of too short a duration would not provide accurate effect estimates. Findings from a recent individual prospective controlled trial comparing BDP at different doses were that the peak effect for a number of outcome measures had plateaued after around six weeks (Busse 1999). For the update of this review, discussions between the reviewers led to a compromise on excluding data from studies of shorter than 4 weeks duration. We would only prospectively include studies that were of at least 4 weeks duration. However, we have retained studies of less than four weeks previously 'included' in the review, but we have removed the data that they contribute from pooled analyses. Delivery could be by pressurised metered dose inhaler (MDI) with or without holding chamber/spacer, breath‐actuated MDI or dry powder inhaler (DPI). Any co‐intervention was acceptable, including the use of oral corticosteroids. We excluded studies concerned with treatment delivered by nebuliser. Since this review was first published there has been considerable research and development concerning CFC‐free pMDI formulations. We have opted not to pool studies in which different propellants were used. For the purposes of continuity and to avoid potentially heterogeneous findings, this review will consider HFA‐BDP and CFC‐BDP separately when compared with placebo. Equivalence between CFC and HFA preparations will not be considered in this review.
Types of outcome measures
We considered all outcome measures. We assessed results reported as both absolute values and change compared to baseline. Outcomes of particular interest considered to be important a priori were as follows:
Primary outcomes: The Outcomes reflecting airway calibre were the main outcomes of interest: (1)‐Forced expiratory volume in one second (FEV1). (2)‐Morning and evening diary card peak expiratory flow rate (PEFR). (3)‐Diurnal variability in diary card PEFR, clinic PEFR
Secondary outcomes: (1) Symptoms: daytime and night‐time symptom score, percentage of symptom free days and nights (2) Rescue bronchodilator use: puffs beta2 agonist/day, puffs beta2 agonist/night (3) Bronchial hyper‐responsiveness (BHR): methacholine challenge (PC20 FEV1, PD20 FEV1); histamine challenge (PC20 FEV1, PD20 FEV1) (4) Health‐related quality of life (5) Asthma exacerbations: hospital admission rates, days off work or school, unscheduled doctor visits due to exacerbation. (6) Safety outcomes: hypothalamo‐pituitary‐adrenal (HPA) axis function reflected in serum and urinary cortisol measures (7) Oropharyngeal side‐effects: hoarseness, sore throat, oropharyngeal Candidiasis We did not consider measures of growth and bone turnover.
Search methods for identification of studies
Electronic searches
Stage 1 We initially searched the Cochrane Airways Group trial register (March 1999). The following search terms were applied:
steroid* OR glucocorticoid* OR corticosteroid* OR beclomethasone OR budesonide OR fluticasone OR triamcinolone OR flunisolide OR Becotide OR Becloforte OR Pulmicort OR Flixotide
We imported the electronic abstracts of citations resulting from this search into a bibliographic database termed the Inhaled Steroid Register (ISR). Two reviewers (NPA and JB) hand‐searched this. We removed duplicate publications.
Stage 2 We searched the ISR using the following terms:
beclomethasone OR Becotide OR Becloforte
Electronic abstracts were exported to a new database and termed the Beclomethasone Register. Citations were initially excluded if it was clear that the study:
(a) Was not concerned with treatment of chronic asthma in humans (b) Was not an RCT (c) Did not include a treatment arm with an inhaled corticosteroid
Where uncertainty existed, we retrieved the publication in full text version.
Stage 3 Subsequent annual update searches were run on the Cochrane Airways Group trial register using the search terms listed under stage 1. Authors were approached when necessary for additional data. These searches were current as of January 2004.
Searching other resources
We searched the bibliographies of all papers retrieved in full text form and relevant narrative reviews for additional publications. We handsearched the British Journal of Clinical Research and the European Journal of Clinical Research, which are not electronically indexed on MEDLINE or EMBASE, for relevant studies. We contacted authors of included studies and asked if they were aware of further studies missed by searching electronic sources. We also contacted the UK headquarters of Glaxo Wellcome manufacturers of Becotide and Becloforte to find details of studies sponsored by them which may have been missed. Finally, we searched the proceedings of meetings of the European Respiratory Society (1997 to 1999), British Thoracic Society (1997/1998) and American Thoracic Society (1997 to 1999) for relevant trials.
Data collection and analysis
Selection of studies
The decision to exclude studies prior to full paper retrieval was made by one author (NPA). The full text papers retrieved were reviewed independently by two authors (NPA and JB). Disagreement as to which papers to include was resolved by consensus. The reviewers who were blinded to the authors names, institution and funding sources, independently assessed the methodological quality of each study. Abstracts from the new search were read and selected for retrieval by the reviewers independently. Trials thought to be eligible for inclusion were retrieved and any disagreements were resolved by discussion in order to arrive at the final list of included studies.
Data extraction and management
One reviewer (NPA) extracted data for each outcome from the published results of included trials. In the case of continuous outcomes such as spirometry: (1) Where outcomes were evaluated at a number of time points, we only used data from the last time point. (2) We extracted data from graphical plots when presented in this form; attempt was made to verify such data by contacting authors. (3) If an intention‐to‐treat analysis (ITT) was not used by the investigators, and it was not explicit in the presentation of results how many subjects (N) were in each group at the time of last evaluation of that outcome, we calculated the appropriate N value for each intervention group by subtracting the number of patients who withdrew in each intervention group from those randomised to each intervention group.
It should be noted that in the graphical displays of this review continuous outcome data such as spirometry and PEFR are displayed using negative figures because the sign convention built into the software interprets smaller numbers as favourable (as is usually the case for dichotomous outcomes such as withdrawal rates). This ensures that results favouring BDP are consistently displayed to the left of the vertical line on the graphical meta‐analysis display. We contacted authors (by mail, fax and/or electronic mail) on at least two occasions to clarify details of randomisation and/or request missing outcome data. An attempt was made to send requests to correct current addresses by searching MEDLINE, EMBASE and hospital World Wide Web (WWW) sites for up‐to‐date contact details. We approached Glaxo Wellcome for data for those trials in which the contact authors did not initially reply or when authors suggested doing so and which had been sponsored by the company.
Data for outcomes reported but which could not be included in the meta‐analysis have been listed in Table 1.
1. Study data not available for inclusion in meta‐analysis.
| Study ID | Data not included |
| Brompton Hospital/MRC Collaborative Trial 1974 | Reduction in dose of oral prednisolone No numerical data available |
| BTTA 1976 | Reduction in dose of oral prednisolone No standard deviation values |
| Bel 1990 | Asthma symptom score No standard deviation values available for above outcome |
| Bergmann 1990 | FEV1 Specific airways resistance Asthma symptom severity score Change in morning PEFR compared to baseline Evening PEFR Inhaled beta2 agonist use Symptom scoresd deviation values for any of the above outcomes No SD values available for above outcomes |
| Carpentiere 1990 | FEV1 (% predicted) Bronchial responsiveness to propranolol (PC20 FEV1) No standard deviation values available |
| Gaddie 1973 | FEV1 FVC Plasma cortisol bronchodilator use No standard deviation values for any of the above outcomes |
| Harvey 1976 | % change in FEV1 % change in FEF50 8am plasma cortisol Daily dose oral prednisolone No standard deviation values for any of the above outcomes |
| Hodson 1974 | FEV1 FVC Clinic PEFR Serum cortisol No standard deviation values available for above outcomes |
| Holst 1974 | Morning wheeze/breathlessness/tightness of breathing scores Night‐time wheeze/breathlessness/tightness of breathing scores FEV1 No numerical data for above outcomes |
| Kerrebijn 1976 | %predicted FEV1 Serum cortisol No standard deviation values for above outcomes |
| Klein 1977 | Weekly symptom score Asthma attack score Additional medication score clinic PEF FEV1 FVC MEF25‐75 No standard deviation values for above outcomes |
| Kraemer 1987 | % change in FEV1 % change in specific airway conductance % change in airway resistance No numerical data for above outcomes |
| Lovera 1975 | Reduction in number of wheezing attacks Days free of wheezing (symptom free days) No standard deviation values for above outcomes Expiratory flow at 25% vital capacity Expiratory flow at 70% TLC Specific airway conductance No units stated for above outcomes |
| Martin 1974 | FEV1 FVC Daily symptom score No numerical data available for above outcomes |
| Nathan 1997 | % Change FEV1 compared to baseline FVC PEFR FEF25‐75% Home PEFR am and pm Patient and physician evaluation of symptoms (wheeziness, tightness in chest, shortness of breath and cough) No numerical data for above outcomes |
| Riordan 1974 | Daily asthma symptom score Morning PEFR Evening PEFR Rescue beta2 agonist use FEV1 FVC Plasma cortisol post ACTH analogue No standard deviation values for above outcomes |
| Smith 1973a | Rescue beta2 agonist use Number of wheezing attacks per day Daily PEFR Basal plasma cortisol pre and post ACTH analogue No usable numerical data for above outcomes |
| Smith 1973b | Percentage change in FEV1 compared to baseline Asthma symptom score No usable numerical data for above outcomes |
| Fanelli 1993 | Bronchial responsiveness to histamine (PC20 FEV1) No standard deviation values |
| Vatrella 1996 | Bronchial responsiveness to methacholine (PC20 FEV1) No standard deviation values |
| Vilsvik 1974 | FEV1 (% predicted) Clinic PEFR Plasma cortisol Eosinophilic cell count Data not presented in a form suitable for analysis |
| Vogt 1976 | Asthma symptom score Aerosol evaluation score physicians and patients Extent of symptom relief score 8 am plasma cortisol No standard deviation values |
| Webb 1977 | FEV1 FVC Plasma cortisol pre and post metyrapone No numerical data for above outcomes |
| Trigg 1994 | Bronchial responsiveness to histamine (PC20FEV1) No standard deviation values for above outcomes |
| Wang 1994 | FEV1 No standard deviation available |
| Berkowitz 1998 | Change in FEV1 Change in FVC Change in clinic PEFR Asthma symptom score Change in FEF25‐75 compared to baseline No standard deviation values available for above outcomes |
| Simons 1997 | Change in FEV1 Change in FVC Change morning and evening PEFR No standard deviation available for above outcomes Methacholine bronchial responsiveness (PC20 FEV1) Numerical data not available % of children not missing school due to asthma, % children not requiring beta2 agonist: data analysed using non‐parametric methods by authors therefore not included in meta‐analysis |
| Bronsky 1998 | Change in FEF25‐75 compared to baseline Change in FVC compared to baseline Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Use of inhaled beta2 agonist Change in daily asthma symptom score compared to baseline Use of inhaled beta2 agonist No standard deviation values available for above outcomes |
| Fahy 1998 | Methacholine bronchial responsiveness (PC20 FEV1) No standard deviation values available |
| Salmeron 1989 | Daily asthma symptom score FVC No numerical data available |
| Radha 1975 | Plasma cortisol Uncertainty regarding units of measurement, no reply from author |
| Lacronique 1991 | Daily asthma symptom scoreUncertainty regarding standard deviation values, no clarification from author |
| Davies 1977 | Blood eosinophil countUnits reported unclear |
| Hoshino 2001 | Change in FEV1 (L), Change in FEV1 (% predicted) |
| Laviolette 1999 | Morning FEVI %, Morning FEV1 (L), daytime asthma symptoms scores, B‐agonists daily use %, morning PEFR (L/min), evening PEFR (L/M), nocturnal awakings nights %, asthma exacerbations %, asthma attacks % |
Assessment of asthma severity is a problematic area. The biological mechanisms of disease are incompletely understood and no molecular or genetic markers of disease severity have been identified. At the current time, severity can only be defined in terms of either (a) the frequency of symptoms, exacerbations and/or the degree of reduction in airway calibre, or (b) the amount of medication required to maintain a given level of control. For the purposes of this review we had originally planned to classify studies according to baseline FEV1 (% predicted) alone. However, we found this measurement was reported inconsistently. An attempt was therefore made to classify asthma severity using current criteria (GINA 1995; NHLBI 1997). This classification of severity is based upon FEV1 (% predicted), frequency of symptoms and exacerbations and diurnal PEFR variability. Four grades of severity are defined: severe, moderate, mild persistent and mild intermittent. However, this classification only applies to untreated patients. The guidelines also recommend a range of daily doses of ICS for disease at each severity grade. For example, BUD 200 to 400 mcg/d for mild persistent disease, 400 to 600 mcg/d for moderate disease, > 600 mcg/d for severe disease in adult patients. Therefore in the case of studies in which patients were using an ICS at the time of enrolment, we attempted to classify severity according to baseline ICS use using these ranges.
Assessment of risk of bias in included studies
The trials were scored using the Cochrane approach: Grade A: adequate allocation concealment Grade B: unclear allocation concealment Grade C: clearly inadequate concealment
We also assessed the studies using a five point scoring instrument (Jadad 1996): (a) Was the study described as randomised? (yes = 1 no = 0) (b) Was the study described as double blind? (yes = 1 no = 0) (c) Was there a description of withdrawals and dropouts? (yes = 1 no = 0) (d) Was the method of randomisation well described and appropriate? (yes = 1 no = 0) (e) Was the method of double blinding well described and appropriate? (yes = 1 no = 0) (f) Deduct 1 point if method of randomisation or blinding inappropriate
We measured inter‐rater agreement using the kappa statistic. Disagreement was resolved by consensus.
Measures of treatment effect
For each outcome for continuous data the mean, the standard deviation, and the number of the patients were required for each assessment. For binary data, such as clinical improvement or no clinical improvement, the relative risk was used to express the treatment effect. For this type of data the number in each group and the numbers experiencing the outcome were sought. Where the mean and standard deviation were not reported for each treatment group they were extracted if available. Data prior the randomisation phase were not used to assess safety or efficacy.
Data synthesis
We calculated a weighted treatment effect across trials using the Cochrane statistical package RevMan Analyses 4.2.6. For continuous outcomes, we calculated a weighted mean difference (WMD) or standardised mean difference (SMD) as appropriate. For binary/dichotomous outcomes, we calculated a relative risk (RR). We assessed heterogeneity with the I‐square statistic as described in Higgins 2003. We used a fixed effect model when homogenous treatment effects were present (I square 0%). We applied a Random Effects model when heterogeneity was present (I square >0%), but we have reported the results of Random Effects modelling where this has resulted in a significant pooled effect estimate becoming non‐significant, or where I square is greater than 25%. We expressed pooled treatments effects with 95% confidence intervals (95% CI).
A number of a priori conditions were established regarding the comparisons made:
(1) Studies were distinguished as those in which patients were (a) not treated with regular oral steroids (OCS), (b) dependent upon regular OCS prior to study. It was recognised a priori that trials assessing the efficacy of ICS in OCS dependent patients may have an 'oral steroid down‐titration' design. In other words they may use reduction in OCS use as an outcome measure, whilst attempting to maintain asthma control. On the other hand studies recruiting non‐OCS treated asthmatics are more likely to have a design aimed at detecting improvements in asthma control. It was inappropriate to combine trials with these different designs and objectives.
(2) It was anticipated that measures of bronchial hyper‐responsiveness (PD20 FEV1, PC20 FEV1) would often be reported as geometric means. Presentation of results in this way indicates that data has been logarithmically transformed prior to analysis by investigators to take account of a skewed distribution. Data for such outcomes was only pooled across studies where the mean and standard deviation of logged values (from which geometric means are derived) could be calculated.
(3) Data from studies that employed pMDI using a CFC propellant were not pooled with those using an HFA propellant. The similarity or otherwise of effects between these two preparations has not been sufficiently established, and there is some evidence from a large prospective study that a dose of 400mcg/d of HFA‐BDP may be equivalent to 800mcg/d of CFC‐BDP (Gross 1999). Although the dose response may be more pronounced at lower doses of HFA‐BDP than at equivalent increments in CFC, we have opted to pool studies assessing the effects of HFA‐BDP in the same categories, performing subgroup analyses where possible. This review is not primarily concerned with dose response, except as a possible explanation of heterogeneity, as we are focusing on efficacy in the first instance.
Sensitivity analysis
We performed sensitivity analyses on the basis of methodological quality. Results were re‐analysed using studies of only the highest quality scores (Jadad 3 to 5). We undertook subgroup analyses based upon patient age, BDP dose, delivery device, study duration and asthma severity where appropriate.
Results
Description of studies
Results of the search
SEARCH ‐ March 1999 Stage 1 electronic search (ISR): 6494 citations retrieved, 2162 original citations
Stage 2 electronic search (BDP Register): 1149 citations retrieved: 379 not RCT; 190 not chronic asthma in humans; 177 no ICS treatment arm; 113 no placebo group comparison (other comp, not review); 290 papers retrieved in full text form; 94 not RCT; 126 no placebo arm; 3 infants; 2 treatment period of less than 1 week; 2 nebuliser delivery device; 4 other reasons (see Excluded study characteristics); 8 awaiting translation; 51 included studies. Additional sources: one study was excluded as a result of contact with authors (Turner 1998).
Agreement between the two independent assessments of study quality were as follows: Randomisation: kappa = 1; Double‐blind: kappa = 0.9; Withdrawals/dropouts: kappa = 0.3; Method of randomisation: kappa = 0.3; Method of blinding: kappa = 0.4. Any disagreement we had was resolved by consensus.
UPDATE ‐ January 2003 Eight studies met the inclusion criteria. Five studies (Gross 1999; Hampel 2000; Hoshino 2001; Laviolette 1999; Malmstrom 1999), assessed the efficacy of CFC‐BDP, and three studies(Gross 1999; Hampel 2000; Nayak 2002) assessed the efficacy of HFA‐BDP. All studies specified race, duration of asthma diagnosis and number of randomised participants. They all used broadly similar inclusion and exclusion criteria, according to smoking, comorbid medical conditions and concomitant medications. Some studies reported previous use of inhaled corticosteroids but the doses and types were not specified in all of the trials.
The language of publication of the great majority of studies was English; one study (Fournier 1990) was translated from French. No studies were excluded on the basis of language.
Seven additional publications require translation and are awaiting assessment. One study (Shen 1991) appears from the English language abstract to be a randomised controlled trial. Two trials (Kudo 1995; Rozniecki 1988) are placebo controlled trials, but it is unclear from the English language abstracts whether subjects were randomised to intervention groups. Four further studies (Boszormeny‐Nagy 1980; Chonabayashi 1986; Iwata 1995; Muittari 1974) were retrieved by the search. Titles of these articles suggest that they may be relevant to this review, but no English language abstract was available.
Included studies
POPULATIONS The studies came from all around the world including the USA, Canada, UK, central Europe, Japan, New Zealand and Australia. Eleven studies were multi centre trials (Berkowitz 1998; Bronsky 1998; Simons 1997; Matthys 1998; Lacronique 1991; Salmeron 1989; Gross 1999; Hampel 2000; Malmstrom 1999; Nathan 2001; Nayak 2002). The great majority of studies were carried out in a secondary care, recruiting participants from hospital outpatient clinics.
Five studies were conducted in children, 33 in adult participants. Eight studies (Bergmann 1990; Radha 1975; Riordan 1974; Vogt 1976), recruited participants from a wider age group including children, adolescents and adults. In two studies (Cameron 1973; Hodson 1974), the age range of participants recruited was not stated, however it is likely that only adult participants were recruited to these trials given the institutions to which the investigators were affiliated. These studies have been classified as 'adult' for the purposes of subgroup analysis.
One study recruited participants with asthma caused by exposure to specific occupational allergens (Maestrelli 1993); one study (Vatrella 1996) recruited participants with asthma caused specifically by seasonal exposure to allergen.
One study (Kerstjens 1994) recruited participants with both chronic obstructive airways disease and asthma. However data concerning the asthmatic participants alone were obtained from the investigators.
ORAL STEROID TREATMENT In fifteen studies (BTTA 1976; Brompton/MRC 1974; Cameron 1973; Davies 1977; Harvey 1976; Hodson 1974; Kerigan 1977; Lacronique 1991; Webb 1977, Hampel 2000; Hoshino 2001; Laviolette 1999; Malmstrom 1999; Nathan 2001; Nayak 2002) the dependence on treatment with regular oral prednisolone for asthma control was an inclusion criterion. In three studies a proportion of enrolled participants were receiving oral steroid treatment but this was not an inclusion criterion. No attempt was made to reduce daily prednisolone dose in these studies. In one study (Kerrebijn 1976) a proportion of participants were receiving oral steroids at enrolment and attempt was made to withdraw oral prednisolone in these participants.
Treatment with oral corticosteroids at the time of enrolment was an exclusion criterion for all included studies except Gross 1999. In this study a seven to 12 day course of oral steroid treatment (prednisolone 30 mg/d) was followed during the run in period and prior to randomisation. In one study (Malmstrom 1999) oral steroids were permitted for asthma exacerbation episodes but patients who suffered from more than two episodes were withdrawn. In contrast, maintenance treatment with an inhaled corticosteroid prior to the screening visit was an entry criterion in four trials (Bernstein 1999; Laviolette 1999; Nathan 2001; Gross 1999). In two studies (Bernstein 1999; Nathan 2001) patients must have been on a stable daily dose of inhaled corticosterioid prior the run‐in period for two and four weeks respectively.
DIAGNOSIS OF ASTHMA In 16 studies (28%) there was no clear indication of the criteria upon which a diagnosis of asthma was made and appears to have been at the discretion of the investigator. All except one of these (Katsunuma 1993) were published in or prior to 1976. In seven studies a diagnosis of asthma was made of the basis of American Thoracic Society criteria, either by 1962 standards (Boner 1991; Fanelli 1993; Salmeron 1989) or 1987 standards (Kerstjens 1994; Lacronique 1991; Laviolette 1994; Hoshino 1998, Hoshino 2001). In two studies, (Matthys 1998, Hampel 2000) diagnosis was made on the basis of current GINA standards. In 14 studies, diagnosis was supported by either demonstrable variability of FEV1 or PEFR over time, or reversibility following inhalation of beta2 agonist. In five studies, diagnosis was supported by the demonstration of bronchial hyper‐responsiveness to histamine (Trigg 1994; Wang 1994, Wiebicke 1990), methacholine (Fahy 1998; Wiebicke 1990) or propranolol (Carpentiere 1990). In three studies the diagnosis was supported by intermittent symptoms of wheeze and/or cough and breathlessness with bronchial hyper‐responsiveness (BHR) to methacholine (Bel 1990) or histamine (Chan 1993; Pennings 1997). In one study a diagnosis of seasonal asthma was made on the basis of positive skin prick tests for Parietaria with a recognised seasonal increase in asthma symptoms (Vatrella 1996). In two studies a diagnosis of occupational asthma was made on the basis of hyper‐responsiveness to occupational allergens (De Marzo 1988; Maestrelli 1993). Skin‐prick test was performed in two papers (Hoshino 2001; Nathan 2001), and tested different allergens.
SEVERITY OF ASTHMA: STUDIES RECRUITING NON‐OCS TREATED PATIENTS (1)MILD In six studies, investigators stated that the severity of asthma was mild. A baseline FEV1 > 60 % predicted was an inclusion criterion in two studies (Wang 1994; Trigg 1994); FEV1 > 80% in one study (Vatrella 1996); > 90% one study (Pennings 1997). No information was given regarding the baseline frequency of asthma symptoms in these studies.
In a further three studies the authors did not give an opinion as to the severity of disease in the participants recruited, or provide information on the frequency of asthma symptoms in subjects at enrolment. However, baseline FEV1 was > 70 % predicted (Boner 1991), > 75% (Maestrelli 1993; Vatrella 1996). In one study (Fanelli 1993) subjects were enrolled who had baseline FEV1 > 90 (% predicted) and who were asymptomatic.
In all of these studies participants were not receiving a regular inhaled steroid at the time of enrolment.
(2) MILD TO MODERATE In nine studies, investigators classed the severity of asthma of enrolled participants as mild to moderate. In one study (Simons 1997) an inclusion criterion was a baseline FEV1 > 70 (% predicted). In the remaining eight studies (Berkowitz 1998; Bronsky 1998; Fahy 1998; Hoshino 1998, Malmstrom 1999; Laviolette 1999; Bernstein 1999; Hampel 2000), participants were recruited with baseline FEV1 of between 50 and 90 (% predicted). In one further study (Bel 1990), investigators did not give an opinion as to the severity of disease, however the range of baseline FEV1 was 54 to 123 (% predicted) suggests participants in this trial had mild to moderate asthma.
Requirement for BDP 336 mcg/d for asthma control was an inclusion criterion for two studies (Berkowitz 1998; Bronsky 1998). In all other studies participants were not receiving a regular inhaled steroid at the time of enrolment.
(3) MODERATELY SEVERE In five studies, asthma severity was classed as moderate by the investigators. In Nathan 1997, baseline FEV1 was between 50 and 80 (% predicted). In the study by Matthys 1998, participants fulfilled the criteria for moderate severity asthma according to the GINA 1995 criteria. In Salmeron 1989, the mean FEV1 of participants at baseline was 50 (% predicted), and it was stated that symptoms were poorly controlled at baseline. In two studies (Gaddie 1973, Messerli 1975), there was no indication of baseline spirometry or frequency of symptoms.
In Nathan 1997 participants were receiving a regular inhaled steroid at the time of enrolment.
In two studies (Nathan 2001; Nayak 2002), investigators ranked the severity of asthma as moderate. In Nathan 2001, the baseline FEV1 value was between 60 and 90 (% predicted). The mean value recorded for baseline FEV1 in Nayak 2002 was 71.70 (% predicted).
(4) MODERATE TO SEVERE In Vogt 1976, investigators considered participants to have moderate to severe asthma. Baseline FEV1 ranged from 20 to 80 (% predicted). No indication was given as to frequency of asthma symptoms. In Radha 1975, the investigators did not give an opinion as to the severity asthma of participants in the trial, however baseline FEV1 ranged from 35 to 75 (% predicted). In Gross 1999, the global Initiative for asthma classification was used, and participants with at least moderate severity asthma were enrolled.
(5) SEVERE In one study (Klein 1977), the investigators considered their patients to have severe asthma, but no information was given as to baseline spirometry or symptom frequency. In one study (Lovera 1975) the investigators considered their patients to have asthma severe enough to warrant consideration of treatment with oral steroids but again no information was given as to baseline spirometry or symptom frequency
(6) MILD TO SEVERE In two studies (Bergmann 1990; Kerstjens 1994), participants were recruited with a spectrum of disease severity, from mild to severe. Baseline FEV1 ranged between 10 and 120 (% predicted).
(7) NO CLEAR INDICATION OF SEVERITY In three studies (Bennati 1989; Fournier 1990; Kraemer 1987) the investigators did not give an opinion as to the severity of asthma of participants. No description of baseline spirometry or the frequency of asthma symptoms was given.
INTERVENTIONS
DAILY DOSE OF CFC‐BDP 32 studies assessed the effects of a daily nominal dose of BDP of 400 mcg or less versus placebo. Nine studies (21%) assessed the effects of 500 to 800 mcg/d of BDP, whilst 16 (28%) assessed the effects of 1000 mcg/d or greater. Four studies (Brompton/MRC 1974; BTTA 1976; Kerrebijn 1976; Nathan 1997) included a comparison of two or more doses of beclomethasone to placebo within the same study.
Seven studies (Bel 1990; Kraemer 1987; Simons 1997, Bernstein 1999, Gross 1999, Laviolette 1999; Malmstrom 1999), included a treatment arm of nedocromil, sodium cromoglycate, salmeterol, montelukast, theophylline, mometasone furate and HFA‐BDP respectively. These treatment arms were not considered in this review
Three studies included a group of participants treated with additional inhaled corticosteroid. Two studies (Berkowitz 1998; Bronsky 1998) included a triamcinolone acetonide treated group and one (BTTA 1976) included a group treated with betamethasone valerate. These interventions have not been evaluated in this review.
DAILY DOSE OF HFA‐BDP Three studies (Gross 1999; Hampel 2000; Nayak 2002), evaluated the effect of HFA‐BDP versus placebo. In Hampel 2000, two different doses of BDP‐HFA (100 mcg/day, 200 mcg/day) were compared to placebo. In Nayak 2002, a daily dose of 80 mcg/day and 160/day mcg versus placebo were tested in asthmatic children. The comparable efficacy of 400 HFA‐BDP mcg/day and 800 mcg/day BDP‐CFC was assessed in one study and compared to placebo (Gross 1999). The two propellants were not compared directly in this review, but will be the subject of a separate review (Malouf 2004). DELIVERY DEVICE 33 studies used a pMDI to deliver study medication. Two studies used a MDI with spacer device. In two studies the Diskhaler (Simons 1997) and the Rotahaler (Kraemer 1987) were used. Aerochamber Spacer device was used in one study, (Malmstrom 1999). In a single study, (Nayak 2002) undertaken in children the Autohaler breath‐actuated device was employed. Beclomethasone and placebo was delivered using the same device in all conducted studies except in (Nathan 2001), where DPI was used to deliver placebo.
LENGTH OF INTERVENTION PERIOD Four studies used a treatment period of four weeks. 30 studies had a treatment period between one to six months. Six studies had a treatment period of six months or longer. Two studies (BTTA 1976; Cameron 1973) used an intervention period of variable length. OUTCOMES ASSESSED A large number of outcome measures were reported, often using different metrics of measurement. All outcomes were considered except the following: (1) A number of studies included outcomes related directly to the biological basis of airway inflammation in asthma. These included inflammatory cell profiles in bronchial epithelium (Hoshino 1998; Wang 1994), bronchoalveolar lavage fluid (Trigg 1994) and induced sputum (Fahy 1998). The expression of pro‐inflammatory cytokines in bronchoalveolar lavage fluid (Trigg 1994) and bronchial wall epithelium (Hoshino 1998; Wang 1994) has also been assessed. In Hoshino 2001 changes in airway mucosal vascularity were estimated. These outcomes involve invasive or semi‐invasive tissue/fluid sampling and are not generally regarded as useful outcome measures to assess the clinical efficacy of ICS in asthma. These outcomes have not been considered in this review.
(2) A number of studies examined detailed lung mechanics in response to bronchial inhalation challenge. One study (Fanelli 1993) assessed minute ventilation, tidal volume, respiratory frequency, inspiratory and expiratory time and mean inspiratory flow rates following inhaled histamine challenge. A further study (Pennings 1997) assessed the change in various parameters related to airway impedance and reactance following isocapnic cold air bronchial challenge measured by a forced oscillation technique. At the current time these outcome measures are not widely used, and because of the uncertainty as to their value in guiding clinical practice they have not been included in this review.
All other efficacy and safety outcomes were considered. A significant amount of outcome data could not be included in the meta‐analysis, usually because standard deviation values for means were not reported. This is listed in Table 1.
Risk of bias in included studies
All studies were randomised and placebo controlled design, and all were double blind with the exception of three single‐blind studies (Bergmann 1990; Fanelli 1993; Gross 1999). The overall quality of studies was high as assessed by the Jadad method, two studies achieved a score of five (Bennati 1989; Pennings 1997). 13 studies achieved a score of four, 21 achieved a score of three. Only 4 had a Jadad score of two or less. Of the 60 included studies 21 described adequate allocation concealment, i.e. Cochrane score A (Davies 1977; Hodson 1974; Holst 1974; Martin 1974; Messerli 1975; De Marzo 1988; Hoshino 1998; Kerstjens 1994; Kraemer 1987; Chan 1993; Maestrelli 1993; Laviolette 1994; Pennings 1997; Simons 1997; Turner 1998; Nathan 2001; Bernstein 1999; Malmstrom 1999; Laviolette 1999; Hampel 2000; Nayak 2002). In 39 studies allocation concealment was unclear.
Effects of interventions
Results from the meta‐analysis are reported by outcome. All comparisons concern BDP versus placebo.
This complex review required a structured approach to reporting the results, therefore a hierarchy of result reporting was established:
(A) Study medication: CFC‐BDP or HFA‐BDP (B) Background therapy: with or without oral corticosteroids (OCS) (C) Outcome measurement unit: FEV1, PEFR etc. (D) Unit of measurement: i.e. absolute, % predicted or change compared to baseline Within each component subgroup analysis by age group, BDP daily dose, asthma severity, delivery device, length of intervention period and study quality are reported, where the data permit. This was structured in the following way:
(a) Comparisons 1 to 3 consider each outcome according to CFC‐BDP dose (400 mcg/d or less, 500 to 800 mcg/d, 1000 mcg/d or greater). The results for these comparisons are discussed in the text of the results first for each outcome. (b) Comparisons 12 to 14 consider each outcome according to HFA‐BDP dose (100 mcg/day or less, 160‐200 mcg/day and 400 mcg/day).
(c) Studies were pooled, irrespective of CFC‐BDP or HFA‐BDP dose. This allowed an overall effect size to be calculated for each outcome. It also permitted an exploration of whether the effect size varied in a consistent manner according to dose. Because studies were divided into subgroups according to daily dose, it allowed an impression to be gained of any dose response relationship by comparing the 95% confidence intervals around the mean effect size for each dose subgroup. These results are also discussed in the text.
(d) The findings of subgroup analyses based on delivery device, study duration and asthma severity are reported (Comparisons 4 to 7).
Comparisons of CFC‐BDP and HFA‐BDP versus placebo were analysed separately. They were also stratified on the basis of duration: i) data at four weeks and ii) data at greater than four weeks depending on the duration of follow‐up in the studies. In all outcomes the overall estimate from a Fixed Effect model was presented and a test for heterogeneity using a standard chi‐square was performed. Where there was evidence of heterogeneity of treatment effect between trials Random Effects (RE) modelling was applied and we report differences between the two models.
ASTHMATICS NOT TREATED WITH ORAL CORTICOSTEROIDS (OCS)
(1) SPIROMETRY
(a) FEV1 and FEV1 (Litres and % predicted combined): Eleven studies assessed three dose levels in adults (400 mcg/d or less, 500 to 800 mcg/d and 1000 mcg/d or greater). Two studies recruited adults and children (Radha 1975; Vogt 1976). There was a significant difference in FEV1 (expressed either as litres or % predicted) compared with placebo. The mean effect size for each of the three doses were:
400 mcg/d or less: SMD 0.38 (95%CI 0.63 to 0.14), 4 studies. This translates to a difference of around 0.3L
500 to 800 mcg/d SMD 0.62 (95% CI 0.32 to 0.93), 4 studies. This translates to a difference of around 0.5L
1000 mcg/d or greater SMD 0.33 (95% CI 0.02 to 0.64), 5 studies. This translates to a difference of around 0.2L
No significant heterogeneity was found for any of these comparisons. A sensitivity analysis based on methodological quality was undertaken. Two studies with Jadad scores of two were excluded from the analyses (Fanelli 1993; Radha 1975), however this did not significantly alter the direction or size of effect.
(b) FEV1 (Litres/min): Data from six studies were available (Fahy 1998; Bronsky 1998; Vogt 1976; Gross 1999; Pennings 1997; Salmeron 1989). Within each steroid dose range, there was a significant effect in favour of CFC‐BDP compared with placebo (<400: 0.34 litres (95% CI 0.11 to 0.56); 5‐800: 0.33 (95% CI 0.14 to 0.52); greater than 800: 0.48 (95% CI 0.15 to 0.82)). When pooled across these doses, there was a significant effect in favour of BDP of 0.36 litres (95% CI 0.22 to 0.49). No significant heterogeneity was observed within each dose group, or across the six studies when they were pooled.
(c) Change in FEV1 (Litres): There was a significant difference in change from baseline in favour of BDP of 0.36 litres (95% CI 0.20 to 0.53 litres). The 95% confidence intervals for each dose subgroup overlapped widely and there was no significant difference between the results from trials of more or less than 4 weeks duration. Additional subgroup analyses based on delivery device and asthma severity did not identify any particular group of studies showing a greater benefit.
(d) FEV1 (% predicted): There was no significant difference between BDP and placebo: WMD: 5.36% (95% CI ‐0.88 to 11.60%). There was significant heterogeneity between studies (I square 61.3%). A sensitivity analysis based on methodological quality resulted in the removal of one low quality study (Fanelli 1993) but did not reduce the heterogeneity. The subgroup analysis based upon asthma severity resulted in the exclusion of Kerstjens 1994, because participants with a very wide range of asthma severity from mild to severe were recruited. Although heterogeneity resolved completely, the effect remained non‐significant (WMD 1.88% (95% CI ‐2.11 to 5.8%)). It would appear that the Kerstjens 1994study contributes significantly to the overall treatment effect (which disappears when we removed the study) but also to the level heterogeneity, although the reasons for this are not clear. (e) Change in FEV1 (%): Two studies (Malmstrom 1999; Laviolette 1999), reported the changes in FEV1% from baseline. The difference in FEV1% between active treatment groups and placebo was significant (WMD 12.41% (95%CI 8.18 to 16.64)). A small study (Trigg 1994), (25 participants) assessing BDP 1000 mcg/d versus placebo did not show any difference between treatment groups. (f) FVC (Litres) Two studies (Radha 1975; Vogt 1976), assessing BDP 400 and 800 mcg/d versus placebo, respectively. No significant difference between treatment groups was apparent. Radha 1975, comprised only 28 participants and was of weak methodological quality (Jadad score 2). One low quality (Jadad score 2) study (Fanelli 1993) evaluating BDP 2000 mcg/d versus placebo reported FVC, no significant difference between treatment groups was apparent.
A single study (Nathan 2001) reported changes in FVC compared to baseline. There was a significant improvement in favour of BDP 400 mcg/d compared with PLA (CFC‐BDP 0.17, SD 0.45 compared with placebo ‐0.22 SD 0.45). (g) Expiratory flow at low lung volume (FEF 25% to 75% of FEV1) Two studies (Fahy 1998, Vogt 1976), assessed FEF 25% to 75% of FEV1. There was a significant effect improvement in this measure in favour of BDP at doses of 400 and 800 mcg/d versus placebo, WMD 0.93 L/second (95% CI 0.58 to 1.28). A subgroup analysis based on treatment duration did not reveal any differences between groups. It is noteworthy that these two studies had treatment periods of four weeks only.
(h) Change in FEF 25% to 75% FEV1 compared with baseline One study which assessed BDP at a daily dose of 400mcg (Nathan 2001) reported change in FEF 25‐75% FEV1 from baseline. A significant effect was seen in favour of BDP over placebo (0.08, SD 0.60 compared with ‐0.22, SD 0.60) L/second.
(2) Peak expiratory flow (PEF) (a) Morning PEF (L/MIN) Two studies undertaken in adults, presented data for home diary card recorded morning PEFR in a form suitable for meta‐analysis (Fahy 1998; Kerstjens 1994). No significant treatment advantage of BDP over placebo was demonstrated for individual doses. When studies were pooled across dose and duration, no significant overall effect in favour of BDP was evident. (b) Change in morning PEF compared with baseline (L/MIN) Four studies (Gross 1999; Matthys 1998; Malmstrom 1999; Nathan 2001), provided data for the change in PEFR compared with baseline. A pooled estimate was derived for two dose categories (<400mcg/d and 500‐800mcg/d). When data from these two dose categories were pooled there was a significant effect in favour of CFC‐BDP of 39.05 L/min (95% CI 31.95 to 46.15). There was a very low level of heterogeneity (Chi2 3.5, df: 3; I2 14%). (c) Evening PEF (L/min) Two studies (Kerstjens 1994; Malmstrom 1999), presented diary card evening PEFR data in a form suitable for analysis. Both assessed adult subjects. Kerstjens 1994, compared BDP 800 mcg/d versus placebo, no difference between treatment groups was found. Malmstrom 1999, reported the changes of evening PEFR from baseline. This study tested the efficacy of BDP 400 mcg/day versus placebo. The data presented a significant benefit of BDP over placebo, (32.10 L/min, SD 63.45 compared with 0.30 L/min SD 62.52). Subgroup analyses based upon delivery device, study duration and asthma severity were undertaken for diary card morning and evening PEFR but did not identify any groups who appeared to preferentially benefit from treatment with BDP.
(c) PEF: other measures One study (Hoshino 1998) reported diary card daily average PEFR. No difference between BDP 800 mcg/d and placebo treatment groups was seen. However, this study did demonstrate a significant effect in favour of BDP for diurnal variability in PEFR, mean difference 11% (95% CI 5 to 18%).
(2) RESCUE BETA2 AGONIST USE
(a) Absolute rescue beta2 agonist use Daily rescue beta2 agonist use was reported with data suitable for analysis in three studies, all of which were in adults (Hoshino 1998; Matthys 1998; Vatrella 1996). There was significantly lower beta2 agonist use for low dose BDP compared to placebo: WMD 0.9 puffs/d (95% CI 0.4 to 1.2). One study assessed BDP 800 mcg/d versus placebo (Hoshino 1998) that was undertaken in 171 asthmatic adults and demonstrated significantly lower beta2 agonist use for BDP, mean difference ‐1.80 puffs/d (95%CI ‐2.21 to ‐1.38). One study (Vatrella 1996) with a small sample size of 20 asthmatics assessed BDP 1000 mcg/d versus placebo and showed significantly lower beta2 agonist use in the BDP treated group, WMD ‐2.9 puffs/d (95% CI ‐3.5 to ‐2.4).
When studies were pooled across all doses, an overall effect in favour of BDP was apparent: WMD ‐1.98 puffs/d (95% CI ‐2.34 to ‐1.62). There was a high degree of heterogeneity when studies were pooled (I square 94%) and therefore a Random Effect model was applied. The resultant pooled effect estimate remained significant (‐2.39 puffs/d (95% CI ‐4.02 to ‐0.75). The 95% confidence intervals for the pooled treatment effect for medium (500‐800 mcg/d) and high dose BDP (1000 mcg/d or greater) did not overlap those of low dose (400 mcg/d or less) BDP, giving the impression of a dose response effect. However this observation needs to be viewed with caution since it is based on only three studies with relatively small numbers of participants in the medium and higher dose range (38/294 participants overall) and is not a randomised comparison. Subgroup analyses based on delivery device, study duration and asthma severity could not account for the heterogeneity.
(b) Change in rescue beta2 agonist use Three studies from different dose ranges (400mcg/d and 500‐800mcg/d) reported data on change in rescue medication usage (400mcg/d: Malmstrom 1999; Nathan 2001; 500‐800 mcg/d: Gross 1999). There was a significant reduction in daily beta agonist use at a dose of 400 mcg/d of ‐2.32 puffs/d (95% CI ‐2.55 to ‐2.09). When pooled with the Gross 1999study there was a significant degree of heterogeneity (72.9%). This may be both a reflection of the pre‐dosing OCS schedule, and the higher dose of CFC‐BDP given to participants in Gross 1999. However, the pooled effect estimate for the subgroups was significantly in favour of CFC‐BDP with both Fixed Effect and Random Effects modelling: FE: ‐2.18 puffs/day (95% CI ‐2.38 to ‐1.97); RE: ‐2.07 puffs/day (95% CI ‐2.60 to ‐1.54). (3) ASTHMA SYMPTOMS (a) Absolute symptom scores The reporting of symptom scores was disparate and the pooled estimates and we have analysed data as SMDs due to the different scales employed. Two small studies reported symptom scores as absolute values (Hoshino 1998; Vatrella 1996), and when pooled there was significant difference of ‐2.81 standard deviations (95% CI ‐3.77 to ‐1.86).
(b) Change in symptom scores Kerstjens 1994 and Malmstrom 1999 reported change in asthma symptom score from baseline. When pooled, BDP led to a significant improvement compared to placebo of SMD 0.19 (95%CI 0.35 to 0.03). However, there was a high level of heterogeneity (I square 72%) which is difficult to interpret given the number of studies and the differences in their characteristics (including sample size, sensitivity of symptom scale used and dose of BDP used in the studies). Further studies would elucidate whether the differences in symptoms are related to the characteristics of the studies described above, or whether additional factors may contribute to different responses such as baseline symptoms. (4) BRONCHIAL HYPER‐RESPONSIVENESS (BHR) BHR to a number of pharmacological stimuli was assessed. Five studies reported data on methacholine challenge. When studies were pooled across all doses an overall effect in favour of treatment with BDP was apparent (SMD: 0.53 (95% CI 0.10 to 0.96)). We performed a subgroup analysis based on duration of treatment. The single study that assessed treatment over a period of four weeks did not demonstrate a significant difference in methacholine BHR between active treatment and placebo groups. However, there was a significantly lower methacholine BHR in the BDP treatment group when the three studies with treatment periods of greater than four weeks were pooled SMD 0.53 (95% CI 0.10 to 0.96). This lends some support to the notion that duration of treatment may be a significant factor modulating the treatment effect of BDP. However, this must be viewed with caution due to the small number of studies and participants included in this analysis. No trend in keeping with a dose response effect was present. Subgroup analyses based upon BDP dose, delivery device, and asthma severity did not identify any groups that appeared to benefit preferentially.
Histamine PC20 FEV1 was significantly greater in BDP 800 mcg/d treated participants compared to placebo in the single study in adults (Kerstjens 1994), that reported this outcome: mean difference log 10 values 1.19 (95% CI 0.66 to 1.71).
One study (Pennings 1997), assessed high dose BDP (1000 mcg/d or greater) used other provoking stimuli. This study evaluated BHR to inhalational cold air challenge (IHCA), assessed by post challenge FEV1 (litres). There was no significant difference between treatment groups. It should be noted however, that within the same study, BHR to IHCA as measured using a forced oscillation technique found a statistically significant improvement in the BDP treated participants compared to placebo (data not shown).
(5) ASTHMA EXACERBATIONS No studies reported hospital admission rates or emergency department attendance rates due to asthma exacerbation. Likewise, no studies assessed days missed from work or school due to asthma exacerbations.
Nine studies (1071 participants) assessed BDP 400 mcg/d compared to placebo (Bel 1990; Bennati 1989; Bergmann 1990; Berkowitz 1998; Boner 1991; Fahy 1998; Matthys 1998; Simons 1997,Nathan 2001). BDP resulted in significantly fewer asthma exacerbation‐related withdrawals than placebo RR 0.29 (95% CI 0.18 to 0.48). This result was not significantly altered by a sensitivity analysis that excluded the single low quality study (Bergmann 1990). Three studies were in children (Bennati 1989; Boner 1991; Simons 1997), four in adults (Bel 1990; Berkowitz 1998; Fahy 1998; Matthys 1998) and two in children and adults (Bergmann 1990; Nathan 2001): there was no heterogeneity in effect size across studies. In only two studies (Berkowitz 1998; Simons 1997) was it clear from the study protocol that a criterion for withdrawal due to asthma exacerbation had been set a priori (see Included Study Characteristics).
Two studies (Hoshino 1998; Vogt 1976) with 117 participants, both in adults compared BDP 800 mcg/d to placebo. No significant difference in withdrawal rates due to exacerbations were apparent between treatment groups, RR 0.34 (95% CI 0.09 to 1.30).
Treatment with BDP 1000 mcg/d or greater resulted in significantly fewer asthma exacerbation‐related withdrawals compared to placebo RR 0.08 (95% CI 0.02 to 0.41). This analysis included three studies in adults (Pennings 1997; Salmeron 1989; Trigg 1994) and one study in children and adults (Fanelli 1993) with a total of 159 subjects. A sensitivity analysis that excluded the single low quality study (Fanelli 1993) produced a similar result. In one study (Salmeron 1989) criteria for exacerbation‐related withdrawal were pre‐defined (see Characteristics of Included Studies). When all 10 studies, (1185 participants) were pooled irrespective of BDP dose a significant effect in favour of BDP was apparent, RR 0.25 (95% CI 0.16 to 0.39). There was no heterogeneity between studies and the 95% confidence intervals for treatment effect for each dose overlapped, no trend suggesting a dose response effect was seen. Subgroup analyses based upon delivery device, study duration and asthma severity did not demonstrate any groups who appeared to benefit preferentially. However, it is noteworthy that when trials were analysed according to treatment duration, a significant effect in favour of BDP was only seen for the subgroup of studies incorporating a treatment period in excess of four weeks. This raises the possibility that treatment duration may have an important influence on effect size for this outcome.
SIDE EFFECTS
(1) OROPHARYNGEAL SIDE EFFECTS
(a) Sore throat and voice change The incidence of sore/dry mouth, throat, hoarseness, dysphonia was reported in five studies, all in adults (Bel 1990; Matthys 1998; Nathan 1997; Pennings 1997; Vogt 1976, Bernstein 1999, Nathan 2001). No significant difference between BDP and placebo was apparent for any of the three dose ranges. Four studies (Bel 1990; Matthys 1998; Nathan 1997, Malmstrom 1999) with a total of 997 subjects assessed BDP 400 mcg/d versus placebo RR 1.44 (95%CI 0.81 to 2.66); one study (Vogt 1976) of 327 subjects assessed 800 mcg/d versus placebo RR 1.58 (95%CI 0.75 to 3.34); two studies (Nathan 1997; Pennings 1997) with 254 subjects assessed BDP 1000 mcg/d or greater versus placebo RR 1.71 (95% CI 0.88 to 3.35). No heterogeneity was present for any comparison. When studies were pooled across all doses no difference between BDP and placebo groups was seen, RR 1.30 (95% CI 0.73 to 2.33). No significant difference in pharyngitis was observed when data from three studies assessing 400mcg/d BDP were pooled (Laviolette 1999, Malmstrom 1999, Nathan 2001): RR 1.27 (95%CI 0.48 to 3.37).
(b) Oral candidiasis Two studies (Bernstein 1999, Nathan 2001) evaluated the effect of BDP 400 mcg/d on the incidence of oral candidiasis. There was no significant difference presented between BDP and placebo, RR 3.75 (95% CI 0.62 to 22.57). One high dose study (Pennings 1997) assessed the effects of 1000 mcg/d on the incidence of oral candidiasis. In this study, diagnosis was based on clinical impression and was not confirmed by swab culture. When studies were pooled across doses, no difference between BDP and placebo was apparent RR 3.56 (95%CI 0.75 to 16.90).
(2) HYPOTHALAMO‐PITUITARY‐ADRENAL (HPA) FUNCTION In a single study of 11 subjects (Maestrelli 1993) no difference in morning plasma cortisol or plasma cortisol levels was found following an insulin tolerance test, when BDP 2000 mcg/d was compared to placebo.
(3) OTHER OUTCOMES One study of 44 subjects (Vatrella 1996) assessed BDP 1000 mcg/d and demonstrated significantly lower serum eosinophil cationic protein (ECP) levels in the BDP group compared to placebo, 285 ± 126 compared with 475 ± 95 %. The change in peripheral blood eosinophil count from baseline was assessed in one study (Malmstrom 1999). No differences were observed between CFC‐BDP 400 mcg/d and placebo (CFC‐BDP: ‐0.07, SD 3.96 cells x 10 3 mic/L versus placebo: ‐0.02, SD 4.01 cells x 10 3 mic/L). ASTHMATICS TREATED WITH OCS: OCS‐SPARING STUDY DESIGN (1) DOSE OF ORAL STEROIDS
(a) Absolute dose of oral steroids BDP 400 mcg/d resulted in a significantly lower daily maintenance dose of oral prednisolone compared to placebo: WMD 6.3 mg/d (95% CI 3.9 to 8.7 mg/d). No heterogeneity in effect size was apparent. The adult patients in the two studies contributing to this analysis (Davies 1977; Webb 1977) had similar baseline oral steroid requirements (mean daily dose 9 to 15 mg).
One study also in adults (Lacronique 1991) demonstrated a significant advantage of BDP 1000 mcg/d compared to placebo: mean difference 6.0 mg/d (95% CI 2.5 to 9.5 mg/d).
When studies were pooled across all doses, a significant effect in favour of BDP was found: WMD 6.2 mg/d (95% CI 4.3 to 8.2 mg/d). The 95% confidence intervals for the dose subgroups overlapped widely. There did not appear to be any trend to suggest a dose response effect.
All studies employed a pMDI and were between one and five months duration. Hence subgroup analyses based on delivery device and study duration were not possible.
(b) Change in dose of oral steroids In two OCS sparing studies (Cameron 1973; Kerigan 1977), assessing BDP 400 mcg/d data were reported as the reduction in daily dose of prednisolone. BDP 400 mcg/d resulted in significant reductions in the daily dose of maintenance oral prednisolone compared to placebo WMD 4.91 mg/d (95% CI 5.9 to 3.9 mg/d). Although both studies reported a significant reduction in prednisolone use there was significant heterogeneity (I square 77%). A sensitivity analysis based on methodological quality resulted in the exclusion of one study (Kerigan 1977), but made no difference to the overall conclusion.
(c) Discontinuation of oral steroids BDP 400 mcg/d resulted in a significantly greater likelihood of discontinuing oral steroids compared to placebo (RR 3.43 (95% CI 2.39 to 4.92). Five studies (174 participants) contributed to this analysis and although significant heterogeneity was apparent (I square 59.1%), Random Effects modelling did not alter the significance of the result.
(2) SPIROMETRY, PEFR AND SYMPTOMS Spirometry, PEFR and symptom scores were reported rarely. Only two studies in oral steroid treated subjects reported numerical data for these outcomes.
A single study in adults over 12 weeks (Davies 1977) that assessed the effect of BDP 400 mcg/d on FEV1 (% predicted) found no advantage of active treatment over placebo.
A single study in adults over 12 weeks (Lacronique 1991) assessed the effects of BDP 1000 mcg/d versus placebo on percentage change in FEV1 compared to baseline. A significant effect in favour of active treatment was found, mean difference 13% (95% CI 5 to 21). The same study also demonstrated a significant advantage of BDP 1000 mcg/d over placebo for clinic measured PEFR, mean difference (95% CI) 8 % predicted (95% CI 2 to 14 % predicted). A significant advantage of BDP over placebo was also apparent for daily asthma symptom score, mean difference 0.7 (95% CI 0.3 to 1.1).
(3) LOCAL SIDE EFFECTS The incidence of oropharyngeal side effects (sore or dry mouth/throat, hoarseness) and oropharyngeal Candidiasis were reported rarely. A single study in adults (BTTA 1976) assessed the effects of both low dose (400 mcg/d) and medium dose (800 mcg/d) BDP versus placebo on the incidence of hoarseness and did not find a significant difference between active intervention and placebo for either dose.
A single study in adults (Brompton/MRC 1974) also assessing low dose (400 mcg/d) and medium dose (800 mcg/d) BDP found a significantly lower likelihood of oral Candidiasis in placebo treated participants compared to those who received both doses: BDP 400 mcg/d RR 4.7 (95% CI 1.5 to 14.8); BDP 800 mcg/d RR 8 (95% CI 2.7 to 24.0). Diagnosis was based upon a combination of clinical features (erythema associated with discrete or confluent white patches), and throat swab culture.
ASTHMATICS TREATED WITH OCS: NON‐OCS SPARING STUDY DESIGN One study (Kerrebijn 1976), undertaken in the early years following the introduction of inhaled BDP for the treatment of chronic asthma. The results from these study had not been included in the meta‐analysis for a number of reasons:
a) there was no attempt to taper OCS use during the treatment period of the trial so the minimum effective dose of OCS was not established.
b) Numerical data in a form suitable for meta‐analysis were not reported in the published papers. The author responded to letters for data requests (Kerrebijn 1976) but were unable to provide missing data.
The characteristics of this study and a description of results were outlined in the Characteristics of Included Studies section, however the reader was referred to the original publications for further details.
ASTHMATICS NOT TREATED WITH ORAL CORTICOSTEROIDS (OCS): HFA‐BDP ‐ doses of 100‐400mcg/day.
Three studies (Gross 1999; Hampel 2000; Nayak 2002) were identified for inclusion. There was an important difference in the design of the Gross 1999 study compared with Hampel 2000 and Nayak 2002. Gross 1999 administered a course of oral steroids to participants, and subsequently maintained participants on a dose of HFA‐BDP that was higher than the dosage used in Hampel 2000 and Nayak 2002. When data were pooled for outcomes such as beta‐agonist use and PEF, the level of heterogeneity was very high. We therefore report the pooled effects of these studies with and without the Gross 1999 data.
We have only performed subgroup analyses according to age. In order to prevent double‐counting participants we have pooled data separately from the lowest dose arms of Hampel 2000 and Nayak 2002 (100mcg ‐ see comparison 12) and from the moderate dose arms (160/200mcg ‐ see comparison 13).
(1) SPIROMETRY
(a) Change in FEV1 (% predicted) HFA‐BDP led to significant improvements in FEV1 (% predicted) from baseline when compared to placebo, for both BDP at a daily dose of 160‐200 mcg (6.36% (95%CI 3.94 to 8.78)) and at a daily dose of 100 mcg/d (5.9% (95% CI 3.40 to 8.78)). (b) FEV1 (L/min) Gross 1999 reported that both groups improved in FEV1 post‐OCS treatment, and that in participants subsequently given HFA‐BDP this improvement was maintained more successfully compared with placebo‐treated participants (P value of the HFA‐BDP and CFC‐BDP groups versus placebo: </=0.003).
(c) Change in peak expiratory flow (L/min) am PEF Hampel 2000 and Gross 1999 reported significant differences in favour of BDP, but when pooled, there was significant heterogeneity (comparison 12: I square: 90%; comparison 13: I square: 86.4%). As there were only two studies, any exploration of heterogeneity would only serve to highlight differences at trial level. The differences do not simply encompass pre‐treatment with oral steroids or dose of inhaled steroid, but also extend to severity and study duration. Both Fixed Effect and Random Effects modelling gave significant effects. However, the Random Effects model gave a confidence interval that was considerably wider than that generated by the Fixed Effect model for both comparisons: Comparison 12 FE: 40.49 L/min (95% CI 30.09 to 50.89); RE: 41.22 L/min (95% CI 8.29 to 74.15); comparison 13: FE: 43.21 L/min (95% CI 32.64 to 53.79), RE: 43.42 L/min (95% CI 14.70 to 72.13).
pm PEF Hampel 2000 reported that HFA‐BDP at both daily doses led to a significant improvement compared to placebo; HFA‐BDP 100 mcg/d 22.7 L/min SD 41.54 L/min versus placebo 6.3 L/min, SD 49.36 L/min; HFA‐BDP 200 mcg/d 22.1 L/min SD 48.06 L/min versus placebo 6.3 L/min, SD 49.36 L/min
(d) FEF25‐75% Hampel 2000 reported a significant improvement in FEF25 to 75% from baseline for active treatment groups (HFA 100 or 200 mcg/d) compared to placebo, HFA‐BDP 100mcg/d 22.7% SD 41.54% versus placebo 6.3% SD 49.36%; HFA‐BDP 200 22.1% SD 48.06% versus placebo 6.3% SD 49.36%). (2) RESCUE BETA‐2 AGONIST USE
(a) Change in rescue‐beta2 agonist use There was a high level of heterogeneity when the studies were pooled (comparison 12: I square: 86.6%; comparison 13: I square: 76.7%). Data pooled with a Fixed Effect model from the three studies indicated that there was a significant reduction in rescue medication by ‐0.64 puffs/day (95% CI ‐0.87 to ‐0.41, comparison 12) or ‐0.81 puffs/day (95% CI ‐1.01 to ‐0.61, comparison 13). With Random Effects modelling the difference remained significant in both instances, but the confidence intervals widened considerably (comparison 12: ‐0.78 puffs/day (‐1.43 to ‐0.13); comparison 13: ‐0.88 puffs/day (‐1.34 to ‐0.41)). In the outcome data assessed under comparison 12, the pooled subgroup effect estimates did not overlap when a Fixed Effect model was applied, which suggested that there may have been a different response in adults in children. However, there was an overlap in comparison 13.
(3) ASTHMA SYMPTOM SCORE
(a) Change in symptoms Asthma symptoms recorded using a daily diary card were reported in two studies (Hampel 2000; Nayak 2002), although we were unable to pool data due to different measurement of outcome. Hampel 2000 adopted a six point scoring system from zero (no symptoms present) to 5 (symptoms prevented normal activities). Nayak 2002 used a four point system, 0=none and 3= severe symptoms. Symptoms were assessed across four domains: Wheezing, cough, shortness of breath and chest tightness. HFA‐BDP for all doses was better than placebo in controlling asthma symptoms. Each individual study reported significant improvements in most domains for both lower and higher dose range comparisons with placebo.
(4) ADVERSE EVENTS Various adverse events were reported. Individual studies reported no significant differences in the numbers of participants reporting side effects between HFA‐BDP treatment groups and placebo for all doses.
Discussion
This review compared the efficacy and safety of HFA‐BDP and CFC‐BDP with placebo in patients with persistent asthma. 60 randomised trials recruiting 6542 adults and children with asthma were included, and the overall methodological quality of studies was high. 57 trials compared CFC‐BDP at varied doses with placebo, and three studies assessed the efficacy of HFA‐BDP compared with placebo. The studies varied in terms of BDP dose, treatment duration, delivery device and outcome measures reported. Asthmatics not treated with oral steroids The majority of studies contributing to the meta‐analysis were in adult participants. CFC‐BDP resulted in statistically significant improvements in FEV1 compared to placebo. The observed differences may be of clinical significance, although the level of clinically significant change for these measures has never been formally established in asthma.
CFC‐BDP treatment led to significantly greater morning PEFR compared to placebo of 46.52 L/min (95% CI 15.1 to 108.21). No active treatment advantage was apparent for evening PEFR or diurnal variability in PEFR. Significantly less rescue beta2 agonist was required in BDP treated patients: 1.98 puffs/d (95% CI 1.62 to 2.34 puffs/day). A wide variety of scales were used to assess symptoms, and the quality of reporting was poor with a substantial number of trials failing to provide standard deviation values for mean treatment effects. The data for daily asthma symptoms, where available, suggest that BDP results in significantly less symptoms. However, because no validated symptom scores were used (with clinically worthwhile thresholds established) and treatment effects had to be expressed using a unitless standardised mean difference, the treatment differences between BDP and placebo are difficult to translate into a clinically meaningful measures.
Methacholine and histamine BHR, which are surrogate markers for the degree of airway inflammation, were significantly improved in BDP treated participants compared to placebo.
Important outcome measures such as hospital admission rates, GP attendance rates and days lost from school/work due to asthma exacerbations were not reported. Withdrawal rates in the context of a clinical trial cannot be considered equivalent to hospital admission rates or GP attendance rates due to exacerbation but may be a surrogate marker for these. The likelihood of withdrawal from study due to asthma exacerbation was significantly less in BDP treated participants compared to placebo; RR 0. 25(95% CI 0.16 to 0.39). However, it is not appropriate to calculate numbers needed to treat as studies contributing to the analysis had widely varying treatment periods and criteria for withdrawal.
Subgroup analyses based on treatment duration raise the possibility that this is an important determinant of overall treatment response. When studies reporting FEV1 and trial withdrawal due to exacerbation were pooled according to treatment period of four weeks or treatment period greater than four weeks, a significant effect in favour of BDP was only seen for studies of longer duration.
No differences in the incidence of local side effects in the form of sore throat, hoarseness or oral candidiasis were apparent. The effects on HPA function were rarely reported. No two studies reported the same outcome measure and conflicting results were found. The studies assessing HPA function by insulin and synthetic ACTH stress testing did not demonstrate a significant difference in response between BDP and placebo treated participants. Too few studies reported such outcomes to draw meaningful conclusions.
Evidence in support of a dose response effect was not apparent for any efficacy outcome. However, caution needs to be exercised in interpreting this finding for two reasons. Firstly, dose response effects are best assessed in the context of individual trials in which participants have been randomised to different doses of active treatment, with comparisons made between treatments. Secondly, it was only possible to include the results of two studies judged to have recruited participants with moderate to severe asthma in the meta‐analysis (Radha 1975; Vogt 1976). A clinically meaningful dose response effect is most likely to be seen in severe asthmatics who will have the greatest margin for improvement in outcomes reflecting the degree of asthma control. This group is under‐represented in this analysis. In the absence of trials, it is not possible to predict whether or not a dose‐response effect will be seen in more severe disease. Although patients with severe asthma have greater room for improvement, it should also be appreciated that even in mild asthma, BDP only ameliorates and does not abolish asthma effects. Another unknown variable is the level of steroid responsiveness. Inadequate control of symptoms through existing therapy is one marker used to define asthma severity. Thus patients with 'severe' asthma have, by and large, failed to respond adequately to inhaled steroids. Hence an alternative possibility is that 'severe' patients may be selected by their lack steroid responsiveness, so may not show a significant dose‐response effect. These possibilities need to be explored by future studies. It is noteworthy that there were differing levels of heterogeneity when data from CFC and HFA‐BDP treatment arms in Gross 1999 were pooled in their respective analyses. It would be inaccurate to conclude that at a dose ratio of 1:2, CFC and HFA‐BDP are equivalent for three reasons. Firstly, such an interpretation would be based on a relatively weak comparison between heterogeneity statistics. Secondly, we only have data that suggest this on change in am PEF, but not on change in rescue medication use as heterogeneity was high in both sets of analyses. Lastly this interpretation assumes that the oral steroid pre‐treatment phase in Gross 1999 produced a similar baseline population to those in the other trials. Findings from two large randomised studies indicate that on certain outcomes such as beta‐agonist use, HFA‐BDP is equivalent to CFC‐BDP at half the dose (Gross 1999; Busse 1999). Whilst this review has not examined head‐to‐head comparisons between doses of BDP, the different levels of heterogeneity in the CFC and HFA‐BDP analyses may be explained in part by a steep dose response curve to HFA‐BDP on certain clinical outcomes. The predosing schedule could also have contributed to the different responses to treatment from this study. Systematic assessments of the equivalence between doses of CFC and HFA‐BDP in randomised comparisons, and the 'optimisation' of oral steroid treatment prior to randomisation are warranted before more comment can be made on these results.
Both CFC‐BDP and HFA‐BDP were well tolerated and there were no drug or propellant related safety concerns. Oral steroid treated asthmatics BDP treatment resulted in a clinically significant oral steroid sparing effect compared to placebo. The end of trial mean daily prednisolone dose was 6 mg/d (95% CI 4 to 8 mg/d) lower in BDP treated participants compared to placebo. Reductions in daily dose of 5 mg/d (95% CI 4 to 6 mg/d) in BDP treated participants were achieved. The likelihood of discontinuing oral steroids was significantly greater for BDP treated participants compared to placebo RR 0.40 (95% CI 0.23 to 0.70). It was not appropriate to calculate numbers needed to treat as trials were of varying duration.
The effects of BDP on measures of airway calibre (FEV1, PEFR), symptoms and local side effects in OCS treated asthmatics were assessed in one trial only. Three studies used different scales reported such outcomes; and conflicting results were found (non‐significant differences or small effects in favour of BDP therapy). No firm conclusions concerning the effects of treatment can be drawn for these outcomes.
It should be noted that only studies conducted in adult patients contributed to these analyses; generalisation to children with oral steroid dependent asthma therefore needs to be made with caution.
Methodological Limitations (1) The possibility of bias related to missed published trials occurs in all systematic reviews. The initial search identified studies conducted over the course of the last three decades (1973 to 1998). Given the wide time period, volume of literature and the fact that eight foreign language publications are still awaiting translation in order to assess their eligibility raises the possibility that studies were missed despite a comprehensive search strategy. This risk is somewhat offset by the fact that only one further study was identified from correspondence with authors and that GSK, sponsors of a large proportion of BDP related studies did not identify any further trials from their own records.
(2) Bias related to missed unpublished 'negative' trials is a further possibility. However, a funnel plot of withdrawal due to asthma exacerbation in the non‐OCS treated parallel group studies showed spread of results to both sides of the overall relative risk. This suggests that there was no major bias of this type.
(3) A large number of efficacy outcomes were reported, using a range of different scales. This factor, combined with a significant proportion of studies in which numerical data were either not presented with standard deviations around mean scores or in which authors were unable or unwilling to provide such data, reduced the power of the analysis.
(4) The majority of studies were conducted in a secondary care setting, with patients attending hospital outpatient clinics for review. In most instances it was not clear how patients were identified as potential recruits (e.g. patients already attending hospital asthma clinics, primary care referrals, advertisement etc). Only one study was carried out in a primary care. Study setting may therefore have biased recruitment towards patients with more symptoms, since they have been largely conducted in secondary care. (5) The identification of three ranges for nominal daily BDP dose are arbitrary. However the range boundaries were felt to generally reflect current dose distinctions made in clinical practice.
Authors' conclusions
Implications for practice.
This review strongly supports the use of inhaled BDP in the treatment of chronic asthma for at least 4 weeks duration. CFC‐BDP and HFA‐BDP improve airflow limitation, reduce symptoms and the need for rescue bronchodilators and reduce the likelihood of exacerbations when compared to placebo. These findings apply to children and adults, with all degrees of disease severity.
In oral steroid treated patients, inhaled CFC‐BDP had a clinically significant oral steroid sparing effect. Although oral steroids are no longer a first line therapy for asthma in developed countries it is likely that in some areas of the world a substantial proportion of asthmatics are receiving oral steroids who could be maintained on inhaled BDP. This review supports a recommendation that all such patients should receive a trial of inhaled BDP for asthma control, for even in patients in whom oral steroids cannot be stopped entirely, worthwhile reductions in dose are possible.
Implications for research.
(1) Consistent methods of reporting symptoms aided by the development of a validated questionnaire (with established thresholds of clinical importance) would greatly assist in the interpretation of symptom changes in response to therapy.
(2) Measurement of economically important outcomes such as hospital admission rates, primary care attendance and days missed from work/school due to asthma exacerbations are needed in any future studies.
(3) Improved reporting of primary study methods (particularly details of how patients are allocated to treatment groups) in line with CONSORT 1996 recommendations would improve the accuracy of systematic reviews.
(4) Assessment of any potential clinically relevant dose response effect for BDP in the treatment of asthma will be best achieved by a further systematic review assessing trials in which patients have been randomised to low and high dose BDP within the same trial. It is hoped that this will include trials recruiting subjects with severe asthma as well as those with mild to moderate disease.
(5) Establish the minimum duration of treatment required for a clinically meaningful treatment response for BDP.
(6) Head‐to‐head comparisons of low‐moderate doses of HFA‐BDP would also help to establish the validity of pooling doses of less than 400mcg/day.
What's new
| Date | Event | Description |
|---|---|---|
| 21 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 22 October 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Anna Bara for running the initial electronic search, Steve Milan for assistance with statistical methods, and Professor Paul Jones for editorial support. We also like to acknowledge the authors who kindly provided additional information and/or data including Dr E.H. Bel, Dr D. Bennati, Professor K.C. Bergmann, Dr I. Broder, Professor A.L. Boner, Dr K.N. Chan, Dr P. Chervinsky, Prof C. Collins‐Williams, Dr N. De Marzo, Professor S. Godfrey, Dr F.E. Hargreave, Professor M. Hodson, Dr K.F. Kerrebijn, Dr R. Kraemer, Dr H.A.M. Kerstjens, Dr M. Laviolette, Dr C.A. Lenney, Dr P. Maestrelli, Dr J.L. Malo, Dr P. Martin, Professor T.V. O'Donnell, Dr R.A. Nathan, Dr H.J. Pennings, Dr M.M. Pizzichini, Professor S.C. Siegel, Professor E. Simons, Professor Dame M. Turner‐Warwick and Dr M.S. Webb. We would like to thank Julia Earnshaw, Veronica Prentice and Karen Richardson (GSK/Allen and Hanbury's) who assisted us by searching company records for any potentially missed trials. Our thanks go to the NHS Research and Development programme for financial support for this project. Finally, our thanks go to Dr Chris Cates for additional editorial support and Kirsty Olsen who copy edited this review. A grant from the Nederlands Astma Fonds to fund the Cochrane Airways Group Respiratory Systematic Review Research Fellow enabled Dr Reem Malouf to work on the update of this review.
Data and analyses
Comparison 1. CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) and FEV1 (% predicted) combined | 4 | 257 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.38 [0.14, 0.63] |
| 1.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 3 | 229 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.41 [0.14, 0.67] |
| 1.3 Children and Adults | 1 | 28 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.54, 0.95] |
| 2 Change in FEV1(L/min) | 2 | 370 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [0.26, 0.46] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 1 | 256 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.26, 0.54] |
| 2.3 Children and Adults | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [0.18, 0.46] |
| 3 Change in FEV1 (%) | 2 | 756 | Mean Difference (IV, Fixed, 95% CI) | 12.41 [8.18, 16.64] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 2 | 756 | Mean Difference (IV, Fixed, 95% CI) | 12.41 [8.18, 16.64] |
| 3.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Children and Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Children and Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in morning PEF (L/min) | 3 | 878 | Mean Difference (IV, Fixed, 95% CI) | 35.95 [27.85, 44.04] |
| 6.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 2 | 764 | Mean Difference (IV, Fixed, 95% CI) | 35.09 [26.29, 43.88] |
| 6.3 Children and Adults | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | 40.7 [20.05, 61.35] |
| 7 Morning PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Change in evening PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 FEF 25 to 75 (L/second) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Change in FEF 25‐75% (L/second) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Children and Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Weekly asthma symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Methacholine bronchial responsiveness (log10 PC20 FEV1) | 3 | 51 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.04, 1.10] |
| 12.1 Children | 2 | 35 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.64 [‐0.05, 1.33] |
| 12.2 Adults | 1 | 16 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.69, 1.29] |
| 12.3 Children and Adults | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Daily use of beta‐2 agonist (puffs/day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Change in daily use of beta‐2 agonist (puffs/day) | 2 | 622 | Mean Difference (IV, Fixed, 95% CI) | ‐2.32 [‐2.55, ‐2.09] |
| 14.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Adults | 1 | 508 | Mean Difference (IV, Fixed, 95% CI) | ‐2.32 [‐2.55, ‐2.09] |
| 14.3 Children and adults | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐2.36 [‐3.41, ‐1.31] |
| 15 Nights without sleep disturbance due to asthma symptoms (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Change in nocturnal awakenings (nights/wk) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Change in daily asthma symptoms score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Change in cough score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 Children and Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Change in wheezing score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.3 Children and Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Change in breathing difficulty score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Children and Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 Change in eosinophil count (cells x 103/micL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 21.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 Total withdrawals | 2 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.30, 0.68] |
| 22.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 Adults | 2 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.30, 0.68] |
| 22.3 Children and Adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Withdrawals due to adverse effects | 2 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.19, 1.06] |
| 23.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 Adults | 1 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.16, 1.32] |
| 23.3 Children and Adults | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 1.98] |
| 24 Withdrawals due to asthma exacerbations | 9 | 928 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.18, 0.48] |
| 24.1 Children | 3 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.71] |
| 24.2 Adults | 4 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.15, 1.11] |
| 24.3 Children and Adults | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.12, 0.51] |
| 25 Numbe of participants with asthma attacks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 25.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26 Worsening asthma | 2 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.38, 0.63] |
| 26.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.2 Adults | 2 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.38, 0.63] |
| 26.3 Children and Adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Influenza | 2 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.77, 2.77] |
| 27.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27.2 Adults | 2 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.77, 2.77] |
| 27.3 Children and Adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Oral candidiasis | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.75 [0.62, 22.57] |
| 28.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.2 Adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.3 Children and Adults | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.75 [0.62, 22.57] |
| 29 Pharyngitis | 3 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.48, 3.37] |
| 29.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.2 Adults | 1 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.46, 8.07] |
| 29.3 Children and Adults | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.20, 3.16] |
| 30 Dysphonia | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.23, 12.80] |
| 30.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 Adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 Children and Adults | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.23, 12.80] |
| 31 Dyspepsia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 31.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 31.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 31.3 Children and Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 32 Cough | 2 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.22] |
| 32.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 32.2 Adults | 1 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.10, 1.62] |
| 32.3 Children and Adults | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.27] |
| 34 Fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 34.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 34.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 34.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 35 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 35.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 35.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 35.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 36 Bronchitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 36.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 36.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 36.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 37 Sinustis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 37.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 37.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 37.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 38 Rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 38.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 38.2 Adults | 1 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.05, 1.15] |
| 38.3 Children and Adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 40 Oropharyngeal side effects | 4 | 997 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.81, 2.56] |
| 40.1 Children | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 40.2 Adults | 4 | 997 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.81, 2.56] |
| 40.3 Children and Adults | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 41 Headache | 4 | 1015 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.91, 1.78] |
| 41.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 41.2 Adults | 2 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.92, 1.83] |
| 41.3 Children and Adults | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.30, 3.48] |
| 42 Upper respiratory tract infection side effects | 2 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.81, 1.49] |
| 42.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 42.2 Adults | 2 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.81, 1.49] |
| 42.3 Children and Adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 43 Alanine aminotransferase (ALT) > normal upper limit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 43.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 43.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 43.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 44 Alanine aminotransferase (ALT) > 3 times of upper limit of normal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 44.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 44.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 44.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 45 Asparate amibotransferase (AST) >upper normal limit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 45.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 45.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 45.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 46 Aspartate aminotransferase (AST) > 3 times upper normal limit | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 46.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 46.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 46.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
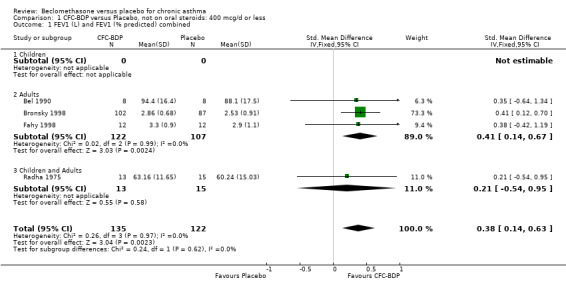
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 1 FEV1 (L) and FEV1 (% predicted) combined.
1.2. Analysis.
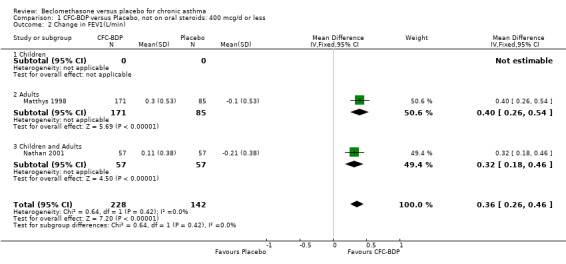
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 2 Change in FEV1(L/min).
1.3. Analysis.
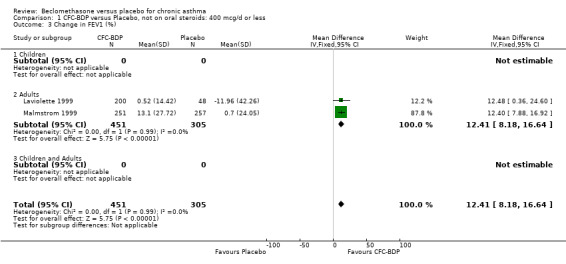
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 3 Change in FEV1 (%).
1.4. Analysis.
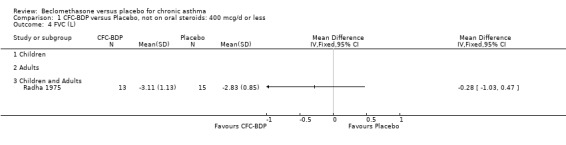
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 4 FVC (L).
1.5. Analysis.
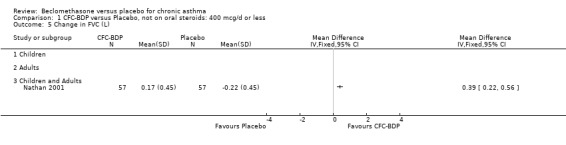
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 5 Change in FVC (L).
1.6. Analysis.
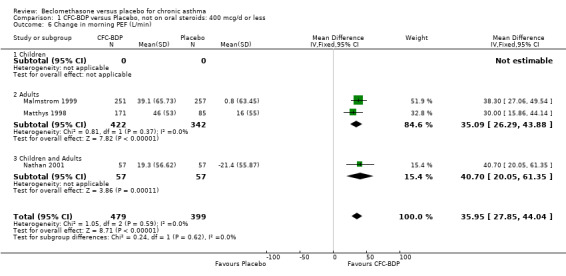
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 6 Change in morning PEF (L/min).
1.7. Analysis.
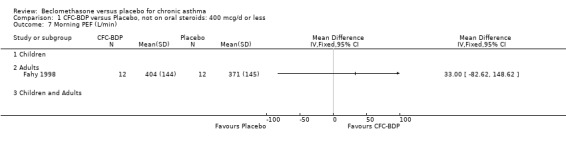
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 7 Morning PEF (L/min).
1.8. Analysis.
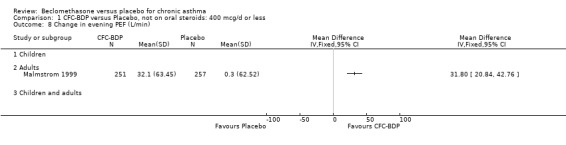
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 8 Change in evening PEF (L/min).
1.9. Analysis.
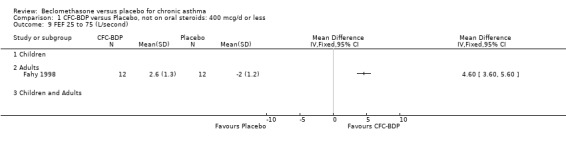
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 9 FEF 25 to 75 (L/second).
1.10. Analysis.
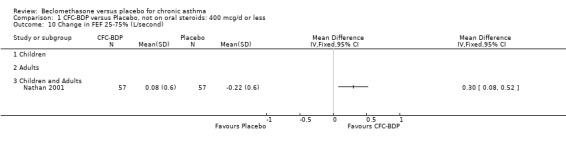
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 10 Change in FEF 25‐75% (L/second).
1.11. Analysis.
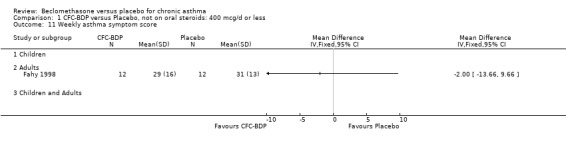
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 11 Weekly asthma symptom score.
1.12. Analysis.
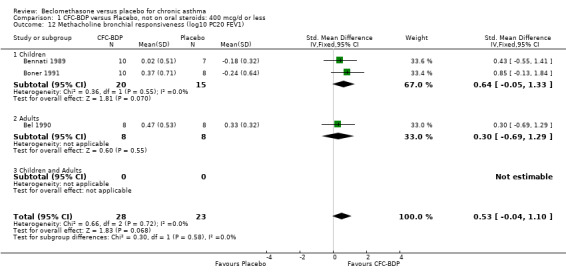
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 12 Methacholine bronchial responsiveness (log10 PC20 FEV1).
1.13. Analysis.
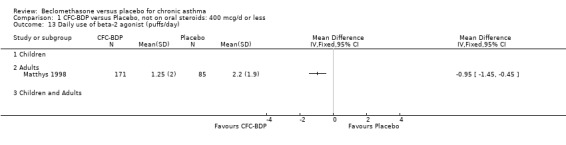
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 13 Daily use of beta‐2 agonist (puffs/day).
1.14. Analysis.
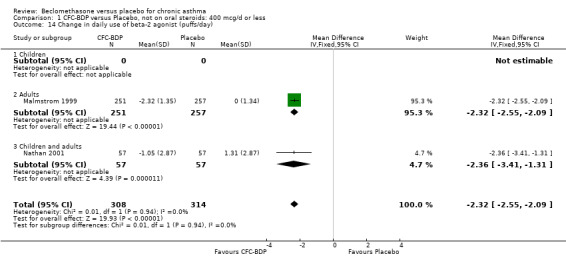
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 14 Change in daily use of beta‐2 agonist (puffs/day).
1.15. Analysis.
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 15 Nights without sleep disturbance due to asthma symptoms (%).
1.16. Analysis.
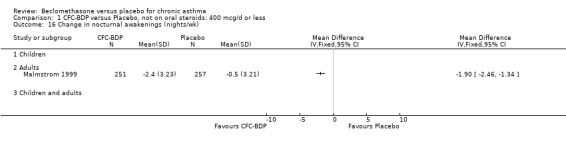
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 16 Change in nocturnal awakenings (nights/wk).
1.17. Analysis.
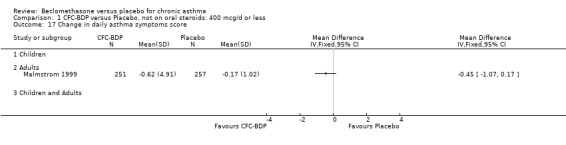
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 17 Change in daily asthma symptoms score.
1.18. Analysis.
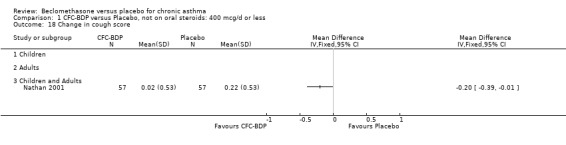
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 18 Change in cough score.
1.19. Analysis.
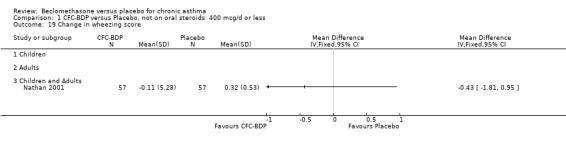
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 19 Change in wheezing score.
1.20. Analysis.
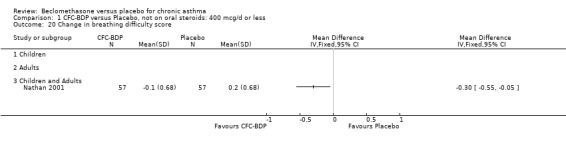
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 20 Change in breathing difficulty score.
1.21. Analysis.
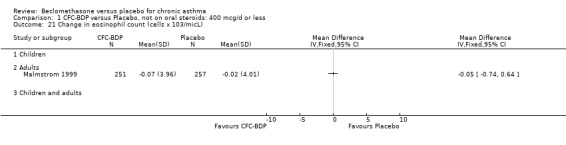
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 21 Change in eosinophil count (cells x 103/micL).
1.22. Analysis.
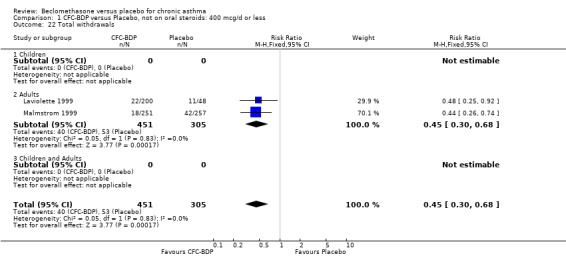
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 22 Total withdrawals.
1.23. Analysis.
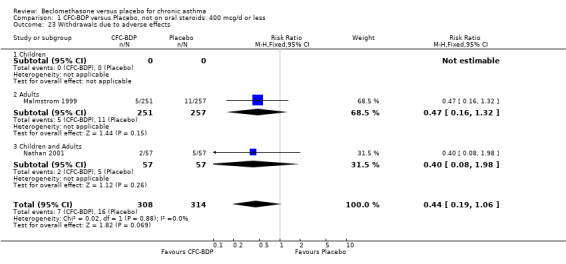
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 23 Withdrawals due to adverse effects.
1.24. Analysis.
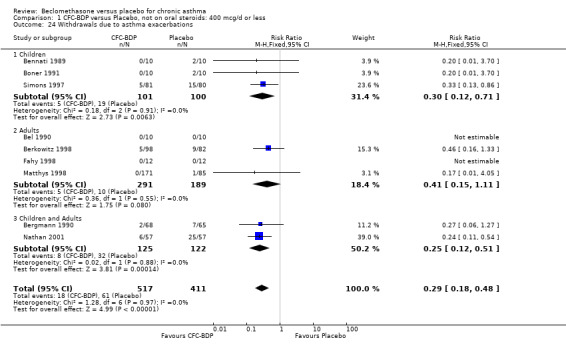
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 24 Withdrawals due to asthma exacerbations.
1.25. Analysis.
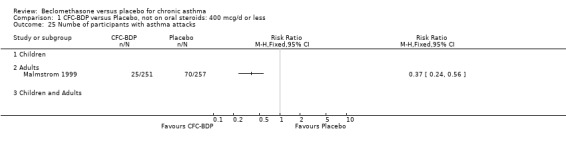
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 25 Numbe of participants with asthma attacks.
1.26. Analysis.
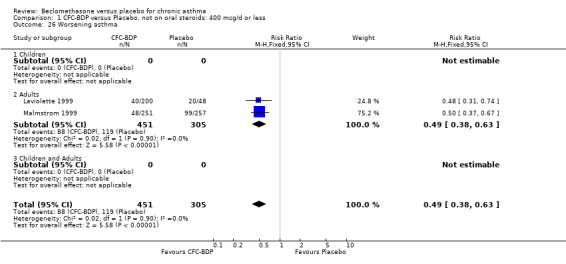
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 26 Worsening asthma.
1.27. Analysis.
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 27 Influenza.
1.28. Analysis.
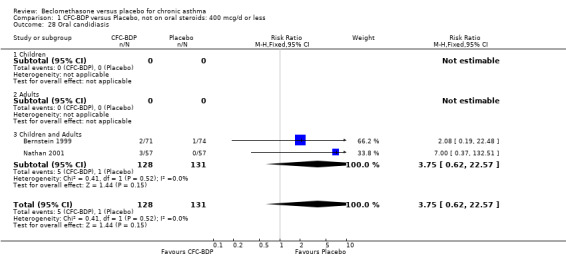
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 28 Oral candidiasis.
1.29. Analysis.
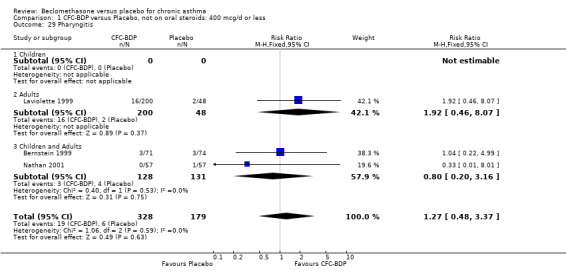
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 29 Pharyngitis.
1.30. Analysis.
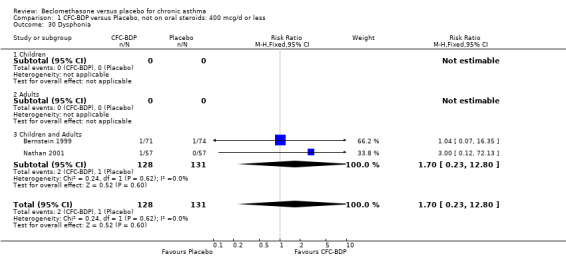
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 30 Dysphonia.
1.31. Analysis.
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 31 Dyspepsia.
1.32. Analysis.
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 32 Cough.
1.34. Analysis.
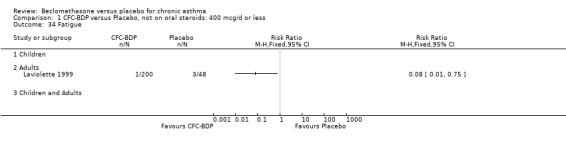
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 34 Fatigue.
1.35. Analysis.
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 35 Nausea.
1.36. Analysis.
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 36 Bronchitis.
1.37. Analysis.
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 37 Sinustis.
1.38. Analysis.
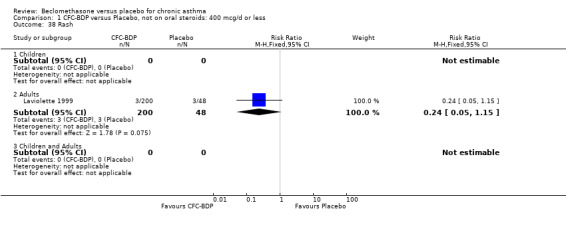
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 38 Rash.
1.40. Analysis.
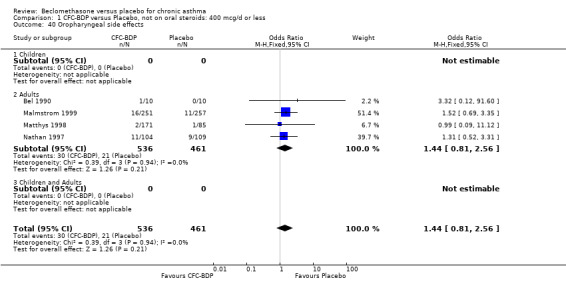
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 40 Oropharyngeal side effects.
1.41. Analysis.
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 41 Headache.
1.42. Analysis.
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 42 Upper respiratory tract infection side effects.
1.43. Analysis.
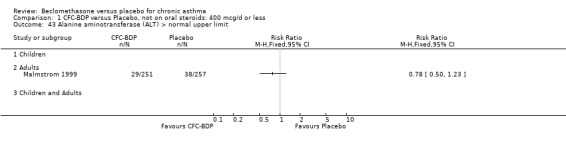
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 43 Alanine aminotransferase (ALT) > normal upper limit.
1.44. Analysis.
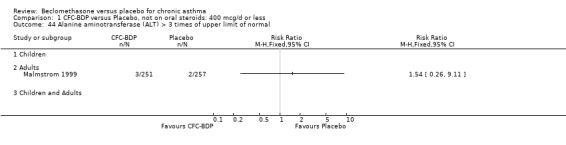
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 44 Alanine aminotransferase (ALT) > 3 times of upper limit of normal.
1.45. Analysis.
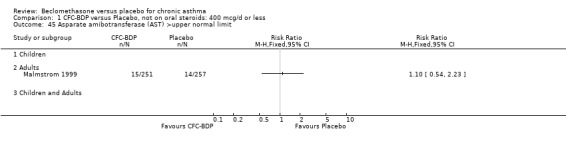
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 45 Asparate amibotransferase (AST) >upper normal limit.
1.46. Analysis.
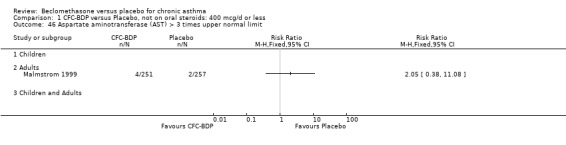
Comparison 1 CFC‐BDP versus Placebo, not on oral steroids: 400 mcg/d or less, Outcome 46 Aspartate aminotransferase (AST) > 3 times upper normal limit.
Comparison 2. CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) and FEV1 (% Predicted) combined | 4 | 411 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.49 [0.30, 0.69] |
| 1.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 3 | 325 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.53 [0.31, 0.76] |
| 1.3 Children and Adults | 1 | 86 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.08, 0.77] |
| 2 FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Children and Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FEF 25 to 75% (L/second) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Children and Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Morning PEFR (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in morning PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Evening PEFR (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Daily PEFR (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Diurnal variation in PEFR (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Histamine bronchial responsiveness (log10 PC20 FEV1) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Methacholine bronchial responsiveness (Dmin) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Daily use of beta2 agonists (puffs/day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Change in daily use of beta‐2 agonists (puffs/day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Daily asthma symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Change in daily asthma symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Nights without disturbance(%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Withdrawal due to worsening asthma symptoms | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1 Children | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Adults | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Children and Adults | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Withdrawal due to asthma exacerbation (No. of patients) | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.30] |
| 17.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Adults | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.30] |
| 17.3 Children and Adults | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.05] |
| 18 At least one side effect reported | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Number of participants with normal plasma cortisol levels | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.3 Adults and children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Dysphonia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 Cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 Adverse inhalation feel/pharyngitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 Adverse inhalation taste | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 23.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24 Laryngitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 Oropharyngeal side‐effects (No. of patients) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 25.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.3 Children and Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
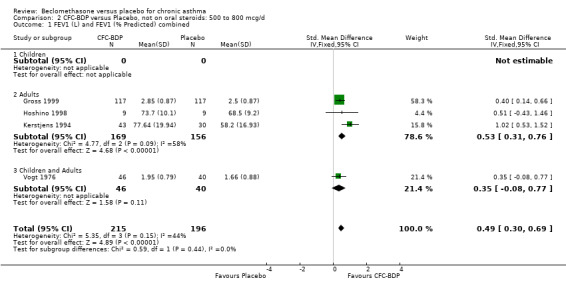
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 1 FEV1 (L) and FEV1 (% Predicted) combined.
2.2. Analysis.
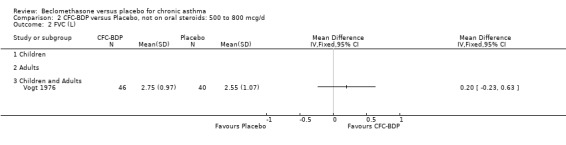
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 2 FVC (L).
2.3. Analysis.
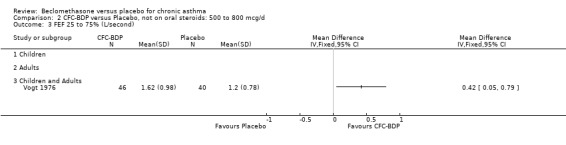
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 3 FEF 25 to 75% (L/second).
2.4. Analysis.
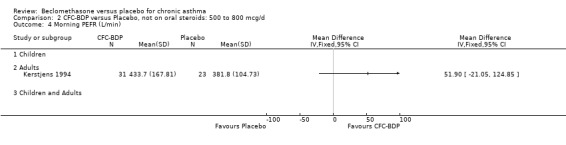
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 4 Morning PEFR (L/min).
2.5. Analysis.
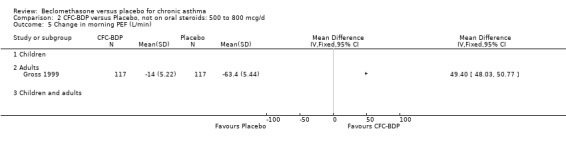
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 5 Change in morning PEF (L/min).
2.6. Analysis.
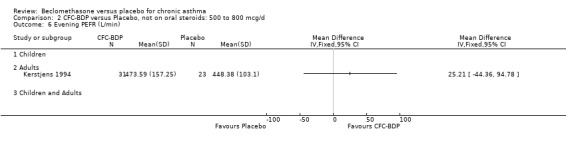
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 6 Evening PEFR (L/min).
2.7. Analysis.
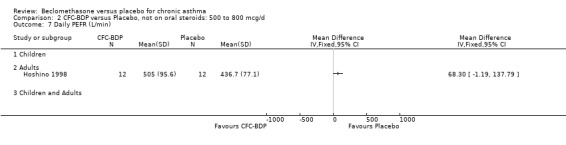
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 7 Daily PEFR (L/min).
2.8. Analysis.
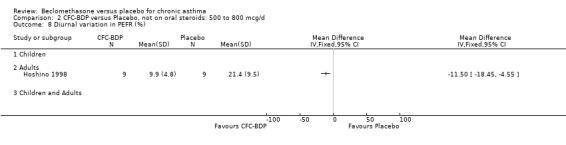
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 8 Diurnal variation in PEFR (%).
2.9. Analysis.
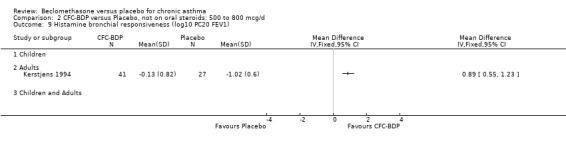
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 9 Histamine bronchial responsiveness (log10 PC20 FEV1).
2.10. Analysis.
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 10 Methacholine bronchial responsiveness (Dmin).
2.11. Analysis.
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 11 Daily use of beta2 agonists (puffs/day).
2.12. Analysis.
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 12 Change in daily use of beta‐2 agonists (puffs/day).
2.13. Analysis.
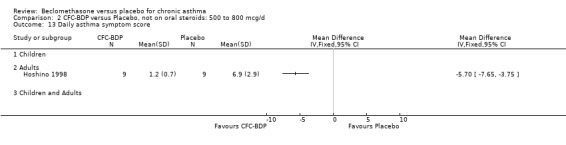
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 13 Daily asthma symptom score.
2.14. Analysis.
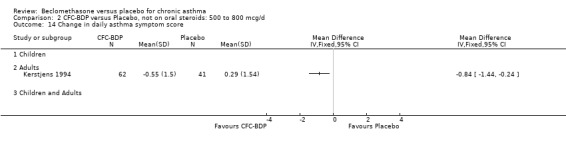
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 14 Change in daily asthma symptom score.
2.15. Analysis.
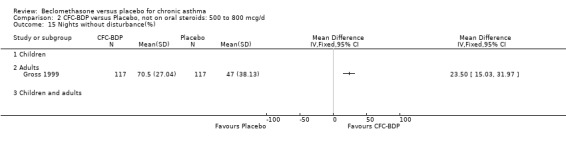
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 15 Nights without disturbance(%).
2.16. Analysis.
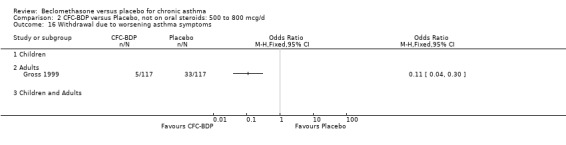
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 16 Withdrawal due to worsening asthma symptoms.
2.17. Analysis.
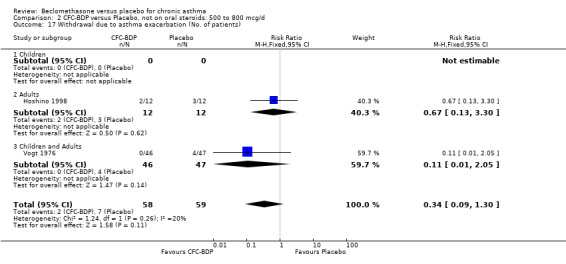
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 17 Withdrawal due to asthma exacerbation (No. of patients).
2.18. Analysis.
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 18 At least one side effect reported.
2.19. Analysis.
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 19 Number of participants with normal plasma cortisol levels.
2.20. Analysis.
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 20 Dysphonia.
2.21. Analysis.
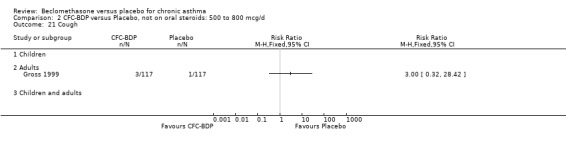
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 21 Cough.
2.22. Analysis.
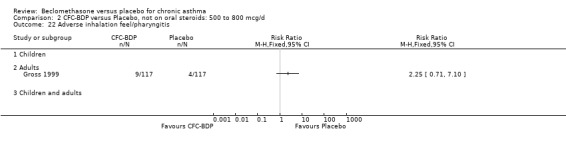
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 22 Adverse inhalation feel/pharyngitis.
2.23. Analysis.
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 23 Adverse inhalation taste.
2.24. Analysis.
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 24 Laryngitis.
2.25. Analysis.
Comparison 2 CFC‐BDP versus Placebo, not on oral steroids: 500 to 800 mcg/d, Outcome 25 Oropharyngeal side‐effects (No. of patients).
Comparison 3. CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) and FEV1 (% predicted) combined | 5 | 142 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.01, 0.68] |
| 1.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 5 | 142 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.01, 0.68] |
| 1.3 Children and Adults | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FVC (% predicted) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Children and Adults | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FEV1/FVC ratio (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in FEV1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Clinic PEFR (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 PEF (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Daily use of beta‐2 agonists (puffs/day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Daily asthma symptom score | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Children and Adults | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Methacholine bronchial responsiveness (log values) | 2 | 38 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.12, 1.18] |
| 9.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 2 | 38 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.12, 1.18] |
| 9.3 Children and Adults | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 plasma cortisol (mmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Plasma cortisol (mmol/L) 120 min after 0.1IU/kg body weight insulin | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Serum eosinophil count (x10 6/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Serum eosinophil catonic protein (ECP) (mcg/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 FEV1 (L) following inhaled cold air challenge | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Inspiratory Vital Capacity (L) following inhaled cold air challenge | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Withdrawal due to asthma exacerbation | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Adults | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Children and Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Oropharyngeal side‐effects | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.88, 3.35] |
| 17.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Adults | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.88, 3.35] |
| 17.3 Children and Adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Oropharyngeal Candidiasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
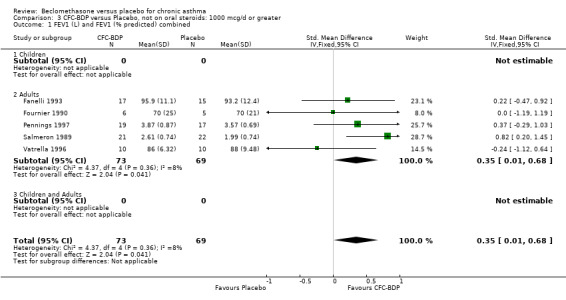
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 1 FEV1 (L) and FEV1 (% predicted) combined.
3.2. Analysis.
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 2 FVC (% predicted).
3.3. Analysis.
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 3 FEV1/FVC ratio (%).
3.4. Analysis.
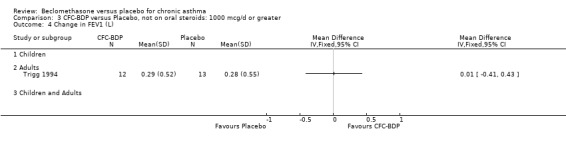
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 4 Change in FEV1 (L).
3.5. Analysis.
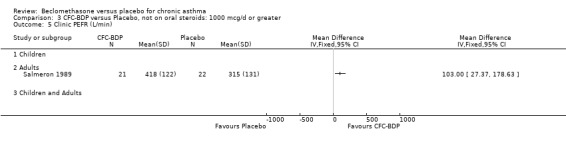
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 5 Clinic PEFR (L/min).
3.6. Analysis.
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 6 PEF (% predicted).
3.7. Analysis.
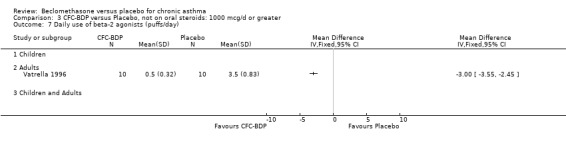
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 7 Daily use of beta‐2 agonists (puffs/day).
3.8. Analysis.
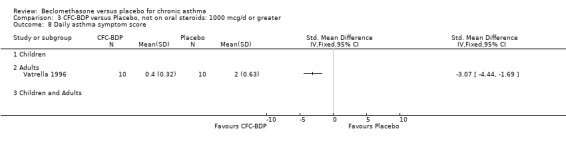
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 8 Daily asthma symptom score.
3.9. Analysis.
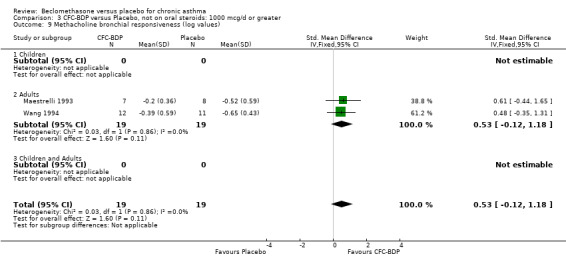
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 9 Methacholine bronchial responsiveness (log values).
3.10. Analysis.
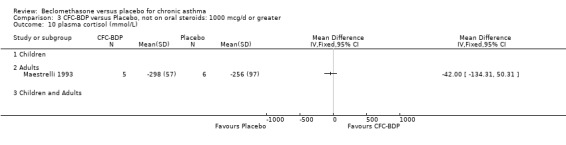
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 10 plasma cortisol (mmol/L).
3.11. Analysis.
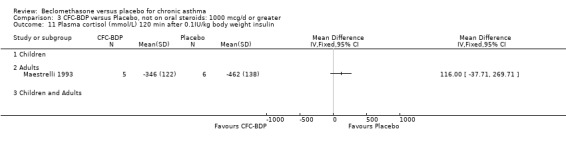
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 11 Plasma cortisol (mmol/L) 120 min after 0.1IU/kg body weight insulin.
3.12. Analysis.
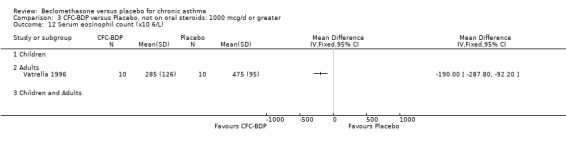
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 12 Serum eosinophil count (x10 6/L).
3.13. Analysis.
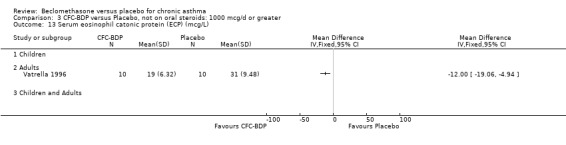
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 13 Serum eosinophil catonic protein (ECP) (mcg/L).
3.14. Analysis.
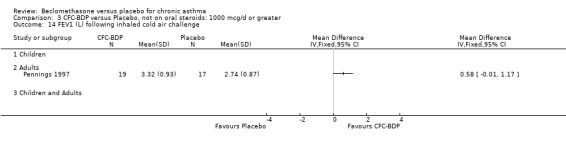
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 14 FEV1 (L) following inhaled cold air challenge.
3.15. Analysis.
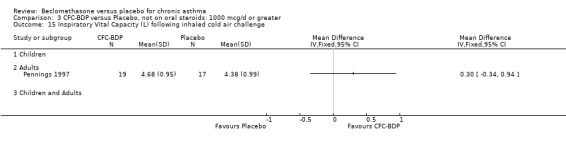
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 15 Inspiratory Vital Capacity (L) following inhaled cold air challenge.
3.16. Analysis.
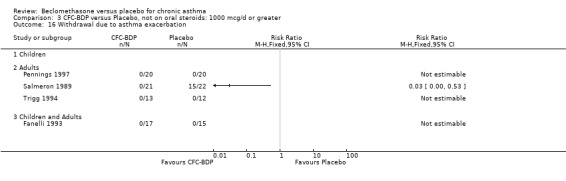
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 16 Withdrawal due to asthma exacerbation.
3.17. Analysis.
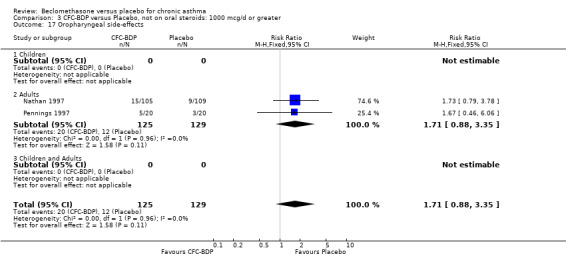
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 17 Oropharyngeal side‐effects.
3.18. Analysis.
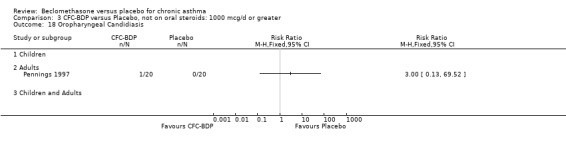
Comparison 3 CFC‐BDP versus Placebo, not on oral steroids: 1000 mcg/d or greater, Outcome 18 Oropharyngeal Candidiasis.
Comparison 4. CFC‐BDP versus Placebo, not on oral steroids: all doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 6 | 612 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [0.22, 0.49] |
| 1.4 400 mcg/day or less | 2 | 213 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.11, 0.56] |
| 1.5 500 to 800 mcg/day | 2 | 320 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.14, 0.52] |
| 1.6 1000 mcg/day or greater | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.15, 0.82] |
| 2 FEV1 (% predicted) | 7 | 198 | Mean Difference (IV, Random, 95% CI) | 5.36 [‐0.88, 11.60] |
| 2.4 400 mcg/day or less | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐4.70, 12.31] |
| 2.5 500 to 800 mcg/day | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 12.39 [‐1.57, 26.34] |
| 2.6 1000 mcg/day or greater | 3 | 63 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐5.25, 5.25] |
| 3 Change in FEV1 (L) | 2 | 370 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [0.26, 0.46] |
| 3.1 BDP 400 mcg/day or less | 2 | 370 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [0.26, 0.46] |
| 3.2 BDP 500 to 800 mcg/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 BDP 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Morning PEFR (L/min) | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | 46.52 [‐15.18, 108.21] |
| 5.1 400 mcg/day or less | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 33.0 [‐82.62, 148.62] |
| 5.3 500 to 800 mcg/day | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 51.90 [‐21.05, 124.85] |
| 5.4 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Change in morning PEF (L/min) | 4 | 1112 | Mean Difference (IV, Fixed, 95% CI) | 39.05 [31.95, 46.15] |
| 6.1 400 mcg/day or less | 3 | 878 | Mean Difference (IV, Fixed, 95% CI) | 35.95 [27.85, 44.04] |
| 6.2 500‐800mcg/day or less | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | 49.4 [34.62, 64.18] |
| 6.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 FVC (L) | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.16, 0.60] |
| 7.1 BDP 400 mcg/day or less | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.47, 1.03] |
| 7.2 BDP 500 to 800 mcg/day | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.23, 0.63] |
| 7.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Methacholine bronchial responsiveness (log 10 values) | 5 | 89 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.53 [0.10, 0.96] |
| 9.3 400 mcg/day or less | 3 | 51 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.04, 1.10] |
| 9.4 500 to 800 mcg/day | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 1000 mg/day or greater | 2 | 38 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.12, 1.18] |
| 10 FEF25‐75% (L/Second) | 2 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.93 [0.58, 1.28] |
| 10.1 400 mcg/day or less | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 4.6 [3.60, 5.60] |
| 10.2 500‐800 mcg/day | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [0.05, 0.79] |
| 10.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Daily asthma symptom score | 2 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.81 [‐3.77, ‐1.86] |
| 12.1 400 mcg/day or less | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 500 to 800 mcg/day | 1 | 18 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐3.90, ‐1.25] |
| 12.4 1000 mcg/day or greater | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.07 [‐4.44, ‐1.69] |
| 13 Daily asthma symptoms score (change from baseline) | 2 | 611 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.35, ‐0.03] |
| 13.1 400 mcg/day or less | 1 | 508 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.30, 0.05] |
| 13.2 500‐800 mcg/day | 1 | 103 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐0.95, ‐0.15] |
| 13.3 1000 mcg/day or greater | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Daily use of beta2 agonists (puffs/day) | 3 | 294 | Mean Difference (IV, Fixed, 95% CI) | ‐1.98 [‐2.34, ‐1.62] |
| 14.1 400 mcg/day or less | 1 | 256 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.45, ‐0.45] |
| 14.3 500 to 800 mcg/day | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐3.4 [‐4.79, ‐2.01] |
| 14.4 1000 mcg/day or greater | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐3.55, ‐2.45] |
| 15 Change in rescue beta‐agonist use | 3 | 856 | Mean Difference (IV, Fixed, 95% CI) | ‐2.18 [‐2.38, ‐1.97] |
| 15.1 400 mcg/day or less | 2 | 622 | Mean Difference (IV, Fixed, 95% CI) | ‐2.32 [‐2.55, ‐2.09] |
| 15.2 500‐800 mcg/day | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | ‐1.64 [‐2.08, ‐1.20] |
| 15.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Withdrawal due to asthma exacerbation | 15 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.16, 0.39] |
| 16.4 400 mcg/day or less | 9 | 928 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.18, 0.48] |
| 16.5 500 to 800 mcg/day | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.30] |
| 16.6 1000 mcg/day or greater | 4 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.53] |
| 17 Oral candidiasis | 4 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.41, 3.33] |
| 17.1 400 mcg/day or less | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.75 [0.62, 22.57] |
| 17.2 500‐800 mcg/day | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.05] |
| 17.3 1000 mcg/day or greater | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 18 Oropharyngeal side‐effects | 5 | 623 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.73, 2.33] |
| 18.1 400 mcg/day or less | 2 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.25, 9.54] |
| 18.4 500 to 800 mcg/day | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.05] |
| 18.5 1000 mcg/day or greater | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.88, 3.35] |
4.1. Analysis.
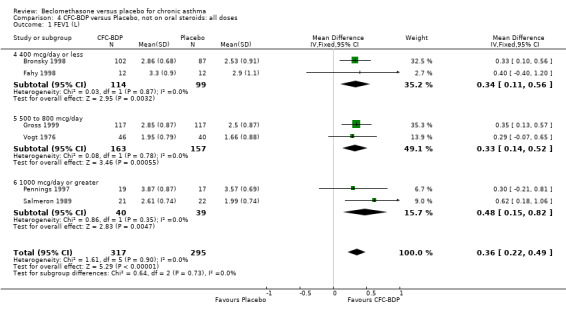
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 1 FEV1 (L).
4.2. Analysis.
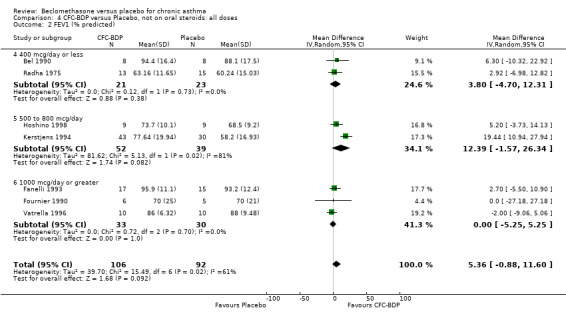
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 2 FEV1 (% predicted).
4.3. Analysis.
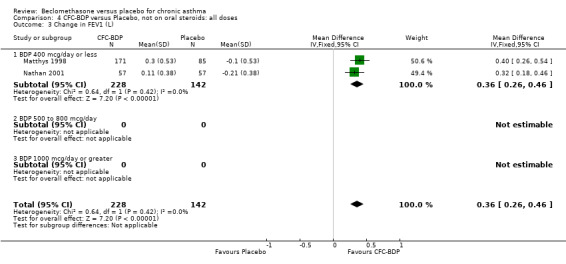
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 3 Change in FEV1 (L).
4.5. Analysis.
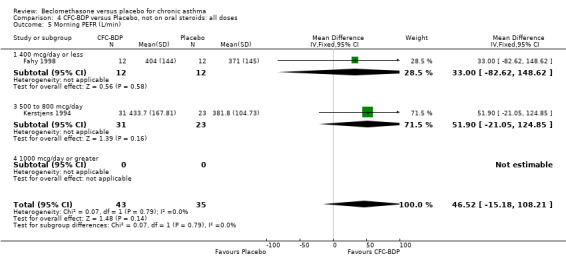
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 5 Morning PEFR (L/min).
4.6. Analysis.
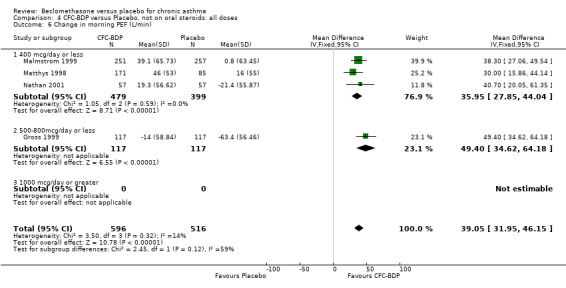
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 6 Change in morning PEF (L/min).
4.7. Analysis.
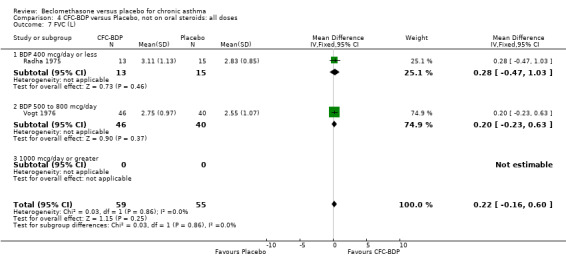
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 7 FVC (L).
4.9. Analysis.
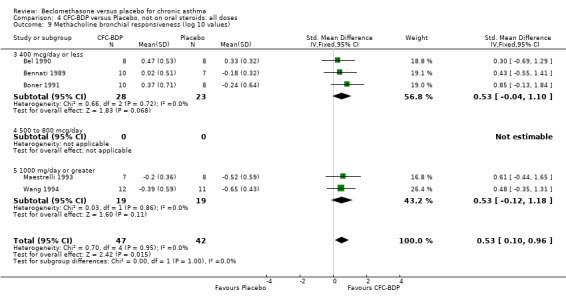
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 9 Methacholine bronchial responsiveness (log 10 values).
4.10. Analysis.
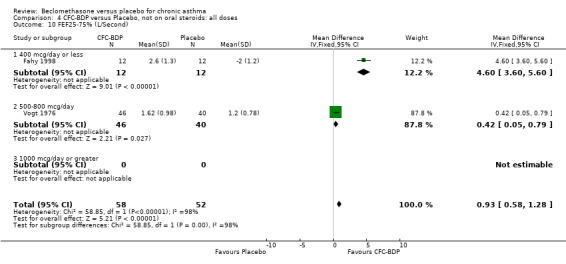
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 10 FEF25‐75% (L/Second).
4.12. Analysis.
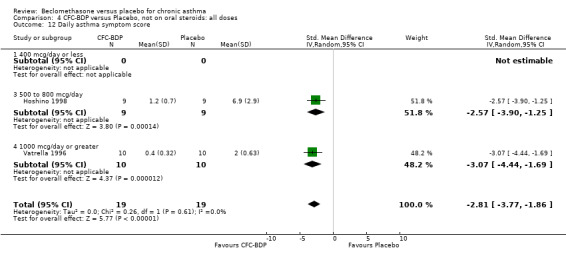
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 12 Daily asthma symptom score.
4.13. Analysis.
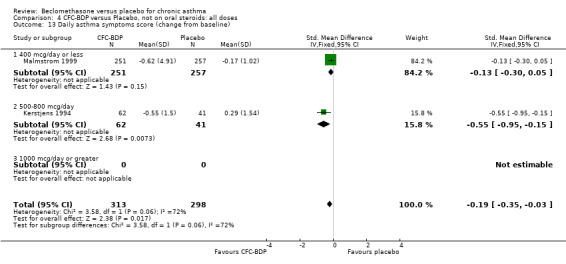
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 13 Daily asthma symptoms score (change from baseline).
4.14. Analysis.
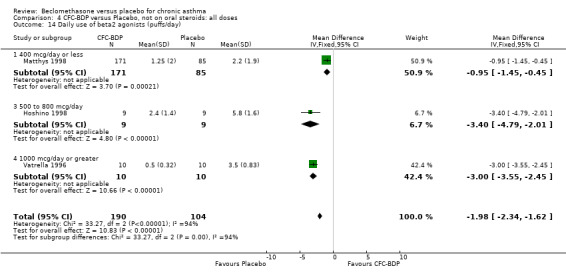
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 14 Daily use of beta2 agonists (puffs/day).
4.15. Analysis.
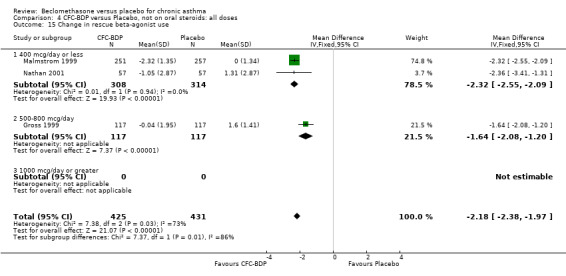
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 15 Change in rescue beta‐agonist use.
4.16. Analysis.
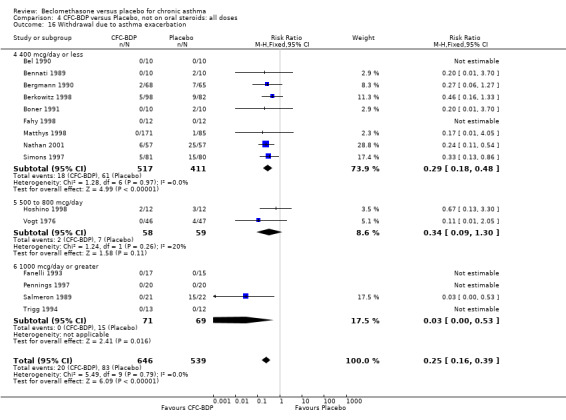
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 16 Withdrawal due to asthma exacerbation.
4.17. Analysis.
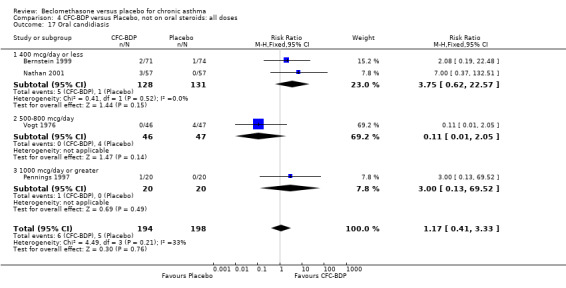
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 17 Oral candidiasis.
4.18. Analysis.
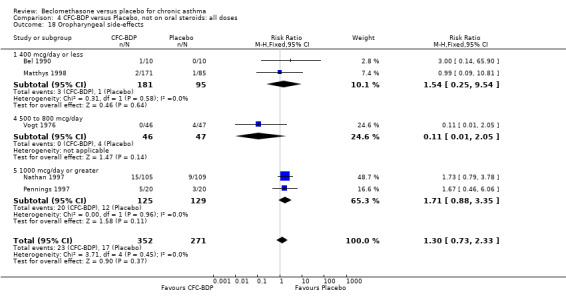
Comparison 4 CFC‐BDP versus Placebo, not on oral steroids: all doses, Outcome 18 Oropharyngeal side‐effects.
Comparison 5. CFC‐BDP versus Placebo, not on oral steroids: all severities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 6 | 612 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [0.22, 0.49] |
| 1.1 Mild | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.21, 0.81] |
| 1.2 Mild to moderate | 2 | 213 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [0.11, 0.56] |
| 1.3 Moderate | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [0.18, 1.06] |
| 1.4 Moderate to Severe | 2 | 320 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.14, 0.52] |
| 2 FEV1(% predicted) | 6 | 125 | Mean Difference (IV, Random, 95% CI) | 1.88 [‐2.11, 5.88] |
| 2.1 Mild | 3 | 63 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐5.25, 5.25] |
| 2.2 Mild to Moderate | 2 | 34 | Mean Difference (IV, Random, 95% CI) | 5.45 [‐2.42, 13.31] |
| 2.3 Moderate | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Moderate to Severe | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 2.92 [‐6.98, 12.82] |
| 4 Change in morning PEF (L/min) | 4 | 1112 | Mean Difference (IV, Fixed, 95% CI) | 39.05 [31.95, 46.15] |
| 4.1 Mild | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Mild to moderate | 1 | 508 | Mean Difference (IV, Fixed, 95% CI) | 38.30 [27.06, 49.54] |
| 4.3 Moderate | 3 | 604 | Mean Difference (IV, Fixed, 95% CI) | 39.55 [30.40, 48.71] |
| 4.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Daily use of beta2 agonist (puffs/day) | 3 | 294 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐4.02, ‐0.75] |
| 5.1 Mild | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐3.55, ‐2.45] |
| 5.2 Mild to moderate | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐3.4 [‐4.79, ‐2.01] |
| 5.3 Moderate | 1 | 256 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.45, ‐0.45] |
| 5.4 Moderate to severe | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Daily asthma symptom score | 2 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.81 [‐3.77, ‐1.86] |
| 6.1 Mild | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.07 [‐4.44, ‐1.69] |
| 6.2 Mild to moderate | 1 | 18 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐3.90, ‐1.25] |
| 6.3 Moderate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Moderate to severe | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Methacholine bronchial responsiveness (log values) | 4 | 72 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.55 [0.08, 1.03] |
| 7.1 Mild | 3 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.63 [0.09, 1.17] |
| 7.2 Mild to Moderate | 1 | 16 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.69, 1.29] |
| 7.3 Moderate | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Moderate to Severe | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Withdrawal due to asthma exacerbation | 13 | 1032 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.16, 0.40] |
| 8.1 Mild | 4 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.70] |
| 8.2 Mild to moderate | 5 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.22, 0.79] |
| 8.3 Moderate | 3 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.08, 0.35] |
| 8.4 Moderate to severe | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.05] |
| 9 Oropharyngeal side‐effects | 5 | 623 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.73, 2.33] |
| 9.1 Mild | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.46, 6.06] |
| 9.2 Mild to moderate | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| 9.3 Moderate | 2 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.78, 3.42] |
| 9.4 Moderate to severe | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.05] |
5.1. Analysis.
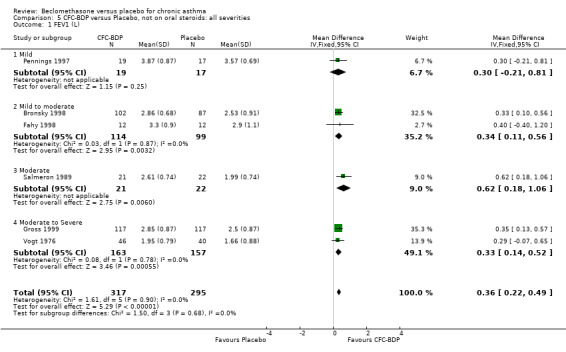
Comparison 5 CFC‐BDP versus Placebo, not on oral steroids: all severities, Outcome 1 FEV1 (L).
5.2. Analysis.
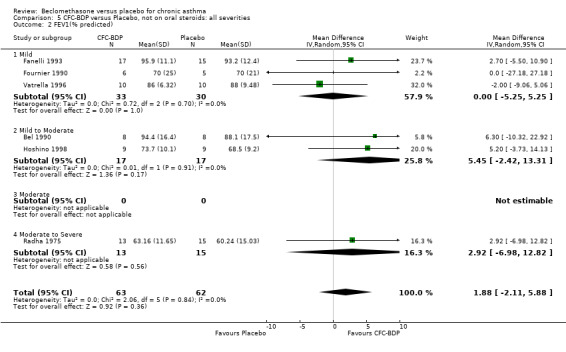
Comparison 5 CFC‐BDP versus Placebo, not on oral steroids: all severities, Outcome 2 FEV1(% predicted).
5.4. Analysis.
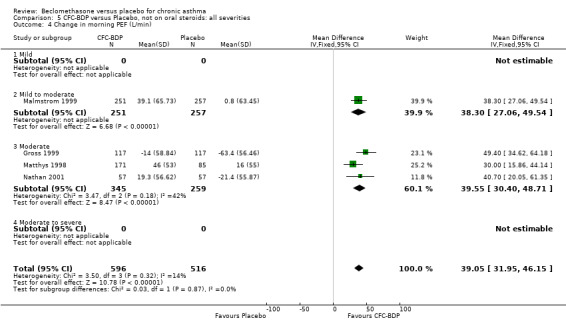
Comparison 5 CFC‐BDP versus Placebo, not on oral steroids: all severities, Outcome 4 Change in morning PEF (L/min).
5.5. Analysis.
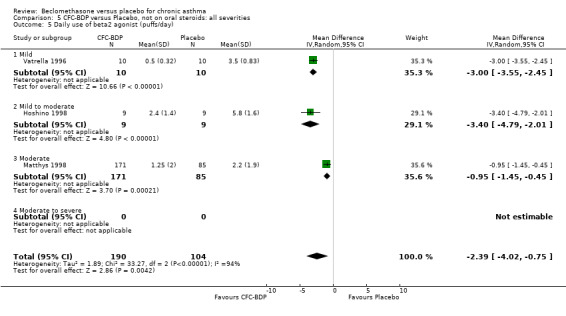
Comparison 5 CFC‐BDP versus Placebo, not on oral steroids: all severities, Outcome 5 Daily use of beta2 agonist (puffs/day).
5.6. Analysis.
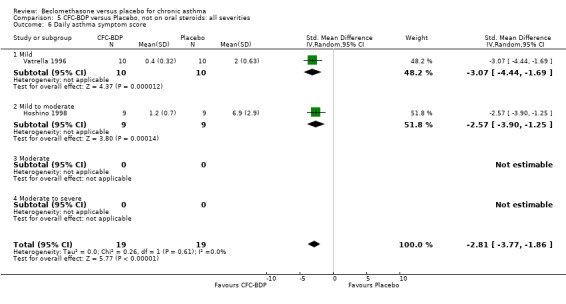
Comparison 5 CFC‐BDP versus Placebo, not on oral steroids: all severities, Outcome 6 Daily asthma symptom score.
5.7. Analysis.
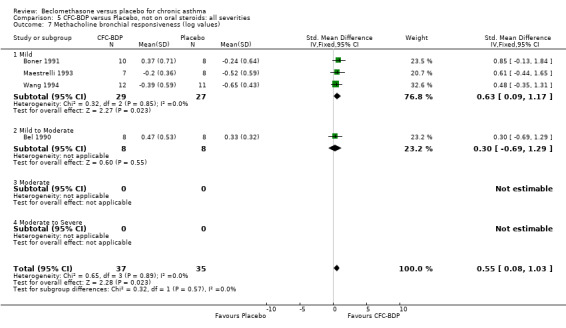
Comparison 5 CFC‐BDP versus Placebo, not on oral steroids: all severities, Outcome 7 Methacholine bronchial responsiveness (log values).
5.8. Analysis.
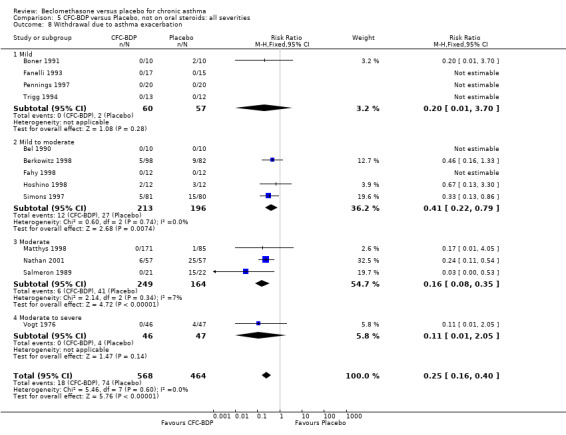
Comparison 5 CFC‐BDP versus Placebo, not on oral steroids: all severities, Outcome 8 Withdrawal due to asthma exacerbation.
5.9. Analysis.
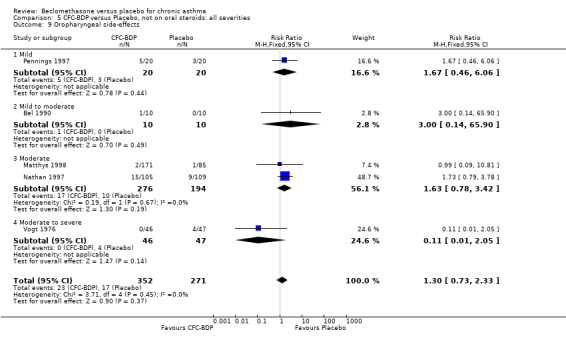
Comparison 5 CFC‐BDP versus Placebo, not on oral steroids: all severities, Outcome 9 Oropharyngeal side‐effects.
Comparison 6. CFC‐BDP versus Placebo, not oral steroids: all delivery devices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV (L) | 6 | 612 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [0.22, 0.49] |
| 1.1 MDI | 5 | 588 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [0.22, 0.49] |
| 1.2 MDI+SPACER | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.40, 1.20] |
| 1.3 DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 FEV1 (% predicted) | 7 | 198 | Mean Difference (IV, Random, 95% CI) | 5.36 [‐0.88, 11.60] |
| 2.1 MDI | 6 | 182 | Mean Difference (IV, Random, 95% CI) | 5.26 [‐1.67, 12.18] |
| 2.2 MDI+SPACER | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 6.30 [‐10.32, 22.92] |
| 2.3 DPI | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Morning PEFR (L/min) | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | 46.52 [‐15.18, 108.21] |
| 3.1 MDI | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 51.90 [‐21.05, 124.85] |
| 3.2 MDI+SPACER | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 33.0 [‐82.62, 148.62] |
| 3.3 DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Change in morning PEF (L/min) | 4 | 1112 | Mean Difference (IV, Fixed, 95% CI) | 39.05 [31.95, 46.15] |
| 4.1 MDI | 3 | 604 | Mean Difference (IV, Fixed, 95% CI) | 39.55 [30.40, 48.71] |
| 4.2 MDI + SPACER | 1 | 508 | Mean Difference (IV, Fixed, 95% CI) | 38.30 [27.06, 49.54] |
| 4.3 DPI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Daily use of beta2 agonists (puffs/day) | 3 | 294 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐4.02, ‐0.75] |
| 5.1 MDI | 3 | 294 | Mean Difference (IV, Random, 95% CI) | ‐2.39 [‐4.02, ‐0.75] |
| 5.2 MDI+SPACER | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 DPI | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Daily asthma symptom score | 2 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.81 [‐3.77, ‐1.86] |
| 6.1 MDI | 2 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.81 [‐3.77, ‐1.86] |
| 6.2 MDI+SPACER | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 DPI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Methacholine bronchial responsiveness (log values) | 5 | 89 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.53 [0.10, 0.96] |
| 7.1 MDI | 4 | 73 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.58 [0.11, 1.06] |
| 7.2 MDI+SPACER | 1 | 16 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.69, 1.29] |
| 7.3 DPI | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Withdrawal due to asthma exacerbation | 15 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.16, 0.39] |
| 11.1 MDI | 11 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.14, 0.39] |
| 11.2 MDI+SPACER | 3 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 DPI | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.13, 0.86] |
| 12 Oropharyngeal side‐effects | 5 | 623 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.73, 2.33] |
| 12.1 MDI | 4 | 603 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.69, 2.27] |
| 12.2 MDI+SPACER | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| 12.3 DPI | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
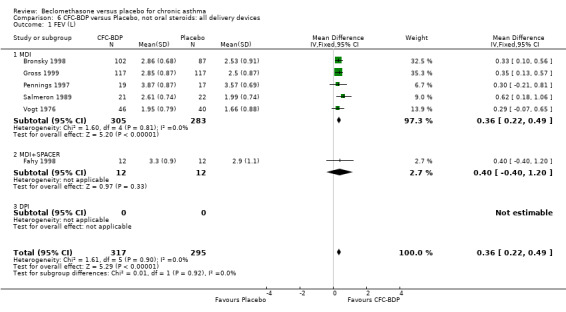
Comparison 6 CFC‐BDP versus Placebo, not oral steroids: all delivery devices, Outcome 1 FEV (L).
6.2. Analysis.
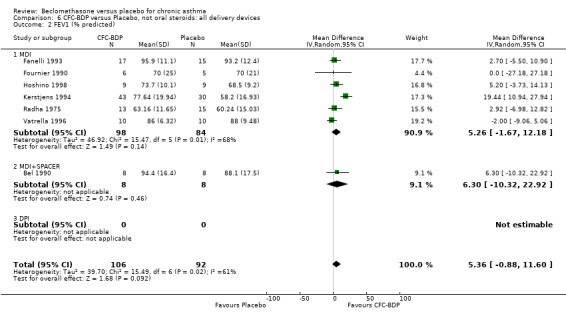
Comparison 6 CFC‐BDP versus Placebo, not oral steroids: all delivery devices, Outcome 2 FEV1 (% predicted).
6.3. Analysis.
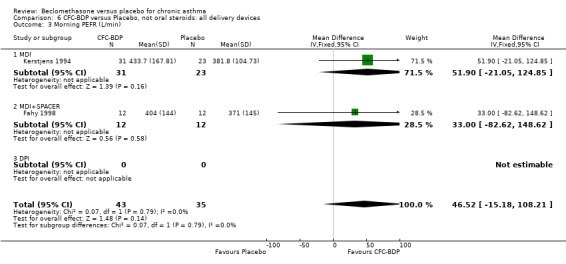
Comparison 6 CFC‐BDP versus Placebo, not oral steroids: all delivery devices, Outcome 3 Morning PEFR (L/min).
6.4. Analysis.
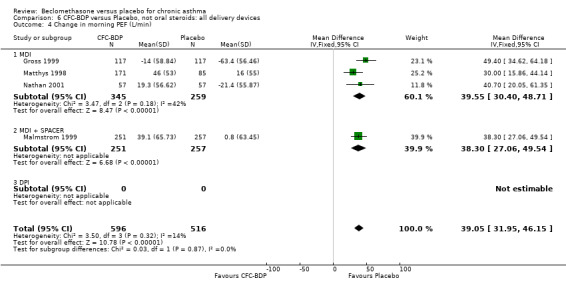
Comparison 6 CFC‐BDP versus Placebo, not oral steroids: all delivery devices, Outcome 4 Change in morning PEF (L/min).
6.5. Analysis.
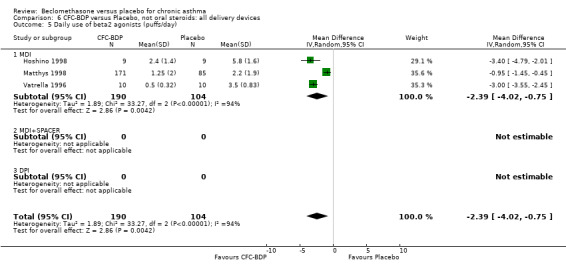
Comparison 6 CFC‐BDP versus Placebo, not oral steroids: all delivery devices, Outcome 5 Daily use of beta2 agonists (puffs/day).
6.6. Analysis.
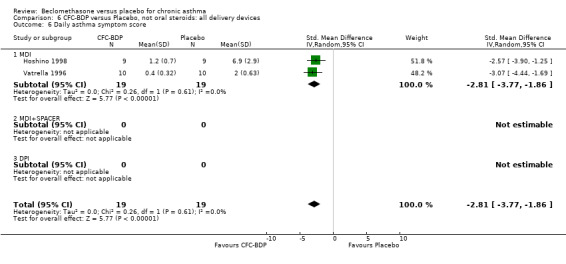
Comparison 6 CFC‐BDP versus Placebo, not oral steroids: all delivery devices, Outcome 6 Daily asthma symptom score.
6.7. Analysis.
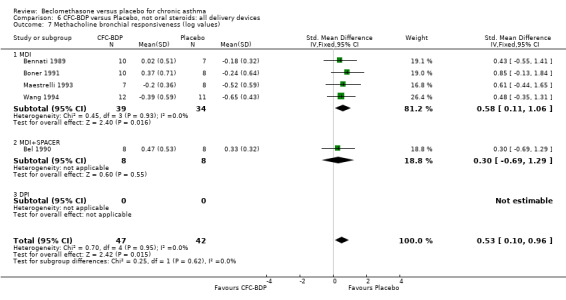
Comparison 6 CFC‐BDP versus Placebo, not oral steroids: all delivery devices, Outcome 7 Methacholine bronchial responsiveness (log values).
6.11. Analysis.
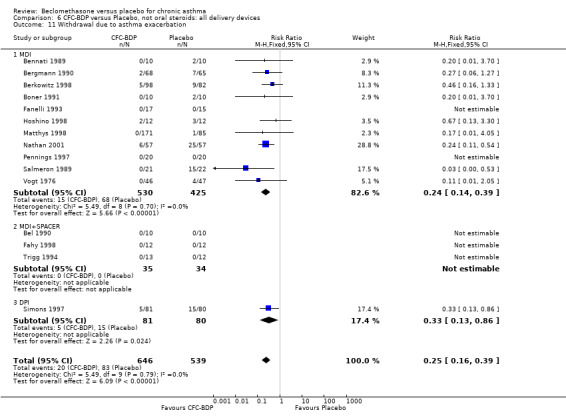
Comparison 6 CFC‐BDP versus Placebo, not oral steroids: all delivery devices, Outcome 11 Withdrawal due to asthma exacerbation.
6.12. Analysis.
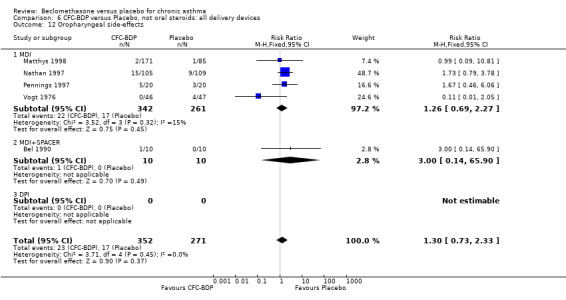
Comparison 6 CFC‐BDP versus Placebo, not oral steroids: all delivery devices, Outcome 12 Oropharyngeal side‐effects.
Comparison 7. CFC‐BDP versus placebo, not on oral steroids: all study durations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 6 | 612 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [0.22, 0.49] |
| 1.1 At week 4 | 2 | 110 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.02, 0.63] |
| 1.2 > 4 weeks | 4 | 502 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [0.22, 0.51] |
| 2 FEV1 (% predicted) | 7 | 198 | Mean Difference (IV, Random, 95% CI) | 5.36 [‐0.88, 11.60] |
| 2.1 At week 4 | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 2.70 [‐5.50, 10.90] |
| 2.2 > 4 weeks | 6 | 166 | Mean Difference (IV, Random, 95% CI) | 5.90 [‐1.75, 13.56] |
| 3 FEV1 % (change from baseline) | 2 | 756 | Mean Difference (IV, Fixed, 95% CI) | 12.15 [8.34, 15.96] |
| 3.1 At week 4 | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 > 4 weeks | 2 | 756 | Mean Difference (IV, Fixed, 95% CI) | 12.15 [8.34, 15.96] |
| 4 Morning PEFR (L/min) | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | 46.52 [‐15.18, 108.21] |
| 4.1 At week 4 | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 33.0 [‐82.62, 148.62] |
| 4.2 > 4 weeks | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 51.90 [‐21.05, 124.85] |
| 5 Change in morning PEF (L/min) | 4 | 1112 | Mean Difference (IV, Fixed, 95% CI) | 39.05 [31.95, 46.15] |
| 5.1 At week 4 | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 > 4 weeks | 4 | 1112 | Mean Difference (IV, Fixed, 95% CI) | 39.05 [31.95, 46.15] |
| 6 Methacholine bronchial responsiveness (log values) | 5 | 89 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.53 [0.10, 0.96] |
| 6.1 At week 4 | 1 | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.55, 1.41] |
| 6.2 > 4 weeks | 4 | 72 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.55 [0.08, 1.03] |
| 7 FEF25‐75% (L/second) | 2 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.28, ‐0.58] |
| 7.1 At week 4 | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐4.6 [‐5.60, ‐3.60] |
| 7.2 > 4 weeks | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐0.79, ‐0.05] |
| 10 Total daily Beta2‐agonist use (change from baseline) | 2 | 622 | Mean Difference (IV, Fixed, 95% CI) | ‐2.32 [‐2.55, ‐2.09] |
| 10.1 At week 4 | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 > 4 weeks | 2 | 622 | Mean Difference (IV, Fixed, 95% CI) | ‐2.32 [‐2.55, ‐2.09] |
| 11 Daily use of Beta2‐ agonists (puffs per day) | 3 | 294 | Mean Difference (IV, Fixed, 95% CI) | ‐1.98 [‐2.34, ‐1.62] |
| 11.1 At week 4 | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 > 4 weeks | 3 | 294 | Mean Difference (IV, Fixed, 95% CI) | ‐1.98 [‐2.34, ‐1.62] |
| 12 Daytime asthma symptoms score (change from baseline) | 2 | 611 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐1.08, ‐0.22] |
| 12.1 At week 4 | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 > 4 weeks | 2 | 611 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐1.08, ‐0.22] |
| 13 Daily asthma symptoms score | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.22, ‐1.37] |
| 13.1 At week 4 | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 > 4 weeks | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.22, ‐1.37] |
| 40 Withdrawals due to asthma exacerbation | 15 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.16, 0.39] |
| 40.1 At week 4 | 4 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.13] |
| 40.2 > 4 weeks | 11 | 1016 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.17, 0.41] |
| 41 Oropharyngel side‐effect | 5 | 623 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.73, 2.33] |
| 41.1 At week 4 | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.59, 2.38] |
| 41.2 > 4 weeks | 3 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.56, 4.65] |
7.1. Analysis.
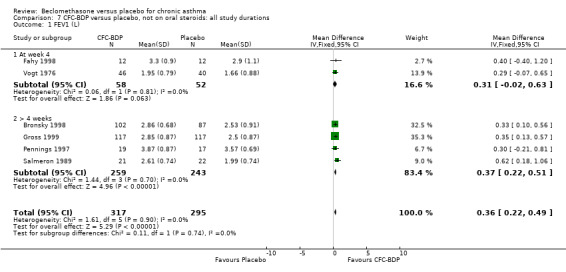
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 1 FEV1 (L).
7.2. Analysis.
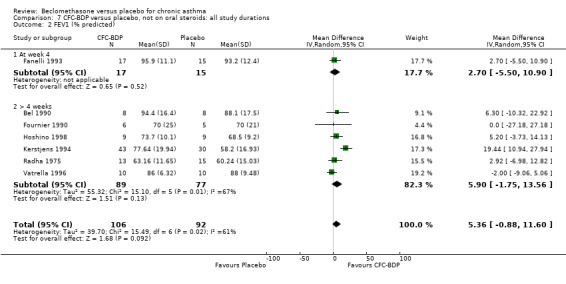
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 2 FEV1 (% predicted).
7.3. Analysis.
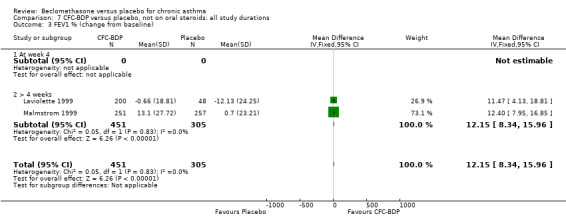
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 3 FEV1 % (change from baseline).
7.4. Analysis.
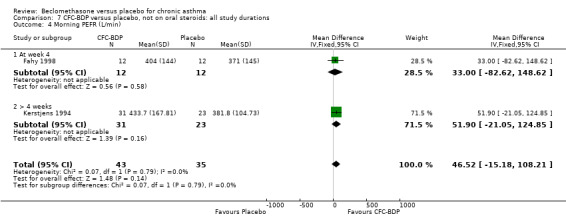
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 4 Morning PEFR (L/min).
7.5. Analysis.
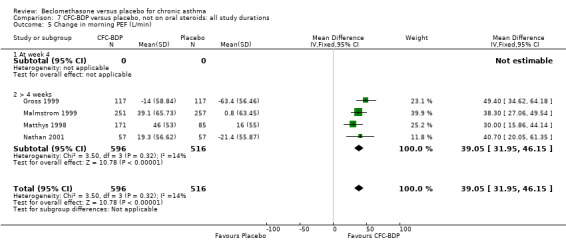
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 5 Change in morning PEF (L/min).
7.6. Analysis.
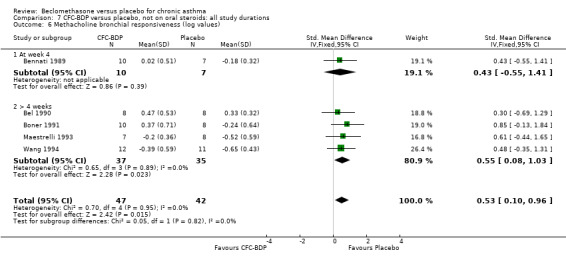
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 6 Methacholine bronchial responsiveness (log values).
7.7. Analysis.
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 7 FEF25‐75% (L/second).
7.10. Analysis.
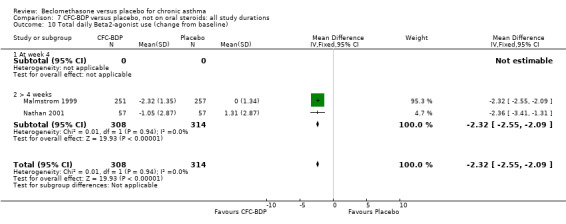
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 10 Total daily Beta2‐agonist use (change from baseline).
7.11. Analysis.
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 11 Daily use of Beta2‐ agonists (puffs per day).
7.12. Analysis.
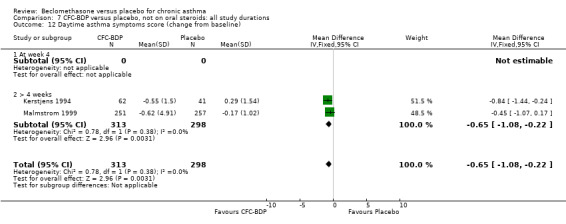
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 12 Daytime asthma symptoms score (change from baseline).
7.13. Analysis.
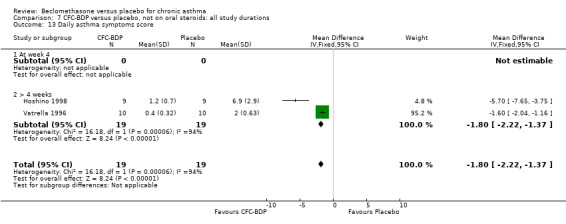
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 13 Daily asthma symptoms score.
7.40. Analysis.
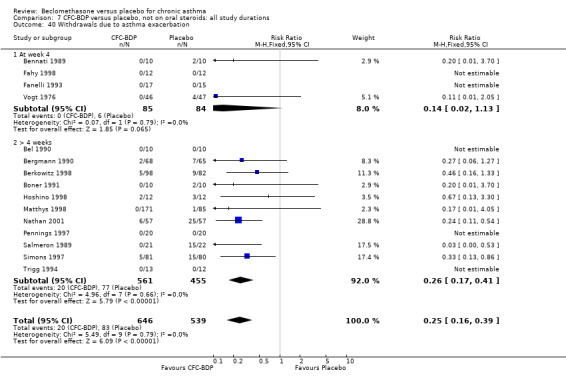
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 40 Withdrawals due to asthma exacerbation.
7.41. Analysis.
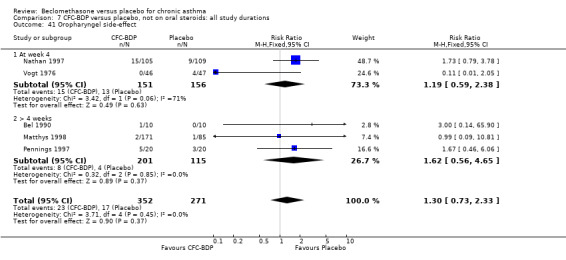
Comparison 7 CFC‐BDP versus placebo, not on oral steroids: all study durations, Outcome 41 Oropharyngel side‐effect.
Comparison 8. CFC‐BDP versus Placebo,on oral steroids: 400 mcg/d or less.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Daily dose of oral prednisiolone (mg) | 2 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐6.31 [‐8.68, ‐3.94] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 2 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐6.31 [‐8.68, ‐3.94] |
| 2.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Reduction in daily dose of oral prednisolone (mg) | 2 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐4.91 [‐5.88, ‐3.94] |
| 3.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 2 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐4.91 [‐5.88, ‐3.94] |
| 3.3 Children and Adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Daytime asthma symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Night‐time asthma symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Plasma cortisol (mmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Oropharyngeal side‐effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Oropharyngeal Candidiasis (No. of patients) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Discontinuation of oral steroids (No. of patients) | 4 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 6.95 [2.79, 17.34] |
| 9.1 Children | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 4 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 6.95 [2.79, 17.34] |
| 9.3 Children and Adults | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
Comparison 8 CFC‐BDP versus Placebo,on oral steroids: 400 mcg/d or less, Outcome 1 FEV1 (% predicted).
8.2. Analysis.
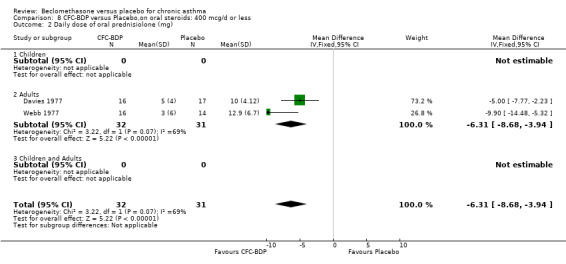
Comparison 8 CFC‐BDP versus Placebo,on oral steroids: 400 mcg/d or less, Outcome 2 Daily dose of oral prednisiolone (mg).
8.3. Analysis.
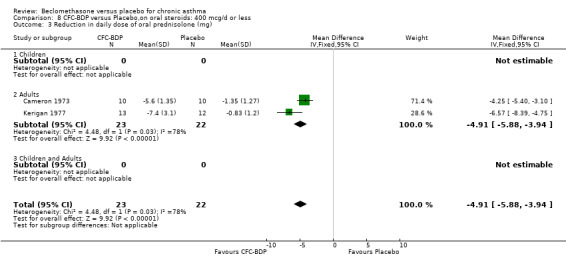
Comparison 8 CFC‐BDP versus Placebo,on oral steroids: 400 mcg/d or less, Outcome 3 Reduction in daily dose of oral prednisolone (mg).
8.4. Analysis.
Comparison 8 CFC‐BDP versus Placebo,on oral steroids: 400 mcg/d or less, Outcome 4 Daytime asthma symptom score.
8.5. Analysis.
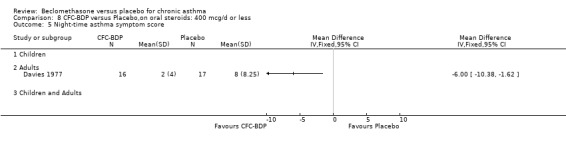
Comparison 8 CFC‐BDP versus Placebo,on oral steroids: 400 mcg/d or less, Outcome 5 Night‐time asthma symptom score.
8.6. Analysis.
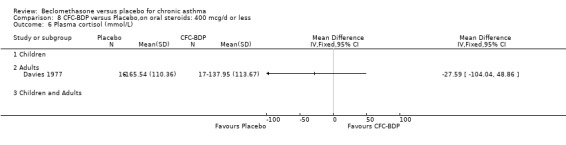
Comparison 8 CFC‐BDP versus Placebo,on oral steroids: 400 mcg/d or less, Outcome 6 Plasma cortisol (mmol/L).
8.7. Analysis.
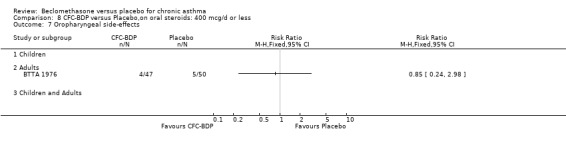
Comparison 8 CFC‐BDP versus Placebo,on oral steroids: 400 mcg/d or less, Outcome 7 Oropharyngeal side‐effects.
8.8. Analysis.
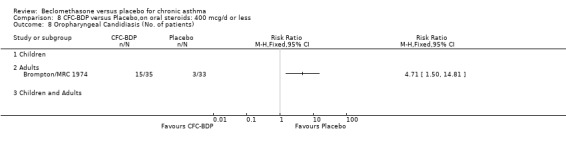
Comparison 8 CFC‐BDP versus Placebo,on oral steroids: 400 mcg/d or less, Outcome 8 Oropharyngeal Candidiasis (No. of patients).
8.9. Analysis.
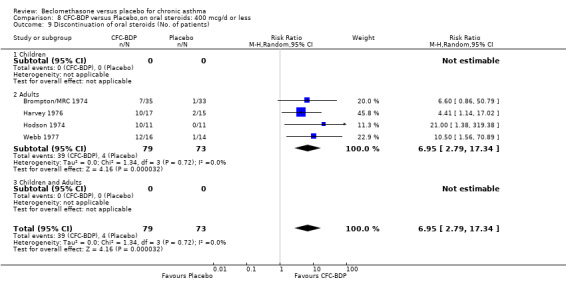
Comparison 8 CFC‐BDP versus Placebo,on oral steroids: 400 mcg/d or less, Outcome 9 Discontinuation of oral steroids (No. of patients).
Comparison 9. CFC‐BDP versus Placebo, on oral steroids: 500‐800 mcg/d.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7 Discontinuation of oral steroids | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Oropharyngeal candidiasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Oropharyngeal side‐effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.7. Analysis.
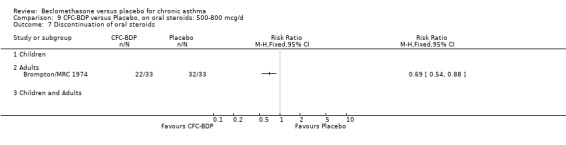
Comparison 9 CFC‐BDP versus Placebo, on oral steroids: 500‐800 mcg/d, Outcome 7 Discontinuation of oral steroids.
9.8. Analysis.
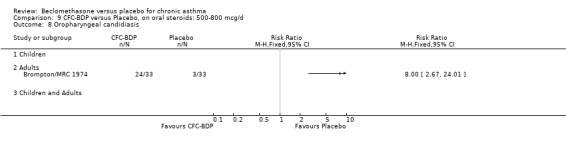
Comparison 9 CFC‐BDP versus Placebo, on oral steroids: 500‐800 mcg/d, Outcome 8 Oropharyngeal candidiasis.
9.9. Analysis.
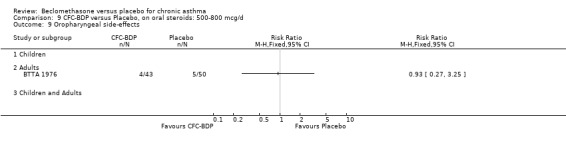
Comparison 9 CFC‐BDP versus Placebo, on oral steroids: 500‐800 mcg/d, Outcome 9 Oropharyngeal side‐effects.
Comparison 10. CFC‐BDP versus Placebo, on oral steroids: 1000 mcg/d or greater.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1(%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PEF (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Daily asthma symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Daily dose of oral prednislone (mg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Discontinuation of oral prednisolone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Children and Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.
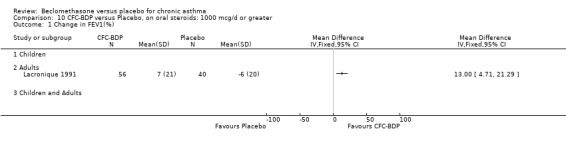
Comparison 10 CFC‐BDP versus Placebo, on oral steroids: 1000 mcg/d or greater, Outcome 1 Change in FEV1(%).
10.2. Analysis.
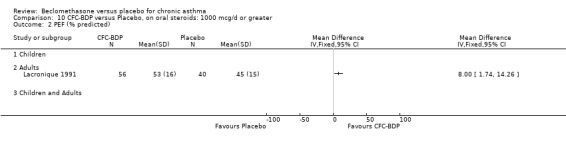
Comparison 10 CFC‐BDP versus Placebo, on oral steroids: 1000 mcg/d or greater, Outcome 2 PEF (% predicted).
10.3. Analysis.
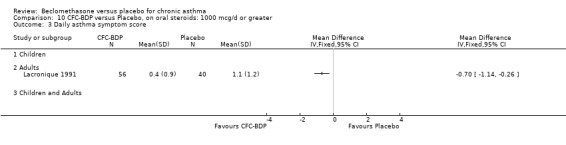
Comparison 10 CFC‐BDP versus Placebo, on oral steroids: 1000 mcg/d or greater, Outcome 3 Daily asthma symptom score.
10.4. Analysis.
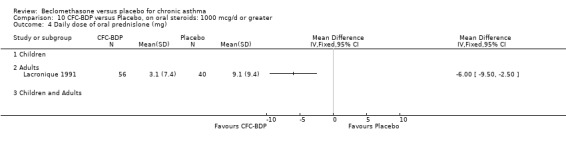
Comparison 10 CFC‐BDP versus Placebo, on oral steroids: 1000 mcg/d or greater, Outcome 4 Daily dose of oral prednislone (mg).
10.5. Analysis.
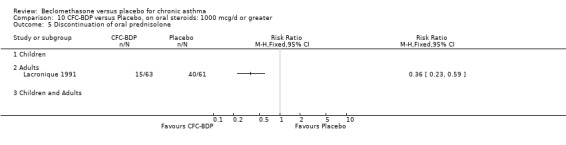
Comparison 10 CFC‐BDP versus Placebo, on oral steroids: 1000 mcg/d or greater, Outcome 5 Discontinuation of oral prednisolone.
Comparison 11. CFC‐BDP versus Placebo, on oral steroids: all doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in daily dose of oral prednisolone (mg) | 2 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐4.91 [‐5.88, ‐3.94] |
| 1.1 400 mcg/day or less | 2 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐4.91 [‐5.88, ‐3.94] |
| 1.2 500 to 800 mcg/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 1000 mcg/day or greater | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Daily dose of oral prednisolone (mg) | 3 | 159 | Mean Difference (IV, Fixed, 95% CI) | ‐6.21 [‐8.18, ‐4.25] |
| 2.1 400 mcg/day or less | 2 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐6.31 [‐8.68, ‐3.94] |
| 2.2 500 to 800 mcg/day | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 1000 mcg/day or greater | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐9.50, ‐2.50] |
| 3 Discontinuation of oral steroids (No. of patients) | 5 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 5.30 [1.95, 14.46] |
| 3.1 400 mcg/day or less | 3 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 7.05 [2.54, 19.57] |
| 3.2 500 to 800 mcg/day | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 11.00 [1.50, 80.43] |
| 3.3 1000 mcg/d or greater | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.52, 3.21] |
11.1. Analysis.
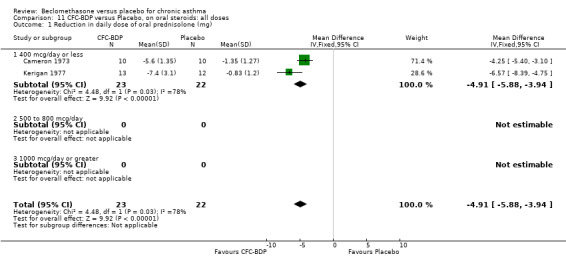
Comparison 11 CFC‐BDP versus Placebo, on oral steroids: all doses, Outcome 1 Reduction in daily dose of oral prednisolone (mg).
11.2. Analysis.
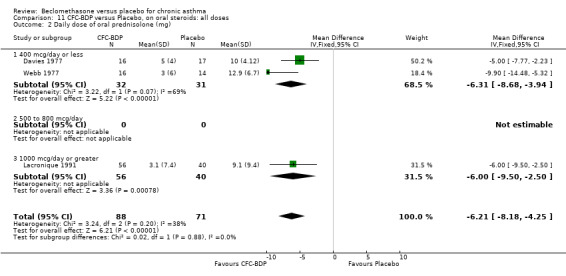
Comparison 11 CFC‐BDP versus Placebo, on oral steroids: all doses, Outcome 2 Daily dose of oral prednisolone (mg).
11.3. Analysis.
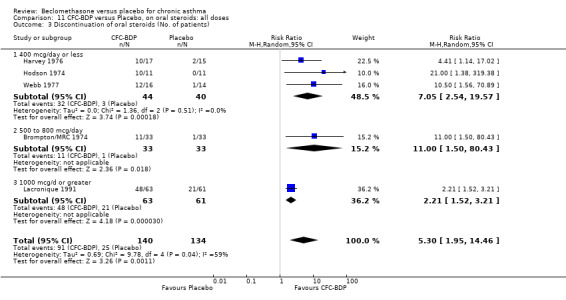
Comparison 11 CFC‐BDP versus Placebo, on oral steroids: all doses, Outcome 3 Discontinuation of oral steroids (No. of patients).
Comparison 12. HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1(% predicted change from baseline) | 2 | 414 | Mean Difference (IV, Fixed, 95% CI) | 5.90 [3.48, 8.31] |
| 1.1 Children | 1 | 236 | Mean Difference (IV, Fixed, 95% CI) | 5.54 [2.23, 8.85] |
| 1.2 Adults | 1 | 178 | Mean Difference (IV, Fixed, 95% CI) | 6.3 [2.77, 9.83] |
| 1.3 Children and adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Change in morning PEF (L/min) | 2 | 408 | Mean Difference (IV, Fixed, 95% CI) | 40.49 [30.09, 50.89] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 2 | 408 | Mean Difference (IV, Fixed, 95% CI) | 40.49 [30.09, 50.89] |
| 2.3 children and adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Change in evening PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 FEF25%‐75% (%change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 FEV1 (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in beta2‐agonist use (puffs/day) | 3 | 644 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.43, ‐0.13] |
| 6.1 Children | 1 | 236 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.61, 0.03] |
| 6.2 Adults | 2 | 408 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.82, ‐0.27] |
| 6.3 Children and adults | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Asthma symptoms score: wheeze (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Asthma symptoms score: cough (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Asthma symptoms score: shortness of breath (Change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Asthma symptoms scores: Chest tightness (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Days free from any asthma symptoms (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Nights without sleep disturbance % | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.4 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.5 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Change in percentage of nights without sleep disturbance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Exacerbations of asthma | 2 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.37, 1.76] |
| 14.1 Children | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.56] |
| 14.2 Adults | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.48, 3.16] |
| 14.3 Children and adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Increased asthma symptoms | 2 | 466 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.07, 1.74] |
| 15.1 Adults | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 0.89] |
| 15.2 Children | 1 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.33, 1.14] |
| 15.3 Children and adults | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Total withdrawals | 2 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.39, 1.18] |
| 16.1 Children | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.41, 1.43] |
| 16.2 Adults | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.15, 1.53] |
| 16.3 Children and adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17.1 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.3 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.4 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Withdrawals due to non‐compliance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 >/= one side‐effect reported | 3 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.15] |
| 19.1 Childern | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.84, 1.17] |
| 19.2 Adults | 2 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.50, 1.55] |
| 19.3 Children and adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Cough | 2 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.32, 1.92] |
| 20.1 Children | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.29, 1.95] |
| 20.2 Adults | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 16.35] |
| 20.3 Children and adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Pharyngitis | 2 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.55, 1.96] |
| 21.1 Children | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.47, 2.00] |
| 21.2 Adults | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.36, 4.70] |
| 21.3 Children and adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 Sinusitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 23.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24 Upper respiratory tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 Low morning plasma cortisol levels | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 25.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26 Abnormal respones to low dose ACTH stimulation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 26.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 28 Change in beta2‐agonist use (puffs/day, predosing schedule subgroup analysis) | 3 | 644 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.43, ‐0.13] |
| 28.1 No OCS predosing schedule | 2 | 414 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.80, ‐0.09] |
| 28.2 OCS Pre‐dosing schedule | 1 | 230 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐1.94, ‐0.96] |
12.1. Analysis.
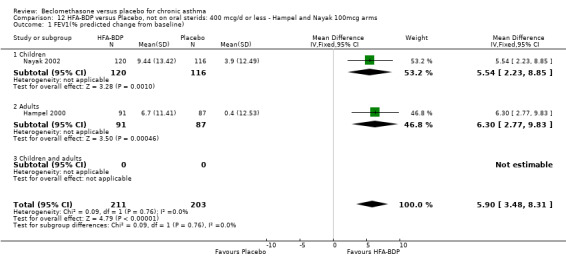
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 1 FEV1(% predicted change from baseline).
12.2. Analysis.
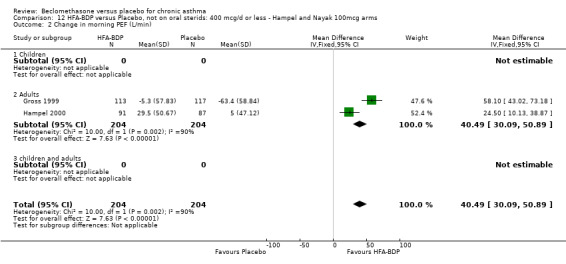
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 2 Change in morning PEF (L/min).
12.3. Analysis.
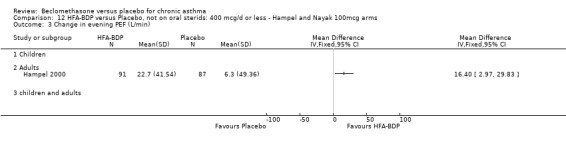
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 3 Change in evening PEF (L/min).
12.4. Analysis.
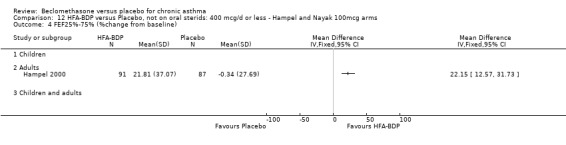
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 4 FEF25%‐75% (%change from baseline).
12.5. Analysis.
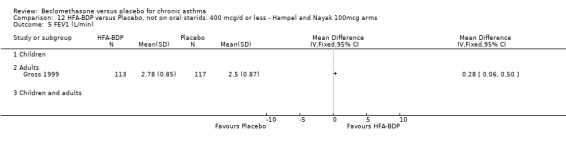
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 5 FEV1 (L/min).
12.6. Analysis.
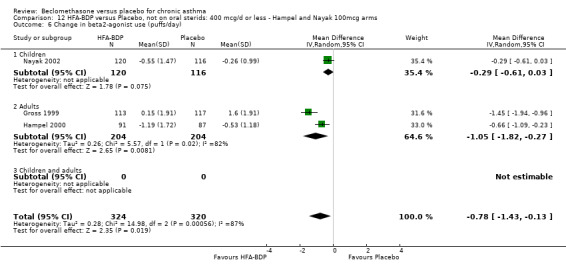
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 6 Change in beta2‐agonist use (puffs/day).
12.7. Analysis.
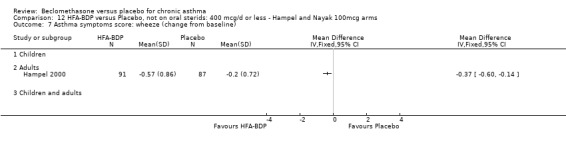
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 7 Asthma symptoms score: wheeze (change from baseline).
12.8. Analysis.
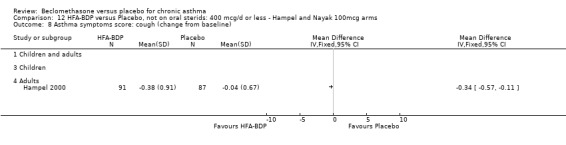
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 8 Asthma symptoms score: cough (change from baseline).
12.9. Analysis.
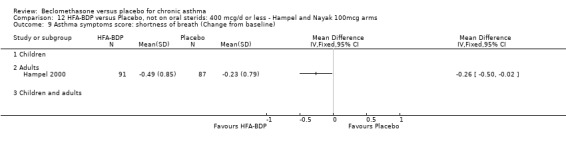
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 9 Asthma symptoms score: shortness of breath (Change from baseline).
12.10. Analysis.
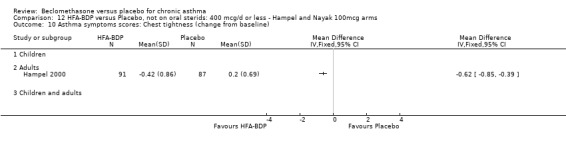
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 10 Asthma symptoms scores: Chest tightness (change from baseline).
12.11. Analysis.
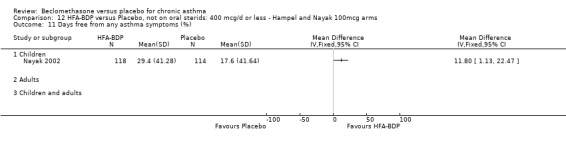
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 11 Days free from any asthma symptoms (%).
12.12. Analysis.
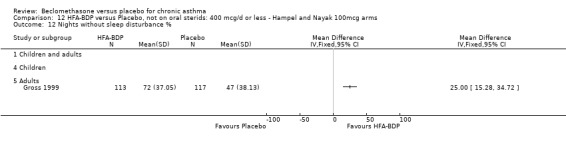
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 12 Nights without sleep disturbance %.
12.13. Analysis.
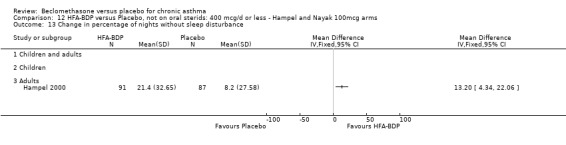
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 13 Change in percentage of nights without sleep disturbance.
12.14. Analysis.
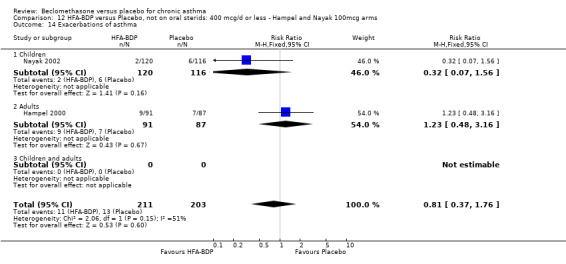
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 14 Exacerbations of asthma.
12.15. Analysis.
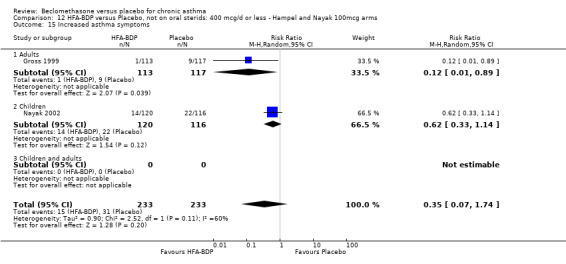
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 15 Increased asthma symptoms.
12.16. Analysis.
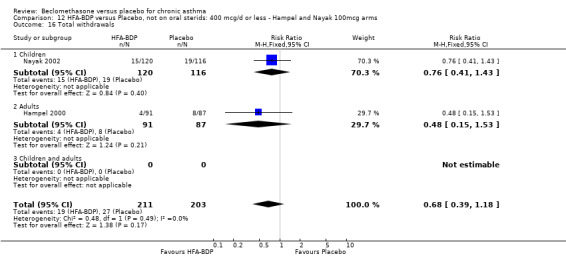
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 16 Total withdrawals.
12.17. Analysis.
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 17 Withdrawals due to adverse events.
12.18. Analysis.
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 18 Withdrawals due to non‐compliance.
12.19. Analysis.
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 19 >/= one side‐effect reported.
12.20. Analysis.
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 20 Cough.
12.21. Analysis.
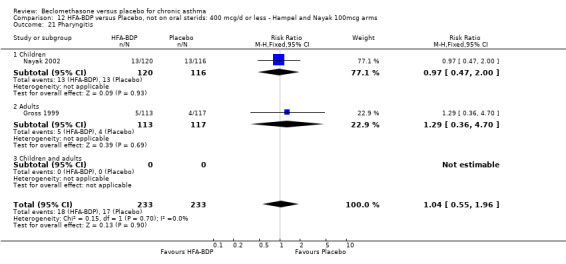
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 21 Pharyngitis.
12.22. Analysis.
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 22 Rhinitis.
12.23. Analysis.
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 23 Sinusitis.
12.24. Analysis.
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 24 Upper respiratory tract infection.
12.25. Analysis.
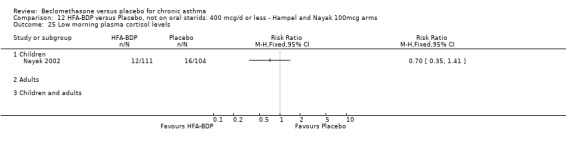
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 25 Low morning plasma cortisol levels.
12.26. Analysis.
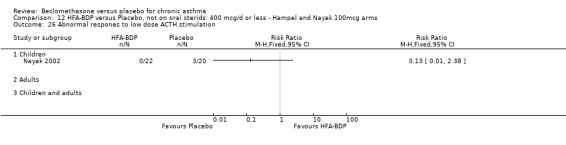
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 26 Abnormal respones to low dose ACTH stimulation.
12.28. Analysis.
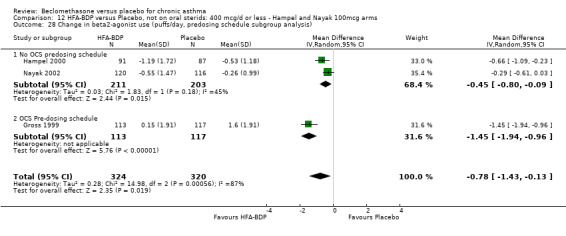
Comparison 12 HFA‐BDP versus Placebo, not on oral sterids: 400 mcg/d or less ‐ Hampel and Nayak 100mcg arms, Outcome 28 Change in beta2‐agonist use (puffs/day, predosing schedule subgroup analysis).
Comparison 13. HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (% predicted) (change from baseline) | 2 | 412 | Mean Difference (IV, Fixed, 95% CI) | 6.36 [3.94, 8.78] |
| 1.1 Children | 1 | 233 | Mean Difference (IV, Fixed, 95% CI) | 4.9 [1.70, 8.10] |
| 1.2 Adults | 1 | 179 | Mean Difference (IV, Fixed, 95% CI) | 8.30 [4.60, 12.00] |
| 1.3 Children and adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Change in morning PEF (L/min) | 2 | 409 | Mean Difference (IV, Fixed, 95% CI) | 43.21 [32.64, 53.79] |
| 2.1 Adults | 2 | 409 | Mean Difference (IV, Fixed, 95% CI) | 43.21 [32.64, 53.79] |
| 2.2 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Children and adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Change in evening PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in FEF25‐75 (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in beta2‐agonist use (puffs/day) | 3 | 642 | Mean Difference (IV, Fixed, 95% CI) | ‐0.81 [‐1.01, ‐0.61] |
| 5.1 Children | 1 | 233 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐0.99, ‐0.49] |
| 5.2 Adults | 2 | 409 | Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐1.25, ‐0.59] |
| 5.3 Children and adults | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Nights without sleep disturbance (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Change in percentage of nights without sleep disturbance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Asthma symptoms score: cough (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Asthma symptoms score: shortness of breath (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Asthma symptoms score: chest tightness (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Asthma symptoms score: wheeze (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Days free from any asthma symptoms (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Children and adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Exacerbations of asthma | 2 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.42, 1.91] |
| 13.1 Children | 1 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.13, 1.94] |
| 13.2 Adults | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.48, 3.16] |
| 13.3 Children and adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Increased asthma symptoms | 2 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.23, 0.77] |
| 14.1 Children | 1 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.28, 1.04] |
| 14.2 Adults | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 0.89] |
| 14.3 Children and adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Total withdrawals | 2 | 412 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.24, 0.92] |
| 15.1 Children | 1 | 233 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.18, 0.98] |
| 15.2 Adults | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.18, 1.81] |
| 15.3 Children and adults | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Withdrawals due to inadequate response | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1 Children | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Adults | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Children and adults | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Withdrawals due to adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17.1 Children | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Adults | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.3 Children and adults | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 >/= one side‐effect reported | 3 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.82, 1.16] |
| 18.1 Children | 1 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| 18.2 Adults | 2 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.55] |
| 18.3 Children and adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Sinusitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Upper respiratory tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 Low morning plasma cortisol levels | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 Abnormal respones to low dose ACTH stimulation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22.1 Children | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Adults | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.3 Children and adults | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 Cough | 2 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.43, 2.32] |
| 23.1 Children | 1 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.41, 2.41] |
| 23.2 Adults | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 16.35] |
| 23.3 Children and adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Pharyngitis | 2 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.42, 1.63] |
| 24.1 Children | 1 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.31, 1.54] |
| 24.2 Adults | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.36, 4.70] |
| 24.3 Children and adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 25.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.3 Children and adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
13.1. Analysis.
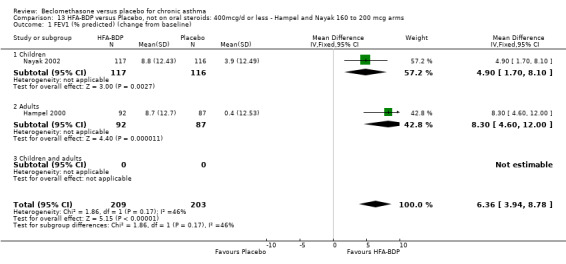
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 1 FEV1 (% predicted) (change from baseline).
13.2. Analysis.
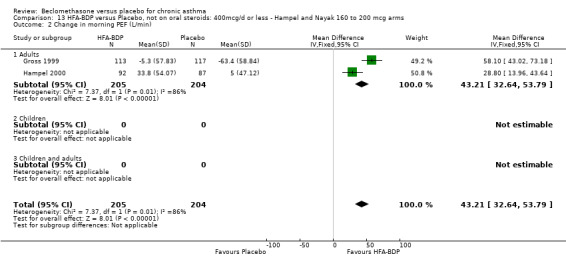
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 2 Change in morning PEF (L/min).
13.3. Analysis.
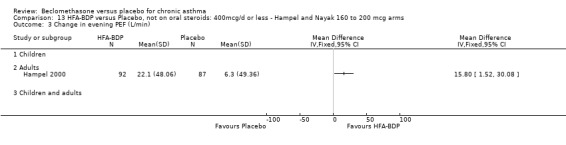
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 3 Change in evening PEF (L/min).
13.4. Analysis.
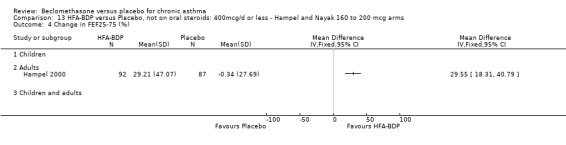
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 4 Change in FEF25‐75 (%).
13.5. Analysis.
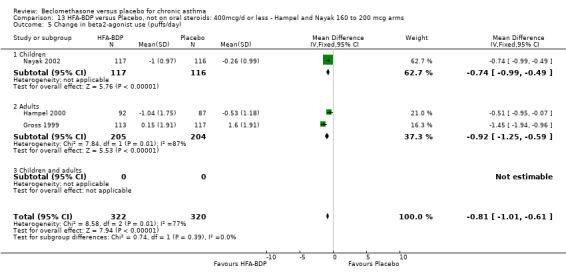
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 5 Change in beta2‐agonist use (puffs/day).
13.6. Analysis.
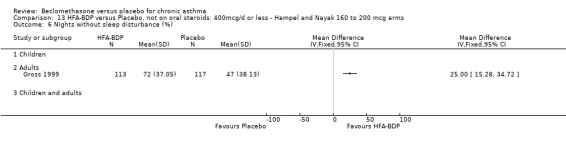
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 6 Nights without sleep disturbance (%).
13.7. Analysis.
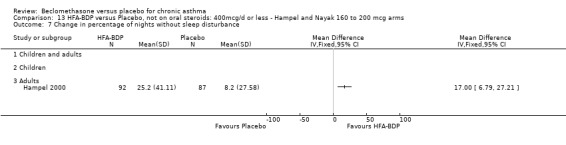
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 7 Change in percentage of nights without sleep disturbance.
13.8. Analysis.
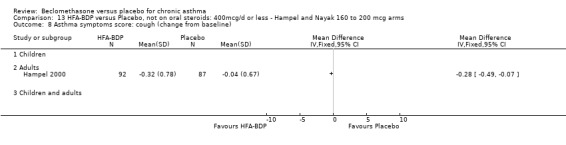
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 8 Asthma symptoms score: cough (change from baseline).
13.9. Analysis.
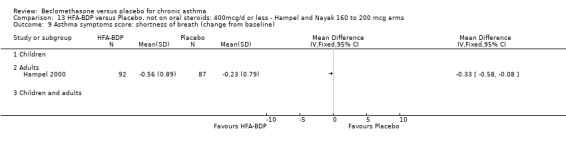
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 9 Asthma symptoms score: shortness of breath (change from baseline).
13.10. Analysis.
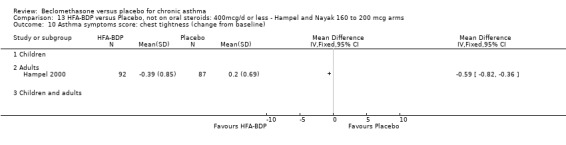
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 10 Asthma symptoms score: chest tightness (change from baseline).
13.11. Analysis.
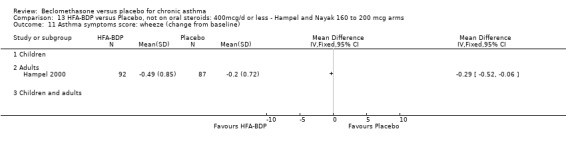
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 11 Asthma symptoms score: wheeze (change from baseline).
13.12. Analysis.
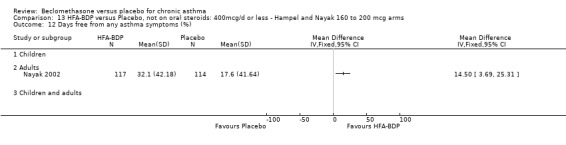
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 12 Days free from any asthma symptoms (%).
13.13. Analysis.
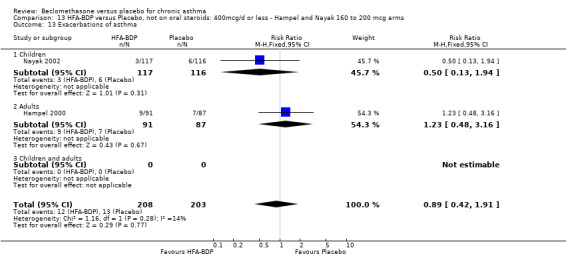
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 13 Exacerbations of asthma.
13.14. Analysis.
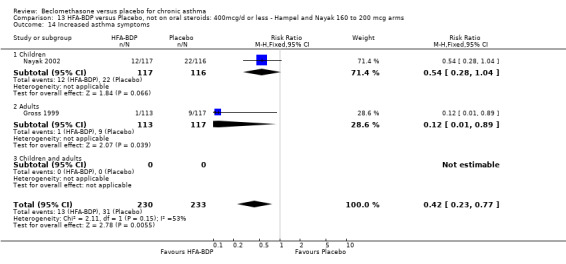
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 14 Increased asthma symptoms.
13.15. Analysis.
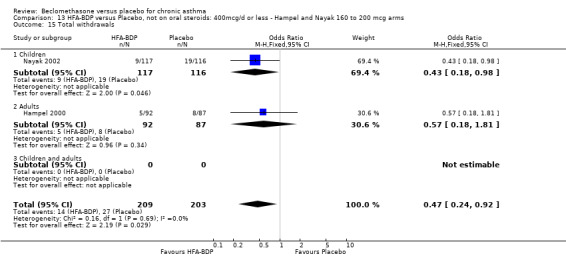
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 15 Total withdrawals.
13.16. Analysis.
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 16 Withdrawals due to inadequate response.
13.17. Analysis.
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 17 Withdrawals due to adverse events.
13.18. Analysis.
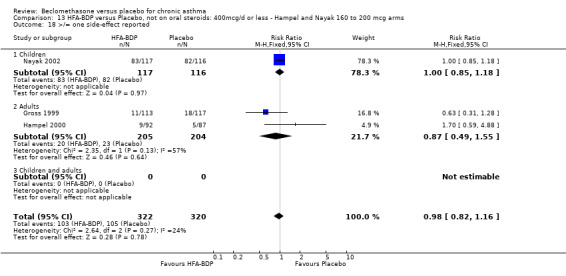
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 18 >/= one side‐effect reported.
13.19. Analysis.
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 19 Sinusitis.
13.20. Analysis.
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 20 Upper respiratory tract infection.
13.21. Analysis.
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 21 Low morning plasma cortisol levels.
13.22. Analysis.
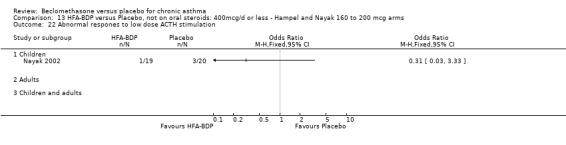
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 22 Abnormal respones to low dose ACTH stimulation.
13.23. Analysis.
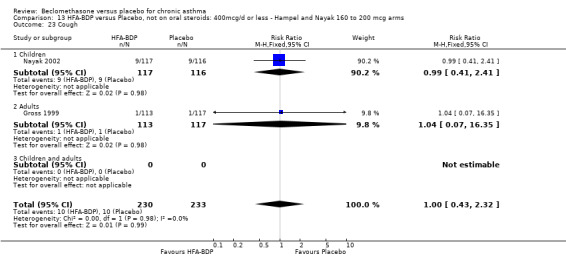
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 23 Cough.
13.24. Analysis.
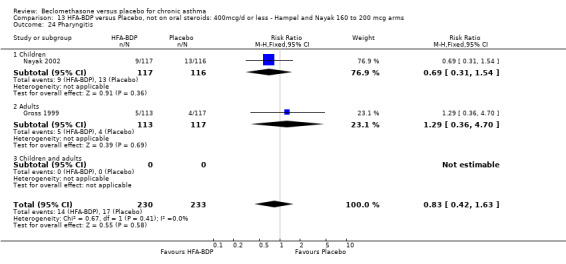
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 24 Pharyngitis.
13.25. Analysis.
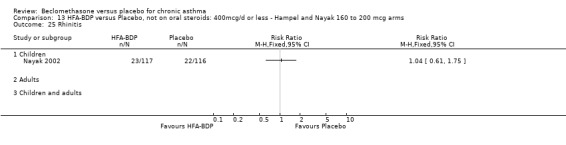
Comparison 13 HFA‐BDP versus Placebo, not on oral steroids: 400mcg/d or less ‐ Hampel and Nayak 160 to 200 mcg arms, Outcome 25 Rhinitis.
Comparison 14. BDP versus Placebo: Crossover studies, not on oral steroids: 400 mcg/d or less.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Daily PEFR (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Daily asthma symptom score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Methacholine bronchial responsiveness (log10 PD20 FEV1) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Histamine bronchial responsiveness (log10 PC20 FEV1) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 8am serum cortisol (mmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 4pm serum cortisol (mmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Serum cortisol 30 mins post 0.25mg cosyntropin (mmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
14.1. Analysis.
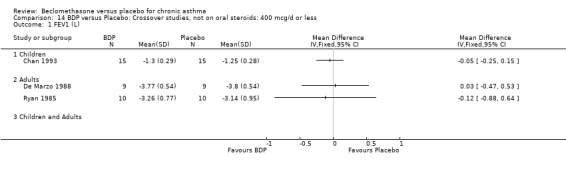
Comparison 14 BDP versus Placebo: Crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 1 FEV1 (L).
14.2. Analysis.
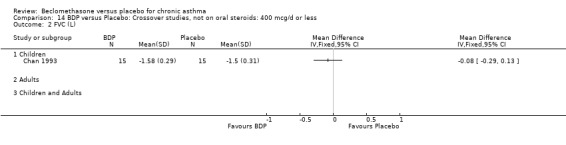
Comparison 14 BDP versus Placebo: Crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 2 FVC (L).
14.3. Analysis.
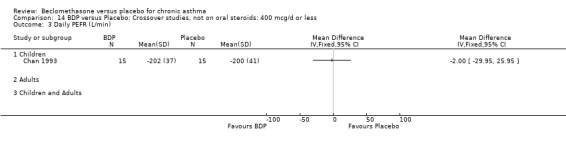
Comparison 14 BDP versus Placebo: Crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 3 Daily PEFR (L/min).
14.4. Analysis.
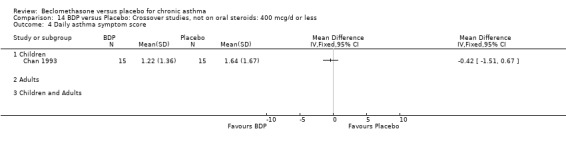
Comparison 14 BDP versus Placebo: Crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 4 Daily asthma symptom score.
14.5. Analysis.
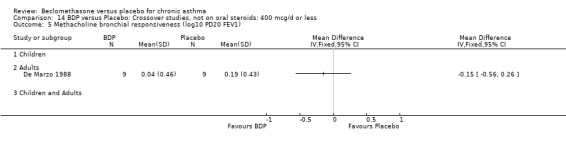
Comparison 14 BDP versus Placebo: Crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 5 Methacholine bronchial responsiveness (log10 PD20 FEV1).
14.6. Analysis.
Comparison 14 BDP versus Placebo: Crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 6 Histamine bronchial responsiveness (log10 PC20 FEV1).
14.7. Analysis.
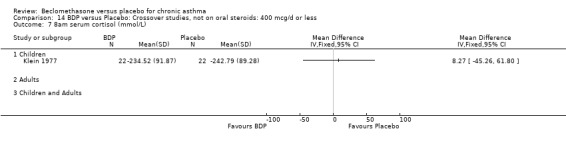
Comparison 14 BDP versus Placebo: Crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 7 8am serum cortisol (mmol/L).
14.8. Analysis.
Comparison 14 BDP versus Placebo: Crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 8 4pm serum cortisol (mmol/L).
14.9. Analysis.
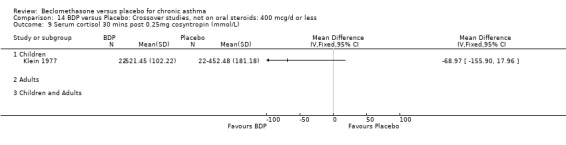
Comparison 14 BDP versus Placebo: Crossover studies, not on oral steroids: 400 mcg/d or less, Outcome 9 Serum cortisol 30 mins post 0.25mg cosyntropin (mmol/L).
Comparison 15. BDP versus Placebo: Crossover studies, not on oral steroids: 1000 mcg/d or greater.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Methacholine bronchial responsivness (log10 PD20 FEV1) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Children and Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
15.1. Analysis.
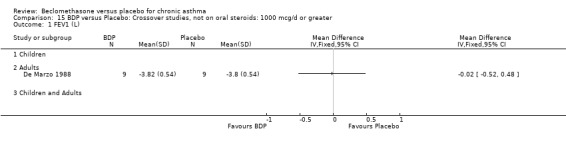
Comparison 15 BDP versus Placebo: Crossover studies, not on oral steroids: 1000 mcg/d or greater, Outcome 1 FEV1 (L).
15.2. Analysis.
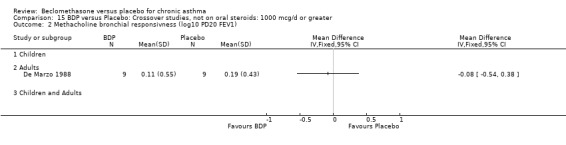
Comparison 15 BDP versus Placebo: Crossover studies, not on oral steroids: 1000 mcg/d or greater, Outcome 2 Methacholine bronchial responsivness (log10 PD20 FEV1).
Comparison 16. BDP versus Placebo: Crossover studies, on oral steroids, 400 mcg/d or less.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient preference for BDP rather than placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Children and Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Physician preference for BDP rather than placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Children and Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
16.1. Analysis.
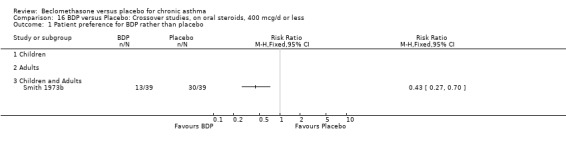
Comparison 16 BDP versus Placebo: Crossover studies, on oral steroids, 400 mcg/d or less, Outcome 1 Patient preference for BDP rather than placebo.
16.2. Analysis.
Comparison 16 BDP versus Placebo: Crossover studies, on oral steroids, 400 mcg/d or less, Outcome 2 Physician preference for BDP rather than placebo.
Comparison 17. BDP versus Placebo: Crossover studies, not on oral steroids: all doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 400 mcg/day or less | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 500‐800 mcg/day | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 1000 mcg/day or greater | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
17.1. Analysis.
Comparison 17 BDP versus Placebo: Crossover studies, not on oral steroids: all doses, Outcome 1 FEV1 (L).
Comparison 18. BDP versus Placebo: Crossover studies, not on oral steroids: all severities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Mild | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Mild to moderate | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Moderate | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Moderate to severe | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
18.1. Analysis.
Comparison 18 BDP versus Placebo: Crossover studies, not on oral steroids: all severities, Outcome 1 FEV1 (L).
Comparison 19. BDP versus Placebo: Crossover studies, not on oral steroids: all delivery devices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 MDI | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 MDI+SPACER | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 DPI | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
19.1. Analysis.
Comparison 19 BDP versus Placebo: Crossover studies, not on oral steroids: all delivery devices, Outcome 1 FEV1 (L).
Comparison 20. BDP versus Placebo: Crossover studies, not on oral steroids: all study durations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 1 to 4 weeks | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 1 to 5 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 6 months or greater | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
20.1. Analysis.
Comparison 20 BDP versus Placebo: Crossover studies, not on oral steroids: all study durations, Outcome 1 FEV1 (L).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bel 1990.
| Methods | Setting: The Netherlands, hospital outpatient clinic Length of intervention period: 4 months Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 31 adults: 14M 17F Age range: 17 to 73 years Inclusion criteria: History of episodic dyspnoea or wheezing either spontaneously or after exercise Exclusion criteria: Use of inhaled steroids or cromoglycate within six months of the study | |
| Interventions | BDP: 50 mcg 2 pfs 4x daily (400 mcg/d) Placebo:2 pfs 4xdaily Delivery device: MDI+spacer |
|
| Outcomes | FEV1 (% predicted) Methacholine BHR (PC20 FEV1) Daily asthma symptom score Withdrawal due to asthma exacerbation (number of patients) Oro‐pharyngeal side effects | |
| Notes | Reply from author but unable to clarify whether allocation concealment was used or provide data for asthma symptom score The study also had a nedocromil arm: results not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bennati 1989.
| Methods | Setting: Italy, paediatric outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, computer generated sequence Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 5 | |
| Participants | 30 children: 24M 6F Age range: 6 to 14 years Inclusion criteria: Patients with allergic asthma: either seasonal allergy to grass pollen or parietaria, or perennial allergy to house dust mite Exclusion criteria: No respiratory infection in 2 months prior to study, no use of inhaled or oral steroids in 2 months prior to study. | |
| Interventions | BDP: 50 mcg 2 pfs 3x daily (300 mcg/d) Placebo: 2 pfs 3xdaily Delivery device: MDI |
|
| Outcomes | Methacholine BHR (PC20 FEV1) Withdrawal due to asthma exacerbation (number of patients) | |
| Notes | Reply from author clarifying method of random order generation, use of allocation concealment still unclear. Study also has a BDP + salbutamol arm: results not included Patients were studied for one month during the maximal exposure period for their specific allergen: in spring for grass pollen and parietaria, autumn‐winter for house dust mite | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bergmann 1990.
| Methods | Setting: Germany, hospital outpatient clinic Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: single blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 2 | |
| Participants | 202 children and adults 124M 78F Age range: 12 to 78 years Inclusion criteria: Patients with a history of asthma, at least 15% difference between morning and evening PEFR on at least 7 days of run‐in period Exclusion criteria: Pregnancy Use of ICS or OCS, sodium cromoglycate or ketotifen. | |
| Interventions | BDP: 100 mcg 4x daily (400 mcg/d) Placebo: 4xdaily Delivery device: MDI |
|
| Outcomes | FEV1
Specific airways resistance
Morning PEFR
Evening PEFR
Daily beta2 agonist use (puffs/day)
aytime/nighttime breathlessness, cough, morning tightness score
All outcomes expresses as change compared to baseline Withdrawal due to asthma exacerbation (number of patients) |
|
| Notes | Reply from author: confirmed MDI delivery device used but unable to clarify details concerning the method of randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Berkowitz 1998.
| Methods | Setting: multicentre study, USA, hospital outpatient clinics Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 339 adults randomised, 274 completed study: 103M 171F Age range: 18 to 65 years Inclusion criteria: Clinical diagnosis of asthma for at least 2 years Requiring BDP 336 mcg/d or equivalent for control of symptoms FEV1 (% predicted) 50 ‐90% 15% or greater improvement in FEV1 following inhaled beta2 agonist Exclusion criteria: Lung disease other than asthma or other significant disease Frequent emergency dept visits or hospitalisations due to asthma exacerbations | |
| Interventions | BDP: 42 mcg 4 pfs 2x daily (336 mcg/d) Placebo: 4 pfs 2xdaily Delivery device: MDI |
|
| Outcomes | % change in FEV1 compared to baseline (as percentage of baseline) Change in FEV1 compared to baseline Change in FEF25‐75 compared to baseline Change in FVC compared to baseline Change in clinic PEFR compared to baseline Daily asthma symptom score Use of inhaled beta2 agonist Withdrawal due to asthma exacerbation (number of patients) Incidence of pharyngitis, dysphonia, dry throat, oral Candidiasis | |
| Notes | No reply from author to clarify details of randomisation method. Study also included a triamcinolone acetonate treatment arm: results not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bernstein 1999.
| Methods | Setting: multicentre study, USA Length of intervention period: 12 weeks Randomisation: yes, using a computer‐generated code Allocation concealment: method was stated Design: Masking: double‐blind, double‐dummy Excluded: Stated Withdrawals: Stated Baseline characteristics: Comparable Jadad score: 4 | |
| Participants | 365 children and adults 222 F 143 M
Age range: 12 to 74 years
Inclusion criteria: Patients with a history of asthma for at least 6 months,
Using an inhaled glucocorticoid daily for at least 30 days
Patients must have a stable of regimen of TAA, BDP or FP
None smokers or stopping in the last 6 months Any medication that interacts with the inhaled corticosteroids was not allowed Increase in the FEV1 >12% within 30 min after two puffs of albuterol
90>FEV1>60 of predicted normal value Exclusion criteria: Pre‐menarche Pregnancy Lactation Immunotherapy unless on a stable maintenance Treatment with oral corticosteroids for >14 days, in the last 6 months Need of ventilator support in the previous 5 year Hospitalization for asthma in the previous 3 months Requirement of >12 puffs a day of short acting B2‐agonist on 2 consecutive days. |
|
| Interventions | 1‐MF 100 mcg bid (200 mcg/day)
2‐MF 200 mcg bid (400 mcg/day)
3‐MF 400 mcg bid (800 mcg/day)
4‐BDP 168 mcg bid (336 mcg/day)
5‐Placebo Delivery device: MDI |
|
| Outcomes | FEV1 (%), FEV1 (%) with FEV1<75% predicted, FEV1 (%) with FEV1 >75% predicted, FVC (%), FEF25‐75%, AM PEFR (%), PM PEFR (%), wheezing score, difficulty breathing scores, cough score, B2‐ agonist use, number of nocturnal awakenings, response to therapy scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Boner 1991.
| Methods | Setting: Italy, paediatric outpatient clinic Length of intervention period: 8 weeks Randomisation: yes, by tossing of coin Allocation concealment: no Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 20 children: 17M 3F Age range: 6 to 12 years Inclusion criteria: Documented asthma as defined by ATS criteria with baseline FEV1 > 70 (% predicted) Allergic to either seasonal allergens (grass pollen and parietaria) or perennial allergens (house dust mite) Exclusion criteria: Not clearly stated, but from demographic data table of participants none receiving regular ICS prior to study. | |
| Interventions | BDP: 50 mcg 2 pfs 3x daily (300 mcg/d) Placebo: 2 pfs 3xdaily Delivery device: MDI |
|
| Outcomes | Methacholine BHR (PC20 FEV1) Withdrawal due to asthma exacerbation (number of patients) | |
| Notes | Reply from author clarifying method of random order generation and that allocation concealment was not employed. Antihistamines, short‐acting theophyllines discontinued 48 hours prior to methacholine challenge, long‐acting theophylline and cromolyn preparations discontinued 1 week prior to challenge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Brompton/MRC 1974.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 7 months Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 108 adults Inclusion criteria: Asthma of sufficient severity to warrant treatment with oral steroids for at least 6 months prior to study Over 16 years of age Mode dose prednisolone at least 5mg per day Exclusion criteria: Bronchopulmonary aspergillosis or positive for Aspergillus precipitins | |
| Interventions | BDP
1. 50 mcg 2 pfs 4xdaily (400 mcg/d)
2. 50 mcg 2 pfs 4xdaily (800 mcg/d) Placebo: 2 or 4 puffs 4xdaily Delivery device: MDI |
|
| Outcomes | Reduction in daily dose of oral prednisolone (number of patients) Discontinuation of oral prednisolone (number of patients) Incidence of oral Candidiasis (throat swab culture) | |
| Notes | Reply from authors but unclear as to whether allocation concealment was employed. 4 week run in period to establish minimum dose oral prednisolone required to keep patients symptomatically stable. Patients grouped according to daily dosage of prednisolone during the last 2 weeks of the run‐in period (5 to 9 mg, 10 to 15 mg, 16 mg or more) before randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bronsky 1998.
| Methods | Setting: multicentre study, USA, hospital outpatient clinics Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 328 adults randomised Age range: 18 to 68 years Inclusion criteria: Clinical diagnosis of asthma for at least 2 years Requiring BDP 336 mcg/d or equivalent for control of symptoms FEV1 50 to 90 (% predicted) 15% or greater improvement in FEV1 following inhaled beta2 agonist Exclusion criteria: Lung disease other than asthma or other significant disease Frequent emergency dept visits or hospitalisations due to asthma exacerbations | |
| Interventions | BDP: 42 mcg 4 pfs 2x daily (336 mcg/d) Placebo: 4 pfs 2xdaily Delivery device: MDI |
|
| Outcomes | FEV1 Change in FEV1 compared to baseline Change in FEF25‐75 compared to baseline Change in FVC compared to baseline Change in morning PEFR compared to baseline Change in evening PEFR compared to baseline Change in daily asthma symptom score compared to baseline Use of inhaled beta2 agonist Incidence of pharyngitis, dysphonia, dry throat, thrush | |
| Notes | Reply from author but did not clarify details of randomisation method. Study also included a triamcinolone acetonate treatment arm: results not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
BTTA 1976.
| Methods | Setting: UK , hospital outpatient clinic Length of intervention period: > 2 years Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Jadad score: 3 | |
| Participants | 189 adults: 91M 67F Mean age: 48.5 years Inclusion criteria: Adults > 16 years of age requiring oral prednisolone for asthma control for at least 1 year Able to use pressurised MDI, willing to give up all bronchodilator therapy other than salbutamol Exclusion criteria: Pregnancy or malignant disease | |
| Interventions | BDP:
1. 50 mcg 2pfs 4xdaily (400 mcg/d)
2. 100 mcg 2 pfs 4x daily (800 mcg/d) Placebo: 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | Reduction/cessation in use of oral prednisolone Recovery of normal tetracosactrin test response Oro‐pharyngeal side effects | |
| Notes | No reply from author to clarify details of randomisation method.
Trial conducted in two phases
Phase I
First week: use of trial inhaler 4x daily, no change in dose of maintenance oral steroid, beta2 agonists as required
Further weeks: (variable for each patient) stepwise reduction in daily dose of maintenance oral steroid to minimal dose necessary to control symptoms Phase II Continuation of treatment with trial inhaler, for further 24 week period during which time daily dose of oral steroid was further reduced according to a priori criteria based on the number of "failure days" (i.e. day on which patient needed to increase dose of oral steroid or take > 8 puffs of salbutamol), and clinical judgement at clinic visit. Study included a betamethasone valerate treatment arm: results not included |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cameron 1973.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: see notes Randomised: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: no separate baseline data Jadad score: 3 | |
| Participants | 20 patients Age range: not stated No inclusion or exclusion criteria stated | |
| Interventions | BDP: 50mcg 2 pfs 4xdaily (400mcg/d) Placebo: 2 pfs 4x daily Delivery device: MDI |
|
| Outcomes | Reduction in daily dose oral prednisolone | |
| Notes | No reply from author to clarify details of randomisation method. Length of study variable. Initial four week period, with daily OCS dose reduced at four week intervals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Carpentiere 1990.
| Methods | Setting: Italy, hospital outpatient clinic Length of intervention period: 4 weeks Randomised: yes, method not stated Allocation concealment: unclear Design: crossover, 2 week washout Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: FEV1 comparable Jadad Score: 2 | |
| Participants | 16 adults: 12M 4F Age range: 20 to 48 years Inclusion criteria: FEV1 > 70 (% predicted) Popranolol BHR (PC20 FEV1 < 8mg/ml) Exclusion criteria: Use of oral steroids, theophylline, cromoglycate or anticholinergics | |
| Interventions | BDP: 250 mcg 2pfs 2x daily (1000mcg/d) Placebo: 2 pfs 2xdaily Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) Propranolol BHR (PC20 FEV1) | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chan 1993.
| Methods | Setting: UK, paediatric outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, computer generated random number sequence Allocation concealment: yes Design: crossover, no washout Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable age and height Jadad score: 5 | |
| Participants | 18 children Age range: 7.7 to 8.7 years Inclusion criteria: Low birth weight Children symptomatic with wheeze or troublesome cough who had a positive airway responsiveness to histamine. Exclusion criteria: Previous regular prophylactic therapy for asthma, course of oral steroids for any reason within last three months | |
| Interventions | BDP: 200 mcg 2x daily (400 mcg/d) Placebo: 2xdaily Delivery device: Diskhaler DPI |
|
| Outcomes | FEV1 FVC Histamine BHR (PC20 FEV1) Daily PEFR Daily asthma symptom score | |
| Notes | Reply from author clarifying method of random order generation, that allocation concealment was employed, numerical data for spirometry and histamine challenge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Davies 1977.
| Methods | Setting: Canada, hospital outpatient clinic Length of intervention period: 12 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes Design: parallel Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 33 adults: 15M 18F Mean age: 55 (BDP group) 43 (placebo group) Inclusion criteria: Requiring oral steroid treatment for asthma control for 1 year or longer 15% or greater improvement in FEV1 after inhaled beta2 agonist Exclusion criteria: Chronic atopic eczema or a history of chronic bronchitis as defined by ATS criteria. | |
| Interventions | BDP 50mcg 2 pfs 4x daily (400mcg/d) Placebo 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) Daytime asthma symptom score Nighttime asthma symptom score Daily dose oral prednisolone Daily beta2 agonist use Blood eosinophil count 2 pm serum cortisol | |
| Notes | Reply from authors clarifying method of random order generation and that allocation concealment was employed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
De Marzo 1988.
| Methods | Setting: Italy, hospital outpatient clinic Length of intervention period: 1 week Randomisation: yes, computer generated random number sequence Allocation concealment: yes Design: crossover, 1 week washout Masking: double blind Excluded: stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 9 adults: 8M 1F Age range: 20 to 48 years Inclusion criteria: Free of respiratory infections for at least 8 weeks or exposure to TDI for at least 2 weeks. Exclusion criteria: Not stated | |
| Interventions | BDP:
1.50 mcg 4 pfs 2x daily (400mcg/d)
2.250 mcg 4 pfs 2xdaily (1000mcg/d) Placebo: 4 pfs 2xdaily Delivery device: MDI |
|
| Outcomes | FEV1 Methacholine BHR (PD20 FEV1) | |
| Notes | Reply from authors clarifying method of random order generation and that allocation concealment was employed. This study examines the effect of low and high dose inhaled steroids and placebo on airway responsiveness to methacholine in TDI sensitized subjects. Patients were treated for 7 days and underwent TDI challenge on day 7. The data for FEV1 and methacholine challenge is for day 6 prior to TDI challenge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Fahy 1998.
| Methods | Setting: USA, newspaper advertisement and hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, random number table Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable, except % predicted FEV1 which was higher in placebo group Jadad score: 4 | |
| Participants | 24 adults: 14M 10F Mean (SD) age: BDP group 34 (9) years, placebo group 32 (9) years Inclusion criteria: A history of asthma symptoms (not otherwise defined) Methacholine BHR (PC20 FEV1 8 mg/ml or less) Exclusion criteria: ICS or OCS use within last 6 weeks Upper respiratory tract infection within last 6 weeks Smoker within last year or 10 or more pack year history | |
| Interventions | BDP: 42 mcg 4 pfs 2x daily (336 mcg/d) Placebo: 4pfs 2xdaily Delivery device: MDI+Ellipse spacer |
|
| Outcomes | FEV1 FEF25‐75% Morning PEFR Methacholine BHR (PC20 FEV1) Weekly beta2 agonist use Weekly asthma symptom score Induced sputum outcomes: % of squamous,epithelial, neutrophil, macrophage, lymphocyte and eosinophils ECP, tryptase, fibrinogen and mucin‐like glycoprotein Withdrawal due to asthma exacerbation (number of patients) | |
| Notes | Reply from author but use of allocation concealment unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fanelli 1993.
| Methods | Setting: Italy, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: single blind Excluded: not stated Withdrawals: not stated Baseline characteristics: comparable Jadad score: 1 | |
| Participants | 32 adolescents/ adults: 16M 16F Age range: 15 to 45 years Inclusion criteria: Clinically stable, asymptomatic asthma (ATS criteria), with severe to moderate level of bronchial hypersensitivity (PC20 FEV1 0.01 to 1.7 mg/ml.) Exclusion criteria: Smokers Use of ICS or OCS | |
| Interventions | BDP: 250 mcg 2 pfs 4x daily (2000 mcg/d) Placebo: 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) FVC (% predicted) FEV1/VC (%) Histamine BHR (PC20 FEV1) Minute ventilation, inspiratory flow rate, tidal volume, respiratory frequency, inspiratory/expiratory time, after histamine challenge Withdrawal due to asthma exacerbation (number of patients) | |
| Notes | No reply from author to clarify details of randomisation method. The randomised study was followed by observational study: results not included here | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fournier 1990.
| Methods | Setting: France, hospital outpatient clinic Length of intervention period: 3 months Randomised: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable except fairly large range for % predicted FEV1 at baseline (53‐104) Jadad score: 3 | |
| Participants | 11 adults: 9M 2F Age range: 23 to 63 years Inclusion criteria: No asthma exacerbation within last month No oral steroid treatment within last month Normal chest xray Non smokers Exclusion criteria: Respiratory tract infection within last month | |
| Interventions | BDP: 250 mcg 2 pfs 2x daily (1000 mcg/d) Placebo: 2 pfs 2xdaily Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) Comparsion of different fixation methods for bronchial biopsies | |
| Notes | French translation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gaddie 1973.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 2 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 15 adults: 8M 7F Age range: 21 to 74 years Inclusion criteria: History of airway obstruction with pulmonary function abnormalities typical of asthma Exclusion criteria: Systemic steroids within last 3 months | |
| Interventions | BDP: 100mcg 1pf 4x daily (400mcg/d) Placebo: 1 pf 4xdaily Delivery device: MDI |
|
| Outcomes | Change in FEV1 Change in FVC 9 am plasma cortisol Daily beta2 agonist use | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gross 1999.
| Methods | Setting: Multicentre study Length of intervention: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: Parallel Masking: double‐blind. Dosing regimens differed in terms of number of puffs of medication per day. There two types of placebo given. Participants were aware that they were in one of two groups with different dosing regimens, but they were not aware as to whether they were being given placebo or active treatment. Excluded: Stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 347 patients F/M 185/162 Age range: 18 to 65 years Inclusion criteria: None smokers Moderate asthma who were symptomatic despite current treatment with bronchodilators and inhaled steroid of 0 to 400 mcg/day FEV1 > 15% after B2 ‐agonist Mean AM PEF of between 50 and 80% of the predicted normal value plus one or more of the following: sleep disturbance >1 night, presence of asthma symptoms >3 days, use of B2agonist at least twice a day to relieve symptoms Exclusion criteria: any clinically significant abnormality or disease; acute or lower respiratory tract infection within 4 weeks before the enrolment; Medication other than SABA prn | |
| Interventions | 1‐HFA‐BDP 50 mcg (4 puffs, 2 x daily ‐ 400 mcg/day)
2‐CFC‐BDP 50 mcg (8 puffs 2 x daily; 800 mcg/day)
3‐HFA‐placebo (4 puffs, 2 x daily, or 8 puffs, 2 x daily) Treatment preceded by short course of oral steroids to optimise asthma control prior to study entry. Delivery device: pMDI |
|
| Outcomes | FEV1; am PEF; Nights without sleep disturbance %; B2‐agonist use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hampel 2000.
| Methods | Setting: multicentre study USA and France Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking; double blind Design: parallel group Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 270 adults Age range 18‐65 years Inclusion criteria: Adults with mild to moderate asthma for at least 12 months No ICS use for previous 3 months ß‐agonist prn only Increase in FEV1 >/= 15% in response to ß‐agonist Non‐smokers Appropriate use of pMDI Exclusion criteria: Cardiac disease; pulmonary disease other than asthma; Hypersensitivity to BDP A history of alcohol or substance abuse medications other than ß‐agonists prn not permitted in the study | |
| Interventions | BDP 50 mcg 2xdaily (100mcg/d)
BDP 100 mcg 2xdaily (200 mcg/d)
Placebo Delivery device: pMDI |
|
| Outcomes | FEV1 Morning PEF Evening PEF FEF25%‐75% Nights without sleep disturbance Asthma symptoms scores Daily beta2‐agonist use All outcomes reported as change compared to baseline | |
| Notes | No reply from the author to clarify method of randomisation and the withdrawal numbers due to side effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Harvey 1976.
| Methods | Setting: USA, hospital outpatient clinic Length of intervention period: 12 weeks Randomisation: yes method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 34 adolescents/adults: 11M 23F Age range: 14 to 75 years Inclusion criteria: Asthmatic patients dependent on oral steroids for at least 6 months, not exceeding 20mg per day. Exclusion criteria: Pregnancy Concurrent severe disease | |
| Interventions | BDP: 50mcg 2 pfs 4xdaily (400mcg/d) Placebo: 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | % change in FEV1 % change in FEF 50 Daily oral prednisolone dose 8am plasma cortisol Discontinuation of oral steroids (number of patients) | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hodson 1974.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 9 weeks Randomisation: yes, method not stated Allocation concealment: yes (assignment to groups by independent pharmacist) Design: parallel group Masking: double blind Excluded: not stated Withdrawal: stated Baseline characteristics: not reported Jadad score: 4 | |
| Participants | 25 patients: no details of age but probably adults only Inclusion criteria: Asthmatic patients requiring prednisolone at least 7mg/day for adequate control Exclusion criteria: Co‐existent chronic obstructive bronchitis | |
| Interventions | BDP: 50 mcg 2 pfs 4x daily (400mcg/d) Placebo 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | No. patients discontinuing oral prednisolone FEV1 FVC Clinic PEFR Plasma cortisol Plasma cortisol post tetracosactrin | |
| Notes | Reply from author but unable to clarify method of random order generation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Holst 1974.
| Methods | Setting: New Zealand, hospital outpatient clinic Length of intervention period: 12 weeks Randomisation: yes, closed box containing cards marked with allocation schedule Allocation concealment: yes Design: crossover, no washout Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 27 children/adults: 7M 20F Age range: 7‐66 years Inclusion criteria: Asthmatic patients with at least 10% reversibility in FEV1 Exclusion criteria: Hospital admission for acute asthma exacerbation within previous 12 months | |
| Interventions | BDP: 400mcg/d Placebo Delivery device: MDI |
|
| Outcomes | FEV1 Morning asthma symptom score Nighttime asthma symptom score | |
| Notes | Reply from authors clarifying method of random order generation and use of allocation concealment. No numerical outcome data in form suitable for meta‐analysis presented. Overall finding of a significant reduction in asthma symptoms and an improvement in FEV1 in BDP treated group compared to placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hoshino 1998.
| Methods | Setting: Japan, hospital outpatient clinic Length of intervention period: 6 months Randomisation: yes, method not stated Allocation concealment: yes (off site allocation of patients by pharmacist with coded schedule) Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 30 adults randomised, 24 completed study: 19M 5F Age range: 16‐48 years Inclusion criteria: Patients with mild to moderate asthma (ATS criteria 1987) Non‐smokers 20% or greater improvement in PEFR or FEV1 spontaneously or after inhaled beta2 agonist Exclusion criteria: Use of inhaled or oral steroids, nedocromil or sodium cromoglycate within last 4 months Respiratory tract infection within last 2 weeks | |
| Interventions | BDP: 400 mcg 2xdaily (800 mcg/d) Placebo: 2xdaily Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) FEV1 Daily PEFR Diurnal variation in PEFR (morning‐evening as % of daily mean) Methacholine BHR (Dmin) Daily asthma symptom score Daily beta2 agonist use Withdrawal due to asthma exacerbation (number of patients) Bronchial biopsy measurements: lamina reticularis thickness, activated eosinophils, T‐lymphocytes, fibroblasts Expression of IGF‐1, TGF‐beta1, PDGF | |
| Notes | Reply from author confirming use of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hoshino 2001.
| Methods | Setting: Japan Length of intervention: 6 months Allocation concealment: unclear Masking: double‐blind Design: parallel‐group Withdrawals: stated Baseline characteristics : comparable Jadad score: 3 | |
| Participants | 28 asthmatic patients
F/M:17/11, asthma diagnosis was according to ATS, 14 people in the BDP group and 14 in the placebo, mean age 29 in the BDP and 30 in the Placebo, there was no significant differences at baseline between the two groups. Inclusion criteria: Non‐smokers, 20% improvement in FEV1 after B2‐agonist or spontaneously, None received inhaled or oral corticosteroids, or any other asthma medication rather than B2‐agonist. Exclusion criteria: Upper or lower respiratory infections for at least 2 weeks before entering the trial |
|
| Interventions | BDP 800mcg/d
Placebo Delivery device: MDI |
|
| Outcomes | FEV1 FEV1 % Airway vascularity | |
| Notes | No reply from the author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Katsunuma 1993.
| Methods | Setting: Japan, hospital outpatient clinic Length of intervention period: 8 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout Masking: single blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 22 children/adults: 13 M 9 F Age range: 9‐24 years Inclusion criteria: Allergic asthma (not further defined) Exclusion criteria: Use of oral steroids currently or in previous 3 months | |
| Interventions | BDP: either 150 mcg 2xdaily or 100 mcg 3xdaily (300 mcg/d) Placebo: 2 or 3 x daily Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) Methacholine BHR (Dmin) | |
| Notes | No reply from author to clarify details of randomisation method. A significant carryover effect was calculated for FEV1 (% predicted) when individual patient data was estimated from the graphical plot for this outcome. It was not possible to assess carryover effect for methacholine challenge Dmin (data presented in graph form using logarithmic scale). The combined period data for this outcome was not therefore included in the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kerigan 1977.
| Methods | Setting: Canada, hospital outpatient clinic Length of intervention period: 2 years Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: comparable Jadad score: 2 | |
| Participants | 25 adults: 11M 14F Age range: 27 to 77 years Inclusion criteria: Asthmatic patients requiring oral prednisolone therapy for asthma control Exclusion criteria: Not stated | |
| Interventions | BDP: 50 mcg 2 pfs 4xdaily (400mcg/d) Placebo: 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | Reduction in daily oral prednisolone dose | |
| Notes | Reply from authors but unable to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kerrebijn 1976.
| Methods | Setting: The Netherlands, paediatric outpatient clinic Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel Masking: single blind (patients blinded) Excluded: not stated Withdrawals: stated Baseline characteristics: symptom scores pre‐trial similar both treatment groups Jadad score: 2 | |
| Participants | 30 children Age range: not stated Inclusion criteria: "Severe and frequent asthma symptoms which failed to show adequate improvement despite treatment with spasmolytics, cromoglycate and in some cases, corticosteroids" Exclusion criteria: Not stated | |
| Interventions | BDP:
1. 50 mcg 1 pf 3xdaily (150 mcg/d)
2. 100 mcg 1 pf 3xdaily (300 mcg/d) Placebo: 1 pf 3xdaily Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) Daily asthma symptom score | |
| Notes | Reply from author but unable to clarify details concerning method of randomisation. 3 month run‐in period during which time all patients received BDP 300 micrograms daily via MDI openly for 3 months. No numerical data in a form suitable for meta‐analysis presented. Overall finding of a significant reduction in asthma symptoms in BDP treated group compared to placebo, no difference in spirometry measures. The study was continued for a further 12 months in an open manner in a smaller group of children who had been receiving BDP 300 mcg/d to assess effects of treatment on height, weight and adrenal function and incidence of oral Candiasis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kerstjens 1994.
| Methods | Setting: The Netherlands, hospital outpatient clinic Length of intervention period: 18 months Randomisation: yes, computer generated random number sequence Allocation concealment: yes (randomisation schedule handled by central independent non‐affiliated telephone centre separate from participating centres) Design: parallel group Masking: double blind Excluded: no separate data for asthmatic patients Withdrawals: stated Baseline characteristics: no separate data for asthmatic patients Jadad score: 4 | |
| Participants | 98 adults: 57M 41F Inclusion criteria: Aged 18 to 60 yrs FEV1 and IVC below predicted normal value Histamine BHR ( PC20 FEV1 <8mg/ml) Reversibility to beta‐2 agonist No ICS or cromoglycates use in last four weeks, antihistamines in last six weeks No separate exclusion criteria | |
| Interventions | BDP: 2 pfs 4xdaily (800mcg/d)+ terbutaline 250mcg 2 pfs 4x daily Placebo: 2 puffs 4x daily+ terbutaline 250mcg 2 pfs 4x daily Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) Histamine BHR (PC20 FEV1) Morning PEFR Afternoon PEFR Diurnal variation in PEFR Change in asthma symptom score | |
| Notes | Reply from author clarifying method of random order generation and use of allocation concealment. Raw data for FEV1, bronchial responsiveness to histamine and PEFR for asthmatic subjects provided by author. Asthma defined by histamine BHR of 8 mg/dL or less and significant reversibility to inhaled beta2 agonist. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Klein 1977.
| Methods | Setting: USA, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, 4 week washout Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 22 children: 19M 3F Mean age: 11 years Inclusion criteria: Severe, chronic asthma requiring "round‐the‐clock" therapy with theophylline, adrenergic agents, and/or sodium cromoglycate 15% or greater improvement in FEV1 or MEF25‐75 after inhaled beta2 agonist Exclusion criteria: Patients not clinically stable at start of trial | |
| Interventions | BDP: 50 mcg 2 puffs 4xdaily (400 mcg/d) Placebo: 2 puffs 4xdaily Delivery device: MDI |
|
| Outcomes | FEV1 FVC FEF25‐75 Clinic PEFR Weekly symptom score Asthma attack score Additional medication score 8 am serum cortisol 4 pm serum cortisol Serum cortisol after rapid ACTH test | |
| Notes | Reply from author but unable to clarify details concerning method of randomisation. It is probable that none of the subjects had been on therapy with either inhaled or oral steroids before the study (confirmation by author). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kraemer 1987.
| Methods | Setting: Germany hospital outpatient clinic Length of intervention period: 8 weeks Randomisation: yes by computer generated random number method (Glaxo Switzerland) Allocation concealment: yes Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 30 children: 14M 16F Age range: 5 to 15 years Inclusion criteria: History of wheeze, elevated IgE levels, positive RAST against one or more perennial allergens and reversible airways obstruction on pulmonary function tests Specific airways conductance < 80 (% predicted). Exclusion criteria: Symptom free at start of study | |
| Interventions | BDP: 1. children < 12 years 100mcg/d 2. children > 12 years 200 mcg/d Delivery device: Rotahaler DPI | |
| Outcomes | % change in FEV1 Lung function via whole body plethysmography Thoracic gas volume Specific airway resistance Specific airway conductance Carbachol BHR (PD100 Raw) | |
| Notes | Reply from author clarifying method of random order generation and use of allocation concealment. Study also included a sodium cromoglycate treatment arm: results not included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lacronique 1991.
| Methods | Setting: multicentre study France, hospital outpatient clinics Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable except for distribution of gender within each treatment arm Jadad score: 3 | |
| Participants | 124 adults 58 M 66 F Mean age 52 years Inclusion criteria: Diagnosis of asthma (ATS criteria) Asthmatic for 10 years or longer Requiring maintenance oral steroids for asthma control Significant improvement in FEV1 after inhaled salbutamol Exclusion criteria: Inhaled steroid treatment in last two months Co‐existent COPD Smokers | |
| Interventions | BDP: 250mcg 1 pf 4x daily (1000mcg/d) Placebo: 1 pf 4xdaily Delivery device: MDI |
|
| Outcomes | Discontinuation of oral prednisolone (number of patients) Daily dose oral prednisolone % change in FEV1 PEFR (% predicted) Daily asthma symptom score | |
| Notes | No reply from author to clarify details of randomisation method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Laviolette 1994.
| Methods | Setting: Canada, hospital outpatient clinic Length of intervention period: 6 weeks Randomisation: yes, computer generated sequence Allocation concealment: yes Design: crossover, no washout Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 12 adults: 6M 6F Inclusion criteria: Diagnosis of asthma (ATS criteria) Beta2 agonist use only Exclusion criteria: Use of oral steroids in previous 6 months | |
| Interventions | BDP: 50 mcg 2 pfs 2x daily (200mcg/d) Placebo: 2 pfs 2xdaily Delivery device: Diskhaler DPI |
|
| Outcomes | FEV1 FVC Morning PEFR Methacholine BHR (PC20 FEV1) Daily use beta‐2 agonists Blood eosinophil count | |
| Notes | Reply from author clarifying method of random order generation and use of allocation concealment. Raw data for FEV1, bronchial responsiveness to methacholine and PEFR provided by authors. A carryover effect is evident, first period data used only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Laviolette 1999.
| Methods | Setting: Multi‐center Length of intervention period:12 to 16 weeks Randomisation: yes, using a computer‐generated schedule Allocation concealment: yes Design: parallel group Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 642 patients, age >15 Inclusion criteria: None smokers A history of intermittent or persistent asthma for at least one year On inhaled corticosteroids for at least 6 weeks before the pre study visit Exclusion criteria: Respiratory disorders other than asthma Upper respiratory infection within the 3 wk before the pre study visit Pregnancy | |
| Interventions | 1‐ montelukast 10 mg /day + BDP 200 mcg x 2/day(16 wk) 2‐ placebo tab + BDP 200mcg x 2/day (16 wk) 3‐ montelukast 10 mg/day + inhaled placebo (12 wk) 4‐ placebo tab/day + inhaled placebo (12 wk) | |
| Outcomes | FEV1 Morning PEFR Evening PEFR Daytime symptoms score B‐agonist use | |
| Notes | Missing data has been requested from the author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lovera 1975.
| Methods | Setting: Canada, hospital outpatient clinic Length of intervention period: 6 weeks Randomisation: yes, using random number table Allocation concealment: unclear Design: crossover, no washout Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: not reported Jadad score: 4 | |
| Participants | 21 children/adolescents: 16M 5F Age range 6 to 20 years Inclusion criteria: Diagnosis of asthma Under consideration for treatment with oral steroids to control asthma Exclusion criteria: Current use of oral steroids | |
| Interventions | BDP: 50 mcg 2 pfs 4x daily (400mcg/d) Placebo: 2 pfs 4 x daily Delivery device: MDI |
|
| Outcomes | Number of symptom free days Number of wheezing attacks Hours of wheezing Medication requirements Total lung capacity Expiratory flow at 25% vital capacity Expiratory flow at 70% TLC Specific airway conductance | |
| Notes | Reply from authors clarifying method of random order generation but use of allocation concealment unclear. First part of paper reports results of non‐randomised open study: results not included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Maestrelli 1993.
| Methods | Setting: Italy, hospital outpatient clinic Length of intervention period: 5 months Randomisation: yes, method not stated Allocation concealment: yes Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable but subjects in BDP group slightly older Jadad score: 3 | |
| Participants | 15 adults: 4M 11F Age range: 21 to 54 years Inclusion criteria: Occupational asthma induced by TDI Methacholine BHR Exclusion criteria: Use of ICS in month prior to study or OCS in 2 months prior to study Smokers | |
| Interventions | BDP: 1mg 2x daily (2000 mcg/d) Placebo: 2xdaily Delivery device: MDI |
|
| Outcomes | Methacholine BHR (PD20 FEV1) Maximum fall in FEV1 after exposure to TDI Plasma cortisol following 0.1IU/kg Insulin at 120 minutes | |
| Notes | Reply from authors confirming use of allocation concealment. Original data for methacholine BHR and plasma cortisol provided by authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Malmstrom 1999.
| Methods | Setting: multicentre study, Europe, Africa, Australia, central America, south America Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Masking: double blind, double dummy Design: parallel group Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 895 patients: 118 M, 182 F
Age range: 15‐85 years. Inclusion criteria: Patients with at least one year history of asthma FEV1 between 50% and 85% of predicted value Increase of at least 15% in absolute FEV1 after inhaled‐B‐agonists Daytime asthma score of at least 64 out of possible 336 Daily use of at least one puff of short acting inhaled B‐agonist. Exclusion criteria: Using inhaled or oral corticosteroids, cromolyn, or nedocromil within the previous 4 weeks Using long acting B‐agonists, antimuscarinics, newly instituted theophylline within the previous 2 weeks. Intermitted use of short acting antihistamines was allowed Immunotherapy was permitted if it had been started at least 6 months before the initial study with a constant dose. |
|
| Interventions | 1. Montelukast 10 mg/day(12 weeks)
2. Beclomethasone 200 mcg x2/day (12 weeks)
3. Placebo Delivery device: spacer |
|
| Outcomes | FEV1 Daytime asthma symptoms Nocturnal awakenings Morning and evening peak expiratory flow rate Daily use of salbutamol | |
| Notes | No reply from the author to clarify method or randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Martin 1974.
| Methods | Setting: New Zealand, hospital outpatient clinic Length of intervention period: 10 days Randomisation: yes, method not stated Allocation concealment: yes (codes retained by pharmacy) Design: crossover, 1 week washout Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: no details Jadad score: 3 | |
| Participants | 18 adults Age range: not stated Inclusion criteria: Asthmatic patients with FEV1 variability of at least 0.5 L Exclusion criteria: none stated | |
| Interventions | BDP: 50 mcg 2 pfs 4x daily (400mcg/d) Placebo: 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | FEV1 FVC Daily asthma symptom score | |
| Notes | No numerical data in a form suitable for meta‐analysis presented. Overall finding of a significant reduction in asthma symptoms and a significant improvement in spirometry in BDP treated group compared to placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Matthys 1998.
| Methods | Setting: multicentre study Germany, hospital outpatient clinic Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind, double dummy Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 256 adult patients: 129M 127F Age range: 18 to 65 years Inclusion criteria: Diagnosis of asthma for at least 4 weeks Moderate severity disease according to GINA criteria Increase in FEV1 of at least 15% following inhaled beta2 agonist Using inhaled beta2 agonist for relief of symptoms No use of inhaled steroids for at least 3 weeks before trial PEFR 50‐80 (% predicted) over last 7 days of 2 week run‐in period Exclusion criteria: Smoker within last 6 months Cardiac or pulmonary disease other than asthma Known hypersensitivity to BDP Respiratory tract infection within last 4 weeks | |
| Interventions | BDP:
1. 50 mcg 4 pfs 2x daily (400 mcg/d)
2. 100 mcg 2pfs 2xdaily (400 mcg/d) Placebo: 2 or 4 pfs 2xdaily Delivery device: HFA propellant MDI |
|
| Outcomes | Change in morning PEFR compared to baseline Change in FEV1 compared to baseline Daily use of beta2 agonist % nights free from sleep disturbance Withdrawal due to asthma exacerbation (number of patients) Oro‐pharyngeal side effects | |
| Notes | Reply from author, request for details of randomisation method forwarded to sponsoring pharmaceutical company but no reply. The investigators pool the results for both groups of patients treated with BDP delivered by different schedules. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Messerli 1975.
| Methods | Setting: Switzerland, hospital outpatient clinic Length of intervention period: 3 weeks Randomisation: yes, method not stated Allocation concealment: yes Design: parallel group Masking: double blind Excluded: stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 16 adults: 10M 6F Age range: 18 to 64 years Inclusion criteria: Stable asthma for more than one year No requirement for oral steroids or corticotrophin treatment in the previous six months Exclusion criteria: Not stated | |
| Interventions | BDP: 50 mcg 2 pfs 4x daily (400mcg/d) Placebo: 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | % change in FEV1 % change in VC % change in clinic PEFR Blood eosinophil count 8am plasma cortisol Daily asthma symptom score daily Daily beta2 agonist use Withdrawal due to asthma exacerbation (number of patients) | |
| Notes | No reply from author to clarify method of random order generation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Nathan 1997.
| Methods | Setting; USA, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 423 children/adults: 170M 251F Age range: 12 to 65 years Inclusion criteria: Asthma for at least two years Increase in FEV1 > 15% after inhaled beta2 agonist FEV1 50‐80 (% predicted) Regular use of ICS Exclusion criteria: Not stated | |
| Interventions | BDP:
1. 42mcg 4 pfs 2x daily (336mcg/d)
2. 84mcg 2 pfs 2xdaily (336mcg/d)
3. 84mcg 8 pfs 2xdaily (1344mcg/d) Placebo: 8 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | % change FEV1 FVC Morning PEFR Evening PEFR FEF25‐75% Patient and physician evaluation of symptoms (wheeziness, tightness in chest, shortness of breath and cough) Oro‐pharyngeal side effects | |
| Notes | Reply from author but unable to clarify details concerning method of randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Nathan 2001.
| Methods | Setting: Multicenter, USA Length of intervention period: 12 weeks Randomisation: yes, using a computer‐genera ted random code Allocation concealment: stated Design: parallel group Masking: double blind, double‐dummy Excluded: Stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 227 children/adults: 78 M 149 F Age range: 12 to 75 years Inclusion criteria: History of asthma at least for the last 6 months Maintenance on prescribed inhaled glucocorticoids at least 30 days before the study entry Baseline FEV1 between 60‐90% of the normal predicted value Increase in absolute FEV1>12% and absolute volume increase of at least 200 ml within 30 minutes of two inhalations of short acting B2 agonist (albuterol) Exclusion criteria: Requiring daily nebulized B2‐adrenergic agonist Two events of emergency hospital treatment in the previous 3 months Requiring intubation for asthma within the last 5 years Clinical evidence of oral candidiasis or other respiratory diseases other than asthma were also excluded Smoking in the last 6 months pregnant and breast feeding women were excluded Women who were using an acceptable methods of birth control were also not enrolled. | |
| Interventions | 1‐MF‐DPI 100 mcgx2day
2‐MF‐DPI 200 mcg/x2day
3‐BDP 168mcgx2day
Placebo Delivery device: MDI |
|
| Outcomes | FEV1 FVC FEF25‐75% Morning PEFR (L/M) Wheezing score Breathing difficulty score Cough score B2‐agonist daily use Nocturnal awakening | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Nayak 2002.
| Methods | Setting: USA, multi‐centre study Length of intervention period: 12 weeks Randomisation: yes, (computer‐produced randomisation schedule) Masking: Double‐blind Study design: parallel‐group Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 353 children, M/F 224/129, age range: 5‐12 years. Comorbid Illnesses: rhinitis, contact dermatitis, otitis media. Withdrawal rate: 15 (BDP 80), 9 (BDP 160), 19 (placebo) Inclusion criteria:Children aged 5 to 12 years with moderate stable asthma, symptomatic asthma for at least 6 months before the enrolment, on B‐agonist inhaler therapy one puff bid, FEV1between 50‐80% of predicted after withholding short acting beta2‐agonist inhaler for 4h, FEV1 >12% after 400 mcg inhalation of B‐agonist. Exclusion criteria: Any other pulmonary diseases rather than asthma, evidence of immunologic, neoplastic, endocrine, hematologic, cardiac, hepatic, renal, GI, neurological and psychiatric abnormalities, Upper or lower respiratory tract infection within 2 or 4 weeks prior the screening phase or during the run in period, a history of BDP hypersensitivity, use of injected, oral or inhaled corticosteroids within 6 months, 8 weeks, and 6 weeks, respectively. Use of other asthma therapies was not allowed. | |
| Interventions | 1‐HFA‐BDP 80 mcg/d(n=120), 2‐HFA‐BDP 160 mcg/d(n=117), 3‐Placebo (n=116) Delivery device: Autohaler breath‐actuated device |
|
| Outcomes | FEV1(% predicted)
Morning PEF
Daily use of rescue beta2‐agonist
Days free from asthma symptoms
Asthma exacerbations Adverse events Acute asthma episode Plasma cortisol levels |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pennings 1997.
| Methods | Setting: The Netherlands, hospital outpatient clinic Length of intervention period: 6 weeks Randomisation: yes, by computer generated sequence Allocation concealment : yes Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: BDP treatment group higher baseline FEV1 than placebo otherwise comparable Jadad score: 5 | |
| Participants | 40 adults: 20M 20F Mean age: 29 (BDP group) 28 (placebo group) Inclusion criteria: Asthma as defined by recurrent attacks of dyspnoea and wheezing Normal bronchial impedance Histamine BHR (PD20 FEV1 < 8 micromols) Positive skin test to at least one aero‐allergen Exclusion criteria: Use of ICS or OCS in last 3 months | |
| Interventions | BDP: 250 mcg 2 pfs 2x daily (1000mcg/d) Placebo: 2 puffs 2xdaily Delivery device: MDI |
|
| Outcomes | FEV1 FEV1 after cold air challenge (IHCA) IVC after cold air challenge (IHCA) Bronchial resistance at 8 Hz (R8) during forced oscillation (FOT) after cold air challenge Frequency dependence of resistance (FD) during FOT after cold air challenge Reactance at 8 Hz (X8) during FOT after cold air challenge Resonant frequency (fO) during FOT after cold air challenge Withdrawal due to asthma exacerbation (number of patients) Oro‐pharyngeal side effects | |
| Notes | Reply from author clarifying method of random order generation and use of allocation concealment. Raw data for all outcomes supplied by authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Radha 1975.
| Methods | Setting: India, hospital inpatients and outpatients Length of intervention period: 6 weeks Randomisation: yes, method not stated Allocation concealment: not stated Design: parallel group Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: comparable Jadad score: 2 | |
| Participants | 30 children/adults: 16M 14F Age range: 11to 53 years Inclusion criteria: Bronchial asthma without satisfactory relief of symptoms using bronchodilators Exclusion criteria: Acute respiratory infection Use of drugs which would interfere with estimation of plasma cortisol | |
| Interventions | BDP: 50mcg 2 pfs 4x daily (400mcg/d) Placebo: 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) FVC Number and severity of asthma exacerbations per week Plasma cortisol (time not specified) | |
| Notes | No reply from author to clarify details of method of randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Riordan 1974.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 2 weeks Randomisation: yes, method not stated Allocation concealment: not stated Design: crossover, no washout Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: no data for patients randomised to each treatment sequence Jadad score: 2 | |
| Participants | 25 children and adults: 8M 17F Age range: 12‐77 years Inclusion criteria: Asthma with significant reversibility of airway obstruction Stable symptoms Exclusion criteria: Not stated | |
| Interventions | BDP: 50 mcg 2 pfs 4x daily (400 mcg/d) Placebo: 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | FEV1 FVC Morning PEFR Evening PEFR Clinic PEFR Daily symptom score Use of additional beta2 agonists | |
| Notes | No reply from author to clarify details of method of randomisation. No numerical data presented in a form suitable for meta‐analysis. Overall finding of significant improvements in PEFR and symptom scores for BDP group compared to placebo, no difference in spirometry or beta2 agonist use. Study also included a betamethasone valerate treatment arm: results not considered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ryan 1985.
| Methods | Setting: Canada, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, 2 week washout Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 10 adults: 2M 8F Age range 22 to 38 years Inclusion criteria: Asthma symptoms controlled on bronchodilators alone, no disturbance of sleep Normal or near normal spirometry Exclusion criteria: Not stated | |
| Interventions | BDP: 50 mcg 4 pfs 2x daily (400 mcg/d) Placebo: 4 pfs 2xdaily Delivery device: MDI |
|
| Outcomes | FEV1 Histamine BHR (PC20 FEV1) | |
| Notes | No reply from author to clarify details of method of randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Salmeron 1989.
| Methods | Setting: multicentre study France, hospital outpatient clinics Length of intervention period: 8 weeks Randomised: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 43 adults Mean age: 49 (BDP group) 44 (placebo group) Inclusion criteria: One or more asthma attacks during observation period FEV1 < 80 (% predicted) that improved by at least 20% following 2 week course oral prednisolone in run‐in period Exclusion criteria: OCS dependency | |
| Interventions | BDP: 250 mcg 2 pfs 3x daily (1500 mcg/d) Placebo: 2 pfs 3xdaily Delivery device: MDI |
|
| Outcomes | FEV1 FVC Clinic PEFR Daily asthma symptom score Withdrawal due to asthma exacerbation (number of patients) | |
| Notes | No reply from author to clarify details of method of randomisation. Study included an initial 2 weeks period during which all patients received 800mcg salbutamol and 20mg theophylline per day followed by a 2 week course of oral prednisolone (0.5mg/kg/per day). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Simons 1997.
| Methods | Setting: multicentre study Canada, hospital outpatient clinics Length of intervention period: 12 months Randomisation: yes, computer generated sequence Allocation concealment: yes (off site body generated list, with coded schedules) Design: parallel group Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 4 | |
| Participants | 241 children: 140M 101F Age range: 6 to 14 years Inclusion criteria: Children with clinically stable asthma (not otherwise defined) No more than 1 month of treatment with corticosteroids for asthma in past No inhaled or oral corticosteroid treatment within last 3 months FEV1 > 70 (% predicted) 10% or greater increase in FEV1 after inhaled beta2 agonist Methacholine BHR (PC20 FEV1 < 8mg/ml) Exclusion criteria: Emergency dept attendance or hospital admission due to asthma exacerbation within last 3 months History of life‐threatening asthma History of adverse reaction to study medications | |
| Interventions | BDP: 200 mcg 2x daily (400 mcg/d) Placebo: 2xdaily Delivery device: Diskhaler DPI |
|
| Outcomes | Methacholine BHR (PC20 FEV1) FEV1 (% predicted) FEV1 FVC FEF 25, FEF 50, FEF 75, FEF25‐75 Morning PEFR Evening PEFR Clinic PEFR Rescue beta2 agonist not required (% of children) Waking at night (% of nights) No school missed because of asthma (% of children) Waking at night (% of nights) Activities affected by asthma (% of days) Height assessment Blood eosinophil count Withdrawal due to asthma exacerbation (number of patients) | |
| Notes | Reply from author clarifying method of random order generation and use of allocation concealment. Exacerbation of asthma defined as the presence of symptoms despite study medication and the use of albuterol as rescue medication. If an exacerbation occurred, children were treated with oral prednisolone 1mg/kg for 5 to 7 days. If symptoms still not controlled children were withdrawn. Also withdrawn if : More than 1 course prednisolone required in month or 4 in year or: Hospitalization for asthma required Non‐parametric tests used to compare symptom outcomes Salmeterol treatment arm: results not included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Smith 1973a.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout Masking: first 8 patients randomised ‐ single blind (patient blinded), remaining 12 patients double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: no details Jadad score: 3 | |
| Participants | 20 adults: 7M 13F Age range: 19 to 54 years Inclusion criteria: Asthma inadequately controlled by bronchodilator therapy, disodium cromoglycate or hyposensitization Exclusion criteria: Not stated | |
| Interventions | BDP: 50 mcg 2 pfs 4x daily (400 mcg/d), MDI Placebo: 2 pfs 4xdaily, MDI | |
| Outcomes | Daily beta2 agonist use Wheezing episodes per day Daily PEFR Plasma cortisol Plasma cortisol post tetracosactrin | |
| Notes | No reply from author to clarify details of method of randomisation. No numerical data presented in a form suitable for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Smith 1973b.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 2 weeks Randomisation: yes, method not stated Allocation concealment: not stated Design: crossover, no washout Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: not stated regarding baseline spirometry or doses of oral steroids used Jadad score: 3 | |
| Participants | 40 children/adults: 26M 14F Age range: 5 to 20 years Inclusion criteria: Asthma requiring maintenance OCS for control Exclusion criteria: Not stated | |
| Interventions | BDP: 50 mcg 2 pfs 4x daily (400/d) Placebo: 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | % change in FEV1 Patient preference for BDP Physician preference for BDP Daily asthma symptom score | |
| Notes | No reply from author to clarify details of method of randomisation. No details of patient baseline characteristics in terms of spirometry or doses of oral steroids used. No details regarding criteria used by physicians to assess which treatment was "better". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Toogood 1977.
| Methods | Setting: Canada, hospital outpatient clinic Length of intervention period: see interventions Randomisation: yes, method not stated Allocation concealment: unclear Design: non‐randomised dose escalation followed by randomised crossover Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: comparable Jadad score: 2 | |
| Participants | 34 adults: 17M 17F Mean age: 45 years Inclusion criteria: OCS dependent chronic asthma Poorly controlled despite oral steroids Exclusion criteria: Maintenance oral steroid therapy for less than one year | |
| Interventions | Inital 8 week treatment period during which all patients received BDP in escalating doses every 2 weeks; initially receiving 200 mcg daily, up to 1600 mcg daily Double blind crossover phase: BDP 1600mcg daily for 4 weeks or placebo for 4 weeks | |
| Outcomes | Arterial oxygen tension RV VC MMFR DLCO Low PEFR of day High PEFR of the day Asthma disability score: Attack frequency, mean severity of attacks, asthma hours, longest attack of the day, bronchodilator tablets, inhaler doses, days of asthma, days of total disability. Illness score by patient Illness score by physician Asthma disability score by patient Asthma disability score by physician | |
| Notes | Unique trial design. No attempt was made to reduce oral prednisolone dose at any stage of the study. Placebo treated patients experienced significantly more symptoms compared to BDP group; results for other outcomes during randomised phase of trial unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Trigg 1994.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 4 months Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 25 adults Age range: 18 to 45 years Inclusion criteria: FEV1 > 60 (% predicted) Histamine BHR (PC20 FEV1 < 2mg/ml) Exclusion criteria: Smokers Respiratory tract infection Other systemic disease | |
| Interventions | BDP: 250 mcg 2 pfs 2x daily (1000mcg/d) Placebo: 2 puffs 2xdaily Delivery device: MDI+spacer |
|
| Outcomes | Change in FEV1 compared to baseline Histamine BHR (PC20 FEV1) Bronchial biopsy Withdrawal due to asthma exacerbation (number of patients) | |
| Notes | No reply from author to clarify details of method of randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Turner 1998.
| Methods | Setting: Canada, primary care and hospital outpatient clinic Length of intervention period: 3 weeks Randomisation: yes, computer generated list sequence Allocation concealment: yes (list generated off‐site and administered by pharmacist) Design: parallel group Masking: double blind Excluded: stated Withdrawals: stated Baseline characteristics: comparable Jadad score: 5 | |
| Participants | 34 adults: 12M 22F Mean age: 36 (BDP group) 38 (placebo group) Inclusion criteria: Asthma as defined by episodic wheezing, chest tightness and/or breathlessness and either FEV1 < 70 (% predicted) or FEV1/FVC ratio < 70 (% predicted) with 15% or greater increase in FEV1 after inhaled beta2 agonist or Methacholine BHR (PC20 FEV1 < 8mg/ml) AND Mild exacerbation of asthma as defined by a history of increasing symptoms for at least 2 weeks that were not spontaneously improving and/or and increased need for an inhaled beta2 agonist. AND Sputum eosinophilia at least 4% (normal 2% or less) All patient on treatment with as required beta2 agonist. Exclusion criteria: ICS (BDP or BUD) > 1000 mcg daily Exacerbation of asthma requiring oral prednisolone, A and E attendance or hospital admission in the previous 3 months | |
| Interventions | BDP: 250 mcg 2 pfs 2x daily (1000 mcg/d) Placebo: 2 puffs 2 x daily Delivery device: MDI+spacer |
|
| Outcomes | FEV1 Morning PEFR Diurnal variation in PEFR Daytime asthma symptom score Daytime use of beta2 agonists Sputum eosinophil count Blood eosinophil count Serum ECP Withdrawal due to asthma exacerbation (number of patients) | |
| Notes | Patients continued their pre‐study treatment during study, including any inhaled steroid. Withdrawal criteria included worsening of asthma symptoms requiring increased short‐acting beta2 agonists of 8 or more puffs daily, if nocturnal symptoms disturbed sleep or if post‐bronchodilator FEV1 fell by more than 10% below baseline value. Salmeterol treatment arm: results not included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Vatrella 1996.
| Methods | Setting: Italy, hospital outpatient clinic Length of intervention period: 5 months Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: unclear Withdrawals: stated (none) Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 20 adults: 8M 12F Age range 20 to 47years Inclusion criteria: Positive skin prick test to Parietaria Seasonal onset of asthma symptoms during pollen season FEV1 > 80 (% predicted) Methacholine BHR None or minimal asthma symptoms outside asthma season Exclusion criteria: ICS use Smokers Respiratory tract infection | |
| Interventions | BDP: 250 mcg 2pfs 2x daily (1000mcg/d) Placebo: 2 puffs 2xdaily Delivery device: MDI |
|
| Outcomes | FEV1 (% predicted) Clinic PEFR (% predicted) Methacholine BHR (PD20 FEV1) Daytime asthma symptom score Daily beta2 agonist use Blood eosinophil count Serum ECP level | |
| Notes | No reply from author to clarify details of method of randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vilsvik 1974.
| Methods | Setting: Norway, hospital outpatient clinic Length of intervention period: 3 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: crossover, no washout Masking: double blind Excluded: stated Withdrawals: not stated Baseline characteristics: comparable Jadad score: 2 | |
| Participants | 27 adults: 18M 9F Age range: 23 to 74 years Inclusion criteria: Asthmatic patients dependent on OCS able to successfully transfer to inhaled BDP during an initial open study Exclusion criteria: Not stated | |
| Interventions | BDP: 100mcg 1 pf 4x daily (400mcg/d) Placebo: 1pf 4xdaily Delivery device: MDI |
|
| Outcomes | FEV1 MEFR Clinic PEFR Plasma cortisol (no time defined) Blood eosinophil count | |
| Notes | No reply from author to clarify details of method of randomisation. First part of study was an open study to determine which patients could successfully reduce their oral steroid intake by using supplemental BDP. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vogt 1976.
| Methods | Setting: USA, hospital outpatient clinic Length of intervention period: 4 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated Baseline characteristics: comparable (but no data to support) Jadad score: 3 | |
| Participants | 93 adolescents/adults: 33M 60F Age range: 14 to 65 years Inclusion criteria: Patients with reversible airways disease (not further defined) requiring continuous use of inhaled bronchodilators, but not requiring oral steroids 15% or greater improvement in FEV1 following inhaled isoproteronol Exclusion criteria: Not stated | |
| Interventions | BDP: 100 mcg 2 pfs 4x daily (800mcg/d) Placebo: 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | FEV1 FVC FEF25t o 75 8 am plasma cortisol Extent of symptom relief score Aerosol evaluation score physicians and patients Withdrawal due to asthma exacerbation (number of patients) Oro‐pharyngeal side effects | |
| Notes | Reply from author but unable to clarify method use of allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wang 1994.
| Methods | Setting: UK, hospital outpatient clinic Length of intervention period: 4 months Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: not stated Baseline characteristics: comparable Jadad score: 2 | |
| Participants | 25 adults 7M 18F Age range: 18 to 45 years Inclusion criteria: Patients with mild asthma (not further defined) FEV1 > 60 (% predicted) Exclusion criteria: Use of ICS, nedocromil or sodium cromoglycate within last 6 months | |
| Interventions | BDP: 250 mcg 2pfs 2x daily (1000mcg/d) Placebo: 2 pfs 2xdaily Delivery device: MDI |
|
| Outcomes | FEV1 Methacholine BHR (PC20 FEV1) Bronchial biopsy: % GM‐CSF expression in bronchial epithelium % GM‐CSF expression in bronchial epithelium Eosinophils per mm2 bronchial epithelium | |
| Notes | No reply from author to clarify details of method of randomisation. Standard deviation values for methacholine bronchial responsiveness (log 10 PC20 FEV1) have been calculated as a weighted average using the two other studies that report this outcome. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Webb 1977.
| Methods | Setting: USA, hospital outpatient clinic Length of intervention period: 12 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: no details Withdrawals: stated (none) Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 30 adults: 17M 13F Mean age: 50 (BDP group) 55 (placebo group) Inclusion criteria: At least six months treatment with prednisolone 5‐20mg daily equivalent Improvement in FEV1 by at least 15% following inhaled isoproteronol Exclusion criteria: Significant co‐existent disease | |
| Interventions | BDP: 50mcg 2 pfs 4x daily (400mcg/d) Placebo: 2 pfs 4xdaily Delivery device: MDI |
|
| Outcomes | Daily dose oral prednisolone No patients discontinuing prednisolone FEV1 FVC Morning plasma cortisol Plasma cortisol post metyrapone | |
| Notes | No reply from author to clarify details of method of randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wiebicke 1990.
| Methods | Setting: Germany, hospital outpatient clinic Length of intervention period: 3 weeks Randomisation: yes, method not stated Allocation concealment: unclear Design: parallel group Masking: double blind Excluded: not stated Withdrawals: stated (none) Baseline characteristics: comparable Jadad score: 3 | |
| Participants | 25 adults: 14M 11F Age range: 18 to 41 years Inclusion criteria: Mild asthma (not further defined) not requiring regular medication FEV1 > 75 (% predicted) Non smokers Methacholine and histamine BHR (PC100 Sraw < 8.0mg/ml). Exclusion criteria: Respiratory tract infection in last 6 weeks | |
| Interventions | BDP: 250 mcg 2 pfs 4x daily (2000 mcg/d) Placebo: 2 pfs 4xdaily Delivery device: MDI+spacer |
|
| Outcomes | Histamine BHR (PC100SRaw) Methacholine BHR (PC100SRaw) Bronchial responsiveness to isocapnic hyperventilation (PV75SRaw) Bronchial responsiveness to isocapnic SO2 hyperventilation (PV75Sraw) Specific airway resistance | |
| Notes | No reply from author to clarify details of method of randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
ATS: American Thoracic Society; BDP: beclomethasone dipropionate; BHR: bronchial hyper‐responsiveness; BUD: budesonide; DLCO: carbon monoxide diffusing capacity; DPI: dry powder inhaler; ECP: eosinophil cationic protein; FEV1: forced expired volume in one second; FEF25‐75: forced expiratory flow at 25 to 75% of FVC; FEF25: maximal expiratory flow at 25% of FVC; FEF50: maximal expiratory flow at 50% of FVC; FEF 75: maximal expiratory flow at 75% of FVC; FVC: forced vital capacity; GINA: Global Initiative for Asthma; GM‐CSF: granulocyte‐macrophage colony stimulating factor; ICS: inhaled corticosteroid; IGF‐1: insulin‐like growth factor; MDI: metered dose inhaler; MMFR: maximum mid‐expiratory flow rate; OCS: oral corticosteroid; PC100 SRaw: concentration of inhalant necessary to increase SRaw by 100%; PC20 FEV1: concentration of inhalant required to produce a 20% fall in FEV1; PD100 Raw: dose of inhalant necessary to increase Raw by 100%; PD20 FEV1: dose of inhalant required to produce a 20% fall in FEV1; PDGF: platelet derived growth factor; PEFR: peak expiratory flow rate; PV75 SRaw: provocative ventilation necessary to increase SRaw by 75%; Raw: airway resistance RV: residual volume; SRaw: specific airway resistance; SO2: sulphur dioxide; TDI: toluene diisocyanate; TGF‐1beta: transforming growth factor one beta; TLC: total lung capacity; VC: vital capacity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Apold 1975 | No placebo treatment arm |
| Baba 2002 | Clinical controlled trial |
| Bentley 1996 | Single dose study |
| Bonnaud 1991 | Not an RCT |
| Burge 1982 | Not an RCT |
| Chang 1998 | Study concerned specifically with recurrent cough. |
| Choovoravech 1977 | No placebo comparison group |
| Cockcroft | Patients were not randomised to a placebo arm at the start of the study |
| Del Bufalo 1989 | No placebo arm included |
| Douay 1976 | RCT (crossover) ‐ excluded due to inadequate duration (3 weeks). |
| Doull 1995a | Study concerned specifically with recurrent (viral‐induced) wheeze in childhood, as opposed to asthma |
| Emel'ionov 1995 | Not an RCT |
| Francia 1994 | Not an RCT |
| Harrison 1999 | Intervention period for 2 weeks (EXCLUDED ACCORDING TO NEW REVIEW ENTRY CRITERIA) |
| Harvald 1976 | Not an RCT |
| Hoshino 1999 | Not an RCT |
| Huang 1993 | Study in infants |
| Juniper 2002 | RCT, comparing 800 mcg/day HFA‐BDP with 1600 mcg/day CFC‐BDP |
| Kaptein 1993 | Not an RCT |
| Konig 1974 | Study not an RCT (confirmed after communication with authors) |
| Malo 1996 | Not an RCT |
| Menz 1986 | Not an RCT |
| Michel 1977 | Not an RCT |
| Nakajima 1979 | Not an RCT |
| Nielsen 1999 | Salmeterol Versus placebo on asthmatic patients already receiving inhaled beclomethasone |
| Nizankowska 1989 | No placebo group |
| Noonan 2001 | Open‐label trial |
| Patakas 1978 | Not an RCT (confirmation after contact with author) |
| Pizzichini 1996 | Single dose study |
| Richards 1978 | The dose of beclomethasone used is not stated |
| Rodriguez 1986 | Not an RCT |
| Rohdewald 1992 | Not an RCT |
| Ronchetti 1976 | Study in infants |
| Ruff 1977 | Not an RCT |
| Sidel'nikov 1985 | Not an RCT (excluded following Russian translation) |
| Sil'vestrov 1983 | Not an RCT (excluded following Russian translation) |
| Silkoff 2001 | Not an RCT |
| Stick 1995 | Study concerned with infant population |
| Storr 1986 | Nebuliser delivery device |
| Webb 1986 | Nebuliser delivery device |
| Yuksel 1992 | Study in infants |
Contributions of authors
Nick Adams retrieved papers identified by electronic search, handsearched additional sources for relevant studies, assessed trials for methodological quality, contacted authors to clarify details of trial design and/or request missing data, extracted data from included trials and wrote text of review.
Janine Bestall retrieved papers identified by search, assessed trials for methodological quality, contacted authors for clarification or trial details and/or request missing data.
January 2003 update: Reem Malouf assessed studies for inclusion, extracted and entered data, write‐up. Toby Lasserson assisted with data extraction and entry, write‐up of findings and interpretation.
Changes made by RM and TL were discussed with and approved by NA, who retains responsibility for the review.
Paul Jones provided editorial support .
Sources of support
Internal sources
NHS Research and Development, UK.
Nederlands Astma Fonds, Netherlands.
External sources
No sources of support supplied
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Bel 1990 {published data only}
- Bel EH, Timmers MC, Hermans J, Dijkman JH, Sterk PJ. The long‐term effects of nedocromil sodium and beclomethasone dipropionate on bronchial responsiveness to methacholine in nonatopic asthmatic subjects. American Review of Respiratory Disease 1990;141(1):21‐8. [DOI] [PubMed] [Google Scholar]
Bennati 1989 {published data only}
- Bennati D, Piacentini GL, Peroni DG, Sette L, Testi R, Boner AL. Changes in bronchial reactivity in asthmatic children after treatment with beclomethasone alone or in association with salbutamol. Journal of Asthma 1989;26(6):359‐64. [DOI] [PubMed] [Google Scholar]
Bergmann 1990 {published data only}
- Bergmann KC, Bauer CP, Overlack A. A placebo‐controlled blinded comparison of nedocromil sodium and beclomethasone dipropionate in bronchial asthma. Lung 1990;168:230‐9. [DOI] [PubMed] [Google Scholar]
- Bergmann KC, Bauer CP, Overlack A. A placebo‐controlled, blind comparison of nedocromil sodium and beclomethasone dipropionate in bronchial asthma. Current Medical Research & Opinion 1989;11(8):533‐42. [DOI] [PubMed] [Google Scholar]
Berkowitz 1998 {published data only}
- Berkowitz R, Rachelefsky G, Harris AG, Chen R. A comparison of triamcinolone acetonide MDI with a built‐in tube extender and beclomethasone dipropionate MDI in adult asthmatics. Chest 1998;114(3):757‐65. [DOI] [PubMed] [Google Scholar]
Bernstein 1999 {published data only}
- Bernstein D, Berkowitz R, Chervinsky P, Dvorin D, Finn A, Gross G, Karetzky M, Kemp J, Laforce C, Lumry W, Mendelson L, Nelson H, Pearlman D, Rachelefsky G, Ranter P, Repsher L, Segal A, Selner J, Settipane G, Wanderer A, Cuss F, Nolop K, Harrison J. Dose ranging study of a new steroid for asthma mometasone furate dry powder inhaler. Respiratory Medicine 1999;93:603‐12. [DOI] [PubMed] [Google Scholar]
Boner 1991 {published data only}
- Boner AL, Piacentini GL, Bonizzato C, Dattoli V, Sette L. Effect of inhaled beclomethasone dipropionate on bronchial hyperreactivity in asthmatic children during maximal allergen exposure. Pediatric Pulmonology 1991;10(1):2‐5. [DOI] [PubMed] [Google Scholar]
Brompton/MRC 1974 {published data only}
- Anonymous. Double‐blind trial comparing two dosage schedules of beclomethasone dipropionate aerosol in the treatment of chronic bronchial asthma. Preliminary report of the Brompton Hospital‐Medical Research Council Collaborative Trial. Lancet 1974;2(7876):303‐7. [PubMed] [Google Scholar]
Bronsky 1998 {published data only}
- Bronsky E, Korenblat P, Harris AG, Chen R. Comparative clinical study of inhaled beclomethasone dipropionate and triamcinolone acetonide in persistent asthma. Annals of Allergy, Asthma, & Immunology 1998;80(4):295‐302. [DOI] [PubMed] [Google Scholar]
BTTA 1976 {published and unpublished data}
- Anonymous. A controlled trial of inhaled corticosteroids in patients receiving Prednisone tablets for asthma. British Journal of Diseases of the Chest 1976;70(2):95‐103. [DOI] [PubMed] [Google Scholar]
Cameron 1973 {published data only}
- Cameron SJ, Cooper EJ, Crompton GK, Hoare MV, Grant IW. Substitution of beclomethasone aerosol for oral prednisolone in the treatment of chronic asthma. British Medical Journal 1973;4(886):205‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpentiere 1990 {published data only}
- Carpentiere G, Castello F, Marino S. Effect of beclomethasone dipropionate on the bronchial responsiveness to propranolol in asthmatics. Chest 1990;98(2):263‐5. [DOI] [PubMed] [Google Scholar]
Chan 1993 {published and unpublished data}
- Chan KN, Silverman M. Increased airway responsiveness in children of low birth weight at school age: Effect of topical corticosteroids. Archives of Disease in Childhood 1993;69(1):120‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 1977 {published data only}
- Davies G, Thomas P, Broder I, et al. Steroid‐dependent asthma treated with inhaled beclomethasone dipropionate. A long‐term study. Annals of Internal Medicine 1977;86(5):549‐53. [DOI] [PubMed] [Google Scholar]
De Marzo 1988 {published data only}
- Marzo N, Fabbri LM, Crescioli S, Plebani M, Testi R, Mapp CE. Dose‐dependent inhibitory effect of inhaled beclomethasone on late asthmatic reactions and increased responsiveness to methacholine induced by toluene diisocyanate in sensitised subjects. Pulmonary Pharmacology 1988;1(1):15‐20. [DOI] [PubMed] [Google Scholar]
Fahy 1998 {published data only}
- Fahy JV, Boushey HA. Effect of low‐dose beclomethasone dipropionate on asthma control and airway inflammation. European Respiratory Journal 1998;11(6):1240‐7. [DOI] [PubMed] [Google Scholar]
Fanelli 1993 {published data only}
- Fanelli A, Maggi E, Stendardi L, Gorini M, Duranti R, Scano G. Preventive effects of beclomethasone on histamine‐induced changes in breathing pattern in asthma. Chest 1993;103(1):122‐8. [DOI] [PubMed] [Google Scholar]
Fournier 1990 {published data only}
- Fournier M, Renon D, Roy‐Ladurie F, Pappo M, Pariente R. Bronchial tolerance to three months' inhalation of beclomethasone dipropionate. Histological and microbiological study by asthmatic patients. Presse Medicale 1990;19(31):1441‐4. [PubMed] [Google Scholar]
Gaddie 1973 {published data only}
- Gaddie J, Reid IW, Skinner C, Petrie GR, Sinclair DJ, Palmer KN. Aerosol beclomethasone dipropionate in chronic bronchial asthma. Lancet 1973;1(7805):691‐3. [DOI] [PubMed] [Google Scholar]
Gross 1999 {published data only}
- Gross G, Thompson PJ, Chervinsky P, Vanden Burgt J. Hydrofluoroalkane‐134a beclomethasone dipropionate, 400 mug, is as effective as chlorofluorocarbon beclomethasone dipropionate, 800 mug, for the treatment of moderate asthma. Chest 1999;115(2):343‐51. [DOI] [PubMed] [Google Scholar]
Hampel 2000 {published data only}
- Hampel F, Lisberg E, Guerin JC. Effectiveness of low doses (50mcg and 100mcg bid) of beclamethasone dipropionate delivered as a CFC‐free extrafine aerosol in adults with mild‐to‐moderate asthma. Journal of Asthma 2000;37(5):389‐98. [DOI] [PubMed] [Google Scholar]
Harvey 1976 {published data only}
- Harvey LL, Nair SV, Kass I. Beclomethasone dipropionate aerosol in the treatment of steroid‐dependent asthma. A 12‐week double‐blind study comparing beclomethasone dipropionate and a vehicle aerosol. Chest 1976;70(03):345‐50. [DOI] [PubMed] [Google Scholar]
Hodson 1974 {published data only}
- Hodson ME, Batten JC, Clarke SW, Gregg I. Beclomethasone dipropionate aerosol in asthma. Transfer of steroid‐dependent asthmatic patients from oral prednisone to beclomethasone dipropionate aerosol. American Review of Respiratory Disease 1974;110(4):403‐8. [DOI] [PubMed] [Google Scholar]
Holst 1974 {published data only}
- Holst PE, O'Donnell TV. A controlled trial of beclomethasone dipropionate in asthma. New Zealand Medical Journal 1974;79(511):769‐73. [PubMed] [Google Scholar]
Hoshino 1998 {published data only}
- Hoshino M, Nakamura Y, Sim JJ, Yamashiro Y, Uchida K, Hosaka K, Isogai S. Inhaled corticosteroid reduced lamina reticularis of the basement membrane by modulation of insulin‐like growth factor (IGF)‐I expression in bronchial asthma [see comments].. Clinical & Experimental Allergy 1998;28(5):568‐77. [DOI] [PubMed] [Google Scholar]
Hoshino 2001 {published data only}
- Hoshino M, Takahashi M, Takai Y, Sim J, Aoike N. Inhaled corticosteroids decrease vascularity of the bronchial mucosa in patients with asthma. Clinical and Experimental Allergy 2001;31:722‐30. [DOI] [PubMed] [Google Scholar]
Katsunuma 1993 {published data only}
- Katsunuma T, Hashimoto K, Akimoto K, Ebisawa M, Iikura Y. Effect of inhaled beclomethasone dipropionate on bronchial responsiveness in patients with asthma. Annals of Allergy 1993;70(2):165‐70. [PubMed] [Google Scholar]
Kerigan 1977 {published data only}
- Kerigan AT, Pugsley SO, Cockcroft DW, Hargreave FE. Subsittution of inhaled beclomethasone dipropionate for ingested prednisone in steroid‐dependent asthmatics. Canadian Medical Association Journal 1977;116(8):867‐71. [PMC free article] [PubMed] [Google Scholar]
Kerrebijn 1976 {published data only}
- Kerrebijn KF. Beclomethasone dipropionate in long‐term treatment of asthma in children. Journal of Pediatrics 1976;89(5):821‐6. [DOI] [PubMed] [Google Scholar]
Kerstjens 1994 {published and unpublished data}
- Kerstjens HA, Brand PL, Hughes MD, et al. A comparison of bronchodilator therapy with or without inhaled corticosteroid therapy for obstructive airways disease. Dutch Chronic Non‐Specific Lung Disease Study Group [see comments]. New England Journal of Medicine 1992;327(20):1413‐9. [DOI] [PubMed] [Google Scholar]
- Kerstjens HA, Brand PL, Jong PM, Koeter GH, Postma DS. Influence of treatment on peak expiratory flow and its relation to airway hyperresponsiveness and symptoms. The Dutch CNSLD Study Group. Thorax 1994;49(11):1109‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 1977 {published data only}
- Klein R, Waldman D, Kershnar H, et al. Treatment of chronic childhood asthma with beclomethasone dipropionate aerosol: I. A double‐blind crossover trial in nonsteroid‐dependent patients. Pediatrics 1977;60(1):7‐13. [PubMed] [Google Scholar]
Kraemer 1987 {published data only}
- Kraemer R, Sennhauser F, Reinhardt M. Effects of regular inhalation of beclomethasone dipropionate and sodium cromoglycate on bronchial hyperreactivity in asthmatic children. Acta Paediatrica Scandinavica 1987;76(1):119‐23. [DOI] [PubMed] [Google Scholar]
Lacronique 1991 {published data only}
- Lacronique J, Renon D, Georges D, Henry‐Amar M, Marsac J. High‐dose beclomethasone: oral steroid‐sparing effect in severe asthmatic patients. European Respiratory Journal 1991;4(7):807‐12. [PubMed] [Google Scholar]
Laviolette 1994 {published and unpublished data}
- Laviolette M, Ferland C, Trepanier L, Rocheleau H, Dakhama A, Boulet LP. Effects of inhaled steroids on blood eosinophils in moderate asthma. Annals of the New York Academy of Science 1994;725:288‐97. [DOI] [PubMed] [Google Scholar]
Laviolette 1999 {published data only}
- Laviolette M, Malmstrom K, Lu S, Chervinsky P, Pujet JC, Peszek I, Zhang J, Reiss TF. Montelukast added to inhaled beclomethasone in treatment of asthma. American Journal of Respiratory & Critical Care Medicine 1999;160(6):1862‐8. [DOI] [PubMed] [Google Scholar]
Lovera 1975 {published data only}
- Lovera J, Collins‐Williams C, Bailey J. Beclomethasone dipropionate by aerosol in the treatment in asthmatic children. Postgraduate Medical Journal 1975;51(Suppl 4):96‐8. [PubMed] [Google Scholar]
- Lovera J, Cooper DM, Collins‐Williams C, Levison H, Bailey JD, Orange RP. Clinical and physiological assessment of asthmatic children treated with beclomethasone dipropionate. Journal of Allergy & Clinical Immunology 1976;57(2):112‐23. [DOI] [PubMed] [Google Scholar]
Maestrelli 1993 {published data only}
- Maestrelli P, Marzo N, Saetta M, Boscaro M, Fabbri LM, Mapp CE. Effects of inhaled beclomethasone on airway responsiveness in occupational asthma. Placebo‐controlled study of subjects sensitized to toluene diisocyanate. American Review of Respiratory Disease 1993;148(2):407‐12. [DOI] [PubMed] [Google Scholar]
Malmstrom 1999 {published data only}
- Malmstrom K, Rodriguez‐Gomez G, Guerra J, Villaran C, Pineiro A, Wei L, Seidenberg B, Reiss. Oral montelukast inhaled beclomethasone and placebo for chronic asthma. Annals of Internal Medicine 1999;130(6):487‐95. [DOI] [PubMed] [Google Scholar]
Martin 1974 {published data only}
- Martin PD, Gebbie T, Salmond CE. A controlled trial of beclomethasone dipropionate by aerosol in chronic asthmatics. New Zealand Medical Journal 1974;79(511):773‐6. [PubMed] [Google Scholar]
Matthys 1998 {published data only}
- Matthys H, Nowak D, Hader S, Kunkel G. Efficacy of chlorofluorocarbon‐free beclomethasone dipropionate 400 micrograms day‐1 delivered as an extrafine aerosol in adults with moderate asthma. Respiratory Medicine 1998;92 Suppl A:17‐22. [DOI] [PubMed] [Google Scholar]
Messerli 1975 {published data only}
- Messerli C, Studer H, Scherrer M. Systemic side effects of beclomethasone dipropionate aerosols (becotide, aldecine, sanasthmyl) in otherwise non steroid treated asthmatic patients. Pneumonologie 1975;153(1):29‐42. [DOI] [PubMed] [Google Scholar]
Nathan 1997 {published data only}
- Nathan RA, Nolop KB, Cuss FM, Lorber RR. A comparison of double‐strength beclomethasone dipropionate (84 microg) MDI with beclomethasone dipropionate (42 microg) MDI in the treatment of asthma [see comments]. Chest 1997;112(1):34‐9. [DOI] [PubMed] [Google Scholar]
Nathan 2001 {published data only}
- Nathan RA, Pinnas JL, Schwartz HJ, Grossman J, Yancey SW, Emmett AH, et al. A six‐month, placebo‐controlled comparison of the safety and efficacy of salmeterol or beclomethasone for persistent asthma. Annals of Allergy 1999;82(6):521‐9. [DOI] [PubMed] [Google Scholar]
Nayak 2002 {published data only}
- Nayak A, Robert L, Weinstein S, Stampone P, Welch M. Efficacy and safety of beclomethasone dipropionate extrafine aerosol in childhood asthma a 12 ‐week randomized double blind placebo controlled study. Chest 2002;122:1956‐65. [DOI] [PubMed] [Google Scholar]
Pennings 1997 {published data only}
- Pennings HJ, Wouters EF. Effect of inhaled beclomethasone dipropionate on isocapnic hyperventilation with cold air in asthmatics, measured with forced oscillation technique. European Respiratory Journal 1997;10(3):665‐71. [PubMed] [Google Scholar]
Radha 1975 {published data only}
- Radha TG, Viswanathan R, Sharma AK. Beclomethasone dipropionate aerosol in asthma: a double blind study. Indian Journal of Medical Research 1975;63(11):1659‐66. [PubMed] [Google Scholar]
Riordan 1974 {published data only}
- Riordan JF, Dash CH, Sillett RW, McNicol MW. A comparison of betamethasone valerate, beclomethasone dipropionate and placebo by inhalation for the treatment of chronic asthma. Postgraduate Medical Journal 1974;50(suppl 4):61‐4. [PubMed] [Google Scholar]
Ryan 1985 {published data only}
- Ryan G, Latimer KM, Juniper EF, Roberts RS, Hargreave FE. Effect of beclomethasone dipropionate on bronchial responsiveness to histamine in controlled nonsteroid‐dependent asthma. Journal of Allergy & Clinical Immunology 1985;75(1 Pt 1):25‐30. [DOI] [PubMed] [Google Scholar]
Salmeron 1989 {published data only}
- Salmeron S, Guerin JC, Godard P, et al. High doses of inhaled corticosteroids in unstable chronic asthma. A multicenter, double‐blind, placebo‐controlled study. American Review of Respiratory Disease 1989;140(1):167‐71. [DOI] [PubMed] [Google Scholar]
Simons 1997 {published data only}
- Simons FE. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate‐Salmeterol Xinafoate Study Group. New England Journal of Medicine 1997;337:1659‐65. [DOI] [PubMed] [Google Scholar]
Smith 1973a {published data only}
- Smith AP, Booth M, Davey AJ. A controlled trial of beclomethasone dipropionate for asthma. British Journal of Diseases of the Chest 1973;67(3):208‐14. [DOI] [PubMed] [Google Scholar]
Smith 1973b {published data only}
- Smith JM. A clinical trial of beclomethasone dipropionate aerosol in children and adolescents with asthma. Clinical Allergy 1973;3(3):249‐53. [DOI] [PubMed] [Google Scholar]
Toogood 1977 {published data only}
- Toogood JH, Lefcoe NM, Haines DS, et al. A graded dose assessment of the efficacy of beclomethasone dipropionate aerosol for severe chronic asthma. Journal of Allergy & Clinical Immunology 1977;59(4):298‐308. [DOI] [PubMed] [Google Scholar]
Trigg 1994 {published data only}
- Trigg CJ, Manolitsas ND, Wang J, et al. Placebo‐controlled immunopathologic study of four months of inhaled corticosteroids in asthma. American Journal of Respiratory & Critical Care Medicine 1994;150(1):17‐22. [DOI] [PubMed] [Google Scholar]
Turner 1998 {published data only}
- Turner MO, Johnston PR, Pizzichini E, Pizzichini MM, Hussack PA, Hargreave FE. Anti‐inflammatory effects of salmeterol compared with beclomethasone in eosinophilic mild exacerbations of asthma: A randomized, placebo controlled trial. Canadian Respiratory Journal 1998;5(4):261‐8. [DOI] [PubMed] [Google Scholar]
Vatrella 1996 {published data only}
- Ponticiello A, Vatrella A, Parrella R, et al. Inhaled beclomethasone dipropionate (BDP) prevents seasonal changes in atopic asthmatics. Monaldi Archives for Chest Disease 1997;52(2):112‐7. [PubMed] [Google Scholar]
- Vatrella A, Ponticiello A, Parrella R, et al. Serum eosinophil cationic protein (ECP) as a marker of disease activity and treatment efficacy in seasonal asthma. Allergy 1996;51(8):547‐55. [DOI] [PubMed] [Google Scholar]
Vilsvik 1974 {published data only}
- Vilsvik JS, Schaanning J. Beclomethasone dipropionate aerosol in adult steroid‐dependent obstructive lung disease. Scandinavian Journal of Respiratory Diseases 1974;55(3):169‐75. [PubMed] [Google Scholar]
Vogt 1976 {published data only}
- Vogt F, Chervinsky P, Dwek J, Grieco M. Beclomethasone dipropionate aerosol in the treatment of chronic bronchial asthma. Journal of Allergy & Clinical Immunology 1976;58(2):316‐21. [DOI] [PubMed] [Google Scholar]
Wang 1994 {published data only}
- Wang JH, Trigg CJ, Devalia JL, Jordan S, Davies RJ. Effect of inhaled beclomethasone dipropionate on expression of proinflammatory cytokines and activated eosinophils in the bronchial epithelium of patients with mild asthma. Journal of Allergy & Clinical Immunology 1994;94(6 Pt 1):1025‐34. [DOI] [PubMed] [Google Scholar]
Webb 1977 {published data only}
- Webb DR. Beclomethasone in steroid‐dependent asthma. Effective therapy and recovery of hypothalamo‐pituitary‐adrenal function. The Journal of the American Medical Association 1977;238(14):1508‐11. [DOI] [PubMed] [Google Scholar]
Wiebicke 1990 {published data only}
- Wiebicke W, Jorres R, Magnussen H. Comparison of the effects of inhaled corticosteroids on the airway response to histamine, methacholine, hyperventilation, and sulfur dioxide in subjects with asthma. Journal of Allergy & Clinical Immunology 1990;86(6 Pt 1):915‐23. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Apold 1975 {published data only}
- Apold J. [Treatment of asthma in children with beclomethasone dipropionate aerosols]. Tidsskrift for Den Norske Laegeforening 1975;95(19‐21):1149‐52. [PubMed] [Google Scholar]
Baba 2002 {published data only}
- Baba K, Sakakibara A, Tagi T, Niwa S, Wakayama H, Takagi K.
- Baba K, Sakakibara A, Tagi T, Niwa S, Wakayama H, Takagi K. Long term observation of the clinical course after step down of corticosteroid inhalation in adult chronic asthmatics correlation with serum levels of eosinophil cationic protein. Respirology 2002;7:225‐66. [DOI] [PubMed] [Google Scholar]
Bentley 1996 {published data only}
- Bentley AM, Walker S, Hanotte F, Vos C, Durham SR. A comparison of the effects of oral cetirizine and inhaled beclomethasone on early and late asthmatic responses to allergen and the associated increase in airways hyperresponsiveness. Clinical & Experimental Allergy 1996;26(8):909‐17. [PubMed] [Google Scholar]
Bonnaud 1991 {published data only}
- Bonnaud F, Desfougeres JL. [Treatment of asthma in the adult using a combination of salbutamol 100 micrograms‐‐beclomethasone 50 micrograms. Results of a study on 1917 patients seen in general medicine]. Allergie et Immunologie 1991;23(7):295‐300. [PubMed] [Google Scholar]
Burge 1982 {published data only}
- Burge PS. The effects of corticosteroids on the immediate asthmatic reaction. European Journal of Respiratory Diseases ‐ Supplement 1982;122:163‐6. [PubMed] [Google Scholar]
Chang 1998 {published data only}
- Chang AB, Phelan PD, Carlin JB, Sawyer SM, Robertson CF. A randomised, placebo controlled trial of inhaled salbutamol and beclomethasone for recurrent cough. Archives of Disease in Childhood 1998;79(1):6‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choovoravech 1977 {published data only}
- Choovoravech P, Choovoravech N, Yongpanich Y, Preeyasombut C. [Beclomethasone dipropionate aerosol in the treatment of chronic bronchial asthma: A preliminary study in Thai subjects]. Journal of the Medical Association of Thailand 1977;60(12):619‐25. [PubMed] [Google Scholar]
Cockcroft {published data only}
- Cockcroft DW, McParland CP, O'Byrne PM, et al. Beclomethasone given after the early asthmatic response inhibits the late response and the increased methacholine responsiveness and cromolyn does not. Journal of Allergy and Clinical Immunology 1993;91(6):1163‐8. [DOI] [PubMed] [Google Scholar]
Del Bufalo 1989 {published data only}
- Bufalo C, Impullitti S, Miniero R, Gunella G. [Clinical controlled study on high doses of beclomethasone dipropionate associated with salbutamol in long term treatment of asthmatic subjects]. Rivista di Patologia e Clinica della Tubercolosi e di Pneumologia 1989;60:137‐55. [Google Scholar]
Douay 1976 {published data only}
- Douay B, Robin H, Racadot A. Study of a novel corticoid (Becotide) in chronic asthma and COPD [Étude d'un nouveau corticoïde local, la Bécotide, dans l'asthme chronique et la bronchite chronique spastique]. Lille Medical 1976;21(7 Suppl 3):623‐8. [PubMed] [Google Scholar]
Doull 1995a {published data only}
- Doull I, Freezer N, Holgate S. Osteocalcin, growth, and inhaled corticosteroids: A prospective study. Archives of Disease in Childhood 1996;74(6):497‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doull IJ, Donovan SJ, Wood PJ, Holgate ST. Bloodspot cortisol in mild asthma: the effect of inhaled corticosteroids. Archives of Disease in Childhood 1995;72(4):321‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doull IJ, Freezer NJ, Holgate ST. Growth of prepubertal children with mild asthma treated with inhaled beclomethasone dipropionate. American Journal of Respiratory & Critical Care Medicine 1995;151(6):1715‐9. [DOI] [PubMed] [Google Scholar]
- Doull IJM, Lampe FC, Smith S, Schreiber J, Freezer NJ, Holgate ST. Effect of inhaled corticosteroids on episodes of wheezing associated with viral infection in school age children: Randomised double blind placebo controlled trial. British Medical Journal 1997;315(7112):858‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doull IJM, Sandall D, Smith S, Schreiber J, Freezer NJ, Holgate ST. Differential inhibitory effect of regular inhaled corticosteroid on airway responsiveness to adenosine 5' monophosphate, methacholine, and bradykinin in symptomatic children with recurrent wheeze. Pediatric Pulmonology 1997;23(6):404‐11. [DOI] [PubMed] [Google Scholar]
- Freezer NJ, Croasdell H, Doull IJ, Holgate ST. Effect of regular inhaled beclomethasone on exercise and methacholine airway responses in school children with recurrent wheeze. European Respiratory Journal 1995;8(9):1488‐93. [PubMed] [Google Scholar]
Emel'ionov 1995 {published data only}
- Emel'ianov AV, Trofimov VI. [The effect of glucocorticoids on mineral metabolism in bronchial asthma patients]. Ter Arkh 1995;67(12):31‐3. [PubMed] [Google Scholar]
Francia 1994 {published data only}
- Francia G, Senna GE, Betteli C, Musumeci C, Fratta‐Pasini A, Piubello G, et al. Pituitary‐adrenal function in asthmatic patients treated with high dose of beclomethasone. Allerg Immunol (Paris) 1994;26(1):11‐5. [PubMed] [Google Scholar]
Harrison 1999 {published data only}
- Harrison LI, Colice GL, Donnell D, Soria I, Dockhorn R. Adrenal effects and pharmacokinetics of CFC‐free beclomethasone dipropionate: A 14‐day dose‐response study. Journal of Pharmacy & Pharmacology 1999;51(3):263‐9. [DOI] [PubMed] [Google Scholar]
Harvald 1976 {published data only}
- Harvald B, Letman H. [Treatment of asthma with beclomethasone]. Ugeskrift for Laeger 1976;138(44):2717‐21. [PubMed] [Google Scholar]
Hoshino 1999 {published data only}
- Hoshino M, Takahashi M, Takai Y, JaeJoon S. Inhaled corticosteroids decrease subepithelial collagen deposition by modulation of the balance between matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 expression in asthma. Journal of Allergy and Clinical Immunology 1999;104(2):356‐63. [DOI] [PubMed] [Google Scholar]
Huang 1993 {published data only}
- Huang JL, Hung IJ, Hsieh KH. Effect of inhaled beclomethasone dipropionate in the treatment of recurrent wheezing in infancy and early childhood. Journal of the Formosan Medical Association 1993;92(12):1066‐9. [PubMed] [Google Scholar]
Juniper 2002 {published data only}
Kaptein 1993 {published data only}
- Kaptein AA, Brand PL, Dekker FW, Kerstjens HA, Postma DS, Sluiter HJ. Quality‐of‐life in a long‐term multicentre trial in chronic nonspecific lung disease: assessment at baseline. The Dutch CNSLD Study Group. European Respiratory Journal 1993;6:1479‐84. [PubMed] [Google Scholar]
Konig 1974 {published data only}
- Konig P, Jaffe P, Godfrey S. Effect of corticosteroids on exercise‐induced asthma. Journal of Allergy & Clinical Immunology 1974;54(1):14‐9. [DOI] [PubMed] [Google Scholar]
Malo 1996 {published and unpublished data}
- Malo JL, Cartier A, Cote J, et al. Influence of inhaled steroids on recovery from occupational asthma after cessation of exposure: an 18‐month double‐blind crossover study. American Journal of Respiratory & Critical Care Medicine 1996;153(3):953‐60. [DOI] [PubMed] [Google Scholar]
Menz 1986 {published data only}
- Menz G. [Treatment of asthma with high doses of beclomethasone]. Schweizerische Rundschau fur Medizin Praxis 1986;75(30‐31):914‐6. [PubMed] [Google Scholar]
Michel 1977 {published data only}
- Michel FB, Clauzel AM, Calvayrac P, Grenier J. [Mechanisms of action of glucocorticoids in the treatment of asthma. Comparative effects of glucocorticoids via the general route and beclomethasone dipropionate via aerial route on plasma levels of cyclic AMP]. Nouvelle Presse Medicale 1977;6(15):1278‐80. [PubMed] [Google Scholar]
Nakajima 1979 {published data only}
- Nakajima S, Fujihara Y, Tsuya Y, Ohishi M, Takagi O. Changes of bronchoconstriction by treatment. Acta Medica Kinki University 1979;4(2):273‐9. [Google Scholar]
Nielsen 1999 {published data only}
- Nielsen LP, Pedersen B, Faurschou P, Madsen F, Wilcke JTR, Dahl R. Salmeterol reduces the need for inhaled corticosteroid in steroid‐dependent asthmatics. Respiratory Medicine 1999;93(12):863‐8. [DOI] [PubMed] [Google Scholar]
Nizankowska 1989 {published data only}
- Nizankowska E, Szczeklik A. Glucocorticosteroids attenuate aspirin‐precipitated adverse reactions in aspirin‐intolerant patients with asthma. Annals of Allergy 1989;63:159‐62. [PubMed] [Google Scholar]
Noonan 2001 {published data only}
- Noonan I, Reiss T, Knorr B, Guerra J, Matz J. Long term asthma control with oral montelukast and inhaled beclomethasone for adults and children 6 yeras and older. Clinical & Experimental Allergy 2001;31:845‐45. [DOI] [PubMed] [Google Scholar]
Patakas 1978 {published data only}
- Patakas DLGSL. Effect of beclomethasone dipropionate inhalation on exercise induced bronchospasm. IRCS Medical Science: Cardiovascular System 1978;6(11):448. [Google Scholar]
Pizzichini 1996 {published and unpublished data}
- Pizzichini MM, Kidney JC, Wong BJ, et al. Effect of salmeterol compared with beclomethasone on allergen‐induced asthmatic and inflammatory responses [published erratum appears in Eur Respir J 1996 Oct;9(10):2190]. European Respiratory Journal 1996;9(3):449‐55. [DOI] [PubMed] [Google Scholar]
Richards 1978 {published data only}
- Richards W, Platzker A, Church JA, Yamamoto F, Foster S. Steroid‐dependent asthma treated with inhaled beclomethasone dipropionate in children. Annals of Allergy 1978;41(5):274‐7. [PubMed] [Google Scholar]
Rodriguez 1986 {published data only}
- Rodriguez Sanchon B, Manresa F, Romero P, Cardona M. [Nocturnal asthma: Treatment with high inhalatory doses of salbutamol and beclomethasone]. Revista Clinica Espanola 1986;178:322‐6. [PubMed] [Google Scholar]
Rohdewald 1992 {published data only}
- Rohdewald P. [Inhaled glucocorticoids. Slight side effects at high effectiveness]. Deutsche Medizinische Wochenschrift 1992;117(10):390‐3. [DOI] [PubMed] [Google Scholar]
Ronchetti 1976 {published data only}
- Ronchetti R, Gentili G. [Beclomethasone in the treatment of infantile asthma]. Minerva Pediatrica 1976;28(7):478‐85. [PubMed] [Google Scholar]
Ruff 1977 {published data only}
- Ruff F, Brouet G. [Four years use of beclomethasone dipropionate aerosols]. Nouvelle Presse Medicale 1977;6(15):1283‐5. [PubMed] [Google Scholar]
Sidel'nikov 1985 {published data only}
- Sidel'nikov VM, Pashun TV, Pomytkina LR. [Comparative evaluation of the effectiveness of different methods of prophylactic treatment for children with bronchial asthma]. Pediatriia 1985;4:34‐6. [PubMed] [Google Scholar]
Sil'vestrov 1983 {published data only}
- Sil'vestrov VP, Surovov Iu A, Pakulin IA, Larin NV. [Effect of treatment with ventolin and becotide on ventilation and hemodynamics in patients with bronchial asthma]. Klinicheskaia Meditsina 1983;61(12):54‐8. [PubMed] [Google Scholar]
Silkoff 2001 {published data only}
- Silkoff P, McClean P, Spino M, Erlich L, Slutsky A, Zamel N. Dose response relationship and peroducibility of the fall in exhaled nitric oxide after inhaled beclomethasone dipropionate therapy in asthma patients. Chest 2001;119:1322‐28. [DOI] [PubMed] [Google Scholar]
Stick 1995 {published data only}
- Stick SM, Burton PR, Clough JB, Cox M, LeSouef PN, Sly PD. The effects of inhaled beclomethasone dipropionate on lung function and histamine responsiveness in recurrently wheezy infants. Archives of Disease in Childhood 1995;73(4):327‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Storr 1986 {published data only}
- Storr J, Lenney CA, Lenney W. Nebulised beclomethasone dipropionate in preschool asthma. Archives of Disease in Childhood 1986;61(3):270‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Webb 1986 {published data only}
- Webb MS, Milner AD, Hiller EJ, Henry RL. Nebulised beclomethasone dipropionate suspension. Archives of Disease in Childhood 1986;61(11):1108‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuksel 1992 {published data only}
- Yuksel B, Greenough A. Randomised trial of inhaled steroids in preterm infants with respiratory symptoms at follow up. Thorax 1992;47(11):910‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Boszormeny‐Nagy 1980 {published data only}
- Boszormenyi‐Nagy G, Herjavecz I. [Clinical effects of beclomethasone dipropionate in bronchial asthma]. Orvosi Hetilap 1980;121(16):947‐50. [PubMed] [Google Scholar]
Chonabayashi 1986 {published data only}
- Chonabayashi N, Yoshimura K, Nakamori Y, Nakatani T, Nakata K, Tanimoto H. [Method of decreasing oral corticosteroids by adding a beclomethasone dipropionate inhaler for steroid‐dependent asthmatic patients]. Nippon Kyobu Shikkan Gakkai Zasshi ‐ Japanese Journal of Thoracic Diseases 1986;24(5):522‐30. [PubMed] [Google Scholar]
Iwata 1995 {published data only}
- Iwata M, Iinuma Y, Sugino Y, Suzuki Y. [Effects and limitations of inhaled high dose beclomethasone dipropionate (2400 micrograms/day) in steroid‐dependent asthmatics]. Arerugi 1995;44(5):562‐6. [PubMed] [Google Scholar]
Kudo 1995 {published data only}
- Kudo K, Hojo M, Kabe J. [Inhaled beclomethasone in long‐term management of asthma: optimal dose and optimal duration of treatment]. Nippon Kyobu Shikkan Gakkai Zasshi 1995;33(9):956‐65. [PubMed] [Google Scholar]
Muittari 1974 {published data only}
- Muittari A, Veneskoski T. [Beclomethasone aerosol in asthma therapy]. Duodecim 1974;90(18):1228‐34. [PubMed] [Google Scholar]
Rozniecki 1988 {published data only}
- Rozniecki J, Szmidt M, Grzelewska‐Rzymowska I, Gorski P, Grabski W. [Clinical evaluation of beclomethasone dipropionate aerosol in patients with bronchial asthma]. Polski Tygodnik Lekarski 1988;43(12‐13):412‐5. [PubMed] [Google Scholar]
Shen 1991 {published data only}
- Shen X, Niu SF, Cai YY. [A clinical trial of treating asthma of moderate severity with beclomethasone dipropionate aerosol]. Chung‐Hua Nei Ko Tsa Chih Chinese Journal of Internal Medicine 1991;30(9):536‐8, 593. [PubMed] [Google Scholar]
Additional references
BTS 1997
- British Thoracic Society. The British Guidelines on asthma management 1995 review and position statement. Thorax 1997;52(Suppl 1):S1‐S21. [Google Scholar]
BTS 2003
- The British Thoracic Society/Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. Thorax 2003;58(Suppl 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
Busse 1999
- Busse W, brazinsky Sh, Jacobson K, stricker W, et al. Efficacy response of inhaled beclomethasone dipropionate in asthma is proportional to dose and is improved by formulation with a new propellant. Journal of Allergy and Clinical Immunology 1999;104(6):1215‐22. [DOI] [PubMed] [Google Scholar]
CONSORT 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. The Journal of the American Medical Association 1996;276:637‐9. [DOI] [PubMed] [Google Scholar]
GINA 1995
- National Asthma Education and Prevention Program. Global strategy for asthma management and prevention NHBLI/WHO workshop report. National Institute of Health, Bethseda, MD 1995, issue NIH Publication No. 95‐3659.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Lipworth 1993
- Lipworth BJ. Clinical pharmacology of corticosteroids in bronchial asthma. Pharmacology Therapy 1993;58:173. [DOI] [PubMed] [Google Scholar]
Malouf 2004
- Malouf R, Wright J. Pressurised‐metred does inhalers versus hand‐held inhalers for the delivery of corticosteroids in asthma. The Cochrane Library 2004, Issue 4. [Google Scholar]
NHLBI 1997
- National Asthma Education and Prevention Program. Guidelines for the Diagnosis and Managment of Asthma, Expert Panel Report No. 2. Bethesda MD: NIH/National Heart, Lung and Blood Institute 1997, issue Publication No. 97‐4051.


