Abstract
During embryonic development in bilaterally symmetric organisms, correct midline crossing is important for the proper formation of functional neural circuits. The aberrant development of neural circuits can result in multiple neurodevelopmental disorders, including horizontal gaze palsy, congenital mirror movement disorder, and autism spectrum disorder. Thus, understanding the molecular mechanisms that regulate proper axon guidance at the midline can provide insights into the pathology of neurological disorders. The signaling mechanisms that regulate midline crossing have been extensively studied in the Drosophila ventral nerve cord and the mouse embryonic spinal cord. In this review, we discuss these axon guidance mechanisms, highlighting the most recent advances in the understanding of how commissural axons switch their responsiveness from attractants to repellents during midline crossing.
Introduction
In bilaterally symmetric organisms, precise wiring of neural circuits at the midline is crucial for the proper coordination of the left and right sides of the body. This process is achieved by commissural interneurons, which project their axons across the midline. In order to connect with their synaptic partners, commissural axons (CAs) navigate through a series of intermediate targets or choice points (de Ramon Francas et al., 2017; Dickson and Zou, 2010; Kaprielian et al., 2001; Neuhaus-Follini and Bashaw, 2015a; Vallstedt and Kullander, 2013). In both the vertebrate spinal cord and the invertebrate ventral nerve cord, conserved families of ligands and cell surface receptors signal locally to reorganize the growth cone cytoskeleton, leading to either axon attraction or axon repulsion (Evans and Bashaw, 2010a) (Figure 1). Growing CAs initially respond to attractive cues that guide them towards the midline. Once they reach the midline, they switch their responsiveness and become sensitive to repulsive cues in order to exit the midline and to prevent re-crossing. This change in responsiveness is important for CAs to form correct connections (Figure 1). In this review, we will provide an overview of the mechanisms that regulate CA guidance in the developing mouse spinal cord and Drosophila ventral nerve cord, highlighting the latest studies that provide new insights into molecular mechanisms that control the switch in CA responsiveness during midline crossing.
Figure 1: Axon guidance receptors and ligands in the Drosophila ventral nerve cord and in the mouse embryonic spinal cord.
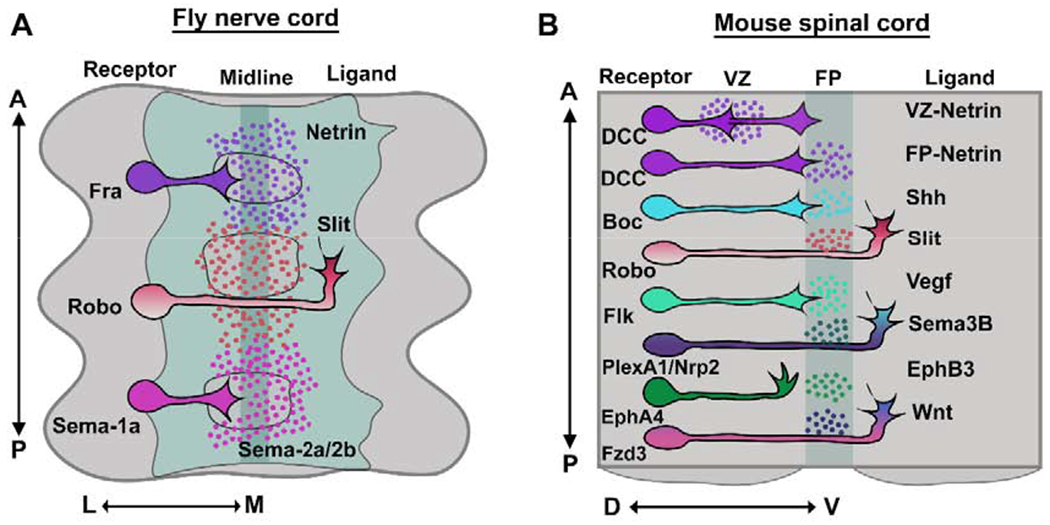
(A) In Drosophila, Fra is the attractive axon guidance receptor that responds to its ligand Netrin, which is secreted from midline cells, while the repulsive axon guidance receptor, Robo mediates repulsion in response to a midline source of Slit. Fra is the Drosophila homolog of DCC and it is referred to by its correct species name throughout this review. In response to secreted Sema-2a and Sema-2b, Sema-1a promotes midline crossing independently of the Fra/Netrin pathway. (B) In the mouse spinal cord, DCC, a vertebrate homolog of Fra promotes midline attraction in response to VZ, as well as FP derived netrin1, while Robo mediates midline repulsion in response to FP derived Slit. In addition, the FP expresses Shh and Vegf, which promotes midline crossing through the interactions with their receptors Boc and Flk, respectively. PlexinA1 and Neuropilin-2 (Nrp2) receptors mediate repulsion in response to Sema3B while Ephrin-A4 facilitates repulsion in response to midline repellent, Ephrin-B3. The proper rostral turning of post-crossing commissural axons is regulated by interactions between Fzd3 receptor and its ligand, Wnt. FP, floor plate; VZ, ventricular zone; A, anterior; P, posterior; L, lateral; M, medial; D, dorsal; V, ventral.
Commissural axon guidance at the ventral midline of the developing spinal cord has been extensively studied. Commissural neurons (CNs) are born in the dorsal spinal cord and extend their axons ventrally towards the floor plate (FP) intermediate target (Dodd et al., 1988; Tulloch et al., 2019). Once they cross, the majority of CAs exit the FP on the contralateral side and turn rostrally towards the brain (Bovolenta and Dodd, 1990). The first spinal CNs are generated in the dorsal spinal cord at embryonic day 9.5 (E9.5). Some CNs extend their axons across the FP at E10.5 and by E12.5, most of the axons have crossed the midline (Pignata et al., 2016). CNs in the spinal cord are a highly heterogeneous population of cells which are subdivided into early-born dI1- dI6 neurons and late-born dILA and dILB neurons (Tulloch et al., 2019). In particular, CA navigation of the dorsal-most dI1 neurons has been widely investigated (Pignata et al., 2016).
Spinal dl1 CA growth is directed by several guidance cues. The roof plate expresses the repellents BMP7 and Draxin, which repel CAs from the dorsal spinal cord (Augsburger et al., 1999; Butler and Dodd, 2003; Islam et al., 2009). In addition, the FP expresses the attractants netrin1, Shh and VEGF which attract CAs to the ventral midline (Charron et al., 2003; Kennedy et al., 1994; Ruiz de Almodovar et al., 2011; Serafini et al., 1996) (Figure 1B). Interestingly, several recent studies suggest that netrin1 expressed in the ventricular zone (VZ), rather than FP derived netrin1, is the primary source of Netrin that promotes CAs to grow into the FP (Dominici et al., 2017; Varadarajan et al., 2017) (Figure 1B). Alternatively, VZ-derived Netrin and FP-derived Netrin could work together to guide CAs; indeed, a more recent study suggests that VZ and FP-derived netrin1 act together to guide spinal CAs towards the ventral midline (Moreno-Bravo et al., 2019). In addition to Netrin-DCC, Shh-Boc (Okada et al., 2006) and VEGF-FLK1 (Ruiz de Almodovar et al., 2011) also contribute to CA attraction to the midline (Figure 1B). Thus, it is the combined action of multiple attractive ligand/receptor interactions that guide CAs to the FP. As they approach and enter the FP, CAs must suppress their responsiveness to multiple repellent pathways, including Slits and their Roundabout (Robo) receptors and Semaphorins and their Plexin and Neuropilin (Npn) receptors (Figure 1B) (Brose et al., 1999; Long et al., 2004; Nawabi et al., 2010; Zou et al., 2000). For instance, Robo3, a divergent Robo family member attenuates Robo1 mediated repulsion in pre-crossing CAs and promotes midline crossing (Sabatier et al., 2004). In addition to antagonizing Robo1 repulsion, Robo3 also contributes to midline attraction by potentiating the activity of DCC (Zelina et al., 2014). Recent evidence suggests that Robo3 also guides CAs towards the midline by mediating repulsion from the motor column through its ligand NELL2 (Jaworski et al., 2015). After reaching the midline, CAs restore repulsion by a variety of mechanisms that we will discuss in this review. Restoring repulsion allows CAs to exit the FP and prevents them from re-entering. Upon reaching the contralateral side of the FP, CAs turn anteriorly in response to Wnt and Shh gradients (Aviles and Stoeckli, 2016; Bourikas et al., 2005; Lyuksyutova et al., 2003; Wilson and Stoeckli, 2013; Yam et al., 2012).
The Drosophila ventral nerve cord is analogous to the vertebrate spinal cord. The ventral nerve cord consists of segmentally repeating neuromeres. The majority of neurons in the Drosophila embryonic CNS are commissural neurons. These CNs extend their axons to the midline, crossing in either the anterior or posterior commissure in each segment (Rickert et al., 2011) (Figure 1A). In comparison to vertebrate systems, there are a smaller number of signaling pathways that regulate axon guidance at the midline in insects. Nevertheless, these fundamental signaling pathways are evolutionarily conserved in other organisms (Evans and Bashaw, 2010a). A particular advantage of the Drosophila nervous system is the availability of a range of genetic tools and molecular markers to label specific subsets of CNs, which allows for precise examination of specific neurons in various genetic backgrounds. In Drosophila, Frazzled (Fra) promotes midline attraction in response to midline derived NetrinA/B while midline repulsion is mediated by Robo1/2 in response to Slit (Figure 1A) (Dickson and Gilestro, 2006; Moore et al., 2007). Fra is the Drosophila homolog of DCC and it is referred to by its correct species name throughout this review. In pre-crossing CAs, Slit-Robo1 mediated repulsion is negatively regulated by Commissureless (Comm) (Keleman et al., 2002; Kidd et al., 1998; Tear et al., 1996) and Robo2 (Evans et al., 2015) (Figures 2A and 3A). Although Netrin-Fra signaling plays an important role in promoting axon growth across the midline, many axons still cross the midline in fra mutants or netrinAB double mutants (Garbe et al., 2007; Mitchell et al., 1996), indicating the existence of additional pathways in controlling midline crossing. Consistently, recent studies have shown that in addition to Netrin-Fra signaling, Sema-1a acts as a receptor in CAs to promote midline crossing in response to midline-secreted Sema-2a and Sema-2b (Hernandez-Fleming et al., 2017) (Figure 1A).
Figure 2: Mechanisms that regulate Slit-Robo repulsion by altering Robo1 levels at the growth cone membrane.
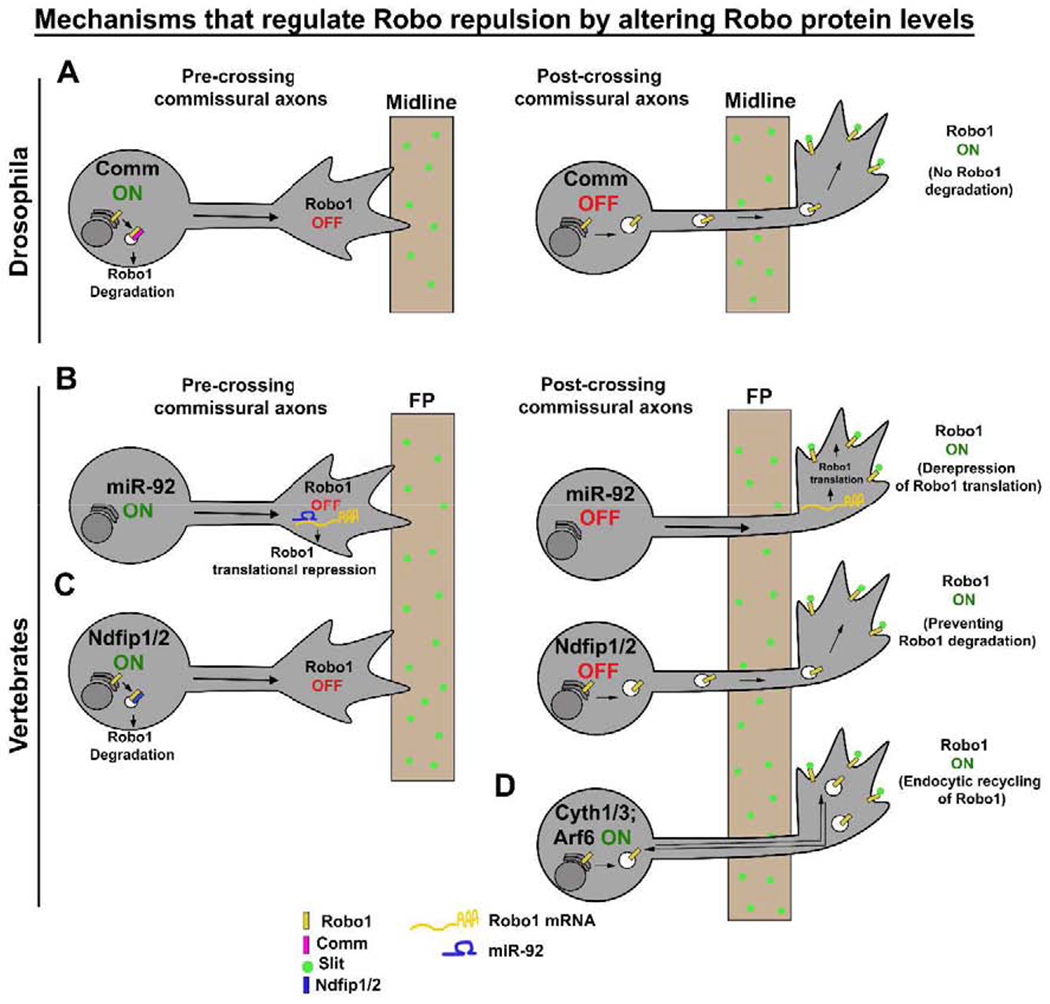
(A) In Drosophila, as CAs approach the midline, they express Comm, which diverts newly synthesized Robo1 to late endosomes, presumably for degradation. After midline crossing, Comm expression is down-regulated and Robo1 is trafficked to the growth cone membrane, thereby restoring Slit repulsion. (B) In chick, pre-crossing CAs suppress Slit repulsion by expressing miR-92, which down-regulates Robo1 by translational repression while post-crossing CAs restore Slit-Robo repulsion by down-regulating miR-92. (C) In mouse, Ndfip1/2 targets Robo1 for endosomal degradation and suppresses Slit repulsion in pre-crossing CAs, while in post-crossing CAs, Ndfip1/2 levels are down-regulated, which allows an increase in Robo1 levels on the growth cone membrane. (D) In mouse, Cytohesin-1/3 activates Arf6 to promote Robo1 endocytic recycling, thereby enhancing Slit-Robo1 repulsion in post-crossing CAs. CAs, commissural axons; FP, floor plate.
Figure 3: Mechanisms that regulate Slit-Robo repulsion without affecting Robo1 levels.
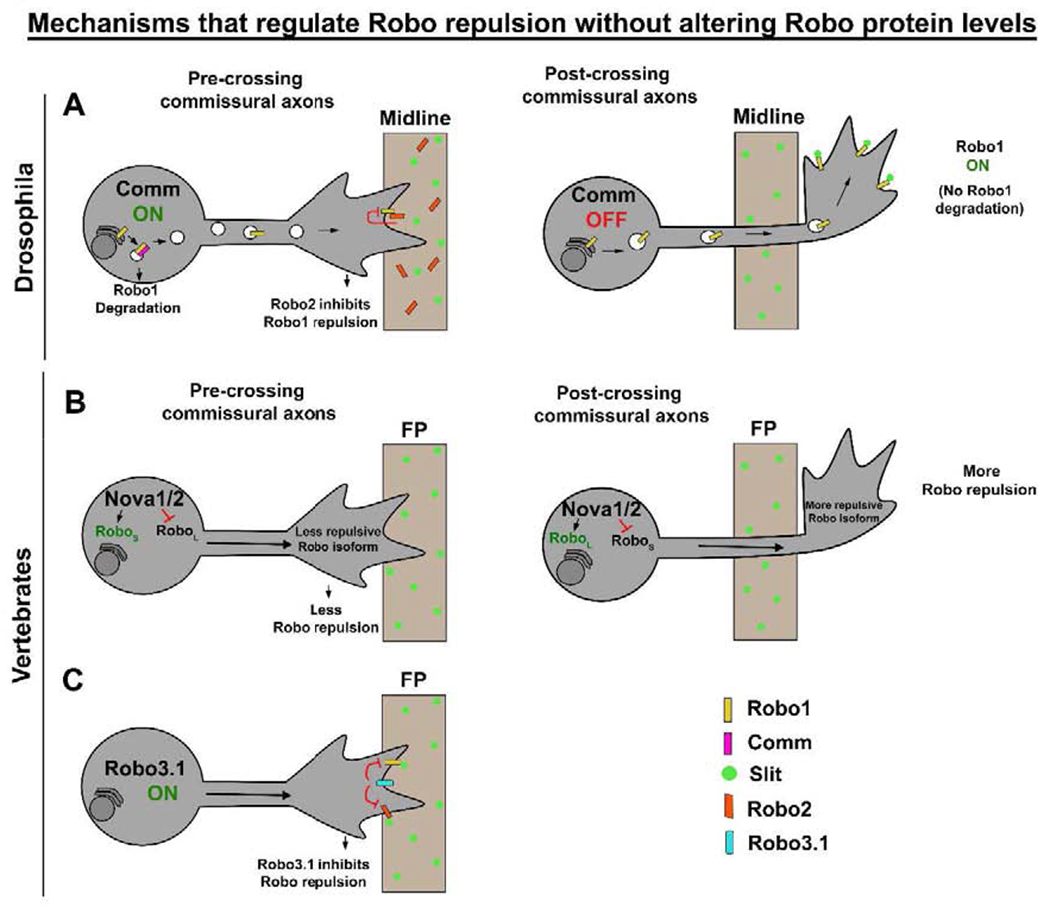
(A) In Drosophila, as CAs approach the midline, Robo2 that is expressed in midline cells binds to Robo1 on the growth cone membrane in trans and inhibits Slit-Robo1 repulsion. In post-crossing CAs, both Comm and Robo2 levels are down-regulated by unknown mechanisms and this re-establishes Slit repulsion. (B) In mouse, Nova1/2 directed alternative splicing generates a less-repulsive Roboshort isoform and promotes midline crossing. As CAs cross the midline, Nova1/2 generates a more-repulsive Robolong isoform. (C) In pre-crossing CAs, Robo3.1 promotes midline crossing by suppressing Robo1/2 mediated Slit repulsion. CAs, commissural axons; FP, floor plate.
In the past several years there has been significant progress in understanding the regulatory mechanisms that modulate CA responsiveness at the midline. In this review, we discuss these regulatory mechanisms, emphasizing recent findings that determine how the activity and expression of axon guidance molecules are regulated spatially and temporally to control CA responsiveness during midline crossing.
Regulation of axon guidance molecules at the midline
When commissural axons reach the midline, they switch their responsiveness by altering the expression and activity of surface receptors. This spatial and temporal regulation of axon guidance receptor expression on the surface of the commissural growth cone is achieved by a diverse array of molecular mechanisms and these regulatory events can occur at the post-transcriptional or the post-translational level. Post-transcriptional mechanisms include alternative splicing, microRNA regulation and local protein synthesis.
Alternative splicing
Alternative splicing events play important roles in regulating CA guidance at the midline in the developing mouse spinal cord (Grabowski and Black, 2001; Li et al., 2007; Zheng, 2020). The NOVA (Neuro-oncological ventral antigen) family of splicing factors control CA attraction by increasing the production of DCClong isoform while decreasing the production of DCCshort isoform (Leggere et al., 2016). In Nova1/2 double knockout (dKO) spinal cords, the majority of CAs fail to cross the midline and this defect is rescued by DCClong expression but not by DCCshort expression (Leggere et al., 2016). DCClong and DCCshort isoforms vary in the extracellular FN4-FN5 linker sequence and both bind to Netrin with similar affinity but in different conformations (Xu et al., 2014). However, it is not clear if and how these different isoform conformations transduce Netrin signal into distinct intracellular actions. In addition, since the Nova dKO midline crossing phenotype is partially rescued by deleting Robo1, it is likely that the failure to cross the midline in Nova dKOs is due in part to enhanced repulsive activity (Johnson et al., 2019).
Recent findings from the same group suggest that NOVA also regulates the temporal production of two Robo1/2 isoforms, e6b+ (Robolong) and e6b− (Roboshort) that differ in a short linker between Ig3 and Ig4 domains (Johnson et al., 2019) (Figure 3B). In the Nova1/2 dKO, production of the Robolong isoform, which has a stronger repulsive activity increases, while the production of the Roboshort isoform decreases. The midline crossing defect in Nova1/2 dKO is partially rescued by reducing Robolong expression (Johnson et al., 2019). Somewhat paradoxically, previous results from this group have shown that expressing Robo3, which is known to inhibit Robo repulsion in pre-crossing CAs, is unable to rescue the Nova1/2 dKO midline crossing phenotype (Leggere et al., 2016). If reducing the expression of Robolong isoform rescues the Nova1/2 dKO midline crossing defects by suppressing repulsion, it is unclear why expression of Robo3 is unable to rescue, since Robo3 attenuates Robo repulsive signaling. In a later study, the same group has also shown that simultaneously removing one copy of Nova1/2 and Robo3 leads to significant defects in midline crossing, suggesting that Nova functions with Robo3 to allow CA midline entry (Johnson et al., 2019). Together these seemingly conflicting observations raise some uncertainty about the precise role of Nova1/2 in midline crossing.
It is worth noting that the protein coding difference between Robolong and Roboshort is 3 and 4 amino acids for Robo1 and Robo2 respectively (Johnson et al., 2019). Both Robo1/2 isoforms have the ability to bind to Slit with similar binding affinity but differ in downstream signaling. Robolong has a stronger effect than Roboshort in activating RAC and inhibiting CDC42 (Johnson et al., 2019). One recent report suggests that active Robo signaling is achieved upon the release of Robo from auto-inhibition and the subsequent Ig4 mediated Robo dimerization, which in turn activates intracellular signaling by an unknown mechanism (Barak et al., 2019). Accordingly, one possibility is that the distinct functions of Robolong and Roboshort isoforms may be due to the differences in their ability to mediate receptor dimerization. An intact linker region along with an Ig4 domain may be crucial for the formation of active Robo dimers in order to mediate a stronger Slit signal. The Roboshort isoform in which the linker region is absent, may fail to form active dimers since the receptor structure is altered. Detailed biochemical characterization of the properties of these Robo isoforms may resolve how these minor sequence changes result in distinct signaling properties.
Robo3 also undergoes alternative splicing and generates two isoforms that differ in their cytoplasmic domains (Chen et al., 2008). Robo3.1 is specifically expressed on pre-crossing CAs, while Robo3.2 is specifically expressed on post-crossing CAs. In vivo experiments suggest that Robo3.1 facilitates midline crossing in pre-crossing CAs by suppressing Slit-mediated repulsion (Figure 3C), whereas Robo3.2 facilitates midline repulsion in post-crossing CAs and prevents re-crossing. It had been hypothesized that Robo3.1 acts as a Slit sink, thereby preventing Robo1/2 from binding to Slit, while Robo3.2 mediates Slit repulsion by functioning as a classical Robo receptor (Chen et al., 2008; Sabatier et al., 2004). However, these models have been excluded in light of reports indicating that mammalian Robo3 proteins do not bind to Slit (Li et al., 2014; Zelina et al., 2014). Thus, it remains unclear how Robo3.1 antagonizes Robo1 activity in pre-crossing CAs and how Robo3.2 mediates repulsion in response to Slit on post-crossing CAs. More recent comparative analysis of Robo3 sequences reveals striking conservation of the exon encoding the cytoplasmic portion of the Robo3.1 isoform across mammalian species (Friocourt and Chedotal, 2017). Strangely, the alternative exon encoding Robo3.2 sequences in mice does not seem to be conserved even between closely related rodent species (Friocourt and Chedotal, 2017) suggesting that Robo3.2 does not play a fundamental conserved role in vertebrate axon guidance.
MicroRNAs
Regulation of the surface expression of axon guidance receptors is also achieved via microRNAs. A recent study suggests that the fine-tuned regulation of Robo1 in developing chick spinal CAs is mediated by miR-92 (Figure 2B). miR-92 binds to the miRNA recognition element in the 3’ untranslated region (3’ UTR) of Robo1 mRNA and causes translational repression (Yang et al., 2018). miR-92 is strongly expressed in pre-crossing CAs and has a reciprocal expression pattern from both Robo1 mRNA and protein. This study also suggests that miR-92 suppresses Robo1 expression in primary neurons from mice (Yang et al., 2018). Despite these observations, there is no in vivo evidence for the role of miR-92 in the regulation of CA guidance in the developing mouse spinal cord. Thus, it remains to be determined whether this mode of regulation is physiologically relevant if so, how miRNA expression and activity are controlled to direct local Robo1 expression in CAs.
Interestingly, it is worth noting that the miR-92 family is evolutionarily conserved and in Drosophila, the miR-92 family consists of miR-92a and miR-92b. Both miRNAs are expressed at high levels in the embryo (Yuva-Aydemir et al., 2015). However, dRobo1 does not have miR-92a/b binding sites in its 3’ UTR (based on miRbase) suggesting that miR-92 mediated Robo1 regulation is confined to vertebrates or that miR-92 target sequences have diverged.
Local protein synthesis
Robo3.1 is specifically expressed in pre-crossing CAs and promotes midline crossing by suppressing Slit-Robo1 repulsion (Sabatier et al., 2004), potentiating DCC-mediated attraction (Zelina et al., 2014) and directing repulsion from the motor column in response to NELL2 (Jaworski et al., 2015; Pak et al., 2020). However, the molecular mechanism that down-regulates Robo3.1 expression in post-crossing CAs is unclear. A recent study proposed that Robo3.1 expression in post-crossing CAs is controlled through translational regulation (Zhuang et al., 2019). This study identified a novel mechanism for the control of Robo3.1 expression involving a “reader” protein called YTH domain-containing family protein (YTHDF), which has been shown to enhance Robo3.1 translational efficiency by binding to the m6A (N6-Methyladenosine) modified Robo3.1 mRNA (Zhuang et al., 2019). Mutation of m6A sites on Robo3.1 mRNA or YTHDF1 knock down cause dramatic reduction in Robo3.1 protein levels and conditional knockdown of YTHDF1 in spinal CAs results in pre-crossing axon guidance errors. This study also provides evidence that FP-derived signals down-regulate YTHDF1 expression to prevent Robo3.1 translation in post-crossing CAs (Zhuang et al., 2019), although the identities of these signals remain unknown.
Additionally, there are also some insights into the mechanisms that regulate the spatial and temporal expression of the Robo3.2 isoform. For example, there is evidence that Robo3.2 is locally translated in post-crossing CAs and Robo3.2 expression appears to be induced in the presence of FP derived signals. In addition, the nonsense-mediated mRNA decay (NMD) pathway induces the degradation of Robo3.2 transcripts in axons that contact the FP (Colak et al., 2013). Nevertheless, the lack of conservation of Robo3.2 sequences in closely related rodent species calls into question the general importance of Robo3.2 in axon guidance.
Regulation at the post-translational level
During midline crossing, the surface expression of axon guidance receptors on the commissural growth cone is regulated by various post-translational mechanisms such as regulated receptor trafficking, regulated receptor endocytosis, regulated proteolytic processing and receptor-receptor interactions.
Receptor trafficking
The specific delivery of axon guidance receptors at the growth cone membrane is required to control axon responsiveness in a temporal manner. For instance, precise temporal regulation of the Robo1 receptor in Drosophila CAs is achieved by Comm (Figure 2A). In Drosophila, Comm controls midline crossing by negatively regulating Robo1 surface levels on pre-crossing CAs, thereby preventing these axons from prematurely responding to the midline repellant Slit (Keleman et al., 2002; Kidd et al., 1998; Tear et al., 1996). Previous studies have suggested that Comm acts as an endocytic sorting receptor for Robo1 and targets newly synthesized Robo1 to the endosomal compartment, presumably for degradation (Keleman et al., 2002; Keleman et al., 2005), and that this activity is correlated with the ability of Comm to physically associate with Robo1 (Figure 2A). In comm mutants, Robo1 is constitutively trafficked to the growth cone surface and prevents CAs from crossing the midline (Keleman et al., 2005).
Surprisingly, Comm can also regulate Robo1 repulsion through a sorting-independent mechanism, since midline guidance still occurs normally when Robo1 is replaced with a version of the receptor that is insensitive to Comm sorting (sorting-defective Robo1- RoboSD) (Gilestro, 2008). The fact that comm, robo1 double mutants are nearly identical to robo1 single mutants (Kidd et al., 1998) strongly argues that Comm’s sole function in promoting midline crossing is through inhibition of Robo1-mediated repulsion. Therefore, the observation that preventing Comm from regulating Robo1 by replacing endogenous Robo1 with RoboSD does not result in a comm mutant phenotype means that Comm must also regulate other cargoes in the Robo1 pathway to prevent Slit-mediated repulsion. Thus, identifying additional cargoes of Comm is of great interest.
As CAs approach the midline, Comm expression is high, allowing axons to cross. Once CAs reach the midline, Comm expression is down regulated through unknown mechanisms, which restores Robo1 mediated Slit sensitivity to prevent re-crossing (Keleman et al., 2002). Thus, the precise temporal regulation of Comm is especially important for proper midline crossing in the Drosophila CNS (Figure 2A). It has been shown that the Netrin receptor, Fra, can be cleaved by gamma secretase and the released Fra intracellular domain (ICD) subsequently translocates to the nucleus and activates transcription of comm (Neuhaus-Follini and Bashaw, 2015b). This non-canonical function of Fra is independent of Netrin, as comm expression is unaltered in NetAB mutants (Yang et al., 2009). The transcriptional activation function of the Fra ICD only partially regulates comm expression, as fra mutants result in an incomplete loss of comm expression and exhibit milder crossing defects compared to comm mutants (Neuhaus-Follini and Bashaw, 2015b), suggesting that there must be an additional pathway(s) to regulate comm expression. Interestingly, Comm does not appear to be conserved outside of dipteran insects (Evans and Bashaw, 2012; Keleman et al., 2002). However, several vertebrate proteins have been identified that may have analogous functions to Drosophila Comm to regulate intracellular trafficking of Robo1 in commissural neurons.
For example, the vertebrate proline-rich and Gla domain containing PRRG proteins have been shown to share some sequence similarity to the functional cytoplasmic LPXY motif of Drosophila Comm (Justice et al., 2017). In vitro experiments suggest that mis-expression of PRRG4 can disrupt the normal plasma membrane localization of rRobo1 and downregulate Robo1 levels in cultured mammalian cells; however, the observed change in Robo1 localization is not consistent with Comm’s effect on Drosophila Robo. PRRG4 appears to trap rRobo1 in the endoplasmic reticulum and Golgi (Justice et al., 2017), while Comm has been shown to redirect dRobo1 into late endosomes (Keleman et al., 2002). Whether PRRG4 shares other properties with Comm, such as the requirement of its PY motifs for Robo1 regulation or the ability to interact with Robo has not been tested. Genetic experiments in Drosophila have shown that the expression of human Robo1 results in a dominant negative phenotype where axons ectopically cross the midline (Justice et al., 2017). It is unclear how hRobo1 expression causes this phenotype, since expression of mouse Robo1 in similar experiments leads to the expected gain of function phenotype where axons are repelled from the midline (Tim Evans, personal communication). Further, this ectopic crossing phenotype is enhanced when PRRG4 and hRobo1 are co-expressed in this context (Justice et al., 2017). If PRRG4 functions to decrease hRobo1 levels, it is unclear why the co-expression of PRRG4 enhances the ectopic crossing phenotype, since decreasing the levels of hRobo1 should reduce the dominant negative activity and suppress the phenotype. Whether PRRG4 is expressed in CAs in the developing spinal cord and has any function during CA guidance has not been investigated.
Recently, we have identified another class of mammalian proteins which has limited sequence similarity to the functional domain of Comm (Gorla et al., 2019). Ndfip1 and Ndfip2 are Nedd4 family interacting proteins, which contain PPXY and LPXY motifs in their structure and serve as adaptor proteins that recruit Nedd4 E3 ligases to specific substrate proteins, which leads to their ubiquitylation and subsequent degradation (Harvey et al., 2002; Mund and Pelham, 2009; Shearwin-Whyatt et al., 2004). These are late endosomal proteins similar to dComm and they can re-localize mammalian Robo1 to these compartments when co-expressed in Cos-7 cells (Gorla et al., 2019). In vitro biochemical data delineates an intracellular trafficking pathway consisting of Ndfip adaptor proteins and HECT domain containing Nedd4 E3 ubiquitin ligases that act together to promote Robo1 ubiquitylation and its subsequent degradation (Figure 2C). Furthermore, Ndfip proteins are expressed in CAs in the developing spinal cord and removal of Ndfip proteins results in an increase in the expression of Robo1 and a failure of some spinal CAs to cross the floor plate (Gorla et al., 2019). Examining the genetic interactions between Ndfip1/2 and Robo1 will further validate the direct role of Ndfip proteins in Robo1 regulation in vivo. Although the existing evidence suggests an important role for Ndfip proteins in regulating Robo1 expression, it is also possible that Ndfip proteins can regulate other pathways to control axon guidance.
In Drosophila, Comm’s ability to regulate the surface levels of Robo1 may depend on its interaction with the WW domain containing Nedd4 E3 ubiquitin ligase (dNedd4), since point mutations in the Nedd4 binding site in Comm disrupt its ability to regulate Robo1 (Myat et al., 2002). However, it is possible that the demonstrated requirement of the proline rich (PY) motifs in dComm may be due to interactions with other WW domain containing proteins, not because of interactions with Nedd4. Indeed, in vivo genetic experiments have suggested that dNedd4 is not required for midline crossing, as Nedd4 zygotic null mutants have no CA guidance defects (Keleman et al., 2005). Still, since Nedd4 is maternally deposited and there are multiple Nedd4 family ligases in Drosophila, the possibility of a role for E3 ubiquitin ligases in Comm mediated Robo1 regulation cannot be excluded. Mammalian Ndfip proteins can also bind to Nedd4 family E3 ligases (Mund and Pelham, 2009) and in vitro biochemical data suggests that E3 ligase activity is important for Ndfip-mediated Robo1 regulation (Gorla et al., 2019). Thus, investigating the expression of Nedd4 E3 ligases and their role in spinal CA guidance would provide more insights into Robo receptor trafficking in vertebrates. In addition, the molecular mechanisms that inhibit the activity of dComm and mammalian Ndfip in post-crossing CAs remains to be investigated.
In chick, RabGDI, a component of the vesicle fusion machinery triggers the membrane insertion of Robo1, thereby changing the CA responsiveness from attraction to repulsion at the midline (Philipp et al., 2012). In cooperation with RabGDI, Calsyntenin-1 transports Robo1 containing Rab11 positive vesicles to the growth cone surface in a precisely regulated manner (Alther et al., 2016). The accumulation of Robo1 at the growth cone membrane elicits an increase in Slit responsiveness, expelling the CAs from the floor plate. In addition to Robo1 trafficking, Calsyntenin-1 also regulates trafficking of Frizzled 3, a guidance receptor in the Wnt pathway and this function of Calsyntenin-1 is independent of RabGDI (Alther et al., 2016).
CAs also become responsive to Wnt once they cross the midline and Wnt-mediated attraction is required for the proper anterior turn after exiting the FP. Shisa2 is a transmembrane protein that interacts with a Wnt receptor, Frizzled 3 (Fzd3) in the ER and inhibits Fzd3 glycosylation, thereby preventing the translocation of Fzd3 to the growth cone plasma membrane (Onishi and Zou, 2017). Pre-crossing CAs express higher levels of Shisa2, which inhibits the cell surface presentation of Fzd3, thereby preventing CAs from sensing the Wnt gradient. After CAs reach the FP, Shisa2 levels decrease, allowing glycosylated Fzd3 to translocate to the surface. When CAs exit the FP, Fzd3 on the growth cone membrane responds to the anterior-high, posterior-low Wnt gradient and allows CAs to turn anteriorly. Interestingly, the spatio-temporal expression of Shisa2 appears to be regulated by Shh-Smo signaling (Onishi and Zou, 2017). The levels of Shisa2 mRNA are increased in the dorsal spinal cord of Smo cKO, which suggests that Shh-Smo signaling controls Shisa2 expression, potentially at the level of transcription (Onishi and Zou, 2017). The ability of Shh-Smo signaling to regulate the levels of Shisa2 provides evidence that there is cross talk between the Shh and Wnt pathways, in which Shh-Smo signaling switches on Wnt/PCP attractive signaling in CAs to allow for correct anterior turning on the contralateral side of the midline. However, whether Shisa2 is a direct transcriptional target of Shh-Smo signaling and whether Shisa2 levels are down-regulated by Gli-dependent transcriptional repression in response to Shh remains to be determined.
Receptor endocytosis
Another way of controlling the surface expression of axon guidance receptors in parallel to trafficking is through regulated endocytosis. In Drosophila, Slit dependent Robo1 endocytosis from the axon surface positively regulates repulsion during midline crossing. Robo1 trafficking from surface to late endosomes is essential for receptor activation and induces repulsion at the midline by allowing the recruitment of the Robo1 downstream effector, Son of sevenless (Sos) (Chance and Bashaw, 2015). In vertebrate commissural neurons, both Robo1 endocytosis and its subsequent recycling are important for modulating Slit sensitivity (Kinoshita-Kawada et al., 2019). Arf6 (ADP-ribosylation factor 6) GTPase and its activators, Cytohesins regulate Robo1 endocytic trafficking spatiotemporally to control Slit responsiveness of commissural neurons (Figure 2D). A decrease in surface Robo1 levels and the suppression of Slit-induced growth cone collapse upon siRNA mediated knockdown of Cytohesin-1/3 and Arf6 in CAs suggests that Arf6 along with Cytohesin-1/3 promotes Robo1 recycling in response to Slit, allowing axons to exit the midline. Cytohesin-2 may play a reciprocal role to Cytohesin-1/3 by negatively regulating the response to Slit in pre-crossing CAs, although the mechanism is unclear (Kinoshita-Kawada et al., 2019). Ex vivo rescue experiments in mouse spinal cord suggests that the guanine nucleotide exchange factor activity of Cytohesin-2 is important to inhibit the Slit response (Kinoshita-Kawada et al., 2019), so it would be interesting to determine if Cytohesin-2 activates other GTPases in pre-crossing CAs to negatively regulate Slit-Robo1 repulsion. Thus, Arf6 in association with distinct Cytohesins contributes to the change in Slit response during midline crossing.
Receptor endocytosis is also important for Shh-mediated growth cone attraction. Shh induces Boc and Ptch1 internalization into Rab5+ early endosomes and this internalization requires the endocytic adapter protein Numb (Ferent et al., 2019). Internalization of the Boc/Ptch1 receptor complex upon Shh binding leads to Smo activation, which in turn is required for the phosphorylation of Src family kinases in growth cones to induce turning. A mutant form of Shh (ShhN E90A), that only binds to Ptch1 but not to Boc, fails to induce Ptch1 internalization and also fails to activate non-canonical Shh signaling, indicating that Shh binding to Ptch1 alone may not be sufficient to trigger Ptch1 internalization (Ferent et al., 2019). Thus, with the help of Numb, Boc links Ptch1 to the endocytic machinery to induce Shh signal transduction.
Proteolytic processing
In addition to limiting Slit responsiveness, pre-crossing CAs also suppress their responsiveness to Sema3B and this process also appears to be regulated at the post-translational level. For instance, PlexinA1, one of the receptors for Sema3B, is degraded by Calpain proteases in pre-crossing CAs (Nawabi et al., 2010). When CAs are approaching the floor plate, they express low levels of PlexinA1. Upon reaching the floor plate, PlexinA1 levels are up-regulated and this temporal expression of Plexin A1 appears to be controlled by Calpain mediated Plexin A1 proteolysis (Figure 4A). Spinal CAs in which endogenous Calpain protease activity is inhibited, undergo growth cone collapse in response to Sema3B. Dorsal spinal cord open book preparations isolated from mice that have been treated with a Calpain inhibitor show pre-crossing CA defects with axons stalling at the FP entry. This suggests that increased PlexinA1 cell surface levels upon Calpain protease inactivation causes CAs to acquire premature sensitivity to Sema3B (Nawabi et al., 2010). As CAs approach the FP, Calpain activity is inhibited by a FP-derived factor, NrCAM (Nawabi et al., 2010). However, the mechanism by which NrCAM antagonizes Calpain protease activity is unknown. Thus, FP contact and Calpain inactivation stabilizes PlexinA1 expression on the growth cone surface, which subsequently gains responsiveness to Sema3B and allows for exit from the FP (Figure 4A).
Figure 4: Mechanisms that regulate Semaphorin signaling in the mouse spinal cord.
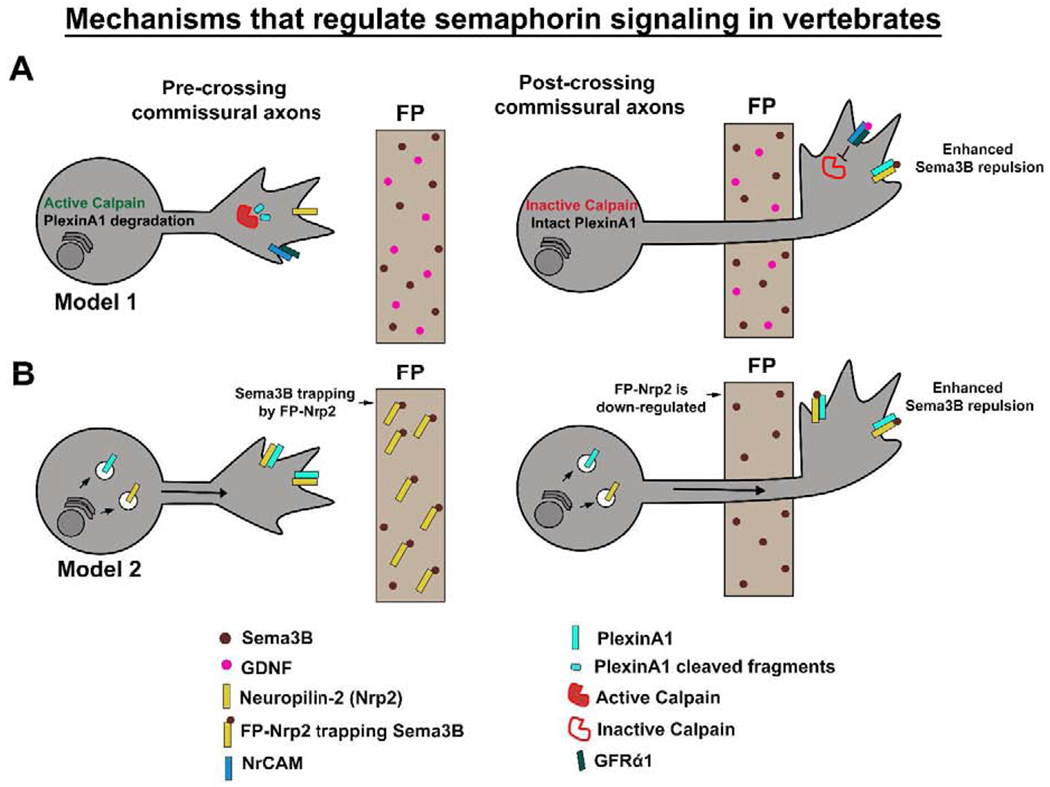
(A) As CAs approach the midline, Calpain cleaves PlexinA1 receptor to reduce Sema3B sensitivity. During midline crossing, Calpain activity is inhibited by FP secreted GDNF through NrCAM and its co-receptor GFRα1. This allows PlexinA1 to reach to the surface. PlexinA1 and Nrp2 then mediate Sema3B repulsion in post-crossing CAs. (B) In pre-crossing CAs, PlexinA1 and Nrp2 are expressed at the growth cone surface and their sensitivity to Sema3B is inhibited by FP-Nrp2. After midline crossing, FP-Nrp2 is down-regulated, which releases Sema3B and allows repulsion. CAs, commissural axons; FP, floor plate.
In contrast to the low levels of PlexinA1 in pre-crossing CAs observed in earlier studies (Nawabi et al., 2010), a subsequent study from the Tran lab suggests that PlexinA1 is strongly expressed in pre-crossing CAs in the mouse spinal cord (Hernandez-Enriquez et al., 2015). This observation raises questions about the role of Calpain mediated PlexinA1 down-regulation in pre-crossing CAs. This study also shows that Neuropilin-2 (Nrp2) and PlexinA1 are co-expressed in pre-crossing CAs and these axons are responsive to Sema3B in vitro (Hernandez-Enriquez et al., 2015). Again, this observation contradicts earlier findings from both the Castellani and Tessier-Lavigne labs, which showed that CAs isolated from pre-crossing stages of spinal cord development are not responsive to Sema3B. Interestingly, Hernandez-Enriquez et al. find that FP-derived, but not axon-derived Nrp2 is required to suppress premature Sema3B induced repulsion in pre-crossing CAs, thereby promoting midline crossing (Figure 4B). Nrp2 is expressed at the FP as early as E9.5 and reaches high levels at E11.5. Nrp2 expression decreases significantly by E13.5, at which point the majority of the CAs have crossed the midline, although it is unclear how the effect of FP-Nrp2 is limited to pre-crossing CAs, since there are still significant levels of FP-expressed NRP-2 at E11.5 and E12.5, when many axons have already crossed. FP specific deletion of Nrp2 causes a reduction in midline crossing, as evidenced by a decrease in commissure thickness. This reduction in commissure thickness is suppressed by the removal of PlexinA1 (Hernandez-Enriquez et al., 2015), suggesting that FP-derived Nrp2 antagonizes Sema3B-PlexinA1 repulsion. Based on these observations, it was proposed that FP-derived Nrp2 functions as a Sema3B sink and that the specific deletion of Nrp2 from the FP results in the release of Sema3B and premature repulsion; however, there is no clear evidence that removal of FP-derived Nrp2 actually results in a change in Sema3B protein distribution. An alternative possibility is that the FP-Nrp2 can facilitate CA midline crossing through interactions with axonal Nrp2. For example, FP-derived Nrp2 may prevent Sema3B responsiveness in pre-crossing CAs by forming trans-interactions with axonal Nrp2 (Chen et al., 1998), similar to the mechanism that has been recently proposed in Drosophila, where Robo2 expressed in midline cells acts to inhibit Slit-Robo1 repulsion (Evans et al., 2015) (Figure 3A). Trans interactions between FP-Nrp2 and axonal Nrp2 could potentially prevent complex formation between axonal Nrp2 and PlexinA1, thereby suppressing Sema3B responsiveness during midline crossing.
The apparent discrepancy in PlexinA1 expression in pre-crossing CAs from these two groups might depend on the epitope specificity of the PlexinA1 antibody used in the study. Nawabi et al. used a PlexinA1 antibody raised against the N-terminal region of PlexinA1, whereas Hernandez-Enriquez et al. used a PlexinA1 antibody that was raised against the C-terminal region. It is possible that the PlexinA1 antibody used by Hernandez-Enriquez et al. might be detecting cleaved PlexinA1 fragments generated by Calpain activity in pre-crossing CAs. It is also possible that these two regulatory mechanisms may act independently in distinct subsets of CAs to control Semaphorin repulsion.
Recently, the spatial and temporal cell surface sorting of repulsive guidance receptors PlexinA1, Nrp2, and Robo1/2 during spinal CA navigation in chick and mouse embryos has been characterized in vivo (Pignata et al., 2019). Using elegant live imaging studies with pHLuorin-tagged receptors, the sequential sorting of repulsive guidance receptors at the commissural growth cone surface was observed, suggesting that this sequential sorting may control specific functions for midline repellents during and after midline crossing (Pignata et al., 2019). Nrp2 is expressed at the growth cone surface throughout CA floor plate navigation, whereas the surface expression of PlexinA1 is detected only when CAs navigate the first half of the FP, thus providing additional evidence for PlexinA1 expression in crossing and post-crossing CAs but not in pre-crossing CAs (Pignata et al., 2019). Robo1 sorts to the growth cone surface when CAs navigate the second half of the FP whereas Robo2 is specifically sorted in post-crossing CAs. Further, super resolution microscopy reveals that PlexinA1 and Robo1 receptors sort to distinct sub-domains in commissural growth cones and that this difference in spatial compartmentalization appears to be regulated at the level of membrane insertion. The FP stalling phenotype in Robo1/2 mutants and the premature turning phenotype in PlxnA1 mutants is rescued by the expression of pHLuorin-Robo1 and pHLuorin-PlexinA1 respectively (Pignata et al., 2019). Importantly, the sorting pattern of these pHLuo-tagged receptors when expressed in mutant commissural growth cones is comparable to when they are expressed in wild-type growth cones, suggesting that the expression level of pHLuorin-tagged receptors is likely to recapitulate endogenous receptor dynamics. This study reveals a unique spatial and temporal sequence of repulsive guidance receptors at the growth cone surface during midline navigation. Yet, the mechanism underlying this differential sorting and how the precise spatio-temporal differences of receptor sorting coordinate with ligand distributions to elicit guidance responses remain to be determined.
Several studies have shown the importance of ectodomain shedding of axon guidance receptors in regulating growth cone responsiveness to guidance cues. In particular, ADAM metalloprotease mediated receptor shedding and its role in axon guidance has been extensively characterized both in invertebrates and vertebrates. Studies in Drosophila have reported that Kuzbanian/ADAM10 acts as a positive regulator for Slit-Robo repulsive signaling (Coleman et al., 2010). The ADAM10 protease mediated shedding of cell adhesion molecules L1CAM and N-Cadherin is important to regulate mouse retinal ganglion cell (RGC) axon pathfinding (Marcos et al., 2015). Furthermore, by targeting both the receptor and ligand in the context of ephrin-Eph signaling, ADAM10 plays a role in the termination of axon extension (Hattori et al., 2000). ADAM10 can promote a developmental switch in responsiveness to the axonal repellant Sema3A by cleaving the extracellular domain of the Neuropilin1 receptor in mouse sensory neurons (Romi et al., 2014). Additionally, many groups have suggested a role for sequential cleavage of axon guidance receptors by metalloproteases and gamma secretases in axon guidance. The best studied axon guidance receptors that undergo this sequential proteolytic cleavage are DCC and Eph (Bai and Pfaff, 2011; Galko and Tessier-Lavigne, 2000; Georgakopoulos et al., 2006; Litterst et al., 2007; Neuhaus-Follini and Bashaw, 2015b; Taniguchi et al., 2003). Nevertheless, the molecular mechanisms that regulate the spatio-temporal distribution and the activity of proteases are not well studied.
Receptor-receptor interactions
Interactions (cis or trans) between axon guidance receptors can also contribute to the change in axon responsiveness in CAs. For instance, in Drosophila, Robo2 binds to Robo1 and acts intrans to inhibit Robo1 repulsion in pre-crossing CAs (Figure 3A). In vivo gain-of-function and rescue experiments suggest that the extracellular domains of Robo2 are required for the Robo1 interaction and specifically, the Ig2 domain of Robo2 is crucial for its ability to promote midline crossing (Evans and Bashaw, 2010b; Evans et al., 2015). In vertebrates, many axon guidance receptors form complexes through direct interactions with each other and respond cooperatively to extracellular guidance cues. For instance, in cultured Xenopus spinal axons, Netrin-DCC mediated chemoattractive response is silenced by the interactions between the cytoplasmic domains of Robo and DCC (Cooper, 2002; Stein and Tessier-Lavigne, 2001). In addition, it has also been suggested that the Netrin-DCC attractive response converts to repulsion by the direct interactions between the cytoplasmic domains of DCC and UNC5 (Hong et al., 1999). However, in vivo importance of these receptor-receptor interactions in CA midline crossing have not been tested. One recent study suggests that Netrin-DCC mediated attractive signaling is enhanced by the intracellular interactions between Robo3 and DCC (Zelina et al., 2014). Recent studies also present evidence for the role of homotypic Eph-Eph receptor interactions in receptor pre-clustering, which ensures the efficient activation of Eph/ephrin signaling (Nikolov et al., 2014). Finally, receptor interactions between PlexinA and Neuropilin are crucial to transduce the Sema3B and Sema3F repulsive guidance response in post-crossing CAs (Hernandez-Enriquez et al., 2015; Nawabi et al., 2010; Rohm et al., 2000).
Conclusion
In the past few years, significant progress has been made in understanding the molecular mechanisms that regulate CA responsiveness during midline crossing, which requires the proper coordination of multiple attractive and repulsive signaling pathways. A number of regulatory molecules have been identified to control the temporal expression and activity of the same axon guidance receptor on the growth cone membrane, suggesting a redundancy in axon guidance regulation. For instance, Slit-Robo1 repulsion in pre-crossing CAs has been shown to be regulated by several molecular mechanisms both in vertebrates and invertebrates, which begs the question of why there is a necessity for multiple mechanisms to control the same guidance receptor? It is possible that these redundant pathways may control the same receptor in distinct subsets of CAs. Analyzing guidance defects by depleting these regulatory molecules specifically in commissural neuron subtypes with subtype-specific Cre drivers will provide a better insight into regional diversity in CA guidance mechanisms.
Furthermore, there is a need to better understand the regulatory mechanisms that determine the differential expression pattern for molecules that modulate axon guidance receptor levels on growth cone membranes. For example, molecules that control surface Robo1 expression such as Comm in Drosophila, miR-92 in chick, or Ndfip in mouse are primarily expressed in pre-crossing CAs to suppress Slit-Robo1 repulsion, and their expression is down-regulated in post-crossing CAs. Since there is evidence for transcriptional regulation of Comm by the intracellular domain of Fra in Drosophila, it will be interesting to determine if transcriptional regulation also plays a role in controlling the expression of these other regulatory molecules in vertebrates. It is possible that the activity of these molecules may also be determined by post-translational modifications. The continued investigation of the regulation of axon responsiveness at intermediate targets in invertebrate and vertebrate model systems will undoubtedly offer novel insights into the complex biology of neural circuit assembly, and should elucidate general regulatory mechanisms that are likely to play fundamental roles in the development and function of diverse types of organs and tissues.
Table 1:
In this table we present a summary of the regulatory factors that are discussed in detail in this review. For each factor, we include the axon guidance receptor which is regulated, the proposed molecular mechanism of regulation, the model system, as well as the type of in vivo evidence that supports the proposed regulatory mechanisms.
| Factor | Receptor | Mechanism | System | in vivo evidence |
|---|---|---|---|---|
| Comm | Robo1 | Down regulates dRobo1 by targetting it to late endosomes | Drosophila | Genetic ablation |
| Ndfip1/2 | Robo1 | Down regulates hRobo1 by targeting it for endosomal degradation | Mouse | Genetic ablation |
| Prrg4 | Robo1 | Down regulates rRobo1 (unknown mechanism) | Rat | Mis-expression in Drosophila |
| miR-92 | Robo1 | Down regulates Robo1 by translational repression | Chick | shRNA |
| Arf6&Cyth1/3 | Robo1 | Promotes Endocytic recycling of Robo1 | Mouse | Genetic ablation |
| Nova1/2 | Robo1 | Regulates Robo1 activity by alternative splicing | Mouse | Genetic ablation |
| Nova1/2 | DCC | Regulates DCC activity by alternative splicing | Mouse | Genetic ablation |
| Robo3.1 | Robo1/2 | Inhibits Robo1/2 repulsion (unknown mechanism) | Mouse | Genetic ablation |
| RabGDI/Cst1 | Robo1 | Regulates repulsion by promoting membrane insertion of Robo1 | Chick | shRNA |
| Robo2 | Robo1 | Inhibits Robo1 repulsion through binding to the Robo1 ectodomain | Drosophila | Genetic ablation |
| Shisa2 | Fzd3 | Prevents Fzd3 membrane presentation | Mouse/Rat | shRNA |
Highlights.
Conserved mechanisms of axon guidance at the midline are explored
Advances in understanding how axons change their responsiveness are highlighted
Diverse strategies for regulation of axon guidance receptors are discussed
Acknowledgements
We thank members of the Bashaw Lab for critical reading of the manuscript. Research in the Bashaw Lab is supported by grants from the NIH (R35 NS097340) and NSF (IOS-1853719).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alther TA, Domanitskaya E, Stoeckli ET, 2016. Calsyntenin 1-mediated trafficking of axon guidance receptors regulates the switch in axonal responsiveness at a choice point. Development 143, 994–1004. [DOI] [PubMed] [Google Scholar]
- Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S, 1999. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron 24, 127–141. [DOI] [PubMed] [Google Scholar]
- Aviles EC, Stoeckli ET, 2016. Canonical wnt signaling is required for commissural axon guidance. Dev Neurobiol 76, 190–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai G, Pfaff SL, 2011. Protease regulation: the Yin and Yang of neural development and disease. Neuron 72, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak R, Yom-Tov G, Guez-Haddad J, Gasri-Plotnitsky L, Maimon R, Cohen-Berkman M, McCarthy AA, Perlson E, Henis-Korenblit S, Isupov MN, Opatowsky Y, 2019. Structural Principles in Robo Activation and Auto-inhibition. Cell 177, 272–285 e216. [DOI] [PubMed] [Google Scholar]
- Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardo M, Stoeckli ET, 2005. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat Neurosci 8, 297–304. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Dodd J, 1990. Guidance of commissural growth cones at the floor plate in embryonic rat spinal cord. Development 109, 435–447. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T, 1999. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96, 795–806. [DOI] [PubMed] [Google Scholar]
- Butler SJ, Dodd J, 2003. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron 38, 389–401. [DOI] [PubMed] [Google Scholar]
- Chance RK, Bashaw GJ, 2015. Slit-Dependent Endocytic Trafficking of the Robo Receptor Is Required for Son of Sevenless Recruitment and Midline Axon Repulsion. PLoS Genet 11, e1005402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M, 2003. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell 113, 11–23. [DOI] [PubMed] [Google Scholar]
- Chen H, He Z, Bagri A, Tessier-Lavigne M, 1998. Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron 21, 1283–1290. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M, 2008. Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron 58, 325–332. [DOI] [PubMed] [Google Scholar]
- Colak D, Ji SJ, Porse BT, Jaffrey SR, 2013. Regulation of axon guidance by compartmentalized nonsense-mediated mRNA decay. Cell 153, 1252–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman HA, Labrador JP, Chance RK, Bashaw GJ, 2010. The Adam family metalloprotease Kuzbanian regulates the cleavage of the roundabout receptor to control axon repulsion at the midline. Development 137, 2417–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HM, 2002. Axon guidance receptors direct growth cone pathfinding: rivalry at the leading edge. Int J Dev Biol 46, 621–631. [PubMed] [Google Scholar]
- de Ramon Francas G, Zuniga NR, Stoeckli ET, 2017. The spinal cord shows the way - How axons navigate intermediate targets. Dev Biol 432, 43–52. [DOI] [PubMed] [Google Scholar]
- Dickson BJ, Gilestro GF, 2006. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol 22, 651–675. [DOI] [PubMed] [Google Scholar]
- Dickson BJ, Zou Y, 2010. Navigating intermediate targets: the nervous system midline. Cold Spring Harb Perspect Biol 2, a002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM, 1988. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron 1, 105–116. [DOI] [PubMed] [Google Scholar]
- Dominici C, Moreno-Bravo JA, Puiggros SR, Rappeneau Q, Rama N, Vieugue P, Bernet A, Mehlen P, Chedotal A, 2017. Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature 545, 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Bashaw GJ, 2010a. Axon guidance at the midline: of mice and flies. Curr Opin Neurobiol 20, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Bashaw GJ, 2010b. Functional diversity of Robo receptor immunoglobulin domains promotes distinct axon guidance decisions. Curr Biol 20, 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Bashaw GJ, 2012. Slit/Robo-mediated axon guidance in Tribolium and Drosophila: divergent genetic programs build insect nervous systems. Dev Biol 363, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Santiago C, Arbeille E, Bashaw GJ, 2015. Robo2 acts in trans to inhibit Slit-Robo1 repulsion in pre-crossing commissural axons. Elife 4, e08407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferent J, Giguere F, Jolicoeur C, Morin S, Michaud JF, Makihara S, Yam PT, Cayouette M, Charron F, 2019. Boc Acts via Numb as a Shh-Dependent Endocytic Platform for Ptch1 Internalization and Shh-Mediated Axon Guidance. Neuron 102, 1157–1171 e1155. [DOI] [PubMed] [Google Scholar]
- Friocourt F, Chedotal A, 2017. The Robo3 receptor, a key player in the development, evolution, and function of commissural systems. Dev Neurobiol 77, 876–890. [DOI] [PubMed] [Google Scholar]
- Galko MJ, Tessier-Lavigne M, 2000. Function of an axonal chemoattractant modulated by metalloprotease activity. Science 289, 1365–1367. [DOI] [PubMed] [Google Scholar]
- Garbe DS, O’Donnell M, Bashaw GJ, 2007. Cytoplasmic domain requirements for Frazzled-mediated attractive axon turning at the Drosophila midline. Development 134, 4325–4334. [DOI] [PubMed] [Google Scholar]
- Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK, 2006. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J 25, 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilestro GF, 2008. Redundant mechanisms for regulation of midline crossing in Drosophila. PLoS One 3, e3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorla M, Santiago C, Chaudhari K, Layman AAK, Oliver PM, Bashaw GJ, 2019. Ndfip Proteins Target Robo Receptors for Degradation and Allow Commissural Axons to Cross the Midline in the Developing Spinal Cord. Cell Rep 26, 3298–3312 e3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski PJ, Black DL, 2001. Alternative RNA splicing in the nervous system. Prog Neurobiol 65, 289–308. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Shearwin-Whyatt LM, Fotia A, Parton RG, Kumar S, 2002. N4WBP5, a potential target for ubiquitination by the Nedd4 family of proteins, is a novel Golgi-associated protein. J Biol Chem 277, 9307–9317. [DOI] [PubMed] [Google Scholar]
- Hattori M, Osterfield M, Flanagan JG, 2000. Regulated cleavage of a contact-mediated axon repellent. Science 289, 1360–1365. [DOI] [PubMed] [Google Scholar]
- Hernandez-Enriquez B, Wu Z, Martinez E, Olsen O, Kaprielian Z, Maness PF, Yoshida Y, Tessier-Lavigne M, Tran TS, 2015. Floor plate-derived neuropilin-2 functions as a secreted semaphorin sink to facilitate commissural axon midline crossing. Genes Dev 29, 2617–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Fleming M, Rohrbach EW, Bashaw GJ, 2017. Sema-1a Reverse Signaling Promotes Midline Crossing in Response to Secreted Semaphorins. Cell Rep 18, 174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E, 1999. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97, 927–941. [DOI] [PubMed] [Google Scholar]
- Islam SM, Shinmyo Y, Okafuji T, Su Y, Naser IB, Ahmed G, Zhang S, Chen S, Ohta K, Kiyonari H, Abe T, Tanaka S, Nishinakamura R, Terashima T, Kitamura T, Tanaka H, 2009. Draxin, a repulsive guidance protein for spinal cord and forebrain commissures. Science 323, 388–393. [DOI] [PubMed] [Google Scholar]
- Jaworski A, Tom I, Tong RK, Gildea HK, Koch AW, Gonzalez LC, Tessier-Lavigne M, 2015. Operational redundancy in axon guidance through the multifunctional receptor Robo3 and its ligand NELL2. Science 350, 961–965. [DOI] [PubMed] [Google Scholar]
- Johnson V, Junge HJ, Chen Z, 2019. Temporal regulation of axonal repulsion by alternative splicing of a conserved microexon in mammalian Robo1 and Robo2. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice ED, Barnum SJ, Kidd T, 2017. The WAGR syndrome gene PRRG4 is a functional homologue of the commissureless axon guidance gene. PLoS Genet 13, e1006865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprielian Z, Runko E, Imondi R, 2001. Axon guidance at the midline choice point. Dev Dyn 221, 154–181. [DOI] [PubMed] [Google Scholar]
- Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, Technau GM, Dickson BJ, 2002. Comm sorts robo to control axon guidance at the Drosophila midline. Cell 110, 415–427. [DOI] [PubMed] [Google Scholar]
- Keleman K, Ribeiro C, Dickson BJ, 2005. Comm function in commissural axon guidance: cell-autonomous sorting of Robo in vivo. Nat Neurosci 8, 156–163. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M, 1994. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78, 425–435. [DOI] [PubMed] [Google Scholar]
- Kidd T, Russell C, Goodman CS, Tear G, 1998. Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron 20, 25–33. [DOI] [PubMed] [Google Scholar]
- Kinoshita-Kawada M, Hasegawa H, Hongu T, Yanagi S, Kanaho Y, Masai I, Mishima T, Chen X, Tsuboi Y, Rao Y, Yuasa-Kawada J, Wu JY, 2019. A crucial role for Arf6 in the response of commissural axons to Slit. Development 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggere JC, Saito Y, Darnell RB, Tessier-Lavigne M, Junge HJ, Chen Z, 2016. NOVA regulates Dcc alternative splicing during neuronal migration and axon guidance in the spinal cord. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liu S, Lei Y, Cheng Y, Yao C, Zhen X, 2014. Robo3.1A suppresses slit-mediated repulsion by triggering degradation of Robo2. J Neurosci Res 92, 835–846. [DOI] [PubMed] [Google Scholar]
- Li Q, Lee JA, Black DL, 2007. Neuronal regulation of alternative pre-mRNA splicing. Nat Rev Neurosci 8, 819–831. [DOI] [PubMed] [Google Scholar]
- Litterst C, Georgakopoulos A, Shioi J, Ghersi E, Wisniewski T, Wang R, Ludwig A, Robakis NK, 2007. Ligand binding and calcium influx induce distinct ectodomain/gamma-secretase-processing pathways of EphB2 receptor. J Biol Chem 282, 16155–16163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M, 2004. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron 42, 213–223. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y, 2003. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302, 1984–1988. [DOI] [PubMed] [Google Scholar]
- Marcos S, Nieto-Lopez F, Sandonis A, Cardozo MJ, Di Marco F, Esteve P, Bovolenta P, 2015. Secreted frizzled related proteins modulate pathfinding and fasciculation of mouse retina ganglion cell axons by direct and indirect mechanisms. J Neurosci 35, 4729–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Doyle JL, Serafini T, Kennedy TE, Tessier-Lavigne M, Goodman CS, Dickson BJ, 1996. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron 17, 203–215. [DOI] [PubMed] [Google Scholar]
- Moore SW, Tessier-Lavigne M, Kennedy TE, 2007. Netrins and their receptors. Adv Exp Med Biol 621, 17–31. [DOI] [PubMed] [Google Scholar]
- Moreno-Bravo JA, Roig Puiggros S, Mehlen P, Chedotal A, 2019. Synergistic Activity of Floor-Plate- and Ventricular-Zone-Derived Netrin-1 in Spinal Cord Commissural Axon Guidance. Neuron 101, 625–634 e623. [DOI] [PubMed] [Google Scholar]
- Mund T, Pelham HR, 2009. Control of the activity of WW-HECT domain E3 ubiquitin ligases by NDFIP proteins. EMBO Rep 10, 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myat A, Henry P, McCabe V, Flintoft L, Rotin D, Tear G, 2002. Drosophila Nedd4, a ubiquitin ligase, is recruited by Commissureless to control cell surface levels of the roundabout receptor. Neuron 35, 447–459. [DOI] [PubMed] [Google Scholar]
- Nawabi H, Briancon-Marjollet A, Clark C, Sanyas I, Takamatsu H, Okuno T, Kumanogoh A, Bozon M, Takeshima K, Yoshida Y, Moret F, Abouzid K, Castellani V, 2010. A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev 24, 396–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus-Follini A, Bashaw GJ, 2015a. Crossing the embryonic midline: molecular mechanisms regulating axon responsiveness at an intermediate target. Wiley Interdiscip Rev Dev Biol 4, 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus-Follini A, Bashaw GJ, 2015b. The Intracellular Domain of the Frazzled/DCC Receptor Is a Transcription Factor Required for Commissural Axon Guidance. Neuron 87, 751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolov DB, Xu K, Himanen JP, 2014. Homotypic receptor-receptor interactions regulating Eph signaling. Cell Adh Migr 8, 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK, 2006. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature 444, 369–373. [DOI] [PubMed] [Google Scholar]
- Onishi K, Zou Y, 2017. Sonic Hedgehog switches on Wnt/planar cell polarity signaling in commissural axon growth cones by reducing levels of Shisa2. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak JS, DeLoughery ZJ, Wang J, Acharya N, Park Y, Jaworski A, Ozkan E, 2020. NELL2-Robo3 complex structure reveals mechanisms of receptor activation for axon guidance. Nat Commun 11, 1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp M, Niederkofler V, Debrunner M, Alther T, Kunz B, Stoeckli ET, 2012. RabGDI controls axonal midline crossing by regulating Robo1 surface expression. Neural Dev 7, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignata A, Ducuing H, Boubakar L, Gardette T, Kindbeiter K, Bozon M, Tauszig-Delamasure S, Falk J, Thoumine O, Castellani V, 2019. A Spatiotemporal Sequence of Sensitization to Slits and Semaphorins Orchestrates Commissural Axon Navigation. Cell Rep 29, 347–362 e345. [DOI] [PubMed] [Google Scholar]
- Pignata A, Ducuing H, Castellani V, 2016. Commissural axon navigation: Control of midline crossing in the vertebrate spinal cord by the semaphorin 3B signaling. Cell Adh Migr 10, 604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert C, Kunz T, Harris KL, Whitington PM, Technau GM, 2011. Morphological characterization of the entire interneuron population reveals principles of neuromere organization in the ventral nerve cord of Drosophila. J Neurosci 31, 15870–15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohm B, Ottemeyer A, Lohrum M, Puschel AW, 2000. Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech Dev 93, 95–104. [DOI] [PubMed] [Google Scholar]
- Romi E, Gokhman I, Wong E, Antonovsky N, Ludwig A, Sagi I, Saftig P, Tessier-Lavigne M, Yaron A, 2014. ADAM metalloproteases promote a developmental switch in responsiveness to the axonal repellant Sema3A. Nat Commun 5, 4058. [DOI] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Fabre PJ, Knevels E, Coulon C, Segura I, Haddick PC, Aerts L, Delattin N, Strasser G, Oh WJ, Lange C, Vinckier S, Haigh J, Fouquet C, Gu C, Alitalo K, Castellani V, Tessier-Lavigne M, Chedotal A, Charron F, Carmeliet P, 2011. VEGF mediates commissural axon chemoattraction through its receptor Flk1. Neuron 70, 966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Le M, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M, 2004. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell 117, 157–169. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M, 1996. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001–1014. [DOI] [PubMed] [Google Scholar]
- Shearwin-Whyatt LM, Brown DL, Wylie FG, Stow JL, Kumar S, 2004. N4WBP5A (Ndfip2), a Nedd4-interacting protein, localizes to multivesicular bodies and the Golgi, and has a potential role in protein trafficking. J Cell Sci 117, 3679–3689. [DOI] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M, 2001. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science 291, 1928–1938. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Kim SH, Sisodia SS, 2003. Presenilin-dependent “gamma-secretase” processing of deleted in colorectal cancer (DCC). J Biol Chem 278, 30425–30428. [DOI] [PubMed] [Google Scholar]
- Tear G, Harris R, Sutaria S, Kilomanski K, Goodman CS, Seeger MA, 1996. commissureless controls growth cone guidance across the CNS midline in Drosophila and encodes a novel membrane protein. Neuron 16, 501–514. [DOI] [PubMed] [Google Scholar]
- Tulloch AJ, Teo S, Carvajal BV, Tessier-Lavigne M, Jaworski A, 2019. Diverse spinal commissural neuron populations revealed by fate mapping and molecular profiling using a novel Robo3(Cre) mouse. J Comp Neurol 527, 2948–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallstedt A, Kullander K, 2013. Dorsally derived spinal interneurons in locomotor circuits. Ann N Y Acad Sci 1279, 32–42. [DOI] [PubMed] [Google Scholar]
- Varadarajan SG, Kong JH, Phan KD, Kao TJ, Panaitof SC, Cardin J, Eltzschig H, Kania A, Novitch BG, Butler SJ, 2017. Netrin1 Produced by Neural Progenitors, Not Floor Plate Cells, Is Required for Axon Guidance in the Spinal Cord. Neuron 94, 790–799 e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NH, Stoeckli ET, 2013. Sonic hedgehog regulates its own receptor on postcrossing commissural axons in a glypican1-dependent manner. Neuron 79, 478–491. [DOI] [PubMed] [Google Scholar]
- Xu K, Wu Z, Renier N, Antipenko A, Tzvetkova-Robev D, Xu Y, Minchenko M, Nardi-Dei V, Rajashankar KR, Himanen J, Tessier-Lavigne M, Nikolov DB, 2014. Neural migration. Structures of netrin-1 bound to two receptors provide insight into its axon guidance mechanism. Science 344, 1275–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam PT, Kent CB, Morin S, Farmer WT, Alchini R, Lepelletier L, Colman DR, Tessier-Lavigne M, Fournier AE, Charron F, 2012. 14-3-3 proteins regulate a cell-intrinsic switch from sonic hedgehog-mediated commissural axon attraction to repulsion after midline crossing. Neuron 76, 735–749. [DOI] [PubMed] [Google Scholar]
- Yang L, Garbe DS, Bashaw GJ, 2009. A frazzled/DCC-dependent transcriptional switch regulates midline axon guidance. Science 324, 944–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Huang H, Shao Q, Yee S, Majumder T, Liu G, 2018. miR-92 Suppresses Robo1 Translation to Modulate Slit Sensitivity in Commissural Axon Guidance. Cell Rep 24, 2694–2708 e2696. [DOI] [PubMed] [Google Scholar]
- Yuva-Aydemir Y, Xu XL, Aydemir O, Gascon E, Sayin S, Zhou W, Hong Y, Gao FB, 2015. Downregulation of the Host Gene jigr1 by miR-92 Is Essential for Neuroblast Self-Renewal in Drosophila. PLoS Genet 11, e1005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelina P, Blockus H, Zagar Y, Peres A, Friocourt F, Wu Z, Rama N, Fouquet C, Hohenester E, Tessier-Lavigne M, Schweitzer J, Roest Crollius H, Chedotal A, 2014. Signaling switch of the axon guidance receptor Robo3 during vertebrate evolution. Neuron 84, 1258–1272. [DOI] [PubMed] [Google Scholar]
- Zheng S, 2020. Alternative splicing programming of axon formation. Wiley Interdiscip Rev RNA, e1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang M, Li X, Zhu J, Zhang J, Niu F, Liang F, Chen M, Li D, Han P, Ji SJ, 2019. The m6A reader YTHDF1 regulates axon guidance through translational control of Robo3.1 expression. Nucleic Acids Res 47, 4765–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M, 2000. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell 102, 363–375. [DOI] [PubMed] [Google Scholar]


